Abstract
Eyeblink and postauricular reflexes to standardized affective images were examined in individuals without (n = 37) and with (n = 20) autism spectrum disorders (ASDs). Affective reflex modulation in control participants replicated previous findings. The ASD group, however, showed anomalous reflex modulation patterns, despite similar self-report ratings of pictures. Specifically, the ASD group demonstrated exaggerated eyeblink responses to pleasant images and exaggerated postauricular responses to unpleasant images. Although ASD is often conceptualized in terms of specific deficits in affective responding in the social domain, the present results suggest a domain-general pattern of deficits in affective processing and that such deficits may arise at an early phase in the stream of information processing.
Keywords: Autism spectrum disorder, Affective startle modulation, Eyeblink, Postauricular
Introduction
Kanner's (1943) classic treatise on autism was titled “Autistic Disturbances of Affective Content.” Clinically, individuals with autism have minimal affective response to social aspects of their environment yet can exhibit extreme affect to ostensibly inconsequential nonsocial aspects of the environment. A child with autism may remain affectively flat even when enthusiastically greeted by his parent; however, the same child may become visibly excited at the sight of swirling water. This range of reactions may be troubling to caregivers, may lead to avoidance by peers, and detracts from a child's ability to learn from his social environment in an experience-dependent manner. Thus, the core features of the disorder may be conceptualized in terms of affective dysregulation. In addition, existing studies of psychiatric features confirm the presence of high rates of affective disturbances in autistic individuals and their families, and mood and associated behavioral problems are frequent targets for pharmacologic treatment in this population (Mazefsky et al. 2008; Bolton et al. 1998).
Despite the potential relation of dysregulated affective processes to the core features of autism, little is known about affective disturbances in this disorder. In contrast, the recent emergence of the field of social cognitive and affective neuroscience has suggested that deficits in affect regulation may characterize virtually all other DSM-IV Axis I and II disorders (Ochsner 2008). Outside of autism research, much is known about affective phenotypes, the neural circuitry involved in basic affective processes, the genetics of affective disorders, and both medical and psychological treatments that can be used to target affective disorders (Arinami et al. 1996; Fowles 1988; Depue and Iacono 1989; Larson et al. 2007). The goal of the present study was to assess basic affective processes in autism via an examination of affective modulation of the startle response, a well validated measure of affective processing. Ultimately, the study of neurobiologically-based responses to affective stimuli in general may elucidate neurobiological systems mediating social interaction deficits in autism spectrum disorders (ASD).
Consistent with a broad affective processing approach to understanding social cognition in ASD, Bachevalier and Loveland (2006) proposed that a critical but neglected component of biological accounts of social cognition deficits in ASD is impaired social-affective behavior that is mediated by the orbitofrontal-amygdala circuit. This circuit includes the ventromedial portion of the prefrontal cortex, the amygdala, and connections with the hypothalamus and brainstem (MacLean 1949; Papez 1937), as well as dorsal and ventral circuits that mediate online processing of sensory events and monitoring of emotional states, respectively. This model suggests that social cognitive deficits in ASD may reflect impaired responses to the broader category of affective stimuli, of which social stimuli are but one (albeit prominent) member. It also implies that measures sensitive to orbitofrontal-amygdala functioning may be well-matched to understanding social processing deficits in ASD.
Affective modulation of the startle reflex is particularly well suited to address the integrity of brain circuits mediating affective processing in ASD because the neurobiological mechanism mediating its modulation has been exquisitely documented (i.e., direct projections from the lateral and central nuclei of the amygdala to the nucleus reticularis pontis caudalis (Davis 1989; Davis et al. 1997; Hitchcock and Davis 1987; Fendt et al. 1994). Additionally, the superb temporal resolution of electromyography (EMG) allows for conclusions regarding functioning of these circuits that does not reflect feedback connections from other brain regions.
In the present study, affective modulation of the startle eyeblink and postauricular reflexes were assessed. The eyeblink reflex is recorded by positioning EMG recording sensors under the eye and measuring the vigor of the obligatory eyeblink response when the subject is mildly startled. A substantial body of nonhuman (Davis et al. 1993) and human (Lang et al. 1993a, b) research has demonstrated that responses to startle probes are modulated by emotional factors. In particular, affective context modulates the magnitude of the startle eyeblink reflex, a phenomenon believed to index defensive-protective tendencies (Lang et al. 1998; Bradley et al. 1993). When nonclinical samples view affective pictures, startle eyeblink response magnitudes are modulated by picture valence: unpleasant pictures potentiate and pleasant pictures attenuate the reflex, relative to neutral pictures (e.g., Bradley et al. 1993; Dichter et al. 2002). This linear1 pattern of valence-dependent startle modulation is thought to reflect the priming of neurobiologically-based defensive and appetitive systems by unpleasant and pleasant stimuli, respectively (Lang et al. 1998).
The startle eyeblink response is primarily an index of defensive priming and is not ideally suited to index approach-oriented states (Dillon and Labar 2005; Dichter and Tomarken 2008). This is at least in part because the inhibition of the startle blink during pleasant pictures reflects the continuation of an early attentional attenuation of startle blink magnitude during emotional pictures (Bradley et al. 1993). The postauricular (PA) reflex, however, appears to be ideally suited to index appetitive emotional states. The PA reflex is a vestigial muscle response in humans that acts to pull the ear backward (Gray 1901/1995; O'Beirne and Patuzzi 1999), and is recorded by positioning EMG recording sensors behind the ear and measuring the vigor of the PA muscle in response to a startle probe.
Because the PA reflex is also evoked by an acoustic probe stimulus, it may be assessed concurrently with the startle eyeblink response. The PA reflex tracks affective predispositions; however, the direction of the reflex-affect relation is generally the opposite to that observed for the eyeblink reflex: approach motivational states potentiate and defensive motivation states attenuate PA reflex magnitudes, respectively (Hess et al. 2007; Benning et al. 2004). It has been theorized that the neural circuit for the PA reflex parallels that of the eyeblink reflex circuit in terms of input (i.e., the cochlear nucleus) and output (i.e., the facial-motor nucleus), but it does not include the nucleus reticularis pontis caudalis—the juncture of the startle circuit at which input is received from the amygdala (Hackley 1993).
Startle eyeblink reflex modulation has received limited attention in the autism literature, and no study to date has investigated PA modulation in ASD. Bernier et al. (2005) reported intact fear-potentiated startle eyeblink responses in individuals with ASD using a fear-conditioning paradigm. The fear-potentiated startle paradigm indexes startle modulation to a cue conditioned to signal threat (e.g., an aversive air puff). These authors concluded that the capacity of the lateral and central nuclei of the amygdaloid nuclei to potentiate the startle reflex is intact in ASD. However, Wilbarger et al. (2009) investigated startle eyeblink modulation during emotional picture presentation in ASD and found that reflexes were larger to both pleasant and unpleasant pictures, compared to neutral pictures. The authors interpreted these findings to reflect an increase in defensive system activity in ASD, despite similar subjective image rating. However, this study did not measure the PA reflex and did not include subjective ratings of image arousal.
In the present study, modulation of the startle eyeblink and PA reflexes were assessed in response to normative pictorial stimuli drawn from the International Affective Picture System (IAPS, Lang et al. 2005). This paradigm extends the Bernier et al. (2005) and Wilbarger (2009) studies by recording both startle eyeblink and PA modulation in response to externally presented, normative emotional stimuli. Given that the PA reflex may be more sensitive to positive emotional states (Benning et al. 2004), a comprehensive assessment of affective startle modulation during both unpleasant and pleasant emotional states would optimally include measurement of PA reflex modulation.
