Abstract
Seven novel diamidino 2,5-bis(aryl)thiazoles (5a–5g) were synthesized and evaluated against Trypanosoma brucei rhodensiense (T. b. r.) and Plasmodium falciparum (P. f). The diamidines were obtained directly from the corresponding bis-nitriles (4a–g) by the action of lithium bis(trimethylsilyl)amide. The bis-nitriles 4a–4f were synthesized in four steps starting with the Stille coupling of 2-tributyltinthiazole with the appropriate cyanoaryl halide. The bis-nitrile 5g was obtained by the palladium facilitated coupling of the mixed tinsilyl reagent 2-trimethylsilyl-5-trimethyltinthiazole with 2-bromo-5-cyanopyridine. The amidoxime potential prodrugs 6a–e, 6g were obtained by the reaction of hydroxylamine with the bis-nitriles. O-Methylation of the amidoximes gave the corresponding N-methoxyamidines 7a–c, 7e, 7g. The diamidines showed strong DNA binding affinity as reflected by ΔTm measurements. Four of the diamidines 5a, 5b, 5d and 5e were highly active in vitro against P. f. giving IC50 values between 1.1 and 2.5 nM. The same four diamidines showed IC50 values between 4 and 6 nM against T. b. r. The selectivity indices ranged from 233 to 9175. One diamidine 5a produced one of four cures at an ip dose of 4 × 5 mg/kg in the STIB900 mouse model for acute African trypanosomiasis. The amidoxime and N-methoxyamidine of 5a were the only produgs to provide cures (1/4 cures) in the same mouse model on oral dosage at 4 × 25 mg/kg.
1. Introduction
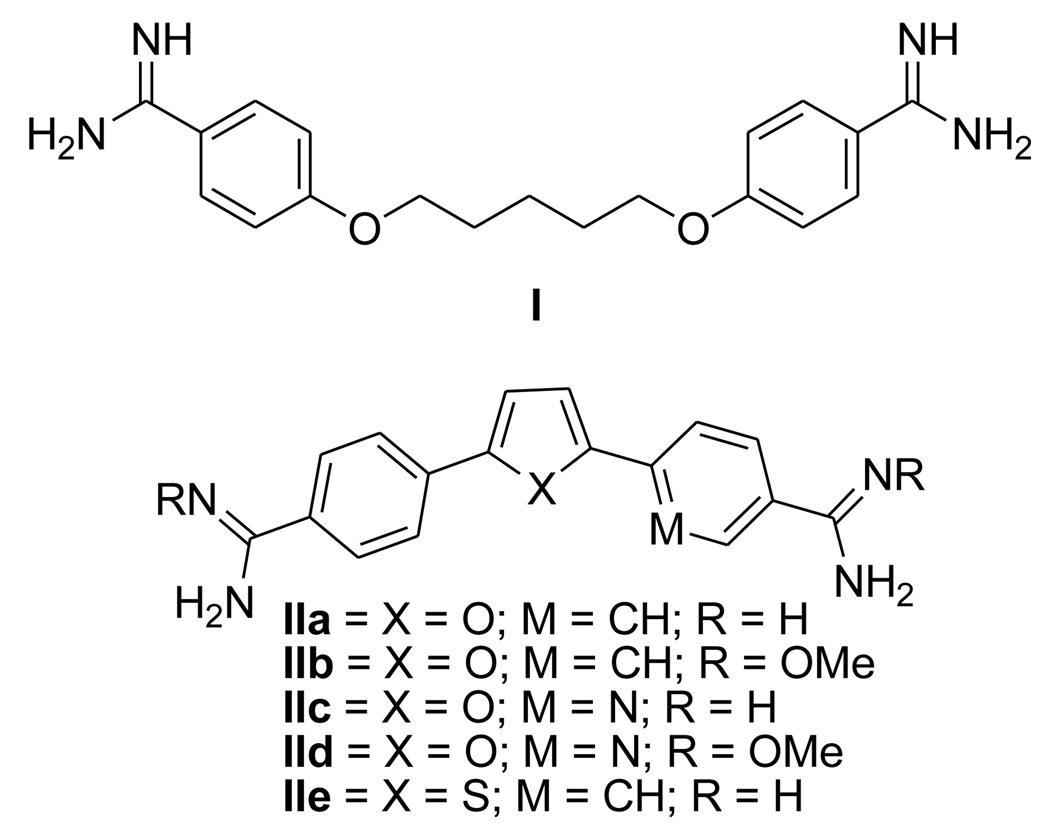
It has been estimated that approximately 2.2 billion people are at risk for the related protozoan diseases malaria and trypanosomiasis.1 Available drugs for treatment of both of these diseases exhibit significant shortcomings. The growth of multi-drug resistance strains of Plasmodium falciparum, the species which causes the most severe cases of malaria, has created an urgent need for new antimalarials with different modes of action.2,3 Presently, only three such compounds are noted as undergoing advanced evaluation.3 Currently four compounds are registered for use against human African trypanosomiasis (HAT); two are used for 1st stage disease [suramin and pentamidine(I)] and two are used against 2nd stage disease [melarsoprol and eflornithine].4,5 All of these drugs are administered by injection and have undesirable side effects. Thus, the need for new drugs to treat diseases caused by these two parasites is evident.
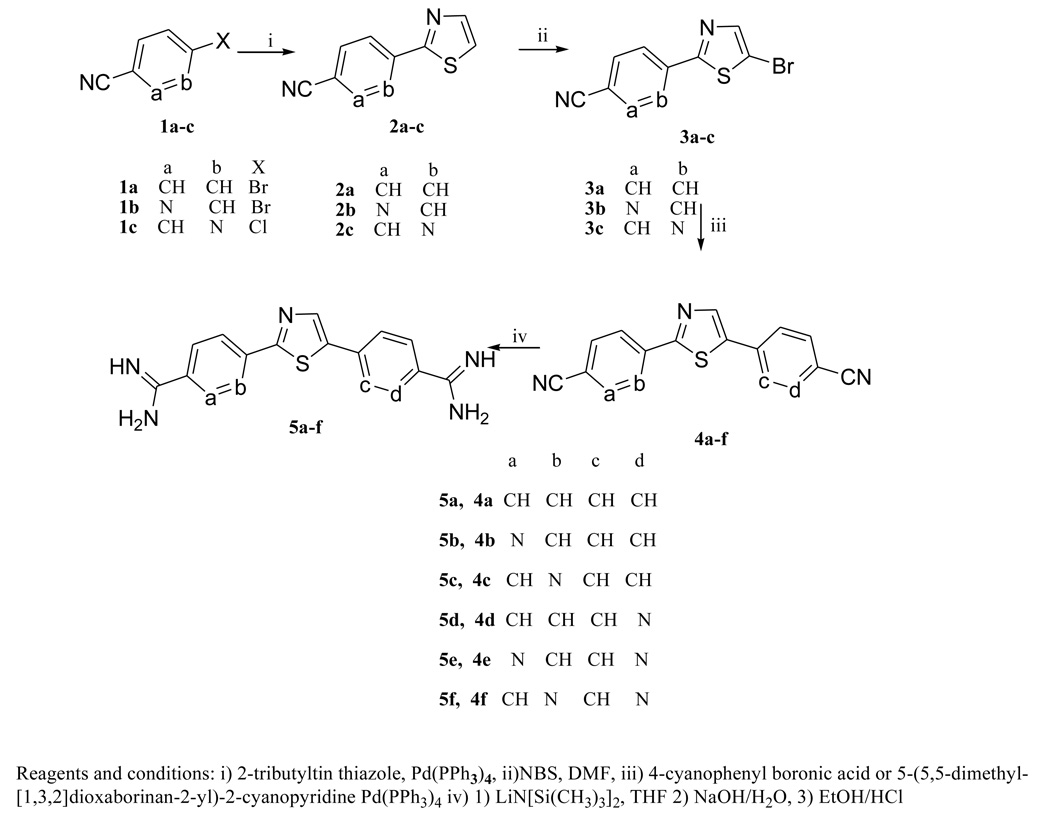
Aromatic diamidines, pentamidine(I) being the preeminent member of the class, have been studied extensively as antiprotozoal agents for nearly eight decades.6 Despite broad spectrum antimicrobial activity of this class, pentamidine remains the only member of this class of compounds to see significant human use. Pafuramidine (IIb), a prodrug of furamidine (IIa)(Figure 1), was subjected to extensive clinical evaluation advancing to Phase III trials against both HAT and AIDS-related Pneumocystsis jiroveci pneumonia and to Phase II trials against malaria.7, 8 Unfortunately, in an additional safety study which paralleled the phase III studies some volunteers exhibited liver and renal toxicities and trials with pafuramidine were discontinued.7 Thus it is important to continue to search for effective and safe members of this highly active class of compounds. We have shown that replacement of the phenyl rings of furamidine with pyridyls leads to compounds which are active in a model for CNS stage HAT.9 Both the azafuramidine IIc and its prodrug IId were effective in mouse models for both stages of the disease.9,10 A number of studies have focused upon replacement of the central furan ring of IIa with other 5-ring heterocyclic systems including pyrrole11,12, thiophene(IIe)11,12, imidazole11, oxazole11,13 thiadiazole13, oxadiazole13, as well as several with several 6-ring heterocyclic units.14,15 Despite these extensive studies it appears that use of a thiazole central linker has not been investigated. This report describes the synthesis and evaluation of diamidino 2,5-diarylthiazoles including several analogues in which the aryl units are pyridyls. Such compounds are expected to have different absorption and distribution properties than IIa and IIe and thus may exhibit different toxicity profiles. Given the locations where these diseases occur it is highly desirable to develop orally effective antiprotozoal drugs.4,16 Due to the dicationic nature of diamidines they typically exhibit quite poor oral bioavailability. This problem has been overcome in several cases by use of amidoxime prodrugs.17,18 Consequently, we also report the synthesis and evaluation of several amidoxime prodrugs in the thiazole series.
Figure 1.
Important antiparasitic compounds.
