Abstract
The responses to low red light/far-red light (R/FR) ratios simulating dense stands were evaluated in wheat (Triticum aestivum L) cultivars released at different times in the 20th century and consequently resulting from an increasingly prolonged breeding and selection history. While tillering responses to the R/FR ratio were unaffected by the cultivars, low R/FR ratios reduced grain yield per plant (primarily grain number and secondarily grain weight per plant) particularly in modern cultivars. Low R/FR ratios delayed spike growth and development, reduced the expression of spike marker genes, accelerated the development of florets already initiated, and reduced the number of fertile florets at anthesis. It is noteworthy that low R/FR ratios did not promote stem or leaf sheath growth and therefore the observed reduction of yield cannot be accounted for as a consequence of divergence of resources towards increased plant stature. It is proposed that the regulation of yield components by the R/FR ratio could help plants to adjust to the limited availability of resources under crop conditions.
Keywords: Grain yield, phytochrome, red/far-red ratio, shade avoidance, spike, tillering, wheat
Introduction
Wheat crops are grown at high plant densities where mutual shading among plants severely reduces the red light (R) to far-red light (FR) ratio (Holmes and Smith, 1977b; Chelle et al., 2007) and, hence, the proportion of the active form of phytochrome (Holmes and Smith, 1977a). In wheat, a low R/FR ratio reduces tillering (Kasperbauer and Karlen, 1986; Casal, 1988; Evers et al., 2007), a response also observed in other cereals such as barley (Skinner and Simmons, 1993), maize (Maddonni et al., 2002), and sorghum (Kebrom et al., 2006). In wheat, low R/FR ratios can enhance the growth of the leaf sheath and of the short basal internodes, but they actually reduce extension growth of the uppermost internode that carries the spike (Casal, 1988, 1993). The initiation of tiller death in wheat crops has been related to a threshold R/FR ratio (Sparkes et al., 2006), and a low R/FR ratio also has a role in determining rooting traits that affect the anchorage capacity of wheat plants and favour lodging (Sparkes and King, 2008). The phytochrome gene family contains three members—PHYA, PHYB, and PHYC—in grasses (Mathews and Sharrock, 1996; Kulshreshtha et al., 2005). Based on observations in maize (Sheehan et al., 2007), sorghum (Childs et al., 1997), rice (Takano et al., 2005), and barley (Hanumappa et al., 1999), the responses to the R/FR ratio appear to be mediated mainly by phytochrome B.
Part of the significant wheat yield increments over the last century reflects the use of cultivars with higher yield potential as a result of breeding and selection for yield per se (Perry and D'Antuono, 1989; Slafer et al., 1994). This selection has largely been conducted without knowledge of the modified physiological traits that caused the increased yield. Therefore, it has been very informative to compare side by side the physiology of cultivars released to the market at different times and reflecting the trend in yield gain. Following this approach, yield increments of bread wheat have been assigned to a higher harvest index, stability, and resistance to lodging and diseases in modern cultivars (Austin et al., 1980, 1989; Cox et al., 1988; Ledent and Stoy, 1988; Calderini et al., 1995). Modern cultivars show improved survival of floret primordia and a stronger allocation of resources to the growing juvenile spike during the stem elongation phase (Siddique et al., 1989; Slafer and Andrade, 1989).
It is often considered that plant responses to R/FR, although critical for the ability to compete with neighbours under natural conditions, are detrimental for the yield of crops. The idea is that the investment of resources into organs that increase competitive ability (e.g. longer stems or leaf sheaths) reduces the allocation of resources to harvestable organs (e.g. grains and leaves) (Smith, 1995; Schmitt, 1997; Franklin and Whitelam, 2005; Sawers et al., 2005; Kebrom and Brutnell, 2007). In sunflower, low R/FR ratio treatments applied to the stem of plants isolated from nearby neighbours promote stem growth and reduce yield (Libenson et al., 2002). Reducing the response to low R/FR ratios by expressing high levels of phytochrome to retain enough active phytochrome even in densely grown plants increased the harvest index in tobacco (Robson et al., 1996), tuber yield in potato (Boccalandro et al., 2003; Schittenhelm et al., 2004), and grain yield in rice (Garg et al., 2006).
Since increased yield can result from reduced stem growth (Siddique et al., 1989) and a low R/FR ratio reduces peduncle growth in wheat (Casal, 1993), a low R/FR ratio could increase, rather than reduce, yield in wheat. It can also be reasoned that breeding and selection for yield have indirectly reduced the promotion of plant stature, the inhibition of tillering, and the predicted negative impact of these responses on yield in plants exposed to a low R/FR ratio. To test these hypotheses, the responses to R/FR ratios were compared in 10 cultivars that are representative of the breeding and selection process in Argentina during the 20th century and the kinetics of spike growth and development were investigated in plants exposed to different R/FR treatments. The light environment of wheat spikes is also described.
Materials and methods
Growth conditions
All the experiments were conducted at the experimental field of the Faculty of Agronomy, University of Buenos Aires, Argentina (34º 35' S, 58º 29' W). Seeds of wheat (Triticum aestivum L.) were germinated on moistened cotton in Petri dishes incubated at 20 °C under 10 μmol m−2 s−1 of fluorescent white light in a growth chamber. When coleoptile length had reached ∼1 cm, single seedlings were transplanted to 6.0 l pots containing a soil–sand–compost mixture and placed outdoors. Pots were placed in a single row leaving a distance of 12 cm between neighbouring plants (Supplementary Fig. S1A available at JXB online). In all the experiments and in different years, the sowing date was between 25 and 28 June, i.e. within the normal sowing date for commercial crops in the same area. The pots were fertilized with urea before and during tillering (total dose equivalent to 100 kg of nitrogen per hectare). Plants were watered daily during high demand periods. Weeds were periodically removed by hand, and eventual diseases and insect infections were controlled chemically.
Experiments 1 and 2: tillering, plant stature, and yield components
Experiments 1 and 2 were designed to test the hypothesis that the inhibition of tillering and the promotion of plant stature by a low R/FR ratio and the detrimental consequences of these responses on grain yield have been reduced by breeding and selection during the 20th century. In experiment 1, plants were exposed to R, FR, R+FR, or no supplemental light to investigate whether the light treatments were perceived by phytochrome. Klein Cacique, which is the most modern cultivar of the series used here, and the older cultivar Klein Rendidor were used. Kinetic data are plotted against thermal time, using a base temperature of 0 °C. In experiment 2, 10 wheat cultivars were selected to investigate any trend in the magnitude of the responses to the R/FR ratio along with breeding for yield during the 20th century. The plants were exposed to R or FR treatments and the experiment was repeated during 3 years. The cultivars were selected based on the following criteria: (i) successful use in commercial crops in Argentina for at least 7 years (Slafer and Andrade, 1989); (ii) similar duration of the growth cycle; and (iii) availability of seeds to cover the period under study (20th century) as homogeneously as possible. The cultivars (with the year of release to the market shown in parentheses) are the following: Klein Favorito (1920), Eureka F.C.S. (1939), Klein Orgullo (1943), General Roca MAG (1954), Klein Rendidor (1958), Buck Manantial (1964), Buck Pucará (1980), Las Rosas (1984), Pro INTA Pigué (1988), and Klein Cacique (1991). In these cultivars, time to anthesis (102, 104, 100, 91, 96, 99, 98, 97, 98, and 105 d, respectively) did not correlate with the year of release. These cultivars have already been included in other studies of physiological traits modified by breeding and selection during the 20th century (Slafer and Andrade, 1989; Calderini et al., 1995; Appendino et al., 2003). All these cultivars were included in each one of the three different years that the experiment was repeated. In these experiments, the R/FR treatments started immediately after transplant and the number of tillers and expanded leaves were recorded twice a week. Dead tillers, defined by fully senescent leaves, were not counted. The final height of the leaf sheaths, the final length of the leaf lamina, and the length of the internodes were also recorded periodically. Grains were harvested at the end of the cycle and weighed after oven drying at 75 °C for 48 h. The weight of 1000 grains was also recorded and the number of grains per plant was calculated by dividing grain weight per plant by the average grain weight from the 1000 grain sample.
