Abstract
Background
Increased consumption of sugar-sweetened beverages (SSB) has been associated with an elevated risk of obesity, metabolic syndrome, and type 2 diabetes. However, the effects of SSB consumption on blood pressure (BP) are uncertain. The objective of this study was to determine the relationship between changes in SSB consumption and changes in BP among adults.
Methods and Results
Prospective analysis of 810 adults who participated in the PREMIER Study (an 18-month behavioral intervention trial). BP and dietary intake (by two 24-h recalls) were measured at baseline, 6-month, and 18-month. Mixed effects models were applied to estimate the changes in BP in responding to changes in SSB consumption. At baseline, mean SSB intake was 0.9 ± 1.0 servings/day (10.5 ± 11.9 fl oz/day), and mean SBP/DBP was 134.9 ± 9.6/84.8 ± 4.2 mmHg. After controlling for potential confounders, a reduction in SSB of 1 serving/day was associated with a 1.8 mmHg (95% CI: 1.2 to 2.4 mmHg) reduction in SBP and 1.1 mmHg (95% CI: 0.7 to 1.4 mmHg) reduction in DBP over 18 months. After additional adjustment for weight change over the same period, a reduction in SSB intake was still significantly associated with reductions in SBP and DBP (P < 0.05). Reduced intake of sugars was also significantly associated with reduced BP. No association was found for diet beverage consumption or caffeine intake and BP. These findings suggest that sugars may be the nutrients that contribute to the observed association between SSB and BP.
Conclusions
Reduced consumption of SSB and sugars were significantly associated with reduced BP. Reducing SSB and sugar consumption may be an important dietary strategy to lower BP.
Keywords: Blood pressure, Hypertension, Glucose, Nutrition, Follow-up studies
INTRODUCTION
Elevated blood pressure (BP) continues to be one of the most common and important health problems in the United States (U.S.). In 2004, 72 million (35%) U. S. adults had hypertension [defined as systolic BP (SBP) ≥ 140 mmHg and/or diastolic blood pressure (DBP) ≥ 90 mmHg, or use of antihypertensive medication] and another 59 million (29%) had prehypertension [defined as SBP from 120 to 140 mmHg, or DBP from 80 mmHg to 90 mmHg]1. Elevated BP is an established risk factor for cardiovascular disease, stroke, kidney disease, all-cause mortality, and shortened life expectancy2.
Sugar-sweetened beverages (SSB) are the most commonly consumed caloric beverage and the leading source of added sugars in the U.S.3. Mean SSB consumption was 28 ± 1 oz/day (2.3 servings/day) for U.S. adults (> 20 y) as reported from the National Health and Nutritional Examination Survey (NHANES) 1999–20044. An emerging body of evidence from prospective studies documented that increased SSB consumption is associated with a higher risk of obesity5–7, type II diabetes7–9, and coronary heart disease10. Experimental studies11–14 found that high consumption of sugary drinks can induce hypertension in animal models. Whether long-term consumption of SSB has a direct effect on BP in humans has not been well investigated. Up to date, three human studies have provided limited data that suggest there may be a positive association between habitual SSB consumption and BP15–17. However, these studies are either cross-sectional16, did not have direct measure of BP17, or failed to show the association was statistically significant15. In addition, it is not clear whether both high consumption of the SSB and diet beverages (sweetened by artificial sweeter, no calories) may increase the risk of high BP.
A relationship between consumption of SSB or diet beverages and BP could have substantial public health implications, given the high prevalence of elevated BP and widespread consumption of these beverages. Therefore, the primary objective of this study is to prospectively examine the relationship between changes in SSB consumption and BP among U.S. adults. Additionally, we evaluate whether change in consumption of diet beverages is associated with BP.
METHODS
Study population
Study participants are from the PREMIER study. PREMIER is a completed, 18-month multicenter randomized trial designed to test the BP-lowering effects of two multi-component behavioral interventions in adults with SBP of 120 to 159 mmHg and DBP of 80 to 95 mmHg18. The study consisted of 810 men and women, aged 25–79, recruited from 4 study centers in the U.S. (Baltimore, MD; Baton Rouge, LA; Durham, NC; and Portland, OR). Information on study design, participant recruitment, and data collection has been previously published19.
Eligible participants were randomly assigned to one of three groups: (A) an “Advice Only” comparison group that received information but no behavioral counseling on weight loss, physical activity, sodium intake, or the DASH (Dietary Approaches to Stop Hypertension) dietary pattern; (B) a behavioral intervention, termed “Established”, that received counseling on how to lose weight, increase physical activity and reduce sodium intake; or (C) a behavioral intervention, termed “Established Plus DASH”, that received counseling on the same lifestyle goals as “Established” along with counseling on the DASH dietary pattern. The weight loss approaches in the Established group focused on increased physical activity and reduced energy intake. By contrast, the weight loss approach in Established Plus DASH group focused on increased physical activity, reduced energy intake, and substitution of high-fat, high-calorie foods with fruits and vegetables. All 810 study participants enrolled at baseline were included in this analysis.
Measurement of BP
BP was measured manually by trained, certified observers at baseline, 3, 6, 12, and 18 months using a standard protocol. After participants sat quietly for 5 minutes, the observer measured BP in the right arm with an appropriately sized cuff. For this analysis, the values of SBP and DBP were calculated by taking the mean of all available measurements at baseline (4 visits), 6 months (3 visits) or 18 months (3 visits). At each visit, a set of 2 BP measurements was obtained. BPs taken in participants who reported using antihypertensive medication within the preceding month were censored; along with missing values, these cases received imputed values by using the BP measured at the preceding visit (last observation carried forward method, LOCF), or using BP values from similar participants in the advice group (single imputation Hot-Deck procedure). Overall, 9% of the BP at 6 months (5% due to antihypertensive medication use and 4% due to loss to follow-up; 3% were imputed using LOCF method) and 17% at 18 months (13% due to antihypertensive medication use and 4% due to loss to follow-up; 5% were imputed using LOCF method) were imputed. Hypertension was defined as an average SBP ≥ 140 mmHg, a DBP ≥ 90 mmHg, or use or antihypertensive medication.