2. Results and discussion
2.1. Chemistry
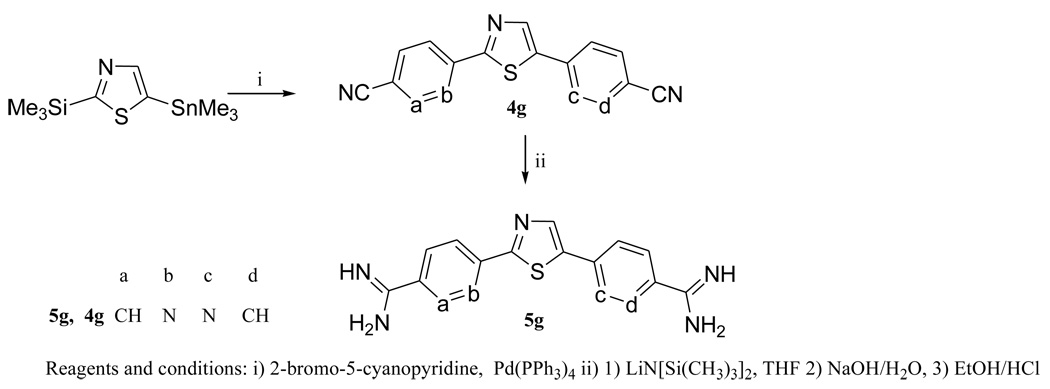
The key step(s) in the synthesis of the various diarylthiazoles employs palladium catalyzed coupling reaction which we have previously extensively used.10,19–21 The 2,5-bis-(amidinophenyl)thiazoles 5a–c were synthesized from 2,5-bis(4-cyanophenyl)thiazoles 4a–c which were obtained in four steps( Scheme 1). The first step involves a Stille coupling reaction between 2-tributylstanyl thiazole and 4-bromocyanophenyl (1a), 5-bromo-2-cyanopyridine (1b) and 6-chloronicotinonitrile (1c), respectively, to form the mono-aryl thiazoles 2a–c. Bromination of 2a–c with N-bromosuccinimide in DMF solution, furnished 3a–c in good yield in the second step. Subsequent Suzuki coupling of 3a–c with 4-cyanophenyl boronic acid gave 4a–c in good yield (Scheme 1). In a similar way, the bis-nitriles 4d–4f were prepared from 2a–c however in this case 5-(5,5-dimethyl-[1,3,2]dioxaborinan-2-yl)-2-cyanopyridine was used to couple with the bromothiazoles 3a–3c. The target bis-amidines 5a–f were obtained from the bis-nitriles 4a–4f by the action of lithium bis(trimethylsilyl)amide in THF.22
Scheme 1.
Synthesis of bis-amidines 5a–f.
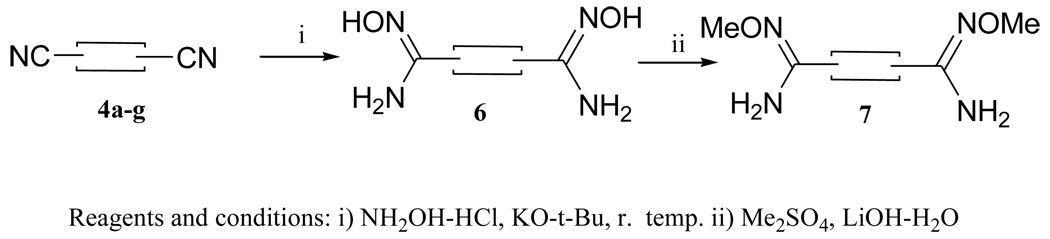
As a part of this study, we also prepared a bis-amidine with both pyridyl nitrogen atoms adjacent to thiazole core( Scheme 2). The synthesis of the bis-amidine 5g required the corresponding bis-nitrile 4g (Scheme 2). The preparation of 4g was achieved by using the mixed tin and silyl reagent 2-trimethylsilyl-5-trimethyltinthiazole23 in a palladium catalyzed coupling reaction with 2-bromo-5-cyanopyridine to give 4g in a modest yield. The bis-amidine 5g was obtained in good yield employing lithium bis(trimethylsilyl)amide as previously described.
Scheme 2.
Synthesis of bis-amidine 5g.
The potential prodrugs of the bis-amidines were prepared as outlined in Scheme 3. The bis-amidoximes 6a–e,g were readily obtained by the reaction of the corresponding bis-nitriles 4 with hydroxylamine. Methylation of the diamidoximes 6 with dimethylsulfate in the presence of aqueous lithium hydroxide gave the N-methoxyamidines 7a–c,e,g in reasonable yield.
Scheme 3.
Synthesis of bis-amidoxime prodrugs.
2.2 Biology
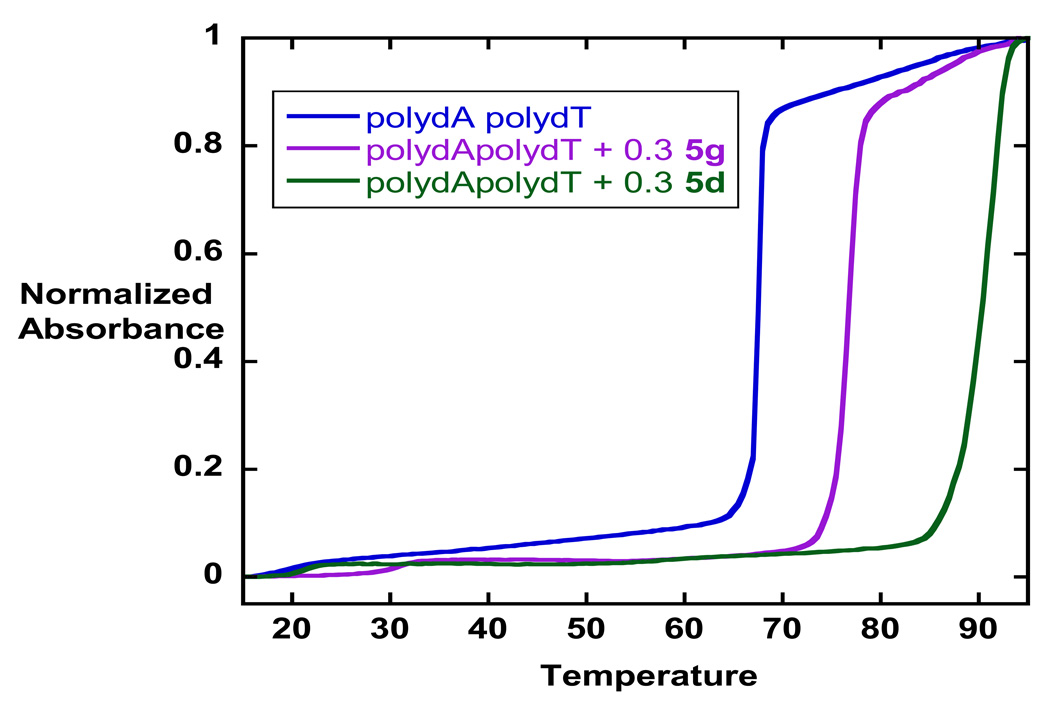
Table 1 summarizes the DNA binding affinities for the thiazole diamidines and the in vitro evaluation of the dications against Plasmodium falciparum (P. f.) and Trypanosoma brucei rhodensiense (T. b. r.). For comparative purposes similar data for furamidine (IIa), azafuramidine (IIc) and the thiophene analogue (IIe) are also provided in Table 1. The former two compounds are included since promising antiprotzoan data has been reported for them and the latter is included due to the close structural analogy with the thiazoles. We have found that the determination of melting temperature increases ΔTm (Tm of complex – Tm of free DNA) provides a rapid and accurate method for ranking the binding affinities for a variety of aryldiamidines .6a,24,25 Typical results are illustrated in Figure 2. The ΔTm values of the thiazole diamidines for their complexes with poly (dA-dT) range from10 to 22.9°C. The ΔTm value for the parent thiazole diamidine 5a is 21.1°C. This value is approximately 20% less than that for both IIa and IIe. The differences in ΔTm values for the furamidine and IIe and 5a reflects the introduction of the nitrogen atom into the five membered ring heterocycle and is consistent with the “N-effect” we have noted for a number of cases of replacement of phenyl rings with pyridyl rings in other triaryl diamidine systems. 26 On binding to DNA, transfer of the aza-analogues from aqueous solution into the less polar environment of the minor groove is less favorable than that for furamidine and IIe.26 The thiazole analogues which contain pyridyl rings replacing one or both of the phenyl rings of 5a generally show a decline in ΔTm values. However, there is not an obvious systematic change in ΔTm with structure which suggests that small effects from subtle differences in van der Waals, H-bonding interactions and molecular geometry are likely contributors to the variation in values. Consistent with other series of aryl diamidines26 generally the compounds with higher DNA affinities exhibit greater antiprotozoan activity.
Table 1.
DNA affinities and in vitro antiprotozoan activity for thiazole analogs.
 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Code | a | b | c | d | DNA affinity ΔTm(°C)a |
T. b. r. IC50(nM) b |
P. f. IC50(nM) b |
cytotox IC50(µM) c |
| 5a | CH | CH | CH | CH | 21.1 | 4 | 2.0 | 36.7 |
| 5b | N | CH | CH | CH | 19.2 | 4 | 2.5 | 1.8 |
| 5c | CH | N | CH | CH | 13.9 | 16 | 12.5 | 102.5 |
| 5d | CH | CH | CH | N | 22.9 | 6 | 2.4 | 40.2 |
| 5e | N | CH | CH | N | 18.2 | 6 | 1.1 | 1.4 |
| 5f | CH | N | CH | N | 12.8 | 38 | 13 | 37. 4 |
| 5g | CH | N | N | CH | 10.0 | 176 | 24.6 | >195.0 |
| IIa | 25 | 3.2 | 13.8 | 6.5 | ||||
| IIc | 19.3 | 7.0 | 6.5 | 77.9 | ||||
| IIe | 25 | 3 | 13.8 | 51.7 | ||||
| I | 12.6 | 2.8 | 46.4 | 46.6 | ||||
Figure 2.