Experiment 3: kinetics of peduncle and spike growth
Experiment 3 was designed to test the hypothesis that since a low R/FR ratio can reduce peduncle (stem) growth, this light condition would favour spike growth by making more resources available for this process. The R/FR treatments started when the first node was visible; the spike and the peduncle were harvested two or three times per week. Peduncle length was measured and then the peduncle and spike were dried at 75 °C for 48 h, before recording their dry weight. Buck Pucará (1980) was selected because experiments with Buck cultivars had shown delayed peduncle growth in response to a low R/FR ratio (Casal, 1988, 1993). This experiment was repeated in two different years.
Experiment 4: spike and floret development
In experiment 4, the R or FR treatments started when the first node was visible and was aimed to investigate whether the differences in grain numbers observed in experiments 1 and 2 were due to effects on floret number and whether floret development was affected. Klein Cacique, which is the most modern cultivar of the series used here, and Buck Manantial, selected because it is an older cultivar within those cultivars still showing both tillering and yield responses in experiment 2, were used. In experiment 4, the number of living florets was recorded in apical, central, and basal spikelets, and florets were assigned a developmental score (Waddington et al., 1993). The results of this experiment were confirmed in an unreported experiment involving less frequent sampling.
Experiment 5: spike transcriptome
Experiment 5 was conducted with plants of Klein Cacique (the cultivar also included in experiments 1, 2, and 4) exposed to R or FR to investigate the changes in gene expression. The spike of the main shoot of wheat plants of cultivar Klein Cacique was harvested on liquid nitrogen during the afternoon (18:00 h) 90 d after transplant (before anthesis). Four spikes were pooled for each one of the three biological replicates used for high and low R/FR ratio conditions. Total RNA was extracted by using the RNeasy Plant Mini Kit (Qiagen). RNA quality was assessed by gel electrophoresis. RNA was processed and hybridized to Affymetrix Wheat GeneChip microarrays following the manufacturer's instructions. The hybridization images were obtained and processed by Affymetrix MAS 5.0. Expression data were normalized to the sum of each microarray (Clarke and Zhu, 2006) and only the genes showing the presence flag for each one of the three replicates of at least one of the two conditions were used for further analysis. Expression data were log10 transformed and used for calculations of probability of true positives or confidence (Grant et al., 2005). The description of the differentially expressed genes was completed by the functional annotation provided by HarvEST: WheatChip Version 1.54, available at http://harvest.ucr.edu/.
To characterize the 117 genes that responded to R/FR treatments, the expression values for these genes in different developmental contexts were obtained from PLEXdb http://www.plexdb.org, experiment TA3 (Schreiber et al., 2009). Log10 intensity data (three replicates per condition) were normalized to the median for each gene across the different stages and grouped by using hierarchical clustering with dchip [http://www.dchip.org/] (Li and Wong, 2003). For comparative purposes, the expression values of a group of 117 randomly selected genes were downloaded.
Light treatments
Natural radiation was supplemented daily with R, FR, or R+FR during 4 h starting during the afternoon and ending ∼15 min after the beginning of full darkness (Casal, 1988). R (10±0.2 μmol m−2 s−1) was provided by 60 W fluorescent tubes (Growlux, Sylvania, Argentina) and red acetate sheets (La Casa del Acetato, Buenos Aires, Argentina). FR (10±0.9 μmol m−2 s−1) was provided by a 60 W internal reflector incandescent lamp (Osram, Buenos Aires, Argentina) filtered with a red acetate sheet and a blue acrylic sheet (Paolini 2031, Buenos Aires, Argentina). R+FR treatment (7+10 μmol m−2 s−1) was provided by sources similar to those described for FR but without the blue acrylic filter. The light sources (all of the same dimensions) were East–West oriented, placed at plant height and towards the South side of the plants to avoid shading the plants (Supplementary Fig. S1A at JXB online). Non-irradiated controls were placed towards the North of mock sources. The spectral distribution of the light provided by the R, FR, and R+FR light was similar to that described earlier (Casal, 1993). The position of the pots was adjusted vertically to ensure that the plants were adequately exposed to the light treatment. The treatments started either immediately after the transplant or at the first visible node stage (∼2 months after sowing) as indicated for each experiment, when plants were randomly assigned to a given light condition and to one of the three light sources of each light quality type placed in the field.
Light measurements
The R and FR treatments were characterized with an R/FR SKR 110 sensor (Skye Instruments Ltd) facing the light sources (Supplementary Fig. S1B at JXB online). To calculate the horizontal integral R/FR ratio received by the plants treated with R or FR only from one side, the sum of R fluence rates measured with the sensor facing towards and against the light source was divided by the sum of FR fluence rates measured with the sensor facing towards and against the light source (Supplementary Fig. S1B, C). Approximately 2 h before sunset, supplementary FR reduced the integral R/FR ratio of light received by the plants from 1.0 (sunlight) to 0.6, and supplementary R increased the integral R/FR ratio to 1.2.
The light within wheat canopies was also measured with the SKR 110 sensor. The sensor was placed facing towards North either within a high density wheat canopy in the field or out of the canopy to characterize the horizontally propagating light that reaches the leaf tissues that surround the spike.
The light received by the spike inside the tube formed by the surrounding leaf sheaths was recorded with a fibre optic of 0.5 mm diameter (Poly-Optics Inc.) connected to the tip (after removing the cosine corrector) of the fibre optic probe of a spectroradiometer (Ocean Optics Inc.). Plants were harvested and immediately taken to the laboratory. Some of the plants were dissected to identify the position of the spike. In the remaining shoots the free end of the fibre optic was transversely introduced into the leaf sheath tube at the position of the spike. The shoots were illuminated from one side with an incandescent lamp facing the tip of the fibre optics (Fig. 1D) and the results of repeated scans were averaged and divided by the light received by the tip of the fibre optic in the absence of the shoot.
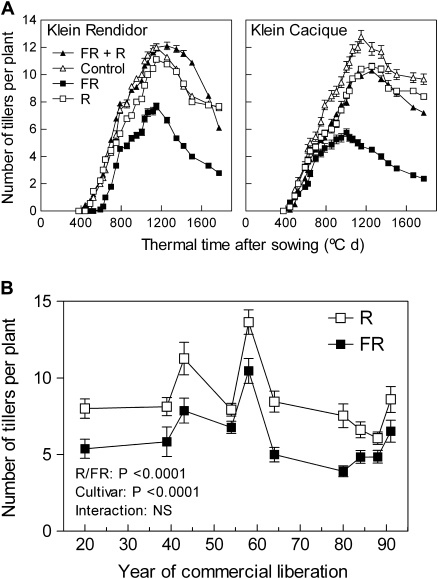
Fig. 1.
