Abstract
Aluminium (Al) rhizotoxicity coincides with low pH; however, it is unclear whether plant tolerance to these two factors is controlled by the same mechanism. To address this question, the Al-resistant alr104 mutant, two Al-sensitive mutants (als3 and als5), and wild-type Arabidopsis thaliana were compared in long-term exposure (solution culture) and in short-term exposure experiments (H+ and K+ fluxes, rhizosphere pH, and plasma membrane potential, Em). Based on biomass accumulation, als5 and alr104 showed tolerance to low pH, whereas alr104 was tolerant to the combined low-pH/Al treatment. The sensitivity of the als5 and als3 mutants to the Al stress was similar. The Al-induced decrease in H+ influx at the distal elongation zone (DEZ) and Al-induced H+ efflux at the mature zone (MZ) were higher in the Al-sensitive mutants (als3 and als5) than in the wild type and the alr104 mutant. Under combined low-pH/Al treatment, alr104 and the wild type had depolarized plasma membranes for the entire 30 min measurement period, whereas in the Al-sensitive mutants (als3 and als5), initial depolarization to around –60 mV became hyperpolarization at –110 mV after 20 min. At the DEZ, the Em changes corresponded to the changes in K+ flux: K+ efflux was higher in alr104 and the wild type than in the als3 and als5 mutants. In conclusion, Al tolerance in the alr104 mutant correlated with Em depolarization, higher K+ efflux, and higher H+ influx, which led to a more alkaline rhizosphere under the combined low-pH/Al stress. Low-pH tolerance (als5) was linked to higher H+ uptake under low-pH stress, which was abolished by Al exposure.
Keywords: Aluminium toxicity, distal root elongation zone, H+ flux, K+ flux, low pH, mature root zone, plasma membrane potential
Introduction
Aluminium (Al) affects root growth in acidic soils. A number of mechanisms responsible for Al tolerance have been characterized in plants, such as (i) release of organic acid anions (e.g. Ma et al., 1997; Kochian et al., 2004; Hoekenga et al., 2006; Liu et al., 2009; Ryan et al., 2009); (ii) release of phenolic compounds (Kidd et al., 2001); (iii) rhizosphere alkalinization (Degenhardt et al., 1998); (iv) internal detoxification of Al by complexation with organic acid anions (Ma et al., 2001; Shen et al., 2002); and (v) redistribution of accumulated Al away from sensitive root tissues (Larsen et al., 2005). However, the low pH itself can affect root growth in various plant species (Arnon and Johnson, 1942; Llugany et al., 1995; Lazof and Holland, 1999; Kidd and Proctor, 2001; Koyama et al., 2001; Kinraide, 2003; Rangel et al., 2005; Iuchi et al., 2007; Sawaki et al., 2009). Nevertheless, our knowledge of proton toxicity and the molecular mechanisms underlying low-pH tolerance is rather limited when compared with Al tolerance.
Low-pH tolerance and K+ nutrition appear to be interlinked in some plants species because the addition of K+ to the external medium alleviated H+ toxicity in maize (Yan et al., 1992), common bean (Rangel et al., 2005), and sugar beet (Lindberg and Yahya, 1994). Also, down-regulation of CIPK23, which encodes the regulatory kinase of a major K+ transporter AKT1, may be responsible for the higher sensitivity of the Arabidopsis stop1 mutant to low pH (Iuchi et al., 2007; Sawaki et al., 2009). Although the detailed mechanism underlying this phenomenon is unclear, the increased internal K+ concentration could be due to changes in K+ transport at the root–soil interface, via either increased K+ uptake or decreased K+ efflux. Lower K+ efflux under Al exposure in comparison with low pH exposure has been reported for soybean cells grown in suspension culture (Stass and Horst, 1995), which might indicate Al-induced changes in the plasma membrane potential (Em) because K+ transport and accumulation in roots is highly dependent on Em. Hence, Em and K+ flux need to be assessed simultaneously to elucidate the role of K+ nutrition in low-pH tolerance.