Measurement of dietary and beverage intake
Dietary intake was measured by unannounced 24-hour dietary recalls conducted by telephone interviews. Two recalls (one on a week day and one on a weekend) per participant were obtained at baseline, 6, and 18 months. A multiple–pass technique and portion size estimation aids (2 Dimensions Food Portion Visual, Nutrition Consulting Enterprises, Framingham, MA) were used during the phone interview. Intakes of total energy, nutrients (e.g. sugar and caffeine) and food groups (e.g. dairy foods and fruits & vegetables) were calculated using the Nutrition Data System for Research (Version NDS-R 1998, University of Minnesota, Minneapolis, MN). For this analysis, participants' daily nutrient, energy, and beverage intake were calculated by taking the average from two 24-hour dietary recalls. SSB was defined as carbonated or uncarbonated drinks that were sweetened with sugars (sucrose or high-fructose corn syrup). These included regular soft drinks, fruit drinks, lemonade, fruit punch, and other sweetened beverages, but excluded diet drinks. Diet beverages were defied as carbonated or uncarbonated drinks that were sweetened with artificial sweeteners (non-calorically sweeteners).
Measurement of covariates
Weight and height were measured with subjects wearing light clothing and no shoes using a calibrated scale and a wall-mounted stadiometer. Fitness was assessed using a 2-stage 10-minute sub-maximal treadmill stress test and defined as the heart rate (beats/minute) at a fixed work load (Stage 2). Physical activity (PA) and estimated energy expenditure (kcal/kg/day) was assessed using a 7-day recall questionnaire20. Urinary excretion of sodium and potassium were obtained from 24-hour urinary collection at baseline, 6 months, and 18 months. Participants' characteristics such as age, sex, race/ethnicity, income, education, employment and marriage status, and smoking habits were collected at baseline. Because the DASH diet includes several dietary components, we used a single index, DASH Index, to measure overall adherence to the DASH diet. The DASH Index is an average of three subindices measuring daily intake of dairy products, fruits and vegetables servings, and percentage of calories from saturated fat. A score of 0–1 indicates that the intake is in the target range of the DASH diet, while scores <0 indicate worse than target and scores >1 indicated better than target. The computational details of the DASH Index have been described previously21.
Statistical analysis
Descriptive data on SSB consumption and BP at each visit were expressed as mean ± standard deviation if not mentioned otherwise. Student's T-test and χ2 test were applied to compare continuous variables and categorical variables, respectively. For the primary analysis, we applied mixed effects models to account for the correlation between repeated measurements, and to incorporate between-individual variability to estimate the overall effect. The main exposure was the change in SSB consumption from baseline to follow-up visits (continuous: Δ = follow-up - baseline). In this way, the regression coefficient of change in SSB consumption represents the longitudinal association between SSB and BP (the average change in BP on the concurrent average change in SSB consumption). Potential confounding factors that were adjusted for included gender, race, baseline age, alcohol intake, randomization assignment, study sites, baseline PA and change in PA, baseline fitness and change in fitness, baseline SSB consumption, baseline dietary intakes of selected foods and nutrients and their changes during follow-up, and baseline body mass index (BMI) and change in weight. The primary analyses were conducted by combining all participants and adding intervention assignment as a covariate in all models. Stratified analyses were performed to evaluate whether the associations of SSB and BP were modified by race (white vs. black), gender (male vs. female) and hypertension status (hypertensive vs. non-hypertension). All statistical analyses were performed using STATA version 9.0 (Stata Corp. College Station, Texas). Statistical significance was set at P ≤ 0.05 (two-tailed).
RESULTS
Baseline characteristics and SSB consumption
At baseline, mean SSB intake in PREMIER participants was 0.9 ± 1.0 servings/day (equal to 10.5 ± 11.9 fl oz/day) and mean diet beverages intake was 0.9 ± 1.2 servings/day (11.2 ± 14.0 fl oz/day). The mean SBP/DBP was 134.9 ± 9.6/84.8 ± 4.2 mmHg. Table 1 displays the sociodemographic characteristics, anthropometric measurements, PA, fitness, dietary intakes of selected foods and nutrients, adherence to DASH Index, urinary sodium and potassium excretions across the baseline SSB consumption quartiles and in the entire study population. Compared with persons in the lowest (1st) quartile, individuals in the higher quartiles were on average younger, less fit, had lower annual household incomes, and drank less alcohol. African Americans drank more SSB than Whites (difference = 4.3 fl oz/day, P < 0.0001) and men drank more than women (difference = 3.7 fl oz/day, P < 0.0001). Participants in the higher quartiles of SSB consumption also had greater body weights, BMIs, and waist circumferences compared with those in the 1st quartile (P < 0.0001 for trend). For dietary intake, there was a trend of higher consumption of total calories, total carbohydrates, glucose, fructose, sucrose, combined sugar (sum of monosaccharide and disaccharide), and lower consumption of protein, dairy foods, fruit and vegetables, dietary fiber, caffeine, calcium, and magnesium with higher SSB intake. Across quartiles of SSB consumption, a slightly significant increase was observed in DBP, but not in SBP or prevalence of hypertension.
Table 1.