Tillering responses to the R/FR ratio perceived by phytochrome in wheat cultivars released to the Argentinean market at different times during the 20th century. (A) Time course of tiller number per plant in two wheat cultivars (Klein Rendidor and Klein Cacique, released to the market in 1958 and 1991. respectively). Supplementary FR reduced tillering compared with the non-light-supplemented controls (P <0.001). Supplementary R, which did not increase tillering when compared with the control, significantly reversed the inhibition caused by supplementary FR when given simultaneously with FR (P <0.001), according to factorial ANOVA (genotype, R/FR) and Bonferroni post-tests for day 79. Data are means and SE of 10 plants (experiment 1). This R/FR reversal indicates phytochrome perception. (B) A low R/FR ratio significantly reduces the number of tillers per plant compared with a high R/FR ratio and this effect is similar in different cultivars tested here and released in Argentina during the 20th century. Data correspond to day 64 (when plants were close to the maximum number of tillers and before the beginning of significant tiller death) and are means and SE of 15 plants from three different years (five plants per year) (experiment 2). Data were analysed by factorial ANOVA (genotype, R/FR, year, Supplementary Table S1 at JXB online). Treatments started at the coleoptile stage.
Results
Tillering and plant stature responses to the R/FR ratio in wheat cultivars released during the 20th century
Figure 1A shows the time course of tiller number per plant in two cultivars. Supplementary FR significantly reduced tillering compared wiht supplementary R or non-light-supplemented controls. Supplementary R did not promote tillering compared with the non-light-supplemented controls but adding R to the FR supplement back-reversed the inhibition caused by FR, indicating that the effect of FR is mediated by phytochrome (Fig. 1A). R was considered a safer high R/FR ratio control than no light supplement because although plant–plant interference was minimal in the present setting, weak FR reflection on distant neighbours could affect plant responses in a very sensitive cultivar and R would correct this eventual deviation.
Figure 1B shows the number of tillers per plant 64 d after sowing (when plants were close to the maximum number of tillers and before the beginning of significant tiller death) for the 10 cultivars plotted against the year of commercial release of these materials to the market. Data are the average of three different years, and factorial analysis of variance (ANOVA) indicates significant effects of year of the experiment, cultivar, and the ratio (Supplementary Table S1 at JXB online). No significant interaction between cultivar and light condition was observed, indicating that the reduction of tillering caused by a low compared with a high R/FR ratio is similar in the cultivars with different breeding and selection history used here. Despite differences in tillering among years, data corresponding to one of the 3 years demonstrate that the pattern of cultivar response to the R/FR ratio is not an artefact of averaging different years (Supplementary Fig. S2 at JXB online).
Low R/FR ratios did not increase plant stature.
The height of the leaf sheaths was unaffected by the R/FR ratio (Supplementary Fig. S3 at JXB online) and this agrees with previous observations where a promotion of leaf growth was observed only for late sowing dates (Casal, 1988). A significant negative correlation between the position of the uppermost node and the year of liberation to the market was observed, indicating (as expected) that modern cultivars are shorter (Supplementary Fig. S4 at JXB online). The position of the uppermost node was unaffected by the R/FR ratio, i.e. the plants exposed to low R/FR were not taller than those grown under high R/FR (Supplementary Fig. S4).
Yield responses to the R/FR ratio in wheat cultivars released during the 20th century
The plants used in the aforementioned experiments were taken to maturity and harvested to investigate the effects of the R/FR ratio on yield components. Supplementary FR reduced grain yield per plant compared with supplementary R or non-light-supplemented controls (Fig. 2A). The treatment where R was given simultaneously with FR produced a significantly higher yield than the FR supplement. This R-reversal of the action of FR indicates the control of yield by phytochrome (Fig. 2A).
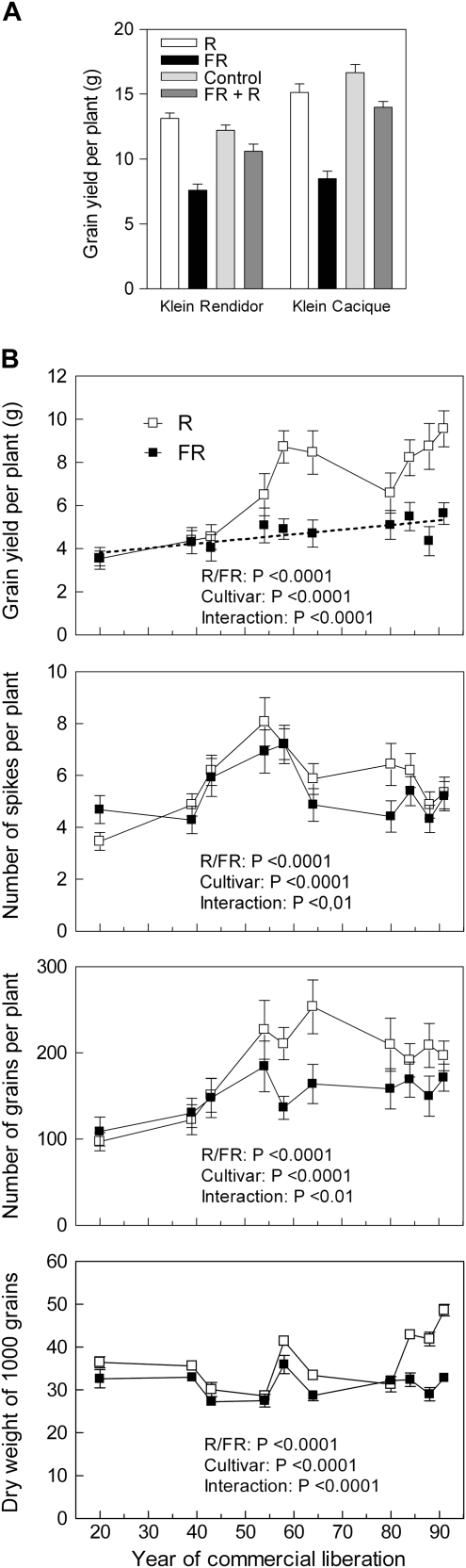
Fig. 2.
Yield component responses to the R/FR ratio perceived by phytochrome in wheat cultivars released to the Argentinean market at different times during the 20th century. (A) Grain yield per plant in two wheat cultivars (Klein Rendidor and Klein Cacique released to the market in 1958 and 1991, respectively). Supplementary FR reduced yield compared with the non-light-supplemented controls (P <0.001). Supplementary R, which did not significantly increase yield when compared with the control, significantly reversed the inhibition caused by supplementary FR when given simultaneously with FR (P <0.001), according to factorial ANOVA (genotype, R/FR) and Bonferroni post-tests. Data are means and SE of 10 plants (experiment 1). This R/FR reversal indicates phytochrome perception. (B) A low R/FR ratio significantly reduces grain yield per plant compared with a high R/FR ratio and this effect is not reduced in more modern cultivars released in Argentina during the 20th century. The same is true for the number of spikes per plant, the number of grains per plant, and the weight of 1000 grains. Data are means and SE of 15 plants from three different years (five plants per year) (experiment 2). Data were analysed by factorial ANOVA (genotype, R/FR, year, Supplementary Table S1 at JXB online). The dotted line in (b) shows the linear regression of yield per plant against year of commercial liberation for low R/FR plants (y=3.38+0.0215x, P <0.01). The high R/FR-treated plants also show a significant linear component (y=2.19+0.0772x, P <0.005). Treatments started at the coleoptile stage.
Figure 2B shows the yield components for the 10 cultivars, and the data are the average of three different years of experimentation. For low R/FR-treated plants, the yield per plant increased steadily with the year of release to the market (Fig. 2B). The average rate is a 0.6% increase per year, which is very similar to the rate obtained in the field (i.e. under a low R/FR ratio) with cultivars released over the same period (Slafer and Andrade, 1989; Calderini et al., 1995). A low R/FR ratio significantly reduced yield per plant, except in the oldest wheat cultivars of this series (Fig. 2B, Supplementary Fig. S2 at JXB online).
