Abstract
As the number of elderly individuals rises, Alzheimer’s disease (AD), marked by amyloid-β deposition, neurofibrillary tangle formation, and low-level neuroinflammation, is expected to lead to an ever-worsening socioeconomic burden. AD pathoetiologic mechanisms are believed to involve chronic microglial activation. This phenomenon is associated with increased expression of membrane-bound CD40 with its cognate ligand, CD40 ligand (CD40L), as well as increased circulating levels of soluble forms of CD40 (sCD40) and CD40L (sCD40L). Here, we review the role of this inflammatory dyad in the pathogenesis of AD. In addition, we examine potential therapeutic strategies such as statins, flavonoids, and human umbilical cord blood transplantation, all of which have been shown to modulate CD40-CD40L interaction in mouse models of AD. Importantly, therapeutic approaches focusing on CD40-CD40L dyad regulation, either alone or in combination with amyloid-β immunotherapy, may provide for a safe and effective AD prophylaxis or treatment in the near future.
INTRODUCTION
Alzheimer’s disease (AD) is the most common form of dementia among the elderly affecting an estimated 5.2 million Americans in 2008 alone, a number that is expected to more than double in decades to come [1]. Age remains the most apparent risk factor for developing AD, as the prevalence for the disease begins to mount at age 65 and plateaus at 50% around age 85–90 [2–4]. However, both the prevalence and incidence of AD are expected to rise as diagnosis of the disease improves and general medicine prolongs longevity. At present, the annual economic impact of AD on the American people is estimated to exceed 100 billion dollars. Undoubtedly, the public health challenge is significant.
AD is pathologically distinguished from other forms of dementia by the presence of both extraneuronal β-amyloid plaques and intraneuronal neurofibrillary tangles. Common pathological features of this chronic neurodegenerative disease also include neuronal injury and low-level, chronic neuroinflammation characterized by activated glial cells. While the “amyloid cascade” hypothesis proposes that the central pathoetiologic mechanism of AD involves the dysmetabolism and deposition of amyloid-β peptides (Aβ) as senile plaques, there are a growing number of reports supporting the notion of neuroinflammation as a disease-perpetrating event [5]. The most compelling data come from epidemiologic studies showing that exposure to non-steroidal anti-inflammatory drugs (NSAIDs) is inversely associated with risk for AD [6–8]. While the only randomized controlled primary prevention trial with NSAIDs initially reported a positive association with risk for AD with an early follow-up period [9], Breitner and colleagues recently presented data from this trial suggesting that NSAIDs actually reduced risk for AD when the follow-up period was extended. Furthermore, the investigation of the role of neuroinflammation in AD has uncovered the importance of glial activation in disease progression. In particular, the activation of microglia, the resident immune cells of the central nervous system (CNS), is believed to significantly contribute to the pathoetiology of AD.
Essential to microglial activation is the stimulatory signal from CD40 ligation. CD40 is a 45- to 50-kDa type I integral membrane glycoprotein and a member of the tumor necrosis factor receptor superfamily. Studies originally identified CD40 on B lymphocytes, where it was shown to mediate T cell-dependent B cell activation and differentiation. It is found on most immune and some non-immune cells including macrophages, dendritic cells, endothelial cells, smooth muscle cells, and B cells, as well as astrocytes and microglia [5,10–15]. Membrane-bound CD40 and its cognate CD40 ligand (CD40L–also known as CD154), a trimeric 33-kDa type II membrane glycoprotein, play a critical role in microglial phenotypic transformation from a ramified (resting), to an activated macrophage morphology [16] and enhance surface expression of inflammatory markers [17,18].
Accordingly, these cell surface molecules have been reported to augment neuroinflammation and reduce clearance of Aβ from the brain. CD40L also occurs in a soluble, secreted form (sCD40L) that retains the ability to bind and activate membrane-bound CD40 [19, 20]. Further, CD40 can also be secreted as a soluble form (sCD40), which may serve to neutralize CD40L [21]. Clearly, CD40 and CD40L are key immunoregulatory molecules as they provide co-stimulatory input to cells from both the innate and adaptive arms of the immune system, substantiating the importance of this duet in the neuroinflammatory component of AD. In this article, we review the implications of this inflammatory dyad for the pathogenesis, diagnosis, and potential treatment of AD.
THE CD40-CD40L DYAD AND NEUROINFLAMMATION IN AD
Over the past two decades, our understanding of AD pathophysiology has greatly expanded, and proponents of the amyloid cascade hypothesis have revised it accordingly. However, one general concept has remained constant – that Aβ peptides are neurotoxic. Notably, a continuous inflammatory cycle exists in the AD brain marked by chronic, low-level secretion of pro-inflammatory cytokines and acute-phase reactants around Aβ plaques. The term “reactive gliosis” has been used to describe this inflammatory cycle wherein glial cells, including microglia and astrocytes, undergo various phenotypic changes indicative of activation. Aβ peptides (both 1–40 and 1–42 isoforms) have traditionally been regarded as potent activators of microglia, and numerous research articles demonstrate neuronal damage by means of this unrestrained inflammatory response [22], which often involves the cytokines tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), interleukin-1β (IL-1β), as well as nitric oxide [23–27]. Importantly, microglia have long been considered to elicit these neuroinflammatory reactions via the CD40-CD40L dyad [23, 28].
In support of this CD40-CD40L neuroinflammatory pathway, we and others have demonstrated that microglia are only modestly activated by Aβ exposure in vitro and that co-stimulation with IFN-γ or CD40L synergistically enhances this activation by upregulation of CD40 expression and ligation, respectively [22, 29–35]. Specifically, when cultured microglia are co-administered Aβ peptide and IFN-γ or CD40L in the presence of primary neurons, microglia secrete appreciably high levels of TNF-α, resulting in neuronal injury [28, 36]. In addition, CD40L treatment of cultured human microglia has been reported to disturb the expression of genes regulating amyloid precursor protein (APP) processing and tau phosphorylation (a process contributing to neurofibrillary tangle formation) [37]. As in vivo validation of this pathway, we found that disruption of CD40-CD40L signaling (via CD40L deficiency) in mice carrying human “Swedish” mutant APP (APPsw, increases the production of Aβ) results in significantly less microgliosis and tau hyperphosphorylation in these animals [36]. Furthermore we have demonstrated that PSAPP AD model mice genetically deficient in CD40L exhibit decreased astrocytosis and microgliosis, which is associated with diminished Aβ levels and β-amyloid plaque load. Treatment of PSAPP mice (bigenic AD mice expressing mutant human presenilin-1 and APPsw) with CD40L-depleting antibody also caused marked attenuation of Aβ/β-amyloid pathology, which was associated with decreased amyloidogenic processing of APP and increased circulating levels of Aβ. Moreover, in neuroblastoma cells overexpressing wild-type human APP, the CD40–CD40L interaction resulted in amyloidogenic APP processing. These studies are key because they demonstrate that blocking CD40-CD40L interaction by genetic or pharmacologic means mitigates β-amyloid plaque deposits in multiple models of AD. Indeed, this work is salient because we can draw the conclusion that CD40L-induced microgliosis is indeed a pathogenic form of microglial activation in the context of AD-like pathology [36].
