Abstract
In angiosperms, a zygote generally divides into a two-celled proembryo consisting of an apical and a basal cell that possess different cell fates. This first division of the zygote is a putative step in the formation of the apical–basal axis of the proembryo. The gamete fusion activates the egg, and the gamete fusion site on the zygote has been reported to provide a possible cue for subsequent zygotic development and/or embryonic patterning in animals and plants. In this study, the gamete fusion site on the rice zygote was labelled by in vitro fertilization of a rice egg cell with a fluorescence-stained sperm cell. The positional relationship between the gamete fusion site and the division plane formed by zygotic cleavage was monitored using a fixed culture of the fusion site-labelled zygote until the two-celled proembryo stage. The results indicate that gamete fusion sites exist on two-celled proembryos with no relation to the position of the first division plane, and that the gamete fusion site on the rice zygote does not function as a determinant for positioning the zygote division plane.
Keywords: Asymmetric division, egg cell, fertilization, rice, sperm cell, zygote
Introduction
In angiosperms, the sporophytic generation is initiated by double fertilization. In this process, one sperm cell fuses with the egg cell and the resultant zygote develops into an embryo, and the central cell fuses with a second sperm cell to form a triploid primary endosperm cell, which develops into the endosperm (reviewed in Sargent, 1900 and in Russell, 1992). It has been shown that the apical cell of the two-celled proembryo produced by zygotic cell division develops into the embryo proper while the basal cell develops into the suspensor and hypophysis (Pritchard, 1964; Schulz and Jensen, 1968; Tykarska, 1976, Schel et al., 1984; Mansfield and Briarty, 1991; reviewed in Lindsey and Topping, 1993). In addition to cytological observations of cleavage of plant zygotes, it has been reported that two putative homeotic genes, WUSCHEL HOMEOBOX2 (WOX2) and WOX8 are specifically expressed in the apical and basal cells of the Arabidopsis two-celled proembryo, respectively, suggesting that the two daughter cells from a zygote possess different transcriptional profiles (Haecker et al., 2004). Moreover, the YODA-dependent MAPKKK signalling pathway (Lukowitz et al., 2004) and the temporal accumulation of a phytohormone auxin via PIN7, an auxin efflux carrier protein (Friml et al., 2003), are thought to be crucial for cell fate specification of the basal and apical cells of the Arabidopsis two-celled proembryo, respectively. Recently, Bayer et al. (2009) indicated that the YODA pathway seems to be activated by the interleukin-1 receptor-associated kinase (IRAK)/Pelle-like kinase that is expressed in sperm cells. These cytological and genetic analyses suggest that a cell division of the zygote is closely related to the cell specification of the two daughter cells and is a step in the formation of the apical–basal axis of the proembryo.
In animals, sperm entry activates the oocyte via cascades of ionic and biochemical events and, in some cases, the sperm entry position provides a cue for subsequent embryonic patterning (Gerhart et al., 1989; Goldstein and Hird, 1996; Bowerman, 1999; Piotrowska and Zernicka-Goetz, 2000). As for plant gamete fusion, Antoine et al. (2000) showed that Ca2+ influx, triggered at the gamete fusion point, propagates in the zygote as a wavefront and that the elevated Ca2+ within the zygote induces a post-fusion event; the rapid formation of the zygotic cell wall. Moreover, using free-living gametes of brown algae, it has been revealed that the sperm entry site functions as positional information for establishing an intermediate default axis of the zygote, which is then overridden by light stimuli that cause the formation of the thallus–rhizoid axis (Kropf, 1997; Hable and Kropf, 2000). These reports suggest that the gamete fusion activates the egg cell, and that the gamete fusion site marks the apical–basal axis orientation of the zygote and proembryo.
The rice in vitro fertilization (IVF) system is a suitable system to elucidate the relationship between the gamete fusion point on the zygote and the position of the cleavage plane in the two-celled proembryo, since the IVF-produced rice zygote divides into an asymmetric two-celled proembryo consisting of a small apical cell with dense cytoplasm and a large basal cell with well-developed vacuoles in a highly similar manner to the zygote within the embryo sac (Uchiumi et al., 2007; Sato et al., 2010). However, since the gamete fusion site cannot be traced using the conventional IVF system, the IVF system requires modification to enable labelling of the gamete fusion site and for subsequent tracing of the fusion site in relation to the first division plane of the zygote.