Our primary hypotheses were that the ASD group would show non-linear affective modulation of the eyeblink and PA reflexes. These hypotheses are based on the findings of Wilbarger et al. (2009) as well as neuroimaging studies documenting anomalous affective modulation in ASD of regions mediating affective responses in a number of contexts (e.g., Baron-Cohen and Belmonte 2005; Schultz et al. 2000). We additionally had specific hypotheses regarding responses to pleasant stimuli based on evidence of reduced voluntary gaze toward social stimuli in ASD (Sasson et al. 2008) and of heightened arousal in response to social stimuli in ASD (Dalton et al. 2005). The conceptualization that social cognition deficits in ASD may reflect a broader dysfunction in processing putatively appetitive stimuli led us to hypothesize blunted startle modulation to pleasant images specifically. Secondary analyses concerned subjective affective responses to IAPS images. Because alterations in affective responding are typically evident in psychophysiological but not subjective measures in other disorders (Dichter and Tomarken 2008; Dichter et al. 2004), we hypothesized that group differences would be evident in psychophysiological, but not subjective, responses.
Method
Participants
Participants with ASD were recruited through the University of North Carolina (UNC) Autism Spectrum Disorders Registry in conjunction with regional TEACCH (Treatment and Education of Autistic and related Communication-handicapped CHildren) clinics. They received a DSM-IV (American Psychiatric Association 1994) clinical diagnosis of an ASD by an experienced clinician and met lifetime criteria for autism or ASD on the Autism Diagnostic Interview-Revised (ADI-R, Lord et al. 1994). Comorbidity information from the ASD sample was not collected. Typically-developing participants were recruited via mass emails sent to UNC faculty and staff, and verified during a phone screen that they did not have a history of any psychiatric or developmental disorder, were not taking psychotropic medications, and did not have an immediate family member with an ASD diagnosis. Participants received $20 for completing the startle session.
Data were not analyzable from four participants with ASD and one neurotypical participant because discomfort with skin abrasion yielded unacceptably high impedances (>50 kΩ). The final ASD sample consisted of 20 participants (3 women; age range: 10.1–17.4 years, mean = 12.4, SD = 1.8; diagnoses: eight autistic disorder, seven As-pergers, three PDD-NOS; two not specified). The final neurotypical sample consisted of 37 participants (4 women; age range: 10.7–30.4 years, mean = 17.4, SD = 4.8). Groups differed with respect to age, t(55) = 3.61, p < .0001 but not gender distribution, χ2(1) = 0.21, p > 0.65. Eight children with ASD were taking psychotropic medications (six were taking one medication and two were taking two medications; six were taking SSRIs, two were taking stimulants, one was taking an atypical antipsychotic, and one was taking an alpha-2 agonist).
Stimulus Materials
Each participant first viewed five neutral images presented with startle probes to allow the startle response to habituate (these trials were not included in analyses), followed by the experimental set which consisted of 50 images. Of these, 30 (10 each of pleasant, neutral, and unpleasant) were chosen from the IAPS (Lang et al. 2005) on the basis of their published affective valence and arousal ratings. To diminish predictability, eight of the ten pictures within each of the three categories were presented with startle probes. The remaining 20 images were created in-house and results from these trials are not reported here.2 Presentation was pseudo-random such that the same picture category was never repeated more than twice in a row and pictures of each category were equally distributed throughout the session. Pictures were displayed on a 17 in color monitor approximately 1.0 m in front of the participant.
Procedure
During the startle session, participants viewed pictures while EMG was recorded. Each trial consisted of 6 s picture presentation followed by an inter-trial (ITI) interval of 10 s. Acoustic startle probes of 50 ms, 100 dB white-noise bursts with instantaneous rise times were binaurally-presented 3,500–4,500 ms after picture onset (varied to reduce the predictability of the timing of the probes) via noise-cancelling headphones. Picture and probe presentation were controlled by the E-Prime v1.1 software package (Psychology Software Tools Inc., Pittsburgh, PA).
To diminish the predictability of the procedure, five probes, equally spaced throughout the session, were presented during ITI's. Pictures were presented in one of two counter-balanced orders to diminish the probability that startle modulation would be affected by the particular order of picture presentation. Pictures were selected on the basis of extreme normative IAPS ratings of valence and arousal separately for males and females, and separate male and female picture sets were used that were matched, picture-by-picture, with respect to published gender-specific normative IAPS ratings of valence and arousal. Two separate age-based sets were used to minimize potentially offensive material for those individuals under 18. This procedure yielded four sets of pictures (i.e., male adult, female adult, male child, and female child), each presented in two different orders. All sets were invariant with respect to the order of image valence categories. The average (SD) normative valence and arousal ratings of images within the four sets are shown in Table 1 and IAPS image numbers are listed in the Appendix.
Table 1.
Mean (SD) normative valence and arousal ratings for IAPS images shown to female adults, female children, male adults, and male children
| Valence | Arousal | |||||
|---|---|---|---|---|---|---|
| Pleasant | Neutral | Unpleasant | Pleasant | Neutral | Unpleasant | |
| Female adult | 7.51 (0.59) | 5.31 (0.87) | 2.17 (0.63) | 6.34 (0.62) | 2.91 (0.87) | 6.81 (0.49) |
| Female child | 7.39 (0.62) | 5.27 (0.67) | 2.87 (0.83) | 4.93 (0.88) | 2.63 (0.42) | 6.46 (0.89) |
| Male adult | 7.42 (0.43) | 5.03 (0.43) | 2.70 (0.50) | 6.56 (0.78) | 2.60 (0.39) | 6.64 (0.39) |
| Male child | 7.15 (0.42) | 4.94 (0.40) | 3.00 (0.86) | 5.02 (1.02) | 2.38 (0.83) | 6.77 (0.90) |
There were no significant differences between images set with respect to either valence or arousal ratings, p's > .05
Pictures were presented twice to each participant. During the first presentation, startle responses were recorded. Next, pictures were presented a second time without startle probes or recording electrodes and participants rated each picture with respect to pleasure and arousal using 9-point Likert scales. During the rating procedures, participants controlled the duration of picture exposure, though viewing time data were not recorded. The range and direction of the ratings for Valence were −4 (extremely unpleasant) to +4 (extremely pleasant) and for Arousal were 0 (not at all aroused) to +8 (extremely aroused).
Physiological Recording and Data Reduction
Startle responses were measured via EMG. Five Ag–AgCl electrodes with 4 mm inner diameter (BIOPAC Systems; Goleta, CA) were used: two were placed on the orbicularis oculi muscle below the left eye to record the eyeblink reflex; two were placed behind the ear to record the PA reflex (one electrode was placed directly adjacent to the tendon on the surface of the pinna, and the other electrode was placed on the scalp over the PA muscle per Sollers and Hackley (1997); and a ground electrode was attached to the forehead. Biopac tensive adhesive gel was used as a conductive electrolyte. The raw EMG signal was sampled at 200 Hz, and the gain was amplified by 2000. High-pass (50-Hz) and low-pass (500-Hz) filters were applied, and the data were rectified and integrated with AcqKnowledge software (BIOPAC Systems; Goleta, CA).
Peak EMG activity was calculated as the maximum EMG response within a window of 10–250 ms for eyeblink responses (Dichter and Tomarken 2008) and 8–30 ms for PA reflex responses (Benning et al. 2004) after probe onset. The baseline was calculated as the average EMG activity during the 50 ms before the onset of the probe. Reflex magnitudes were converted to T-scores on a within-participant basis for each measure (i.e., eyeblink and PA reflexes) to adjust for between-participants differences in response and baseline EMG amplitude (Funayama et al. 2001).3 Magnitude measures were averaged across each of the three levels of the Valence factor for each startle measure for each participant.