The number of grains per plant was significantly reduced by a low R/FR ratio (Fig. 2B). The number of grains per plant is the component that most closely follows the pattern of grain yield across cultivars and light conditions (R2=0.69, P <0.0001), followed by the weight of 1000 grains (R2=0.49, P=0.0006), while spike number was not significantly correlated with grain yield (R2=0.14, P=0.11). It is interesting to note that the R/FR ratio had a significant effect on averaged individual grain weight (Fig. 2B), which is a remarkably stable component (Slafer et al., 1994; Sadras, 2007). The effect was particularly obvious for the most modern cultivars, which come from different seed companies. In some years, the plants exposed to a high R/FR ratio retained more fertile tillers than the plants exposed to a low R/FR ratio (last date in Fig. 1A, experiment 1) but this effect was not very stable. Actually, the effect of the R/FR ratio on the number of spikes per plant showed a strong interaction between cultivar, R/FR condition, and year of experiment (Fig. 2B, experiment 2, P <0.0001, Supplementary Table S1 at JXB online). Since in many cultivars a high R/FR ratio, compared with a low R/FR ratio, increased grain number but not spike number and in those cases where a high R/FR ratio did increase spike number the additional spikes were small and contributed little to grain yield, the differences in number of grains per plant were largely due to differences in the number of grains per spike. Given the significant impact of the R/FR ratio on grain number, the subsequent experiments reported here concentrate on the pre-anthesis phase of spike development, when the number of grains is defined (Fischer, 1984).
Low R/FR ratios delay spike and stem growth
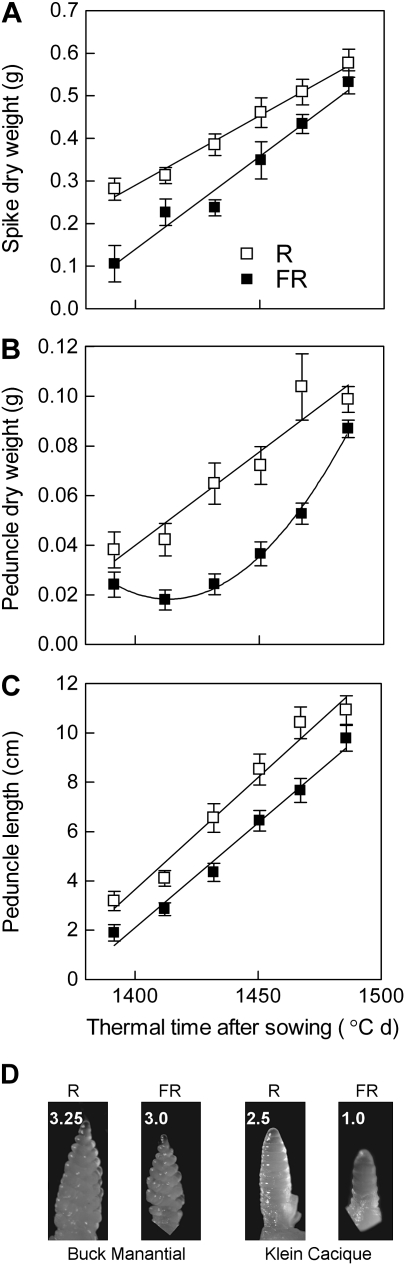
In contrast to the typical acceleration of stem growth by a low R/FR ratio observed in many species, the uppermost internode (peduncle, which spans from the uppermost node to the base of the spike) of wheat shows delayed extension growth under low compared with high R/FR ratios perceived by phytochrome (Casal, 1993). To test whether delayed peduncle growth caused increased spike growth by reducing competition for resources under a low R/FR ratio, plants of wheat grown outdoors were frequently harvested during the pre-anthesis phase of spike development in the main shoot, in two different years. High, compared with low R/FR ratios increased spike dry weight, peduncle dry weight, and peduncle length (P <0.0001 of R/FR main effect in the factorial ANOVA including R/FR and harvest time, Fig. 3). The analysis of regression indicates that the difference in spike dry weight was already established at the beginning of the period of observation, and the subsequent rate of growth was actually higher in plants exposed to a low R/FR ratio (slopes significantly different at P <0.05 in Fig. 3A). Peduncle dry weight accumulation was delayed in plants exposed to low, compared with high R/FR ratios, but a low R/FR ratio accelerated peduncle dry matter gain at later stages [a quadratic term was significant (P <0.0001) only for plants exposed to a low R/FR ratio, Fig. 3B]. Peduncle length increments were also delayed in a low, compared with a high R/FR ratio (parallel lines in Fig. 3C). Therefore, the plants exposed to a high R/FR ratio accumulated more dry matter both in the peduncle and in the ear at early stages of the growth of these organs, but higher rates of dry matter accumulation in plants exposed to low R/FR ratios at least partially compensated these differences at later stages. Low R/FR ratios also caused some delay of spike development (Fig. 3D).
Fig. 3.
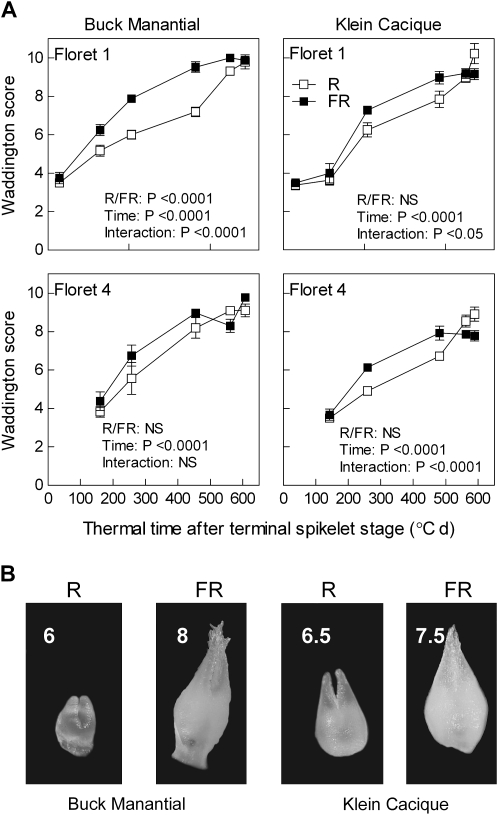
Low, compared with high, R/FR ratios delay peduncle (uppermost internode) and spike growth in wheat plants of the cultivar Buck Pucará (year of commercial release: 1980). Spike dry weight (A), peduncle dry weight (B), and peduncle length (C) are plotted against thermal time after sowing. At 1370 °C days the spikes were detected by touch (∼2 cm length). Each datum point is the mean and SE of eight different plants (destructive measurements) from two different years (four plants per point, per year) (experiment 3). Data were subjected to factorial ANOVA (time, R/FR) and regression analysis. (D) Representative spikes (the Waddington score is indicated) from 49-day-old plants from experiment 4 with cultivars Buck Manantial and Klein Cacique (year of commercial release: 1964 and 1991, respectively). Treatments started at the first visible node stage (i.e. after the transition to the reproductive phase).