In the present study, the gamete fusion site on the rice zygote was fluorescence-labelled, and the positional relationship between the gamete fusion site and the zygotic first division plane was monitored to judge whether the gamete fusion point functions as a putative positional cue for the positioning of the zygote cleavage plane.
Materials and methods
Plant materials, isolation of gametes, and IVF
Oryza sativa L. cv. Nipponbare was grown in environmental chambers (K30-7248, Koito Industries, Yokohama, Japan) at 26 °C in a 13/11 h light/dark cycle. Isolation of rice egg and sperm cells and electrofusion-mediated IVF using the isolated gametes were conducted as described by Uchiumi et al. (2007).
Culture of zygotes
Two methods were used for zygote culture. One was a Millicell-based culture (Uchiumi et al., 2007). Briefly, zygotes produced by IVF were transferred onto the membranes of 12-mm-diameter Millicell-CM dishes (Millipore, Billerica, MA, USA). These dishes were placed in 35-mm-diameter plastic dishes filled with 2 ml of N6Z medium (Kumlehn et al., 1998). To these dishes, 50 μl aliquots of a suspended rice cell culture (Line Oc; RIKEN Bio-Resource Center, Tsukuba, Japan) were added as feeder cells. After overnight culture of zygotes at 26 °C in the dark without shaking, cultures were continued with gentle shaking (40 rpm).
The other culture method, ‘in-drop zygote culture’, was used for tracing the fluorescence-labelled fusion point on the zygote during the development and division of the cell. One hundred microlitres of a suspended rice cell culture was added to 35-mm-diameter plastic dishes filled with 2 ml of N6Z medium and cultured at 26 °C with gentle shaking (40 rpm) overnight. Droplets of approximately 5 μl of this conditioned medium were placed on a coverslip covered with mineral oil (Sigma-Aldrich, St Louis, MO, USA). Then, 5–10 cell aggregates of cultured cells were transferred from the cultured dishes into a culture droplet, as mentioned above, under an inverted microscope (Olympus BX-71, Tokyo, Japan). IVF-produced zygotes were transferred into the culture droplet, and cultured at room temperature (22–26 °C). Observations and digital imaging of zygotes and their resulting embryos were conducted using a BX-71 inverted microscope equipped with a cooled charge-coupled device (CCD) camera (Penguin 600CL; Pixcera, Los Gatos, CA, USA) and InStudio software (Pixcera).
Fluorescent labelling of the gamete fusion site
The plasma membrane of isolated sperm cells was stained with concanavalin A lectin conjugated with Alexa Fluor 488 (ConA 488; Invitrogen, Carlsbad, CA, USA) according to Sun et al. (2002) with some modifications. Isolated sperm cells were transferred into mannitol drops (370 mOsmol kg−1 H2O) containing 0.5 μg ml−1 ConA 488. After incubation for 1–2 min, cells were washed by transferring them into fresh mannitol droplets three times and a fluorescent-labelled sperm cell was fused with an egg cell as described above. The resulting zygotes were washed by transferring them into fresh mannitol droplets (450 mOsmol kg−1 H2O) twice and then cultured using a Millicell-based culture or an in-drop culture system as described above. In a Millicell or droplet, one to three zygotes were cultured. Fluorescent observation of the sperm cells or the fusion point on the zygote was conducted using a BX-71 inverted fluorescence microscope with 460–490 nm excitation and 510–550 nm emission wavelengths (U-MWIBA2 mirror unit; Olympus).
Fluorescent staining of zygotes
IVF-produced zygotes were transferred into mannitol drops (450 mOsmol kg−1 H2O) containing 0.5 μg ml−1 ConA 488. After incubation for 1–2 min, cells were moved into mannitol drops containing 10 ng ml−1 FM4-64 (Invitrogen) and then incubated for 5 min. The zygotes were washed by transferring them into fresh mannitol droplets (450 mOsmol kg−1 H2O) twice, and were then incubated in fresh mannitol droplets for 30–60 min and observed with an LSM5 Exciter confocal laser scanning microscope (Carl Zeiss, Jena, Germany) with 488 nm excitation and 505–530 nm emission wavelengths for ConA 488, and with 543 nm excitation and >560 nm emission wavelengths for FM4-64.
