Abstract
How the fetal “allograft” avoids rejection during pregnancy remains a major unresolved immunological paradox. Recent work has suggested that fetomaternal tolerance is in fact maintained by a number of redundant mechanisms, but their relative importance has remained poorly defined. Here, I discuss an emerging controversy regarding the ability of maternal T cells to mediate fetal rejection at a time when they appear to be ignorant of fetal and placental antigens. This paradox-within-a-paradox highlights two major research directions in the field of reproductive immunology, that when ultimately reconciled, promise to give significant insight into mechanisms of impaired fertility and compromised fetal and maternal health.
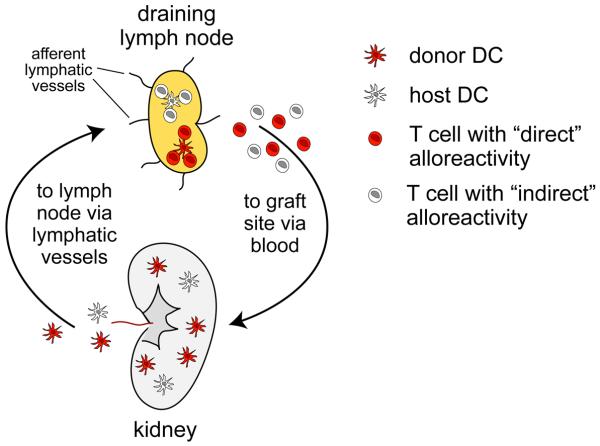
The immunological rejection of organ transplants classically unfolds as a multistep process involving multiple cell types acting at discrete anatomical locations (Figure 1). The process is initiated when dendritic cells (DCs), which are the highly potent antigen presenting cells stationed as immunological sentinels throughout the body, become activated and migrate via lymphatic vessels from the site of the transplant to the draining lymph nodes (LN). These cells have two potential sources: they can be of donor origin, which implies that they will present graft-derived peptides on donor major histocompatibility complex (MHC) molecules, or they can be of host origin, which implies that they will present graft-derived peptides on host MHC molecules. These distinct antigen presentation pathways will activate non-overlapping sets of host T cells, which will undergo a stereotypical response of proliferation and differentiation to generate large numbers of effector T cells of varying specificities. These T cells will then leave the LN, migrate via the blood to the site of the transplant, and, upon arrival, express their cytolytic and cytokine-producing effector function to induce the tissue damage that ultimately causes graft necrosis.
Figure 1.
An overview of solid organ transplant rejection. The rejection of a solid organ transplant, such as the kidney depicted here, is initiated by migratory DCs of both donor and host origin. Donor DCs (red) are transplanted along with the graft itself, and upon their surgery-induced activation migrate to the draining LN via lymphatic vessels. These cells stimulate host T cells that react with graft-derived peptides presented by donor haplotype MHC molecules, a pathway termed “direct” allorecognition [41]. In contrast, host DCs (white) differentiate de novo within the graft from precursors recruited from host blood. Upon their subsequent migration to the LN, these cells stimulate host T cells that react with graft-derived peptides presented by host haplotype MHC molecules, a pathway termed “indirect” allorecognition. Both sets of activated T cells proliferate and differentiate within the LN to generate large numbers of effector T cells. These cells then exit the LN, migrate via the blood to the graft site, and then perform the effector functions that induce graft necrosis. These effector functions include the direct killing of graft cells by CD8+ cytotoxic T cells (CTLs) that possess direct alloreactivity to donor MHC class I molecules, as well as the elaboration of inflammatory cytokines such as IFN-γ and TNF-α by CD8+ and CD4+ T cells that possess either direct or indirect graft alloreactivity. These cytokines impair graft survival through a variety of mechanisms, including their direct killing of transplanted cells or host endothelial cells, or their activation of other effector cells of the immune system such as macrophages.
Since pregnancy involves the close apposition of two disparate tissues, the uterus and the placenta, the tenets of transplantation immunology predict that the placenta, along with the fetus, would be rejected like all genetically mismatched organ transplants. The fact that this does not normally occur has provided an ongoing source of fascination to immunologists and reproductive biologists alike since the time this paradox was first articulated over 50 years ago [1]. Given the multistep nature of organ transplant rejection, there are many potential opportunities for preventing rejection of the fetal allograft, and the reader is referred to several excellent reviews for a comprehensive overview of the field and the multiple mechanisms currently implicated in maintaining fetomaternal tolerance [2-4]. Here, I will focus on recent data in mice suggesting that one such mechanism involves attenuating immune surveillance of the maternal/fetal interface during the first half of gestation so that the initial exposure of maternal T cells to fetal/placental antigens is minimized. These data present a conundrum, however, as other recent data suggest that T cell-mediated fetal rejection can indeed be induced early in gestation in a true “allospecific” fashion, meaning that it occurs in allogeneic but not syngeneic mating combinations. How can T cells mediate allospecific fetal rejection at a time when they are not even aware of fetal/placental alloantigens? After describing the essentials of these two sets of data, I will propose several ways that they might be reconciled. In particular, I discuss the possibilities that pregnancy failure results either from T cell priming to antigens present in semen rather than the implanted conceptus, or from aberrant NK cell activation at the maternal/fetal interface leading to bystander T cell activation. In both cases, I suggest that the T cells contributing to fetal loss might not actually have specificity towards fetal/placental antigens.