Thermal melting curves (Absorbance at 260nm versus temperature in degrees C) are shown for poly dA•dT and its complexes with 5g and 5d. ΔTm values were determined from the peak in first derivative plots (dA260/dT) and are collected in Table 1.
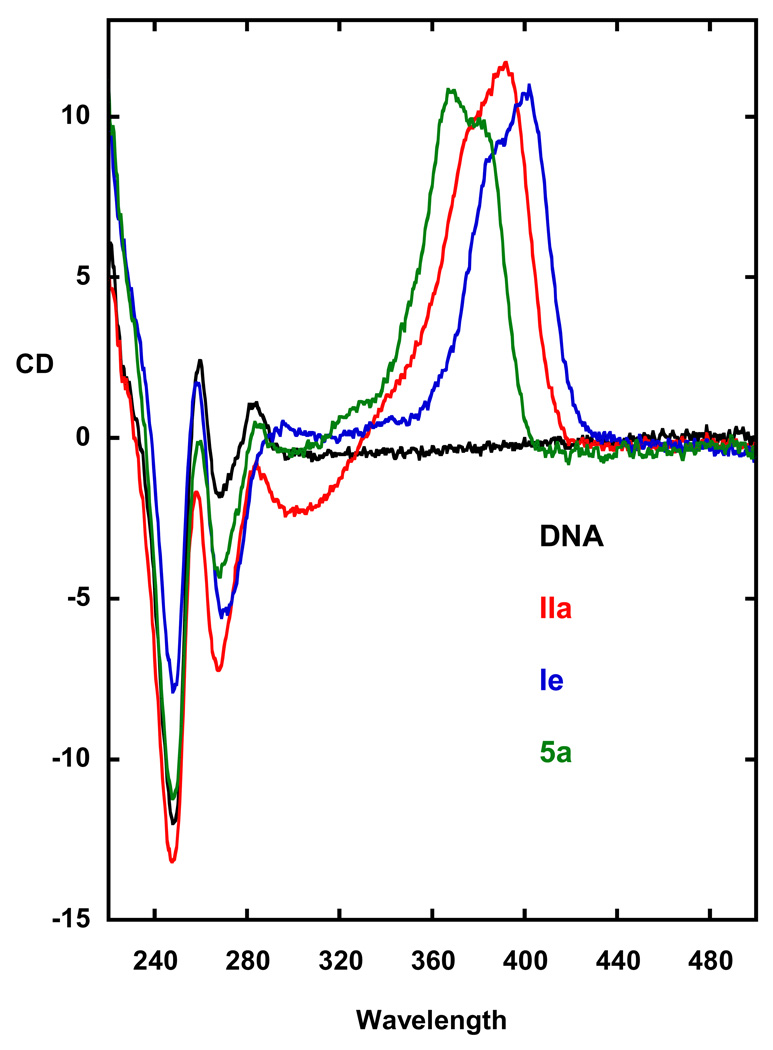
In order to determine if these thiazole analogues are binding in the DNA minor groove, we acquired CD spectra for 5a along with IIa and IIe, for comparison, on binding to DNA (see Figure 3). Minor groove binding compounds give a large positive induced CD signal on binding to AT DNA sequences and cause only small changes in the pattern of the DNA CD spectrum.27 Such a pattern is observed on adding IIa and IIe to polydA.polydT (Figure 3) with a strong, positive induced signal between 350 to 430 nm smaller changes in the DNA spectral region below 300 nm. As can be seen in Figure 2, 5a exhibits a similar CD spectra with that of IIa and IIe. These results clearly support a minor groove binding mode for the thiazole 5a.27 A detailed analysis of the effect of structure induced variation by thiazoles analogues awaits the results of the biophysical studies which are in progress.
Figure 3.
Circular dichroism plots (CD versus wavelength) are shown for poly dA•dT (DNA) and its complexes with IIa, IIe and 5a. DNA without bound compond does not have any CD signal above 300 nm while the unbound compounds are not chiral. On binding to DNA the compounds exhibit a large positive CD spectrum above 300nm hat is characteristic of minor groove binding. The changes below 300 are due to induced changes in the compound CD as well as slight changes in the DNA spectrum.
The four thiazoles 5a, 5b, 5d, 5e were highly active against P. f. exhibiting IC50 values between 1.1 and 2.5 nM which are about nearly a factor of ten more active than IIa and IIe. These low nanomolar values meet our group’s criteria of IC50 values of less than 100 nM to enter animal studies and the properties of these compounds are in general agreement with criteria suggested by others.16 The thiazoles 5a–5f are highly active in vitro versus T. b. r. giving IC50 values ranging between 4 and 38 nM. Four (5a, 5b, 5d, 5e) of the seven new diamidines gave IC50 values (4–6 nM) comparable to furamidne (3.2 nM) and IIe(3 nM) and pentamidine (2.8 nM). The selectivity indices for the four most active compounds against T. b. r. are reasonable ranging from 233 to 9175, however they are not as large as that of pentamidine(16,643). Based upon activity and selectivity the compounds were advanced to the rigorous T. b. r. STIB900 mouse model of infection.
Data for the parent diamidines 5a–5f as well as data for the prodrugs 6a–6e, 6g and 7a–c, 7e, 7g obtained from the STIB900 mouse model for the acute phase of African trypanosomiasis are presented in Table 2. The parent diamidines were administered by intraperitoneal injection at the screening dose of 4 × 5 mg/kg whereas the potential prodrugs were given orally at the screening dose of 4 × 25 mg/kg. At this low dose level only the parent diamidine 5a cured 1 of four infected test animals. This result is comparable to that for IIa and inferior to that for IIc and IIe which gave 3/4 and 2/4 cures, respectively, at this dose level. Only two thiazole prodrugs 6e and 7e, prodrugs of 5e, cured; 1/4 infected mice. The results for 6e and 7e are inferior to that for pafuramidine and significantly less effective than those for IId which gave 4/4 cures at both 25mg/kg and the quite low dose of 5 mg/kg. This relatively modest in vivo activity may be due to poor PK. Currently, we are using IId as the lead compound that new compounds must match or surpass in efficacy to be taken into preclinical evaluations for 2nd stage HAT, none of the thiazoles meet this standand and consequently they will not be pursued further.
Table 2.
Antitrypanosomal evaluation of the thiazole analogues in the STIB900 mouse model.
| Code | Dosage routeb | Dosage(4 × mg/kg) | Curesc | Survival (daysd) |
|---|---|---|---|---|
| 5a | ip | 5 | 1/4 | >44.5 |
| 6a | po | 25 | 0/4 | 35 |
| 7a | po | 25 | 0/4 | 41 |
| 5b | ip | 5 | 0/4 | 44.5 |
| 6b | po | 25 | 0/4 | 37.5 |
| 7b | po | 25 | 0/4 | 42.75 |
| 5c | ip | 5 | 0/4 | 30.25 |
| 6c | po | 25 | 0/4 | 24 |
| 7c | po | 25 | 0/4 | 35 |
| 5d | ip | 5 | 0/4 | >36 |
| 6d | po | 25 | 0/4 | 36.75 |
| 5e | ip | 5 | 0/4 | 50.5 |
| 6e | po | 25 | 1/4 | >37.5 |
| 7e | po | 25 | 1/4 | >47.25 |
| 5f | ip | 5 | 0/4 | 29.25 |
| 5g | ip | 5 | 0/4 | 21.5 |
| 6g | po | 25 | 0/4 | 9 |
| 7g | po | 25 | 0/4 | 9 |
| I | ip | 5 | 2/4 | .45 |
| IIa | ip | 5 | 1/4 | >46 |
| IIb | po | 25 5 |
4/4 0/4 |
>60 30 |
| IIc | ip | 5 | 3/4 | >54.5 |
| IId | po | 25 5 |
4/4 4/4 |
>60 >60 |
| IIe | ip | 5 | 2/4 | >48.75 |
3. Experimental
3.1 Biology
3.1.1 Efficacy evaluations
In vitro assays28 with T. b. r. STIB 900 and P. f. K1 strain as well as the efficacy study in an acute mouse model for T. b. r. STIB 90029 were carried out as previously reported.
3.1.2 Tm Measurements
Thermal melting experiments were conducted with a Cary 300 spectrophotometer. Cuvettes for the experiment are mounted in a thermal block and the solution temperatures are monitored by a thermistor in the reference cuvette. Temperatures were maintained under computer control and are increased at 0.5 °C/min. The experiments were conducted in 1 cm path length quartz curvettes in CAC 10 buffer (cacodylic acid 10mM, EDTA 1mM, NaCl 100mM with NaOH added to give pH = 7.0). The concentrations of DNA were determined by measuring the absorbance at 260nm. A ratio of 0.3 moles compound per mole of DNA was used for the complex and DNA with no compound was used as a control.30 ΔTm values are determined by the peak in first derivative curves (dA/dT).