Results
Fluorescent labelling of the gamete fusion site on the rice zygote
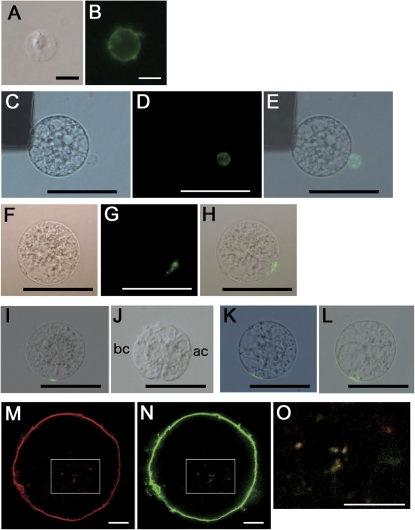
The plasma membrane of isolated sperm cells was stained with concanavalin A conjugated with Alexa Fluor 488 (ConA 488) as described in the Materials and methods (Fig. 1A, B). Subsequent IVF of an egg cell with a labelled sperm cell allowed visualization of the gamete fusion site on the zygote via the fluorescent probe (Fig. 1C–H). However, the fluorescent signal disappeared during culture of the IVF-zygotes into two-celled embryos (Fig. 1I, J). In our rice IVF system, approximately 90% of IVF-zygotes generally divide into two-celled proembryos within 24 h of gamete fusion and 10% remain as single cells (Uchiumi et al., 2007). Interestingly, the ConA 488-derived signal from the fusion site was detectable in the undivided zygotes even after 24 h of culture (Fig. 1K, L). These observations suggest that the fluorescent signal of the gamete fusion site disappeared from cells capable of division, whereas that on zygotes incapable of division remained detectable even after cell culture.
Fig. 1.
Fluorescent labelling of the sperm cell membrane and the gamete fusion site (A–H), traces of the fusion site (I–L), and putative endocytotic vesicles in zygotes (M–O). (A, B) A sperm cell stained with concanavalin A conjugated with Alexa Fluor 488 (ConA 488). (A, B) Bright-field and fluorescent images, respectively. (C–E) Alignment of an egg cell with a ConA 488-stained sperm cell on one of the electrodes under an alternating current field in a fusion droplet. Bright-field and fluorescent images are presented in (C) and (D), respectively. (E) A merged image of (C) and (D). (F–H) A zygote produced by IVF using a sperm cell labelled with ConA 488. Bright-field and fluorescent images are presented in (F) and (G), respectively. (H) A merged image of (F) and (G). (I, J) A fusion site-labelled zygote in (I) was cultured in a Millicell insert, and divided into a two-celled embryo as shown in (J). No ConA 488-derived signal was detectable on the two-celled embryo. Bright-field and fluorescent images are merged. (K, L) A fusion site-labelled zygote in (K) was cultured for 24 h and remained as a single cell, as shown in (L) where a ConA 488 signal from the fusion site can still be observed. Bright-field and fluorescent images are merged. (M–O) A zygote stained with ConA 488 for 1–2 min and FM4-64 for 5 min was observed using confocal microscopy. The fluorescent signals from FM4-64 and ConA 488 are presented in (M) and (N), respectively. Areas enclosed by white squares in (M) and (N) are merged in (O). Abbreviations: ac, apical cell; bc, basal cell. Bars (A, B, M–O)10 μm; (C–L) 50 μm.
The disappearance of the fluorescent signal was investigated next. An IVF-zygote was stained with ConA 488 and FM4-64, a fluorescent probe for plasma membrane and endocytotic vesicles, and was observed after 30–60 min incubation. Small foci derived from FM4-64 or ConA 488 were visible in the zygote (Fig. 1M, N) and fluorescent signals from these foci had mostly merged (Fig. 1O). This suggests that the fusion site disappeared through putative endocytosis-dependent membrane metabolism and/or recycling.
In-droplet culture of zygotes
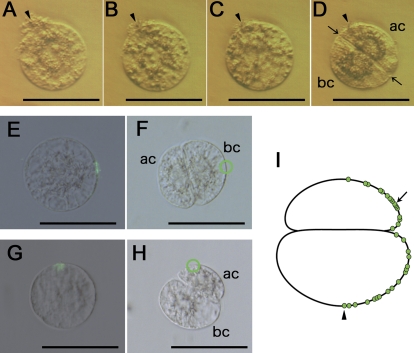
The disappearance of the labelled fusion site during zygote culture indicated that the fusion site could not be traced on two-celled proembryos, which were cultured using the Millicell based procedure. This is partly because both the zygotes and the two-celled proembryos move on the Millicell-membrane during culture. Therefore, an in-drop culture system was established as described in the Materials and methods. During isolation procedures, several egg cells that are attached to cell or tissue debris derived from ovular cells or tissues can be obtained. To check whether the position of the zygote is fixed or not in the droplet during culture, such egg cells were used for IVF and the resulting IVF-zygotes were incubated using the in-drop culture system. Continuous observation of the zygotes revealed that the position of debris on each zygote shifted only slightly during zygote development and division (Fig. 2A–D). The Supplementary Movie 1 at JXB online shows continuous images of development and division of the zygote with debris to illustrate the slightly shifted position of debris on the zygote and its resulting proembryo. While establishing the rice IVF system, it had been observed that addition of Ca2+ to the fusion droplets resulted in attachment of the egg cell to the surface of the coverslip (data not shown). Because the zygote culture medium also contains Ca2+, zygotes can be attached or fixed to the surface of the coverslip via a possible effect of Ca2+.