Low R/FR ratios reduce the number of fertile florets at anthesis
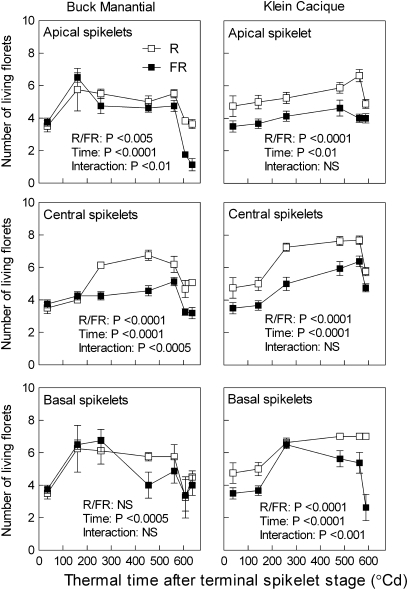
The number of developing florets was recorded in upper, central, and lower spikelets of the main shoot spikes between the terminal spikelet stage and anthesis. For all these positions and cultivars except basal spikelets of Buck Manantial, low R/FR ratios reduced the number of florets at anthesis (final harvest, Fig. 4). The R/FR ratio apparently affected both floret differentiation and floret decay. For instance, in the upper and central spikelets of the cultivar Klein Cacique, the effect of the R/FR ratio was already present very soon after the terminal spikelet stage (Fig. 4), when floret death is not taking place. Therefore, the R/FR ratio is affecting the differentiation of florets. Conversely, for the apical spikelets of Buck Manantial and the basal spikelet of Klein Cacique, the decay in floret number was steeper in low R/FR ratio-treated plants [there is a significant (P <0.01) interaction between the R/FR ratio and harvest time for the last three harvests, indicating that the effect was larger for the last harvest; Fig. 4]. This steeper decay took place when new florets were no longer differentiating, indicating that the R/FR ratio also affects the rate of floret death.
Fig. 4.
Low, compared with high, R/FR ratios reduce the number of fertile florets per spikelet in wheat plants of the cultivars Buck Manantial and Klein Cacique (year of commercial release: 1964 and 1991, respectively). Spikes were harvested at different times after terminal spikelet stage and the florets were counted in upper, central, and lower spikelets. Each datum point is the mean and SE of five different plants (destructive measurements, experiment 4). Data were subjected to factorial ANOVA (time, R/FR). Treatments started at the first visible node stage.
Low R/FR ratios accelerate floret development
Low R/FR ratios significantly accelerated the development of the basal floret (floret 1) and a more distant floret (floret 4) from central spikelets of the main shoot spike (Fig. 5). Floret 4 of plants of the cultivar Klein Cacique grown under a low R/FR ratio interrupted their developmental progression before reaching Waddington stage 9–10. These florets are likely to die before reaching anthesis.
Fig. 5.
Low, compared with high, R/FR ratios accelerate floret development in central spikelets of wheat spikes of the cultivars Buck Manantial and Klein Cacique (year of commercial release: 1964 and 1991, respectively). (A) Spikes were harvested at different times after the terminal spikelet stage, and the status of the florets (Waddington score) was recorded in central spikelets. Each datum point is the mean and SE of five different plants (destructive measurements, experiment 4). Data were subjected to factorial ANOVA (time, light). (B) Ovaries of representative basal florets (the Waddington score is indicated) harvested 14 d after the terminal spikelet stage. Treatments started at the first visible node stage.
Changes in gene expression in the spike of plants exposed to different R/FR ratios
Since low R/FR ratios delayed spike growth and development and reduced the number of fertile florets at anthesis, it was decided to investigate whether the spike transcriptome was affected. Plants of wheat of the cultivar Klein Cacique were grown outdoors and transferred to the low R/FR treatment when the first internode was visible (58 d after the transplant). Other plants were randomly assigned to the control condition, exposed to a high R/FR ratio. The spike was harvested during the afternoon (18:00 h) 32 d later (before anthesis), when the length of the spike was 9.7±0.2 cm (mean ±SE of 12 spikes) in the high R/FR ratio condition and 8.7±0.1 cm in the low R/FR ratio condition (P <0.0001). The length of the peduncle was 10.7±0.4 cm under the high R/FR ratio and 6.6±0.5 cm under the low R/FR ratio (P <0.0001). A total of 117 genes showing a confidence value (Grant et al., 2005) >0.8 were identified (Supplementary Table S2 at JXB online). All these genes showed higher expression under high than under low R/FR ratios.
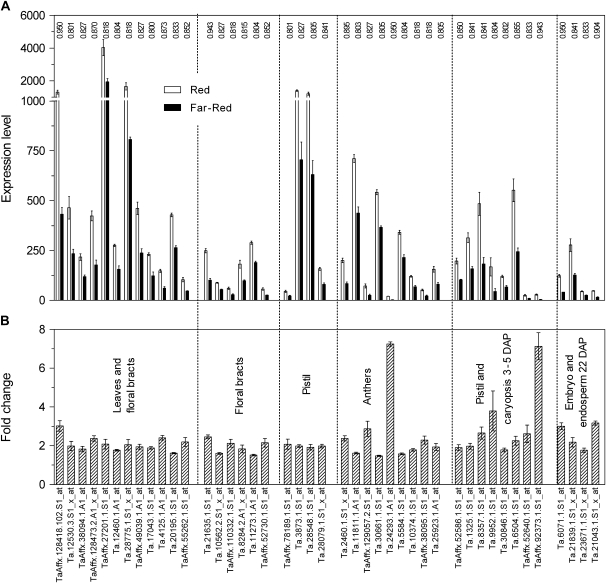
To investigate the functional nature of the genes affected by the R/FR ratio, their expression values were downloaded from a publicly available wheat expression atlas involving different developmental contexts (Schreiber et al., 2009). The genes were then grouped in clusters according to their expression patterns. A large proportion of the genes affected by the R/FR ratio (43 of 117) showed expression mainly in reproductive organs (Supplementary Fig. S5 at JXB online). These include leaf and floral bract, floral bract, pistil, anther, pistil and caryopsis, and embryo and endosperm genes (Supplementary Figs S5 and S6 at JXB online). For comparative purposes, the expression values of a set of 117 randomly selected genes were also downloaded, and the latter included only 13 genes expressed mainly in reproductive organs (Supplementary Fig. S6 at JXB online), indicating that the genes expressed mainly in reproductive organs are over-represented among those affected by the R/FR ratio (χ2 Fischer's exact test, P <0.0001). On average, the expression of genes of significantly affected reproductive organs was 2.4 times higher under high, compared with low R/FR ratios (Fig. 6). In addition to these spike-enriched genes, three ATP synthase genes, two photosystem II-related genes, and a plastidic fibrillin (Monte et al., 1999) were also promoted by high compared with low R/FR ratios and might be related to the impending emergence of the spike.
Fig. 6.
Low, compared with high R/FR ratios, reduce the expression of genes that are expressed mainly in the spike. (A) Absolute expression values in the spike of high and low R/FR-treated plants. (B) Ratio between the expression under high and low R/FR ratios. Data are the means and SE of three biological replicates from experiment 5. The confidence levels (Grant et al., 2005) are indicated in (A).