Data Analysis
The omnibus analyses of interest were mixed measures multivariate regressions performed separately on startle eyeblink and PA magnitudes with Group (neurotypical, ASD) as the between-participants factor and Valence (Unpleasant, Neutral, Pleasant) as the within-participants factor. Primary a priori analyses tested whether groups differed on planned linear Valence contrasts. For eyeblink responses, the linear trend coefficients were −1, 0, and +1, respectively, for pleasant, neutral, and unpleasant marginal means. Conversely, for PA and pleasure ratings responses, the linear trend coefficients were +1, 0, and −1, respectively, for pleasant, neutral, and unpleasant marginal means. Linear trend tests were followed by tests of differential modulation to unpleasant and pleasant images, each relative to neutral images, and finally tests of group differences in modulation to each image category alone were conducted.
For valence ratings, the a priori planned contrast was a linear trend test as well (trend coefficients: −1, 0, and +1). For arousal ratings, the a priori planned contrast was a quadratic, rather than linear, trend test (trend coefficients: +1, −2, and +1).
Results
Gender and Age Effects on Reflex Modulation
There were no significant main effects or interactions involving gender, age, and picture set on the primary dependent measures. In addition, age was not correlated with eyeblink or PA startle responses to the three picture categories or to differences between any picture categories, p's > .20. Thus, age and gender were excluded from all analyses reported below. However, because groups differed in age, we present selected supplementary omnibus analyses excluding neurotypical participants age 17 and older (n = 20).
Eyeblink Modulation
Figures 1 and 2 depict average startle and PA reflex responses, respectively, for both diagnostic groups. The Group × Valence ANOVA on eyeblink magnitudes revealed no main effect of Valence, F(2,54) = 0.58, p > .55, or Group, F(1,55) = 0.21, p > .65, but a Group × Valence interaction, F(2,54) = 3.78, p < .03. The overall linear Valence trend was non-significant, F(1,55) = 0.01, p > .97, but the Group × linear Valence trend interaction was significant, F(1,55) = 5.71, p < .05. Between groups t-tests on eyeblink differences during pleasant and unpleasant images, relative to during neutral images, revealed that groups differed in modulation during pleasant images, p < .05, but not unpleasant images, p > .50.
Fig. 1.
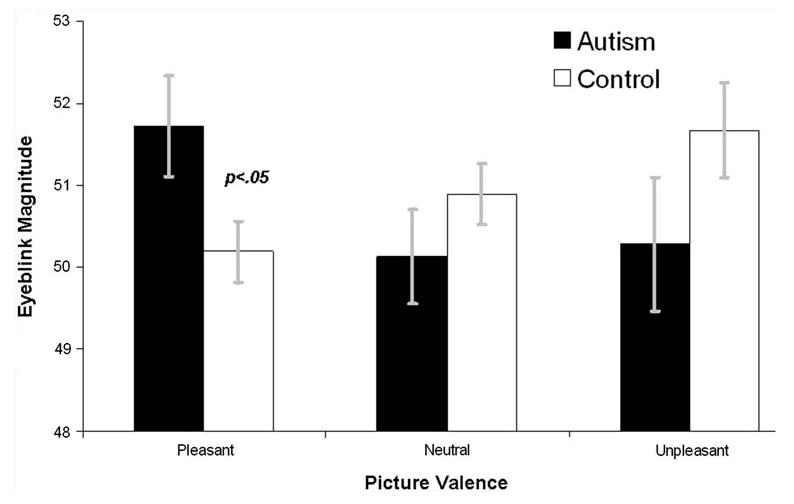
Startle eyeblink reflex responses during affective pictures by both diagnostic groups. Error bars are standard errors of the mean. Magnitude is expressed as within subjects T-scores
Fig. 2.
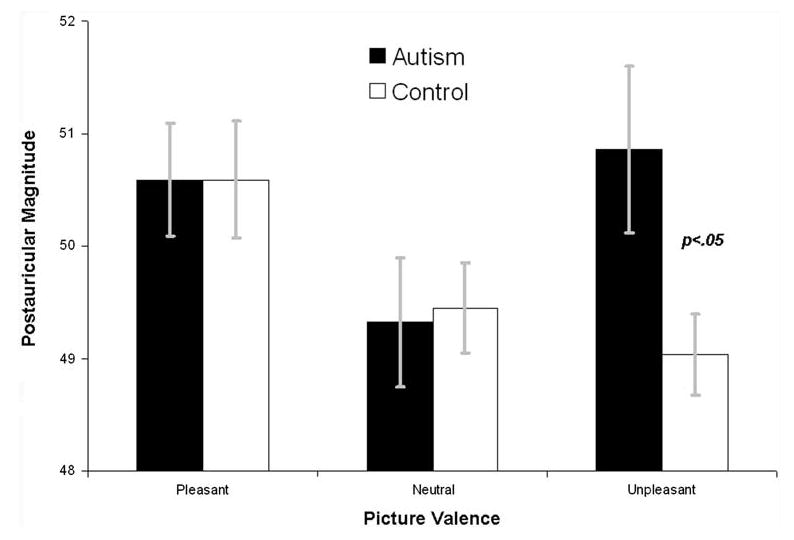
Postauricular reflexes during affective pictures by both diagnostic groups. Error bars are standard errors of the mean. Magnitude is expressed as within subjects T scores
The significant Group × linear Valence trend interaction was followed up with linear trend tests and category-specific modulation tests within each group. Within the neurotypical group, there was a significant linear Valence trend, F(1,36) = 4.46, p < .05, that reflected a significant difference between startle blink magnitudes during neutral and pleasant images, t(36) = 2.11, p < .05, but not during neutral and unpleasant images, t(36) = 1.17, p > .25. Within the ASD group, there was no significant linear Valence trend, F(1,19) = 1.91, p > .15. Exploratory follow-up tests revealed no significant difference between neutral and pleasant images in the ASD group, t(19) = 1.38, p > .15, nor a significant difference between neutral and unpleasant images, t(19) = 0.17, p > .85. Finally, groups did not differ in responses to unpleasant and neutral images, p's >.15, but did differ in responses to pleasant images, t(55) = 2.28, p < .05.
In summary, neurotypical participants exhibited typical linear valence modulation of the eyeblink reflex, whereas individuals with ASD did not, and group differences on Valence startle eyeblink modulation were significant. Groups differed in potentiation of the eyeblink reflex to pleasant pictures and in overall magnitude to pleasant pictures.
Eyeblink Modulation Excluding Older Neurotypical Controls
When controls 17 and older were excluded, eyeblink modulation findings were nearly identical: The Group × Valence ANOVA on eyeblink magnitudes revealed no main effect of Valence, F(2,37) = 0.11, p > .90, or Group, F(1,38) = 0.16, p < .70, but there was a Valence × Group interaction, F(2,37) = 3.89, p < .03. The linear Valence trend was non-significant as well, F(1,38) = 0.00, p > .98, but the Group × linear Valence trend interaction was significant, F(1,38) = 6.84, p < .02.
Postauricular Modulation
The Group × Valence ANOVA on PA magnitudes revealed no main effect of Valence, F(2,54) = 2.08, p > .10, a trend towards a main effect of Group, F(1,55) = 3.50, p < .07, but no Valence × Group interaction, F(2,54) = 1.68, p < .15. There was also no linear Valence trend, F(1,55) = 1.10, p < .30, or Group × linear Valence trend interaction, F(1,55) = 2.21, p > .10. Exploratory between groups t-tests on PA differences during pleasant and unpleasant images, relative to during neutral images, revealed that groups differed in modulation during unpleasant images, p < .05, but not pleasant images, p >.50.