Similar to our studies above, Todd Roach and colleagues [35] showed that PSAPP mice given a regimen of anti-CD40L antibody (commencing at an age when initial Aβ deposition occurs) exhibited superior spatial as well as non-spatial memory compared to non-treatment control animals. Furthermore, Laporte and colleagues [33] demonstrated that APPsw mice deficient for CD40 experience reduced reactive gliosis (microgliosis and astrocytosis) and cerebral Aβ burden compared to CD40-sufficient littermates. Altogether, these studies suggest that CD40-CD40L interaction is essential for neuroinflammatory responses and, in particular, microglial activation, which may significantly contribute to AD pathogenesis.
Perhaps the most convincing clinical evidence of the role of CD40-CD40L signaling in inflammatory AD-like neurodegeneration comes from clinical studies demonstrating a mild increase of circulating sCD40L in mild cognitive impairment (MCI), which likely represents a prodrome to AD as it is positively associated with increased risk of AD incidence and rapid cognitive decline [35, 38]. This clinical association between sCD40L and AD is also strengthened by evidence of sCD40L engagement of CD40 on endothelial cells resulting in various pro-inflammatory responses, including expression of adhesion molecules and tissue factors, release of cytokines/chemokines and expression of vessel-remodeling metalloproteinases [29,31,38]. Interaction between CD40L and CD40 activates various signaling cascades via the tumor necrosis factor receptor-associated factor family members [12, 21, 39–42]. As sCD40L also negatively affects re-endothelizing functions of endothelial cells [43], it has even further potential to interfere with microcirculation. Thus taken together, the soluble or membrane-bound CD40L and CD40 on smooth muscle and endothelial cells seem to work in concert with Aβ peptides to promote a feed-forward, self-perpetuating inflammatory cascade in both the vascular wall and brain parenchyma which, in turn, may initiate AD pathogenesis and/or propagate its progression [40,44].
Aβ-induced CD40 is incorporated into the cell membrane at constitutively low levels on vascular smooth muscle and endothelial cells [5, 45, 46]. Therefore, not surprisingly, the risk relationship between sCD40L and AD mirrors the positive correlation between sCD40L levels and vascular disease. This is because endothelial cells are a key component of the blood-brain barrier, suggesting that there could be alterations of this physiological unit under conditions of increased sCD40L expression or function. Accordingly, we previously demonstrated that Aβ increases both CD40 mRNA transcription and CD40 protein expression in cultured human vascular endothelial cells within 48 hours of exposure [47–49]. These findings further suggest that factors that increase the risk of developing vascular disease also increase the risk for AD [45, 49]. In fact, cerebral perfusion abnormalities are even seen in the earliest phases of AD [50, 51]. This is not surprising, as the majority (>95%) of circulating CD40L exists in platelets [52]. CD40L is rapidly translocated to the platelet surface after stimulation and is then cleaved, generating sCD40L over a period of minutes to hours [53]. Taken together, these data suggest CD40-sCD40L/CD40L dyad is a central mediator of both vascular and AD.
In contrast to their role as elicitors of neuronal damage, a number of studies have suggested a neuroprotective role for microglia in AD [54]. The theories of neuroprotection or neurotoxicity regarding microglia are not mutually exclusive, however. Activated microglia augment Aβ clearance and Aβ vaccination [55–57] or recruitment of bone marrow-derived microglia [58, 59] can result in neuroprotection. Moreover, the low-density lipoprotein-related protein receptors on microglia may promote the export of soluble Aβ from the brain. However, these same receptors on neurons have also been found to increase intraneuronal uptake and toxicity of Aβ [60]. Similarly, carrier proteins such as apolipoprotein E, apolipoprotein J, and α2-macroglobulin can transport Aβ either to microglia, promoting Aβ clearance, or to neurons, promoting neurodegeneration [61].
Additional data confirming microglia-associated neuroprotection comes from studies demonstrating the attenuation of microgliosis with concomitant secretion of anti-inflammatory cytokines such as IL-4, IL-10, and IL-13 [62]. Indeed, just as IL-1β can activate neurotoxic effects of microglia, IL-4 and other anti-inflammatory cytokines promote an alternate activation state of microglia [63]. Microglia, much like peripheral macrophages, consist of a heterogeneous population of effector-type cells. Accordingly, several subsets of microglia have been characterized by differential expression of alloantigen molecules; primarily CD40 and = major histocompatibility complex class II, during establishment of cell lines [64–66]. The putative anti-inflammatory, neuroprotective subset of microglia or Th2-responsive cells are induced by IL-4, reduction of CD40 expression, and various other anti-inflammatory cytokines [10]. Such Th2-type microglia are distinct from Th1-type (characterized by pro-inflammatory cytokine production) in terms of the expression of at least two cell surface molecules: Th2-microglia express no CD40 and/or low CD86, both of which are expressed by antigen presenting cells to function in communication with CD4+ Th cells. However, further differentiation of Th1 and Th2-responsive microglia may be necessary [64–66]. Nevertheless, in order to mitigate the chronic inflammatory cycle that exists in AD, it may be necessary to promote these Th2-type (anti-inflammatory cytokine producing) microglial responses as opposed to Th1-type responses. Indeed, a current popular hypothesis we and other have suggested is that transitioning from Th1- to Th2-type responses may not only suppress neuroinflammation, but also enhance Aβ clearance and neuroprotective mechanisms [67].
However, it should be noted that a recent study employing TgCRND8 and APPsw AD model mice suggests the converse, that Th1-type responses may be beneficial. IL-6 is a well-established pro-inflammatory cytokine, which is elevated in AD patients. Interestingly, Chakrabarty and colleagues [68] found that murine IL-6 expression resulted in extensive reactive gliosis that concurrently reduced amyloid burden in TgCRND8 mice. This reduction was accompanied by up-regulation of glial phagocytic markers in vivo. Murine IL-6 also enhanced microglia-mediated phagocytosis of Aβ aggregates in vitro. Importantly, murine IL-6-induced neuroinflammation had no effect on APP processing in TgCRND8 and had no effect on APP processing or steady-state levels of Aβ in young APPsw mice, suggesting that murine IL-6-mediated gliosis may be beneficial early in the disease process by potentially enhancing clearance of β-amyloid [67, 69].
CD40 AS A BIOMARKER FOR AD
CD40 protein induction on microglia and vascular endothelial cells occurs in the presence of low doses of soluble forms of Aβ, suggesting that microgliosis may occur early in disease progression, prior to Aβ deposition [68]. For this reason, a number of studies have examined levels of gliosis-associated cytokines present in the cerebrospinal fluid (CSF) of patients with AD, in an attempt to identify objective, quantitative markers of this neuropsychiatric illness. Unfortunately, these types of studies have been plagued by problems, as data from the literature about intrathecal or circulating cytokine levels are often discordant. One explanation for this discordance is the nature of cytokines in general, which are characterized by short half-lives and signaling that occurs mainly in autocrine or paracrine fashions. Additionally, cytokines may be buffered by soluble receptors in the periphery, further confounding results. Furthermore, cytokines released in the CNS may act in similar manners across neurological disorders, amplifying certain neuropathological events. Thus, no specific defined cytokine release pattern in the circulation or CSF has as yet been identified for AD.