T cell-mediated fetal “rejection” during the first half of murine gestation
The first clear example of allospecific, T cell-mediated abortion came from a seminal study on the effect of 1-methyl-tryptophan on murine pregnancy [5]. In this study, systemic administration of 1-methyl-tryptophan, which had previously been characterized as a substrate of the tryptophan-catabolizing enzyme idoleamine 2,3-dioxygenase (IDO), induced wholesale fetal deterioration by embryonic day (E) 9.5 in female mice mated to allogeneic males but not syngeneic males. Moreover, abortion was not induced when the females were on a T and B cell-deficient Rag1−/− background. Interestingly, intercrosses of mice with targeted deletions of the gene encoding IDO later revealed that IDO itself was not required for successful allogeneic pregnancy [6]. Nonetheless, these matings were resistant to the abortion-inducing effects of 1-methyl-tryptophan. A likely scenario was that 1-methyl-tryptophan catabolism by IDO generated metabolic products that induced abortion in an allospecific and T cell- (and/or perhaps B-cell-) dependent fashion.
T cell alloresponses have also been implicated in a model of murine abortion induced by regulatory T cell (Treg) deficiency [7]. In this model, T cell-deficient nu/nu females were reconstituted with Treg-depleted polyclonal T cells and then immediately mated to syngeneic or allogeneic males. When the mating partners were syngeneic males, pregnancy rates and litter sizes were normal. In contrast, allogeneic matings gave no living pups when pregnancy was assessed between E11.5 and E19.5. Logically, this implied that Treg deficiency induces wholesale fetal loss prior to E11.5 in an allospecific fashion. A separate study gave similar allospecific results when Tregs were depleted one day prior to mating with antibodies towards CD25, a Treg cell-surface marker [8]. Conversely, Treg injection prevented fetal loss in the abortion-prone DBA/2 male X CBA/J female mating combination, which otherwise shows a high percentage (~20-30%) of resorbing pups within each litter as early as E10.5-12.5 [9]. Although the T cell dependence of abortion in this latter model has not been firmly established, the known ability of Tregs to suppress T cell-mediated alloresponses is consistent with antigen-specific T cells contributing to fetal death.
A third example of allospecific early pregnancy loss comes from a study on the gestational function of Galectin-1 (Gal-1), a secreted glycan-binding protein with pleiotropic effects on T cells [10]. Gal-1-deficient females showed increased rates of fetal resorption by E13.5 when mated to allogeneic, but not syngeneic, males, while administration of recombinant Gal-1 to DBA/2-mated CBA/J females conversely prevented fetal loss under conditions of sound stress. Lastly, allospecific abortion by E13.5 has been observed following antibody blockade of programmed death ligand 1 (PD-L1, also called B7-H1), an inhibitory costimulatory molecule expressed by a number of cell types including placental cells, and whose receptor PD-1 is expressed by T cells [11]. Here too, abortion was T cell- (and/or B cell-) dependent, as it was not observed in Rag1-deficient females. Furthermore, PD-L1−/− mice on a mixed genetic background had reduced number of implantation sites when crossed to allogeneic males. This result is controversial, however, as inbred mice with targeted deletions of either PD-L1 or PD-1 show normal pregnancy rates in both allogeneic and syngeneic mating combinations [12].
Evidence for T cell ignorance of the fetus and placenta during the first half of gestation
Taken at face value, the above results indicate that T cell allorecognition plays a central role in immune-mediated early fetal loss. As mentioned, however, this interpretation conflicts with recent attempts to directly document T cell responses to the early mouse conceptus (i.e. prior to ~E12.5). Notably, these attempts date back to the mid-1990s, when an influential paper by Tafuri et al. showed that maternal T cell receptor (TCR) transgenic T cells with specificity for an allogeneic paternal MHC I molecule underwent dramatic phenotypic changes (TCR and CD8 downregulation) when females were mated to allogeneic males of the appropriate strain background, but not to syngeneic or third-party allogeneic males [13]. However, these results could not be repeated in two separate studies that used other strains of TCR transgenic mice also with direct reactivity towards paternal MHC I molecules [14, 15]. Despite the natural concern over interpreting negative data, and the possibility that the various transgenic TCRs used in these experiments might have substantially different avidities towards their respective peptide/MHC complexes, these latter results were more consistent with maternal T cells being ignorant of intact paternal MHC I molecules.