Consistent with our a priori data analysis strategy, linear trend tests and category-specific modulation tests within each group were also conducted. Within the neurotypical group, there was a significant linear Valence trend, F(1,36) = 4.54, p < .05, a significant difference between neutral and pleasant images, t(36) = 2.13, p < .05, but no difference between neutral and unpleasant images, t(36) = 0.69, p > .45. Within the ASD group, there was no linear Valence trend, F(1,19) = 0.07, p > .75, and no significant difference between neutral and pleasant images, t(19) = 0.27, p > .78, or neutral and unpleasant images, t(19) = 1.40, p > .15. Finally, groups did not differ in responses to pleasant and neutral images, p's > 85, but did differ in responses to unpleasant images, t(55) = 2.47, p < .05.
In summary, neurotypical participants showed the expected affective modulation of the PA reflex, but participants with ASD did not. Groups differed in potentiation of the PA reflex to unpleasant images.
Postauricular Modulation Excluding Older Neurotypical Controls
When controls 17 and older were excluded, results were highly similar: The Group × Valence ANOVA on PA magnitudes revealed no main effect of Valence, F(2,37) = 1.95, p > .15, no main effect of Group, F(1,38) = 0.62, p > .44, and no Valence × Group interaction, F(2,37) = 1.11, p > .34. There was also no linear Valence trend, F(1,38) = 0.96, p > .30, but a trend towards a Group × linear Valence trend interaction, F(1,38) = 4.00, p > .06.
Relations between Eyeblink and Postauricular Startle Measures
To test relations between eyeblink and PA startle measures, correlations were conducted on responses to the three image categories as well as modulation to pleasant and unpleasant images. There were no significant relations within or across groups, r's < .14, p's > .30.
Self-Report Responses to Pictures
Figures 3 and 4 depict ratings of pleasure and arousal, respectively, for both diagnostic groups. The Group (neurotypical, ASD) × Valence (Unpleasant, Neutral, Pleasant) regression performed on pleasure ratings revealed a main effect of Valence, F(2,54) = 90.87, p < .0001, and a significant linear Valence trend, F(1,55) = 127.64, p < .0001, but no main effect or interactions with Group, p's > .75. Similar analyses of arousal data revealed a main effect of Valence, F(2,54) = 82.58, p < .0001, and a significant quadratic Valence trend, F(1,55) = 91.54, p < .0001, but no main effect or interaction with Group, p's > .10. In summary, there was no difference between neurotypical and ASD participants in their affective ratings of images.
Fig. 3.
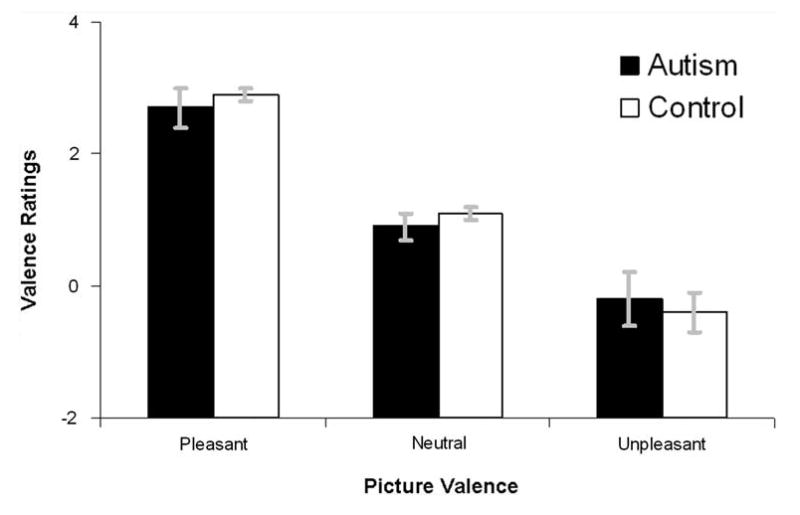
Valence ratings of both diagnostic groups. Error bars are standard errors of the mean. The range and direction of the ratings are −4 (extremely unpleasant) to +4 (extremely pleasant)
Fig. 4.
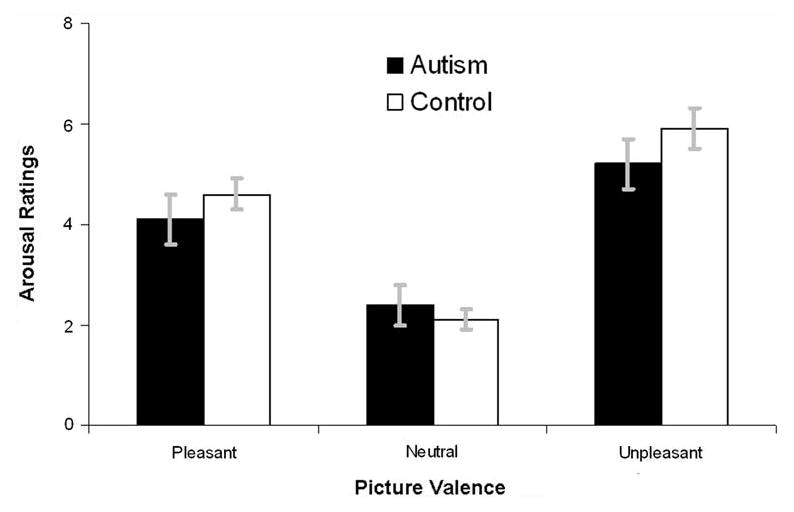
Arousal ratings of both diagnostic groups. Error bars are standard errors of the mean. The range and direction of the ratings are 0 (not at all aroused) to +8 (extremely aroused)
Discussion
The present study assessed affective startle eyeblink and PA modulation by normative emotional pictures in participants with ASDs and a neurotypical comparison group. The comparison group replicated the classic linear modulation patterns by valence on both eyeblink and PA reflexes. However, the patterns of data in the ASD group were non-linear, and there were group differences in eyeblink modulation to pleasant images and postauricular modulation to unpleasant images. These results imply one of two conclusions: (a) ASD is characterized by anomalous neurobiological systems mediating approach and withdrawal motivation to exteroceptive stimuli; or (b) the IAPS images had differential emotional impact on ASD and neurotypical participants. Our finding that groups did not differ in subjective ratings of valence and arousal argue for the former interpretation.
Patterns of reflex modulation suggest two interpretations of affect modulation in ASD. First, ASD may be characterized by an overall lack of modulation to affective stimuli. This pattern is consistent with the lack of startle blink modulation that has been observed in major depressive disorder (Dichter et al. 2004; Dichter and Tomarken 2008; Kaviani et al. 2004) and may indicate a deficit in basic emotional reactivity in ASD. The lack of any substantial emotional modulation of the PA reflex in our ASD sample is consistent with this interpretation, which would also suggest a dissociation between psychobiological measures and subjective ratings of emotion in ASD.
Alternatively, modulation patterns to both pleasant and unpleasant image categories indicated that ASD may be characterized by reflex potentiation in affective contexts where inhibition would be neurotypical (i.e., eyeblink modulation in pleasant contexts and PA modulation in unpleasant contexts were both potentiated relative to the comparison group). Startle eyeblink potentiation has been observed during internal visualization of personally relevant emotional scenes (Miller et al. 2002) and enhancement of emotional states due to emotion regulation (Dillon and Labar 2005). Thus, individuals with ASD may engage in greater internalizing of pleasant stimuli, although this interpretation must be tempered by the overall lack of modulation in the ASD group.
