Abstract
Recent reports indicate that aerobic exercise improves the overall physical fitness and health of asthmatic patients. The specific exercise-induced improvements in the pathology of asthma and the mechanisms by which these improvements occur, however, are ill-defined; thus, the therapeutic potential of exercise in the treatment of asthma remains unappreciated. Using an OVA-driven mouse model, we examined the role of aerobic exercise in modulating inflammatory responses associated with atopic asthma. Data demonstrate that moderate intensity aerobic exercise training decreased leukocyte infiltration, cytokine production, adhesion molecule expression, and structural remodeling within the lungs of OVA-sensitized mice (n = 6–10; p < 0.05). Because the transcription factor NF-κB regulates the expression of a variety of genes that encode inflammatory mediators, we monitored changes in NF-κB activation in the lungs of exercised/sensitized mice. Results show that exercise decreased NF-κB nuclear translocation and IκBα phosphorylation, indicating that exercise decreased NF-κB activation in the lungs of sensitized mice (n = 6). Taken together, these results suggest that aerobic exercise attenuates airway inflammation in a mouse model of atopic asthma via modulation of NF-κB activation. Potential exists, therefore, for the amelioration of asthma-associated chronic airway inflammation through the use of aerobic exercise training as a non-drug therapeutic modality.
Allergic or atopic asthma, the most common form of asthma, is identified by the presence of characteristic clinical symptoms of wheezing, chest tightness, dyspnea and cough, and by the presence of reversible airway narrowing and/or airway hyperresponsiveness to a variety of inhaled bronchoconstrictor stimuli (1). Although multifactorial in origin, atopic asthma is characterized as an inflammatory process that is the result of an inappropriate immune response to common allergens; this inflammatory process can be illustrated in a two-phase airway response. The first or early phase, an immediate reaction triggered upon allergen contact, involves the cross-linking of allergen-specific IgE to FcεRI on submucosal pulmonary mast cells resulting in degranulation and release of both preformed and newly synthesized inflammatory mediators. Early-phase events such as increased microvascular permeability, increased mucus secretion, and smooth muscle contraction are largely attributed to the actions of histamine. The second or late phase, a delayed and sustained inflammatory response, involves the response to cysteinyl leukotrienes, PGs, cytokines (e.g., IL-4, IL-5, IL-13), chemokines (e.g., IL-8, monocyte chemoattractant protein 1 (MCP-1),2 RANTES), adhesion molecules (i.e., VCAM-1), and matrix metalloproteinases. These factors are either released by, recruit, and/or promote the inflammatory cascade of macrophages and various leukocytes such as Th2 lymphocytes, eosinophils, and neutrophils. Notably, gene expression of many of these proinflammatory factors is regulated by the transcription factor NF-κB (2). Sequelae from this phase perpetuate further tissue damage and airway remodeling via epithelial tissue sloughing, smooth muscle proliferation and contraction, and basement membrane thickening.
The increase in prevalence, severity, and mortality of asthma highlights the complexity of the disease in relation to environmental and genetic factors as well as the need for more efficacious drug and non-drug interventions (3). Given that exercise modulates immune responses in healthy individuals (4–6), consideration of the clinical ramifications of exercise in the modification of a disease for which the immune system has a role is of vital importance. Several clinical studies suggest that aerobic exercise training improves the overall physical fitness and health of asthmatics through increasing ventilatory capacity and lessening of asthma-related symptoms (7–11). American Thoracic Society guidelines for pulmonary rehabilitation programs, in accordance with these clinical studies, recommend the implementation of low to moderate intensity aerobic exercise training for those with chronic respiratory disease, including asthma (12). Despite these studies and recommended guidelines, it remains unclear whether physical training can attenuate asthmatic inflammatory responses and disease progression (13, 14).
The hypothesis of this study was that moderate aerobic exercise would attenuate inflammatory responses associated with the asthmatic airway. Studies described herein indicate that moderate intensity aerobic exercise training attenuated lung inflammatory responses in an OVA-driven mouse model of atopic asthma. Exercise-mediated decreases in NF-κB nuclear translocation and IκBα phosphorylation indicate that attenuation of NF-κB activation may facilitate the observed anti-inflammatory effects of exercise. In light of these findings, potential may exist for the reduction of asthma-associated chronic airway inflammation through the use of aerobic exercise training as a non-drug therapy.
Materials and Methods
Animals
Sixty female BALB/cJ mice (3–5 wk old; The Jackson Laboratory, Bar Harbor, ME), a strain susceptible to OVA-induced IgE responses (15, 16), were used. Mice were housed in a pathogen-free containment facility and maintained in autoclaved Microisolator cages (Lab Products, Maywood, NJ). Mice were provided with food (Teklad, Madison, WI) and water ad libitum. Two animals were withdrawn from the exercised nonsensitized group for failure to exercise. All animal treatments were approved by the University of Alabama at Birmingham Institutional Animal Care and Use Committee and were in accordance with the National Institutes of Health recommended guidelines.
OVA sensitization and exercise protocol
At the start of the protocol, mice (six to nine per group) were assigned randomly to one of the following four experimental groups: sedentary and nonsensitized (S), sedentary and OVA sensitized (SO), exercised and non-sensitized (E), and exercised and OVA sensitized (EO). OVA-sensitized mice (SO and EO) were injected i.p. (200 µl/mouse) on days 0 and 14 with 50 µg of alum-precipitated chicken egg OVA (Imject Alum; Pierce, Rock-ford, IL; grade VII OVA, ≥98% pure, Sigma-Aldrich, St. Louis, MO). Limulus amebocyte lysate analysis demonstrated that this commercial OVA solution contained ~50 endotoxin units (EU)/ml endotoxin (data not shown). Following these injections and beginning on day 21, mice were exposed to aerosolized OVA at a concentration of 5 mg/ml (in 0.85% NaCl solution) for 30 min/day for 5 consecutive days. Thereafter, aerosolized boosters of OVA (at the same concentration) were administered for 10 min/day, 5 consecutive days/wk for the remainder of the study. Mice assigned to the nonsensitized groups (S and E groups) received i.p. injections and aerosolizations of saline only.
One to 2 h postaerosolization, exercised mice (E and EO groups) underwent bouts of aerobic exercise on a motorized treadmill (Exer 6M; Columbus Instruments, Columbus, OH). This mode of exercise was used because exercise intensity and duration could be manipulated experimentally, unlike voluntary running or swimming. Exercise bouts were performed three times per wk for a total time period of 4 wk. During the first week of the exercise regimen, treadmill speed and exercise duration were progressively increased from 10.0 m/min for 30 min to 13.5 m/min for 45 min to acclimate to and train mice on the treadmill. In the remainder of the protocol (weeks 2–4), mice exercised for 45 min at 13.5 m/min (0% grade). All exercise bouts included brief warm-up and cool-down periods so that the total treadmill time was ~60 min. Previous studies have defined moderate intensity aerobic exercise as brief (15–60 min) bouts of treadmill running at 50–75% maximum O2 consumption or ~15–22 m/min (17–21).Because the EO group received an inflammatory-promoting pulmonary treatment, treadmill parameters were set at the lower end of the max O2 consumption range so as to reduce the chance of exercise-induced bronchospasm. Repetitive aerobic exercise was tailored to mimic the classic parameters of traditional pulmonary rehabilitation programs; such parameters include intermittent weekly sessions of moderately intense physical aerobic training, with emphasis on the lower extremities, for a period of 1 mo (14, 22, 23). Twenty-four hours after completion of the sensitization/ exercise protocol, mice were euthanized via i.p. injection of ketamine (8.7 mg/100 g body wt) mixed with xylazine (1.3 mg/100 g body wt) (24).