The pattern of expression of both CD40 and CD40L has been reported to be altered in the brains of AD patients as well as in several animal models of AD [45]. Recently, sCD40L was quantified at baseline in 136 subjects with MCI and 30 age-matched controls. Of the MCI patients, sixty of the 136 cases converted to AD (MCI-AD) during a follow-up period of 4–7 years. It was found that baseline levels of sCD40, but not sCD40L, were elevated in MCI-AD cases compared to age-matched controls. Furthermore, MCI patients who were cognitively stable or developed vascular dementia during follow-up did not have significantly increased levels of sCD40 or sCD40L compared to controls. The levels of sCD40 correlated with decreased baseline performance on mini-mental state examination in both controls and plasma levels of sCD40 correlated with the levels of soluble amyloid precursor protein-α and -β (sAPP-α and sAPP-β) in CSF [70, 71]. An earlier study (73 AD patients compared to 102 controls) found sCD40 and sCD40L are increased in AD which in turn, correlated positively Aβ1–40 and Aβ1–42, respectively. When they combined sCD40, sCD40L, Aβ and apolipoprotein E for a diagnostic predictor of AD, they found that this panel had high sensitivity and specificity (>90%) [28]. The consistent findings in the above studies make a compelling case for using both sCD40 and sCD40L as plasma biomarkers of AD; however, it should be noted that these measures would likely need to be combined with others in order to attain the requisite sensitivity, specificity, and predictive value to be useful biomarkers [see ref. 72 for a review].
CD40-CD40L TARGETED AD THERAPEUTIC STRATEGIES
Statins
Statins belong to a class of drugs that inhibit the activity of HMG-CoA reductase, the rate-limiting enzyme of the mevalonate pathway of cholesterol synthesis. For that reason, statins have been prescribed primarily for the purposes of lowering cholesterol levels. By inhbiting HMG-CoA reductase, these compounds not only decrease cholesterol synthesis, but also increase synthesis of low-density lipoprotein receptors, resulting in an increased clearance of low-density lipoprotein from the bloodstream [29]. Interestingly, some epidemiological studies, although preliminary, suggest that statin administration might be protective against the development of AD [73–75].
In light of these studies, we examined how statins may mechanistically afford protection against AD. We initially found that lovastatin suppresses IFN-γ-induced CD40 expression. Additionally, lovastatin markedly inhibits IFN-γ intracellular signaling. Furthermore, lovastatin suppressed TNF-α, interleukin IL-1β and IL-6 production promoted either by IFN-γ or by Aβ peptide challenge in the presence of CD40 stimulation. Moreover, data revealed that lovastatin markedly attenuates CD40-mediated inhibition of microglial phagocytosis of Aβ peptide, causing a deficit in amyloid clearance from the brain [76]. Importantly, in a small clinical pilot study, patients with mild to moderate AD who were administered 80 mg atorvastatin daily over 1 year experienced a significant clinical improvement [77]. As sCD40L is involved in both activation of microglia and induction of vascular inflammation, this clinical benefit could be secondary not only to cholesterol-dependent reduction of vascular risk factors, but also because of reduced Aβ deposition in brain parenchyma, as demonstrated in our experimental models [78] and by others, as well [77]. Additionally the lipid-independent anti-inflammatory properties of statins [79], including reduction of circulating sCD40L levels in different clinical conditions [80] may have also led to this outcome.
Flavonoids
While several anti-inflammatory drugs have been found to prevent microglial-mediated inflammation, their underlying mechanisms remain unclear and the search for more effective practical compounds continues. Recent research has focused on the analysis of flavonoids, which epidemiological studies suggest are beneficial agents that militate against neurodegeneration and aging [81]. Flavonoids, a group of phenolic phytochemicals, are common in vascular plants and are abundant in particular spices, vegetables, and fruits. They are considered important constituents in the human diet, although their daily intake varies with dietary habits [82]. Several medicinal properties have been ascribed to flavonoids, notably anti-oxidant [83, 84], anti-carcinogenic [84, 85], and anti-inflammatory activity [86]. One such flavonoid, apigenin, and its phase I metabolite, luteolin, have been found to reduce CD40 and CD40L expression on dendritic cells and basophils, respectively. Previous research has also shown apigenin’s ability to inhibit pro-inflammatory cytokine production by monocytes, macrophages, and microglia and further substantiates this compound as a versatile immunomodulator [87].
In a recent study, we investigated the potential anti-inflammatory effects and mechanisms of the flavonoids, apigenin and luteolin, in cultured microglia. Both apigenin and luteolin significantly reduced CD40 expression in N9 and murine-derived primary microglia cell lines induced by IFN-γ. This reduction was paralleled by significant decreases in the release of the pro-inflammatory cytokines IL-6 and TNF-α by microglia. Furthermore, we demonstrated that apigenin and luteolin treatments achieve these reductions through inactivation of the signal transducer and activator of transcription-1 signaling pathway, which plays a central role in IFN-γ-induced microglial CD40 expression [88, 89]. In this manner, flavonoids may prove to be effective modulators of CD40-CD40L interactions and consequently effective therapeutics for AD-like neurodegeneration [90].
Human Umbilical Cord Blood Cell Transplantion
A major clinical challenge is finding a suitable cell source for the regeneration of damaged tissues. Human umbilical cord blood cells (HUCBCs) have unique immunomodulatory potential. In addition to fulfilling the function of tissue reconstruction, the immune properties of HUCBCs are advantageous for clinical applications where an anti-inflammatory, Th2 response is therapeutic. Previous work has indicated that HUCBCs may be immune-privileged cells as the surface antigen expression characteristics of the cells may enable them to avoid rejection or induction of graft versus host disease. Previous analysis has demonstrated HUCBCs do not express = major histocompatibility complex class II molecules or CD40 required for Th1-cell activation thought to be responsible for both transplant rejection and AD progression [91, 92]. Also, HUCBCs do not elicit a proliferative response of allogeneic peripheral bone marrow cells during coculture in vitro [93]. Thus, an additional potential anti-CD40 treatment is human umbilical cord blood cell transplantion.
Previous experiments in our laboratory have shown they alter Alzheimer-like pathology after infusion into the PSAPP mouse model. Specifically, we observed marked reductions in Aβ levels/β-amyloid plaques and associated astrocytosis following multiple low-dose infusions of HUCBCs. HUCBC infusions also reduced cerebral vascular Aβ deposits in the APPsw mouse model. All of these effects were associated with suppression of the CD40-CD40L interaction, as evidenced by decreased circulating and brain sCD40L, elevated systemic immunoglobulin M levels, attenuated CD40L-induced inflammatory responses, and reduced surface expression of CD40 on microglia. Importantly, deficiency in CD40 abolishes the effect of HUCBCs on elevated plasma Aβ levels. Moreover, microglia isolated from HUCBC-infused PSAPP mice demonstrated increased phagocytosis of Aβ. Furthermore, sera from HUCBC-infused PSAPP mice significantly increased microglial phagocytosis of the Aβ peptide while inhibiting IFN-γ-induced microglial CD40 expression. Increased microglial phagocytic activity in this scenario was inhibited by addition of recombinant CD40L protein [94–96]. While the findings above testament of the beneficial effects of human umbilical cord blood cell transplantion, it is important to note that to date the exact mechanism(s) of action that confer these reductions in amyloidosis coincident with CD40 dyad modulation has yet to be established. For review of the various theories the reader is referred to Kostrzewa and Segura-Aguilar [97].