Baseline characteristics of PREMIER study participants by quartile of sugar-sweetened beverages consumption
| Sugar-Sweetened Beverage Consumption Quartiles |
||||||
|---|---|---|---|---|---|---|
| Variables | 1st | 2nd | 3rd | 4th | P for trend | All Participants |
| SSB intake, fl oz/day | ||||||
| Mean (SD) | 0 | 6.3 (2.3) | 12.0 (1.8) | 27.0 (10.7) | 10.5 (11.9) | |
| Median (range) | 0 (0–0) | 6.3 (0.2–8.5) | 12.4 (8.6–16.4) | 25.0 (16.5–75.4) | 8.5 (0–75.4) | |
| Age, year | 52.2 (8.4) | 50.3 (9.3) | 49.4 (8.9) | 47.1 (8.5) | <0.001 | 50.0 (8.9) |
| Female, % | 63.3 | 72.0 | 64.5 | 50.5 | 0.01 | 61.5 |
| Africa American, % | 19.0 | 34.0 | 44.7 | 46.5 | <0.001 | 34.0 |
| Education (have college degree or above), % | 59.4 | 65.0 | 57.4 | 50.0 | 0.06 | 57.2 |
| Annual household income (above $45,000/year), % | 76.4 | 65.0 | 65.5 | 67.0 | 0.01 | 70.0 |
| Marriage status (married), % | 69.2 | 65.0 | 62.9 | 65.0 | 0.50 | 66.1 |
| Current smoking (yes), % | 4.4 | 3.0 | 4.1 | 7.1 | 0.50 | 4.8 |
| Current alcohol intake (yes), % | 58.4 | 56.0 | 42.1 | 34.5 | <0.001 | 48.1 |
| Physical activity (time spent on moderate or hard activity), min/week† | 210 | 180 | 145 | 175 | 0.19 | 173.5 |
| Fitness, mean (SD): heart beats/min | 129.0 (13.6) | 130.3 (14.6) | 130.0 (14.4) | 133.2 (15.7) | 0.009 | 130.5 (14.5) |
| Anthropometric measurements, mean (SD) | ||||||
| Weight, lbs | 204.3 (40.1) | 193.9 (40.0) | 211.3 (39.4) | 224.7 (42.7) | <0.001 | 209.8 (41.5) |
| BMI, kg/m2 | 32.4 (5.7) | 31.2 (5.2) | 33.5 (5.8) | 34.5 (5.9) | <0.001 | 33.1 (5.8) |
| Waist Circumference, cm | 106.7 (14.8) | 102.5 (14.6) | 107.9 (15.2) | 111.3 (15.5) | <0.001 | 107.6 (15.2) |
| Dietary intake, mean (SD) | ||||||
| Total energy, kcal/day | 1785.8 (567.2) | 1755.2 (509.8) | 1988.4 (611.3) | 2180.0 (683.5) | <0.001 | 1904.5 (625.2) |
| Total protein, % of TEI | 17.3 (4.5) | 16.0 (3.6) | 15.2 (3.4) | 14.4 (3.1) | <0.001 | 15.9 (4.0) |
| Total fat, % of TEI | 33.9 (8.5) | 32.1 (7.7) | 32.8 (7.0) | 32.7 (6.7) | 0.13 | 33.1 (7.6) |
| Saturated fat, % of TEI | 11.2 (3.5) | 10.9 (3.0) | 10.7 (3.0) | 10.7 (2.8) | 0.35 | 10.9 (3.2) |
| Monounsaturated fat, % of TEI | 13.4 (4.1) | 12.6 (3.5) | 12.8 (3.3) | 12.9 (3.2) | 0.17 | 13.2 (3.6) |
| Polyunsaturated fat, % of TEI | 7.2 (2.8) | 6.5 (2.5) | 7.1 (2.4) | 7.0 (2.4) | 0.75 | 7.0 (2.6) |
| Trans fat, % of TEI | 2.3 (1.2) | 2.3 (1.1) | 2.4 (1.1) | 2.3 (1.2) | 0.96 | 2.3 (1.2) |
| Total carbohydrate, % of TEI | 48.6 (10.3) | 52.1 (8.9) | 52.4 (9.2) | 53.7 (8.3) | <0.001 | 51.3 (9.6) |
| Glucose, g/day | 18.3 (10.2) | 24.0 (11.0) | 29.2 (11.7) | 42.1 (17.4) | <0.001 | 27.5 (15.8) |
| Fructose, g/day | 16.0 (10.8) | 20.1 (9.0) | 28.1 (11.6) | 42.1 (17.6) | <0.001 | 25.9 (16.5) |
| Sucrose, g/day | 42.6 (28.1) | 44.5 (23.1) | 49.8 (29.1) | 59.0 (31.9) | <0.001 | 48.7 (29.5) |
| Total sugar, g/day | 92.3 (42.7) | 104.6 (41.2) | 122.1 (44.3) | 156.8 (52.2) | <0.001 | 116.9 (52.4) |
| Dairy foods, serving/day† | 1.5 | 1.5 | 1.4 | 1.3 | 0.01 | 1.4 |
| Fruit and vegetable, serving/day | 4.7 (2.3) | 4.5 (2.3) | 4.5 (2.3) | 4.2 (2.4) | 0.003 | 4.5 (2.3) |
| Dietary fiber, g/day | 18.0 (7.7) | 16.4 (7.8) | 16.3 (7.5) | 16.0 (7.7) | 0.001 | 16.9 (7.7) |
| Caffeine, mg/day† | 157.8 | 110.9 | 79.1 | 101.4 | <0.001 | 111.2 |
| Calcium, mg/day† | 711.4 | 633.5 | 666.2 | 651.1 | 0.049 | 672.5 |
| Magnesium, mg/day | 287.2 (98.1) | 261.7 (102.7) | 265.9 (105.5) | 261.2 (114.6) | 0.001 | 272.0 (105.3) |
| Folate, ug/day | 346.2 (157.2) | 333.1 (148.0) | 332.5 (165.2) | 332.2 (162.8) | 0.70 | 337.6 (158.4) |
| DASH Index | −3.2 (2.3) | −3.3 (2.2) | −3.2 (2.1) | −3.4 (1.9) | 0.68 | −3.3 (2.1) |
| Urinary excretion, mean (SD) | ||||||
| Sodium, mmol/24h | 174.7 (72.4) | 155.7 (55.9) | 169.4 (71.2) | 189.1 (80.4) | 0.21 | 174.5 (72.7) |
| Potassium, g/24h | 70.1 (25.0) | 65.7 (23.9) | 63.8 (24.8) | 65.2 (29.6) | 0.002 | 66.6 (26.0) |
| Blood pressure, mean (SD) | ||||||
| SBP, mmHg | 135.2 (9.5) | 135.7 (10.0) | 135.6 (9.7) | 133.2 (9.6) | 0.57 | 134.9 (9.6) |
| DBP, mmHg | 84.2 (3.8) | 84.9 (4.0) | 85.4 (4.6) | 85.0 (4.2) | 0.01 | 84.8 (4.2) |
| Prevalence of hypertension, % | 35.4 | 41.0 | 41.2 | 34.0 | 0.31 | 37.5 |
Median was shown instead of mean because of the highly skewed distribution of that variable
P for trend by Wilcoxon rank-sum test
TEI: total energy intake
At 18-months, 94% of study participants had at least one BP measurement and 90% had at least one dietary recall. For all participants, mean SBP declined 9.8 ± 9.4 mmHg at 6 months and 8.2 ± 9.9 mmHg at 18 months, each net of baseline. For DBP, corresponding declines were 5.4 ± 6.5 mmHg and 5.6 ± 6.8 mmHg, respectively. Compared to baseline, the mean reduction in SSB consumption was 0.5 ± 1.1 servings/day (6.0 ± 13.0 fl oz/day) at 6 months, and 0.2 ± 1.0 servings/day (2.8 ± 12.0 fl oz/day) at 18 months. The consumption of diet beverages was reduced by 0.2 ± 1.1 servings/day (2.3 ± 13.6 fl oz/day) at 6 months but increased by 0.1 ± 1.2 servings/day (1.5 ± 14.0 fl oz/day) at 18 months.