Histopathology and immunohistochemistry
Lungs fixed in 70% alcoholic Formalin and paraffin embedded were stained with Alcian blue-periodic acid-Schiff and hematoxylin as described previously (25). After random coding, the degree of inflammation in the periodic acid-Schiff and hematoxylin analyses was assessed subjectively; in particular, the extent of leukocyte infiltrate, epithelial cell hypertrophy, and mucus production was assessed. A semiquantitative rating scale ranged from 0 (none) to maximal (4). Each average score per category was multiplied by the extent of appearance in the lungs (also rated 0–4) to devise an overall index of inflammation. In addition, Formalin-fixed paraffin-embedded sections were stained with Abs specific for ICAM-1 (R&D Systems, Minneapolis, MN), VCAM-1 (Santa Cruz Biotechnology, Santa Cruz, CA), or the NF-κB subunit p65 (Santa Cruz Biotechnology). Formalin-preserved sections were treated with citrate buffer (pH 6.0) to facilitate Ag retrieval and then stained with the respective primary Ab followed by the appropriate Alexa Fluor 564 secondary Ab (Molecular Probes, Eugene, OR) as described previously (26).
Lavage and serum protein analysis
Mice were lavaged (24) or cardiac punctured (27) as described previously. Briefly, mice were lavaged with 1 ml × 4 of 0.9% saline and collected bronchoalveolar lavage fluid (BALF) was centrifuged to pellet the cellular fraction; the first milliliter collected was used for cytokine analysis. Cell viability was determined via trypan blue exclusion and cell types were differentiated on cytospin preps using Diff-Quik stain. Cell differentials were determined from at least 500 leukocytes using standard hematological criteria. Total protein concentrations in BALF were determined via detergent-compatible assay (Bio-Rad, Hercules, CA). BALF samples were analyzed for KC, MCP-1, RANTES, IL-4, and IL-5 protein content via ELISA (BioSource International, Camarillo, CA). Serum samples were analyzed for total IgE and OVA-specific IgE via ELISA (BD PharMingen, San Diego, CA).
Analysis of IκBα phosphorylation
Lungs were extracted, homogenized, and lysed in a buffer containing 10 µM Tris, 0.15 mM NaCl, 1% Triton X-100, and the protease inhibitors aprotinin, leupeptin, pepstatin A (100 µg/ml each), and 10 µM PMSF. Equivalent amounts of protein (50 µg/lane) for each sample were electro-phoresed and transferred to a polyvinylidene difluoride membrane. Blots were then immunoblotted with a polyclonal rabbit Ab directed against IκBα-Ser32 (diluted 1/1000 in TBS containing 0.1% Tween 20 and 5% BSA; Cell Signaling, Beverly, MA) followed by a donkey anti-rabbit IgG Ab conjugated to HRP (diluted 1/2000 in TBS containing 0.1% Tween 20 and 5% BSA). Immunoblots were developed using chemiluminescence. Blots were then stripped (0.2 N NaOH for 5 min at room temperature) and reprobed with a polyclonal rabbit Ab against IκBα (diluted 1/1000 in TBS containing 0.1% Tween 20 and 5% BSA; Cell Signaling) to verify IκBα protein expression in each sample.
Statistical analysis
Data were analyzed using SPSS Version 11.0 (SPSS, Chicago, IL). Results are reported as group means ±SEM. ANOVA determined differences among the group means and the Tukey post hoc analysis determined which group means differed significantly (at a level of p ≤ 0.05).
Results
OVA sensitization and exercise protocol