3.1.3 Circular Dichroism (CD)
CD spectra were collected with a Jasco J-810 spectrometer at different ratios of compound to DNA at 25 °C in MES 10 buffer. A DNA solution in a 1-cm quartz cuvette was first scanned over a desired wavelength range. Compounds IIa, IIc and 5a at increasing ratios were then titrated into the same cuvette and the complexes rescanned under same conditions. 31
3.2 Chemistry Experimental Section
Melting points were recorded using a Mel-Temp capillary melting apparatus and are uncorrected. TLC analysis was carried out on silica gel 60 F254 precoated aluminum sheets and detected under UV light. 1H and 13C NMR spectra were recorded employing a Bruker 400 Ultrashield™ or Varian Unity Plus™ 300 spectrometer and chemical shifts (δ) are in ppm relative to TMS as internal standard. Mass spectra were recorded on an Applied Biosystems MALDI-TOF-TOF MS (4800)spectrometer. Elemental analyses were obtained from Atlantic Microlab Inc. (Norcross, GA) and are within ±0.4 of the theoretical values. The compounds reported as salts frequently analyzed correctly for fractional moles of water and/or ethanol of solvation. In each case proton NMR showed the presence of indicated solvent (s). All chemicals and solvents were purchased from Aldrich Chemical Co., VWR, Fischer Scientific, or Frontier and were used as received. The synthesis of 2-trimethylsilyl-5-trimethyltin-thiazole has been previously reported.23
5-(5,5-Dimethyl-[1,3,2]dioxaborinan-2-yl)-2-cyanopyridine
To a round-bottom flask were added 2.35 g (12.8 mmol) 5-bromo-2-cyanopyridine in 45 mL DMSO, 3.76 g (38.4 mmol) potassium acetate, 3.5 g (15.4 mmol) bis(neopentylglycolato)diboron and 0.282 g (3 mol%) PdCl2(dppf). The mixture was heated at 80°C for 2 days. The reaction solution was cooled to room temperature and poured into ice-water. The mixture was extracted with ethyl acetate, washed with saturated brine, dried over MgSO4, and concentrated under reduced pressure. The residue was purified by crystallization from a mixture of EtOAc/hexane to give 0.98 g (36%),white solid, mp 126°C. 1H NMR (300 MHz, CDCl3) δ: 9.05 (s, 1H), 8.20 (d, 1H, J = 7.5 Hz), 7.68 (d, 1H, J = 7.5 Hz), 3.83 (s, 4H), 1.07 (s, 6H). 13C NMR (75 MHz, CDCl3) δ: 22.1, 31.2, 32.3, 72.7, 117.7, 127.8, 135.4, 142.6, 156.1. Anal. Calcd for C11H13N2BO2 C, 61.15; H, 6.07; N, 12.97. Found: C, 61.39; H, 6.07, N, 12.93.
General procedure for Stille coupling reaction
To a round-bottom flask were added 5.49 g (30 mmol) 4-bromo-2-cyanopyridine in 70 mL 1,4-dioxane, 0.69 g (2 mol%) Pd(PPh3)4 and 11.22 g (30 mmol) 2-tributylstannanylthiazole. The mixture was allowed to reflux overnight. The solvent was removed under reduced pressure. Hexane was added to the residue. The precipitate was filtered. The solid was dissolved in EtOAc and washed with 5% NaF. The organic layer was dried over Na2SO4. The solvent was evaporated, and the crude product was purified by column chromatography using hexane/EtOAc (20:1).
2-(Benzonitrile-4-yl)thiazole (2a)
The physical properties observed are consistent with those previously reported.32
2-(2-Cyanopyridin-5-yl)thiazole (2b)
Yield (82%), white solid, mp 167°C. 1H NMR (400 MHz, CDCl3) δ: 7.51(d, 1H, J = 3.2 Hz), 7.8(dd, 1H, J1 = 0.8, J2 = 8 Hz), 8.01(d, 1H, J = 3.2 Hz), 8.40(dd, 1H, J1 = 2.4, J2 = 5.6 Hz), 9.29(d, 1H, J = 2.4 Hz). 13C NMR (100 MHz, CDCl3) δ: 117.3, 121.8, 128.9, 132.5, 134.2, 134.4, 145.2, 149.0, 162.8. Anal. Calcd for C9H5N3S: C, 57.74; H, 2.69; N, 22.44. Found: C, 57.64; H, 2.73; N, 22.31.
2-(3-Cyanopyridin-6-yl)thiazole (2c)
Yield (74%), white solid, mp 167°C. 1H NMR (300 MHz, CDCl3) δ: 7.60(d, 1H, J = 3 Hz), 8.03(d, 1H, J = 3 Hz), 8.09(dd, 1H, J1 = 2.1, J2 = 8.4 Hz), 8.35(d, 1H, J = 8.1 Hz), 8.89(d, 1H, J = 2.1 Hz). 13C NMR (75 MHz, CDCl3) δ: 110.2, 116.8, 119.7, 123.8, 140.6, 145.2, 152.5, 154.3, 167.2. Anal. Calcd for C9H5N3S: C, 57.74; H, 2.69; N, 22.44. Found: C, 57.92; H, 2.70; N, 22.55.
General procedure for bromination of (2a–c)
To a round-bottom flask were added 2a (3.55 g, 19 mmol, 1 eq) in anhydrous DMF (45 mL) and (4.1 g, 23 mmol, 1.2 eq) NBS. The mixture was stirred overnight at room temperature and was poured into ice-water. The precipitate was filtered and washed with water. The crude product was crystallized from a mixture of EtOAc: hexane.
5-Bromo-2-(4-cyanophenyl)thiazole (3a)
Yield (89%), white solid, mp 149°C. 1H NMR (300 MHz, CDCl3) δ: 7.77(d, 2H, J = 8.1 Hz), 7.85 (s, 1H), 8.00 (d, 2H, J = 8.1 Hz). 13C NMR (75 MHz, CDCl3) δ: 110.7, 113.6, 118.2, 126.6, 132.9, 136.7, 145.6, 166.8. Anal. Calcd for C10H5BrN2S-0.1H2O: C, 44.99; H, 1.96; N, 10.49. Found: C, 44.89; H, 1.79; N, 10.26.
5-Bromo-2-(2-cyanopyridin-5-yl)thiazole (3b)
Yield (78%), white solid, mp 159–160°C. 1H NMR (300 MHz, CDCl3) δ: 7.77(d, 1H, J = 8.1 Hz), 7.85 (s, 1H), 8.00 (d, 2H, J = 8.1 Hz). 13C NMR (75 MHz, CDCl3) δ: 112.1, 117.1, 128.9, 131.9, 134.1, 134.6, 146.4, 148.6, 163.8. Anal. Calcd for C9H4BrN3S-0.1H2O: C, 40.35; H, 1.58; N, 15.68. Found: C, 40.49; H, 1.52; N, 15.53.
5-Bromo-2-(3-cyanopyridin-6-yl)thiazole (3c)
Yield (78%), white solid, mp 208–209°C. 1H NMR (300 MHz, CDCl3) δ: 7.90 (s, 1H), 8.09 (dd, 1H, J1 = 2.1 J2 = 8.4 Hz), 8.26(d, 1H, J = 8.1 Hz), 8.86(s, 1H). 13C NMR (75 MHz, CDCl3) δ: 110.4, 114.8, 116.7, 119.1, 140.7, 146.4, 152.6, 153.6, 168.3. Anal. Calcd for C9H4BrN3S: C, 40.62; H, 1.52; N, 15.79. Found: C, 40.67; H, 1.61; N, 15.63.
General procedure for Suzuki coupling of 3a–c
A mixture of 3a (2.65 g, 10 mmol), 4-cyanophenyl boronic acid or 5-(5,5-dimethyl-[1,3,2]dioxaborinan-2-yl)-2-cyanopyridine (1.65 g, 12 mmol), Pd (PPh3)4 (0.23 g, 2 mol%) and K2CO3 (4.14 g, 30 mmol) in 50 mL anhydrous 1,4-dioxane was allowed to reflux overnight. After cooling to room temperature, the mixture was poured into water and extracted with ethyl acetate. The organic layer was washed with brine, dried over MgSO4, and concentrated. The crude product 4a was purified by crystallization from a mixture of EtOAc:hexane for 4a and from DMF for 4b–g.
2,5-Bis(4-cyanophenyl)thiazole (4a)
Yield (77%), yellow solid, mp 276–277°C. 1H NMR (300 MHz, CDCl3) δ: 7.76 (s, 4H), 7.80(d, 2H, J = 8.4 Hz), 8.11(d, 2H, J = 8.4 Hz), 8.22 (s, 1H). 13C NMR (75 MHz, CDCl3) δ: 118.6, 127.2, 127.4, 133.2, 133.3, 137.2, 139.2, 141.8. Anal. Calcd for C17H9N3S-0.1H2O: C, 70.62; H, 3.21; N, 14.53. Found: C, 70.63; H, 3.03; N, 14.25.
5-(4-Cyanophenyl)-2-(2-cyanopyridin-5-yl)thiazole (4b)
Yield (87%), yellow solid, mp 308°C. 1H NMR (300 MHz, DMSO) δ: 8.0(s, 4H), 8.22(d, 1H, J = 8.1 Hz), 8.60(d, 1H, J = 8.1Hz), 8.74(s, 1H), 9.36 (s, 1H). Anal. Calcd for C16H8N4S-0.3H2O: C, 65.42; H, 2.95; N, 19.07. Found: C, 65.57; H, 2.73; N, 19.09.
5-(4-Cyanophenyl)-2-(3-cyanopyridin-6-yl)thiazole (4c)
Yield (87%), yellow solid, mp 357°C. 1H NMR (300 MHz, DMSO) δ: 9.14 (s, 1H), 8.72 (d, 1H, J = 6.8 Hz), 8.33 (d, 1H, J = 9.6 Hz), 8.31(d, 1H, J = 9.6 Hz), 8.00 (dd, 4H, J1 = 4.4 Hz, J2 = 2.0 Hz). Anal. Calcd for C16H8N4S-0.3H2O: C, 65.42; H, 2.95; N, 19.07. Found: C, 65.65; H, 2.79; N, 19.13.
2-(4-Cyanophenyl)-5-(2-cyanopyridin-5-yl)thiazole (4d)
Yield (27%), yellow solid, mp 292–293°C. 1H NMR (400 MHz, DMSO) δ: 8.02(d, 2H, J = 8.4 Hz), 8.16(d, 1H, J = 8 Hz), 8.18(d, 2H, J = 8.4 Hz), 8.43(dd, 1H, J1 = 2.4, J2 = 7.6Hz), 8.75(s, 1H), 9.19(d, 1H, J = 2Hz). 13C NMR (100 MHz, DMSO) δ: 113.6, 118.8, 119.2, 127.4, 130.0, 130.8, 132.1, 133.9, 135.4, 135.8, 136.7, 144.4, 149.0, 165.5. Anal. Calcd for C16H8N4S-0.2H2O: C, 65.83; H, 2.90; N, 19.19. Found: C, 65.70; H, 2.85, N, 19.03.