The R/FR ratio received by the spike and its surrounding leaf tissues in wheat crops
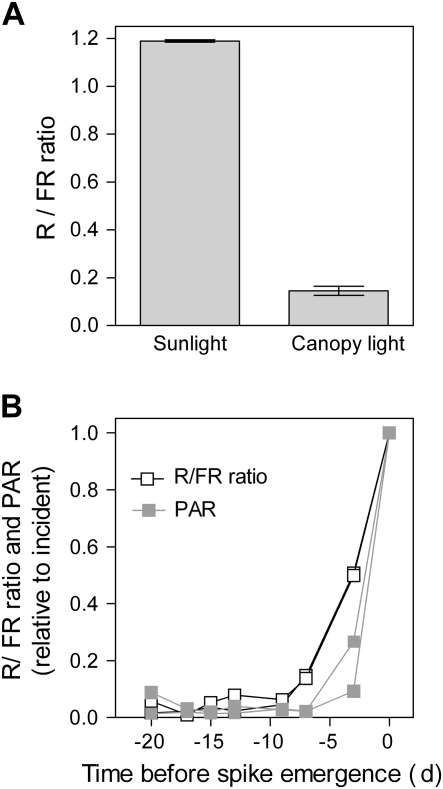
Given the aforementioned effects of the R/FR ratio on spike growth and floret development, the R/FR signals experienced by the leaf tissues surrounding the spike were investigated in adjacent wheat canopies sown at commercial densities (240 plants m−2). When the peduncle is starting to elongate, the leaf sheath tissues that surround the spike (located 10–20 cm beneath the top of the canopy) receive a low R/FR ratio caused by the shade imposed by neighbouring plants (Fig. 7A).
Fig. 7.
R /FR signals received by the spike and its surrounding leaf tissues in wheat crops. (A) The R/FR ratio received by the leaf tissues surrounding the spike within the crop. The sensor was placed facing towards the North either outside the canopy or inside a dense canopy (240 plants m−2) at the height where the spike was developing. (B) Increased R/FR ratios anticipate increased photosynthetically active radiation (PAR, 400–700 nm) reaching the spike emerging from the leaf sheath tube. The PAR and R/FR ratio measured with a fibre optic placed at the position of the spike (usually inside the leaf sheath tube) expressed relative to the values for incident radiation. Ear emergence occurred ∼3 months after sowing. Each datum point corresponds to a spike.
The photosynthetically active radiation (400–700 nm) and R/FR ratio reaching the spike are further reduced by the green leaf sheath tube that surrounds the spike (Fig. 7B). Both photosynthetically active radiation and the R/FR ratio reaching the spike increased before spike emergence because at late stages the spike is covered by a single leaf sheath (belonging to the uppermost leaf). It is interesting to note that the R/FR ratio increased earlier than photosynthetically active radiation (Fig. 7B). This is because the covering leaf tissue also causes a reduction of FR (denominator of the R/FR ratio), less intense than that of photosynthetically active radiation or R but still significant. The anticipated rise of the R/FR ratio could provide an early signal of the impending emergence of the spike.
Discussion
Although the increased growth of the wheat spike achieved by breeding and selection during the 20th century has been related to reduced stem growth (Siddique et al., 1989), low, compared with high, R/FR ratios simultaneously delayed the rate of growth of the spike and of the stem in wheat plants (Fig. 3). Low R/FR ratios delayed the development of the spike even if applied after the transition of the apex to the reproductive stage (Fig. 3) and concomitantly reduced the expression of genes expressed preferentially in the spike (Fig. 6), including three putative zinc-finger proteins (Kobayashi et al., 1998; Nakagawa et al., 2004), a BURP-domain protein gene (Batchelor et al., 2002; Van Son et al., 2009), ABSCISIC ACID INSENSITIVE 5 ABI5 (Gutierrez et al., 2007), a dehydrin (Black et al., 1999), and DOF1 (Mena et al., 1998), previously linked to floral organ or seed development. A low R/FR ratio reduced the number of fertile florets at anthesis (Fig. 4), the number of grains per plant (Fig. 2), and the dry weight of 1000 grains (Fig. 2). These observations involving R/FR signals are consistent with the results of field experiments showing a reduced total number of floret primordia initiated, increased floret abortion, and a reduced number of kernels per spike in response to increasing density (Zhen-Wen et al., 1988). The triple phyA phyB phyC mutant of rice also shows reduced seed production, but this effect is caused by impaired dehiscence of the anther wall and poor pollination (Takano et al., 2009).
The comparison of 10 cultivars released to the Argentinean market throughout the 20th century did not reveal any obvious temporal trend for the magnitude of the tillering response to the R/FR ratio and actually showed a larger response for grain number and weight of the more modern cultivars. The three oldest cultivars tested here lack a yield response to the R/FR treatments, suggesting that breeding during the 20th century has not only failed to eliminate these responses but has actually increased its magnitude. These findings totally contradict the hypothesis that since the responses to low R/FR ratios are detrimental for yield, selection for yield should reduce them.
A widely accepted idea is that the negative effects of a low R/FR ratio on the growth of leaves, roots, or reproductive structures are the consequence of enhanced stem growth because plant resources are allocated to the stem ‘at the expense’ of other organs (Smith, 1995; Schmitt, 1997; Franklin and Whitelam, 2005; Sawers et al., 2005; Kebrom and Brutnell, 2007). In wheat, the delay of spike growth by a low R/FR ratio occurred simultaneously with a delay (rather than a promotion) of stem growth (Fig. 3). Neither the leaf sheaths that extend during the spike growth period (Casal, 1993; and Supplementary Fig. S2 at JXB online) nor the internodes that extend at early stages (Supplementary Fig. S4 at JXB online) were longer under low than high R/FR ratios. In agreement with these observations, the triple phyA phyB phyC mutant of rice is not taller than its wild type (Takano et al., 2009). Since a low R/FR ratio did not increase plant stature in wheat, the observed reduction of grain yield by a low R/FR ratio cannot be regarded as the consequence of investing extra resources in plant stature. A more direct control of grain number by the R/FR ratio is proposed, consistent with the changes in the expression of genes potentially involved in spike development. While low R/FR ratios delayed spike growth, they accelerated the development of the florets already initiated (Fig. 5), indicating a shift in the developmental patterns rather than a general limitation of developmental progression. Long, compared with short days also accelerate floret development and reduce the number of fertile florets at anthesis, but, in contrast to low R/FR ratios, long days accelerate spike growth (Ghiglione et al., 2008). Detailed kinetic studies of spike and stem growth have also suggested that floret death is controlled by a developmental process rather than a trophic limitation (Bancal, 2008).
In wheat crops, the spikes and their surrounding leaf tissues develop in environments with low R/FR ratios (Fig. 7) and these signals correlate with the availability of resources limited by crowding. Conversely, when distant plants are exposed to low R/FR treatments provided by supplementary FR, as in the experiments reported here, the resources are not limited along with these signals. Therefore, while the low R/FR treatments down-regulate yield below the potential of isolated plants, the reduction of yield by the low R/FR ratio of wheat canopies could adjust the generation of yield to the availability of resources. In commercial wheat crops, the early reduction of grain number by a low R/FR ratio might leave a number of grains closer to the number that can be adequately filled (Sadras, 2007) with the resources available later on in crop development. This early regulation by signals that anticipate resource scarcity might prevent an artificial bubble in the generation of yield components followed by a disorderly collapse. In favour of the latter idea, in experiments with more restrictive conditions due to the use of smaller pots, low R/FR ratios actually promoted yield, probably by preventing the generation of unproductive tillers (Casal, 1988) that compete with spike-bearing shoots. Furthermore, in field crops the effect of density on kernel number per spike is determined early in floral development (Zhen-Wen et al., 1988).
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Experimental set-up and light measurement procedures.
Figures S2–S4. Number of tillers per plant, grain yield per plant, number of spikes per plant, number of grains per plant, and weight of 1000 grains (Fig. S2), final height of the sheath of the different leaves (Fig. S3), and final height of the uppermost node (Fig. S4), plotted against the year of release of each cultivar in the 20th century as affected by a high or low R/FR ratio, for one of the three years of experimentation included in Figs 1B and 2B.