To examine the effects of aerobic exercise on allergen-mediated airway responses, BALB/cJ mice were primed with OVA via two rounds of i.p. injections followed by a series of challenges with aerosolized OVA; nonsensitized mice were administered saline alone. Throughout the challenge phase of the protocol, mice that exercised did so repeatedly on a motorized treadmill; sedentary mice continued spontaneous activity. At the conclusion of the sensitization/exercise protocol, mice were analyzed for differences in lung architecture and inflammatory responses.
Exercise attenuated allergen-mediated changes in lung architecture and inflammation
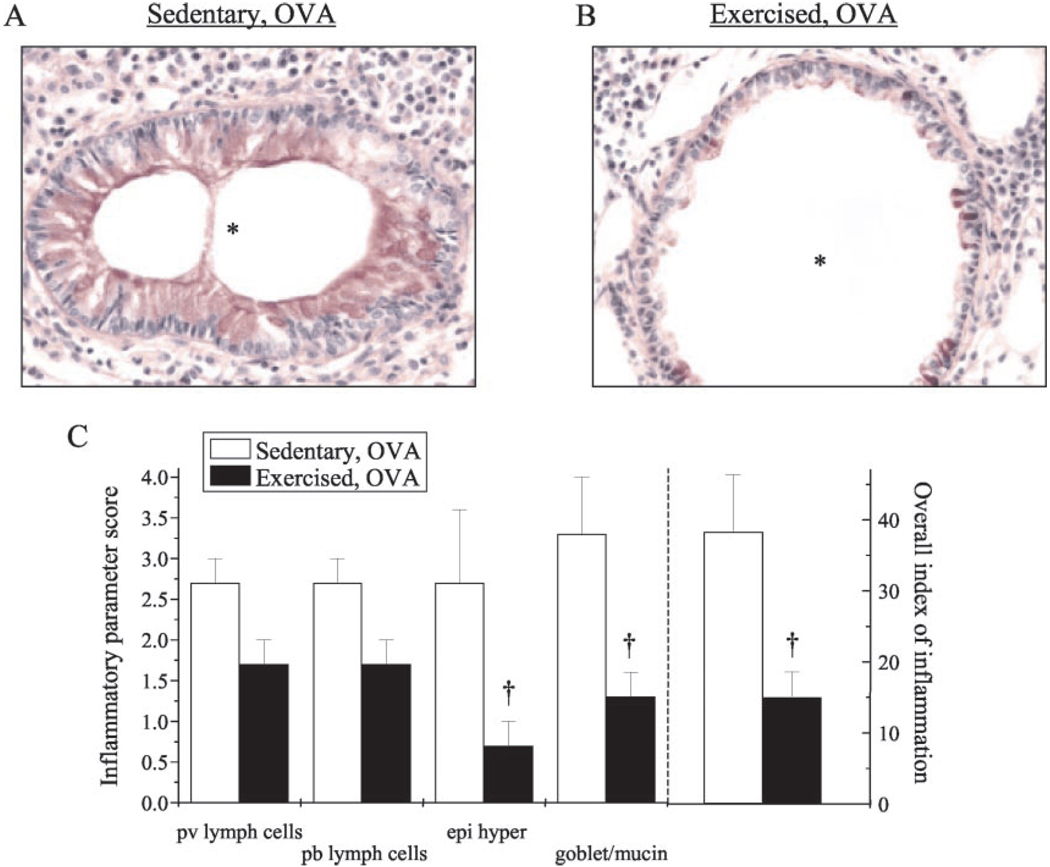
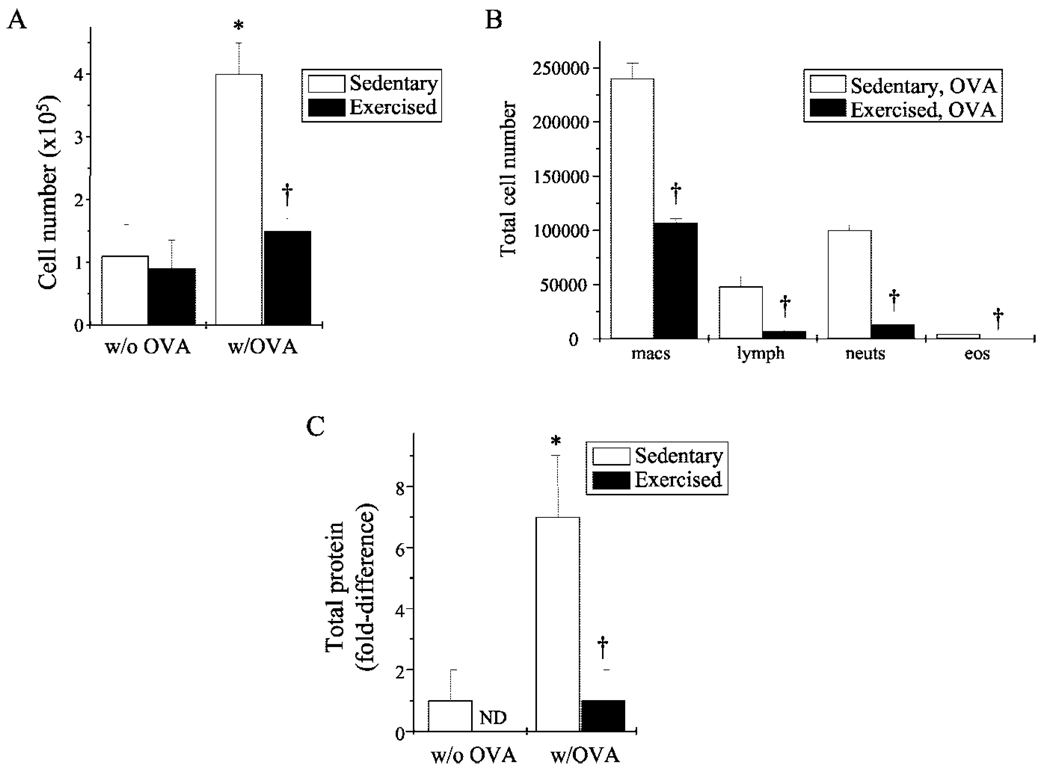
Chronic inflammation in the atopic asthmatic airway leads to characteristic changes in lung architecture. Specifically, changes include increased mucus production, epithelial cell hypertrophy, basement membrane thickening, and inflammatory cell infiltrate, which includes eosinophils and neutrophils (28). To assess the effect of exercise on these parameters in OVA-sensitized mice, lungs were excised and/or lavaged and then prepared for analysis. Normal lung histology was observed in the absence of sensitization (data not shown). In contrast, OVA sensitization stimulated significant increases in inflammatory cellular infiltrate, mucus production, and epithelial hypertrophy (Fig. 1, A and C); exercise attenuated each of these parameters (Fig. 1, B and C). These exercise-mediated modifications were congruently reflected in the decreased total number and composition of infiltrating cells (Fig. 2, A and B) and the decreased protein levels (Fig. 2C) observed in the lung BALF of exercised OVA-sensitized mice as compared with their sedentary counterparts. Importantly, exercise reduced OVA-mediated increases in BALF cell number and total protein to baseline levels observed in nonsensitized mice (Fig. 2, A and C).
FIGURE 1.
Exercise lessened sensitization-induced changes in leukocyte infiltration and lung architecture. A and B, Mice were sensitized/exercised and lungs were prepared for analysis of leukocyte infiltrate, epithelial hypertrophy, and mucus production as described in Materials and Methods. Representative results of five to six separate experiments are shown (*, airway lumen; magnification, ×40). C, Tissue sections were coded randomly and scored subjectively for the inflammatory parameters of: 1) perivascular (pv) and peribronchial (pb) lymphoid accumulation; 2) hypertrophy/hyperplasia of the mucosal epithelium (epi hyper); 3) goblet cell and mucin production; and 4) overall index of inflammation. Grading scale: 0, none; 1, <25% 2, 25–50% 3, 50–75%; 4, >75%. Scores are represented as average scores per group (n = 5–6/group; †, p < 0.05 as compared with SO). Each average score per category was multiplied by the extent of appearance in the lungs (also rated 0–4) to devise an overall index of inflammation. Scores from nonsensitized groups totaled zero (data not shown).
FIGURE 2.
Exercise decreased cellular infiltrate and total lavage protein in sensitized mice. A, Cells collected from BALF of mice were analyzed for differences in total cell count as described in Materials and Methods. Results are reported as cell number (×105) (n = 5–6 for each group; *, p < 0.05 as compared with S; †, p < 0.05 as compared with SO). B, Differential BALF cell counts were performed using standard hematological criteria. Results are reported as percentage of total cell number (n = 3–4 for each group; †, p < 0.05 as compared with SO). C, BALF was monitored for changes in total protein concentrations via detergent-compatible assay. Results are reported as fold differences in total protein, as compared with nonsensitized mice (n = 3–5; *, p < 0.05 as compared with S; †, p < 0.05 as compared with SO; ND, none detected).