Fig. 2.
In-drop culture of a zygote (A–D) and plotting of putative gamete fusion sites on two-celled embryos (E–I). (A–D) A zygote attached to cellular or tissue debris was cultured using the in-drop culture system. Images of the zygote or its resulting two-celled embryo cultured for 0, 5, 11, and 18 h after fusion are presented in (A), (B), (C), and (D), respectively. Arrowheads indicate debris on the zygote or two-celled embryo. Arrows mark the first division plane. (E, F) A zygote with the ConA 488-labelled fusion site in (E) was cultured using the in-drop culture system. The zygote divided to produce a two-celled embryo as shown in (F). A putative trace of the gamete fusion site is enclosed by a green circle. Bright-field and fluorescent images are merged in (E). (F) A bright-field image. (G, H) A zygote with the ConA 488-labelled fusion site in (G) was cultured using the in-drop culture system. The zygote divided to produce a two-celled embryo as shown in (H). The green circle indicates a possible trace of the gamete fusion site on the two-celled embryo. (I) A schematic diagram of a two-celled embryo on which possible gamete fusion sites obtained from 33 independent experiments were plotted. An arrowhead and an arrow indicate the plotted fusion sites derived from (F) and (H), respectively. Abbreviations: ac, apical cell; bc, basal cell. Bars, 50 μm.
Relationship between the gamete fusion site on the zygote and position of the division plane in the two-celled proembryo
Fluorescent labelling of the putative gamete fusion site on the IVF-zygote and subsequent in-drop zygote culture allowed the position of the fusion site on the two-celled proembryo to be traced. In one case, the fusion site was traced to the basal cell far from the division plane (Fig. 2E, F), and in another case it was positioned on the apical cell near the division plane (Fig. 2G, H). The position of putative fusion sites from observations of 33 independent fusion site-labelled zygotes and their resultant two-celled proembryos were plotted on a representative two-celled proembryo on the basis of the relative distance from the first cell division plane (Fig. 2I). The sites were positioned randomly, indicating that there is no relationship between the gamete fusion site on the zygote and the position of the division plane in the two-celled proembryo.
Discussion
The present results from monitoring the positional relationship between the gamete fusion site and the first division plane of zygotes suggested two possibilities; that the gamete fusion point does not function as a determinant for positioning the zygote cleavage plane, and that the rice zygote has an intrinsic potential to divide autonomously into an asymmetric two-celled proembryo.
The rice egg cell embedded in an embryo sac possesses cell polarity with cytoplasm, nucleus, and possible starch granules at the micropylar end and vacuoles at the chalazal side (Dong and Yang, 1989; Maeda and Maeda, 1990). The cytological polarity is maintained in the egg cell, which is probably important for zygote development, even after isolation of the cell from the embryo sac (Uchiumi et al., 2007). In planta, the first division plane of the rice zygote is formed parallel to or slightly obliquely to the synergid attaching surface/region and the position of future division planes and the putative apical–basal axis appears to be predetermined in the egg cell (Sato et al., 2010).