Aβ VACCINATION
Immunization with Aβ peptide efficiently reduces amyloid plaque load and memory impairment in transgenic mouse models of AD. Active Aβ “immunotherapy” has also yielded favorable results in a subset of AD patients. However, a small percentage of patients developed severe aseptic meningoencephalitis associated with brain inflammation and infiltration of T cells. We recently demonstrated genetic or pharmacologic interruption of the CD40-CD40L dyad enhances Aβ1–42 immunization efficacy to reduce cerebral amyloidosis in the APPsw and PSAPP mouse models of AD. Potentially deleterious pro-inflammatory immune responses seen in the previous Elan/Wyeth AN-1792 trial, cerebral amyloid angiopathy and cerebral microhemorrhage, were reduced or absent in these combined approaches. Furthermore, pharmacologic blockade of CD40L decreased T-cell neurotoxicity to Aβ-producing neurons. Further reduction of cerebral amyloidosis in Aβ-immunized PSAPP mice completely deficient for CD40 occurred in the absence of Aβ immunoglobulin G antibodies or efflux of Aβ from brain to blood, but was rather correlated with anti-inflammatory cytokine profiles and reduced plasma sCD40L. These results suggest that CD40-CD40L dyad blockade promotes anti-inflammatory cellular immune responses, likely resulting in promotion of microglial phagocytic activity and Aβ clearance without generation of neurotoxic Aβ-reactive T-cells. Thus, combined approaches of Aβ immunotherapy and CD40-CD40L dyad blockade may provide for a safer and more effective Aβ vaccine in the future [97–100].
CONCLUDING REMARKS
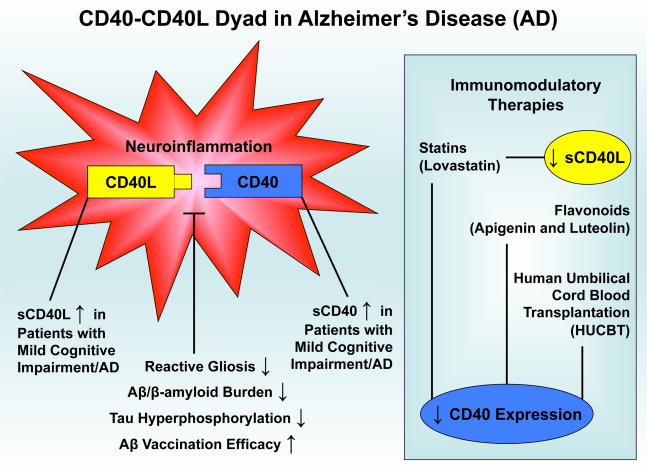
Neuroinflammation is a complex process that employs signaling pathways crucial to the dynamic interplay between glial cells and neurons, which is required to maintain neural health. Complete abolishment of CD40-CD40L interactions between microglia, astrocytes, and neurons may prove to be hazardous, as we have previously shown that ligation of CD40 protected neuronal cells from nerve growth factor-β or serum withdrawal-induced injury and promoted differentiation [101]. For this reason, modulation specifically directed toward neuroinflammatory reactions in CNS would appear to be a preferable way to treat neurodegenerative disease. In this regard, we have summarized such neuroinflammatory events, as well as biomarker correlates, in AD that may be ameliorated by immunotherapy, including statin and flavonoid treatment, Aβ vaccination, and human umbilical cord blood cell transplantion (Fig. 1). Importantly, modulation of the CD40-CD40L dyad is central to this immunotherapy for AD.
Fig. 1.
Model for CD40-CD40L inflammatory dyad in the pathogenesis and potential treatment of AD. See text for references.
Acknowledgments
B. Giunta is supported by an NIH/NIMH Clinical Scientist Award (1 K08 MH082642-01A1). J. Tan is supported by NIH grants 1R41AG031586-01, 1R43AG033417-01, and 1R43AT004871-01.
ABBREVIATIONS
- Aβ
Amyloid beta/beta amyloid
- AD
Alzheimer’s disease
- APP
Amyloid precursor protein
- CNS
Central nervous system
- CSF
Cerebrospinal fluid
- CD40L
CD40 ligand
- HUCBCs
Human umbilical cord blood cells
- IFN
Interferon
- IL
Interleukin
- MCI
Mild cognitive impairment
- NSAIDs
Non-steriodal anti-inflammatory drugs
- sCD40
Soluble CD40
- sCD40L
Soluble CD40 ligand
- Th
T helper cell
- TNF
Tumor necrosis factor
References
- 1.Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol. 2003;60:1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- 2.Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB, Burke JR, Hurd MD, Potter GG, Rodgers WL, Steffens DC, Willis RJ, Wallace RB. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology. 2007;29:125–132. doi: 10.1159/000109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tanzi RE. Alzheimer’s disease and related dementias: the road to intervention. Exp Gerontol. 2000;35:433–437. doi: 10.1016/s0531-5565(00)00109-1. [DOI] [PubMed] [Google Scholar]
- 4.Petersen RC. Challenges of epidemiological studies of mild cognitive impairment. Alzheimer Dis Assoc Disord. 2004;18:1–2. doi: 10.1097/00002093-200401000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Tan J, Town T, Crawford F, Mori T, DelleDonne A, Crescentini R, Obregon D, Flavell RA, Mullan MJ. Role of CD40 ligand in amyloidosis in transgenic Alzheimer’s mice. Nat Neurosci. 2002;5:1288–1293. doi: 10.1038/nn968. [DOI] [PubMed] [Google Scholar]
- 6.in t’Veld BA, Ruitenberg A, Hofman A, Launer LJ, van Duijn CM, Stijnen T, Breteler MM, Stricker BH. Nonsteroidal antiinflammatory drugs and the risk of Alzheimer’s disease. N Engl J Med. 2001;345:1515–1521. doi: 10.1056/NEJMoa010178. [DOI] [PubMed] [Google Scholar]
- 7.Hanyu H, Abe S, Arai H, Asano T, Iwamoto T, Takasaki M, Suzuki T. Diagnostic accuracy of single photon emission CT in Alzheimer-type dementia. Nippon Ronen Igakkai Zasshi. 1992;29:463–468. doi: 10.3143/geriatrics.29.463. [DOI] [PubMed] [Google Scholar]
- 8.Szekely CA, Town T, Zandi PP. NSAIDs for the chemoprevention of Alzheimer’s disease. Subcell Biochem. 2007;42:229–248. doi: 10.1007/1-4020-5688-5_11. [DOI] [PubMed] [Google Scholar]
- 9.ADAPT Research, G. Lyketsos CG, Breitner JC, Green RC, Martin BK, Meinert C, Piantadosi S, Sabbagh M. Naproxen and celecoxib do not prevent AD in early results from a randomized controlled trial. Neurology. 2007;68:1800–1808. doi: 10.1212/01.wnl.0000260269.93245.d2. [DOI] [PubMed] [Google Scholar]
- 10.Townsend KP, Vendrame M, Ehrhart J, Faza B, Zeng J, Town T, Tan J. CD45 isoform RB as a molecular target to oppose lipopolysaccharide-induced microglial activation in mice. Neurosci Lett. 2004;362:26–30. doi: 10.1016/j.neulet.2004.01.082. [DOI] [PubMed] [Google Scholar]
- 11.Abdel-Haq N, Hao HN, Lyman WD. Cytokine regulation of CD40 expression in fetal human astrocyte cultures. J Neuroimmunol. 1999;101:7–14. doi: 10.1016/s0165-5728(99)00124-1. [DOI] [PubMed] [Google Scholar]
