Abstract
Tissue engineering approaches that harvest the stimulatory power of platelet-rich plasma have produced encouraging results in anterior cruciate ligament (ACL) repair. However, a number of recent studies have demonstrated age-dependent differences in cellular responses to such an approach. Identifying the reasons for these differences would allow counteracting them and consequently improve outcomes. In this study we hypothesized that these age-related effects are caused by differences in the expression of the receptors for growth factors released from platelet-rich plasma (PRP). Porcine ACL fibroblasts from a predetermined number of animals of different ages were obtained, and mRNA levels of the receptors of platelet-derived growth factor (PDGF), transforming growth factor β (TGF-β), fibroblast growth factor (FGF), and vascular endothelial growth factor (VEGF) were determined. Expression levels were compared across age groups (young and adolescent) and regressed on age in days. While no significant difference was seen across groups, the regression analysis showed decreases in receptor expression with increasing age. These differences were statistically significant for TGF-β receptor 1, FGF receptor, and VEGF receptor 2; and borderline significant for TGF-β receptor 3 and PDGF receptor. The only receptor that was not associated with age was VEGF receptor 1, a regulator of VEGF receptor 2. These findings suggest that the decrease in growth factor receptor expression as a likely reason for reduced PRP action with increasing age.
Keywords: ACL repair, tissue engineering, platelet-rich plasma, growth factor receptor, gene expression
INTRODUCTION
Tears of the anterior cruciate ligament (ACL) are among the most important disease entities in orthopedics and sports medicine due to their high incidence in today’s highly active population and the associated, substantial increase in risk of subsequent osteoarthritic degeneration. Recently, evidence has been accumulated that osteoarthritic degeneration remains to be a problem for a large number of patients even after the current gold standard in ACL management, ACL reconstruction.1–6
Recent advances in orthopedic research have produced an intriguing new approach to ACL treatment: biological regeneration by tissue engineering enhanced primary ACL repair.7,8 Current studies document the success of such new procedures in in vitro and in vivo testing.7,9–17 These procedures use a collagenous bio-material in combination with platelet concentrates, also known as platelet-rich plasma (PRP) for short. PRP serves for two purposes, firstly the formation of a clot as structural support for wound healing, and secondly, and maybe more importantly, as a source for a plethora of stimulating growth factors, such as TGF, VEGF, FGF, and many more, that induce and enhance remodeling and healing.18–21 Compensating for the ACL’s lack of intrinsic healing capacity also holds much promise for improving long-term outcomes, especially avoiding early onset of osteoarthritis.8
Previous research has shown that ACL fibroblasts respond differently to stimulation by PRP, at different ages.22,23 These studies following this phenomenon in vitro as well as in vivo have shown differences in proliferation and migration on the cellular levels relating to differences in clinical outcomes in histology and biomechanics in large animal models of young, adolescent, and adult animals (Murray MM, Magarian EM, Harrison SL, et al. 2009. Skeletal maturity significantly affects functional healing of the anterior cruciate ligament, submitted).22,23 More specifically, these studies showed significantly better results in young or immature animals, that is, with radiologically open physes, compared to adolescent and adult animals, that is, those with closing or completely closed physes. Between the latter, the differences were less clear and mostly not significant.22,23
These findings imply that different clinical results may be expected in individuals of different ages treated with platelet-enhanced ACL repair. Identification of the underlying reasons for these differences may lead the way to potential remedies. Given the fact that PRP can be produced to a chosen concentration with much reliability, one of the most likely reasons for different responses to stimulation by PRP are differences in the expression of receptors for the main agents of PRP function, growth factors such as platelet-derived growth factor (PDGF), transforming growth factor β (TGF-β), fibroblast growth factor (FGF), or vascular endothelial growth factor (VEGF), which are released as a constant, widely age-independent function of the number of platelets.20,24,25
Thus it was the first hypothesis of this study that there are differences in the expression of the receptors of PDGF, TGF-β, FGF, and VEGF (Table 1) in ACL fibroblasts from young and adolescent individuals, reflecting the differences seen earlier in cellular behavior in these two age groups. The second hypothesis of this study was that absolute age, rather than age group, is a predictor of growth factor receptor expression.