In the embryo sac, the egg cell is generally covered with cell wall at the micropylar portion, and the cell wall is incomplete over the chalazal one-third to two-thirds of the cell, exposing a large area of the egg cell plasma membrane adjacent to the synergids and central cell (Jensen, 1965; reviewed in Huang and Russell, 1992; reviewed in Russell, 1993). Regarding the gamete fusion site on the egg embedded in the embryo sac, it has been shown that the egg cell appears to fuse with a sperm cell in the zone where the synergid cells degenerate (reviewed in Dresselhaus, 2006; reviewed in Spielman and Scott, 2008). However, it has remained unclear whether there is a restricted or specific gamete fusion point on the charazal-side plasma membrane of an egg cell located in the embryo sac. It is possible that gamete fusion can occur around the chalazal surface of the egg cell, except for where it is attached to synergids, because the egg and sperm cells exist as hemi-protoplast and protoplasts in the embryo sac, respectively, to allow membrane fusion between gametes. GENERATIVE CELL SPECIFIC 1 (GCS1) was identified as a key male transmembrane protein for the gamete fusion/recognition process (Mori et al., 2006). In the embryo sac, two sperm cells with a gcs mutation remained attached to an egg cell without cell fusion, and, notably, the attachment of the sperm cell was not restricted to the egg cell surface at points of degenerating synergid contact but was randomly observed on the egg cells. These observations may indicate that there is no specific or restricted point on the egg cell surface for gamete fusion. Our IVF study suggested that, even if gamete fusion occurs at any point on the surface of an egg cell, the resulting IVF-zygote can still divide into a two-celled proembryo in a highly similar manner to a zygote within the embryo sac.
Taking these division profiles of IVF-zygotes and zygotes located within the embryo sac into account, we suggest a possibility that, at least in rice, sperm cell fusion is only needed for the activation of the egg cell and the deliverance of the male genome to the female gamete. The resulting zygote then autonomously establishes cell polarity along the apical–basal axis, which is putatively predetermined in the egg cell. The apical–basal axis is then fixed by division of the zygote into a two-celled proembryo.
Supplementary data
Supplementary data can be found at JXB online.
Supplementary Movie S1. An IVF-derived zygote attached to tissue or cell debris was cultured using the in-drop culture system.
Supplementary Material
Acknowledgments
This work was supported, in part, by a Grant-in-Aid from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (No. 21112007 to TO), from the Japan Society for the Promotion of Science (No. 20570206 to TO), and by a Grant for Basic Sciences from the Sumitomo Foundation (No. 060533 to TO). We thank Drs S Hisanaga and A Asada (Tokyo Metropolitan University) for experiments with confocal laser scanning microscopy and RIKEN Bio Resource Center (Tsukuba, Japan) for providing cultured rice cells (Oc line).
Glossary
Abbreviations
- ConA 488
concanavalin A conjugated with Alexa Fluor 488
- IVF
in vitro fertilization
References
- Antoine AF, Faure JE, Cordeiro S, Dumas C, Rougier M, Feijo JA. A calcium influx is triggered and propagates in the zygote as a wavefront during in vitro fertilization of flowering plants. Proceedings of the National Academy of Sciences, USA. 2000;97:10643–10648. doi: 10.1073/pnas.180243697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer M, Nawy T, Giglione C, Galli M, Meinnel T, Lukowitz W. Paternal control of embryonic patterning in Arabidopsis thaliana. Science. 2009;323:1485–1488. doi: 10.1126/science.1167784. [DOI] [PubMed] [Google Scholar]
- Bowerman B. Maternal control of polarity and patterning during embryogenesis in C. elegans. In: Moody SA, editor. Cell lineage and fate determination. San Diego: Academic Press; 1999. pp. 97–117. [Google Scholar]
- Dong J, Yang HY. An ultrastructural study embryo sac in Oryza sativa L. Acta Botanica Sinica. 1989;31:81–88. [Google Scholar]
- Dresselhaus T. Cell–cell communication during double fertilization. Current Opinion of Plant Biology. 2006;9:41–47. doi: 10.1016/j.pbi.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Friml J, Vieten A, Sauer M, Weijers D, Schwarz H, Hamann T, Offringa R, Jürgens G. Efflux-dependent auxin gradients establish the apical–basal axis of Arabidopsis. Nature. 2003;426:147–153. doi: 10.1038/nature02085. [DOI] [PubMed] [Google Scholar]
