Abstract
Purpose
To investigate the incidence and clinical characteristics of angioedema associated with the use of angiotensin-converting enzyme inhibitors (ACEIs) in an outpatient allergy department.
Methods
A retrospective review of medical records of new patients seen in an allergy clinic. Demographic and clinical data of patients with ACEI-induced angioedema were analyzed.
Results
Nine (0.37%) out of 2,421 new patients attending the allergy clinic developed ACEI-associated angioedema. Enalapril was the drug most frequently incriminated. The onset of the angioedema was as early as after the first dose or as late as 2 years after beginning treatment. Six patients experienced life-threatening angioedema involving the tongue, oropharynx, or larynx, and two patients required transfer to the intensive care unit. One patient required a tracheostomy.
Conclusions
Angiotensin-converting enzyme inhibitor treatment is often responsible for angioedema, especially involving the upper airways. Due to the high proportion of the population exposed to ACEIs and to the severity of this adverse effect, it is important that physicians consider ACEIs as possible inducers when evaluating patients with acute or recurrent angioedema.
Keywords: Angiotensin-converting enzyme inhibitors, angioedema, bradykinin, captopril, enalapril
INTRODUCTION
Angiotensin-converting enzyme inhibitors (ACEIs) are widely used to treat patients with hypertension and refractory cardiac failure. It has been estimated that more than 40 million people worldwide are currently receiving ACEIs, and their use is expected to continue increasing.
Cutaneous adverse effects caused by these drugs include urticaria, maculopapular and lichenoid eruptions, pityriasis rosealike rash, pemphigus, photosensitivity, and linear IgA dermatosis. The most common adverse effects observed in patients treated with ACEIs are a dry nonproductive cough occurring in 15-30% of patients and angioedema.
Angioedema was first described by Milton in 18761 and was termed angioneurotic angioedema by Quincke in 1882.2 Drug-induced angioedema has been associated with the use of various medications, including nonsteroidal anti-inflammatory drugs (NSAIDs), ACEIs, radiocontrast media, angiotensin II receptor antagonists, antibiotics, proton pump inhibitors, statins, fibrinolytic agents, estrogens, diuretics, calcium channel blockers, beta blockers, and psychotropic drugs (serotonin reuptake inhibitors). The drugs most frequently involved are NSAIDs and ACEIs.3,4
Angioedema induced by ACEIs is present in 0.1-0.7% of treated patients5 and more often involves the head, neck, face, lips, tongue and larynx. In rare cases, it can involve visceral organs such as the gut. Life-threatening edema of the upper airway, which is present in 25-39% of cases of ACEI angioedema,6,7 can be resistant to treatment and even fatal.8,9
The mechanism of angioedema in patients taking ACEIs involves the inhibition of ACE, which blocks the conversion of angiotensin, reduces the catabolism of bradykinin, and increases its activity.10 Decreased aminopeptidase P (APP) activity and dipeptidyl peptidase P in the substance P degradation pathways also seem to play a role.11 Furthermore, a polymorphism of XPNPEP2 (the -2399 A variant), a candidate gene encoding membrane-bound APP, is associated with reduced APP activity and a higher incidence of ACEI-induced angioedema.12
Angioedema associated with ACEIs is most commonly observed at the beginning of treatment, but it may also develop long after the drug has been started.13-15 Sometimes, angioedema is present in patients taking ACEIs and other concomitant drugs such as NSAIDs.16,17 This paper presents clinical data from a group of patients with ACEI-induced angioedema attending an allergy clinic between January 2005 and December 2009.
MATERIALS AND METHODS
This is a retrospective review of the medical records of patients attending an allergy clinic in Caracas, Venezuela, for 5 years, from January 2005 to December 2009, with the aim of determining the incidence of angioedema associated with the use of ACEIs. Records of all new patients seen at the Allergy and Clinical Immunology Department of Clínica El Avila during this period were reviewed, and only those with a definitive diagnosis of ACEI-induced angioedema were included in the study. Clinical data retrieved from the history included age, gender, race, history of other medical or allergic conditions, anatomical distribution of angioedema, severity, concomitant therapy, time of onset, responsible ACEI, and management. For ethical considerations, no challenge or re-exposure tests with ACEIs were carried out, as the angioedema in these patients may be life threatening. The diagnosis was based on the clinical picture, temporal relationship to drug exposure, absence of other possible causes of angioedema, and disappearance of the clinical manifestations after discontinuing the drug.