Table 1.
Studied Receptors and Their Binding Growth Factors
| Receptorsa | Description | Growth Factor | Concentration in PRP (pg/ml)b | Function in Fibroblasts |
|---|---|---|---|---|
| TGF-β R1 | Similar binding affinities, high affinity for TGF-β1, low affinity for TGF-β2, equivalent to TGF-β receptor 2 | Various subforms; TGF-β1 enhances fibroblast proliferation and differentiation and acts as stimulus for type I collagen and firbonection production; TGF-β2 more important for embryonic development | ||
| TGF-β-R2a | TGF-β | 9184.0 | ||
| TGF-β-R3 | High affinity for TGF-β1 and TGF-β2 | |||
| PDGF-R | Dimeric receptor in three different conformations (αα, αβ, ββ) | PDGF | 3192.9 | Enhances fibroblast proliferation and collagen synthesis |
| FGFR | Including all splicing variants 48 isoforms | FGF | Traces | Potent stimulus of fibroblast proliferation |
| VEGF-R1 | Binds VEGF-A, thought to modulate VEGF-R2 signaling | VEGF | 61.6 | Expressed in fibroblasts, but role in fibroblast activity still unclear |
| VEGF-R2 | Binds VEGF-A, VEGF-E, VEGF-C; main mediator of VEGF function | |||
| VEGF-R3a | Binds VEGF-C, VEGF-D; lymphangiogenetic |
MATERIALS AND METHODS
Study Design
Previous studies have shown an effect size of 2 when comparing immature and adolescent animals for multiple endpoints in cell behavior, histology, and biomechanics.22,23 Based on these prior findings we calculated the required samples size to be n = 8 per group (effect size 2, alpha = 0.05, equal group sizes, with at least 95% power). This same sample size can detect smaller effects, while maintaining more than 80% power until effect size drops below 1.5. Additionally, to allow testing for prediction of growth factor receptor expression by absolute age using linear regression, we added a group of adult animals (n = 3) to increase the range of values for our independent variable age.
Tissue Procurement
ACL biopsies were obtained aseptically from female Yucatan pigs undergoing IACUC approved surgery for another experiment. Age grouping was based on physeal status, with young pigs showing open physes, and adolescent pigs showing closing, but not yet completely closed physes. Their mean ages were 7.4 ± 1.5 months for the young group and 13.4 ± 0.4 months for the adolescent group. Biopsies were brought to the lab, washed in sterile PBS, and used to establish explant cultures. Briefly, the tissue was carefully examined for any contamination with nonligamentous tissue, which, if found, was generously removed. Subsequently, ACL tissue was cut into pieces of approximately 1 mm3 volume and cultured in 100 mm Petri dishes with a standard medium containing DMEM (Mediatech, Manassas, VA), 10% FBS (HyClone, South Logan, UT), and 100 IU/ml penicillin, 100 mg/ml streptomycin, and 0.25 μg/ml amphotericin B (Mediatech) to allow cellular outgrowth from the tissue onto the cell culture plate. ACL fibroblast cells were collected at confluence for RNA extraction. Care was taken to keep cell culture as short, and exposure to FBS as low as possible to avoid negative, or undue effects of these on the receptor expression.
Total RNA Extraction and mRNA Purification
Cells were homogenized in TRIzol® (Invitrogen), mixed with chloroform, and RNA was precipitated with isopropanol. After quantification, the RNA integrity was checked by agarose gel electrophoresis. Oligotex® (Qiagen), mRNA purification reagent, was used to purify mRNA from total RNA according to standard protocols.
