Abstract
Apple fruit are well known for their storage life, although a wide range of flesh softening occurs among cultivars. Loss of firmness is genetically coordinated by the action of several cell wall enzymes, including polygalacturonase (PG) which depolymerizes cell wall pectin. By the analysis of ‘Fuji’ (Fj) and ‘Mondial Gala’ (MG), two apple cultivars characterized by a distinctive ripening behaviour, the involvement of Md-PG1 in the fruit softening process was confirmed to be ethylene dependent by its transcript being down-regulated by 1-methylcyclopropene treatment in MG and in the low ethylene-producing cultivar Fj. Comparing the PG sequence of MG and Fj, a single nucleotide polymorphism (SNP) was discovered. Segregation of the Md-PG1SNP marker within a full-sib population, obtained by crossing Fj and MG, positioned Md-PG1 in the linkage group 10 of MG, co-located with a quantitative trait locus (QTL) identified for fruit firmness in post-harvest ripening. Fruit firmness and softening analysed in different stages, from harvest to post-storage, determined a shift of the QTL from the top of this linkage group to the bottom, where Md-ACO1, a gene involved in ethylene biosynthesis in apple, is mapped. This PG–ethylene-related gene has beeen positioned in the apple genome on chromosome 10, which contains several QTLs controlling fruit firmness and softening, and the interplay among the allelotypes of the linked loci should be considered in the design of a marker-assisted selection breeding scheme for apple texture.
Keywords: Apple ripening, cell wall metabolism, ethylene, gene expression profiling, polygalacturonase, QTL mapping
Introduction
Ripening of fleshy fruit comprises a complex series of genetically programmed events leading to edible fruit with desirable aspects (Brummell and Harpster, 2001). However, excessive softening during fruit ripening causes undesirable post-harvest deterioration, limiting storability, transportability, and shelf life (Hadfield and Bennett, 1998; Callahan et al., 2004), with a consequent reduction in commercial value.
Softening involves physiological modification of the cell wall polysaccharide architecture (Fischer and Bennett, 1991). Pectin, one of the major components of the primary cell wall, exists as a wide range of acidic heteropolysaccharides linked together to create a pectin network (Willats et al., 2001). Polygalacturonase (PG), encoded by a multigene family (Sitrit and Bennett, 1998; Atkinson et al., 2002), is well established as one of the major enzymes involved in pectin disassembly by biochemically catalysing the hydrolytic cleavage of α(1–4) galacturonan. PG mRNA accumulation, translation, and enzyme activity is known to be ethylene dependent. A basal level of ethylene is sufficient to induce PG transcription, and its accumulation is directly regulated by the level of the hormone (Brummell and Harpster, 2001).
Another climacteric fruit where cell wall degradation has been extensively examined is peach, which belongs to the Rosaceae family that includes apple. In peach, an initial slow decrease in firmness is typically followed by a brief period of rapid softening known as the melting phase, which coincides closely with the climacteric respiration and ethylene burst (Lester et al., 1996). The physiological phenomenon of melting is known to be associated with PG activity, and non-melting cultivars which soften only gradually to a rubbery texture lack high PG activity (Lester et al., 1994; Callahan et al., 2004). Genetic investigation in peach targeted a PG locus, for which four functional alleles were identified and associated with the phenotypes of freestone/clingstone and melting/non-melting flesh (Peace et al., 2005; Peace and Norelli, 2009). The involvement of PG in fruit ripening has also been reported in other fruit crops, including kiwifruit (Atkinson and Gardner, 1993), capsicum (Rao and Paran, 2003), melon (Hadfield et al., 1998; Rose et al., 1998), pear (Rosas-Cárdenasa et al., 2007), avocado (Kutsunai et al., 1993), tomato (Sitrit et al., 1998), and strawberry (Redondo-Nevado et al., 2001), as well as apple (Atkinson et al., 2002).
A recent functional assessment of four genes related to climacteric ripening in 14 cultivars of apple identified an association between Md-PG1 expression and ripening behaviour (Wakasa et al., 2006). Cultivars characterized by a lower firmness loss (i.e. less softening) during ripening immediately after harvest including Fuji and its female parent Ralls Janet had much lower Md-PG1 expression than normal softening cultivars. Low softening in Fuji can be explained by homozygosity at two ethylene biosynthesis genes (Md-ACS1 and Md-ACO1) for alleles conditioning low ethylene production (Costa et al., 2005). While ethylene was required for Md-PG1 expression, the fruit of four cultivars with normal ethylene levels maintained their firmness like Fj, apparently delayed by unknown modification of Md-PG1 expression. In addition, Fj fruit after 3 months storage had equivalent Md-PG1 expression to low firmness Golden Delicious at harvest, indicating that a delayed Md-PG1 expression accounts for the physiological behaviour of Fj. The authors concluded that softening during ripening may depend on Md-PG1 expression (Wakasa et al., 2006).
Genetic mapping studies in the last decade have reported several quantitative trait loci (QTLs) for fruit texture in the apple genome. QTL intervals are typically defined by simple sequence repeat (SSR) markers (Liebhard et al., 2003; Kenis et al., 2008), and in most cases the genes responsible for these QTLs are not known.
The Md-PG1 expression profile was characterized during ripening of two apple cultivars, Mondial Gala (MG) and Fuji (Fj), as well as in a comparison between normal and ethylene-impaired ripening obtained by 1-methylcyclopropene (1-MCP) treatment.
In this study Md-PG1 has been genetically mapped by a single nucleotide polymorphism (SNP), and its location is presented relative to QTLs for fruit firmness measured at harvest, after 2 months of cold storage, after 30 days (d) of ambient condition ripening following harvest, and for softening after the two ripening periods.
Materials and methods
Plant material and phenotyping
Trees of the two apple cultivars MG and Fj from which fruit and DNA were sampled were located in the Experimental Orchard of the Fruit Tree and Woody Plant Science Department, University of Bologna, Italy. These two cultivars are distinguished by a very different ripening behaviour, particularly related to ethylene production and evolution of firmness. For this reason these two cultivars wer used to create a controlled population comprised of 176 individuals.
Genomic DNA was isolated from these materials using the protocol described in Doyle and Doyle (1989).