RESULTS
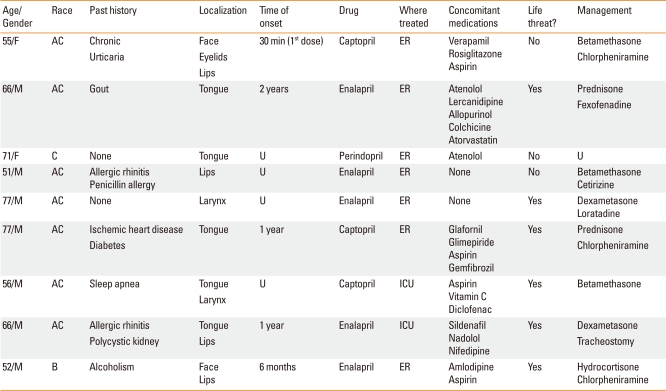
During the study period, 2,421 new patients were seen in the outpatient clinic of the Allergy and Immunology Department. Nine patients (0.37%) developed angioedema related to ACEI treatment. The demographic and clinical data of those patients are presented in Table 1.
Table 1.
Demographic and clinical data in patients with ACEI-induced angioedema
AC, American Caucasoid; C, Caucasian; B, Black; U, unknown; ER, Emergency room; ICU, Intensive care unit.
Seven patients were males, and two were female; their mean age was 63.4±10.3 years (range 51-77 years). Seven patients were American Caucasoid, one was Caucasian, and one was black. The angioedema was localized to the tongue in 5 patients, lips in 4, diffusely on the face in 2, larynx in 2, and eyelids in 1 (Fig. 1). The time of onset of the angioedema in relation to ACEI therapy was obtained for five patients and ranged from 30 minutes after the first drug exposure to 2 years.
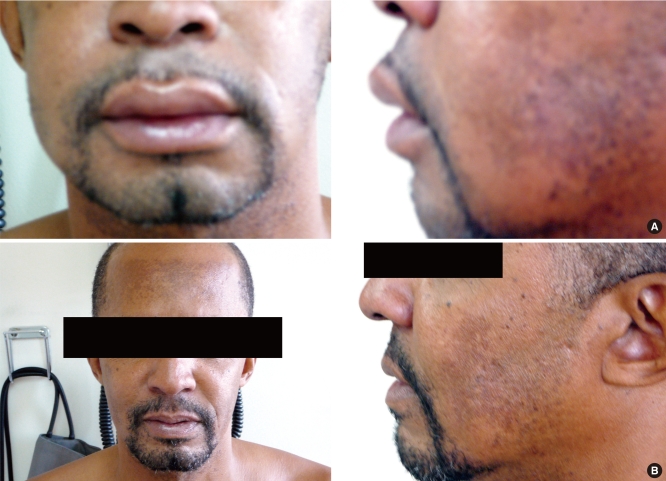
Fig. 1.
(A) A male, 51 years old black patient who experienced diffuse facial and lip angioedema during treatment with enalapril. (B) The same patient as in (A). Notice angioedema resolution after 6 days of enalapril discontinuation.
Enalapril was responsible for the angioedema in 5 patients, captopril in 3, and perindopril in 1. Seven patients were treated in the emergency department and two in the intensive care unit. According to the severity and degree of compromise of airway patency, six patients experienced life-threatening angioedema involving the tongue, oropharynx or larynx. Treatment consisted of parenteral and oral corticosteroids and antihistamines in all patients, and a tracheostomy was performed in one patient. In none of the patients was the diagnosis of ACEI-associated angioedema made by the medical services involved in managing the acute episode.