Reverse Transcription of mRNA and Quantitative
Real-Time RT-PCR
RETROscript™ (Ambion), first-strand synthesis kit, was used to reverse transcribe the mRNA to cDNA according to standard protocols. In brief, mRNA (100 ng) was reverse transcribed with M-MLV reverse transcriptase in 20 μl. The cDNA was diluted 5× and used as template for RT-PCR. Real-time quantitative RT-PCR was used to evaluate mRNA levels using an ABI PRISM® 7700 sequence detection system (Applied Biosystems). We have previously described the real-time RT-PCR conditions.26,27 Briefly, the reactions were run in triplicate, each containing 1 μl cDNA along with 2.5 pmol of gene-specific primers (Table 2) in a final volume of 25 μl with the following thermal profile: 5 min initial denaturing step to activate the DNA polymerase, then 40 cycles each at 95°C for 35 s, 60°C for 45 s, and 72°C for 45 s. Levels of GAPDH transcript proved to be stable under the experimental trials; therefore, it was selected as a reference gene.
Table 2.
Primer Sequences
| Target Gene | Forward Primer Sequence | Reverse Primer Sequence |
|---|---|---|
| TGFBR3 | gcg acg tca cgc tgt gta cc | ccg tca gca gag ctc cga tc |
| TGFBR1 | tgt tcc att ggt gga att cat g | tgg ctg ctc cat tgg cat ac |
| PDGFR | agg ccc aga tct cct tcc ag | cgc agt gtg ctc acc acc tc |
| FGFR2 | gcc ttc ctc cgt acc cac ag | gcc tcc cct gct cag tac ac |
| VEGFR1 | agc cca agg cct cac tca ag | acg gga ggg ctg cac tac ag |
| VEGFR2 | ctg gtt ctg gcc caa caa tc | tgg tcc cca gac atg gaa tc |
| GAPDH | aag ggc atc ctg ggc tac ac | ggt cca ggg gct ctt act cc |
Statistical Evaluation
To test our first hypothesis, differences in growth factor receptor expression between young and adolescent animals, we used two-tailed t-tests to compare the mean mRNA expression, normalized for GAPDH, of each group. To test our second hypothesis, age being a predictor of growth factor expression, we employed linear regression using age as a continuous variable (age in days) instead of a categorized one (young vs. adolescent vs. adult). We added a group of adult animals (n = 3, age 49.1 ± 13.6 months) to extend ages from a range of 174–1,762 days. For TGF-β and VEGF we tested for more than one receptor, since it had been shown that different receptor subtypes mediate different signals. Thus, both receptor expression levels were included into a multivariate regression model to adjust for mutual influences.
All results are presented as means ± SD with 95% confidence intervals (95% CI). Results from the regression analyses are given with the regression coefficient for age in months instead of age in days (i.e., the average change in receptor expression adjusted by GAPDH per 1 month change in age). An alpha of 5% was considered significant but adjusted with the Bonferroni method for multiple testing when appropriate. All calculations were done using intercooled STATA 10 (StataCorp. LP, College Station, TX).
RESULTS
All ACL cell samples showed expression of the studied growth factor receptors. The strongest expression was seen for VEGF receptors, with VEGF-R2 expressed at higher levels than VEGF-R1. Receptors for FGF and TGF-β, too, showed high expression, while the expression of PDGF receptors was the lowest.
When compared between young and adolescent animals, there were no significant differences in the levels of expression for any of the studied receptors, although expression of VEGF-R1 was borderline to significantly higher in the group of young animals (p = 0.07). Table 3 summarizes details on the mean receptor expression per group, mean differences, and statistical significance.
Table 3.