Resolving these contradictory data, and indeed revealing the minimal extent of maternal T cell awareness of the fetus and placenta, only became possible with the development of a model system that, ironically, provided the first unequivocal example of a strong anti-fetal/placental alloresponse [16]. This system is based on use of the Act-mOVA transgenic mouse line, which expresses a transmembrane form of ovalbumin (OVA) from the ubiquitously active β-actin promoter. Fortuitously, the Act-mOVA transgene is expressed by the labyrinthine trophoblasts of the placenta, and at particularly high levels by invasive trophoblasts directly at the maternal/fetal interface [16]. Thus, mating male mice hemizygous for the Act-mOVA transgene to non-transgenic females creates a situation where OVA is highly expressed as a surrogate placental antigen in 50% of the concepti. Maternal T cell responses to placental OVA can then be detected by injecting a small number of fluorescently-labeled TCR transgenic T cells with known specificity towards OVA-derived peptides. If these cells encounter the appropriate OVA peptide/MHC complex, they initiate a proliferative response that can be easily detected by flow cytometry as the serial two-fold dilution of their fluorescence intensity. The approach allows for a high resolution analysis of the timing and the locale of fetal/placental antigen presentation during pregnancy.
Using this approach, two groups have now demonstrated robust proliferative responses of OVA-specific CD4 and CD8 T cells in female mice bearing OVA-expressing concepti [16, 17]. In addition to the uterine LN, these responses were apparent in the spleen and subcutaneous LN, most likely because the passive shedding of OVA-associated membranous debris directly into the maternal circulation gave it access to DCs already resident within the spleen and LN. Both groups also demonstrated that fetal/placental OVA presentation was mediated exclusively by antigen presenting cells (APCs) of maternal rather than fetal origin. The logical extension of this key finding, and an idea that was directly demonstrated in the case of CD8 T cells and one paternal MHC haplotype [16], is that maternal T cells indeed remain unaware of intact paternal MHC molecules during pregnancy, as previously suggested [14, 15]. The situation during pregnancy is thus very different than what is seen following organ transplantation, where T cell responses are initiated by APCs of both host and donor origin (Figure 1). In fact, donor DCs migrating from the site of a surgical organ transplant to the draining LN are considered to be the primary instigators of graft rejection because their expression of donor MHC molecules induces the activation of up to ~0.5-7.0% of host T cells [18, 19]. The non-involvement of an analogous population of “donor” APCs in the anti-fetal/placental T cell response, most likely because the developing mouse embryo simply does not generate DCs until late gestation [20], means that any T cell-mediated alloresponse would have to be initiated by maternal DCs presenting minor histocompatibility antigens. Since the precursor frequency of T cells with such “indirect” alloreactivity are thought to be ~10X less that the precursor frequency of T cells capable of directly recognizing donor MHC molecules (i.e. with “direct” alloreactivity) [18], the developmental limitations in generating fetal DCs in essence reduces the numerical T cell threat to fetal survival by 90%.
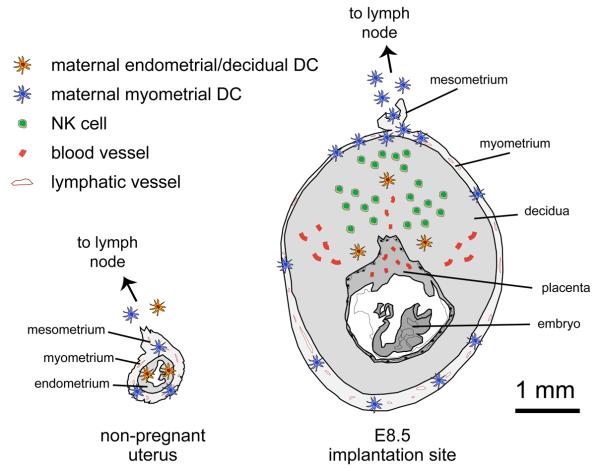
Compounding the lack of involvement of donor APCs in fetal allorecognition, these studies also found that robust OVA presentation commenced only at midgestation (around E10.5), despite clear expression of OVA by cells of the developing placenta as early as E7.5 [16, 17]. This absent early response was explained by a more recent study demonstrating that endometrial DCs become trapped within the tissue upon the transformation of the endometrium into the decidua, the specialized stromal structure formed upon blastocyst implantation to encase the fetus and placenta [21] (Figure 2). Remarkably, these “decidual” DCs remained immobile even after their maturation was induced in situ via intravenous LPS injection, a powerful proinflammatory agent and direct stimulant of DC activation [21]. The entrapment of decidual DCs was furthermore linked to the specialized anatomy of the pregnant mouse uterus and the 1-2 mm thickness of decidual tissue that separates the embryo and early placenta from the overlying smooth musculature (the myometrium), which is the only tissue layer of the mouse uterus containing lymphatic vessels [22]. As a result of the entrapment of decidual DCs, T cell recognition of fetal/placental OVA, even in the draining uterine LN, was mediated by the passive processes mentioned above that presumably only start at midgestation, or only then become first detectable. Since T cells are poorly primed when presented with antigen exclusively by LN-resident DCs [23, 24], the lack of participating migratory DCs in the regional T cell response was likely a major reason that fetal/placental OVA presentation failed to induce T cell priming, even though it induced T cell proliferation. Indeed, the proliferative response to fetal/placental OVA during the second half of gestation was associated with T cell clonal deletion, which is a frequently documented mechanism of T cell peripheral tolerance induction.