Figure S5. Genes with expression in the spike affected by the R/FR treatments grouped in clusters according to their expression patterns in different organs.
Figure S6. Randomly selected genes grouped in clusters according to their expression patterns in different organs.
Table S1. Output of the factorial ANOVA of the results shown in Figs 1B and 2B.
Table S2. Genes showing differential expression in the spike of wheat plants exposed to high or low R/FR ratios and their confidence values.
Supplementary Material
Acknowledgments
We thank L. Gallo-Mendoza for excellent technical assistance. This work was supported by grants from the International Centre for Genetic Engineering and Technology (ICGEB, CRP/ARG07-02), University of Buenos Aires (G044), and CONICET (PIP5958) to JJC.
References
- Appendino ML, Bartoloni N, Slafer GA. Vernalization response and earliness per se in cultivars representing different eras of wheat breeding in Argentina. Euphytica. 2003;130:61–69. [Google Scholar]
- Austin RB, Bingham J, Blackwell RD, Evans LT, Ford MA, Morgan CL, Taylor M. Genetic improvements in winter wheat yields since 1900 and associated physiological changes. Journal of Agricultural Science. 1980;94:675–689. [Google Scholar]
- Austin RB, Ford MA, Morgan CL. Genetic improvement in the yield of winter wheat: a further evaluation. Journal of Agricultural Science. 1989;112:295–301. [Google Scholar]
- Bancal P. Positive contribution of stem growth to grain number per spike in wheat. Field Crop Research. 2008;105:27–39. [Google Scholar]
- Batchelor AK, Boutilier K, Miller SS, Hattori J, Bowman LA, Hu M, Lantin S, Johnson DA, Miki BLA. SCB1, a BURP-domain protein gene, from developing soybean seed coats. Planta. 2002;215:523–532. doi: 10.1007/s00425-002-0798-1. [DOI] [PubMed] [Google Scholar]
- Black M, Corbineau F, Gee H, Côme D. Water content, raffinose, and dehydrins in the induction of desiccation tolerance in immature wheat embryos. Plant Physiology. 1999;120:463–472. doi: 10.1104/pp.120.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccalandro HE, Ploschuk EL, Yanovsky MJ, Sanchez RA, Gatz C, Casal JJ. Increased phytochrome B alleviates density effects on tuber yield of field potato crops. Plant Physiology. 2003;133:1539–1546. doi: 10.1104/pp.103.029579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderini DF, Dreccer MF, Slafer GA. Genetic improvement in wheat yield and associated traits. A re-examination of previous results and latest trends. Plant Breeding. 1995;114:108–112. [Google Scholar]
- Casal JJ. Light quality effects on the appearance of tillers of different order in wheat (Triticum aestivum) Annals of Applied Biology. 1988;112:167–173. [Google Scholar]
- Casal JJ. Novel effects of phytochrome status on reproductive shoot growth in Triticum aestivum L. New Phytologist. 1993;123:45–51. [Google Scholar]
- Chelle M, Evers JB, Combes D, Varlet-Grancher C, Vos J, Andrieu B. Simulation of the three-dimensional distribution of the red:far-red ratio within crop canopies. New Phytologist. 2007;176:223–234. doi: 10.1111/j.1469-8137.2007.02161.x. [DOI] [PubMed] [Google Scholar]
- Childs KL, Miller FR, Cordonnier-Pratt M-M, Pratt LH, Morgan PW, Mullet JE. The Sorghum bicolor photoperiod sensitivity gene, Ma3, encodes a phytochrome B. Plant Physiology. 1997;113:611–619. doi: 10.1104/pp.113.2.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke JD, Zhu T. Microarray analysis of the transcriptome as a stepping stone towards understanding biological systems: practical considerations and perspectives. The Plant Journal. 2006;45:630–650. doi: 10.1111/j.1365-313X.2006.02668.x. [DOI] [PubMed] [Google Scholar]
- Cox TS, Shroyer JP, Ben-Hui L, Sears RG, Martin TJ. Genetic improvement in agronomic traits of hard red winter wheat cultivars from 1919 to 1987. Crop Science. 1988;28:756–760. [Google Scholar]
- Evers JB, Vos J, Chelle M, Andrieu B, Fournier C, Struik PC. Simulating the effects of localized red:far-red ratio on tillering in spring wheat (Triticum aestivum) using a three-dimensional virtual plant model. New Phytologist. 2007;176:325–336. doi: 10.1111/j.1469-8137.2007.02168.x. [DOI] [PubMed] [Google Scholar]
- Fischer RA. 1984. Wheat. In: Smith WH, Banta JJ, ed. Potential productivity of field crops under different environments. Los Baños: IRRI, 129–154. [Google Scholar]
- Franklin KA, Whitelam GC. Phytochromes and shade-avoidance responses in plants. Annals of Botany. 2005;96:169–175. doi: 10.1093/aob/mci165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg AK, Sawers RJ, Wang H, Kim JK, Walker JM, Brutnell TP, Parthasarathy MV, Vierstra RD, Wu RJ. Light-regulated overexpression of an Arabidopsis phytochrome A gene in rice alters plant architecture and increases grain yield. Planta. 2006;223:627–636. doi: 10.1007/s00425-005-0101-3. [DOI] [PubMed] [Google Scholar]
- Ghiglione H, Gonzalez F, Serrago R, Maldonado S, Chilcott C, Curá J, Miralles D, Zhu T, Casal JJ. Autophagy regulated by daylength sets the number of fertile florets in wheat. The Plant Journal. 2008;55:1010–1024. doi: 10.1111/j.1365-313X.2008.03570.x. [DOI] [PubMed] [Google Scholar]
- Grant GR, Liu J, Stoeckert CJJ. A practical false discovery rate approach to identifying patterns of differential expression in microarray data. Bioinformatics. 2005;21:2684–2690. doi: 10.1093/bioinformatics/bti407. [DOI] [PubMed] [Google Scholar]
- Gutierrez L, Van Wuytswinke O, Castelain M, Bellini C. Combined networks regulating seed maturation. Trends in Plant Science. 2007;12:294–300. doi: 10.1016/j.tplants.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Hanumappa M, Pratt LH, Cordonnier-Pratt M-M, Deitzer GF. A photoperiod-insensitive barley line contains a light-labile phytochrome B. Plant Physiology. 1999;119:1033–1040. doi: 10.1104/pp.119.3.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes MG, Smith H. The function of phytochrome in the natural environment—III. Measurement and calculation of phytochrome photoequilibria. Photochemistry and Photobiology. 1977a;25:547–550. [Google Scholar]
- Holmes MG, Smith H. The function of phytochrome in the natural environment—II. The influence of vegetation canopies on the spectral energy distribution of natural daylight. Photochemistry and Photobiology. 1977b;25:539–545. [Google Scholar]
- Kasperbauer MJ, Karlen DL. Light-mediated bioregulation and photosynthate partitioning in wheat. Physiologia Plantarum. 1986;66:159–163. [Google Scholar]
- Kebrom TH, Brutnell TP. The molecular analysis of the shade avoidance syndrome in the grasses has begun. Journal of Experimental Botany. 2007;58:3079–3089. doi: 10.1093/jxb/erm205. [DOI] [PubMed] [Google Scholar]