Association between change in SSB consumption and change in BP
Change in SSB consumption was strongly and positively associated with SBP and DBP in both age-adjusted and multivariate-adjusted models (Table 2). In a multivariate-adjusted model that did not include weight change (model 1), a reduction of 1serving/day (12 fl oz) in SSB consumption was associated with reduced SBP (β = 1.8; 95% CI: 1.2 to 2.4 mmHg) and DBP (β = 1.1; 95% CI: 0.7 to 1.5 mmHg). With additional adjustment for concurrent change in weight (model 2), the associations between SSB intake and BPs were attenuated, but still statistically significant with a reduction of 1 serving/day in SSB consumption being associated with a decrease of 0.7 mmHg in SBP (95% CI: 0.12 to 1.25 mmHg) and 0.4 mmHg (95% CI: 0.02 to 0.75 mmHg) in DBP. Results were similar in sensitivity analyses that adjusted for change in total energy instead of change in weight (data not shown). These results suggest that change in SSB consumption is positively associated with BP, in dependent of weight change and other risk factors for BP. In a sensitivity analysis using non-imputed BP, the results were virtually unchanged, even slightly stronger: a reduction of 1serving/day in SSB consumption was associated with reduced SBP of 2.0 mmHg (95% CI: 1.4 to 2.6 mmHg) and DBP of 1.2 mmHg (95% CI: 0.9 to 1.6 mmHg) in model 2.
Table 2.
Associations of blood pressure with change in sugar-sweetened beverage among PREMIER participants: results from mixed effects models
| Systolic Blood Pressure |
Diastolic Blood Pressure |
|||||
|---|---|---|---|---|---|---|
| β | 95% C | P | β | 95% C | P | |
| All participants | ||||||
| Age-adjusted model | 1.99 | 1.46, 2.52 | <0.001 | 1.12 | 0.78, 1.45 | <0.001 |
| Multivariate model 1 | 1.76 | 1.17, 2.35 | <0.001 | 1.08 | 0.69, 1.47 | <0.001 |
| Multivariate model 2 | 0.70 | 0.15, 1.25 | 0.01 | 0.38 | 0.02, 0.75 | 0.04 |
| Hypertensive status at baseline | ||||||
| Hypertensive | ||||||
| Age-adjusted model | 2.26 | 1.32, 3.21 | <0.001 | 1.33 | 0.75, 1.90 | <0.001 |
| Multivariate model 1 | 1.94 | 0.82, 3.06 | 0.001 | 1.42 | 0.71, 2.14 | <0.001 |
| Multivariate model 2 | 0.76 | −0.30, 1.82 | 0.16 | 0.62 | −0.05, 1.29 | 0.07 |
| Non-hypertensive | ||||||
| Age-adjusted model | 1.61 | 1.05, 2.17 | <0.001 | 0.91 | 0.53, 1.31 | <0.001 |
| Multivariate model 1 | 1.60 | 0.94, 2.20 | <0.001 | 0.88 | 0.43, 1.33 | <0.001 |
| Multivariate model 2 | 0.58 | −0.02, 1.17 | 0.06 | 0.19 | −0.24, 0.61 | 0.34 |
| Race | ||||||
| White | ||||||
| Age-adjusted model | 2.29 | 1.62, 2.95 | <0.001 | 1.29 | 0.87, 1.70 | <0.001 |
| Multivariate model 1 | 1.76 | 1.17, 2.35 | <0.001 | 1.08 | 0.69, 1.47 | <0.001 |
| Multivariate model 2 | 0.70 | 0.15, 1.25 | 0.01 | 0.38 | 0.02, 0.75 | 0.04 |
| Black | ||||||
| Age-adjusted model | 1.38 | 0.48, 2.29 | 0.003 | 0.72 | 0.14, 1.31 | 0.016 |
| Multivariate model 1 | 1.54 | 0.52, 2.56 | 0.003 | 0.92 | 0.23, 1.62 | 0.009 |
| Multivariate model 2 | 0.60 | −0.42, 1.63 | 0.25 | 0.15 | 0.53, 0.83 | 0.67 |
| Gender | ||||||
| Male | ||||||
| Age-adjusted model | 2.01 | 1.33, 2.68 | <0.001 | 1.29 | 0.84, 1.74 | <0.001 |
| Multivariate model 1 | 1.75 | 0.98, 1.52 | <0.001 | 1.14 | 0.60, 1.67 | <0.001 |
| Multivariate model 2 | 0.78 | 0.09, 1.47 | 0.03 | 0.50 | 0.01, 1.00 | 0.05 |
| Female | ||||||
| Age-adjusted model | 1.84 | 1.01, 2.68 | <0.001 | 0.96 | 0.46, 1.62 | <0.001 |
| Multivariate model 1 | 1.84 | 0.93, 1.75 | <0.001 | 1.04 | 0.46, 1.61 | <0.001 |
| Multivariate model 2 | 0.61 | −0.27, 1.48 | 0.01 | 0.21 | −0.34, 0.76 | 0.45 |
β:change in mmHg per change in servings/day
Multivariate model 1: Adjusted for gender, race, family history of hypertension, randomization assignment, site, baseline age, alcohol drinking, and BMI, baseline SSB intake, baseline fitness and change in fitness, baseline physical activity and change in physical activity, baseline urinary sodium excretion and change in urinary sodium excretion, baseline DASH index and change in DASH index.
Multivariate model 2: Model 1 plus change in body weight.
A similar pattern was evident in subgroups (Table 2) defined by baseline hypertension status (hypertensive/non-hypertensive), race (blacks/whites), and gender (female/male). While the pattern of subgroup analyses was similar to that of the overall analyses, not all results were statistically significant, likely because of reduced sample size. Test for interactions showed that the association between BP and change in SSB consumption was not modified by baseline hypertension status, race, or gender (each P for interaction > 0.05).
To examine the dose-response relationship, we divided participants into tertiles based on their 18-month change in SSB consumption. Table 3 shows the baseline sociodemographic characteristics and changes in selected variables across the tertiles of 18-month change in SSB consumption. There is a linear trend in weight loss and reductions in intakes of total energy, glucose, fructose, sucrose, and combined sugars across the tertiles.
Table 3.