Figure 2.
DC-mediated immune surveillance of the non-pregnant and pregnant uterus. (a) In the non-pregnant uterus, DCs are present within both the endometrium (orange cells) and the myometrium (blue cells). Even though the lymphatic vessels of the uterus are confined to the myometrium, maturing endometrial DCs reach these vessels by migrating through the ~0.2-0.4 mm that separates the uterine lumen from the myometrium [21]. This migration allows endometrial DCs to access the draining LN so they can presumably orchestrate immune responses to uterine pathogens. In contrast, the transformation of the endometrium into the decidua upon embryo implantation traps DCs the maternal/fetal interface (b), and thus prevents them from contributing towards T cell recognition of the fetus and placenta [21]. Fetal and placental antigens are instead only transported by DC-independent processes, to be presented by DCs already resident within the LN (and spleen). These latter cells tend to be less inherently immunogenic than DCs stationed within peripheral tissues. Most likely, DC migration from the maternal/fetal interface is severely limited because decidual DCs close to the placenta have to migrate through up to ~1-2 mm of interstitial tissue before encountering a lymphatic vessel. In contrast, myometrial DCs in both the pregnant and non-pregnant uterus have direct access to the myometrial lymphatics and can easily exit the uterus. The 1 mm scale bar applies to both the E8.5 implantation site cross-section as well as to the identically-scaled cross-section of the non-pregnant uterus. The lymphatic vessels of the uterus exit via the mesometrium, a membrane that runs longitudinally along its length. The E8.5 implantation site diagram also depicts the large number of dNK cells located towards the mesometrial pole of the decidua, as well as some major vascular structures.
Immune surveillance of the maternal/fetal interface: a controversy
The idea that T cells can induce fetal rejection in early gestation thus runs against well-controlled evidence of T cell ignorance over the same time period, and against evidence for the tissue entrapment of those DCs that directly survey the maternal/fetal interface. How can this contradiction be explained? Clearly, one possibility is that some natural alloantigens, in contrast to OVA, are able to passively reach the LN prior to E10.5 to serve as peptide sources for T cell priming under abortion-inducing conditions. The expected candidates for these alloantigens would be polymorphic MHC molecules of paternal origin since they provide a rich source of allopeptides for presentation on maternal MHC molecules. While attractive in principle, however, this idea runs into the immediate difficulty that polymorphic MHC molecules are not expressed at appreciable protein levels by cells of the early mouse conceptus [16, 25-27]. Thus, we would have to postulate that rejection was primarily driven by a natural minor histocompatibility antigen with greater immunogenicity and/or access to LN-resident DCs than the transmembrane form of OVA expressed by the Act-mOVA transgene.
Along the same lines, allospecific abortion-inducing manipulations might somehow release DCs from their decidual entrapment, thus allowing them to present alloantigens in the draining LN. This is a testable hypothesis as it predicts, for example, that 1-methyl-tryptophan administration to Act-mOVA-mated females, or Treg depletion in these mice, would induce premature anti-OVA T cell proliferative responses that lead to T cell priming rather than clonal deletion. Indeed, combining the Act-mOVA mating system with experimental manipulations that induce allospecific abortion could conversely be used to dissect the specific pathways that initiate the anti-fetal response. Such an approach has been taken in a recent study that showed that anti-OVA T cells deficient in PD-1 accumulated in the uterine LN more than wild-type anti-OVA T cells [12]. The significance of this finding, however, is unclear since this study was the same one already mentioned that demonstrated normal allogeneic pregnancy rates in mice deficient in PD-L1 or PD-1.
Invoking premature fetal/placental presentation to explain pathological early pregnancy loss, however, still runs into the difficulty that the fastest known time period for naïve T cells to induce graft rejection is ~10 days, as seen with skin grafts. Thus, it is unclear how a classical rejection response could be mounted by E9.5-13.5 in virgin mice, given that implantation occurs ~E4.5. One way to accommodate this issue is to conjecture that manipulations that lead to allospecific abortion do so by inducing paternal MHC expression by trophoblast giant cells surrounding the early conceptus, which in turn induces the immediate embryotoxic effector function of memory T cells that happen to be trafficking through the uterus at the time of implantation, and that happen to possess TCRs with the appropriate alloreactivity. A more likely alternative is that the events leading to T cell priming actually take place prior to implantation and are driven by alloantigens present in semen. Such priming could be the result of semen exposure within the vagina or cervix, or, as recently suggested using the Act-mOVA system [17], the result of semen exposure within the uterus itself. As might be expected, this study also showed that the presentation of seminal antigens in the uterine LN is mediated exclusively by maternal APCs, just like the presentation of fetal/placental antigens. However, the extent to which this response involves the migration of endometrial DCs from the uterus the draining LN is currently unknown. It therefore remains possible that prior to their decidual entrapment, endometrial DCs, addition to vaginal and cervical DCs, have the opportunity to stimulate T cell responses towards antigens potentially shared by the conceptus itself. Certain abortion-inducing manipulations might foster anti-fetal/placental T cell priming by activating these semen-exposed migratory DCs, or, in principle, by inhibiting any ability they might have to actively induce T cell tolerance (for recent work on the tolerogenic effects of semen, see reference [28]). Indeed, anti-OVA T cells in the uterine LN express low but detectable levels of IFN-γ and IL-2 in the pre-implantation period [17], suggesting that their exposure to seminal OVA is not quite as tolerogenic as exposure to fetal/placental OVA during gestation proper. As discussed below, it is also important to note that T cell responses to seminal antigens might induce pregnancy failure even if these antigens are not expressed by the conceptus itself.