Differential startle eyeblink modulation to pleasant images is consistent with the conceptualization that the social cognition deficits that are characteristic of ASDs (American Psychiatric Association 1994) may reflect a broader dysfunction in neurobiological systems mediating approach towards putatively appetitive stimuli. We note that differences in responses to pleasant images was observed only in eyeblink modulation patterns but was not evident in PA reflex modulation, our presumed measure of appetitive dispositions, and thus this finding bears replication. Another unexpected finding was group differences in PA reflex responses to unpleasant stimuli, suggesting the possibility that the ASD group found this category of stimuli to be more approach-motivating than the comparison group. Though the precise reasons for this result are unclear, this finding is broadly consistent with the idea that ASD may be characterized by atypical responses to affective stimuli.
Bernier et al. (2005) reported intact startle eyeblink potentiation to threat cues (i.e., internal states) in a classical conditioning paradigm in ASD, suggesting that the startle modification circuit in response to internal emotional states is unaffected in ASD. The present findings, however, suggest a dysfunction of the startle modification circuit in response to exteroceptive stimuli. It thus appears that the methods used to generate emotional states in the laboratory could have profound effects on the patterns of results in ASD samples. It may be the case that stimuli that symbolize threat potential do not activate the defensive system indexed by the startle blink reflex in individuals with ASD, yet actual aversive outcomes, such as those delivered in a conditioning paradigm, mobilize defensive emotional processing in ASD during contexts that typically elicit defensive activation.
Eyeblink modulation patterns in the present study demonstrate both similarities and differences with those presented in Wilbarger et al. (2009), where individuals with ASD showed startle potentiation to both positive and negative stimuli. In the present study, individuals with ASD demonstrated eyeblink potentiation to pleasant stimuli; however, they did not show eyeblink potentiation to unpleasant stimuli. The unpleasant pictures in our study featured relatively more scenes of interpersonal victimization, whereas those in Wilbarger et al. (2009) included accidents and scenes of contamination. It may be the case that scenes of interpersonal violence activate the defensive system less than scenes of accidents in ASD. In this regard, both the Wilbarger et al. (2009) study and the present data point towards anomalous reflexive responses to startle probes that may be most pronounced in response to normatively pleasant stimuli. Additionally, both studies found no group differences in valence ratings of images, and the present study found no differences in arousal ratings as well.
Although eyeblink and PA affective modulation response profiles are generally the inverse of each other (i.e., greater during unpleasant than pleasant for the eyeblink response and the inverse for the PA response), we note that these two measures are, in fact, uncorrelated (Benning et al. 2004; Sandt et al. 2009), indicating that they measure independent dimensions of emotion. Potentiation of the eyeblink reflex represents activation of the defensive emotional system which, in turn, promotes the withdrawal from frightening or threatening stimuli. In contrast, potentiation of the PA reflex represents activation of the appetitive emotional system, which drives people to approach pleasant or life-sustaining stimuli. Thus, these measures are not redundant in their meaning, but rather represent complementary means to address fundamental questions about the biological basis of motivational priming.
The present findings suggest anomalous responses to normative emotional stimuli in ASD. These findings are generally supportive of the model of Bachevalier and Loveland (2006) that ASD may be characterized by impaired functioning of brain circuitry mediating social-affective behavior, and that anomalous responses to social stimuli in ASD may reflect broader affective dysregulation. Infants and children with ASD demonstrate decreased social attention and social orienting to social stimuli (see Dawson et al. 2002 for a review), and it has been argued that such behavior reflects the lack of sensitivity to social reward (Dawson et al. 2001; Mundy and Neal 2001). The present findings indicate one potential neurobiologic system that may mediate this behavior and suggest that putatively rewarding stimuli may not be processed as such. Blunted responses to negative affect have been reported in ASD as well (Sigman et al. 1992; Corona et al. 1998), and support the present findings that ASD is characterized by generally anomalous neurobiologic responses to emotional stimuli.
Another noteworthy finding of the present study is the dissociation between neurobiologic and self-report responses to emotional stimuli: whereas reflex modulation responses were clearly different in the ASD group relative to the neurotypical comparison group, self-report ratings of both Valence and Arousal were nearly identical across groups. This pattern suggests that variations in reflex modulation may be a more sensitive index of differential affective responses linked to ASDs than subjective self-report. A number of other disorders are characterized by dissociation between profiles of affective modulation of the startle response and subjective responses to emotional stimuli, including psychopathy, borderline personality disorder (Herpertz et al. 2001), and unipolar depression (Dichter and Tomarken 2008; Dichter et al. 2004), highlighting the utility of reflex modulation to provide unique information about affective states.
Though the present study clearly did not directly measure amygdala function, findings implicate alterations in the startle eyeblink modification circuit, which is mediated by direct projections from the lateral and central nuclei of the amygdala to the nucleus reticularus pontis caudalis (Davis 1989; Davis et al. 1997; Hitchcock and Davis 1987; Fendt et al. 1994). The functions of the amygdala include assessing the emotional significance and reward value of a stimulus (Gaffan 1992; Malkova et al. 1997) as well as enhancing memory for emotional stimuli (Phelps and Anderson 1997). It has been argued that amygdala dysfunction may be a core feature of ASD (Baron-Cohen et al. 2000; Schultz et al. 2000) that results in disruption of the ability to assign meaning to social stimuli (Fein et al. 1987) and to acquire the normal motivational and emotional significance of stimuli (Fotheringham 1991).
There are a number of caveats associated with the present study. One limitation is the age difference between groups. In this regard we note that age was not related to startle responses within the neurotypical group, age did not moderate startle responses overall, and results with a subset of participants matched on age were highly similar. Furthermore, startle eyeblink modulation has been demonstrated in samples of children as young as 4–8 years old (Waters et al. 2008) and 4–7 years old (Essex et al. 2003). However, it is possible that affective modulation of the startle responses may be affected by development during ages 17–30, and future studies with age-matched groups are necessary.
Additionally, recent data suggest the potential effects of pubertal status on the startle responses (Quevedo et al. 2009). Pubertal status was not assessed in the present study, and the age ranges of ASD sample likely included participants who were pre-pubertal. Moreover, other psychophysiological indices of affective responses, such as skin conductance, heart rate, and corrugator and zygomatic muscle activity were not recorded. In particular, psychophysiological indices of affective arousal would be necessary to determine whether differential responses to stimulus valence or arousal are driving the present findings. Finally, valence and arousal ratings of IAPS were relatively moderate compared to published studies using the same image set (e.g., Dichter et al. 2004; Dichter and Tomarken 2008).
We also note that eight of the 20 children with ASD were taking psychotropic medications (i.e., SSRIs, stimulants, an atypical antipsychotic, and an alpha-2 agonist). Though the uneven distribution of medication use in this relatively small sample does not allow for a meaningful statistical analysis of the effects of these agents on startle modulation patterns, we note that the literature does not suggest a systematic bias towards greater or lesser startle modulation as a result of taking these agents. Specifically, the effects of selective serotonin reuptake inhibitors on startle responses differ based on whether administration is acute or chronic; i.e., single doses increase startle potentiation to aversive pictures (Browning et al. 2007) whereas more chronic administration blunts startle potentiation (Harmer et al. 2004; but see Harmer et al. 2006). Though there are no data regarding the effects of antipsychotic and psychostimulant medications on startle potentiation, these agents do not appear to affect overall startle blink magnitude or habituation (Graham et al. 2004; Wynn et al. 2007). Anticonvulsants may reduce startle potentiation (Khan and Liberzon 2004), whereas olanzapine, halperidol, risperidone, and quetiapine (Graham et al. 2004; Wynn et al. 2007) as well as psychostimulants (Hanlon et al. 2009) do not affect startle magnitudes. Finally, the effects of these agents on PA modulation are unknown.