DISCUSSION
Various risk factors for ACEI-induced angioedema have been identified, including black race, female gender, previous drug rash,14 smoking habit,18 age older than 65 years, seasonal allergies, recent initiation of ACEIs (first week of therapy), obesity, upper airway surgery or trauma, sleep apnea and immunosuppression in cardiac and renal transplant recipients.19
The issue of adverse effects of ACEIs is clinically relevant due to the large number of subjects exposed to these drugs, which is increasing. Various ACEIs, including enalapril, lisinopril, captopril, ramipril, imidapril, benazepril, trandolapril and perindopril, are in use in many countries. Since angioedema is a drug class effect, it is very important that physicians consider these drugs in the differential diagnosis of angioedema and give proper advice on avoiding all ACEIs to these patients.
All nine patients seen in our allergy clinic had severe angioedema, which was considered life threatening in approximately two-thirds of the cases. ACEIs were rarely considered as possible etiologic factors by the treating physicians in the emergency room or hospital ward. Only symptomatic treatment with corticosteroids and antihistamines was given before referral to the allergy specialist. Many of the patients had repeated episodes of angioedema before being referred for further evaluation.
Regarding the management of patients with ACEI angioedema, the most important recommendation is to avoid ACEIs and modify the treatment, generally with the agreement of the treating physician. Antihypertensive medications from other pharmacologic groups are indicated. In our patients, calcium channel blockers were often recommended. According to Malde et al.,20 only 8% of patients who experienced angioedema from ACEIs previously develop angioedema with angiotensin receptor blockers (ARB). During the 5-year period of our investigation, one patient, a 64-year-old male, developed eyelid and lip angioedema after 2 years of treatment with losartan, an ARB; this resolved after the drug was stopped and therapy was switched to amlodipine.
Recently, Weber et al.,21 proposed a new treatment for ACEI-associated angioedema. They postulated that since bradykinin is a major mediator of angioedema from ACEIs, the bradykinin inhibitor icatibant, presently used in patients with hereditary angioedema, could be effective for these patients.
Footnotes
There are no financial or other issues that might lead to conflict of interest.
References
- 1.Cicardi M, Agostoni A. Hereditary angioedema. N Engl J Med. 1996;334:1666–1667. doi: 10.1056/NEJM199606203342510. [DOI] [PubMed] [Google Scholar]
- 2.Quincke H. Über akutes umschriebenes Hautödem. Monatsh Prakt Dermatol. 1882;1:129–131. [Google Scholar]
- 3.Lombardi C, Crivellaro M, Dama A, Senna G, Gargioni S, Passalacqua G. Are physicians aware of the side effects of angiotensin-converting enzyme inhibitors?: a questionnaire survey in different medical categories. Chest. 2005;128:976–979. doi: 10.1378/chest.128.2.976. [DOI] [PubMed] [Google Scholar]
- 4.Howes LG, Tran D. Can angiotensin receptor antagonists be used safely in patients with previous ACE inhibitor-induced angioedema? Drug Saf. 2002;25:73–76. doi: 10.2165/00002018-200225020-00001. [DOI] [PubMed] [Google Scholar]
- 5.Miller DR, Oliveria SA, Berlowitz DR, Fincke BG, Stang P, Lillienfeld DE. Angioedema incidence in US veterans initiating angiotensin-converting enzyme inhibitors. Hypertension. 2008;51:1624–1630. doi: 10.1161/HYPERTENSIONAHA.108.110270. [DOI] [PubMed] [Google Scholar]
- 6.Sarkar P, Nicholson G, Hall G. Brief review: angiotensin converting enzyme inhibitors and angioedema: anesthetic implications. Can J Anaesth. 2006;53:994–1003. doi: 10.1007/BF03022528. [DOI] [PubMed] [Google Scholar]