- 12.Chakrabarti S, Rizvi M, Pathak D, Kirber MT, Freedman JE. Hypoxia influences CD40-CD40L mediated inflammation in endothelial and monocytic cells. Immunol Lett. 2009;122:170–184. doi: 10.1016/j.imlet.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 13.Dubois B, Vanbervliet B, Fayette J, Massacrier C, Van Kooten C, Briere F, Banchereau J, Caux C. Dendritic cells enhance growth and differentiation of CD40-activated B lymphocytes. J Exp Med. 1997;185:941–951. doi: 10.1084/jem.185.5.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mach F, Schonbeck U, Sukhova GK, Bourcier T, Bonnefoy JY, Pober JS, Libby P. Functional CD40 ligand is expressed on human vascular endothelial cells, smooth muscle cells, and macrophages: implications for CD40-CD40 ligand signaling in atherosclerosis. Proc Natl Acad Sci USA. 1997;94:1931–1936. doi: 10.1073/pnas.94.5.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim KM, Min HY, Jung SH, Lee TH, Kim JG, Kang CY. Characterization of an immunosuppressive anti-CD40 ligand monoclonal antibody. Hybridoma. 1998;17:463–470. doi: 10.1089/hyb.1998.17.463. [DOI] [PubMed] [Google Scholar]
- 16.Ponomarev ED, Shriver LP, Dittel BN. CD40 expression by microglial cells is required for their completion of a two-step activation process during central nervous system autoimmune inflammation. J Immunol. 2006;176:1402–1410. doi: 10.4049/jimmunol.176.3.1402. [DOI] [PubMed] [Google Scholar]
- 17.Aloisi F, De Simone R, Columba-Cabezas S, Penna G, Adorini L. Functional maturation of adult mouse resting microglia into an APC is promoted by granulocyte-macrophage colony-stimulating factor and interaction with Th1 cells. J Immunol. 2000;164:1705–1712. doi: 10.4049/jimmunol.164.4.1705. [DOI] [PubMed] [Google Scholar]
- 18.D’Aversa TG, Weidenheim KM, Berman JW. CD40-CD40L interactions induce chemokine expression by human microglia: implications for human immunodeficiency virus encephalitis and multiple sclerosis. Am J Pathol. 2002;160:559–567. doi: 10.1016/S0002-9440(10)64875-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Obradovic SD, Antovic JP, Antonijevic NM, Ratkovic NG, Vojvodic DV, Subota VS, Gligic BL, Obradovic DV, Marinkovic JM, Wallen HN. Elevations in soluble CD40 ligand in patients with high platelet aggregability undergoing percutaneous coronary intervention. Blood Coagul Fibrinolysis. 2009;20(4):283–289. doi: 10.1097/MBC.0b013e328329f28c. [DOI] [PubMed] [Google Scholar]
- 20.Lessiani G, Dragani A, Falco A, Fioritoni F, Santilli F, Davi G. Soluble CD40 ligand and endothelial dysfunction in aspirin-treated polycythaemia vera patients. Br J Haematol. 2009;145(4):538–540. doi: 10.1111/j.1365-2141.2009.07636.x. [DOI] [PubMed] [Google Scholar]
- 21.Chen K, Huang J, Gong W, Zhang L, Yu P, Wang JM. CD40/CD40L dyad in the inflammatory and immune responses in the central nervous system. Cell Mol Immunol. 2006;3:163–169. [PubMed] [Google Scholar]
- 22.Mocali A, Cedrola S, Della Malva N, Bontempelli M, Mitidieri VA, Bavazzano A, Comolli R, Paoletti F, La Porta CA. Increased plasma levels of soluble CD40, together with the decrease of TGF beta 1, as possible differential markers of Alzheimer disease. Exp Gerontol. 2004;39:1555–1561. doi: 10.1016/j.exger.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 23.McGeer PL, McGeer EG. The inflammatory response system of brain: implications for therapy of Alzheimer and other neurodegenerative diseases. Brain Res Brain Res Rev. 1995;21:195–218. doi: 10.1016/0165-0173(95)00011-9. [DOI] [PubMed] [Google Scholar]
- 24.McGeer PL, Rogers J, McGeer EG. Inflammation, anti-inflammatory agents and Alzheimer disease: the last 12 years. J Alzheimers Dis. 2006;9:271–276. doi: 10.3233/jad-2006-9s330. [DOI] [PubMed] [Google Scholar]
- 25.Itagaki S, McGeer PL, Akiyama H, Zhu S, Selkoe D. Relationship of microglia and astrocytes to amyloid deposits of Alzheimer disease. J Neuroimmunol. 1989;24:173–182. doi: 10.1016/0165-5728(89)90115-x. [DOI] [PubMed] [Google Scholar]
- 26.McGeer PL, Akiyama H, Itagaki S, McGeer EG. Immune system response in Alzheimer’s disease. Can J Neurol Sci. 1989;16:516–527. doi: 10.1017/s0317167100029863. [DOI] [PubMed] [Google Scholar]
- 27.Heneka MT, O’Banion MK. Inflammatory processes in Alzheimer’s disease. J Neuroimmunol. 2007;184:69–91. doi: 10.1016/j.jneuroim.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 28.Meda L, Cassatella MA, Szendrei GI, Otvos L, Jr, Baron P, Villalba M, Ferrari D, Rossi F. Activation of microglial cells by beta-amyloid protein and interferon-gamma. Nature. 1995;374:647–650. doi: 10.1038/374647a0. [DOI] [PubMed] [Google Scholar]
- 29.Buchhave P, Janciauskiene S, Zetterberg H, Blennow K, Minthon L, Hansson O. Elevated plasma levels of soluble CD40 in incipient Alzheimer’s disease. Neurosci Lett. 2009;450:56–59. doi: 10.1016/j.neulet.2008.10.091. [DOI] [PubMed] [Google Scholar]
- 30.Ait-ghezala G, Abdullah L, Volmar CH, Paris D, Luis CA, Quadros A, Mouzon B, Mullan MA, Keegan AP, Parrish J, Crawford FC, Mathura VS, Mullan MJ. Diagnostic utility of APOE, soluble CD40, CD40L, and Abeta1–40 levels in plasma in Alzheimer’s disease. Cytokine. 2008;44:283–287. doi: 10.1016/j.cyto.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 31.Desideri G, Cipollone F, Necozione S, Marini C, Lechiara MC, Taglieri G, Zuliani G, Fellin R, Mezzetti A, di Orio F, Ferri C. Enhanced soluble CD40 ligand and Alzheimer’s disease: evidence of a possible pathogenetic role. Neurobiol Aging. 2008;29:348–356. doi: 10.1016/j.neurobiolaging.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 32.Nikolic WV, Hou H, Town T, Zhu Y, Giunta B, Sanberg CD, Zeng J, Luo D, Ehrhart J, Mori T, Sanberg PR, Tan J. Peripherally administered human umbilical cord blood cells reduce parenchymal and vascular beta-amyloid deposits in Alzheimer mice. Stem Cells Dev. 2008;17(3):423–439. doi: 10.1089/scd.2008.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laporte V, Ait-Ghezala G, Volmar CH, Mullan M. CD40 deficiency mitigates Alzheimer’s disease pathology in transgenic mouse models. J Neuroinflamm. 2006;3:3. doi: 10.1186/1742-2094-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Townsend KP, Town T, Mori T, Lue LF, Shytle D, Sanberg PR, Morgan D, Fernandez F, Flavell RA, Tan J. CD40 signaling regulates innate and adaptive activation of microglia in response to amyloid beta-peptide. Eur J Immunol. 2005;35:901–910. doi: 10.1002/eji.200425585. [DOI] [PubMed] [Google Scholar]