Exercise decreased selectively the production of chemokines and adhesion molecules in the OVA-sensitized lung
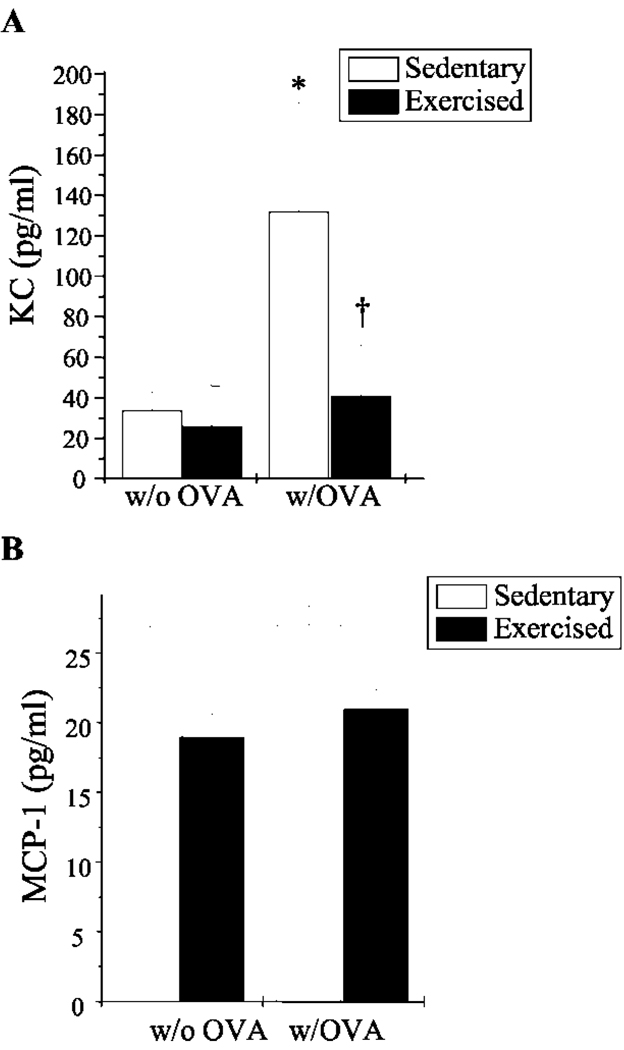
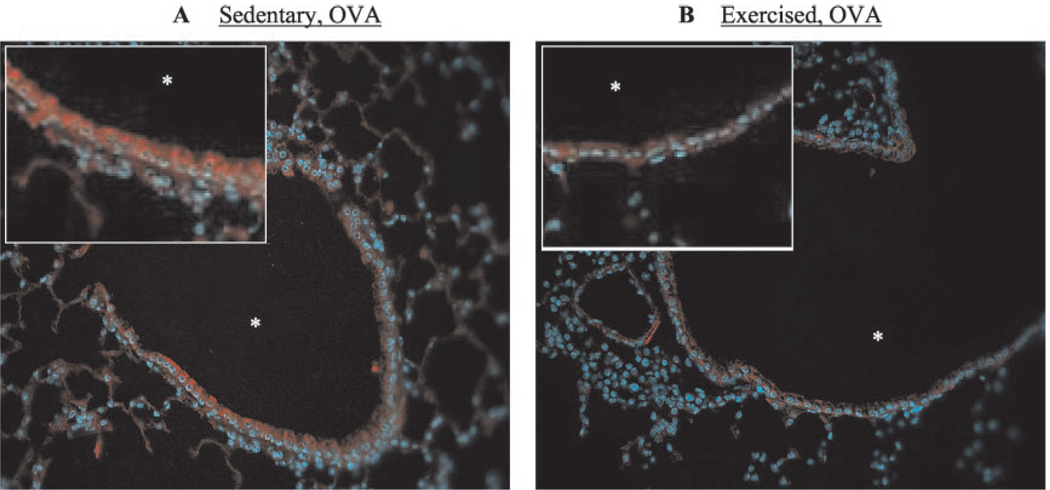
Chemokine production and adhesion molecule expression play important roles in the trafficking of immune cells into the bronchial mucosa and their subsequent activation during an inflammatory response. Increased levels of the chemokines KC (murine homologue of human IL-8), MCP-1, and RANTES and the adhesion molecules ICAM-1 and VCAM-1 have been observed repeatedly in murine models of the asthmatic lung (29–31). To examine the effect of aerobic exercise on chemokine production and adhesion molecule expression in this mouse model of atopic asthma, lungs were lavaged and/or excised and analyzed for changes in secreted KC, MCP-1, and RANTES protein levels and ICAM-1 and VCAM-1 surface protein expression. The lungs of sedentary OVA-sensitized mice contained a 4-fold increase in KC as compared with nonsensitized mice (Fig. 3A); surprisingly, OVA sensitization had little observed effect on MCP-1 levels (Fig. 3B). Importantly, KC levels in exercised OVA-sensitized mice decreased to baseline levels observed in nonsensitized mice. MCP-1 levels tended also to decrease in exercised OVA-sensitized mice, although the difference was not significant statistically as compared with nonsensitized mice. Secreted RANTES protein was not detected in either the nonsensitized or sensitized groups (data not shown). Interestingly, ICAM-1 expression did not differ between the OVA-sensitized groups (data not shown). In contrast, however, exercise reduced VCAM-1 expression in the lung epithelial structures of OVA-sensitized mice (Fig. 4, B and C); VCAM-1 expression was not observed in the lungs of sedentary (Fig. 4A)orexercised (data not shown) nonsensitized mice.
FIGURE 3.
Exercise decreased KC- and MCP-1-secreted protein levels in the lungs of OVA-sensitized mice. BALF was analyzed for KC (A)- and MCP-1-secreted protein levels (B) via ELISA. Results are reported as picograms per milliliter of respective protein (n = 5–6; *, p < 0.05 as compared with S; †, p < 0.05 as compared with SO).
FIGURE 4.
Exercise decreased VCAM-1 surface expression in the lungs of OVA-sensitized mice. Formalin-fixed, paraffin-embedded sections from SO (A) and EO (B) mice were analyzed for VCAM-1 expression as described in Materials and Methods. Tissue was counterstained with Hoescht to detect nuclei (blue). Representative results from three independent experiments are shown (*, airway lumen; magnification, ×25; inset, ×50).