Baseline characteristics and changes in selected variables from baseline to 18 month according to the tertiles of change in SSB intake at 18 months
| Tertiles of change in SSB intake at 18 months |
||||
|---|---|---|---|---|
| 1st | 2nd | 3rd | P for trend | |
| Baseline Characteristics | ||||
| Age, years | 49.3 (8.6) | 51.4 (8.8) | 49.8 (9.0) | 0.5 |
| Female, % | 59.6 | 63.5 | 58.7 | 0.8 |
| African Americans, % | 33.8 | 28.9 | 37.2 | 0.3 |
| Education, (have college degree or above), % | 57.6 | 58.3 | 58.3 | 0.8 |
| Annual household income (> $45,000/year), % | 66.7 | 75.1 | 66.1 | 0.8 |
| Marriage status (married), % | 62.1 | 69.1 | 66.5 | 0.4 |
| Changes (18 months - baseline) | ||||
| SSB intake, fl oz/day | 9.5 (7.4) | −0.9 (1.6) | −15.3 (9.9) | <0.001 |
| Total energy intake, kcal/day | −81.2 (534.1) | −199.8 (563.0) | −350.6 (615.4) | <0.001 |
| DASH Index | 1.1 (2.6) | 1.2 (2.6) | 1.4 (2.7) | 0.3 |
| Glucose intake, g/day | 7.4 (13.9) | 0.9 (13.6) | −7.5 (18.7) | <0.001 |
| Fructose intake, g/day | 9.3 (15.9) | 1.4 (13.5) | −8.2 (19.6) | <0.001 |
| Sucrose intake, g/day | −1.6 (33.4) | −6.1 (30.9) | −9.5 (34.2) | 0.001 |
| Combined sugars intake, g/day | 14.4 (50.0) | −3.3 (49.7) | −23.4 (55.9) | <0.001 |
| Urinary excretion of sodium, mmol/24h | −10.1 (80.4) | −17.4 (76.8) | −18.2(101.1) | 0.2 |
| Urinary excretion of potassium, g/24h | 2.1 (28.6) | 0.5 (28.7) | 4.7 (31.7) | 0.3 |
| Physical activity (time spent on moderate or hard activity), min/week† | 0 | 0 | −10.0 | 0.2 |
| Body weight, lbs | −3.9 (9.2) | −6.4 (12.7) | −10.9 (16.4) | <0.001 |
Data are shown in mean (SD) if not mentioned otherwise
Median was shown instead of mean because of the highly skewed distribution of that variable
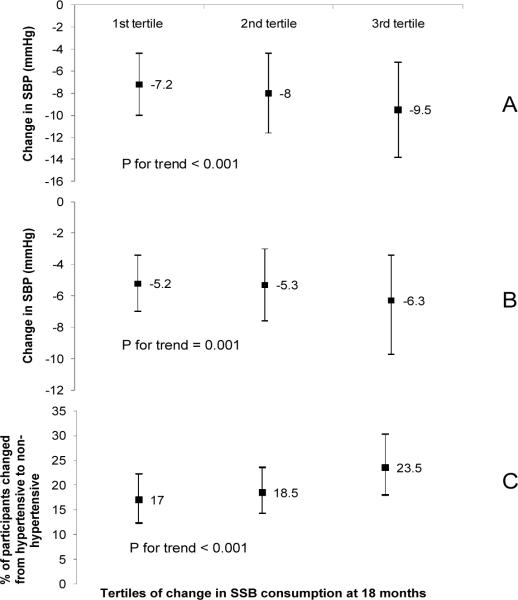
We calculated the model adjusted mean changes in SBP and DBP as well as the proportion of participants who moved from hypertensive at baseline to non-hypertensive at 18 months by tertile of 18-month change in SSB consumption (Figure 1). The mean change in SSB consumption across the tertiles was 9.5 ± 7.4, −0.9 ± 1.6, and −15.3 ± 9.9 fl oz/day (persons in the 3rd tertile had the greatest reduction in SSB consumption). Adjustment variables were the same as those in model 2 in the Table 2. At 18 months, participants in the 3rd tertile had a significantly greater reduction in SBP as compared with individuals in the 1st and 2nd tertiles: the mean reduction in SBP across the tertiles was 7.2 ± 4.3, 8.0 ± 4.3, and 9.5 ± 4.3 mmHg, respectively (P for trend < 0.001). There was also a statistically significant decline in DBP across tertiles (−5.2 ± 1.8, −5.3 ± 2.3, −6.3 ± 2.9 mmHg, respectively; P for trend = 0.001). A trend of increase in the proportion of individuals who moved from the hypertensive at baseline to non-hypertensive at 18 months across tertiles was also observed (17.0%, 18.5%, 23.5%, respectively: P for trend < 0.001).
Figure 1.
Model adjusted mean blood pressure changes (Panel A: SBP; Panel B: DBP) and proportion of participants (%) who moved from hypertensive at baseline to non-hypertensive at 18 months (Panel C) by tertiles of change in sugar-sweetened beverage (SSB) consumption (fl oz/day) from baseline to 18 months (18-mo – baseline). The mean change in SSB consumption across the tertiles was 9.5 ± 7.4, −0.9 ± 1.6, and −15.3 ± 9.9 fl oz/day (persons in the 3rd tertile had the greatest reduction in SSB). Covariates in the model included gender, race, family history of hypertension, randomization assignment, site, baseline age, alcohol drinking, and BMI, baseline SSB intake, baseline fitness and change in fitness, baseline physical activity and change in physical activity, baseline urinary sodium excretion and change in urinary sodium excretion, baseline DASH Index and change in DASH Index, and change in body weight from baseline to 18 months.
Association between change in diet beverages consumption and change in BP
We also examine the relationship between consumption of diet beverages and BP. Change in consumption of diet beverages was not associated with either SBP or DBP in both age-adjusted and multivariate-adjusted models (Table 4).
Table 4.
Associations of blood pressure with change in diet beverages among PREMIER participants: results from mixed effects models
|
Systolic Blood Pressure |
Diastolic Blood Pressure |
|||||
|---|---|---|---|---|---|---|
| β | 95% C | P | β | 95% C | P | |
| All participants | ||||||
| Age-adjusted model | 0.14 | −0.35, 0.64 | 0.57 | −0.18 | −0.49, 0.14 | 0.09 |
| Multivariate model 1 | −0.20 | −0.41, 0.89 | 0.73 | −0.34 | −0.69, 0.01 | 0.08 |
| Multivariate model 2 | 0.25 | −0.24, 0.74 | 0.99 | −0.05 | −0.38, 0.27 | 0.75 |
β:change in mmHg per change in servings/day
Multivariate model 1: Adjusted for gender, race, family history of hypertension, randomization assignment, site, baseline age, alcohol drinking, and BMI, baseline SSB intake, baseline fitness and change in fitness, baseline physical activity and change in physical activity, baseline urinary sodium excretion and change in urinary sodium excretion, baseline DASH index and change in DASH index.
Multivariate model 2: Model 1 plus change in body weight.