- Gerhart J, Danilchik M, Doniach T, Roberts S, Rowning B, Stewart R. Cortical rotation of the Xenopus egg: consequences for the anteroposterior pattern of embryonic dorsal development. Development. 1989;107:37–51. doi: 10.1242/dev.107.Supplement.37. [DOI] [PubMed] [Google Scholar]
- Goldstein B, Hird SN. Specification of the anteroposterior axis in Caenorhabditis elegans. Development. 1996;122:1467–1474. doi: 10.1242/dev.122.5.1467. [DOI] [PubMed] [Google Scholar]
- Hable WE, Kropf DL. Sperm entry induces polarity in fucoid zygotes. Development. 2000;127:493–501. doi: 10.1242/dev.127.3.493. [DOI] [PubMed] [Google Scholar]
- Haecker A, Gross-Hardt R, Geiges B, Sarkar A, Breuninger H, Herrmann M, Laux T. Expression dynamics of WOX genes mark cell fate decisions during early embryonic patterning in Arabidopsis thaliana. Development. 2004;131:657–668. doi: 10.1242/dev.00963. [DOI] [PubMed] [Google Scholar]
- Huang BQ, Russell SD. Female germ unit: organization, reconstruction and isolation. International Review of Cytology. 1992;140:233–293. [Google Scholar]
- Jensen WA. The ultrastructure and composition of the egg and central cell of cotton. American Journal of Botany. 1965;52:781–797. [PubMed] [Google Scholar]
- Kropf DL. Induction of polarity in fucoid zygotes. The Plant Cell. 1997;9:1011–1020. doi: 10.1105/tpc.9.7.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumlehn J, Lörz H, Kranz E. Differentiation of isolated wheat zygotes into embryos and normal plants. Planta. 1998;205:327–333. [Google Scholar]
- Lindsey K, Topping JE. Embryogenesis: a question of pattern. Journal of Experimental Botany. 1993;259:359–374. [Google Scholar]
- Lukowitz W, Roeder A, Parmenter D, Somerville C. A MAPKK kinase gene regulates extra-embryonic cell fate in Arabidopsis. Cell. 2004;116:109–119. doi: 10.1016/s0092-8674(03)01067-5. [DOI] [PubMed] [Google Scholar]
- Maeda E, Maeda K. Ultrastructure of egg apparatus of rice (Oryza sativa) after anthesis. Japanese Journal of Crop Science. 1990;59:179–197. [Google Scholar]
- Mansfield SG, Briarty LG. Early embryogenesis in Arabidopsis thaliana. II. The developing embryo. Canadian Journal of Botany. 1991;69:461–467. [Google Scholar]
- Mori T, Kuroiwa H, Higashiyama T, Kuroiwa T. GENERATIVE CELL SPECIFIC 1 is essential for angiosperm fertilization. Nature Cell Biology. 2006;8:64–71. doi: 10.1038/ncb1345. [DOI] [PubMed] [Google Scholar]
- Piotrowska K, Zernicka-Goetz M. Role for sperm in spatial patterning of the early mouse embryo. Nature. 2000;409:517–521. doi: 10.1038/35054069. [DOI] [PubMed] [Google Scholar]
- Pritchard NH. A cytochemical study of embryo development in Stellaria media. American Journal of Botany. 1964;51:472–479. [Google Scholar]
- Russell SD. Double fertilization. International Review of Cytology. 1992;40:357–390. [Google Scholar]
- Russell SD. The egg cell: development and role in fertilization and early embryogenesis. The Plant Cell. 1993;5:1349–1359. doi: 10.1105/tpc.5.10.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent E. Recent work on the results of fertilization in angiosperms. Annals of Botany. 1900;14:689–712. [Google Scholar]
- Sato A, Toyooka K, Okamoto T. Asymmetric cell division of rice zygotes located in embryo sac and produced by in vitro fertilization. Sexual Plant Reproduction. 2010 doi: 10.1007/s00497-009-0129-9. doi:10.1007/s00497-009-0129-9. [DOI] [PubMed] [Google Scholar]
- Schel JHN, Kieft H, van Lammeren AAM. Interactions between embryo and endosperm during early developmental stage of maize caryopses (Zea mays) Canadian Journal of Botany. 1984;62:2842–2853. [Google Scholar]
- Schulz R, Jensen WA. Capsella embryogenesis: the egg, zygote and young embryo. American Journal of Botany. 1968;55:807–819. [Google Scholar]
- Spielman M, Scott RJ. Polyspermy barriers in plants: from preventing to promoting fertilization. Sexual Plant Reproduction. 2008;21:53–65. [Google Scholar]
- Sun M-X, Kranz E, Moscatelli A, Yang H-Y, Lörz H, Cresti M. A reliable protocol for direct detection of lectin binding sites on the plasma membrane of a single living sperm cell in maize. Sexual Plant Reproduction. 2002;15:53–55. [Google Scholar]
- Tykarska T. Rape embryogenesis. I. The proembryo development. Acta Societatis Botanicorum Poloniae. 1976;45:3–16. [Google Scholar]
- Uchiumi T, Uemura I, Okamoto T. Establishment of an in vitro fertilization system in rice (Oryza sativa L.) Planta. 2007;226:581–589. doi: 10.1007/s00425-007-0506-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.