Fruit samples from parents and progeny were harvested at the starch value of 7 (on a 1–10 scale, where 10 corresponds to complete starch hydrolysis) during three successive years. In the first year, only the two parent cultivars were harvested to measure fruit firmness (with a digital fruit firmness tester equipped with a 11.2 mm probe; T.R. Turoni s.r.l., Italy) and fruit internal ethylene concentrations (gas chromatography) at ∼5 d intervals of room temperature ripening for a total period of 30 d. Ethylene production was assessed as reported in Costa et al. (2005), measuring the hormone concentration in the headspace of five sealed jars/sample, each jar containing a single fruit. At harvest, a subset of fruit of MG was treated with 1 ppm of 1-MCP for 12 h at room temperature, in a sealed and ventilated container.
In the two following years (year 2 and 3), a cold storage experiment was conducted with fruit from MG, Fj, and the Fj×MG population. Five fruits were phenotyped for fruit firmness at each stage: at harvest and after 2 months of cold storage. In the last year, fruit firmness was also measured for all plant material after 30 d of room temperature ripening immediately after harvest.
Md-PG1 transcript profiling
To evaluate Md-PG1 gene expression, quantitative PCR was performed in MG fruit for five physiological stages in year 1: at harvest, after 6 d of room temperature ripening with and without 1-MCP treatment at harvest, and after 20 d of room temperature ripening with and without 1-MCP. MG fruit were evaluated at all five stages, while Fj fruit were not treated with 1-MCP and were evaluated at harvest and after 20 d. Without an ethylene burst, a measurement at 6 d was considered unnecessary for Fj fruit. At each stage, cortexes of three fruits with skin and seeds removed were mixed, frozen, and stored at –80 °C until needed, whereupon 3 g of tissue was ground in liquid nitrogen.
RNA was isolated using the following buffer: 1 M TRIS-HCl pH 8, 10% SDS, 2.5 M NaCl, 0.5 M EDTA pH 8, 1% (w/v) polyvinylpyrrolidone (PVP), 1% (w/v) polyvinylpolypyrrolidone (PVPP), 0.5 M ascorbic acid, 2.5% (w/v) β-mercaptoethanol, and 1% (w/v) proteinase K, with an equal volume of phenol/chloroform/isoamyl alcohol. Final nucleic acid precipitation was carried out with cold ethanol and 8 M LiCl. A 5 mg aliquot of total RNA was treated with 5 U of DNase I (Gibco-Life Technology). RNA (1 mg) was further retrotranscribed into cDNA with oligo(dT)25, following the SuperScript II Reverse Transcriptase protocol (Invitrogen Life Technology).
Quantitative real-time PCR was performed using SYBR Green RT-PCR master kit (PE Applied Biosystems) and two Md-PG1-specific primers (Md-PG1rt-PCR_for_ACCGGTGGGATAGCAACATC and Md-PG1rt-PCR_rev_ATTCCCTTTAGCTCCAAAAT). Each sample was amplified three times, with 1 μl of cDNA, 0.1 μM of each primer, and 12.5 μl of SYBR Green PCR master mix (in a final volume of 25 μl). Quantitative PCR was carried out with the ABI Prism 7000 Sequence Detection System (Applied Biosystems): 95 °C for 10 min, then 40 cycles of 95 °C for 15 s and 60 °C for 1 min. Expression levels were expressed in terms of 2–ΔΔCt (according to the User Bulletin, PE Applied Biosystems), based on the difference in threshold cycles of Md-PG1 and 18S Ct (primers: 18Sfor, GAGAAACGGCTACCACATCCA; and 18Srev, TCCCCGTGTCAGGATTGG, used as reference).
Md-PG1SNP identification
As a first step towards polymorphism detection for Md-PG1, gene sequences were retrieved from the NCBI database (www.ncbi.nlm.nih.gov) for three related climacteric species: apple (L27743), pear (AB084461), and peach (X77231). Next, conserved regions were identified by sequence alignment, using ClustalW (http://www.ebi.ac.uk/Tools/clustalw/), and primers were designed using Primer3 (http://frodo.wi.mit.edu/primer3/) for conserved regions of the gene across the three species: Md-PGfor_ TCCTTCATACACGGACACCA and Md-PGrev_CCTTCCATGCCTTCACAAAT. PCRs were performed in a final volume of 25 μl with 10× PCR buffer, 1 mM MgCl2, 0.25 mM of each dNTP, 0.2 μM of each primer, 1 U of Taq polymerase (Amersham Pharmacia), and 50 ng of DNA. The amplification thermal profile started with an initial denaturation of 94 °C for 120 s, 32 cycles of 94 °C for 40 s, 60 °C for 45 s, 72 °C for 90 s, with a final extension of 72 °C for 6 min, performed with a PTC-200 Peltier Thermal Cycler (MJ Research). Amplicons from MG and Fj were cloned in a pGEM Easy Vector (Promega) and transformed into Escherichia coli DH5α. Plasmid purification and sequencing were performed by Greenomics™, a facility of the Wageningen University and Research Centre (http://www.greenomics.wur.nl/UK). To provide definitive allelotypes, calling of MG and Fj, sequencing was conducted on three biological replicates (amplicons) of each cultivars.
Md-PG1SNP specific primer design
The above-obtained sequences were aligned and examined for SNPs. For the SNP identified, a specific primer pair was developed for which the 3' end of the forward primer matched the SNP ‘G’ allele of MG. To increase specificity, a directed artificial nucleotide mismatch was included (Drenkard et al., 2000) just before the SNP, substituting the matching G base with a non-matching C. This directed error created just a single mismatch with the targeted ‘G’ allele of MG, which can usually be overcome by polymerase enzymes, and two mismatches to the non-targeted ‘T’ allele of Fj which usually prevents amplification due to the formation of an open structure in the primer during the annealing phase. The primers thus became: Md-PG1for_CGGACACCATAGAAGGTTTAAACG, and Md-PG1rev_GGTTCAACCAAATTAAACGCT. In the forward primer, the underlined base indicates the directed mismatch, while the nucleotide in bold is at the SNP site. The PCR mix was identical to that described above. PCR conditions were slightly modified as a hot-start approach was applied to improve specificity for amplification of the ‘G’ allele. A ‘jump’ thermal profile was adopted, with an initial step of high melting temperature, to allow annealing only in the template DNA with better base matching. The thermal cycling profile was characterized by an initial denaturation of 94 °C for 150 s, four cycles of 94 °C for 45 s, 64 °C for 45 s, 72 °C for 90 s, followed by 34 cycles of 94 °C for 30 s, 60 °C for 45 s, 72 °C for 90 s, and a final extension of 72 °C for 6 min.