Association between changes in sugar or caffeine consumption and change in BP
In order to investigate which specific nutrients might be responsible for the observed association between SSB and BP, we examined the associations of change in consumption of sugars (glucose, fructose, sucrose, or combined sugar from all foods and beverages) or caffeine (from all foods and beverages) with change in BP (Table 5). In model 1, without weight change as a covariate, change in BP was significantly and positively associated with changes in glucose, fructose, sucrose, and combined sugars. A 10 g/day reduction in glucose, fructose, sucrose, or combined sugar was associated with reductions in SBP of 0.6 mmHg (95% CI: 0.2 to 1.0), 0.5 mmHg (95% CI: 0.1 to 0.8), 0.4 mmHg (95% CI: 0.2 to 0.6), and 0.3 mmHg (95% CI: 0.1 to 0.5). Corresponding data for DBP were 0.5 mmHg (95% CI: 0.3 to 0.8), 0.4 mmHg (95% CI: 0.2 to 0.6), 0.3 mmHg (95% CI: 0.2 to 0.5); 0.3 mmHg (95% CI: 0.2 to 0.3), respectively. Further adjustment for weight loss attenuated these associations; however, in most instances, they were still statistically significant (Table 5). There was no significant relationship between change in caffeine consumption and change in BP.
Table 5.
Associations of blood pressure with change in sugars or caffeine among PREMIER participants: results from mixed effects models
| Systolic Blood Pressure |
Diastolic Blood Pressure |
|||||
|---|---|---|---|---|---|---|
| β | 95% C | P | β | 95% C | P | |
| Sugars | ||||||
| Glucose (per 10 g/day) | ||||||
| Multivariate model 1 | 0.62 | 0.24, 1.00 | 0.001 | 0.54 | 0.29, 0.78 | <0.001 |
| Multivariate model 2 | 0.32 | −0.02, 0.67 | 0.07 | 0.33 | 0.10, 0.56 | 0.005 |
| Fructose (per 10 g/day) | ||||||
| Multivariate model 1 | 0.46 | 0.01, 0.83 | 0.01 | 0.40 | 0.16, 0.64 | 0.001 |
| Multivariate model 2 | 0.15 | −0.19, 0.48 | 0.39 | 0.18 | −0.04, 0.40 | 0.11 |
| Sucrose (per 10 g/day) | ||||||
| Multivariate model 1 | 0.43 | 0.23, 0.62 | <0.001 | 0.32 | 0.19, 0.45 | <0.001 |
| Multivariate model 2 | 0.29 | 0.11, 0.47 | 0.002 | 0.22 | 0.11, 0.34 | <0.001 |
| Combined Sugars (per 10 g/day) | ||||||
| Multivariate model 1 | 0.30 | 0.17, 0.42 | <0.001 | 0.24 | 0.16, 0.32 | <0.001 |
| Multivariate model 2 | 0.17 | 0.06, 0.28 | 0.003 | 0.15 | 0.08, 0.23 | <0.001 |
| Caffeine (per 100 mg/day) | ||||||
| Multivariate model 1 | 0.35 | −;0.09, 0.80 | 0.12 | 0.06 | −0.23, 0.34 | 0.70 |
| Multivariate model 2 | 0.36 | −0.04, 0.76 | 0.08 | 0.08 | −0.18, 0.34 | 0.56 |
β: mmHg per unit of exposure
Multivariate model 1: Adjusted for gender, race, family history of hypertension, randomization assignment, site, baseline age, alcohol drinking, and BMI, baseline SSB intake, baseline fitness and change in fitness, baseline physical activity and change in physical activity, baseline urinary sodium excretion and change in urinary sodium excretion, baseline DASH index and change in DASH index.
Multivariate model 2: Model 1 plus change in body weight.
DISCUSSION
In this prospective study of 810 men and women with prehypertension and stage I hypertension, there was a positive association between change in SSB consumption and change in BP. After controlling for potential confounders, an average reduction in SSB intake by 1 serving (12 fl oz) per day was associated with a 1.8 mmHg (95% CI: 1.2 to 2.4 mmHg) reduction in SBP and 1.1 mmHg (95% CI: 0.7 to 1.5 mmHg) reduction in DBP over 18 months. This association was partially mediated through weight change. Specifically, after controlling for weight change over the same period, the association between SSB intake and BP was attenuated by approximately 61% (0.7 mmHg/per serving for SBP and 0.4 mmHg/serving for DBP), but still statistically significant (P < 0.05, each), suggesting that reducing SSB intake has a BP lowering effect which is independent of weight loss. We also observed significant, positive associations of BP with change in consumption of sugars (glucose, fructose, sucrose, and combined sugar), but not with change in consumption of caffeine. No association was found for diet beverage consumption and BP. These data suggest that sugars may be the nutrients in SSB that contribute to the observed association between SSB and BP.
Our results are supported by data from two large prospective studies and one cross-sectional study suggesting a positive association between SSB consumption and the risk of hypertension. Data from the Nurses' Health Study17 showed a strong positive association between cola beverage intake and hypertension risk (P for trend < 0.001). Additionally, an analysis of data from the Framingham Offspring Study15 also found that consumption of soft drinks (regular and diet soda combined) was associated with an increased risk of high BP, although not statistically significant. In addition, cross-sectional findings from NHANES (1999–2004) data among adolescents (12–18 years) indicated a positive association between SSB consumption and directly measured BP16.
Our results provide additional evidence supporting a relationship between higher SSB consumption and elevated BP. First, the data show that SSB affects BP in part via mechanisms that are independent of weight change. Second, the relationship is evident in both non-hypertensive and hypertensive, suggesting reduced SSB should have a role in both preventing and treating hypertension. In contrast with the above-mentioned two studies which observed an increased hypertension risk associated with both SSB and diet soft drinks15, 17, we found no association between diet beverages and BP in present study (Table 4).
The mechanism by which higher intake of SSB may increase BP is uncertain. It is well documented that ingestion of caffeine has an acute pressor effect22, 23. However, tolerance to the caffeine-induced pressor effect develops within days23. We found no association between 18-month change in caffeine intake and BP in present study. Studies in a variety of animal models, including rats, dogs, and primates11–14, have shown that diets high in glucose, fructose, or sucrose can induce hypertension. There are few similar studies in humans, and one study has reported that a diet high in sucrose, consumed for 6 weeks, causes a significant elevation in BP24.
A possible mechanism for the pressor effect of sugars may be enhanced sympathetic nervous system activity. An acute increase in catecholamine secretion has been shown after ingestion of sugar during euglycemic clamp studies25. Another mechanism may be a reduction in sodium excretion, as documented in animal and human studies26. Recent evidence suggests that fructose consumption might increase BP by raising serum uric acid16, 27, which can decrease endothelial nitric oxide and/or activate the renin-angiotensin system28.