In Arabidopsis, low pH (H+ toxicity) causes irreversible damage to primary and lateral roots, with the pattern of damage being different from the one caused by Al rhizotoxicity (Koyama et al., 1995). Furthermore, an Arabidopsis quantitative trait locus (QTL) analysis revealed that Al tolerance and H+ tolerance are controlled by different genetic factors (Ikka et al., 2007). In contrast, the proton-hypersensitive Arabidopsis stop1 (sensitive to proton rhizotoxicity 1) mutant is also hypersensitive to Al (Iuchi et al., 2007; Sawaki et al., 2009). Therefore, it appears that H+ and Al3+ toxicities and tolerances are controlled by some separate and some common mechanisms, which would need to be elucidated.
Based on this hypothesis, low pH tolerance of an Al-resistant mutant, alr104, which has higher rhizosphere alkalinizing capacity than the wild-type (Degenhardt et al., 1998; Larsen et al., 1998), and two Al-sensitive mutants, als3 [defective in an ABC transporter-like protein (Larsen et al., 2005)] and als5 [defective in Al exclusion (Larsen et al., 1996)], were studied. The Al stress is inevitably studied in combination with the low-pH stress; as a result, genotypes more sensitive to one stress were occasionally classified as being tolerant to the other stress (for references, see Lazof and Holland, 1999). Hence, the effects of low pH were separated from combined low-pH/Al effects and Al susceptibility or tolerance of these Arabidopsis mutants was re-examined by measuring rhizosphere alkalinization capacity, internal K+ concentration, changes in the plasma membrane potential (Em), and the H+ and K+ net fluxes. It was found that the als5 mutant was tolerant to low pH but sensitive to Al, whereas alr104 was tolerant and als3 was sensitive to both low pH and Al.
Materials and methods
Long-term exposure experiments: hydroponic culture
Arabidopsis thaliana L. seeds were surface sterilized with 1% (v/v) calcium hypochlorite for 10 min. Seeds were then sown on rock-wool strips (1–2 mm thick and 5–6 cm long) that were placed into 250 ml plastic containers containing 1/10 Hoagland solution. The containers were kept at 4 °C for 2 d to achieve synchronized germination. Seedlings were then moved to a growth cabinet with 16 h light (150 μmol m−2 s−1) and 8 h dark at 20±1 °C. Three-week-old seedlings were used to conduct two sets of long-term exposure experiments. During the first set of experiments, A. thaliana L. wild-type ecotype Col-0 and Al-sensitive mutant (als3 and als5) seedlings were exposed to either pH 5.5 or 4.2 with or without 0.5 mM homo-PIPES buffer for 10 d in quadruplicate. Nutrient solutions were changed daily. During the treatment period, the bulk solution pH was measured on the second day at 12 h and 24 h after the nutrient solution was changed.
During the second set of experiments, 3-week-old seedlings [the wild type (ecotype Col-0), als3, als5, and alr104] were exposed to pH 5.5 and a range of Al concentrations (0, 10, 25, 50, 75, 100, or 250 μM AlCl3; pH 4.2) in 0.5 mM homo-PIPES buffer for 7 d. The treatments were performed in triplicate, and the treatment solutions were changed daily.
At the end of the experiments, shoots and roots were separately harvested, washed with 100 μM CaSO4, rinsed with deionized water, dried in an oven at 70 °C for 72 h, and weighed. Dried shoots were digested with a HNO3:HClO4 (10:1) mixture. The K+ concentration was analysed using inductively coupled plasma–mass spectrometry (ICP-MS).
Short-term exposure experiments
Arabidopsis thaliana L. wild- type (ecotype Col-0), als3, als5, and alr104 seeds were surface sterilized with 1% (w/v) calcium hypochlorite and seedlings were grown in 90 mm Petri dishes under constant fluorescent light (150 μmol m−2 s−1) and temperature (23–25 °C) in 0.8% (w/w) agar medium containing basal salt medium (BSM) with 0.1 mM CaCl2, 1 mM KCl, and 0.2 mM MgCl2, pH 5.5. The Petri dishes were oriented upright, so the roots grew down along the agar surface without penetrating it. However, roots were anchored in the agar by root hairs.
Four- to five-day-old seedlings of the wild type (ecotype Col-0) and als3, als5, and alr104 mutants were conditioned in BSM at pH 5.5 for 20 min followed by either low pH (pH 4.2) or combined low-pH/50 μM AlCl3 treatment. All measurements were made at the root distal elongation zone (DEZ; 200 μm away from the root cap) and the mature zone (MZ; 700 μm from the root cap).