- 35.Todd Roach J, Volmar CH, Dwivedi S, Town T, Crescentini R, Crawford F, Tan J, Mullan M. Behavioral effects of CD40-CD40L pathway disruption in aged PSAPP mice. Brain Res. 2004;1015:161–168. doi: 10.1016/j.brainres.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 36.Tan J, Town T, Paris D, Mori T, Suo Z, Crawford F, Mattson MP, Flavell RA, Mullan M. Microglial activation resulting from CD40-CD40L interaction after beta-amyloid stimulation. Science. 1999;286:2352–2355. doi: 10.1126/science.286.5448.2352. [DOI] [PubMed] [Google Scholar]
- 37.Ait-Ghezala G, Mathura VS, Laporte V, Quadros A, Paris D, Patel N, Volmar CH, Kolippakkam D, Crawford F, Mullan M. Genomic regulation after CD40 stimulation in microglia: relevance to Alzheimer’s disease. Brain Res Mol Brain Res. 2005;140:73–85. doi: 10.1016/j.molbrainres.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 38.Boyle PA, Wilson RS, Aggarwal NT, Tang Y, Bennett DA. Mild cognitive impairment: risk of Alzheimer disease and rate of cognitive decline. Neurology. 2006;67:441–445. doi: 10.1212/01.wnl.0000228244.10416.20. [DOI] [PubMed] [Google Scholar]
- 39.Henn V, Slupsky JR, Grafe M, Anagnostopoulos I, Forster R, Muller-Berghaus G, Kroczek RA. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature. 1998;391:591–594. doi: 10.1038/35393. [DOI] [PubMed] [Google Scholar]
- 40.Cipollone F, Chiarelli F, Davi G, Ferri C, Desideri G, Fazia M, Iezzi A, Santilli F, Pini B, Cuccurullo C, Tumini S, Del Ponte A, Santucci A, Cuccurullo F, Mezzetti A. Enhanced soluble CD40 ligand contributes to endothelial cell dysfunction in vitro and monocyte activation in patients with diabetes mellitus: effect of improved metabolic control. Diabetologia. 2005;48:1216–1224. doi: 10.1007/s00125-005-1750-2. [DOI] [PubMed] [Google Scholar]
- 41.van Kooten C, Banchereau J. CD-40-CD-40 ligand. J Leukoc Biol. 2000;67:2–17. doi: 10.1002/jlb.67.1.2. [DOI] [PubMed] [Google Scholar]
- 42.Schonbeck U, Libby P. The CD40/CD154 receptor/ligand dyad. Cell Mol Life Sci. 2001;58:4–43. doi: 10.1007/PL00000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mukundan L, Bishop GA, Head KZ, Zhang L, Wahl LM, Suttles J. TNF receptor-associated factor 6 is an essential mediator of CD40-activated proinflammatory pathways in monocytes and macrophages. J Immunol. 2005;174:1081–1090. doi: 10.4049/jimmunol.174.2.1081. [DOI] [PubMed] [Google Scholar]
- 44.Cipollone F, Ferri C, Desideri G, Paloscia L, Materazzo G, Mascellanti M, Fazia M, Iezzi A, Cuccurullo C, Pini B, Bucci M, Santucci A, Cuccurullo F, Mezzetti A. Preprocedural level of soluble CD40L is predictive of enhanced inflammatory response and restenosis after coronary angioplasty. Circulation. 2003;108:2776–2782. doi: 10.1161/01.CIR.0000103700.05109.0D. [DOI] [PubMed] [Google Scholar]
- 45.Tan J, Town T, Suo Z, Wu Y, Song S, Kundtz A, Kroeger J, Humphrey J, Crawford F, Mullan M. Induction of CD40 on human endothelial cells by Alzheimer’s beta-amyloid peptides. Brain Res Bull. 1999;50:143–148. doi: 10.1016/s0361-9230(99)00122-7. [DOI] [PubMed] [Google Scholar]
- 46.Town T, Tan J, Mullan M. CD40 signaling and Alzheimer’s disease pathogenesis. Neurochem Int. 2001;39:371–380. doi: 10.1016/s0197-0186(01)00044-4. [DOI] [PubMed] [Google Scholar]
- 47.Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, O’Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T. Inflammation and Alzheimer’s disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Suo Z, Humphrey J, Kundtz A, Sethi F, Placzek A, Crawford F, Mullan M. Soluble Alzheimers beta-amyloid constricts the cerebral vasculature in vivo. Neurosci Lett. 1998;257:77–80. doi: 10.1016/s0304-3940(98)00814-3. [DOI] [PubMed] [Google Scholar]
- 49.Suo Z, Tan J, Placzek A, Crawford F, Fang C, Mullan M. Alzheimer’s beta-amyloid peptides induce inflammatory cascade in human vascular cells: the roles of cytokines and CD40. Brain Res. 1998;807:110–117. doi: 10.1016/s0006-8993(98)00780-x. [DOI] [PubMed] [Google Scholar]
- 50.Breteler MM. Vascular risk factors for Alzheimer’s disease: an epidemiologic perspective. Neurobiol Aging. 2000;21:153–160. doi: 10.1016/s0197-4580(99)00110-4. [DOI] [PubMed] [Google Scholar]
- 51.de la Torre JC. Critically attained threshold of cerebral hypoperfusion: the CATCH hypothesis of Alzheimer’s pathogenesis. Neurobiol Aging. 2000;21:331–342. doi: 10.1016/s0197-4580(00)00111-1. [DOI] [PubMed] [Google Scholar]
- 52.Johnson KA, Albert MS. Perfusion abnormalities in prodromal AD. Neurobiol Aging. 2000;21:289–292. doi: 10.1016/s0197-4580(00)00137-8. [DOI] [PubMed] [Google Scholar]
- 53.Andre P, Nannizzi-Alaimo L, Prasad SK, Phillips DR. Platelet-derived CD40L: the switch-hitting player of cardiovascular disease. Circulation. 2002;106:896–899. doi: 10.1161/01.cir.0000028962.04520.01. [DOI] [PubMed] [Google Scholar]
- 54.Urbich C, Dernbach E, Aicher A, Zeiher AM, Dimmeler S. CD40 ligand inhibits endothelial cell migration by increasing production of endothelial reactive oxygen species. Circulation. 2002;106:981–986. doi: 10.1161/01.cir.0000027107.54614.1a. [DOI] [PubMed] [Google Scholar]