Normalized Growth Factor Receptor Expression (Ctreceptor – CtGAPDH)
| Receptor | Young (Mean ±SD) | Adolescent (Mean ± SD) | Mean Difference ± SD | 95% CI | p-Valuea | |
|---|---|---|---|---|---|---|
| TGF-β-R3 | 8.2 ± 0.9 | 8.4 ± 0.6 | −0.2 ± 1.0 | −2.3 | 1.9 | 0.8450 |
| TGF-β-R1 | 11.7 ± 0.7 | 12.6 ± 0.4 | −0.9 ± 0.8 | −2.4 | 0.7 | 0.2553 |
| PDGFR | 8.1 ± 0.6 | 7.9 ± 0.7 | 0.2 ± 0.9 | −1.6 | 2.0 | 0.8300 |
| FGFR2 | 12.8 ± 0.7 | 12.8 ± 0.6 | 0 ± 0.8 | −1.7 | 1.7 | 0.9910 |
| VEGFR1 | 13.0 ± 0.5 | 12.0 ± 0.3 | 1.1 ± 0.6 | −0.1 | 2.2 | 0.0748 |
| VEGFR2 | 19.5 ± 1.0 | 19.7 ± 0.9 | −0.2 ± 1.4 | −2.9 | 2.6 | 0.8912 |
95% CI, 95% confidence interval of mean difference between groups.
This table shows the details of the receptor expression in the young and adolescent groups.
From two-tailed t-test.
Our regression analysis revealed that age is a predictor of some of the studied receptors. All receptors showed a negative correlation with age, that is, a decrease in expression levels over time (Fig. 1). We observed a significant association of TGF-R1 with age (p = 0.005, coefficient −0.08, 95% CI −0.13 to −0.02), while there was no association between TGF-R3 and age (p = 0.082, coefficient −0.05, 95% CI −0.11 to 0.01). In the multivariate model adjusting for TGF-R3 level, the association between TGF-R1 and age was attenuated but remained statistically significant (p = 0.033). VEGF receptors showed no significant association with age, although the association withVEGF-R2 was borderline significant (p = 0.052, coefficient −0.18, 95% CI −0.17 to 0.001), in contrast to VEGF-R1 (p = 0.235, coefficient −0.03, 95% CI −0.17 to 0.02). Again, in the multivariate regression model adjusting for VEGF-R1 levels the association between age and VEGF-R2 changed only marginally (p = 0.066). There was a significant association between FGF-R and age (p <0.001, coefficient −0.12, 95% CI −0.17 to −0.07), while the association of PDGF-R with age was not statistically significant (p = 0.092).
Figure 1.
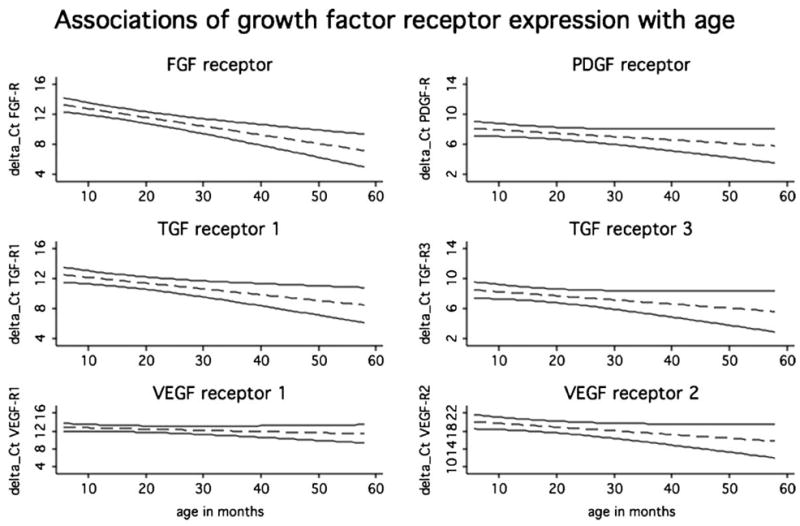
The fitted lines from the linear regression of growth factor expression on age (dashed lines). The solid lines give the upper and the lower limits of the corresponding 95% CIs. Statistically significant association with age was found for TGF-β-R1 (p = 0.01), FGF-R (p <0.01), and VEGF-R2 (p = 0.05). Association with age can be considered borderline significant for TGF-β-R3 (p = 0.08) and PDGF-R (p = 0.09).
DISCUSSION
In this study we hypothesized that age-related differences in the response of ACL fibroblasts to exposure to a collagen–platelet composite are caused by differences in the expression of receptors for the growth factors released by PRP. While we found no difference between the mean growth factor expression in young and adolescent animals, we observed a negative correlation between age and levels of mRNA expression. These associations were statistically significant or at least borderline significant for almost all studied genes, suggesting that growth factor expression plays a role in the observed age-related differences.