In the present study, reflex magnitudes were assessed during the so-called “late valence” phase of the affective response by probes presented 3.5–4.5 s after image onset. Reflex modulation patterns may be assessed throughout the full chronometry of affective responses (Dichter and Tomarken 2008), and studies that incorporate startle probes presented at different latencies could assess whether the affective startle modulation differences observed in ASD in the present context extend to probes indexing affective anticipation (i.e., by presenting startle probes during cues signaling the valence of forthcoming affective images) and early attentional processes (i.e., by presenting startle probes < 500 ms after picture onset) as well.
Despite these limitations, the present findings indicate anomalous affective startle modulation in ASD. These data are consistent with cognitive neuroscience models of brain function that conceptualize social cognition within the broader framework of affective processing, and future startle modulation research should assess response patterns to social stimuli specifically in ASD. These findings also suggest that studies of social cognition in ASD should incorporate conditions that asses responses to affective stimuli more broadly to test the specificity of finding with respect to social information processing.
Acknowledgments
This research was supported by R01 MH073402 (Bodfish) and R21 MH085254 (Dichter & Benning). G. Dichter was supported by NIH/NCRR K12 RR023248 and NIMH K23 MH081285. Assistance for this study was provided by the Subject Registry and Biostatistics Cores of the UNC Neurodevelopmental Disorders Research Center (P30 HD03110). We thank Jeffrey Sibrack for assistance with programming and data collection and Michele Poe, PhD for assistance with various aspects of this project.
Appendix
International Affective Picture System (Lang et al. 2008) Image Numbers:
Female Adults
Neutral: 1450, 5130, 5510, 5910, 7000, 7002, 7006, 7031, 7035, 7060, 7096, 7100, 7140, 7150, 7175, 7185, 7207, 7217, 7490; Pleasant: 1710, 4608, 4670, 5450, 5629, 7270, 7330, 8030, 8041, 8090, 8190, 8470, 8490, 8496; Unpleasant: 1300, 1301, 3500, 3530, 6230, 6250, 6260, 6313, 6350, 6360, 6540, 6560, 6821, 9630, 9810.
Female Children
Neutral 1450, 5030, 5130, 5510, 5731, 7000, 7004, 7006, 7009, 7010, 7020, 7040, 7050, 7080, 7090, 7175, 7185, 7187, 7500; Pleasant: 1463, 1610, 1710, 1999, 2030, 5460, 5470, 7250, 7270, 7325, 7390, 7400, 7410, 7470, 8496; Unpleasant: 1052, 1120, 1200, 1201, 1230, 1270, 1280, 1301, 1321, 1390, 6230, 6350, 6360, 6510, 6821.
Male Adults
Neutral: 1450, 2850, 2880, 5030, 7006, 7009, 7025, 7035, 7040, 7050, 7052, 7080, 7185, 7217, 7233, 7491, 7500; Pleasant: 1650, 4232, 5260, 5460, 5621, 5700, 5950, 8030, 8170, 8180, 8190, 8200, 8260, 8380, 8501; Unpleasant: 1300, 3500, 3530, 6230, 6250, 6260, 6350, 6510, 6540, 6550, 6560, 6570, 9630, 9800, 9810.
Male Children
Neutral: 1450, 5030, 5130, 5510, 5731, 7000, 7004, 7006, 7009, 7010, 7020, 7040, 7090, 7110, 7150, 7185, 7187, 7224, 7233; Pleasant: 1463, 1610, 1710, 2030, 5460, 5470, 7250, 7260, 7270, 7330, 7340, 7390, 7400, 8470, 8496; Unpleasant: 1052, 1120, 1200, 1201, 1230, 1270, 1280, 1300, 1301, 1321, 1390, 6350, 6360, 6510, 6821.
Footnotes
The term “linear” in this context refers to the first-order polynomial trend test that is commonly used in the startle modulation literature to evaluate the effects of picture valence, which is typically divided into the categories of unpleasant, neutral, and pleasant, on reflex responses. Thus, a “linear” pattern of responses indicates either [unpleasant > neutral > pleasant] or [pleasant > neutral > unpleasant] patterns of responding.
In-house experimental images depicted common circumscribed interests and images of faces with neutral expressions. The mood states induced by IAPS images are very brief: in nonclinical samples, affective startle modulation is not evident 1.5–2.5 s after image offset (Dichter et al. 2002). Thus, there are likely no effects of the inclusion of other images on startle responses, given the 10 s ITI employed.
Other researchers have noted highly similar results obtained with both raw and standardized scores (e.g., Grillon and Ameli 2001; Dichter and Tomarken 2008).
Contributor Information
Gabriel S. Dichter, Email: dichter@biac.duke.edu, Carolina Institute for Developmental Disabilities, University of North Carolina at Chapel Hill School of Medicine, 101 Manning Drive, CB# 3366, Chapel Hill, NC 27599-7160, USA; Department of Psychiatry, University of North Carolina at Chapel Hill School of Medicine, CB# 7160, Chapel Hill, NC 27599-7160, USA; Duke-UNC Brain Imaging and Analysis Center, Duke University Medical Center, Durham, NC 27710, USA; Department of Psychiatry and Behavioral Sciences, Duke University Medical Center, Box 3026, Durham, NC 27710, USA.