2,5–Bis(2-cyanopyridin-5-yl)thiazole (4e)
Yield (63 %), yellow solid, mp 333°C. 1H NMR (400 MHz, DMSO) δ: 7.96 (d, 1H, J = 6.8 Hz), 8.16 (d, 1H, J = 8.0 Hz), 8.40 (dd, 1H, J1 = 2.4, J2 = 8.4 Hz), 8.57 (dd, 1H, J1 = 2.4, J2 = 8.4 Hz), 8.72 (s, 1H), 9.17(d, 1H, J = 2.4 Hz), 9.33 (d, 1H, J = 2.0 Hz). ESI-MS m/z: (M -H ) 288. Anal. Calcd for C15H7N5S -0.6H2O: C, 60.03, H, 2.75, N, 23.33. Found: C, 60.33, H, 2.49, N, 22.98.
5–(2-Cyanopyridin-5-yl)-2-(3-cyanopyridin-6-yl)thiazole (4f)
Yield (61%), yellow solid, mp 368°C. 1H NMR (400 MHz, DMSO) δ: 9.26 (d, 1H, J = 2.4 Hz), 9.15(d, 1H, J = 2.0 Hz), 8.82 (s, 1H), 8.52 (dd, 1H, J1 = 2.0, J2 = 8.0 Hz), 8.48 (dd, 1H, J1 = 2.4, J2 = 8.4 Hz), 8.33 (dd, 1H, J1 = 0.8, J2 = 8.4 Hz), 8.18 (dd, 1H, J1 = 0.8, J2 = 8.0 Hz). ESI-MS m/z: (M-H) 288 Anal. Calcd for C15H7N5S-0.2H20: C, 61.51, H, 2.55, N, 23.91. Found: C, 61.42, H, 2.43, N, 23.71.
Synthesis of 2,5-bis(3-cyanopyridin-6-yl)thiazole (4g)
To a round-bottom flask were added 2-trimethylsilyl-5-trimethyltinthiazole1 (1.5 g, 4.73 mmol) in dioxane( 50 mL), 2-bromo-5-cyanopyridine (1.7 g, 9.45 mmol) and Pd(PPh3)4 (2 mol%). The reaction mixture was allowed to reflux overnight. The solvent was evaporated and the crude product was crystallized from DMF to give 0.38 g (28%), yellow solid. mp 366°C. 1H NMR (400 MHz, DMSO) δ: 8.33 (s, 1H), 8.35 (s, 1H), 8.46(dd, 1H, J1 = 1.2, J2 = 8.4 Hz), 8.50 (dd, 1H, J1 = 2.0, J2 = 8.4 Hz), 8.95 (s, 1H), 9.08 (d, 1H, J = 2 Hz), 9.15(d, 1H, J = 1.6 Hz). Anal. Calcd for C15H7N5S-0.3H2O: C, 61.13; H, 2.60; N, 23.76. Found: C, 61.10; H, 2.54, N, 23.65.
General procedure for conversion of dinitriles 4a–g to diamidines 5a–g
2,5-Bis(4-amidinophenyl)thiazole dihydrochloride (5a)
The bis-nitrile 4a (0.28 g, 1 mmol) was suspended in anhydrous THF (8 mL) and LiN[(CH3)3Si]2 solution 1.0 M THF (5 mL) was added to the flask and the mixture was stirred overnight at room temperature, saturated ethanolic HCl (10 mL) was carefully added, and the mixture was stirred overnight. The precipitate was filtered, washed with ether, and dried. The salt of 5a was put into water, basified with aqueous NaOH 10%, and stirred vigorously for 24 h. The precipitate was then filtered, washed with water, ether, and dried to afford 5a (free base) as a yellow solid, which was placed in a 250 mL flask, EtOH was added to the flask, and the solution was chilled in an ice-bath. After passing dry HCl gas for 15 minutes the mixture was stirred overnight. The precipitate was filtered, washed with ether to give a pale yellow solid in 84% yield. mp. >330°C. 1H NMR (300 MHz, CDCl3) δ: 7.97(d, 2H, J1 = 8.4 Hz), 8.02(d, 2H, J = 8.4 Hz), 8.03(d, 2H, J = 8.4 Hz), 8.23(d, 2H, J = 8.4 Hz), 8.67(s, 1H), 9.26(brs, 2H), 9.30(brs, 2H), 9.50(brs, 2H), 9.55(brs, 2H). 13C NMR (100 MHz, CDCl3) δ: 126.5, 126.8, 126.9, 127.1, 127.2, 129.1, 129.8, 135.9, 137.5, 142, 143.1, 165.5, 166. ESI-MS m/z: (M+ +H) 322. Anal. Calcd for C17H15N5S-2HCl-1.0H2O-0.2EtOH: C, 49.57; H, 4.83; N, 16.61. Found: C, 49.58; H, 4.52; N, 16.36.
5-(4-Amidinophenyl)-2-(2-amidinopyridin-5-yl)thiazole dihydrochloride (5b)
Yield 73 % yellow solid, mp >320°C. 1H NMR (300 MHz, DMSO) δ: 7.98(d, 2H, J = 8.4 Hz), 8.05(d, 2H, J = 8.4 Hz), 8.48(d, 1H, J = 8.5 Hz), 8.72(dd, 1H, J1 = 2.2, J2 = 8.0 Hz), 8.75(s, 1H), 9.21(brs, 2H), 9.38(d, 1H, J = 2.4 Hz), 9.48(brs, 4H), 9.71(brs, 2H). 13C NMR (100 MHz, DMSO) δ: 124.4, 127.4, 128.4, 129.7, 133, 135.8, 135.9, 140.2, 143.1, 145.4, 147.2 162.3, 163.2, 165.7. ESI-MS m/z: (M+ +H) 323. Anal. Calcd for C16H14N6S-2HCl-0.2EtOH-1.3H2O: C, 46.02; H, 4.66; N, 19.63. Found: C, 45.97; H, 4.27; N, 19.31.
5-(4-Amidinophenyl)-2-(3-amidinopyridin-6-yl)thiazole dihydrochloride (5c)
Yield 74 % pale yellow solid, mp >350°C. 1H NMR (400 MHz, DMSO) δ: 7.95(d, 2H, J = 6.3 Hz), 8.08(d, 2H, J = 8.8 Hz), 8.35(d, 1H, J = 8.4 Hz), 8.41(dd, 1H, J1 = 2.4, J2 = 8.4 Hz), 8.73(s, 1H), 9.06(d, 1H, J = 2.0 Hz), 9.14(brs, 2H), 9.32(brs, 2H), 9.45 (s, 2H), 9.65(s, 2H). 13C NMR (100 MHz, DMSO) δ: 119.1, 126.0, 127.3 128.3, 129.7, 136.0, 138.6, 141.1, 143.6, 149.9, 154, 163.9, 165.2, 167.4. HRMS: m/z calcd. C16H15N6S: 323.1079: found 323.1082. Anal. Calcd for C16H14N6S-3.5HCl-0.4H2O-0.2EtOH: C, 42.23; H, 4.21; N, 18.02. Found: C, 42.53; H, 4.06; N, 17.63.
2-(4-Amidinophenyl)-5-(2-amidinopyridin-5-yl)thiazole dihydrochloride (5d)
Yield 67% pale yellow solid, mp >350°C. 1H NMR (400 MHz, DMSO) δ: 8.02 (d, 2H, J = 8.4 Hz), 8.24 (d, 2H, J = 8.4 Hz), 8.46(d, 1H, J = 8.4 Hz), 8.56 (d, 1H, J = 8.4 Hz), 8.81 (s, 1H), 9.23 (d, 1H, J = 2 Hz), 9.28(brs, 2H), 9.46 (brs, 2H), 9.55(brs, 2H), 9.67 (brs, 2H). 13C NMR (100 MHz, DMSO) δ: 124.3, 127, 129.9, 130.3, 131.5, 135.6, 135.8, 137.3, 143.2, 144.4, 147.5, 161.7, 165.4, 167.1. Anal. Calcd for C16H14N6S-3HCl-0.5EtOH-1.1H2O: C, 43.02, H, 4.71, N, 17.70. Found: C, 43.24, H, 4.33, N, 17.92.
2,5-Bis(2-amidinopyridin-5-yl)thiazole dihydrochloride (5e)
Yield 71% pale yellow, mp >300°C. 1H NMR (400 MHz, DMSO) δ: 8.51(d, 1H, J = 8.4 Hz), 8.55(d, 1H, J = 8.0 Hz), 8.59(dd, 1H, J1 = 2Hz, J2 = 7.6 Hz), 8.72(dd, 1H, J1 = 2.0 Hz, J2 = 8.0 Hz), 8.88(s, 1H), 9.23(d, 1H, J = 2 Hz), 9.39(d, 1H, J = 2 Hz), 9.50(brs, 2H), 9.60(brs, 2H), 9.73(brs, 2H), 9.78(brs, 2H). 13C NMR (100 MHz, DMSO) δ: 124.3, 124. 5, 131.2, 132.5, 135.8, 136, 136.1, 136.6, 143.5, 143.8, 144.6, 145.5, 147.3, 161.8, 164.2. Anal. Calcd for C15H13N7S-2HCl-0.5EtOH-1.2H2O: C, 43.58, H, 4.66, N, 22.23. Found: C, 43.94, H, 4.26, N, 21.85.
2-(3-Amidinopyridin-6-yl)-5-(2-amidinopyridin-5-yl)thiazole dihydrochloride (5f)
Yield 70%, pale yellow solid, mp>350°C. 1H NMR (400 MHz, DMSO) δ: 8.35 (d, 1H, J = 8.4 Hz), 8.43 (dd, 1H, J1 = 2Hz, J2 = 7.6 Hz), 8.49(d, 1H, J = 8.4 Hz), 8.61 (dd, 1H, J1 = 2.4 Hz, J2 = 8.4 Hz), 8.86 (s, 1H), 9.00 (d, 1H, J = 2 Hz), 9.27(d, 1H, J = 2 Hz), 9.47 (brs, 2H), 9.51(brs, 2H), 9.73 (brs, 2H), 9.76 (brs, 2H). 13C NMR (100 MHz, DMSO) δ: 124.5, 126.9, 127.3, 129.6, 131.3, 135, 135.7, 139.3, 140.3, 141.9, 142.8, 147.3, 154.4, 165.8, 167.7. HRMS: m/z calcd. C15H14N7S: 324.1031: found 324.1044. Anal. Calcd for C15H13N7S-2HCl-0.2EtOH-1.6H2O: C, 42.59, H, 4.50, N, 22.59. Found: C, 42.64, H, 4.05, N, 22.20.