A second major possibility for explaining the allospecific nature of early pregnancy loss invokes a role for decidual natural killer (dNK) cells. These cells constitute the major population of lymphocytes at the maternal/fetal interface, where they regulate placental development through their production of several growth factors and cytokines, including the inflammatory cytokine IFN-γ (reviewed in [29]). dNK cells also express a variety of activating and inhibitory receptors, many of which are polymorphic and whose ligands include both classical and non-classical MHC I molecules. Interestingly, one such class of polymorphic receptors in humans, the killer cell immunoglobulin-like receptors (KIRs), show an expression pattern that is biased towards recognition of HLA-C, the only classical MHC I molecule expressed by placental trophoblasts in direct contact with uterine tissue [30]. Furthermore, epidemiological work suggests that certain obstetrical complications, namely preeclampsia and recurrent miscarriage, might be associated with specific combinations of maternal KIR haplotype and fetal HLA-C haplotype, although this idea remains controversial [29]. Interestingly, the KIR/HLA-C haplotype combinations associated with poor pregnancy outcome are amongst those that would be expected to limit, rather than foster, dNK activation, an observation that is likely explained by the key role of dNK cells in regulating placental development and the associated remodeling of the uterine vasculature [31, 32]. Nonetheless, these data strongly suggest some level of allorecognition of the human placenta by dNK cells.
Interestingly, E9.5-10.5 mouse dNK cells also show a biased receptor expression pattern compared to peripheral NK cells [33]. Unlike the situation in humans, however, it is unclear how this could be taken as an indication of interactions with paternal MHC molecules given the lack of intact MHC I protein expression by early mouse conceptus. Instead, it has recently been suggested that early dNK cell activation might be regulated by engagement of NKG2D, an NK cell receptor that binds a number of stress-induced non-classical MHC I ligands [29]. Thus, the coordinated process of placental development in different strain combinations might be inherently associated with different levels of early dNK cell activation as a result of differential expression of these ligands by either placental or decidual cells. Such a conjecture would explain the curious finding that CBA/J females are abortion prone when mated to DBA/2 males but not when mated to BALB/c males, even though both male strains have the same MHC haplotype; this theory is also consistent with previous work implicating dNK cells as the major instigators of fetal loss in the DBA/2 × CBA/J model [34] and the recent observation that dNK cells show an activated phenotype even at baseline [35]. Indeed, mRNA expression of a number of NKG2D ligands has been detected at the human maternal/fetal interface [36], and protein expression for one of these ligands, RAE-1, has been detected in both decidual and placental cells of mouse implantation sites as early as E6.5 [37]. It is unknown, however, whether expression of these ligands is altered in pathological human pregnancies or in different mating combinations of mice. Nonetheless, these data raise the possibility that dNKs mediate murine abortion as a byproduct of their ability to detect developmental events rather than true allorecognition.
Antigen non-specific inflammation as an effector mechanism in early pregnancy loss
In considering immunological mechanisms of early fetal loss, it is important to note that a number of different immune cell types might produce inflammatory cytokines that, in combination, generate overall levels of systemic inflammation that are either directly toxic to the fetus, placenta, or decidua, or are incompatible with a functioning endocrine system. This latter possibility emerged from a study where agonistic monoclonal antibodies were used to systemically activate CD40, a costimulatory molecule expressed by DCs and B cells critically involved in the ability of CD4 T cells to provide help to CD8 T cells. Strikingly, CD40 activation caused pregnancy failure within four days when the antibody was administered in the immediate post-implantation period [38]. However, the effect was not due to a break in T cell tolerance since abortion was apparent in both allogeneic and syngeneic mating combinations, as well as in B and T cell-deficient Rag2−/− mice. Instead, fetal loss required systemic inflammation, and in particular TNF-α production that in turn inhibited ovarian synthesis of progesterone, the dominant hormone of pregnancy required to maintain decidual viability. Indeed, exogenous progesterone administration rescued pregnancy failure in mice treated with agonistic anti-CD40 antibodies [38].