Md-PG1SNP and QTL mapping
Md-PG1SNP genetic mapping was performed by testing this marker on 176 seedlings of the Fj×MG population. A linkage map (F Costa et al. unpublished) of this population was created with SSR (Liebhard et al., 2002; Silfverberg-Dilworth et al., 2006) as well as AFLP markers within the HiDRAS EU project (www.hidras.unimi.it). Markers were grouped and ordered using JoinMap 3.0 (Van Ooijen and Voorips, 2001) with a Kosambi mapping function. The LOD threshold for mapping was set at 3.0, and the recombination frequency at 0.45. A visual display of the map was created with MapChart software (Voorips, 2001). QTL mapping of firmness and softening was performed with MapQTL 4.0 software (Van Ooijen et al., 2002). The interval mapping method was initially used to determine map locations and specific markers significantly associated with the traits. Markers associated at P <0.02 after automatic cofactor selection were then used for MapQTL's MQM computation to determine the effects of each significant QTL and remove redundant effects. Genome-wide and chromosome-wide LOD thresholds for QTL significance were calculated by performing 1000 iterations with MapQTL's Permutation Test.
Results
Phenotypic characterization of ‘Mondial Gala’ and ‘Fuji’ fruit ripening
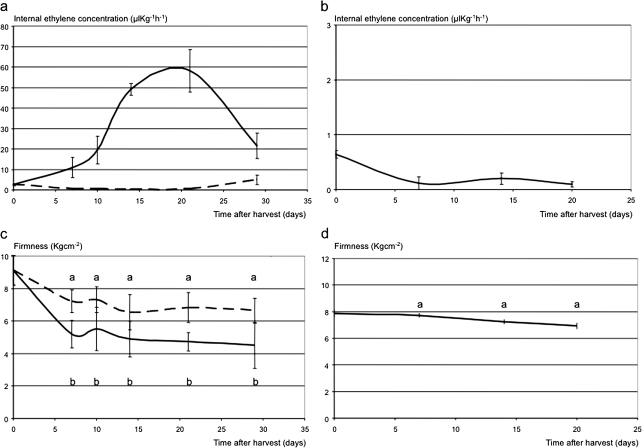
Ethylene production and firmness of MG and Fj fruit demonstrated distinctive patterns over 30 d of room temperature ripening (Fig. 1a, b). At harvest, both cultivars had a minimal level of ethylene production, typical of the first of two phases of ethylene biosynthesis systems (Barry et al., 2000). Only in MG, ethylene production increased dramatically at ∼20 d after harvest to 58 μl kg−1 h−1 (Fig. 1a). After the same period, Fj fruit still produced only 0.10 μl kg−1 h−1, and MG fruit treated with 1-MCP had similarly low detectable ethylene, at 0.45 μl kg−1 h−1. Although collected at the same physiological stage at harvest, MG fruit began with a slightly higher firmness than Fj fruit (9.1 kg cm−2 and 7.9 kg cm−2, respectively; Fig. 1c, d). MG fruit softened by 4.4 kg cm−2 after 20 d to 4.7 kg cm−2, whereas 1-MCP-treated MG fruit softened only half as much, by 2.3 kg cm−2 after 20 d. Even after 20 d of ripening at room temperature, Fj fruit had lost only an average of 0.9 kg cm−2, thus remaining firm (7.0 kg cm−2). This same pattern was observed two seasons later when MG softened from 9.0 kg cm−2 at harvest to 6.5 kg cm−2 after 30 d at room temperature, whereas Fj did not show any loss of firmness (6.4 kg cm−2 at harvest and 6.8 kg cm−2 after ripening).
Fig. 1.
Ethylene evolution and firmness dynamics in Mondial Gala (a, c) and Fuji (b, d) apple fruit. For Mondial Gala, the continuous lines represent the normal ethylene and softening physiology, while the dashed lines indicate the distorted ripening in 1-MCP-treated fruit (a and c). 1-MCP was not applied to Fuji fruit, thus only normal ripening is shown. Standard errors are shown by vertical lines. Points of the ethylene production curves (Mondial Gala control/Mondial Gala 1-MCP-treated/Fuji) characterized by the same letter are not significantly different (LSD test P ≤0,05). In all graphs the days of assessment are reported on the x-axes.
Firmness after 60 days of cold storage, as assessed in two sequential seasons, showed a similar difference between the cultivars. Similar to year 1, MG fruit started at a higher firmness at harvest and showed a stronger softening during storage. MG fruit started at 9.4 and 9.0 kg cm−2 respectively in years 2 and 3, and softened to 7.6 kg cm−2 in both years, thus softening 1.8 kg cm−2 in year 2 and 1.4 kg cm−2 in year 3. In contrast, Fj fruit started at 7.1 and 6.4 kg cm−2, and did not show any significant softening during 60 days of cold storage, ending up at 7.3 and 6.2 kg cm−2.
Md-PG1 expression is ethylene dependent
To reveal the possible involvement of PG in apple climacteric ripening, gene expression was detected by quantitative PCR.
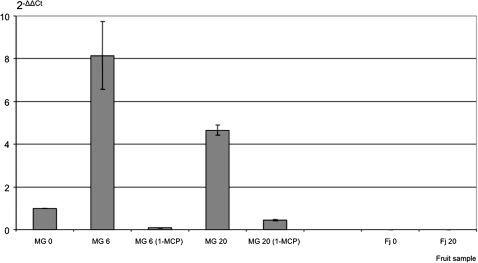
Initially Md-PG1 expression was examined by comparing mRNA accumulation of the two apple cultivars. For MG, three periods were selected: day 0, 6, and 20; while for Fj only day 0 and 20, because for this cultivar an intermediate sample was not necessary, due to the fact that no change in ethylene production was observed, and the firmness slowly decreased during the experiment. Md-PG1 transcription in MG showed a definite peak at day 6 (Fig. 2), coinciding with the onset of increased ethylene production and softening (Fig. 1a). In Fj, which had minimal ethylene production and softening evolution (Fig. 1b), Md-PG1 transcript accumulation was not detectable (Fig. 2). Md-PG1 expression was further analysed in MG treated with 1-MCP. The inhibitory effect was evident in terms of blocked ethylene production as well as the reduced firmness. The impact of 1-MCP on PG was confirmed by the expression analysis carried out in the two MG samples treated at days 6 and 20, where the transcript accumulation was greatly reduced with respect to the control sample.