2,5-Bis-(3-amidinopyridin-6-yl)thiazole dihydrochloride (5g)
Yield 82%, plae yellow solid, mp >350°C. 1H NMR (400 MHz, DMSO) δ: 8.34 (d, 1H, J = 8.4 Hz), 8.37 (d, 1H, J = 8.8 Hz), 8.42(dd, 1H, J1 = 2.0, J2 = 8.8 Hz), 8.44 (dd, 1H, J1 = 2.4, J2 = 8.4 Hz), 9.07 (d, 1H, J = 1.6 Hz), 9.11(d, 1H, J = 2 Hz), 8.95(s, 1H), 9.49(brs, 2H), 9.54(brs, 2H), 9.77(brs, 2H), 9.82(brs, 2H). 13C NMR (100 MHz, DMSO) δ: 119.3, 120, 123.7, 125.8, 138.0, 138.6, 142.5, 145.1, 145.2, 149.9, 154, 154.1, 163.8, 163.9, 169.4. Anal. Calcd for C15H13N7S-3HCl-0.8H2O-0.3C2H5OH: C, 40.64; H, 4.24; N, 21.26. Found: C, 40.94; H, 4.15, N, 20.88.
General procedure for the synthesis of N-hydroxyamidines
To a solution of hydroxylamine hydrochloride (0.695 g, 10 mmol) in anhydrous DMSO (15 mL) was added t-BuOK (1.12 g, 10 mmol) in a few portions at 5°C and stirred for 30 minutes. To this solution was added the bis-nitrile (1 mmol) and the reaction mixture was stirred overnight. The reaction mixture was poured into ice water (100 mL) and the light yellow precipitate was filtered, washed with water and dried under reduced pressure. The free base was converted to the hydrochloride salt by treatment with ethanol saturated with HCl gas.
General procedure for the synthesis of N-methoxyamidines
A mixture of N-dihydroxyamidine (1 mmol) in DMF (10 mL) was stirred at room temp. A solution of lithium hydroxide monohydrate [LiOH·H2O] (6 mmol) was dissolved in H2O (3 mL) and added dropwise to the reaction mixture and stirring was continued for 30 min. Dimethylsulfate (5 mmol) was added dropwise and the mixture was allowed to stir overnight. The reaction mixture was poured slowly into ice water (100 mL). The precipitate formed was filtered washed with water and dried under reduced pressure. The crude product was purified by column chromatography using a mixture EtOAc/hexane (1:1), (3:1) and (4:1) to give a pale yellow compound. The free base was converted to the hydrochloride salt by treatment with ethanol saturated with HCl gas.
2,5-Bis-(4-N-hydroxyamidinophenyl)thiazole dihydrochloride (6a)
Free base: yield (97%), mp 191–192°C. 1H NMR (400 MHz, DMSO) δ: 5.89(s, 2H), 5.92(s, 2H), 7.74(d, 2H, J = 8.4 Hz), 7.78(d, 2H, J = 8.4 Hz), 7.83(d, 2H, J = 8.4 Hz), 7.97(d, 2H, J = 8.4 Hz), 8.39(s, 1H), 9.76(s, 1H), 9.83(s, 1H). 13C NMR (100MHz, DMSO) δ: 126.3, 126.6, 126.6, 131.5, 133.6, 133.7, 135.5, 139.0, 140.9, 150.7, 166.0.
Salt: Yield (89%), mp>260 °C. 1H NMR (DMSO, 400 MHz) δ: 7.85(m, 6H), 8.16(d, 2H, J=8.4Hz), 8.63(s, 1H), 9.25(bs, 2H). 13C NMR (DMSO, 100MHz) δ: 125.8, 126.8, 126.9, 127.6, 129.5, 129.7, 137.2, 139.1, 141.8, 142.8, 159, 166.1, 167.7. Anal. Calcd for C17H15N5O2S-2HCl-1.4H2O-0.2C2H5OH: C, 45.36; H, 4.59; N, 15.19. Found: C, 45.49; H, 4.40, N, 15.01.
2,5-Bis-(4-N-methoxyamidinophenyl)thiazole dihydrochloride (7a)
Free base: yield, (41%), pale yellow solid, mp 186 °C. 1H NMR (400 MHz, DMSO) δ: 3.77(s, 3H), 3.78(s, 3H), 6.14(s, 2H), 6.17 (s, 2H), 7.76(s, 4H), 7.82(d, 2H, J = 8.8 Hz), 7.98(d, 2H, J = 8.4 Hz), 8.41(s, 1H). 13C NMR (400MHz, DMSO) δ: 61.2, 61.3, 126.3, 126.6, 127.0, 134.0, 141.0, 150.8.
Salt: Yield, (90%), pale yellow solid, mp >230 °C.1H NMR (400 MHz, DMSO) δ: 3.83(s, 3H), 3.84(s, 3H), 7.83(d, 2H, J = 8.4 Hz), 7.87(d, 2H, J = 8.0 Hz), 7.89(d, 2H, J = 7.2 Hz), 8.07(d, 2H, J = 8.4 Hz), 8.53(s, 1H). 13C NMR (100MHz, DMSO) δ: 61.9, 62.8, 119.1, 127.8, 129, 130.5, 134.4, 135.7, 140.7, 142.1, 147.9, 150.9, 155.5, 166.2, 168. Anal. Calcd for C19H19N5O2S-2HCl-1.2H2O: C, 47.94; H, 4.95; N, 14.71. Found: C, 48.26; H, 4.85; N, 14.33.
5-(4-N-hydroxyamidinophenyl)-2(2-N-hydroxyamidinopyridin-5-yl)thiazole dihydrochloride (6b)
Free base: yield, (90%), mp 242 °C. 1H NMR (400 MHz, DMSO) δ: 5.95(s, 2H), 5.93(s, 2H), 7.78(q, 4H, J1 = 8.8, J2 = 14.8 Hz), 8.00(d, 1H, J = 8.4Hz), 8.33(dd, 1H, J1 = 2.4, J2 = 8.4 Hz), 8.47(s, 1H), 9.14(dd, 1H, J1 = 0.8, J2 = 2.4 Hz), 9.77(s, 1H), 10.14(s, 1H). 13C NMR (100 MHz, DMSO) δ: 120.3, 126.7, 129.4, 131.2, 133.9, 134.3, 139.8, 141.1, 146.0, 149.6, 150.6, 151.5, 163.1.
Salt: yield, (93%), yellow solid, mp >220 °C. 1H NMR (400 MHz, DMSO) δ: 7.89(d, 2H, J = 8.8 Hz), 8.00(d, 2H, J = 8.4 Hz), 8.27(d, 1H, J = 8.4 Hz), 8.57(dd, 1H, J1 = 2.4, J2 = 8.4 Hz), 8.69(s, 1H), 9.18(bs, 2H), 9.29(dd, 1H, J1 = 0.4, J2 = 2.0 Hz). 13C NMR (100 MHz, DMSO) δ: 119.3, 123.37, 124.3, 131.5, 135.9, 137.7, 138.7, 143.8, 144.9, 147.6, 149.9, 153.8, 163.9, 168.5. Anal. Calcd for C16H14N6O2S-2HCl-1.95H2O: C, 41.55; H, 4.33; N, 18.17. Found: C, 41.88; H, 4.01; N, 17.83.
5-(4-N-methoxyamidinophenyl)-2-(2-N-methoxyamidinopyridin-5-yl)thiazole dihydrochlodide (7b)
Free base: yield (62.5%), yellow solid, mp 211°C. 1H NMR (400 MHz, DMSO) δ: 3.77(s, 3H), 3.84(s, 3H), 6.15(s, 2H), 6.19 (s, 2H), 7.78(s, 4H), 7.99(d, 1H, J = 8 Hz), 8.35(d, 1H, J = 8 Hz), 8.48(s, 1H), 9.15(s, 1H). 13C NMR (100 MHz, DMSO) δ: 61.2, 61.7, 120.7, 126.7, 127.0, 129.7, 131.6, 133.1, 134.5, 139.8, 141.3, 146.1, 149.45, 150.8, 163.0.
Salt: yield (93%), yellow solid, mp >240 °C. 1H NMR (400 MHz, DMSO) δ: 3.85(s, 3H), 3.87(s, 3H), 7.88(d, 2H, J = 8 Hz), 7.93(d, 2H, J = 7.2 Hz), 8.05(d, 1H, J = 8.4 Hz), 8.39(d, 1H, J = 8.4 Hz), 8.61(s, 1H), 9.18(s, 1H). 13C NMR (100 MHz, DMSO) δ: 62.2, 63.4, 120, 125.2, 127.1, 129, 129.9, 135, 139.2, 142.1, 143.6, 148.4, 151.8, 167.9, 169.9. Anal. Calcd for C18H18N6O2S-2HCl-0.4C2H5OH: C, 47.66; H, 4.76; N, 17.74. Found: C, 47.94; H, 4.66; N, 18.02.