The case of CD40 activation-induced abortion thus demonstrates the ability of non-specific inflammation to act as a general effector mechanism in pregnancy failure, independent of the inciting stimulus. Indeed, these experiments also revealed that systemic NK cell activation in the absence of T cells is sufficient to generate enough inflammation to inhibit ovarian function, which in turn suggests a way that pathological dNK cell activation alone might lead to pregnancy failure. However, there is no a priori reason to believe that inflammatory cytokines produced by T cells, even as the result of their bystander activation, would not be able to contribute to overall levels of inflammation and thus potentially amplify processes initiated by T cell-independent pathways. Combined with the conjecture that dNK cells might respond to different degrees of cellular stress at the maternal/fetal interface intrinsic to different mating combinations, this idea sets the stage for situations where pregnancy failure shows both an allospecific component as well as T cell dependence, yet does not require actual T cell awareness of the fetus and placenta. Similar arguments can be made with respect to the idea that inappropriate T cell responses to semen might lead to pregnancy failure, as the responding T cells need not show reactivity towards fetus- or placenta-specific antigens in order to threaten fetal survival. Testing whether exogenous progesterone rescues pregnancy failure in any of these situations would assess the possibility that ovarian insufficiency was the final cause of fetal demise.
Implications
Understanding how uterine DCs and NK cells survey the female reproductive tract has significant implications for disorders of human reproduction. Although rapid progress towards this goal is currently being made, the need for further work is highlighted by the paradox discussed here concerning the induction of T cell-dependent allospecific abortion in early mouse pregnancy despite the apparent minimal extent of concurrent T cell awareness of placental alloantigens. A role for dNK cells in fetal/placental allorecognition is already more established in humans as a result of the epidemiological associations between certain pregnancy complications and specific combinations of fetal and maternal HLA-C and KIR haplotypes. Elucidating the pathogenesis of these complications, however, will require the ability to test hypotheses in tractable animal models, and one limitation of using the mouse as a model organism is the current lack of knowledge regarding how mouse dNK cell activation might be regulated by developmental events at the maternal/fetal interface, and whether these events might be strain combination-specific. Indeed, the activation status of dNK cells in several of the allospecific murine abortion models discussed herein (1-methyl-tryptophan administration, Treg deficiency, Gal-1 deficiency) has also not yet been described. On the other hand, the mouse has recently been used to study the T cell response to semen, and to demonstrate that the uterine adaptation to pregnancy minimizes T cell awareness of the fetus and placenta by locally impairing DC migration. For these insights to be relevant towards human pregnancy, there needs to be greater insight into the function and intrinsic migratory potential of human decidual DCs (for a review of human decidual DCs, see reference [39]), as well as into the developmental differences in decidualization between mice and humans that might extrinsically limit or facilitate DC migration. For example, the presence of lymphatic vessels in the human but not mouse decidua [40] suggests a greater potential for human uterine DCs to survey the maternal/fetal interface, which in turn suggests that pathologies of human decidual development or simply inflammation at the human maternal/fetal interface might foster anti-fetal/placental T cell priming. Such T cell priming would increase the risk of true fetal “rejection” as well as the risk of increasing systemic level of inflammation deleterious to pregnancy outcome.
Acknowledgements
The author thanks the members of his lab for many stimulating discussions, and acknowledges grant support from the NIH (RO1-AI062980).
Glossary
- Major histocompatibility complex (MHC) molecules
The set of highly polymorphic transmembrane proteins that present protein antigen-derived peptides to T cells. Class I MHC molecules present antigens to CD8 T cells, while class II MHC molecules present antigens to CD4 T cells.
- Minor histocompatibility antigens
These antigens are non-MHC polymorphic proteins capable of inducing graft rejection. They can be expressed either ubiquitously or in a tissue-specific fashion. In the case of the fetal allograft, they would also include proteins expressed exclusively by the placenta.
- Dendritic cells (DCs)
DCs are bone marrow-derived cells that play a central role in initiating T cell responses. The cells are specialized for the uptake and presentation of exogenous antigens on both MHC class I and MHC class II molecules. When exposed to infectious agents or inflammation, they also upregulate their expression of T cell costimulatory molecules, making them highly potent antigen presenting cells. DCs are located throughout all peripheral tissues, in addition to the lymph nodes and spleen. In order to stimulate naïve T cells, a peripheral tissue DC must first migrate towards, and then enter, a nearby lymphatic vessel, which will carry it to the draining LN.
- Maternal/fetal interface
The interface between the decidua, which is maternal in origin and derived from the endometrial stroma, and the placenta and other trophectodermal derivatives (i.e. trophoblast giant cells in the mouse) that form the external surface of the implanted conceptus.
- Allogeneic versus syngeneic mating combinations
An allogeneic mating combination involves mating mice of two different strain backgrounds; a syngeneic mating combination involves mating mice of the same strain background. Only with allogeneic mating combinations is there the potential that paternal MHC molecules would be recognized as immunologically foreign.