- 55.Paresce DM, Ghosh RN, Maxfield FR. Microglial cells internalize aggregates of the Alzheimer’s disease amyloid beta-protein via a scavenger receptor. Neuron. 1996;17:553–565. doi: 10.1016/s0896-6273(00)80187-7. [DOI] [PubMed] [Google Scholar]
- 56.Chung H, Brazil MI, Soe TT, Maxfield FR. Uptake, degradation, and release of fibrillar and soluble forms of Alzheimer’s amyloid beta-peptide by microglial cells. J Biol Chem. 1999;274:32301–32308. doi: 10.1074/jbc.274.45.32301. [DOI] [PubMed] [Google Scholar]
- 57.Wyss-Coray T, Lin C, Yan F, Yu GQ, Rohde M, McConlogue L, Masliah E, Mucke L. TGF-beta1 promotes microglial amyloid-beta clearance and reduces plaque burden in transgenic mice. Nat Med. 2001;7:612–618. doi: 10.1038/87945. [DOI] [PubMed] [Google Scholar]
- 58.Schenk DB, Seubert P, Grundman M, Black R. A beta immunotherapy: lessons learned for potential treatment of Alzheimer’s disease. Neurodegener Dis. 2005;2:255–260. doi: 10.1159/000090365. [DOI] [PubMed] [Google Scholar]
- 59.Schenk D. Amyloid-beta immunotherapy for Alzheimer’s disease: the end of the beginning. Nat Rev Neurosci. 2002;3:824–828. doi: 10.1038/nrn938. [DOI] [PubMed] [Google Scholar]
- 60.El Khoury J, Toft M, Hickman SE, Means TK, Terada K, Geula C, Luster AD. Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat Med. 2007;13:432–438. doi: 10.1038/nm1555. [DOI] [PubMed] [Google Scholar]
- 61.Harris-White ME, Frautschy SA. Low density lipoprotein receptor-related proteins (LRPs), Alzheimer’s and cognition. Curr Drug Targets CNS Neurol Disord. 2005;4:469–480. doi: 10.2174/156800705774322102. [DOI] [PubMed] [Google Scholar]
- 62.Hammad SM, Ranganathan S, Loukinova E, Twal WO, Argraves WS. Interaction of apolipoprotein J-amyloid beta-peptide complex with low density lipoprotein receptor-related protein-2/megalin. A mechanism to prevent pathological accumulation of amyloid beta-peptide. J Biol Chem. 1997;272:18644–18649. doi: 10.1074/jbc.272.30.18644. [DOI] [PubMed] [Google Scholar]
- 63.Szczepanik AM, Funes S, Petko W, Ringheim GE. IL-4, IL-10 and IL-13 modulate A beta(1--42)-induced cytokine and chemokine production in primary murine microglia and a human monocyte cell line. J Neuroimmunol. 2001;113:49–62. doi: 10.1016/s0165-5728(00)00404-5. [DOI] [PubMed] [Google Scholar]
- 64.Goerdt S, Orfanos CE. Other functions, other genes: alternative activation of antigen-presenting cells. Immunity. 1999;10:137–142. doi: 10.1016/s1074-7613(00)80014-x. [DOI] [PubMed] [Google Scholar]
- 65.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 66.Duffield JS. The inflammatory macrophage: a story of Jekyll and Hyde. Clin Sci (Lond) 2003;104:27–38. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 67.Shimizu E, Kawahara K, Kajizono M, Sawada M, Nakayama H. IL-4-induced selective clearance of oligomeric beta-amyloid peptide(1–42) by rat primary type 2 microglia. J Immunol. 2008;181:6503–6513. doi: 10.4049/jimmunol.181.9.6503. [DOI] [PubMed] [Google Scholar]
- 68.Chakrabarty P, Jansen-West K, Beccard A, Ceballos-Diaz C, Levites Y, Verbeeck C, Zubair AC, Dickson D, Golde TE, Das P. Massive gliosis induced by interleukin-6 suppresses Abeta deposition in vivo: evidence against inflammation as a driving force for amyloid deposition. FASEB J. 2010;24(2):548–559. doi: 10.1096/fj.09-141754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Town T, Nikolic V, Tan J. The microglial “activation” continuum: from innate to adaptive responses. J Neuroinflamm. 2005;2:24. doi: 10.1186/1742-2094-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Calingasan NY, Erdely HA, Altar CA. Identification of CD40 ligand in Alzheimer’s disease and in animal models of Alzheimer’s disease and brain injury. Neurobiol Aging. 2002;23:31–39. doi: 10.1016/s0197-4580(01)00246-9. [DOI] [PubMed] [Google Scholar]
- 71.Togo T, Akiyama H, Kondo H, Ikeda K, Kato M, Iseki E, Kosaka K. Expression of CD40 in the brain of Alzheimer’s disease and other neurological diseases. Brain Res. 2000;885:117–121. doi: 10.1016/s0006-8993(00)02984-x. [DOI] [PubMed] [Google Scholar]
- 72.Rezai-Zadeh K, Gate D, Szekely CA, Town T. Can peripheral leukocytes be used as Alzheimer’s disease biomarkers? Expert Rev Neurother. 2009;11:1623–1633. doi: 10.1586/ern.09.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mener DJ, Cambio A, Stoddard DG, Martin BA, Palapattu GS. The impact of HMG-CoA reductase therapy on serum PSA. Prostate. 2009 doi: 10.1002/pros.21095. In Press. [DOI] [PubMed] [Google Scholar]
- 74.Relja B, Lehnert M, Seyboth K, Bormann F, Hohn C, Czerny C, Henrich D, Marzi I. Simvastatin reduces mortality and hepatic injury after hemorrhage/resuscitation in rats. Shock. 2009 doi: 10.1097/SHK.0b013e3181cd8d05. In Press. [DOI] [PubMed] [Google Scholar]
- 75.Sharma KK, Gupta R, Agrawal A, Roy S, Kasliwal A, Bana A, Tongia RK, Deedwania PC. Low use of statins and other coronary secondary prevention therapies in primary and secondary care in India. Vasc Health Risk Manag. 2009;5:1007–1014. doi: 10.2147/vhrm.s8017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wolozin B, Kellman W, Ruosseau P, Celesia GG, Siegel G. Decreased prevalence of Alzheimer disease associated with 3-hydroxy-3-methyglutaryl coenzyme A reductase inhibitors. Arch Neurol. 2000;57:1439–1443. doi: 10.1001/archneur.57.10.1439. [DOI] [PubMed] [Google Scholar]
- 77.Townsend KP, Shytle DR, Bai Y, San N, Zeng J, Freeman M, Mori T, Fernandez F, Morgan D, Sanberg P, Tan J. Lovastatin modulation of microglial activation via suppression of functional CD40 expression. J Neurosci Res. 2004;78:167–176. doi: 10.1002/jnr.20234. [DOI] [PubMed] [Google Scholar]
- 78.Sparks DL, Sabbagh MN, Connor DJ, Lopez J, Launer LJ, Browne P, Wasser D, Johnson-Traver S, Lochhead J, Ziolwolski C. Atorvastatin for the treatment of mild to moderate Alzheimer disease: preliminary results. Arch Neurol. 2005;62:753–757. doi: 10.1001/archneur.62.5.753. [DOI] [PubMed] [Google Scholar]