Fig. 2.
Md-PG1 transcript accumulation in control and treated (1-MCP) fruit samples for Mondial Gala (MG), and non-treated Fuji (Fj) fruit. Standard errors are shown by vertical lines.
Md-PG1 sequence analysis and genome positioning
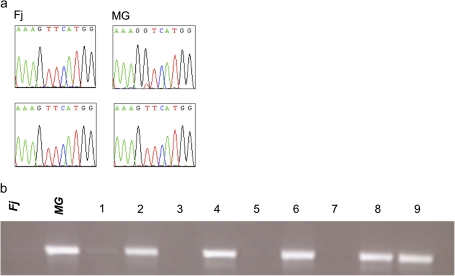
Md-PG1 genomic sequence alignment between Fj and MG revealed the presence of one SNP. The SNP was polymorphic in MG (G/T), while it was monomorphic (T/T) in Fj (Fig. 3a). Domain motif annotation of the Md-PG1 full-length genomic DNA sequence revealed that the SNP was located in the first predicted exon. Amino acid translation revealed a semi-conserved substitution from valine (codon GTC) to phenylalanine (codon TTC).
Fig. 3.
Md-PG1SNP polymorphism in Fuji and Mondial Gala and segregation in their progeny. (a) DNA sequence particulars at the polymorphic SNP site for the parent cultivars. (b) SNP segregation revealed by electrophoresis on agarose gel. The two parental cultivars are indicated with Fj for Fuji and MG for Mondial Gala, while the seedlings are coded with numbers from 1 to 9. (This figure is available in colour at JXB online.)
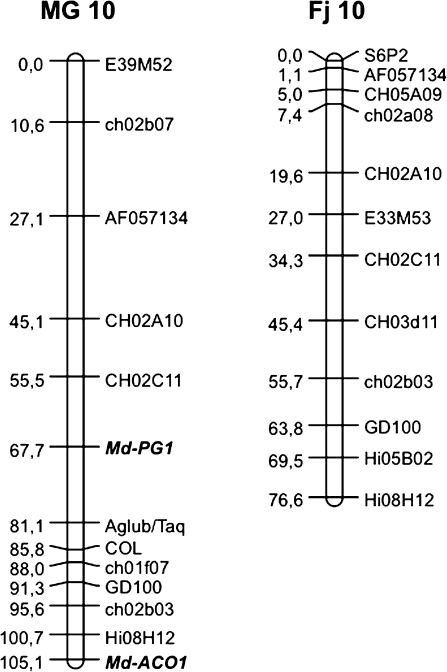
An Md-PG1SNP functional marker was developed for the ‘G’ allele of MG and applied to the Fj×MG population, where it was seen to segregate (Fig. 3b). The presence of the band occurred in individuals sharing the heterozygous Md-PG1 allelotype of MG (G/T), while the absence of amplification occurred in individuals sharing the Fj allelotype (T/T). A 1:1 segregation ratio was observed: 83 G/T and 93 T/T (χ2=0.56 < P=10%). The marker mapped on linkage group (LG) 10 of MG, at 67.7 cM from the top, and 37 cM from the previously mapped candidate gene for ethylene synthesis, Md-ACO1 (Fig. 4).
Fig. 4.
Apple linkage group 10 for Mondial Gala and Fuji developed from the Fj×MG population. For Mondial Gala, two mapped functional markers based on candidate genes are shown in bold: Md-PG1 and Md-ACO1.
QTL identification
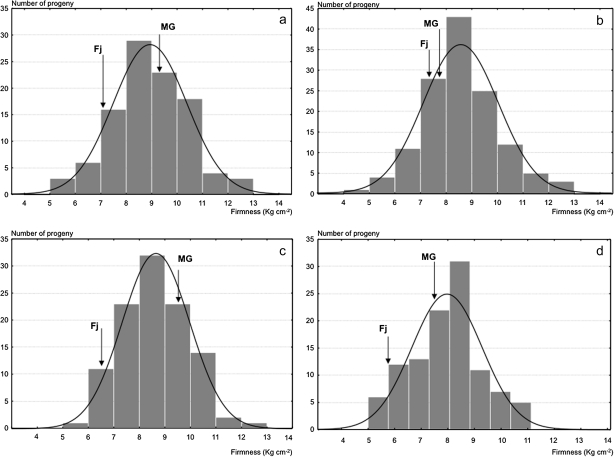
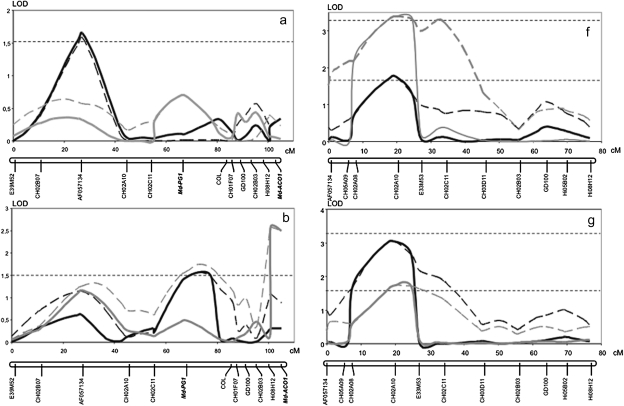
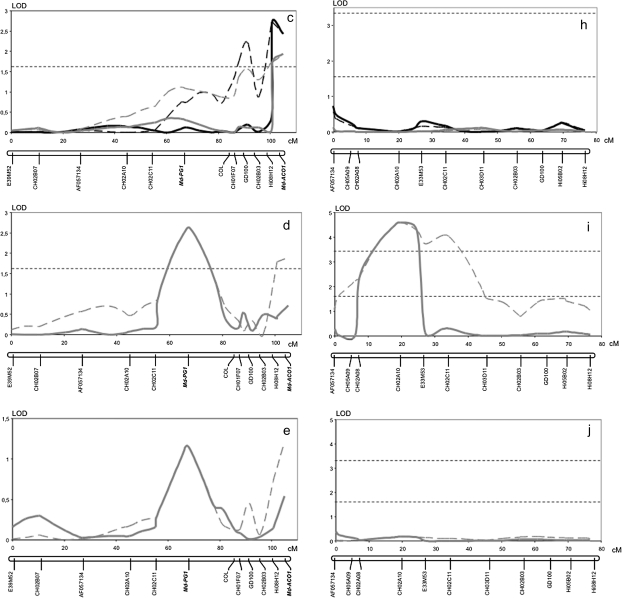
Firmness and softening measured in two years (years 2 and 3) on the 176 fruiting seedlings of the Fj×MG population exhibited quantitative segregation with normal distribution, typical of the traits quantitatively inherited and controlled by several genes (Fig. 5). QTL analysis for these data identified three QTLs for MG and one for Fj in LG10, and not elsewhere in the genomes of the two parent cultivars. In LG10 of MG, significant QTL effects were detected in three distinct regions, with the QTL peaks co-localizing with the marker AF057134 and the genes Md-PG1 and Md-ACO1 (Fig. 6a–e). The QTL on AF057134 was associated with firmness measured at harvest and was just above the LOD threshold (year 2: LOD 1.6, 7.4% variance; Fig. 6a). The QTL on Md-ACO1 for softening was detected after 60 d of cold storage (year 2, LOD 2.4, 11.4% variance; and year 3, LOD 1.94, 8.3% variance; Fig. 6c). This same QTL also tended to show up when firmness was considered instead of softening, but statistical relevance was less evident (Fig. 6b). These data also gave some first indications for a QTL on Md-PG1. The QTL on Md-PG1 was evident for firmness after 30 d of shelf life (LOD 2.62, 10.7%; Fig. 6d). QTLs for softening over this period were not significant, although a peak was observed at the Md-PG1 locus (Fig. 6e).
Fig. 5.
Frequency distributions for fruit firmness measured in the Fuji×Mondial Gala population at harvest for year 2 (a) and 3 (b), and after 2 months of cold storage for year 2 and 3, respectively (c and d).
Fig. 6.