- Regulatory T cell (Treg)
A CD4+ T cell that can suppress activation of other T cells.
- T cell clonal deletion
T cells start proliferating upon TCR engagement but undergo apoptosis in the absence of appropriate costimulation. This stereotypical response leads to the deletion of T cell clones with specificity towards the inducing antigen.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosure
The author declares no financial conflicts of interest.
References
- 1.Medawar PB. Some immunological and endocrinological problems raised by the evolution of viviparity in vertebrates. Symp. Soc. Exp. Biol. 1953;7:320–338. [Google Scholar]
- 2.Trowsdale J, Betz AG. Mother's little helpers: mechanisms of maternal-fetal tolerance. Nat Immunol. 2006;7:241–246. doi: 10.1038/ni1317. [DOI] [PubMed] [Google Scholar]
- 3.Moffett A, Loke C. Immunology of placentation in eutherian mammals. Nat Rev Immunol. 2006;6:584–594. doi: 10.1038/nri1897. [DOI] [PubMed] [Google Scholar]
- 4.Taglauer ES, et al. The hidden maternal-fetal interface: events involving the lymphoid organs in maternal-fetal tolerance. Int J Dev Biol. 2009;54:421–430. doi: 10.1387/ijdb.082800et. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Munn DH, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–1193. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 6.Baban B, et al. Indoleamine 2,3-dioxygenase expression is restricted to fetal trophoblast giant cells during murine gestation and is maternal genome specific. J Reprod Immunol. 2004;61:67–77. doi: 10.1016/j.jri.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Aluvihare VR, et al. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol. 2004;5:266–271. doi: 10.1038/ni1037. [DOI] [PubMed] [Google Scholar]
- 8.Darrasse-Jeze G, et al. CD4+CD25+ regulatory/suppressor T cells prevent allogeneic fetus rejection in mice. Immunol Lett. 2006;102:106–109. doi: 10.1016/j.imlet.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Zenclussen AC, et al. Abnormal T-cell reactivity against paternal antigens in spontaneous abortion: adoptive transfer of pregnancy-induced CD4+CD25+ T regulatory cells prevents fetal rejection in a murine abortion model. Am J Pathol. 2005;166:811–822. doi: 10.1016/S0002-9440(10)62302-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blois SM, et al. A pivotal role for galectin-1 in fetomaternal tolerance. Nat Med. 2007;13:1450–1457. doi: 10.1038/nm1680. [DOI] [PubMed] [Google Scholar]
- 11.Guleria I, et al. A critical role for the programmed death ligand 1 in fetomaternal tolerance. J Exp Med. 2005;202:231–237. doi: 10.1084/jem.20050019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taglauer ES, et al. Maternal PD-1 regulates accumulation of fetal antigen-specific CD8+ T cells in pregnancy. J Reprod Immunol. 2009;80:12–21. doi: 10.1016/j.jri.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tafuri A, et al. T cell awareness of paternal alloantigens during pregnancy. Science. 1995;270:630–633. doi: 10.1126/science.270.5236.630. [DOI] [PubMed] [Google Scholar]
- 14.Rogers AM, et al. Maternal-fetal tolerance is maintained despite transgene-driven trophoblast expression of MHC class I, and defects in Fas and its ligand. Eur J Immunol. 1998;28:3479–3487. doi: 10.1002/(SICI)1521-4141(199811)28:11<3479::AID-IMMU3479>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 15.Zhou M, and Mellor AL. Expanded cohorts of maternal CD8+ T-cells specific for paternal MHC class I accumulate during pregnancy. J Reprod Immunol. 1998;40:47–62. doi: 10.1016/s0165-0378(98)00030-8. [DOI] [PubMed] [Google Scholar]
- 16.Erlebacher A, et al. Constraints in antigen presentation severely restrict T cell recognition of the allogeneic fetus. J Clin Invest. 2007;117:1399–1411. doi: 10.1172/JCI28214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moldenhauer LM, et al. Cross-presentation of male seminal fluid antigens elicits T cell activation to initiate the female immune response to pregnancy. J Immunol. 2009;182:8080–8093. doi: 10.4049/jimmunol.0804018. [DOI] [PubMed] [Google Scholar]
- 18.Benichou G, et al. Contributions of direct and indirect T cell alloreactivity during allograft rejection in mice. J Immunol. 1999;162:352–358. [PubMed] [Google Scholar]
- 19.Suchin EJ, et al. Quantifying the frequency of alloreactive T cells in vivo: new answers to an old question. J Immunol. 2001;166:973–981. doi: 10.4049/jimmunol.166.2.973. [DOI] [PubMed] [Google Scholar]
- 20.Dakic A, et al. Development of the dendritic cell system during mouse ontogeny. J Immunol. 2004;172:1018–1027. doi: 10.4049/jimmunol.172.2.1018. [DOI] [PubMed] [Google Scholar]