Our study has several strengths. First, both diet and BP were measured frequently by trained, certified staff. Second, our study had precise, objective measurements of potential confounders including weight, urinary sodium excretion, physical activity, fitness, and other covariates. Third, the follow-up rate was high and missing data were uncommon. Furthermore, the BP status of our study population is comparable to the BP of two-thirds of the U.S. population. Our study is limited in that it included few Hispanics and Asians. Additionally, given the observational nature of our study, it cannot prove causality or completely rule out residual confounding. Randomized controlled trials are needed to confirm the observation and to determine whether interventions that target SSB or sugar consumption can lower BP among adults.
Our study has important public health implications. In view of the direct, progressive relationship of BP with CVD, even small reductions in BP are projected to have substantial health benefits. For example, it has been estimated that a 3 mmHg reduction in systolic BP should reduce stroke mortality by 8% and CHD mortality by 5%29. Such reductions in SBP would be anticipated by reducing SSB consumption by an average of 2 servings per day. Currently, the average intake of SSB is 2.3 servings per day for U.S. adults. In our study, one third of participants reduced SSB consumption on average of 1.3 servings/day over the 18 months and had an average of 1.5 mmHg more reduction in SBP compared with participants who did not change their SSB consumption, suggesting such reduction in SSB consumption should be achievable and could be beneficial.
In summary, findings from this prospective study suggest a positive association between SSB consumption and BP. These findings warrant future studies, particularly randomized controlled trials, to establish the causal relationship.
Clinical Summary.
Consumption of sugar-sweetened beverages (SSB) has increased dramatically in the United States (U.S.). While high SSB consumption has been linked with excess calorie intake and overweight/obesity, SSB may have other adverse effects. In a prospective study of 810 U.S. adults with prehypertension and stage I hypertension, we found that reducing SSB consumption was associated with significant reductions in blood pressures (BP). On average, a reduction in SSB intake of 1 serving/day (12 ounces/day) was associated with a 1.8 mmHg reduction in SBP and 1.1 mmHg reduction in DBP over 18 months. A positive association was also found for dietary sugar intake and BP. No association was found for diet beverage consumption or caffeine intake and BP. These findings have important clinical and public health implications. It has been estimated that a 3 mmHg reduction in SBP should reduce stroke mortality by 8% and CHD mortality by 5%. Such reductions in SBP would be anticipated by reducing SSB consumption by an average of 2 servings per day. Currently, the average intake of SSB is 2.3 servings per day for U.S. adults. National wide, 72 million (35%) U. S. adults have hypertension and another 59 million (29%) have prehypertension. Given the high prevalence of both SSB consumption and hypertension in the U.S. and throughout much of the world, even small reductions in the SSB consumption should have a beneficial public health impact. In conclusion, our data suggest that reducing SSB and sugar consumption may be an important dietary strategy to lower BP.
Acknowledgements
We thank the PREMIER participants and staff for their contributions to the study. The author responsibilities were as follow-all authors: conception and design of the study, interpretation of analyses, and revision of the manuscript; and LC: conduct of the analyses and the first draft of the manuscript.
Funding Sources The PREMIER Trial was supported by the National Heart, Lung, and Blood Institute, NIH grants UO1 HL60570, UO1 HL60571, UO1 HL60573, UO1 HL60574, and UO1 HL62828. The present study is supported in part from the School of Public Health, Louisiana State University Health Science Center and from the Center for Human Nutrition, Johns Hopkins Bloomberg School of Public Health.
Footnotes
Clinical Trial Registration Information: The Clinical Trial Registration No. for the PREMIER Trial is NCT00000616. URL: http://clinicaltrials.gov/ct2/show/NCT00000616
Disclosures None of the authors had any personal or financial conflict of interests.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- (1).Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, Haase N, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O'Donnell CJ, Roger V, Rumsfeld J, Sorlie P, Steinberger J, Thom T, Wasserthiel-Smoller S, Hong Y, American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics--2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- (2).Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–23. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- (3).Bray GA, Nielsen SJ, Popkin BM. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr. 2004;79:537–43. doi: 10.1093/ajcn/79.4.537. [DOI] [PubMed] [Google Scholar]
- (4).Bleich SN, Wang YC, Wang Y, Gortmaker SL. Increasing consumption of sugar-sweetened beverages among US adults: 1988–1994 to 1999–2004. Am J Clin Nutr. 2009;89:372–81. doi: 10.3945/ajcn.2008.26883. [DOI] [PubMed] [Google Scholar]
- (5).Berkey CS, Rockett HR, Field AE, Gillman MW, Colditz GA. Sugar-added beverages and adolescent weight change. Obes Res. 2004;12:778–88. doi: 10.1038/oby.2004.94. [DOI] [PubMed] [Google Scholar]
- (6).Ludwig DS, Peterson KE, Gortmaker SL. Relation between consumption of sugar-sweetened drinks and childhood obesity: a prospective, observational analysis. Lancet. 2001;357:505–8. doi: 10.1016/S0140-6736(00)04041-1. [DOI] [PubMed] [Google Scholar]
- (7).Schulze MB, Manson JE, Ludwig DS, Colditz GA, Stampfer MJ, Willett WC, Hu FB. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA. 2004;292:927–34. doi: 10.1001/jama.292.8.927. [DOI] [PubMed] [Google Scholar]