A collection of recent studies has described the method and early results of tissue engineering enhanced ACL repair.13,14,17 Among the key factors for the success of this new technique is the stimulating power of platelet concentrate (Fleming BC, Magarian E, Harrison S, et al. 2009. Collagen scaffold supplementation does not improve functional properties of the repaired anterior cruciate ligament, submitted).10 Prior research has identified some of the PDGFs, including TGF-β, VEGF, and FGFs.20,24,28 ACL fibroblasts have been shown to respond strongly and favorably to both a typical cocktail of growth factors released by PRP as well as individual growth factors alone, by increasing proliferative and biosynthetic activity.29 Yet it has been shown that the response of ACL fibroblast differs depending on age, implying that older patients might respond less favorably to such procedures.22,23 However, the release of growth factors from PRP is independent from age and gender, and a constant function of platelet concentration, which can be chosen with much reliability and reproducibility.20,24,25 Thus, growth factor concentrations are an unlikely reason for age-dependent differences, and we hypothesized that it is rather the levels of growth factor receptors that might vary and cause the age-related differences seen in cell response to PRP.
Our first hypothesis was that there would be a difference in the mean expression of the studied receptors between young and immature animals. This hypothesis is based on reported observations showing that most of the differences in outcomes are seen between these groups, while the differences between adolescent and adult animals were mostly nonsignificant. Yet in contrast to what we originally expected, there were no significant differences between these groups. However, it is a well-documented fact that categorization of a continuous variable, such as age in our case, causes the loss of approximately one-third of information, thus a reduction in statistical power.34,35 Also, care should be taken when choosing a parameter to describe age. Stratification by physeal status of the knee, as done in the first part of this study, might not be the most appropriate, or most important, surrogate for cellular aging. Therefore, we also studied the association between age as continuous variable and growth factor receptor expression directly.
The regression analysis of growth factor receptor expression on age showed that all levels of expression decreased with age. These decreases were statistically significant or at least borderline. Thus, for example, VEGF-R2, which has a nonsignificant p-value of 0.052, can be considered at least meaningful. The only receptor without an association with age was VEGF-R1. However, while the function of VEGF-R1 is not unequivocally defined yet, it is thought to modulate VEGF-R2 activity.36 Thus, as a modulator of VEGF-R2, which shows age-dependent expression, it would make sense if VEGF-R1 expression were age-independent. It should be considered though that VEGF action, which is commonly associated with vascularization, might not be as important for ACL fibroblasts, which thrive in a rather hypoxic and hypovascular environment. Those receptors with strong statistical evidence for an association with age bind TGF-β and FGF, well-known stimulators of mesenchymal cell growth and migration.24,37 These findings support earlier studies that showed reduced proliferation and migration in fibroblast from older individuals.22,23 The differences seen between TGF-β-R1 and -R3 can be explained by their different functions. TGF-β-R1 has a high affinity for TGF-β-1, which is a known stimulator of proliferation and differentiation.37,38 In this function TGF-β-R1 and is almost equal with TGF-β-R2, which we therefore did not study. Also, much like the interaction between VEGF-R1 and VEGF-R2, TGF-β-R2 seems to modulate the function of TGF-β-R1.37 TGF-β-R3 has a high affinity for TGF-β-2, the function of which seems to lie mostly in embryonic development and proliferation of stromal cells, has been suggested to be less important for fibroblasts.38,39
Our study has potential shortcomings. First of all, we analyzed a few selected receptors, but we chose those that are well known and recognized for their association with cellular growth and biosynthetic activity. We included different subtypes of receptors if it had been shown that they were associated with different functions, such as VEGF receptor 1 modulating the activity of VEGF receptor 2. However, our findings can be interpreted as indicators of the general trend for other receptors. Secondly, a point could be made that our sample is somewhat small. However, we based our sample size on data reported in a number of independent data sets published in the literature. Finally, it should be considered that our study produces a snapshot of baseline growth factor receptor expression, which is a very dynamic process that might very well change in interaction with PRP.31–33 However, all such changes depend on the cells initial ability to detect growth factors, which leads back to our study question.