Fruit firmness and softening QTL dynamics over linkage group 10 of Mondial Gala and Fuji. LOD profiles are plotted for Mondial Gala and Fuji with regard to the fruit firmness at harvest (a and f), fruit firmness after 2 months of cold storage (b, g), fruit softening after 2 months of cold storage (c, h), firmness after 30 d of room temperature ripening following harvest (d, i), and relative softening (e, j). Black and grey lines represent year 2 and 3, respectively. Dotted lines indicate the interval mapping computation, and the solid lines show multiple QTL mapping (MQM) profiles. The analysis of firmness and softening measured after 30 d of room temperature was carried out only in year 3. The two horizontal dashed lines at LOD 1.6 and 3.3 indicate chromosome-wide and genome-wide thresholds after a permutation test.
For Fj, just a single QTL was observed, the peak of which co-localized with the CH02a10 SSR marker and was thus located in between the MG QTLs of AF057134 and Md-PG1. This QTL was detected for firmness for each of the three periods (harvest, storage, and shelf life; Fig. 6f, g, and i). No indications for softening-related QTLs were observed (Fig. 6h, j). Absence of the QTL at Md-PG1 and Md-ACO1 for Fj is consistent with their allelic constitution, as Fj is homozygous for both these loci and thus further substantiates these candidate genes as being the causal genes.
Discussion
Fruit firmness changes during apple climacteric ripening
Apple is a common climacteric fruit where the impact of the hormone ethylene on the entire ripening process is well characterized (Yang and Hoffman, 1984; White et al., 1994; Bleecker and Kende, 2000; Giovannoni, 2001; Hrazdina et al., 2003). In this work, climacteric ripening of two apple cultivars, Fj and MG, producing different ethylene levels and ripening responses, was examined. Previous studies have related cultivar differences to allelotypes of two ethylene biosynthesis genes: Md-ACS1 and Md-ACO1. Surveys of apple cultivar panels have associated specific alleles of these genes with fruit ethylene production during ripening, and consequently with the rate of fruit softening (Harada et al., 2000; Oraguzie et al., 2004; Costa et al., 2005). In the present study, fruit were allowed to ripen at room temperature for 30 d or in cold storage for 60 d, and differences in fruit ethylene production and softening between MG and Fj matched expectations for these two cultivars from previous reports. Both cultivars are homozygous for the Md-ACS1 allele associated with lower ethylene production. Fj is also homozygous for the lower ethylene allele of Md-ACO1 while MG is heterozygous, having that allele as well as the normal ethylene allele. Correspondingly, a linear and basal ethylene level was observed over the entire period of measurements, and MG displayed a typical climacteric rise in ethylene production. As expected, MG fruit softened considerably at room temperature or during storage, while Fj fruit did not soften over the ripening periods investigated.
The relationship between ethylene and softening in apple fruit was studied in greater detail by treating MG with the ethylene competitor 1-MCP (Sisler and Serek, 1997), a compound used commercially (SmartFresh™) to delay post-harvest ripening during the storage of some climacteric fruits (Watkins, 2006). This treatment was aimed at eliminating differences in genetic background for ethylene biosynthesis of the two cultivars, highlighting only the effect on fruit firmness of removing the ethylene response during MG fruit ripening. 1-MCP applied once just after harvest produced a severe block in ethylene production for the entire period of monitored fruit ripening, which strongly reduced fruit softening such that MG fruit treated with 1-MCP showed physiological kinetics more similar to those observed in Fj fruit (Fig. 1). In normal ripening of MG, heterozygous for the observed SNP, maximum Md-PG1 transcript accumulation occurred at the beginning of the ethylene burst some time during the 6 d after harvest (Fig. 2) which was also when maximum loss of firmness occurred. Transcription was decreased afterwards but was still elevated at the ethylene peak 14 d later. The regulation of Md-PG1 by ethylene was validated by the comparison between MG and Fj fruit and between 1-MCP-treated and untreated MG fruit. In both cases, reduction in ethylene production caused negative Md-PG1 regulation.