5-(4-N-hydroxyamidinophenyl)-2-(3-N-hydroxyamidinopyridin-6-yl)thiazole dihydrochloride (6c)
Free base: yield (88%), yellow solid, mp 225 °C. 1H NMR (400 MHz, DMSO) δ: 5.89(s, 2H), 6.08(s, 2H), 7.78(s, 4H), 8.15(d, 1H, J = 8.0 Hz), 8.20(dd, 1H, J1 = 2.0, J2 = 8.4 Hz), 8.45(s, 1H), 8.92(d, 1H, J = 2.0 Hz), 9.77(s, 1H), 10.03(s, 1H). 13C NMR (400 MHz, DMSO) δ: 119.0, 126.6, 129.7, 130.5, 131.6, 134.7, 141.0, 141.3, 147.2, 149.0, 150.7, 150.8, 167.3
Salt: yield (86%), yellow solid, mp>220 °C. 1H NMR (400 MHz, DMSO) δ: 7.89(d, 2H, J = 8.4 Hz), 8.04(d, 2H, J = 8.4 Hz), 8.32(d, 1H, J = 8.4 Hz), 8.37(dd, 1H, J1 = 2.4, J2 = 8.4 Hz), 8.70(s, 1H), 9.02(d, 1H, J = 1.2 Hz), 9.33(brs, 2H). 13C NMR (100 MHz, DMSO) δ: 123.2, 126, 127.3, 129.6, 131.5, 135.2, 135.3, 139.7, 142.8, 146.9, 154.2, 159, 163.5, 167.3. Anal. Calcd for C16H14N6O2S-2HCl-2H2O: C, 41.47; H, 4.35; N, 18.13. Found: C, 41.79; H, 3.95; N, 17.74.
5-(4-N-methoxyamidinophenyl)-2-(3-N-methoxyamidinopyridin-6-yl)thiazole dihydrochloride (7c)
Free base: yield (25%), yellow solid, mp 203°C. 1H NMR (400 MHz, DMSO) δ: 3.77(s, 3H), 3.80(s, 3H), 6.14(s, 2H), 6.35(s, 2H), 7.79(q, 4H, J1 = 6.4, J2 = 8.4 Hz), 8,16(d, 1H, J = 8.4 Hz), 8.19(dd, 1H, J1 = 2, J2 = 6.4 Hz), 8.48(s, 1H), 8.90(d, 1H, J = 2 Hz). 13C NMR (100MHz, DMSO) δ: 61.1, 61.2, 119.0, 126.7, 127.0, 129.9, 132.0, 132. 9, 135.2, 141.1, 141.5, 147.4, 149.2.
Salt: yield (87%), yellow solid, mp >200 °C. 1H NMR (400 MHz, DMSO) δ: 3.81(s, 3H), 3.99(s, 3H), 7.81(d, 2H, J = 8.4Hz), 7.95(d, 2H, J = 8Hz), 8.19(d, 1H, J = 8 Hz), 8.22(dd, 1H, J1 = 2, J2 = 8.4 Hz), 8.57(s, 1H), 8.92(s, 1H). 13C NMR (100 MHz, DMSO) δ: 61.9, 64.3, 119, 122.9, 124.4, 128.2, 128.6, 129.9, 135.8, 141.1, 146.4, 147.7, 150.2, 155.4, 164.2, 165.8. Anal. Calcd for C18H18N6O2S-2HCl-1.8H2O-0.2C2H5OH: C, 44.46, H, 5.03; N, 16.90. Found: C, 44.55; H, 4.73; N, 16.59.
2-(4-N-hydroxyamidinophenyl)-5-(2-N-hydroxyamidinopyridin-5yl)thiazole dihydrochloride (6d)
Free base: yield (82%), yellow solid mp 249 °C. 1H NMR (400 MHz, DMSO) δ: 5.58(s, 2H), 5.93(s, 2H), 7.83(d, 2H, J = 8.4 Hz), 7.94(d, 1H, J = 8.4 Hz), 8.00(d, 1H, J = 8Hz), 8.15(dd, 1H, J1 = 2.4 J2 = 8Hz), 8.95(d, 1H, J = 2Hz), 9.84 (s, 1H), 10.05(s, 1H). 13C NMR (100 MHz, DMSO) δ: 120.2, 126.4, 126. 7, 127.5, 133.4, 134. 6, 135.5, 135.7, 142.0, 146.0, 149.6, 150.0, 150.6, 167.0.
Salt: yield (81%), yellow solid, mp>350 °C. 1H NMR (400 MHz, DMSO) δ: 7.92(d, 2H, J = 8.4 Hz), 8.18(d, 1H, J = 8.4 Hz), 8.22(d, 1H, J = 8.4 Hz), 8.42(dd, 1H, J1 = 2.4, J 2= 8.4 Hz), 8.77(s, 1H), 8.73(s, 1H), 9.14(d, 1H, J = 1.6 Hz). 13C NMR (100 MHz, DMSO) δ: 123.5, 127, 129.7, 130.4, 135.5, 135.8, 136.8, 143.8, 144, 147.2, 154.9, 158.9, 166.7, 167. Anal. Calcd for C16H14N6O2S-3HCl-1.0H2O: C, 39.88; H, 3.97; N, 17.44. Found: C, 40.06; H, 4.19; N, 17.04.
2,5-Bis-(2-N-hydroxyamidinopyridin-5-yl)thiazole dihydrochloride (6e)
Free base: yield (93%), yellow solid, mp >200 °C. 1H NMR (400 MHz, DMSO) δ: 5.90(s, 2H), 5.94(s, 2H), 7.94(d, 2H, J = 8.4 Hz), 8.00(d, 2H, J = 8.4 Hz), 8.16(d, 1H, J = 8 Hz), 8.17(d, 1H, J = 8 Hz), 8.58(s, 1H), 8.97(s, 1H), 9.15(s, 1H), 10.07(s, 1H), 10.16(s, 1H). 13C NMR (100 MHz, DMSO) δ: 120.2, 120.4, 127.3, 129.2, 134.7, 136.3, 142.2, 146.1, 146.1, 149.6, 150.2, 151.7, 164.1.
Salt: yield (50%), yellow solid, mp >280 °C. 1H NMR (400 MHz, DMSO) δ: 8.19–8.21(m, 2H), 8.44(dd, 1H, J1 = 2.0, J2 = 8.0 Hz), 8.56(dd, 1H, J1 = 2.0, J2 = 8.4 Hz), 8.77(s, 1H), 9.15(s, 1H), 9.29(s, 1H). 13C NMR (100 MHz, DMSO) δ: 119.5, 121.4, 122, 123.5, 130.3, 131.5, 136, 136.6, 137, 144, 147, 147.3, 155, 165.4, 166.8. Anal. Calcd for C15H13N7O2S-4HCl-0.7H2O-0.2C2H5OH: C, 35.36; H, 3.77; N, 18.76. Found: C, 35.64; H, 3.98; N, 18.47.
2,5-Bis(2-N-methoxyamidinopyridin-5-yl)thiazole dihydrochloride (7e)
Free base: yield (39%), yellow solid, mp 191 °C. 1H NMR (400 MHz, DMSO) δ: 3.83(s, 3H), 3.84(s, 3H), 6.16(brs, 2H), 6.20(brs, 2H), 7.94(d, 1H, J = 8.4 Hz), 8.00(d, 1H, J = 8.4 Hz), 8.18(dd, 1H, J1 = 2.4, J2 = 8.4 Hz), 8.36(dd, 1H, J1 = 2, J2 = 8.0Hz), 8.60(s, 1H, ), 8.98(d, 1H, J = 2.0 Hz), 9.16(d, 1H, J = 2 Hz). 13C NMR (DMSO, 100 MHz) δ: 61.6, 61.7, 120.6, 120.8, 127.7, 129.5, 134.6, 134.8, 136.3, 142.4, 146.2, 149.2, 149.4, 150.8, 164.0.
Salt: yield, (84%), yellow solid, mp >280 °C. 1H NMR (400 MHz, DMSO) δ: 3.83(s, 3H), 3.84(s, 3H), 7.99(d, 1H, J = 8.0 Hz), 8.04(d, 1H, J = 8.4 Hz), 8.22(dd, 1H, J1 = 2.4, J2 = 8.4 Hz), 8.40(dd, 1H, J1 = 2, J2 = 8.4 Hz), 8.64(s, 1H), 9.01(d, 1H, J = 2.4 Hz), 9.18(d, 1H, J = 2.4 Hz). 13C NMR (100 MHz, DMSO) δ: 61.9, 62.7, 121, 127, 128.5, 129.3, 130, 133.8, 134.6, 139.4, 141.9, 146.2, 150, 150.4, 155, 163.7, 164.1. Anal. Calcd for C17H17N7O2S-2HCl-1.0H2O: C, 43.04; H, 4.46; N, 20.67. Found: C, 43.27; H, 4.62; 20.67.
2,5-Bis(3-N-hydroxyamidinopyridin-6-yl)thiazole dihydrochloride (6g)
Free base:yield, (97%), yellow solid, mp >250 °C. 1H NMR (400 MHz, DMSO) δ: 6.03(s, 2H), 6.07(s, 2H), 8.10–8.21(m, 4H), 8.90(d, 1H, J = 1.6 Hz), 8.94(d, 1H, J = 2 Hz), 9.94(s, 1H), 10.03(s, 1H). 13C NMR (100MHz, DMSO) δ: 111.3, 119.2, 119.8, 128.7, 130.6, 134.3, 134.7, 142.3, 142.8, 147.2, 149.0, 150.4, 150.9, 169.3.
Salt: yield, (78%), yellow solid, mp >300 °C. 1H NMR (400 MHz, DMSO) δ: 8.26(dd, 1H, J1 = 2.0, J2 = 8.4 Hz), 8.33(s, 2H), 8.91(s, 1H), 8.95 (d, 1H, J = 2.0 Hz), 9.00(s, 1H). 13C NMR (100 MHz, DMSO) δ: 118.4, 120.2, 120.5, 122.8, 126.3, 128.6, 129.4, 131.6, 144.3, 144.5, 156.7, 160.3, 160.6, 165.8, 168. Anal. Calcd for C15H13N7O2S-2HCl-0.3H2O-0.2C2H5OH: C, 41.76; H, 3.82; N, 22.13. Found: C, 41.86; H, 4.03; 22.01.