In summary, our study showed that the levels of expression of the receptors for the most important growth factors released by PRP decrease with age. Our findings suggest that growth factor receptor expression is not the only reason for the differences in responses of ACL fibroblasts at different ages but plays an important role. Future studies might show that targeted up-regulation of PRP receptors might proof more valuable than higher concentrations of PRP to optimize treatment effects in older individuals.
Acknowledgments
This study was funded by the NIH NIAMS grant R01 AR052772 and NIH grant AR054099. The senior author is a founder and stockholder in Connective Orthopaedics, the first author is a consultant for Connective Orthopaedics. Each author certifies that he or she has no commercial associations (e.g., consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article.
References
- 1.Louboutin H, Debarge R, Richou J, et al. Osteoarthritis in patients with anterior cruciate ligament rupture: a review of risk factors. Knee. 2009;16:239–244. doi: 10.1016/j.knee.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Oiestad BE, Engebretsen L, Storheim K, et al. Knee osteoarthritis after anterior cruciate ligament injury: a systematic review. Am J Sports Med. 2009;37:1434–1443. doi: 10.1177/0363546509338827. [DOI] [PubMed] [Google Scholar]
- 3.Gillquist J, Messner K. Anterior cruciate ligament reconstruction and the long-term incidence of gonarthrosis. Sports Med. 1999;27:143–156. doi: 10.2165/00007256-199927030-00001. [DOI] [PubMed] [Google Scholar]
- 4.Lohmander LS, Ostenberg A, Englund M, et al. High prevalence of knee osteoarthritis, pain, and functional limitations in female soccer players twelve years after anterior cruciate ligament injury. Arthritis Rheum. 2004;50:3145–3152. doi: 10.1002/art.20589. [DOI] [PubMed] [Google Scholar]
- 5.Meunier A, Odensten M, Good L. Long-term results after primary repair or non-surgical treatment of anterior cruciate ligament rupture: a randomized study with a 15-year follow-up. Scand J Med Sci Sports. 2007;17:230–237. doi: 10.1111/j.1600-0838.2006.00547.x. [DOI] [PubMed] [Google Scholar]
- 6.von Porat A, Roos EM, Roos H. High prevalence of osteoarthritis 14 years after an anterior cruciate ligament tear in male soccer players: a study of radiographic and patient relevant outcomes. Ann Rheum Dis. 2004;63:269–273. doi: 10.1136/ard.2003.008136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murray MM. Current status and potential of primary ACL repair. Clin Sports Med. 2009;28:51–61. doi: 10.1016/j.csm.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vavken P, Murray MM. Translational studies in ACL repair. Tissue Eng Part A. 2009 doi: 10.1089/ten.teb.2009.0147. [DOI] [PubMed] [Google Scholar]
- 9.Murray MM, Martin SD, Spector M. Migration of cells from human anterior cruciate ligament explants into collagen-glycosaminoglycan scaffolds. J Orthop Res. 2000;18:557–564. doi: 10.1002/jor.1100180407. [DOI] [PubMed] [Google Scholar]
- 10.Murray MM, Palmer M, Abreu E, et al. Platelet-rich plasma alone is not sufficient to enhance suture repair of the ACL in skeletally immature animals: an in vivo study. J Orthop Res. 2009;27:639–645. doi: 10.1002/jor.20796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murray MM, Spector M. The migration of cells from the ruptured human anterior cruciate ligament into collagen-glycosaminoglycan regeneration templates in vitro. Biomaterials. 2001;22:2393–2402. doi: 10.1016/s0142-9612(00)00426-9. [DOI] [PubMed] [Google Scholar]