- Kebrom TH, Burson BL, Finlayson SA. Phytochrome B represses Teosinte Branched1 expression and induces sorghum axillary bud outgrowth in response to light signals. Plant Physiology. 2006;140:1109–1117. doi: 10.1104/pp.105.074856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi A, Sakamoto A, Kubo K, Rybka Z, Kanno Y, Takatsuji H. Seven zinc-finger transcription factors are expressed sequentially during the development of anthers in petunia. The Plant Journal. 1998;13:571–576. doi: 10.1046/j.1365-313x.1998.00043.x. [DOI] [PubMed] [Google Scholar]
- Kulshreshtha R, Kumar N, Balyan HS, Gupta PK, Khurana P, Tyagi AK, Khurana JP. Structural characterization, expression analysis and evolution of the red/far-red sensing photoreceptor gene, phytochrome C (PHYC), localized on the ‘B’ genome of hexaploid wheat (Triticum aestivum L.) Planta. 2005;221:675–689. doi: 10.1007/s00425-004-1473-5. [DOI] [PubMed] [Google Scholar]
- Ledent JF, Stoy V. Yield of winter wheat. A comparison of genotypes from 1910 to 1976. Cereal Research Communications. 1988;16:151–156. [Google Scholar]
- Li C, Wong WH. DNA-Chip Analyzer (dChip) In: Parmigiani G, Garrett ES, Irizarry R, Zeger S, editors. The analysis of gene expression data: methods and software. New York: Springer; 2003. pp. 120–141. [Google Scholar]
- Libenson S, Rodriguez V, Sánchez RA, Casal JJ. Low red to far-red ratios reaching the stem reduce grain yield in sunflower. Crop Science. 2002;42:1180–1185. [Google Scholar]
- Maddonni GA, Otegui ME, Andrieu B, Chelle M, Casal JJ. Maize leaves turn away from neighbors. Plant Physiology. 2002;130:1181–1189. doi: 10.1104/pp.009738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews S, Sharrock RA. The phytochrome gene family in grasses (Poaceae): a phylogeny and evidence that grasses have a subset of the loci found in dicot Angiosperms. Molecular Biology and Evolution. 1996;13:1141–1150. doi: 10.1093/oxfordjournals.molbev.a025677. [DOI] [PubMed] [Google Scholar]
- Mena M, Vicente-Carbajosa J, Schmidt RJ, Carbonero P. An endosperm-specific DOF protein from barley, highly conserved in wheat, binds to and activates transcription from the prolamin-box of a native B-hordein promoter in barley endosperm. The Plant Journal. 1998;16:53–62. doi: 10.1046/j.1365-313x.1998.00275.x. [DOI] [PubMed] [Google Scholar]
- Monte E, Ludevid D, Prat S. Leaf C40.4: a carotenoid-associated protein involved in the modulation of photosynthetic efficiency? The Plant Journal. 1999;19:399–410. doi: 10.1046/j.1365-313x.1999.00537.x. [DOI] [PubMed] [Google Scholar]
- Nakagawa H, Ferrario S, Angenent GC, Kobayashi A, Takatsuji H. The petunia ortholog of arabidopsis SUPERMAN plays a distinct role in floral organ morphogenesis. The Plant Cell. 2004;16:920–932. doi: 10.1105/tpc.018838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry MW, D'Antuono MF. Yield improvement and associated characteristics of some Australian spring wheat cultivars introduced between 1860 and 1982. Australian Journal of Agricultural Research. 1989;40:457–472. [Google Scholar]
- Robson PRH, McCormac AC, Irvine AS, Smith H. Genetic engineering of harvest index in tobacco through overexpression of a phytochrome gene. Nature Biotechnology. 1996;14:995–998. doi: 10.1038/nbt0896-995. [DOI] [PubMed] [Google Scholar]
- Sadras VO. Evolutionary aspects of the trade-off between seed size and number in crops. Field Crops Research. 2007;100:125–138. [Google Scholar]
- Sawers RJH, Sheehan MJ, Brutnell TB. Cereal phytochromes: targets of selection, targets for manipulation? Trends in Plant Science. 2005;10:138–143. doi: 10.1016/j.tplants.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Schittenhelm S, Menge-Hartmann U, Oldenburg E. Photosynthesis, carbohydrate metabolism, and yield of phytochrome-B-overexpressing potatoes under different light regimes. Crop Science. 2004;44:131–143. [Google Scholar]
- Schmitt J. Is photomorphogenic shade avoidance adaptive? Perspectives from population biology. Plant, Cell and Environment. 1997;20:826–830. [Google Scholar]
- Schreiber AW, Sutton T, Caldo RA, et al. Comparative transcriptomics in the Triticeae. BMC Genomics. 2009;10:285. doi: 10.1186/1471-2164-10-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan MJ, Kennedy LM, Costich DE, Brutnell TP. Subfunctionalization of PhyB1 and PhyB2 in the control of seedling and mature plant traits in maize. The Plant Journal. 2007;49:338–353. doi: 10.1111/j.1365-313X.2006.02962.x. [DOI] [PubMed] [Google Scholar]
- Siddique KHM, Perry MW, Kirby EJM. Ear:stem ratio in old and modern wheat varieties; relationship with improvement in number of grains per ear and yield. Field Crops Research. 1989;21:59–78. [Google Scholar]
- Skinner RH, Simmons SR. Modulation of leaf elongation, tiller appearance and tiller senescence in spring barley by far-red light. Plant, Cell and Environment. 1993;16:555–562. [Google Scholar]
- Slafer GA, Andrade FH. Genetic improvement in bread wheat (Triticum aestivum L.) yield in Argentina. Field Crops Research. 1989;21:289–296. [Google Scholar]
- Slafer GA, Satorre H, Andrade FH. Increases in grain yield in bread wheat from breeding and associated physiological changes. In: Slafer GA, editor. Genetic improvement of field crops. NY: Marcel Dekker, Inc.,; 1994. pp. 1–68. [Google Scholar]
- Smith H. Physiological and ecological function within the phytochrome family. Annual Review of Plant Physiology. 1995;46:289–315. [Google Scholar]
- Sparkes DL, Holme SJ, Gaju O. Does light quality initiate tiller death in wheat? European Journal of Agronomy. 2006;24:212–217. [Google Scholar]
- Sparkes DL, King M. Disentangling the effects of PAR and R: FR on lodging-associated characters of wheat (Triticum aestivum) Annals of Applied Biology. 2008;152:1–9. [Google Scholar]
- Takano M, Inagaki N, Xie X, Kiyota S, Baba-Kasai A, Tanabata T, Shinomura T. Phytochromes are the sole photoreceptors for perceiving red/far-red light in rice. Proceedings of the National Academy of Sciences, USA. 2009;106:14705–14710. doi: 10.1073/pnas.0907378106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano M, Inagaki N, Xie X, et al. Distinct and cooperative functions of phytochromes A, B, and C in the control of deetiolation and flowering in rice. The Plant Cell. 2005;17:3311–3325. doi: 10.1105/tpc.105.035899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Son L, Tiedemann J, Rutten T, Hillmer S, Hinz G, Zank T, Manteuffel R, Bäumlein H. The BURP domain protein AtUSPL1 of Arabidopsis thaliana is destined to the protein storage vacuoles and overexpression of the cognate gene distorts seed development. Plant Molecular Biology. 2009;71:319–329. doi: 10.1007/s11103-009-9526-6. [DOI] [PubMed] [Google Scholar]
- Waddington SR, Cartwright PM, Wall PC. A quantitative scale of spike initial and pistil development in barley and wheat. Annals of Botany. 1993;51:119–130. [Google Scholar]
- Zhen-Wen Y, Van Sanford DA, Egli DB. The effect of population density on floret initiation, development and abortion in winter wheat. Annals of Botany. 1988;62:295–302. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.