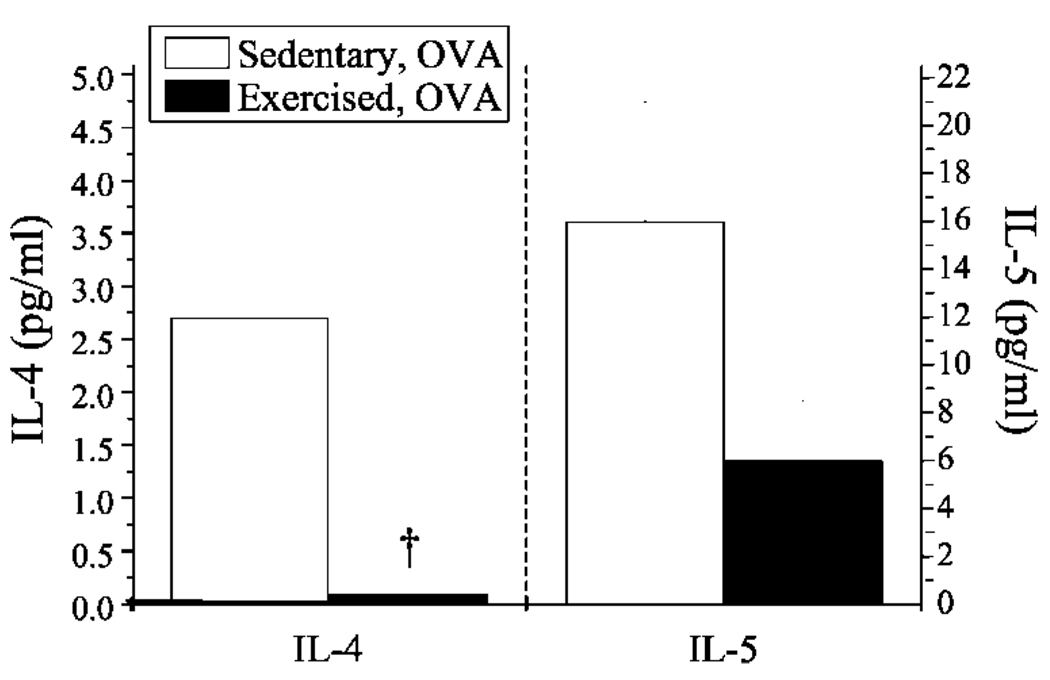
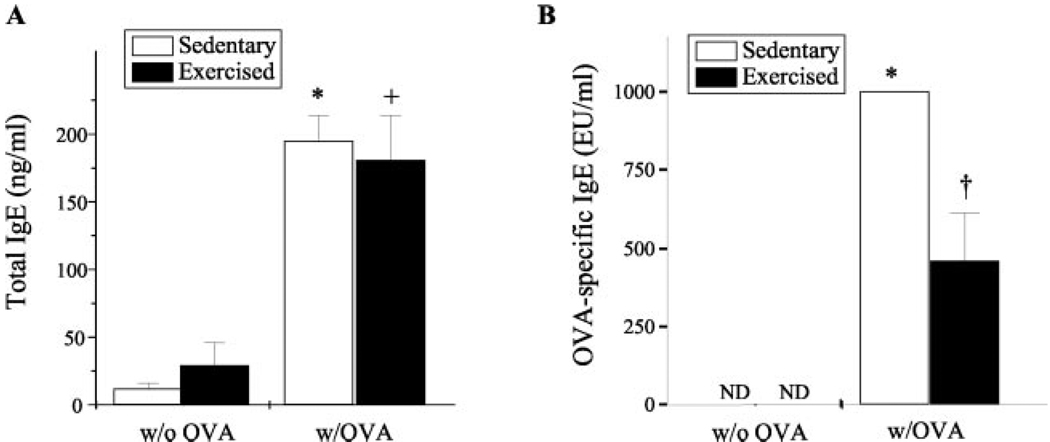
Exercise decreased detectable levels of the Th2-derived lymphokines IL-4, IL-5, and OVA-specific IgE
IgE production and allergen-induced aggregation of IgE bound to FcεRI on submucosal pulmonary mast cells promote inflammatory responses within the atopic asthmatic lung. Th2 cells secrete the lymphokines IL-4 and IL-5, which are important in mediating IgE production and eosinophilia, respectively. Because atopic asthma likely involves these processes, it is considered to be a Th2-driven disease (32). To determine the effect of aerobic exercise on the levels of lavagate IL-4 and IL-5 and serum IgE in OVA-sensitized mice, lavagates were harvested from lungs, serum was collected via intracardiac puncture, and resulting samples were monitored for changes in IL-4 and IL-5 protein content and total and OVA-specific IgE accordingly. In the sensitized airway, exercise decreased the levels of both IL-4- and IL-5-secreted protein by ~13- and 3-fold, respectively (Fig. 5). Little or no IL-4 or IL-5 protein was detected in the absence of OVA sensitization (data not shown). In addition, OVA sensitization induced a significant increase in the serum levels of total (Fig. 6A) and OVA-specific IgE content (Fig. 6B). Interestingly, exercise decreased OVA-specific IgE levels significantly, yet had no discernable effect on total IgE levels (Fig. 6B).
FIGURE 5.
Exercise decreased IL-4-and IL-5-secreted protein levels in the lungs of OVA-sensitized mice. BALF samples were analyzed for differences in secreted IL-4 and IL-5 protein via ELISA. Results are reported as picograms per milliliter of respective protein (n = 5–6; †, p < 0.05 as compared with SO; IL-5 results approached significance with p = 0.06).
FIGURE 6.
Exercise decreased OVA-specific IgE levels but not total IgE levels in the serum of OVA-sensitized mice. Serum samples were monitored for changes in total IgE (A) and OVA-specific IgE (B) levels via ELISA as described in Materials and Methods. Results for total IgE levels are reported as nanograms per milliliter protein; results for OVA-specific IgE totals are reported as EU/ml, with OVA-induced IgE arbitrarily assigned 1000 EU/ml (n = 6–9; *, p < 0.05 as compared with S; +, p < 0.05 as compared with E; †, p < 0.05 as compared with SO; ND, none detected).
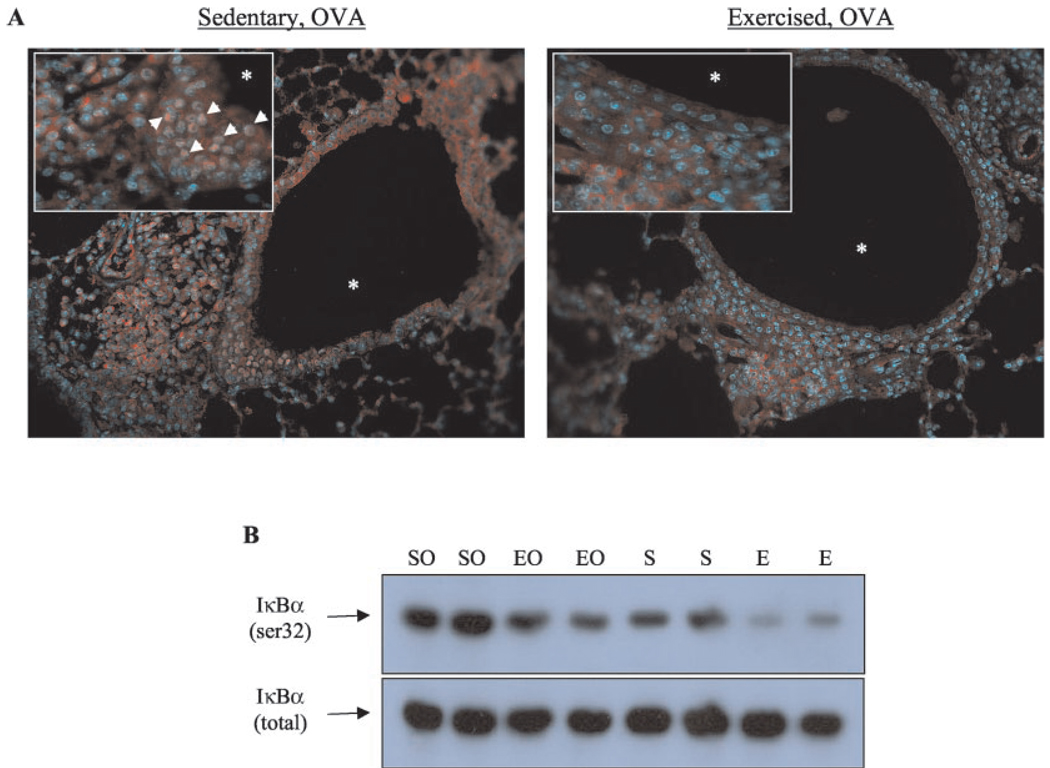
Exercise may attenuate asthmatic responses via modulation of NF-κB activation
NF-κB regulates the gene expression of many inflammatory mediators, including KC and VCAM-1 (2). NF-κB is retained in the cytoplasm of unactivated cells through interaction with members of the IκB inhibitor family, including IκBα (33). Phosphorylation (at serines 32 and 36) and subsequent degradation of IκBα releases NF-κB and allows NF-κB to translocate to the nucleus and activate transcription (33). To investigate the possible mechanisms underlying the effects of exercise on allergen-mediated inflammatory responses, therefore, IκBα phosphorylation and NF-κB nuclear translocation were examined. As shown in Fig. 7, exercise decreased both the phosphorylation of IκBα (Fig. 7A) and translocation of the NF-κB subunit p65 (Fig. 7B) in the lungs of sensitized mice as compared with sedentary controls.