2,5-Bis(3-N-methoxyamidinopyridin-6-yl)thiazole dihydrochloride (7g)
Free base:yield (67%), yellow solid mp>280 °C. 1H NMR (400 MHz, DMSO) δ: 3.79(s, 3H), 3.80(s, 3H), 6.30(s, 2H), 6.35(s, 2H), 8.12(d, 2H, J = 1.6 Hz), 8.19–8.22(m, 2H), 8.71(s, 1H), 8.87(s, 1H), 8.92(s, 1H). 13C NMR (100 MHz, DMSO) δ: 61.3, 61.4, 119.2, 119.8, 127.9, 129.9, 134.7, 135.2, 142.3, 147.5, 149.2, 149.3, 150.7, 150.9, 151.3, 163.7, 169.4.
Salt yield: (83%), yellow solid, mp >300 °C. 1H NMR (400 MHz, DMSO) δ: 3.80(s, 3H), 3.98(s, 3H), 8.14–8.21(m, 2H), 8.74(s, 1H), 8.86(s, 1H), 8.93(s, 1H). 13C NMR (100 MHz, DMSO) δ: 62.9, 63.3, 119.3, 120, 123, 125.8, 135.6, 136.9, 140.5, 142.1, 143.7, 148.0, 148.3, 151.9, 152.8, 167.8, 169.6. Anal. Calcd for C17H17N7O2S-2HCl-0.8H2O-0.2C2H5OH: C, 43.54; H, 4.57; N, 20.43. Found: C, 43.26; H, 4.45; 20.25.
Acknowledgement
We thank Guy Riccio of STI for the technical assistance with the T .b .r. animal model. This work was supported by the Bill and Melinda Gates Foundation and by NIH grant AI46365.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Watkins BM. Trends Parasitology. 2003;19:477. doi: 10.1016/j.pt.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 2.Ridley RG. Nature. 2002;414:686. doi: 10.1038/415686a. [DOI] [PubMed] [Google Scholar]
- 3.Schlitzer M. Arch. Pharm. Chem. Life Sci. 2008;341:149. doi: 10.1002/ardp.200700184. [DOI] [PubMed] [Google Scholar]
- 4.Barrett MP, Boykin DW, Brun R, Tidwell RR. Brit. J. Pharmacol. 2007;152:1155. doi: 10.1038/sj.bjp.0707354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barrett MP, Gilbert IH. Adv. Parasitol. 2006;63:125. doi: 10.1016/S0065-308X(06)63002-9. [DOI] [PubMed] [Google Scholar]
- 6.a Tidwell RR, Boykin DW. Dicationic DNA Minor Groove Binders as Antimicrobial Agents. In: Demeunynck M, Bailly C, Wilson WD, editors. Small Molecule DNA and RNA Binders: From Synthesis to Nucleic Acid Complexes. Vol. 2. New York: Wiley-VCH; 2003. pp. 414–460. [Google Scholar]; b Wilson WD, Nguyen B, Tanious FA, Mathis A, Hall JE, Stephens CE, Boykin DW. Current Medicinal Chemistry-Anti-Cancer Agents. 2005;5:389. doi: 10.2174/1568011054222319. [DOI] [PubMed] [Google Scholar]; c Soeiro MNC, de Souza EM, Stephens CE, Boykin DW. Expert Opin Invest Drugs. 2005;14:957. doi: 10.1517/13543784.14.8.957. [DOI] [PubMed] [Google Scholar]; d Dardonville C. Expert Ther. Pat. 2005;15:1241. [Google Scholar]; e Werbovetz KA. Curr. Opin. Invest. Drugs. 2006;7:147. [PubMed] [Google Scholar]
- 7.a Fairlamb AH. Trends Parasitol. 2003;19:488. doi: 10.1016/j.pt.2003.09.002. [DOI] [PubMed] [Google Scholar]; b Bouteille B, Oukem O, Bisser S, Dumas M. Fundam. Clin. Pharmacol. 2003;17:171. doi: 10.1046/j.1472-8206.2003.00167.x. [DOI] [PubMed] [Google Scholar]; b Yeramian PD, Castagnini LA, Allen JA, Umesh L, Gotuzzo E. Efficacy and Safety of DB289, a New Oral Drug for Treatment of Pneumocystis carinii pneumonia (PCP) in AIDS Patients. Presented at the 43rd Annual Interscience Conference on Antimicrobial Agents and Chemotherapy Meeting; September 14–17, 2003; Chicago, IL. [Google Scholar]; c Wenzler T, Boykin DW, Ismail MI, Hall JE, Tidwell RR, Brun R. Antimicrob. Agents Chemother. 2009;53:4185. doi: 10.1128/AAC.00225-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yeramian P, Meshnick SR, Krudsood S, Chalermrut K, Silchamroon U, Tangpukdee N, Allen J, Brun R, Kwick JJ, Tidwell R, Looareesuwan S. J. Infect. Dis. 2005;192:319. doi: 10.1086/430928. [DOI] [PubMed] [Google Scholar]
- 9.Ansede JH, Voyksner RD, Ismail MA, Boykin DW, Tidwell RR, Hall JE. Xenobiotica. 2005;35:211. doi: 10.1080/00498250500087671. [DOI] [PubMed] [Google Scholar]
- 10.Ismail MA, Brun R, Easterbrook JD, Tanious FA, Wilson WD, Boykin DW. J. Med. Chem. 2003;46:4761. doi: 10.1021/jm0302602. [DOI] [PubMed] [Google Scholar]
- 11.Dann O, Fick H, Pietzner B, Walkenhorst E, Fernbach R, Zeh D. Liebigs Ann. Chem. 1975:160. [Google Scholar]
- 12.Das BP, Boykin DW. J. Med.Chem. 1977;20:1219. doi: 10.1021/jm00219a023. [DOI] [PubMed] [Google Scholar]
- 13.Das BP, Wallace RA, Boykin DW. J. Med.Chem. 1980;23:578. doi: 10.1021/jm00179a022. [DOI] [PubMed] [Google Scholar]
- 14.Kumar A, Rhodes RA, Spychala J, Wilson WD, Boykin DW, Tidwell RR, Dykstra CC, Hall JE, Jones SK, Schinazi RK. Eur. J. Med. Chem. 1995;30:99. doi: 10.1016/0223-5234(96)88214-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.a Kumar A, Boykin DW, Wilson WD, Jones SK, Bender BK, Dykstra CC, Hall JE, Tidwell RR. Eur. J. Med. Chem. 1996;31:767. doi: 10.1016/0223-5234(96)83970-5. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Boykin DW, Kumar A, Bajic M, Wilson WD, Bender BK, Hall JE, Tidwell RR. Eur. J. Med. Chem. 1997;32:965. [Google Scholar]
- 16.Nwaka S, Ramirez B, Brun R, Maes L, Douglas F, Ridley R. PLOS Neg. Trop. Dis. 2009;3:1. doi: 10.1371/journal.pntd.0000440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boykin DW, Kumar A, Bender BK, Hall JE, Tidwell RR. Bioorg. Med. Chem Lett. 1996;6:3017. [Google Scholar]
- 18.Fylaktaidou KC, Hadjipavlou-Litina DJ, Litinas KE, Varella EA, Nicolaides DN. Curr. Pharm. Design. 2008;14:1001. doi: 10.2174/138161208784139675. [DOI] [PubMed] [Google Scholar]
- 19.Kumar A, Stephens CE, Boykin DW. Heterocyclic Comm. 1999;5:301. [Google Scholar]
- 20.Batistia-Parra A, Venkitachalam S, Wilson WD, Boykin DW. Heterocycles. 2003;60:1367. [Google Scholar]
- 21.Ismail MA, Arafa RK, Brun R, Wenzler T, Miao Y, Wilson WD, Boykin DW. J. Med. Chem. 2006;49:5324. doi: 10.1021/jm060470p. [DOI] [PubMed] [Google Scholar]
- 22.Chackal-Catoen S, Miao Y, Wilson WD, Wenzler T, Brun R, Boykin DW. Bioorg. Med. Chem. 2006;14:7434. doi: 10.1016/j.bmc.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 23.Dondoni A, Mastellari AR, Medici A, Negrini E, Pedrini P. Synthesis. 1986:757. [Google Scholar]
- 24.Mallena S, Lee MPH, Bailly C, Neidle S, Kumar A, Boykin DW, Wilson WD. J. Am. Chem. Soc. 2004;126:13659. doi: 10.1021/ja048175m. [DOI] [PubMed] [Google Scholar]
- 25.Czarny A, Boykin DW, Wood A, Nunn C, Neidle S, Zhao M, Wilson WD. J. Am. Chem. Soc. 1995;117:4716. [Google Scholar]
- 26.Wilson WD, Tanious FA, Mathis A, Tevis D, Hall JE, Boykin DW. Biochimie. 2008;90:999. doi: 10.1016/j.biochi.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.a Rodger A, Norden B. Circular Dichroism and Linear Dichroism. New York: Oxford University Press; 1997. [Google Scholar]; b Lyng R, Rodger A, Norden B. Biopolymers. 1992;32:1201. doi: 10.1002/bip.360320910. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez F, Rozas I, Kaiser M, Brun R, Nguyen B, Wilson WD, Garcia RN, Dardonville C. J. Med. Chem. 2008;51:909. doi: 10.1021/jm7013088. [DOI] [PubMed] [Google Scholar]
- 29.Thuita JK, Karanja SM, Wenzler T, Mdachi RE, Ngotho JM, Kagira JM, Tidwell RR, Brun R. Acta Trop. 2008;108:6. doi: 10.1016/j.actatropica.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 30.Wilson WD, Tanious FA, Fernandez-Saiz M, Rigl CT. Drug-DNA Interaction Protocols. Evaluation of Drug-Nucleic Acid Interactions by Thermal Melting Curves. In: Fox K, editor. Methods in Mol. Biol. Vol. 90. 1997. p. 219. [DOI] [PubMed] [Google Scholar]
- 31.McCoubrey A, Latham HC, Cook PR, Rodger A, Lowe G. FEBS Lett. 1996;380:73. doi: 10.1016/0014-5793(95)01537-x. [DOI] [PubMed] [Google Scholar]
- 32.Akihiko T, Yumiko K, Hiroyuki K, Itaru T, Manabu Y, Toshihiko N. Chem. Pharm. Bull. 1997;45:1169. [Google Scholar]