- 21.Collins MK, et al. Dendritic cell entrapment within the pregnant uterus inhibits immune surveillance of the maternal/fetal interface in mice. J Clin Invest. 2009;119:2062–2073. doi: 10.1172/JCI38714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Red-Horse K. Lymphatic vessel dynamics in the uterine wall. Placenta. 2008;29(Suppl A):S55–59. doi: 10.1016/j.placenta.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Itano AA, et al. Distinct dendritic cell populations sequentially present antigen to CD4 T cells and stimulate different aspects of cell-mediated immunity. Immunity. 2003;19:47–57. doi: 10.1016/s1074-7613(03)00175-4. [DOI] [PubMed] [Google Scholar]
- 24.Allenspach EJ, et al. Migratory and lymphoid-resident dendritic cells cooperate to efficiently prime naive CD4 T cells. Immunity. 2008;29:795–806. doi: 10.1016/j.immuni.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaffe L, et al. Analysis of beta 2-microglobulin gene expression in the developing mouse embryo and placenta. J Immunol. 1990;145:3474–3482. [PubMed] [Google Scholar]
- 26.Jaffe L, et al. Distinct patterns of expression of MHC class I and beta 2-microglobulin transcripts at early stages of mouse development. J Immunol. 1991;147:2740–2749. [PubMed] [Google Scholar]
- 27.Mattsson R, et al. In vivo treatment with interferon-gamma during early pregnancy in mice induces strong expression of major histocompatibility complex class I and II molecules in uterus and decidua but not in extra-embryonic tissues. Biol Reprod. 1992;46:1176–1186. doi: 10.1095/biolreprod46.6.1176. [DOI] [PubMed] [Google Scholar]
- 28.Robertson SA, et al. Seminal fluid drives expansion of the CD4+CD25+ T regulatory cell pool and induces tolerance to paternal alloantigens in mice. Biol Reprod. 2009;80:1036–1045. doi: 10.1095/biolreprod.108.074658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Riley JK, Yokoyama WM. NK cell tolerance and the maternal-fetal interface. Am J Reprod Immunol. 2008;59:371–387. doi: 10.1111/j.1600-0897.2008.00593.x. [DOI] [PubMed] [Google Scholar]
- 30.Sharkey AM, et al. Killer Ig-like receptor expression in uterine NK cells is biased toward recognition of HLA-C and alters with gestational age. J Immunol. 2008;181:39–46. doi: 10.4049/jimmunol.181.1.39. [DOI] [PubMed] [Google Scholar]
- 31.Guimond MJ, et al. Absence of natural killer cells during murine pregnancy is associated with reproductive compromise in Tge26 mice. Biol Reprod. 1997;56:169–179. doi: 10.1095/biolreprod56.1.169. [DOI] [PubMed] [Google Scholar]
- 32.Hanna J, et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med. 2006;12:1065–1074. doi: 10.1038/nm1452. [DOI] [PubMed] [Google Scholar]
- 33.Yadi H, et al. Unique receptor repertoire in mouse uterine NK cells. J Immunol. 2008;181:6140–6147. doi: 10.4049/jimmunol.181.9.6140. [DOI] [PubMed] [Google Scholar]
- 34.Gendron RL, Baines MG. Infiltrating decidual natural killer cells are associated with spontaneous abortion in mice. Cell Immunol. 1988;113:261–267. doi: 10.1016/0008-8749(88)90025-1. [DOI] [PubMed] [Google Scholar]
- 35.Mallidi TV, et al. Murine endometrial and decidual NK1.1+ natural killer cells display a B220+CD11c+ cell surface phenotype. Biol Reprod. 2009;81:310–318. doi: 10.1095/biolreprod.109.076448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Apps R, et al. Natural-killer cell ligands at the maternal-fetal interface: UL-16 binding proteins, MHC class-I chain related molecules, HLA-F and CD48. Hum Reprod. 2008;23:2535–2548. doi: 10.1093/humrep/den223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xie X, et al. Pathways participating in activation of mouse uterine natural killer cells during pregnancy. Biol Reprod. 2005;73:510–518. doi: 10.1095/biolreprod.104.033951. [DOI] [PubMed] [Google Scholar]
- 38.Erlebacher A, et al. Ovarian insufficiency and early pregnancy loss induced by activation of the innate immune system. J Clin Invest. 2004;114:39–48. doi: 10.1172/JCI20645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kammerer U, et al. Role of dendritic cells in the regulation of maternal immune responses to the fetus during mammalian gestation. Immunol Invest. 2008;37:499–533. doi: 10.1080/08820130802191334. [DOI] [PubMed] [Google Scholar]
- 40.Red-Horse K, et al. Cytotrophoblast induction of arterial apoptosis and lymphangiogenesis in an in vivo model of human placentation. J Clin Invest. 2006;116:2643–2652. doi: 10.1172/JCI27306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Game DS, Lechler RI. Pathways of allorecognition: implications for transplantation tolerance. Transpl Immunol. 2002;10:101–108. doi: 10.1016/s0966-3274(02)00055-2. [DOI] [PubMed] [Google Scholar]