FIGURE 7.
Exercise decreased NF-κB nuclear translocation and IκBα in OVA-sensitized mice. A, Translocation of the NF-κB subunit p65 (denoted by arrows) was monitored in SO (left) and EO (right) mice via immunohistochemical analysis as described in Materials and Methods. Representative photomicrographs of six separate experiments are shown (*, airway lumen; magnification, ×25; inset, ×50). B, Lung IκBα phosphorylation was analyzed via immunoblotting as described in Materials and Methods. Representative results from six independent experiments are shown.
Discussion
The data presented above demonstrate that moderate intensity aerobic exercise attenuated lung inflammatory responses in a mouse model of atopic asthma. Specifically, exercise lessened the following proinflammatory parameters in mice that were sensitized with OVA: 1) mucus production and epithelial cell hypertrophy in lung tissue; 2) cellular infiltrate and total protein concentration in the airway lumen; 3) secretion of the proinflammatory mediators KC, IL-4, and IL-5 into the airway lumen; 4) expression of the adhesion molecule VCAM-1 in intact lung tissue; and 5) production of OVA-specific IgE in serum. Data also demonstrate that exercise decreased NF-κB nuclear translocation and IκBα phosphorylation in the lungs of OVA-sensitized mice as compared with sedentary controls. Taken together, these data suggest that moderate intensity aerobic exercise training reduces asthmatic-related airway inflammation and that this reduction may occur via inhibition of NF-κB activation.
In the lungs of OVA-sensitized mice, the observed cellular infiltrate was composed primarily of macrophages and to a lesser degree of neutrophils, lymphocytes, and eosinophils. Interestingly, a greater number of neutrophils than eosinophils were observed. Such results are in accordance with a growing body of literature which suggests that, in chronic asthmatics, neutrophils are a predominant inflammatory leukocyte in the lung and contribute to airway remodeling and the development of nonreversible airway obstruction (34–36). Moreover, studies that subjected sensitized animals to inhaled OVA challenge suggest that inflammatory-cell migration into the lung is characterized by an acute neutrophil influx (37) followed by a chronic airway eosinophilia (36, 38, 39). Exercise decreased the numbers of each of the observed infiltrating cell types. In general, exercise appeared to decrease cellular infiltration by improving the integrity of the epithelial layer as indicated by reduced BALF total protein levels. A reduction in BALF total protein levels suggests that the airway epithelial layer was intact and, thereby, more resistant to cellular influx. In addition, the exercise-mediated reduction in neutrophil and eosinophil recruitment may be the result of decreased KC and VCAM-1 expression; KC chemoattracts neutrophils while VCAM-1 facilitates migration of eosinophils (40).
Because Th2 cells secrete the lymphokines IL-4 and IL-5, which are important in mediating IgE production and eosinophilia, these cells have been implicated in the pathogenesis of allergic asthma (reviewed in Ref. 32). In support of this hypothesis, increased levels of IL-4 and IL-5 protein have been detected in the luminal fluids of asthmatics (32). Data presented herein replicate these findings of increased IL-4 and IL-5 in BALF secretions as well as increased IgE in the serum, suggesting that the employed OVA sensitization model is a valid one for the study of atopic asthma. In this model, exercise decreased BALF levels of IL-4 and IL-5 as well as the extent of eosinophilic infiltrate. In addition, exercise decreased OVA-specific IgE levels in the serum of sensitized mice; however, exercise had no affect on serum total IgE content. Although the reasons underlying such a discrepancy in the modulation of IgE production are unclear, these results suggest that exercise attenuates Th2-mediated responses and subsequent humoral responses that lead to the generation of allergen-specific IgE Abs.
The mechanisms responsible for the effects of exercise on immune responses in the asthmatic lung remain ill defined; however, data presented herein suggest that such mechanisms may involve exercise-induced changes in NF-κB activation. Specifically, we show that exercise decreased NF-κB subunit p65 nuclear translocation and IκBα phosphorylation in OVA-sensitized mice. Recent studies have implicated NF-κB activation in the pathogenesis of asthma. Poynter et al. (36) demonstrated recently that NF-κB activity is increased in the airways of an OVA-driven murine model of asthma. In addition, Yang et al. (41) reported that the NF-κB subunit p50 is required for the induction of eosinophilia in an OVA-driven murine model of asthma. As noted earlier, the expression of many immune molecules, including IL-8 and VCAM-1, is regulated by the NF-κB (2). Other possible mechanisms that may underlie the effects of exercise include exercise-induced changes in endogenous neuroendocrine factors, including corticosterone and catecholamines (42). Each of these neuroendocrine factors can modulate immune-related events, such as cytokine production, surface molecule expression, and lymphocyte proliferation (4, 42) as well as NF-κB activation. Through binding to corticosteroid receptors, corticosterone can inhibit NF-κB activation by either interacting physically with NF-κB subunits (43) and/or inducing the gene expression of the NF-κB inhibitor IκB (44). Catecholamines, such as epinephrine, may exert bronchodilatory effects that lessen compressive pressures and thereby decrease stress-induced NF-κB activation in the airways (45, 46). Lastly, aerobic exercise may modulate the efficiency of Ag presentation within the lung and, therefore, subsequent allergen-induced responses in the airways. In support of this possible mechanism, Ceddia et al. (6) demonstrated that acute, exhaustive exercise enhanced macrophage phagocytosis and chemotaxis yet reduced macrophage MHC II expression and Ag presentation ability.
Previous clinical studies have examined the effects of exercise on the asthmatic lung. Emtner et al. (7) have reported that adults with mild–moderate asthma who underwent a physical training program for 10 wk exhibited increased ventilatory capacity, decreased exercise-induced bronchospasm, and decreased asthma-related symptoms. Long-term follow-up of these asthmatic patients indicated that patients who maintained a moderate intensity level of exercise for 3 years following the initial training program reduced their number of asthma-related emergency room visits during that time (8). Similarly, Henriksen and Nielsen (19) demonstrated a beneficial effect of endurance training on exercise-induced bronchoconstriction and working capacity. Additional studies have reported similar findings with regard to exercise-mediated improvement in the ventilatory capacity of asthmatic patients (9, 11). It is interesting to note that several of these studies observed improvements in exercise-induced bronchoconstriction following physical training (7, 9, 10). In light of these observations, there may exist an inverse dose response for the efficacy of exercise in the treatment of asthma. For example, training at a moderate intensity may provide beneficial, anti-inflammatory effects as our data suggest; however, strenuous training may exacerbate disease parameters. Alternatively, improved physical fitness and decreased inflammatory load as a consequence of moderate intensity training may increase the threshold of exercise intensity that triggers bronchoconstriction. Taken together, our data and the findings of previous clinical studies suggest that instituting a protocol of moderate intensity aerobic exercise training early in the course of asthma may provide a protective effect against associated airway inflammation, attenuate disease progression, and decrease asthmatic symptomatology.