QTL dynamics over apple LG10 and the role of Md-PG1
The genetic locations and effects of different genetic mechanisms for fruit firmness and softening between MG and Fj fruit were examined by candidate gene and QTL analyses, using a segregating population created by crossing these two cultivars. In recent years, targeting polymorphisms within candidate genes has often proven successful for identifying genes controlling traits of interest. For fruit firmness in apple, four functional genetic markers have been developed to help dissect phenotypic variability among cultivars, based on the Md-ACS1, Md-ACS3a, Md-ACO1, and Md-Exp7 genes, located on apple LGs 15, 10, and 1, respectively (Harada et al., 2000; Costa et al., 2005, 2008; Wang et al., 2009). In this study, a new putative functional gene was added to this set: Md-PG1.
Through the strategy of artificial mismatch, Md-PG1SNP was mapped to the central region of LG10 in MG. The mapping of both Md-PG1 and Md-ACO1 on this linkage group, together with phenotypic data for a segregating population resulting from a cross between Fj and MG covering relevant periods of post-harvest ripening, allowed exploration of firmness and softening QTL dynamics over this LG. One QTL, at the distal end of the LG and co-locating with Md-ACO1, was associated with firmness and particularly softening after 60 d cold storage. This QTL segregated in MG only, as expected from the reported allelotype of MG for Md-ACO1. Md-PG1 was particularly associated with a QTL peak for firmness after 30 d of room temperature post-harvest ripening.
Therefore, Md-PG1 appears to be more related to firmness changes occurring during ripening under ambient conditions, whereas Md-ACO1 appears more associated with firmness changes during and after cold storage. This differential association may be attributed to the physiological response of ethylene production at different temperatures. Yang and Hoffman (1984) found that in cucumber, ethylene can be stimulated after a period of chilling. In apple, such ethylene stimulation was observed in Fj (Jobling et al., 1995). In this same cultivar, Wakasa et al. (2006) detected that after 3 months of cold storage at 4 °C the transcript accumulation of four ripening-related genes (Md-ACS1, Md-ACO1, Md-PG1, and Md-Exp3) matched the level of ‘Golden Delicious’ fruit at harvest, a cultivar well known to produce high ethylene levels. Phenotypic responses observed for MG, Fj, and their progeny may therefore result from differential temperature-dependent ethylene regulation of ethylene-responsive genes related to cell wall disassembly, including Md-PG1. The initial ripening inhibition due to the effect of cold temperature, and the subsequent ethylene burst occurring after the restoration of the normal temperature might activate the expression of other genes involved in cell wall metabolism.
In addition to the QTLs associated with Md-PG1 and Md-ACO1, a third novel QTL was detected in MG, positioned near the top of LG10 and coincident with the AF057134 locus. We are not aware of candidate genes in this region, but the prospects of identifying the gene are enhanced by the expected availability of the full apple genome sequence in the near future.
From the Fj genetic background, only one region possibly involved in the control of fruit firmness but not fruit softening was detected, also on LG10. The single Fj-derived QTL was located in the upper part of LG10, where CH02a10 is mapped, with the peak occurring between the loci where the QTLs for firmness at harvest (marked by AF057134) and after 30 d of postharvest ripening (Md-PG1) were identified in MG. This QTL in Fj accounted for firmness measured at all stages: at harvest, after post-harvest ripening in ambient conditions, and after cold storage.
QTLs for firmness were also reported by King et al. (2000) and refined by Maliepaard et al. (2001), using a cross between ‘Prima’ and ‘Fiesta’, which appear to correspond to the Md-PG1 QTL in this study. The other three QTLs in this study therefore correspond to three novel genomic regions influencing various physiological aspects of texture.
The undesirable QTL alleles were associated with the Md-PG1SNP-G allele (lower firmness) and the Md-ACO1-2 allele (greater softening). Costa et al. (2008) and Zhu and Barritt (2008) presented the effects of allelic dosage for Md-ACO1, which were in agreement with the present study as cultivars homozygous for the ‘2’ allele exhibited the greatest softening, and heterozygous individuals were also softer than homozygous ‘1’ allele cultivars. Here, the contrast between ‘1/2’ and ‘1/1’ Md-ACO1 allelotypes was observed. For Md-PG1, the effect of the ‘G’ allele was also only observed in a heterozygous state with the ‘T’ allele, in contrast to the effects of homozygous ‘TT’. To have more complete information about allelic dosage effects for this gene, investigation of a wider range of germplasm is needed.
The relatively small degree of texture phenotypic variance explained in MG×Fj by Md-PG1 (up to 10.7%) and Md-ACO1 (up to 11.4%) is consistent with the hypothesis from tomato studies that apple fruit texture changes require activation of multiple enzymes, where each component exhibits genetic variation among cultivars and can be variously influenced by environmental factors. To identify further functional alleles of Md-PG1 and other LG10 QTLs, and interactions among these and other genomic locations, a large and diverse germplasm set is under investigation in the framework of the HiDRAS project using the pedigree-based analysis approach to integrate analyses across pedigree-linked apple populations (Van de Weg et al., 2005).
In this work Md-PG1 was added to the growing list of functional genes for apple texture. Using an SNP observed in the gene coding sequence, this gene was mapped on LG10 and it was associated with fruit firmness and softening by QTL analysis. Effectively, this study presents the first case of a QTN (quantitative trait nucleotide; Morgante and Salamini, 2003) in apple, the effect of which explains part of the complex cell wall-related genetic variability among apple cultivars. Across LG10 four texture QTLs with a dynamic dependent on fruit ripening conditions were identified. The candidate gene approach proved here to be a useful strategy in determining the likely gene underlying a QTL, but for complex traits a single candidate gene might not be sufficient to dissect the comprehensive phenotypic variability. Five functional genes are associated with apple fruit firmness and softening (Md-ACS1, Md-ACS3a, Md-ACO1, Md-Exp7, and Md-PG1). Together, functional markers for these genes can be used to constitute a first molecular tool kit supporting breeding programmes targeting high firmness and a long storage and shelf life.