Net fluxes of H+ and K+ and rhizosphere pH were measured 40 μm away from the root surface using the non-invasive MIFE® system (University of Tasmania, Hobart, Australia) as described by Newman (2001). Microelectrodes were pulled from borosilicate glass capillaries (GC 150-10, SDR Clinical Technology, Middle Cove, Australia), oven dried at 230 °C for ∼5 h, and silanized using tributylchlorosilane (Fluka catalogue no. 90796). Electrodes with an external tip diameter of 2–3 μm were used. The electrodes were back-filled with the appropriate solution (0.15 mM NaCl and 0.4 mM KH2PO4 adjusted to pH 6.0 using NaOH for the H+ electrode and 0.5 M KCl for the K+ electrode). The electrode tips were then front-filled with ionophore cocktails (Fluka catalogue no. 95297 for H+ and no. 60031 for K+). The prepared electrodes were calibrated with a set of standards (pH from 3.2 to 6.5; K+ from 0.5 mM to 10 mM). Electrodes with slopes of <50 mV per 10 units were discarded.
The roots of an intact 4- to 5-day-old Arabidopsis seedling were gently secured horizontally in a measuring chamber with a Parafilm strip and small plastic blocks. The seedling was then placed in the measuring chamber containing BSM and conditioned for at least 20 min. The Em measurements were conducted according to the procedure outlined in Cuin and Shabala (2005). The borosilicate glass microelectrodes (Shabala and Lew, 2002) (Clarke Electrochemical Instruments, Reading, UK) were filled with 1 M KCl, connected to an IE-251 electrometer (Warner Instruments, Hampden, CT, USA) via the Ag–AgCl half-cell, and inserted into the root tissue (DEZ or MZ) with a manually operated micromanipulator (MMT-5, Narishige, Tokyo, Japan). The Em was monitored continually using the CHART software (for details, see Newman, 2001). Once a stable measurement of Em was obtained for 1 min, treatments with either low pH (pH 4.2) or combined low-pH/50 μM AlCl3 were initiated, and the Em was measured for ∼30 min. Eight to 12 plants were measured for every treatment, and the data were averaged.
Results
Long-term exposure experiments: low pH and low-pH/Al stresses in buffered and non-buffered media
Alkalinization capacity and growth of Arabidopsis mutants under low pH:
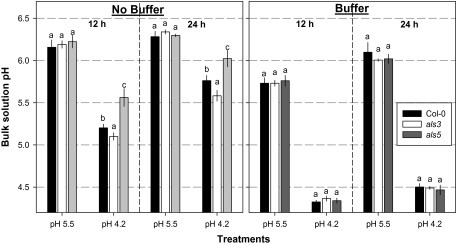
Measurements of the bulk solution pH were made after 12 h and 24 h (Fig. 1). The results revealed that 0.5 mM homo-PIPES buffer was sufficient to keep the pH at ∼4.2 or 5.5 (depending on the treatment) for at least 24 h. In non-buffered medium, within 12 h the plants increased the media pH from 4.2 to 5.1–5.6 depending on the genotype (Fig. 1). The als5 mutant was the most and the als3 mutant was the least effective of the three genotypes tested in increasing the media pH.
Fig. 1.
Bulk solution pH of Arabidopsis thaliana genotypes with and without 0.5 mM homo-PIPES buffer measured on the second day at 12 h and 24 h after the nutrient solution change. Mean ±SE (n=4 replicates). Within each buffer×pH treatment, different letters represent significant difference by LSD test at P <0.001. Arabidopsis seedlings were grown in diluted (1/10) Hoagland solution for 3 weeks; treatments were then imposed for 10 d.
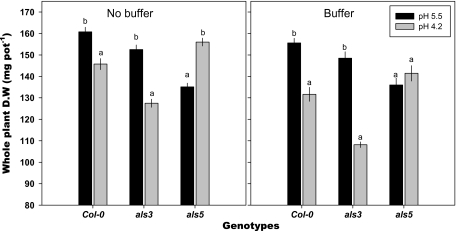
After 10 d in non-buffered medium, low-pH treatment decreased the biomass of the wild type and the als3 mutant, whereas low-pH stress enhanced growth of the als5 mutant (Fig. 2); there were no differences in growth of als5 at the two pHs in the buffered medium. For the wild type and als3 mutant, biomass was similar in the buffered and non-buffered media.