- 79.Fassbender K, Simons M, Bergmann C, Stroick M, Lutjohann D, Keller P, Runz H, Kuhl S, Bertsch T, von Bergmann K, Hennerici M, Beyreuther K, Hartmann T. Simvastatin strongly reduces levels of Alzheimer’s disease beta -amyloid peptides Abeta 42 and Abeta 40 in vitro and in vivo. Proc Natl Acad Sci USA. 2001;98:5856–5861. doi: 10.1073/pnas.081620098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ray KK, Cannon CP. The potential relevance of the multiple lipid-independent (pleiotropic) effects of statins in the management of acute coronary syndromes. J Am Coll Cardiol. 2005;46:1425–1433. doi: 10.1016/j.jacc.2005.05.086. [DOI] [PubMed] [Google Scholar]
- 81.Li J, Zhao SP, Peng DQ, Xu ZM, Zhou HN. Early effect of pravastatin on serum soluble CD40L, matrix metalloproteinase-9, and C-reactive protein in patients with acute myocardial infarction. Clin Chem. 2004;50:1696–1699. doi: 10.1373/clinchem.2003.030940. [DOI] [PubMed] [Google Scholar]
- 82.Dai Q, Borenstein AR, Wu Y, Jackson JC, Larson EB. Fruit and vegetable juices and Alzheimer’s disease: the Kame Project. Am J Med. 2006;119:751–759. doi: 10.1016/j.amjmed.2006.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nielsen SE, Young JF, Daneshvar B, Lauridsen ST, Knuthsen P, Sandstrom B, Dragsted LO. Effect of parsley (Petroselinum crispum) intake on urinary apigenin excretion, blood antioxidant enzymes and biomarkers for oxidative stress in human subjects. Br J Nutr. 1999;81:447–455. doi: 10.1017/s000711459900080x. [DOI] [PubMed] [Google Scholar]
- 84.van Acker SA, van den Berg DJ, Tromp MN, Griffioen DH, van Bennekom WP, van der Vijgh WJ, Bast A. Structural aspects of antioxidant activity of flavonoids. Free Radic Biol Med. 1996;20:331–342. doi: 10.1016/0891-5849(95)02047-0. [DOI] [PubMed] [Google Scholar]
- 85.Saija A, Scalese M, Lanza M, Marzullo D, Bonina F, Castelli F. Flavonoids as antioxidant agents: importance of their interaction with biomembranes. Free Radic Biol Med. 1995;19:481–486. doi: 10.1016/0891-5849(94)00240-k. [DOI] [PubMed] [Google Scholar]
- 86.Kandaswami C, Lee LT, Lee PP, Hwang JJ, Ke FC, Huang YT, Lee MT. The antitumor activities of flavonoids. In Vivo. 2005;19:895–909. [PubMed] [Google Scholar]
- 87.Ferrandiz ML, Gil B, Sanz MJ, Ubeda A, Erazo S, Gonzalez E, Negrete R, Pacheco S, Paya M, Alcaraz MJ. Effect of bakuchiol on leukocyte functions and some inflammatory responses in mice. J Pharm Pharmacol. 1996;48:975–980. doi: 10.1111/j.2042-7158.1996.tb06016.x. [DOI] [PubMed] [Google Scholar]
- 88.Ueda H, Yamazaki C, Yamazaki M. A hydroxyl group of flavonoids affects oral anti-inflammatory activity and inhibition of systemic tumor necrosis factor-alpha production. Biosci Biotechnol Biochem. 2004;68:119–125. doi: 10.1271/bbb.68.119. [DOI] [PubMed] [Google Scholar]
- 89.Smolinski AT, Pestka JJ. Modulation of lipopolysaccharide-induced proinflammatory cytokine production in vitro and in vivo by the herbal constituents apigenin (chamomile), ginsenoside Rb(1) (ginseng) and parthenolide (feverfew) Food Chem Toxicol. 2003;41:1381–1390. doi: 10.1016/s0278-6915(03)00146-7. [DOI] [PubMed] [Google Scholar]
- 90.Rezai-Zadeh K, Ehrhart J, Bai Y, Sanberg PR, Bickford P, Tan J, Shytle RD. Apigenin and luteolin modulate microglial activation via inhibition of STAT1-induced CD40 expression. J Neuroinflamm. 2008;5:41. doi: 10.1186/1742-2094-5-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nguyen VT, Benveniste EN. Involvement of STAT-1 and ets family members in interferon-gamma induction of CD40 transcription in microglia/macrophages. J Biol Chem. 2000;275:23674–23684. doi: 10.1074/jbc.M002482200. [DOI] [PubMed] [Google Scholar]
- 92.Leonard WJ, O’Shea JJ. Jaks and STATs: biological implications. Annu Rev Immunol. 1998;16:293–322. doi: 10.1146/annurev.immunol.16.1.293. [DOI] [PubMed] [Google Scholar]
- 93.Wang M, Yang Y, Yang D, Luo F, Liang W, Guo S, Xu J. The immunomodulatory activity of human umbilical cord blood-derived mesenchymal stem cells in vitro. Immunology. 2009;126:220–232. doi: 10.1111/j.1365-2567.2008.02891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Augello A, Tasso R, Negrini SM, Amateis A, Indiveri F, Cancedda R, Pennesi G. Bone marrow mesenchymal progenitor cells inhibit lymphocyte proliferation by activation of the programmed death 1 pathway. Eur J Immunol. 2005;35:1482–1490. doi: 10.1002/eji.200425405. [DOI] [PubMed] [Google Scholar]
- 95.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 96.Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, Hardy W, Devine S, Ucker D, Deans R, Moseley A, Hoffman R. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42–48. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- 97.Kostrzewa RM, Segura-Aguilar J. Novel mechanisms and approaches in the study of neurodegeneration and neuroprotection. a review. Neurotox Res. 2003;5:375–383. doi: 10.1007/BF03033166. [DOI] [PubMed] [Google Scholar]
- 98.O’Brien TA, Tiedemann K, Vowels MR. No longer a biological waste product: umbilical cord blood. Med J Aust. 2006;184:407–410. doi: 10.5694/j.1326-5377.2006.tb00292.x. [DOI] [PubMed] [Google Scholar]
- 99.Ende N, Chen R, Ende-Harris D. Human umbilical cord blood cells ameliorate Alzheimer’s disease in transgenic mice. J Med. 2001;32:241–247. [PubMed] [Google Scholar]
- 100.Obregon D, Hou H, Bai Y, Nikolic WV, Mori T, Luo D, Zeng J, Ehrhart J, Fernandez F, Morgan D, Giunta B, Town T, Tan J. CD40L disruption enhances Abeta vaccine-mediated reduction of cerebral amyloidosis while minimizing cerebral amyloid angiopathy and inflammation. Neurobiol Dis. 2008;29:336–353. doi: 10.1016/j.nbd.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tan J, Town T, Mori T, Obregon D, Wu Y, DelleDonne A, Rojiani A, Crawford F, Flavell RA, Mullan M. CD40 is expressed and functional on neuronal cells. EMBO J. 2002;21:643–652. doi: 10.1093/emboj/21.4.643. [DOI] [PMC free article] [PubMed] [Google Scholar]