Acknowledgments
This study was carried out with financial support from the Commission of the European Communities (contract no. QLK5-CT-2002-01492), Directorate-General Research–Quality of Life and Management of Living Resources Programme. It does not necessarily reflect the Commission's views and in no way anticipates its future policy in this area. Its content is the sole responsibility of the authors. The involvement of Cameron P. Peace was supported by the National Research Initiative of the USDA Cooperative State Research, Education and Extension Service, grant numbers 2005-35300-15463 and 2008-35300-04435.
References
- Atkinson RG, Gardner RC. A polygalacturonase gene from kiwifruit (Actinidia deliciosa) Plant Physiology. 1993;103:669–670. doi: 10.1104/pp.103.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson RG, Schröder R, Hallett IC, Cohen D, MacRae EA. Overexpression of polygalacturonase in transgenic apple trees leads to a range of novel phenotypes involving changes in cell adhesion. Plant Physiology. 2002;129:122–133. doi: 10.1104/pp.010986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry CS, Llop-Tous MI, Grierson D. The regulation of 1-aminocyclopropane-1-carboxylic acid synthase gene expression during the transition from system-1 to system-2 ethylene synthesis in tomato. Plant Physiology. 2000;123:979–986. doi: 10.1104/pp.123.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleecker A, Kende H. Ethylene: a gaseous signal molecule in plants. Annual Review of Cell and Developmental Biology. 2000;16:1–18. doi: 10.1146/annurev.cellbio.16.1.1. [DOI] [PubMed] [Google Scholar]
- Brummell DA, Harpster MH. Cell wall metabolism in fruit softening and quality and its manipulation in transgenic plants. Plant Molecular Biology. 2001;47:311–340. [PubMed] [Google Scholar]
- Callahan AM, Scorza R, Bassett C, Nickerson M, Abeles FB. Deletions in an endopolygalacturonase gene cluster correlate with non-melting flesh texture in peach. Functional Plant Biology. 2004;31:159–168. doi: 10.1071/FP03131. [DOI] [PubMed] [Google Scholar]
- Costa F, Stella S, Van de Weg WE, Guerra W, Cecchinel M, Dallavia J, Koller B, Sansavini S. Role of the genes Md-ACO1 and Md-ACS1 in ethylene production and shelf life of apple (Malus domestica Borkh) Euphytica. 2005;141:181–190. [Google Scholar]
- Costa F, Van de Weg WE, Stella S, Dondini L, Pratesi D, Musacchi S, Sansavini S. Map position and functional allelic diversity of Md-Exp7, a new putative expansin gene associated with fruit softening in apple (Malus × domestica Borkh.) and pear (Pyrus communis) Tree Genetics and Genomes. 2008;4:575–586. [Google Scholar]
- Doyle JJ, Doyle JL. Isolation of plant DNA from fresh tissue. Focus. 1989;12:13–15. [Google Scholar]
- Drenkard E, Richter BG, Rozen S, Stutius LM, Angell NA, Mindrinos M, Cho RJ, Oefner PJ, Davis RW, Ausubel FM. A simple procedure for the analysis of single nucleotide polymorphisms facilitates map-based cloning in Arabidopsis. Plant Physiology. 2000;124:1483–1492. doi: 10.1104/pp.124.4.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer RL, Bennett AB. Role of cell wall hydrolases in fruit ripening. Annual Review of Plant Physiology and Plant Molecular Biology. 1991;42:675–703. [Google Scholar]
- Giovannoni J. Molecular biology of fruit maturation and ripening. Annual Review of Plant Physiology and Plant Molecular Biology. 2001;52:725–49. doi: 10.1146/annurev.arplant.52.1.725. [DOI] [PubMed] [Google Scholar]
- Hadfield KA, Bennett AB. Polygalacturonases: many genes in search of a function. Plant Physiology. 1998;117:337–343. doi: 10.1104/pp.117.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadfield KA, Rose JKC, Yaver DS, Berka RM, Bennett AB. Polygalacturonase gene expression in ripe melon fruit supports a role for polygalacturonase in ripening-associated pectin disassembly. Plant Physiology. 1998;117:363–373. doi: 10.1104/pp.117.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada T, Sunako T, Wakasa Y, Soejima J, Satoh T, Niizeki M. An allele of the 1-aminocyclopropane-1-carboxylate synthase gene (Md-ACS1) accounts for the low level of ethylene production in climacteric fruits of some apple cultivars. Theoretical and Applied Genetics. 2000;101:742–746. [Google Scholar]
- Hrazdina G, Kiss E, Galli Z, Rosenfield C, Norelli JL, Aldwinckle HS. Down-regulation of ethylene production in Royal Gala apples. Acta Horticulturae. 2003;628:239–251. [Google Scholar]
- Jobling J, McGlasson WB. Chilling at 0°C in air induces ethylene production in Fuji and Lady Williams apples. Australian Journal of Experimental Agriculture. 1995;35:651–655. [Google Scholar]
- Kenis K, Keulemans J, Davey MW. Identification and stability of QTLs for fruit quality traits in apple. Tree Genetics and Genomes. 2008;4:647–661. [Google Scholar]
- King GJ, Maliepaard C, Lynn JR, et al. Quantitative genetic analysis and comparison of physical and sensory descriptors relating to fruit flesh firmness in apple (Malus pumila Mill.) Theoretical and Applied Genetics. 2000;100:1074–1084. [Google Scholar]
- Kutsunai SY, Lin A-C, Percival FW, Laties GG, Christoffersen RE. Ripening-related polygalacturonase cDNA from avocado. Plant Physiology. 1993;103:289–290. doi: 10.1104/pp.103.1.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester DR, Sherman WB, Atwell BJ. Endopolygalacturonase and the melting flesh (M) locus in peach. Journal of the American Society for Horticultural Science. 1996;121:231–235. [Google Scholar]
- Lester DR, Speirs J, Orr G, Brady CJ. Peach (Prunus persica) endopolygalacturonase cDNA isolation and mRNA analysis in melting and nonmelting peach cultivars. Plant Physiology. 1994;105:225–231. doi: 10.1104/pp.105.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebhard R, Gianfranceschi L, Koller B, Ryder CD, Tarchini R, Van de Weg E, Gessler C. Development and characterisation of 140 new microsatellites in apple (Malus×domestica Borkh.) Molecular Breeding. 2002;10:217–241. [Google Scholar]