Fig. 2.
Biomass of Arabidopsis thaliana genotypes at pH 4.2 with and without 0.5 mM homo-PIPES buffer. Mean ±SE (n=4 replicates). In each graph, different letters within each genotype represent significant difference between pH 5.5 and pH 4.2 by t-test at P ≤0.001.
Effect of low pH and Al on root biomass and shoot K+ concentration:
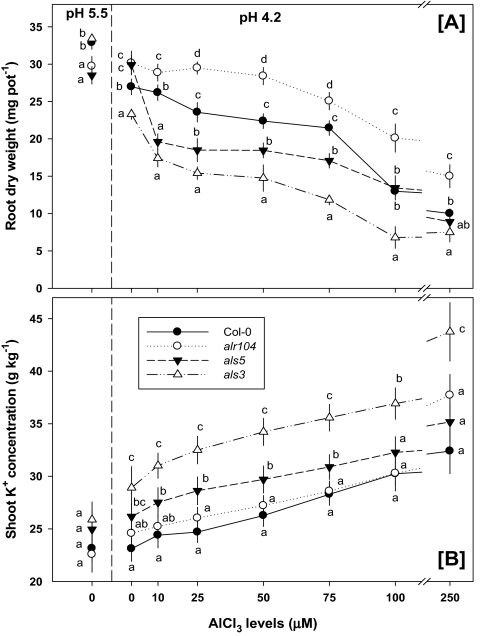
In the homo-PIPES-buffered medium, the low-pH treatment retarded root biomass of the wild type and als3 mutant, whereas root biomass of the alr104 and als5 mutants was unaffected. The als3 mutant showed a greater reduction in root biomass under low pH treatment compared with the wild type (Fig. 3A). The addition of Al severely inhibited root biomass of the Al-sensitive mutants (als3 and als5), even at a low concentration (10 μM), whereas root biomass reduction in the wild type was observed at Al concentrations ≥25 μM. Root biomass of the Al-resistant mutant alr104 was inhibited only at ≥75 μM Al (Fig. 3A). Al-related root biomass reduction was greater in the sensitive mutants (als3 and als5) compared with the wild type and was the lowest in the resistant mutant (alr104).
Fig. 3.
Effect of low pH and Al stresses on Arabidopsis thaliana root biomass [A] and shoot K+ concentration [B]. Means ±SE (n=3 replicates). In each AlCl3 concentration, genotypes sharing a common letter are not significantly different by LSD test at P ≤0.05. Arabidopsis seedlings were grown in diluted (1/10) Hoagland solution for 3 weeks, and treatments were then imposed in buffered medium (0.5 mM homo-PIPES) for 7 d.
The shoot K+ concentration did not differ among the genotypes in the pH 5.5 treatment (Fig. 3B). Under low pH treatment, the Al-sensitive mutants (als5 and especially als3) had higher shoot K+ concentrations compared with the wild type. Interestingly, the shoot K+ concentration of the Al-resistant mutant alr104 did not differ from that of the wild type under any treatment. In all Al treatments, the Al-sensitive mutant als3 had higher shoot K+ concentrations than any of the other genotypes.
Short-term exposure experiments: microelectrode measurements during low pH and low-pH/Al stresses
Rhizosphere pH:
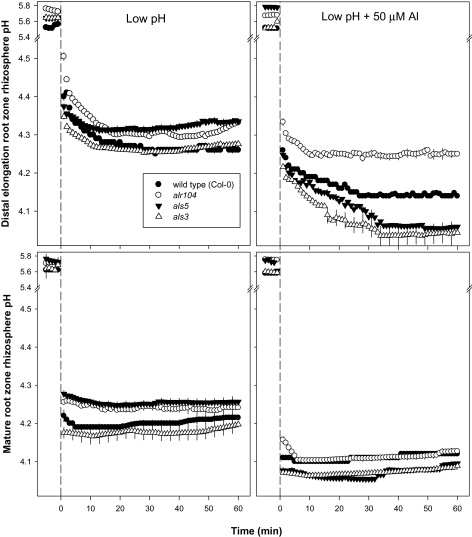
Under the low-pH treatment, the alr104 and als5 mutants had higher rhizosphere pH than the wild type and als3 mutant in both the DEZ and MZ. No significant rhizosphere pH change was observed between the wild type and als3 mutant in both root zones, except for the first 12 min in the DEZ when the als3 mutant demonstrated a slightly lower pH than the wild type (Fig. 4).