- (8).Montonen J, Jarvinen R, Knekt P, Heliovaara M, Reunanen A. Consumption of sweetened beverages and intakes of fructose and glucose predict type 2 diabetes occurrence. J Nutr. 2007;137:1447–54. doi: 10.1093/jn/137.6.1447. [DOI] [PubMed] [Google Scholar]
- (9).Palmer JR, Boggs DA, Krishnan S, Hu FB, Singer M, Rosenberg L. Sugar-sweetened beverages and incidence of type 2 diabetes mellitus in African American women. Arch Intern Med. 2008;168:1487–92. doi: 10.1001/archinte.168.14.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Fung TT, Malik V, Rexrode KM, Manson JE, Willett WC, Hu FB. Sweetened beverage consumption and risk of coronary heart disease in women. Am J Clin Nutr. 2009;89:1037–42. doi: 10.3945/ajcn.2008.27140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Hwang IS, Ho H, Hoffman BB, Reaven GM. Fructose-induced insulin resistance and hypertension in rats. Hypertension. 1987;10:512–6. doi: 10.1161/01.hyp.10.5.512. [DOI] [PubMed] [Google Scholar]
- (12).Preuss HG, Zein M, MacArthy P, Dipette D, Sabnis S, Knapka J. Sugar-induced blood pressure elevations over the lifespan of three substrains of Wistar rats. J Am Coll Nutr. 1998;17:36–47. doi: 10.1080/07315724.1998.10720453. [DOI] [PubMed] [Google Scholar]
- (13).Reaven GM, Ho H. Sugar-induced hypertension in Sprague-Dawley rats. Am J Hypertens. 1991;4:610–4. doi: 10.1093/ajh/4.7.610. [DOI] [PubMed] [Google Scholar]
- (14).Sanchez-Lozada LG, Tapia E, Jimenez A, Bautista P, Cristobal M, Nepomuceno T, Soto V, Avila-Casado C, Nakagawa T, Johnson RJ, Herrera-Acosta J, Franco M. Fructose-induced metabolic syndrome is associated with glomerular hypertension and renal microvascular damage in rats. Am J Physiol Renal Physiol. 2007;292:F423–F429. doi: 10.1152/ajprenal.00124.2006. [DOI] [PubMed] [Google Scholar]
- (15).Dhingra R, Sullivan L, Jacques PF, Wang TJ, Fox CS, Meigs JB, D'Agostino RB, Gaziano JM, Vasan RS. Soft drink consumption and risk of developing cardiometabolic risk factors and the metabolic syndrome in middle-aged adults in the community. Circulation. 2007;116:480–8. doi: 10.1161/CIRCULATIONAHA.107.689935. [DOI] [PubMed] [Google Scholar]
- (16).Nguyen S, Choi HK, Lustig RH, Hsu CY. Sugar-sweetened beverages, serum uric acid, and blood pressure in adolescents. J Pediatr. 2009;154:807–13. doi: 10.1016/j.jpeds.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Winkelmayer WC, Stampfer MJ, Willett WC, Curhan GC. Habitual caffeine intake and the risk of hypertension in women. JAMA. 2005;294:2330–5. doi: 10.1001/jama.294.18.2330. [DOI] [PubMed] [Google Scholar]
- (18).Appel LJ, Champagne CM, Harsha DW, Cooper LS, Obarzanek E, Elmer PJ, Stevens VJ, Vollmer WM, Lin PH, Svetkey LP, Stedman SW, Young DR, Writing Group of the PREMIER Collaborative Research Group Effects of comprehensive lifestyle modification on blood pressure control: main results of the PREMIER clinical trial. JAMA. 2003;289:2083–93. doi: 10.1001/jama.289.16.2083. [DOI] [PubMed] [Google Scholar]
- (19).Svetkey LP, Harsha DW, Vollmer WM, Stevens VJ, Obarzanek E, Elmer PJ, Lin PH, Champagne C, Simons-Morton DG, Aickin M, Proschan MA, Appel LJ. Premier: a clinical trial of comprehensive lifestyle modification for blood pressure control: rationale, design and baseline characteristics. Ann Epidemiol. 2003;13:462–71. doi: 10.1016/s1047-2797(03)00006-1. [DOI] [PubMed] [Google Scholar]
- (20).Blair SN, Haskell WL, Ho P, Paffenbarger RS, Jr., Vranizan KM, Farquhar JW, Wood PD. Assessment of habitual physical activity by a seven-day recall in a community survey and controlled experiments. Am J Epidemiol. 1985;122:794–804. doi: 10.1093/oxfordjournals.aje.a114163. [DOI] [PubMed] [Google Scholar]
- (21).Obarzanek E, Vollmer WM, Lin PH, Cooper LS, Young DR, Ard JD, Stevens VJ, Simons-Morton DG, Svetkey LP, Harsha DW, Elmer PJ, Appel LJ. Effects of individual components of multiple behavior changes: the PREMIER trial. Am J Health Behav. 2007;31:545–60. doi: 10.5555/ajhb.2007.31.5.545. [DOI] [PubMed] [Google Scholar]
- (22).Casiglia E, Bongiovi S, Paleari CD, Petucco S, Boni M, Colangeli G, Penzo M, Pessina AC. Haemodynamic effects of coffee and caffeine in normal volunteers: a placebo-controlled clinical study. J Intern Med. 1991;229:501–4. doi: 10.1111/j.1365-2796.1991.tb00385.x. [DOI] [PubMed] [Google Scholar]
- (23).Robertson D, Frolich JC, Carr RK, Watson JT, Hollifield JW, Shand DG, Oates JA. Effects of caffeine on plasma renin activity, catecholamines and blood pressure. N Engl J Med. 1978;298:181–6. doi: 10.1056/NEJM197801262980403. [DOI] [PubMed] [Google Scholar]
- (24).Israel KD, Michaelis OE, Reiser S, Keeney M. Serum uric acid, inorganic phosphorus, and glutamic-oxalacetic transaminase and blood pressure in carbohydrate-sensitive adults consuming three different levels of sucrose. Ann Nutr Metab. 1983;27:425–35. doi: 10.1159/000176714. [DOI] [PubMed] [Google Scholar]
- (25).Rowe JW, Young JB, Minaker KL, Stevens AL, Pallotta J, Landsberg L. Effect of insulin and glucose infusions on sympathetic nervous system activity in normal man. Diabetes. 1981;30:219–25. doi: 10.2337/diab.30.3.219. [DOI] [PubMed] [Google Scholar]
- (26).Rebello T, Hodges RE, Smith JL. Short-term effects of various sugars on antinatriuresis and blood pressure changes in normotensive young men. Am J Clin Nutr. 1983;38:84–94. doi: 10.1093/ajcn/38.1.84. [DOI] [PubMed] [Google Scholar]
- (27).Johnson RJ, Segal MS, Sautin Y, Nakagawa T, Feig DI, Kang DH, Gersch MS, Benner S, Sanchez-Lozada LG. Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am J Clin Nutr. 2007;86:899–906. doi: 10.1093/ajcn/86.4.899. [DOI] [PubMed] [Google Scholar]
- (28).Feig DI, Kang DH, Nakagawa T, Mazzali M, Johnson RJ. Uric acid and hypertension. Curr Hypertens Rep. 2006;8:111–5. doi: 10.1007/s11906-006-0005-z. [DOI] [PubMed] [Google Scholar]
- (29).Stamler R. Implications of the INTERSALT study. Hypertension. 1991;17:I16–I20. doi: 10.1161/01.hyp.17.1_suppl.i16. [DOI] [PubMed] [Google Scholar]