- 12.Murray MM, Spindler KP, Abreu E, et al. Collagen-platelet rich plasma hydrogel enhances primary repair of the porcine anterior cruciate ligament. J Orthop Res. 2007;25:81–91. doi: 10.1002/jor.20282. [DOI] [PubMed] [Google Scholar]
- 13.Murray MM, Spindler KP, Ballard P, et al. Enhanced histologic repair in a central wound in the anterior cruciate ligament with a collagen-platelet-rich plasma scaffold. J Orthop Res. 2007;25:1007–1017. doi: 10.1002/jor.20367. [DOI] [PubMed] [Google Scholar]
- 14.Murray MM, Spindler KP, Devin C, et al. Use of a collagen-platelet rich plasma scaffold to stimulate healing of a central defect in the canine ACL. J Orthop Res. 2006;24:820–830. doi: 10.1002/jor.20073. [DOI] [PubMed] [Google Scholar]
- 15.Fleming BC, Carey JL, Spindler KP, et al. Can suture repair of ACL transection restore normal anteroposterior laxity of the knee? An ex vivo study. J Orthop Res. 2008;26:1500–1505. doi: 10.1002/jor.20690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fleming BC, Spindler KP, Palmer M, et al. Collagen-platelet composites improve the biomechanical properties of healing ACL grafts in a porcine model. AJSM. 2009 doi: 10.1177/0363546509332257. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spindler KP, Murray MM, Carey JL, et al. The use of platelets to affect functional healing of an anterior cruciate ligament (ACL) autograft in a caprine ACL reconstruction model. J Orthop Res. 2009;27:631–638. doi: 10.1002/jor.20785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anitua E, Sanchez M, Nurden AT, et al. Autologous fibrin matrices: a potential source of biological mediators that modulate tendon cell activities. J Biomed Mater Res A. 2006;77:285–293. doi: 10.1002/jbm.a.30585. [DOI] [PubMed] [Google Scholar]
- 19.Creaney L, Hamilton B. Growth factor delivery methods in the management of sports injuries: the state of play. Br J Sports Med. 2008;42:314–320. doi: 10.1136/bjsm.2007.040071. [DOI] [PubMed] [Google Scholar]
- 20.Eppley BL, Woodell JE, Higgins J. Platelet quantification and growth factor analysis from platelet-rich plasma: implications for wound healing. Plast Reconstr Surg. 2004;114:1502–1508. doi: 10.1097/01.prs.0000138251.07040.51. [DOI] [PubMed] [Google Scholar]
- 21.Sanchez M, Anitua E, Azofra J, et al. Comparison of surgically repaired Achilles tendon tears using platelet-rich fibrin matrices. Am J Sports Med. 2007;35:245–251. doi: 10.1177/0363546506294078. [DOI] [PubMed] [Google Scholar]
- 22.Mastrangelo A, Magarian E, Palmer M, et al. The effect of skeletal maturity on the regenerative function of intrinsic ACL cells. J Orthop Res. 2009 doi: 10.1002/jor.21018. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mastrangelo A, Haus B, Vavken P, et al. Immature animals have denser anterior cruciate ligament wound site cell repopulation than adolescent or adult animals. J Orthop Res. 2009 doi: 10.1002/jor.21070. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Everts PA, Knape JT, Weibrich G, et al. Platelet-rich plasma and platelet gel: a review. J Extra Corpor Technol. 2006;38:174–187. [PMC free article] [PubMed] [Google Scholar]
- 25.Weibrich G, Kleis WK, Hafner G, et al. Growth factor levels in platelet-rich plasma and correlations with donor age, sex, and platelet count. J Craniomaxillofac Surg. 2002;30:97–102. doi: 10.1054/jcms.2002.0285. [DOI] [PubMed] [Google Scholar]