Stephen D. Benning, Department of Psychology, College of Arts & Sciences, Vanderbilt University, 301 Wilson Hall, Nashville, TN 37203, USA
Tia N. Holtzclaw, Carolina Institute for Developmental Disabilities, University of North Carolina at Chapel Hill School of Medicine, 101 Manning Drive, CB# 3366, Chapel Hill, NC 27599-7160, USA
James W. Bodfish, Carolina Institute for Developmental Disabilities, University of North Carolina at Chapel Hill School of Medicine, 101 Manning Drive, CB# 3366, Chapel Hill, NC 27599-7160, USA; Department of Psychiatry, University of North Carolina at Chapel Hill School of Medicine, CB# 7160, Chapel Hill, NC 27599-7160, USA; Center for Development and Learning, University of North Carolina at Chapel Hill, CB # 7255, Chapel Hill, NC 27599-7255, USA
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV. 4th. Washington, DC: 1994. [Google Scholar]
- Arinami T, Li L, Mitsushio H, Itokawa M, Hamaguchi H, Toru M. An insertion/deletion polymorphism in the angiotensin converting enzyme gene is associated with both brain substance P contents and affective disorders. Biological Psychiatry. 1996;40(11):1122–1127. doi: 10.1016/s0006-3223(95)00597-8. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Loveland KA. The orbitofrontal-amygdala circuit and self-regulation of social-emotional behavior in autism. Neuroscience and Biobehavioral Reviews. 2006;30:97–117. doi: 10.1016/j.neubiorev.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Belmonte MK. Autism: a window onto the development of the social and the analytic brain. Annual Review of Neuroscience. 2005;28:109–126. doi: 10.1146/annurev.neuro.27.070203.144137. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Ring HA, Bullmore ET, Wheelwright S, Ashwin C, Williams SC. The amygdala theory of autism. Neuroscience and Biobehavioral Reviews. 2000;24(3):355–364. doi: 10.1016/s0149-7634(00)00011-7. [DOI] [PubMed] [Google Scholar]
- Benning SD, Bernat EM, Starr MJ, Gewirtz JC, Patrick CJ. Post-auricular reflex potentiation during relief stimuli in differential aversive conditioning: A construct validation study. Psychophysiology. 2004a;41:S3–S4. [Google Scholar]
- Benning SD, Patrick CJ, Lang AR. Emotional modulation of the post-auricular reflex. Psychophysiology. 2004b;41(3):426–432. doi: 10.1111/j.1469-8986.00160.x. [DOI] [PubMed] [Google Scholar]
- Bernier R, Dawson G, Panagiotides H, Webb S. Individuals with autism spectrum disorder show normal responses to a fear potential startle paradigm. Journal of Autism and Developmental Disorders. 2005;35:575–583. doi: 10.1007/s10803-005-0002-0. [DOI] [PubMed] [Google Scholar]
- Bolton PF, Pickles A, Murphy M, Rutter M. Autism, affective and other psychiatric disorders: Patterns of familial aggregation. Psychological Medicine. 1998;28(2):385–395. doi: 10.1017/s0033291797006004. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Cuthbert BN, Lang PJ. Pictures as prepulse: Attention and emotion in startle modification. Psychophysiology. 1993;30(5):541–545. doi: 10.1111/j.1469-8986.1993.tb02079.x. [DOI] [PubMed] [Google Scholar]
- Browning M, Reid C, Cowen PJ, Goodwin GM, Harmer CJ. A single dose of citalopram increases fear recognition in healthy subjects. Journal of Psychopharmacology. 2007;21(7):684–690. doi: 10.1177/0269881106074062. [DOI] [PubMed] [Google Scholar]
- Corona R, Dissanayake C, Arbelle S, Wellington P, Sigman M. Is affect aversive to young children with autism? Behavioral and cardiac responses to experimenter distress. Child Development. 1998;69(6):1494–1502. [PubMed] [Google Scholar]
- Dalton KM, Nacewicz BM, Johnstone T, Schaefer HS, Gernsbacher MA, Goldsmith HH, et al. Gaze fixation and the neural circuitry of face processing in autism. Nature Neuroscience. 2005;8(4):519–526. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. Neural systems involved in fear-potentiated startle. Annals of the New York Academy of Sciences. 1989;563:165–183. doi: 10.1111/j.1749-6632.1989.tb42197.x. [DOI] [PubMed] [Google Scholar]
- Davis M, Falls WA, Campeau S, Kim M. Fear-potentiated startle: A neural and pharmacological analysis. Behavioural Brain Research. 1993;58(1–2):175–198. doi: 10.1016/0166-4328(93)90102-v. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Lee Y. Amygdala and bed nucleus of the stria terminalis: Differential roles in fear and anxiety measured with the acoustic startle reflex. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 1997;352(1362):1675–1687. doi: 10.1098/rstb.1997.0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depue RA, Iacono WG. Neurobehavioral aspects of affective disorders. Annual Review of Psychology. 1989;40:457–492. doi: 10.1146/annurev.ps.40.020189.002325. [DOI] [PubMed] [Google Scholar]
- Dichter GS, Tomarken AJ. The chronometry of affective startle modulation in unipolar depression. Journal of Abnormal Psychology. 2008;117(1):1–15. doi: 10.1037/0021-843X.117.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Tomarken AJ, Baucom B. Startle modulation before, during and after exposure to emotional stimuli. International Journal of Psychophysiology. 2002;43(2):191–196. doi: 10.1016/s0167-8760(01)00170-2. [DOI] [PubMed] [Google Scholar]
- Dichter GS, Tomarken AJ, Shelton RC, Sutton SK. Early- and late-onset startle modulation in unipolar depression. Psychophysiology. 2004;41(3):433–440. doi: 10.1111/j.1469-8986.00162.x. [DOI] [PubMed] [Google Scholar]
- Dillon DG, Labar KS. Startle modulation during conscious emotion regulation is arousal-dependent. Behavioral Neuroscience. 2005;119(4):1118–1124. doi: 10.1037/0735-7044.119.4.1118. [DOI] [PubMed] [Google Scholar]
- Essex MJ, Goldsmith HH, Smider NA, Dolski I, Sutton SK, Davidson RJ. Comparison of video- and EMG-based evaluations of the magnitude of children's emotion-modulated startle response. Behavior Research Methods, Instruments, & Computers. 2003;35(4):590–598. doi: 10.3758/bf03195538. [DOI] [PubMed] [Google Scholar]
- Fein D, Pennington B, Waterhouse L. Implications of social deficits in autism for neurological dysfunction. In: Schopler E, Mesibov GB, editors. Neurobiological issues in autism. New York: Plenum Press; 1987. pp. 127–144. [Google Scholar]
- Fendt M, Koch M, Schnitzler HU. Lesions of the central gray block the sensitization of the acoustic startle response in rats. Brain Research. 1994;661(1–2):163–173. doi: 10.1016/0006-8993(94)91193-2. [DOI] [PubMed] [Google Scholar]
- Fotheringham JB. Autism: Its primary psychological and neurological deficit. Canadian Journal of Psychiatry. 1991;36(9):686–692. doi: 10.1177/070674379103600913. [DOI] [PubMed] [Google Scholar]
- Fowles DC. Psychophysiology and psychopathology: A motivational approach. Psychophysiology. 1988;25(4):373–391. doi: 10.1111/j.1469-8986.1988.tb01873.x. [DOI] [PubMed] [Google Scholar]
- Funayama ES, Grillon C, Davis M, Phelps EA. A double dissociation in the affective modulation of startle in humans: Effects of unilateral temporal lobectomy. Journal of Cognitive Neuroscience. 2001;13(6):721–729. doi: 10.1162/08989290152541395. [DOI] [PubMed] [Google Scholar]
- Gaffan D. Amygdala and the memory of reward. In: Aggleton JO, editor. The amygdala: Neurobiological aspects of emotion, memory, and dysfunction. New York: Wiley-Liss; 1992. [Google Scholar]
- Graham SJ, Scaife JC, Balboa Verduzco AM, Langley RW, Bradshaw CM, Szabadi E. Effects of quetiapine and haloperidol on prepulse inhibition of the acoustic startle (eyeblink) response and the N1/P2 auditory evoked response in man. Journal of Psychopharmacology. 2004;18(2):173–180. doi: 10.1177/0269881104042615. [DOI] [PubMed] [Google Scholar]
- Gray H. Anatomy: Descriptive and surgical. 15. New York: Barnes and Noble Books, Inc.; 1901/1995. [Google Scholar]
- Grillon C, Ameli R. Conditioned inhibition of fear-potentiated startle and skin conductance in humans. Psychophysiology. 2001;38(5):807–815. [PubMed] [Google Scholar]