- 7.Slater EE, Merrill DD, Guess HA, Roylance PJ, Cooper WD, Inman WH, Ewan PW. Clinical profile of angioedema associated with angiotensin converting-enzyme inhibition. JAMA. 1988;260:967–970. [PubMed] [Google Scholar]
- 8.Dean DE, Schultz DL, Powers RH. Asphyxia due to angiotensin converting enzyme (ACE) inhibitor mediated angioedema of the tongue during the treatment of hypertensive heart disease. J Forensic Sci. 2001;46:1239–1243. [PubMed] [Google Scholar]
- 9.Cupido C, Rayner B. Life-threatening angio-oedema and death associated with the ACE inhibitor enalapril. S Afr Med J. 2007;97:244–245. [PubMed] [Google Scholar]
- 10.Mlynarek A, Hagr A, Kost K. Angiotensin-converting enzyme inhibitor-induced unilateral tongue angioedema. Otolaryngol Head Neck Surg. 2003;129:593–595. doi: 10.1016/S0194-59980300724-1. [DOI] [PubMed] [Google Scholar]
- 11.Byrd JB, Touzin K, Sile S, Gainer JV, Yu C, Nadeau J, Adam A, Brown NJ. Dipeptidyl peptidase IV in angiotensin-converting enzyme inhibitor associated angioedema. Hypertension. 2008;51:141–147. doi: 10.1161/HYPERTENSIONAHA.107.096552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duan QL, Nikpoor B, Dube MP, Molinaro G, Meijer IA, Dion P, Rochefort D, Saint-Onge J, Flury L, Brown NJ, Gainer JV, Rouleau JL, Agostoni A, Cugno M, Simon P, Clavel P, Potier J, Wehbe B, Benarbia S, Marc-Aurele J, Chanard J, Foroud T, Adam A, Rouleau GA. A variant in XPNPEP2 is associated with angioedema induced by angiotensin I-converting enzyme inhibitors. Am J Hum Genet. 2005;77:617–626. doi: 10.1086/496899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oike Y, Ogata Y, Higashi D, Matsumura T, Numata Y. Fatal angioedema associated with enalapril. Intern Med. 1993;32:308–310. doi: 10.2169/internalmedicine.32.308. [DOI] [PubMed] [Google Scholar]
- 14.Kostis JB, Kim HJ, Rusnak J, Casale T, Kaplan A, Corren J, Levy E. Incidence and characteristics of angioedema associated with enalapril. Arch Intern Med. 2005;165:1637–1642. doi: 10.1001/archinte.165.14.1637. [DOI] [PubMed] [Google Scholar]
- 15.Chin HL, Buchan DA. Severe angioedema after long-term use of an angiotensin-converting enzyme inhibitor. Ann Intern Med. 1990;112:312–313. doi: 10.7326/0003-4819-112-4-312_2. [DOI] [PubMed] [Google Scholar]
- 16.Kampitak T. Recurrent severe angioedema associated with imidapril and diclofenac. Allergol Int. 2008;57:441–443. doi: 10.2332/allergolint.C-08-61. [DOI] [PubMed] [Google Scholar]
- 17.Kamata Y, Iwamoto M, Kamimura T, Kanashiki E, Yoshio T, Okazaki H, Morita T, Minota S. Repeated massive tongue swelling due to the combined use of estramustine phosphate and angiotensin-converting enzyme inhibitor. J Investig Allergol Clin Immunol. 2006;16:388–390. [PubMed] [Google Scholar]
- 18.Morimoto T, Gandhi TK, Fiskio JM, Seger AC, So JW, Cook EF, Fukui T, Bates DW. An evaluation of risk factors for adverse drug events associated with angiotensin-converting enzyme inhibitors. J Eval Clin Pract. 2004;10:499–509. doi: 10.1111/j.1365-2753.2003.00484.x. [DOI] [PubMed] [Google Scholar]
- 19.Abbosh J, Anderson JA, Levine AB, Kupin WL. Angiotensin converting enzyme inhibitor-induced angioedema more prevalent in transplant patients. Ann Allergy Asthma Immunol. 1999;82:473–476. doi: 10.1016/S1081-1206(10)62723-8. [DOI] [PubMed] [Google Scholar]
- 20.Malde B, Regalado J, Greenberger PA. Investigation of angioedema associated with the use of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. Ann Allergy Asthma Immunol. 2007;98:57–63. doi: 10.1016/S1081-1206(10)60860-5. [DOI] [PubMed] [Google Scholar]
- 21.Weber MA, Messerli FH. Angiotensin-converting enzyme inhibitors and angioedema: estimating the risk. Hypertension. 2008;51:1465–1467. doi: 10.1161/HYPERTENSIONAHA.108.111393. [DOI] [PubMed] [Google Scholar]