- Liebhard R, Kellerhals M, Pfammatter W, Jertmini M, Gessler C. Mapping quantitative physiological traits in apple (Malus×domestica Borkh.) Plant Molecular Biology. 2003;52:511–526. doi: 10.1023/a:1024886500979. [DOI] [PubMed] [Google Scholar]
- Maliepaard C, Sillanpää MJ, van Ooijen JW, Jansen RC, Arjas E. Bayesian versus frequentist analysis of multiple quantitative trait loci with an application to an outbred apple cross. Theoretical and Applied Genetics. 2001;103:1243–1253. [Google Scholar]
- Morgante M, Salamini F. From plant genomics to breeding practice. Current Opinion in Biotechnology. 2003;14:214–219. doi: 10.1016/s0958-1669(03)00028-4. [DOI] [PubMed] [Google Scholar]
- Oraguzie NC, Iwanami H, Soejima J, Harada T, Hall A. Inheritance of the Md-ACS1 gene and its relationship to fruit softening in apple (Malus × domestica Borkh.) Theoretical and Applied Genetics. 2004;108:1526–1533. doi: 10.1007/s00122-003-1574-8. [DOI] [PubMed] [Google Scholar]
- Peace CP, Crisosto CH, Gradziel TM. Endopolygalacturonase: a candidate gene for Freestone and Melting flesh in peach. Molecular Breeding. 2005;16:21–31. [Google Scholar]
- Peace CP, Norelli JL. Genomics approaches to crop improvement in Rosaceae. In: Folta KM, Gardiner SE, editors. Genetics and genomics of Rosaceae. Vol. 6. Berlin: Springer; 2009. pp. 19–53. [Google Scholar]
- Rao GU, Paran I. Polygalacturonase: a candidate gene for the soft flesh and deciduous fruit mutation in Capsicum. Plant Molecular Biology. 2003;51:135–141. doi: 10.1023/a:1020771906524. [DOI] [PubMed] [Google Scholar]
- Redondo-Nevado J, Moyano E, Medina-Escobar N, Caballero JL, Munoz-Blanco J. A fruit-specific and developmentally regulated endopolygalacturonase gene from strawberry (Fragaria×ananassa cv. Chandler) Journal of Experimental Botany. 2001;52:1941–1945. doi: 10.1093/jexbot/52.362.1941. [DOI] [PubMed] [Google Scholar]
- Rosas-Cárdenas F de F, Valderrama-Cháirez ML, Cruz-Hernández A, Paredes-López O. Prickly pear polygalacturonase gene: cDNA cloning and transcript accumulation during ethylene treatment, cold storage and wounding. Postharvest Biology and Technology. 2007;44:254–259. [Google Scholar]
- Rose JKC, Hadfield KA, Labavitch JM, Bennett AB. Temporal sequence of cell wall disassembly in rapidly ripening melon fruit. Plant Physiology. 1998;117:345–361. doi: 10.1104/pp.117.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silfverberg-Dilworth E, Matasci CL, Van de Weg WE, et al. Microsatellite markers spanning the apple (Malus × domestica Borkh.) genome. Tree Genetics and Genomes. 2006;2:202–224. [Google Scholar]
- Sisler EC, Serek M. Inhibitors of ethylene responses in plants at the receptor level: recent developments. Physiologia Plantarum. 1997;100:577–582. [Google Scholar]
- Sitrit Y, Bennett AB. Regulation of tomato fruit polygalacturonase mRNA accumulation by ethylene: a re-examination. Plant Physiology. 1998;116:1145–1150. doi: 10.1104/pp.116.3.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ooijen JW, Boer JW, Jansen RC, Maliepaard C. MapQTL 4.0, Software for the calculation of QTL position on genetic maps. Wageningen, The Netherlands: Plant Research International; 2002. [Google Scholar]
- Van Ooijen JW, Voorrips RE. JoinMap Version 3.0, Software for the calculation of genetic linkage maps. Wageningen, The Netherlands: Plant Research International; 2001. [Google Scholar]
- Voorrips RE. MapChart version 2.0. Windows software for the graphical presentation of linkage maps and QTLs. Wageningen. The Netherlands: Plant Research International; 2001. [Google Scholar]
- Wakasa Y, Kudo H, Ishikawa R, Akada S, Senda M, Niizeki M, Harada T. Low expression of an endopolygalacturonase gene in apple fruit with long-term storage potential. Postharvest Biology and Technology. 2006;39:193–198. [Google Scholar]
- Wang A, Yamakake J, Kudo H, Wakasa Y, Hatsuyama Y, Igarashi M, Kasai A, Li T, Harada T. Null mutation of the MdACS3, coding for ripening-specific 1-aminocyclopropane-1-carboxylate synthase, leads to long shelf life in apple fruit. Plant Physiology. 2009;151:391–399. doi: 10.1104/pp.109.135822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins CB. The use of 1-methylcyclopropene (1-MCP) on fruits and vegetables. Biotechnology Advances. 2006;24:389–409. doi: 10.1016/j.biotechadv.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Van de Weg WE, Voorrips RE, Finkers HJ, Kodde LP, Meulenbroek EJ, Jansen J, Bink MCAM. Pedigree genotyping: a new pedigree-based approach of QTL identification and allele mining by exploiting breeding material. Acta Horticulturae. 2005;708:483–488. [Google Scholar]
- White MF, Vasquez J, Yang SF, Kirsch JF. Expression of apple 1-aminocyclopropane-l-carboxylate synthase in Escherichia coli: kinetic characterization of wild-type and active-site mutant forms. Proceedings of the National Academy of Sciences, USA. 1994;91:12428–12432. doi: 10.1073/pnas.91.26.12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willats WGT, McCartney L, Mackie W, Knox P. Pectin: cell biology and prospects for functional analysis. Plant Molecular Biology. 2001;47:9–27. [PubMed] [Google Scholar]
- Yang SF, Hoffman NE. Ethylene biosynthesis and its regulation in higher plants. Annual Review of Plant Physiology. 1984;35:155–89. [Google Scholar]
- Zhu Y, Barritt BH. Md-ACS1 and Md-ACO1 genotyping of apple (Malus×domestica Borkh.) breeding parents and suitability for marker-assisted selection. Tree Genetics and Genomes. 2008;4:555–562. [Google Scholar]