- Hackley SA. An evaluation of the automaticity of sensory processing using event-related potentials and brain-stem reflexes. Psychophysiology. 1993;30(5):415–428. doi: 10.1111/j.1469-8986.1993.tb02065.x. [DOI] [PubMed] [Google Scholar]
- Hanlon MC, Karayanidis F, Schall U. Intact sensorimotor gating in adult attention deficit hyperactivity disorder. The International Journal of Neuropsychopharmacology. 2009;12(5):701–707. doi: 10.1017/S1461145708009711. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Reid CB, Ray MK, Goodwin GM, Cowen PJ. 5HT(3) antagonism abolishes the emotion potentiated startle effect in humans. Psychopharmacology (Berl) 2006;186(1):18–24. doi: 10.1007/s00213-006-0337-z. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Shelley NC, Cowen PJ, Goodwin GM. Increased positive versus negative affective perception and memory in healthy volunteers following selective serotonin and norepinephrine reuptake inhibition. American Journal of Psychiatry. 2004;161(7):1256–1263. doi: 10.1176/appi.ajp.161.7.1256. [DOI] [PubMed] [Google Scholar]
- Herpertz SC, Werth U, Lukas G, Qunaibi M, Schuerkens A, Kunert HJ, et al. Emotion in criminal offenders with psychopathy and borderline personality disorder. Archives of General Psychiatry. 2001;58(8):737–745. doi: 10.1001/archpsyc.58.8.737. [DOI] [PubMed] [Google Scholar]
- Hess U, Sabourin G, Kleck RE. Postauricular and eyeblink startle responses to facial expressions. Psychophysiology. 2007;44(3):431–435. doi: 10.1111/j.1469-8986.2007.00516.x. [DOI] [PubMed] [Google Scholar]
- Hitchcock JM, Davis M. Fear-potentiated startle using an auditory conditioned stimulus: Effect of lesions of the amygdala. Physiology & Behavior. 1987;39(3):403–408. doi: 10.1016/0031-9384(87)90242-3. [DOI] [PubMed] [Google Scholar]
- Kanner L. Autistic disturbances of affective contact. The Nervous Child. 1943;2:217–250. [Google Scholar]
- Kaviani H, Gray JA, Checkley SA, Raven PW, Wilson GD, Kumari V. Affective modulation of the startle response in depression: Influence of the severity of depression, anhedonia, and anxiety. Journal of Affective Disorders. 2004;83(1):21–31. doi: 10.1016/j.jad.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Khan S, Liberzon I. Topiramate attenuates exaggerated acoustic startle in an animal model of PTSD. Psychopharmacology (Berl) 2004;172(2):225–229. doi: 10.1007/s00213-003-1634-4. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Emotion, motivation, and anxiety: Brain mechanisms and psychophysiology. Biological Psychiatry. 1998;44(12):1248–1263. doi: 10.1016/s0006-3223(98)00275-3. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Digitized photographs, instruction manual and affective ratings Technical Report A-6. Gainesville, FL: University of Florida; 2005. [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Affective ratings of pictures and instruction manual Technical Report A-8. Gainesville, FL: University of Florida; 2008. [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN, Patrick CJ. Emotion and psychopathology: A startle probe analysis. Progress in Experimental Personality and Psychopathology Research. 1993a;16:163–199. [PubMed] [Google Scholar]
- Lang PJ, Greenwald MK, Bradley MM, Hamm AO. Looking at pictures: Affective, facial, visceral, and behavioral reactions. Psychophysiology. 1993b;30(3):261–273. doi: 10.1111/j.1469-8986.1993.tb03352.x. [DOI] [PubMed] [Google Scholar]
- Larson CL, Nitschke JB, Davidson RJ. Common and distinct patterns of affective response in dimensions of anxiety and depression. Emotion. 2007;7(1):182–191. doi: 10.1037/1528-3542.7.1.182. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism diagnostic interview—Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- MacLean PD. Psychosomatic disease and the visceral brain; recent developments bearing on the Papez theory of emotion. Psychosomatic Medicine. 1949;11(6):338–353. doi: 10.1097/00006842-194911000-00003. [DOI] [PubMed] [Google Scholar]
- Malkova L, Gaffan D, Murray EA. Excitotoxic lesions of the amygdala fail to produce impairment in visual learning for auditory secondary reinforcement but interfere with reinforcer devaluation effects in rhesus monkeys. Journal of Neuroscience. 1997;17(15):6011–6020. doi: 10.1523/JNEUROSCI.17-15-06011.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazefsky CA, Folstein SE, Lainhart JE. Overrepresentation of mood and anxiety disorders in adults with autism and their first-degree relatives: What does it mean? Autism Research. 2008;1(3):193–197. doi: 10.1002/aur.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MW, Patrick CJ, Levenston GK. Affective imagery and the startle response: Probing mechanisms of modulation during pleasant scenes, personal experiences, and discrete negative emotions. Psychophysiology. 2002;39(4):519–529. doi: 10.1017/s0048577202394095. [DOI] [PubMed] [Google Scholar]
- O'Beirne GA, Patuzzi RB. Basic properties of the sound-evoked post-auricular muscle response (PAMR) Hearing Research. 1999;138(1–2):115–132. doi: 10.1016/s0378-5955(99)00159-8. [DOI] [PubMed] [Google Scholar]
- Ochsner KN. The social-emotional processing stream: Five core constructs and their translational potential for schizophrenia and beyond. Biological Psychiatry. 2008;64(1):48–61. doi: 10.1016/j.biopsych.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papez JW. A proposed mechanism of emotion. Journal of Neuropsychiatry and Clinical Neurosciences. 1937;7(1):103–112. doi: 10.1176/jnp.7.1.103. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Anderson AK. Emotional memory: What does the amygdala do? Current Biology. 1997;7(5):R311–R314. doi: 10.1016/s0960-9822(06)00146-1. [DOI] [PubMed] [Google Scholar]
- Quevedo KM, Benning SD, Gunnar MR, Dahl RE. The onset of puberty: Effects on the psychophysiology of defensive and appetitive motivation. Development and Psychopathology. 2009;21(1):27–45. doi: 10.1017/S0954579409000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandt AR, Sloan DM, Johnson KJ. Measuring appetitive responding with the postauricular reflex. Psychophysiology. 2009;46(3):491–497. doi: 10.1111/j.1469-8986.2009.00797.x. [DOI] [PubMed] [Google Scholar]
- Sasson N, Turner-Brown L, Holtzclaw T, Lam KS, Bodfish J. Children with autism demonstrate circumscribed attention during passive viewing of complex social and nonsocial picture arrays. Autism Research. 2008;1:31–42. doi: 10.1002/aur.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz RT, Gauthier I, Klin A, Fulbright RK, Anderson AW, Volkmar F, et al. Abnormal ventral temporal cortical activity during face discrimination among individuals with autism and Asperger syndrome. Archives of General Psychiatry. 2000;57(4):331–340. doi: 10.1001/archpsyc.57.4.331. [DOI] [PubMed] [Google Scholar]
- Sigman MD, Kasari C, Kwon JH, Yirmiya N. Responses to the negative emotions of others by autistic, mentally retarded, and normal children. Child Development. 1992;63(4):796–807. [PubMed] [Google Scholar]
- Sollers JJ, 3rd, Hackley SA. Effects of foreperiod duration on reflexive and voluntary responses to intense noise bursts. Psychophysiology. 1997;34(5):518–526. doi: 10.1111/j.1469-8986.1997.tb01738.x. [DOI] [PubMed] [Google Scholar]
- Waters AM, Neumann DL, Henry J, Craske MG, Ornitz EM. Baseline and affective startle modulation by angry and neutral faces in 4–8 year-old anxious and non-anxious children. Biological Psychology. 2008;78(1):10–19. doi: 10.1016/j.biopsycho.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Wilbarger JL, McIntosh DN, Winkielman P. Startle modulation in autism: positive affective stimuli enhance startle response. Neuropsychologia. 2009;47(5):1323–1331. doi: 10.1016/j.neuropsychologia.2009.01.025. [DOI] [PubMed] [Google Scholar]
- Wynn JK, Green MF, Sprock J, Light GA, Widmark C, Reist C, et al. Effects of olanzapine, risperidone and haloperidol on prepulse inhibition in schizophrenia patients: A double-blind, randomized controlled trial. Schizophrenia Research. 2007;95(1–3):134–142. doi: 10.1016/j.schres.2007.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]