Footnotes
Abbreviations used in this paper: MCP-1, monocyte chemoattractant protein 1; S, sedentary and nonsensitized; SO, sedentary and OVA sensitized; E, exercised and nonsensitized; EO, exercised and OVA sensitized; EU, endotoxin units; BALF, bronchoalveolar lavage fluid.
References
- 1.O’Byrne PM. Airway inflammation and asthma. Aliment. Pharmacol. Ther. 1996;10:18. doi: 10.1046/j.1365-2036.1996.22164016.x. [DOI] [PubMed] [Google Scholar]
- 2.Christman JW, Sadikot RT, Blackwell TR. The role of nuclear factor-κB in pulmonary diseases. Chest. 2000;117:1482. doi: 10.1378/chest.117.5.1482. [DOI] [PubMed] [Google Scholar]
- 3.Mannino DM, Homa DM, Pertowski CA, Ashizawa A, Nixon LL, Johnson CA, Ball LB, Jack E, Kang DS. Surveillance for asthma—United States, 1960–1995. MMWR. 1998;47:1. [PubMed] [Google Scholar]
- 4.Pedersen BK, Hoffman-Goetz L. Exercise and the immune system: regulation, integration, and adaptation. Physiol. Rev. 2000;80:1055. doi: 10.1152/physrev.2000.80.3.1055. [DOI] [PubMed] [Google Scholar]
- 5.Woods JA, Lu Q, Ceddia MA, Lowder T. Exercise-induced modulation of macrophage function. Immunol. Cell Biol. 2000;78:545. doi: 10.1111/j.1440-1711.2000.t01-9-.x. [DOI] [PubMed] [Google Scholar]
- 6.Ceddia MA, Voss EW, Woods JA. Intracellular mechanisms are responsible for the exhaustive exercise-induced suppression of macrophage antigen presentation. J. Appl. Physiol. 2000;88:804. doi: 10.1152/jappl.2000.88.2.804. [DOI] [PubMed] [Google Scholar]
- 7.Emtner M, Herala M, Stalenheim G. High-intensity physical training in adults with asthma: a 10-week rehabilitation program. Chest. 1996;109:323. doi: 10.1378/chest.109.2.323. [DOI] [PubMed] [Google Scholar]
- 8.Emtner M, Finne M, Stalenheim G. A three year follow-up of asthmatic patients participating in a ten week rehabilitation program with emphasis on physical training. Arch. Phys. Med. Rehabil. 1998;79:539. doi: 10.1016/s0003-9993(98)90070-3. [DOI] [PubMed] [Google Scholar]
- 9.Matsumoto I, Araki H, Tsuda K, Odajima H, Nishima S, Higaki Y, Tanaka H, Tanaka M, Shindo M. Effects of swimming training on aerobic capacity and exercise induced bronchoconstriction in children with bronchial asthma. Thorax. 1999;54:196. doi: 10.1136/thx.54.3.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henriksen JM, Nielsen TT. Effect of physical training on exercise-induced bronchoconstriction. Acta Paediatr. Scand. 1983;72:31. doi: 10.1111/j.1651-2227.1983.tb09659.x. [DOI] [PubMed] [Google Scholar]
- 11.Hallstrand TS, Bates PW, Schoene RB. Aerobic conditioning in mild asthma decreases the hyperpnea of exercise and improves exercise and ventilatory capacity. Chest. 2000;118:1460. doi: 10.1378/chest.118.5.1460. [DOI] [PubMed] [Google Scholar]
- 12.American Thoracic Society. Pulmonary rehabilitation—1999. Am. J. Respir. Crit. Care Med. 1999;159:1666. doi: 10.1164/ajrccm.159.5.ats2-99. [DOI] [PubMed] [Google Scholar]
- 13.Satta A. Exercise training in asthma. J. Sports Med. Phys. Fitness. 2000;40:277. [PubMed] [Google Scholar]
- 14.Cooper CB. Exercise in chronic pulmonary disease: limitations and rehabilitation. Med. Sci. Sports Exer. 2001;33:S643. doi: 10.1097/00005768-200107001-00001. [DOI] [PubMed] [Google Scholar]
- 15.Duguet A, Biyah K, Minshall E, Gomes R, Wang C, Taoudi-benchekroun M, Bates JHT, Eidelman DH. Bronchial responsiveness among inbred mouse strains. Am. J. Respir. Crit. Care Med. 2000;161:839. doi: 10.1164/ajrccm.161.3.9906054. [DOI] [PubMed] [Google Scholar]
- 16.Herz U, Lumpp U, Daser A, Gelfand EW, Renz H. Murine animal models to study the central role of T cells in immediate-type hypersensitivity responses. Adv. Exp. Med. Biol. 1996;409:25. doi: 10.1007/978-1-4615-5855-2_4. [DOI] [PubMed] [Google Scholar]
- 17.Fernando P, Bonen A, Hoffman-Goetz L. Predicting submaximal oxygen consumption during treadmill running in mice. Can. J. Physiol. Pharmacol. 1993;71:854. doi: 10.1139/y93-128. [DOI] [PubMed] [Google Scholar]
- 18.Woods JA, Davis JM, Mayer EP, Ghaffar A, Pate RR. Exercise increases inflammatory macrophage antitumor cytotoxicity. J. Appl. Physiol. 1993;75:879. doi: 10.1152/jappl.1993.75.2.879. [DOI] [PubMed] [Google Scholar]
- 19.Woods JA, Davis JM, Mayer EP, Ghaffar A, Pate RR. Effects of exercise on macrophage activation for antitumor cytotoxicity. J. Appl. Physiol. 1994;76:2177. doi: 10.1152/jappl.1994.76.5.2177. [DOI] [PubMed] [Google Scholar]
- 20.Schefer V, Talan MI. Oxygen consumption in adult and aged C57BL/6J mice during acute treadmill exercise of different intensity. Exp. Gerontol. 1996;31:387. doi: 10.1016/0531-5565(95)02032-2. [DOI] [PubMed] [Google Scholar]
- 21.MacNeil B, Hoffman-Goetz L. Effect of exercise on natural cytotoxicity and pulmonary tumor metastases in mice. Med. Sci. Sports Exer. 1993;25:922. [PubMed] [Google Scholar]
- 22.ACCP/AACVPR Pulmonary Guidelines Panel. Pulmonary rehabilitation:joint ACCP/AACVPR evidence-based guidelines. Chest. 1997;112:1363. [PubMed] [Google Scholar]
- 23.British Thoracic Society Standards of Care Subcommittee. Pulmonary rehabilitation: British Thoracic Society Standards of Care Subcommittee on pulmonary rehabilitation. Thorax. 2001;59:827. [Google Scholar]
- 24.Hickman-Davis JM, Lindsey JR, Zhu S, Matalon S. Surfactant protein A mediates mycoplasmacidal activity of alveolar macrophages. Am. J. Physiol. 1998;274:L270. doi: 10.1152/ajplung.1998.274.2.L270. [DOI] [PubMed] [Google Scholar]