- 26.Hofstaetter JG, Saad FA, Samuel RE, et al. Differential expression of VEGF isoforms and receptors in knee joint menisci under systemic hypoxia. Biochem Biophys Res Commun. 2004;324:667–672. doi: 10.1016/j.bbrc.2004.09.103. [DOI] [PubMed] [Google Scholar]
- 27.Hofstaetter JG, Wunderlich L, Samuel RE, et al. Systemic hypoxia alters gene expression levels of structural proteins and growth factors in knee joint cartilage. Biochem Biophys Res Commun. 2005;330:386–394. doi: 10.1016/j.bbrc.2005.02.168. [DOI] [PubMed] [Google Scholar]
- 28.van den Dolder J, Mooren R, Vloon AP, et al. Platelet-rich plasma: quantification of growth factor levels and the effect on growth and differentiation of rat bone marrow cells. Tissue Eng. 2006;12:3067–3073. doi: 10.1089/ten.2006.12.3067. [DOI] [PubMed] [Google Scholar]
- 29.Murray M, Rice K, Wright RJ, et al. The effect of selected growth factors on human anterior cruciate ligament cell interactions with a three-dimensional collagen-GAG scaffold. J Orthop Res. 2003;21:238–244. doi: 10.1016/S0736-0266(02)00142-0. [DOI] [PubMed] [Google Scholar]
- 30.El-Sharkawy H, Kantarci A, Deady J, et al. Platelet-rich plasma: growth factors and pro- and anti-inflammatory properties. J Periodontol. 2007;78:661–669. doi: 10.1902/jop.2007.060302. [DOI] [PubMed] [Google Scholar]
- 31.Bonner JC, Badgett A, Lindroos PM, et al. Transforming growth factor beta 1 downregulates the platelet-derived growth factor alpha-receptor subtype on human lung fibro-blasts in vitro. Am J Respir Cell Mol Biol. 1995;13:496–505. doi: 10.1165/ajrcmb.13.4.7546780. [DOI] [PubMed] [Google Scholar]
- 32.Ichiki Y, Smith E, LeRoy EC, et al. Different effects of basic fibroblast growth factor and transforming growth factor-beta on the two platelet-derived growth factor receptors’ expression in scleroderma and healthy human dermal fibroblasts. J Invest Dermatol. 1995;104:124–127. doi: 10.1111/1523-1747.ep12613617. [DOI] [PubMed] [Google Scholar]
- 33.Messadi DV, Le A, Berg S, et al. Effect of TGF-beta 1 on PDGF receptors expression in human scar fibroblasts. Front Biosci. 1998;3:a16–a22. doi: 10.2741/A246. [DOI] [PubMed] [Google Scholar]
- 34.Altman DG, Royston P. The cost of dichotomising continuous variables. Br Med J. 2006;332:1080. doi: 10.1136/bmj.332.7549.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Royston P, Altman DG, Sauerbrei W. Dichotomizing continuous predictors in multiple regression: a bad idea. Stat Med. 2006;25:127–141. doi: 10.1002/sim.2331. [DOI] [PubMed] [Google Scholar]
- 36.Petrova TV, Makinen T, Alitalo K. Signaling via vascular endothelial growth factor receptors. Exp Cell Res. 1999;253:117. doi: 10.1006/excr.1999.4707. [DOI] [PubMed] [Google Scholar]
- 37.Clark DA, Coker R. Molecules in focus transforming growth factor-beta (TGF-[beta]) Int J Biochem Cell Biol. 1998;30:293. doi: 10.1016/s1357-2725(97)00128-3. [DOI] [PubMed] [Google Scholar]
- 38.Ellis IR, Schor SL. Differential mitogenic and biosynthetic response of fetal and adult skin fibroblasts to TGF-beta isoforms. Cytokine. 1998;10:281–289. doi: 10.1006/cyto.1997.0294. [DOI] [PubMed] [Google Scholar]
- 39.Kottler UB, Junemann AG, Aigner T, et al. Comparative effects of TGF-beta 1 and TGF-beta 2 on extracellular matrix production, proliferation, migration, and collagen contraction of human Tenon’s capsule fibroblasts in pseudoexfoliation and primary open-angle glaucoma. Exp Eye Res. 2005;80:121–134. doi: 10.1016/j.exer.2004.08.018. [DOI] [PubMed] [Google Scholar]