- 25.Trifilieff A, El-Hashim A, Bertrand C. Time course of inflammatory and remodeling events in a murine model of asthma: effect of steroid treatment. Am. J. Physiol. 2000;279:L1120. doi: 10.1152/ajplung.2000.279.6.L1120. [DOI] [PubMed] [Google Scholar]
- 26.Tousson A, Van Tine BA, Naren AP, Shaw GM, Schwiebert LM. Characterization of CFTR expression and chloride channel activity in human endothelia. Am. J. Physiol. 1998;275:C1555. doi: 10.1152/ajpcell.1998.275.6.C1555. [DOI] [PubMed] [Google Scholar]
- 27.Nemzek JA, Bolgos GL, Williams BA, Remick DG. Difference in normal values for murine white blood cell counts and other hematological parameters based on sampling site. Inflamm. Res. 2001;50:523. doi: 10.1007/PL00000229. [DOI] [PubMed] [Google Scholar]
- 28.Laitinen LA, Laitinen A. Remodeling of the asthmatic airways by glucocorticosteroids. J. Allergy Clin. Immunol. 1996;97:153. doi: 10.1016/s0091-6749(96)80215-6. [DOI] [PubMed] [Google Scholar]
- 29.Lee NA, Gelfand EW, Lee JJ. Pulmonary T cells and eosinophils: coconspirators or independent triggers of allergic respiratory pathology? J. Allergy Clin. Immunol. 2001;107:945. doi: 10.1067/mai.2001.116002. [DOI] [PubMed] [Google Scholar]
- 30.Tomkinson A, Cieslewicz G, Duez C, Larson K, Lee JJ, Gelfand EW. Temporal association between airway hyperresponsiveness and airway eosinophilia in ovalbumin-sensitized mice. Am. J. Respir. Crit. Care Med. 2001;163:721. doi: 10.1164/ajrccm.163.3.2005010. [DOI] [PubMed] [Google Scholar]
- 31.Wells TNC, Proudfoot AEI. Chemokine receptors and their antagonists in allergic disease. Inflamm. Res. 1999;48:353. doi: 10.1007/s000110050472. [DOI] [PubMed] [Google Scholar]
- 32.Wills-Karp M. Immunologic basis of antigen-induced airway hyperresponsiveness. Annu. Rev. Immunol. 1999;17:255. doi: 10.1146/annurev.immunol.17.1.255. [DOI] [PubMed] [Google Scholar]
- 33.Baldwin AS., Jr. The NFκB and IκB proteins: new discoveries and insights. Annu. Rev. Immunol. 1996;14:649. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 34.Wenzel SE, Schwarz LB, Langmack EL, Halliday JL, Trudeau JB, Gibbs RL, Chu HW. Evidence that severe asthma can be divided pathologically into two inflammatory subtypes with distinct physiologic and clinical characteristics. Am. J. Respir. Crit. Care Med. 1999;160:1001. doi: 10.1164/ajrccm.160.3.9812110. [DOI] [PubMed] [Google Scholar]
- 35.Ordonez CL, Shaughnessy TE, Matthay MA, Fahy JV. Increased neutrophil numbers and IL-8 levels in airway secretions in acute severe asthma: clinical and biologic significance. Am. J. Respir. Crit. Care Med. 2000;161:1185. doi: 10.1164/ajrccm.161.4.9812061. [DOI] [PubMed] [Google Scholar]
- 36.Poynter ME, Irvin CG, Janssen-Heininger YM. Rapid activation of nuclear factor-κB in airway epithelium in a murine model of allergic airway inflammation. Am. J. Pathol. 2002;160:1325. doi: 10.1016/s0002-9440(10)62559-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taube C, Dakhama A, Rha Y, Takeda K, Joetham A, Park J, Balhorn A, Takai T, Poch KR, Nick JA, Gelfand EW. Transient neutrophil infiltration after allergen challenge is dependent on specific antibodies and FcγIII receptors. J. Immunol. 2003;170:4301. doi: 10.4049/jimmunol.170.8.4301. [DOI] [PubMed] [Google Scholar]
- 38.Wolyniec WW. Reduction ofantigen-induced airway hyperreactivity and eosinophilia in ICAM-1-deficient mice. Am. J. Respir. Crit. Care Med. 1998;18:777. doi: 10.1165/ajrcmb.18.6.3056. [DOI] [PubMed] [Google Scholar]
- 39.Uyama OD. Role of eosinophils and cell adhesion molecules in the allergen-induced asthmatic response of rats. Res. Commun. Mol. Pathol. Pharmacol. 1995;90:3. [PubMed] [Google Scholar]
- 40.Schwiebert LM, Stellato C, Schleimer RP. The epithelium as a target of glucocorticoid action in the treatment of asthma. Am. J. Respir. Crit. Care Med. 1996;154:S16. doi: 10.1164/ajrccm/154.2_Pt_2.S16. [DOI] [PubMed] [Google Scholar]
- 41.Yang L, Cohn L, Zhang d, Homer R, Ray A, Prabir R. Essential role of nuclear factor κB in the induction of eosinophilia in allergic airway inflammation. J. Exp. Med. 1998;188:1739. doi: 10.1084/jem.188.9.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nagatomi R, Kaifu T, Okutsu M, Zhang X, Kanemi O, Ohmori H. Modulation of the immune system by the autonomic nervous system and its implication in immunological changes after training. Exerc. Immunol. Rev. 2000;6:54. [PubMed] [Google Scholar]
- 43.Adcock IM, Caramor G. Cross-talk between pro-inflammatory transcription factors and glucocorticoids. Immunol. Cell Biol. 2001;79:376. doi: 10.1046/j.1440-1711.2001.01025.x. [DOI] [PubMed] [Google Scholar]
- 44.Auphan N, DiDonato JA, Rosette C, Helmberg A, Karin M. Immunosuppression by glucocorticoids: inhibition of NF-κB activity through induction of IκB synthesis. Science. 1995;270:286. doi: 10.1126/science.270.5234.286. [DOI] [PubMed] [Google Scholar]
- 45.Tschumperlin DJ, Shively JD, Swartz MA, Silverman ES, Haley KJ, Raab G, Drazen JM. Mechanotransduction in the lung: bronchial epithelial compression regulates MAP kinase signaling and HB-EGF-like growth factor expression. Am. J. Physiol. 2002;282:L904. doi: 10.1152/ajplung.00270.2001. [DOI] [PubMed] [Google Scholar]
- 46.Kumar A, Lnu S, Malya R, Barron D, Moore J, Corry DB, Boriek AM. Mechanical stretch activates nuclear factor-κB, activator protein-1, and mitogen-activated protein kinases in lung parenchyma: implications in asthma. FASEB J. 2003;17:1800. doi: 10.1096/fj.02-1148com. [DOI] [PubMed] [Google Scholar]