Fig. 4.
Effect of low-pH and combined low-pH/Al treatments on rhizosphere pH at the distal elongation zone (top panel) and mature zone (bottom panel) of 4- to 5-day-old Arabidopsis thaliana roots. The low-pH and combined low-pH/Al treatments were imposed at time=0; the data recorded in the first 5 min before time=0 represent rhizosphere pH at pH 5.5. Error bars indicate ±SE (n=10–12 seedlings). Arabidopsis seedlings were conditioned in basal salt medium (BSM; 0.1 mM CaCl2+1 mM KCl+0.2 mM MgCl2, pH 5.5) for 20 min before treatments were imposed in unbuffered BSM.
The combined low-pH/50 μM Al treatment resulted in a lower rhizosphere pH than the low-pH treatment alone in both root zones of all genotypes tested. However, the Al-resistant alr104 mutant had a rhizosphere pH that was 0.1±0.01 units higher than that of the wild type in the DEZ. In the MZ, the alr104 mutant demonstrated a higher pH than the wild type for the first 4 min, but there was no significant difference afterwards. Both of the Al-sensitive mutants had lower rhizosphere pH compared with the wild type and alr104 in both root zones (Fig. 4).
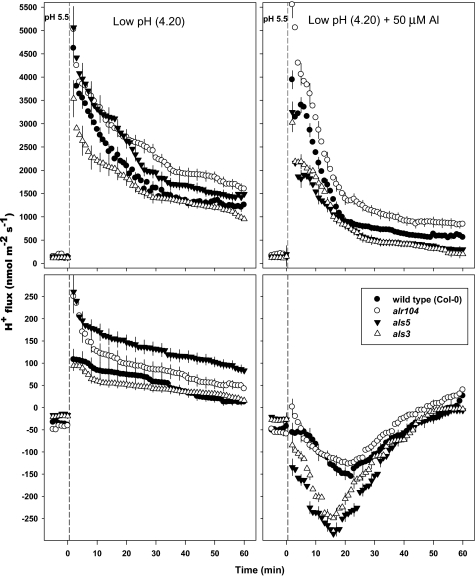
H+ flux:
At pH 5.5, a small net H+ influx in the DEZ and a small net H+ efflux in the MZ were observed in all the genotypes tested (Fig. 5). The low-pH treatment induced a net H+ influx in both root zones of all genotypes, but the magnitude was greater in the DEZ than in the MZ. The alr104 and als5 mutants exhibited higher H+ influxes than the wild type and als3 at both root zones. Under the low-pH treatment, als3 and the wild type had similar H+ influxes in both root zones, except for the first 12 min in the DEZ, where a lower level of H+ influx was observed in the als3 mutant compared with the wild type.
Fig. 5.
Effect of low pH and combined low pH/50 μM Al on H+ fluxes measured at the distal elongation zone (top panel) and the mature zone (bottom panel) of 4- to 5-day-old Arabidopsis thaliana roots. The low-pH and combined low-pH/Al treatments were imposed at time=0; the data recorded in the first 5 min before time=0 represent H+ fluxes at pH 5.5. Negative H+ flux values indicate efflux, and positive values influx. Error bars are ±SE (n=10–12 seedlings). Arabidopsis seedlings were conditioned in basal salt medium (BSM; 0.1 mM CaCl2+1 mM KCl+0.2 mM MgCl2, pH 5.5) for 20 min before treatments were imposed in unbuffered BSM.
In the low-pH/Al treatment (50 μM), the Al-resistant alr104 mutant maintained the highest H+ influx in the DEZ, followed by the wild type; H+ influx was the lowest in the Al-sensitive mutants (als3 and als5; Fig. 5). In the MZ, the 50 μM Al treatment induced H+ efflux in all genotypes tested for the first 45 min. The highest levels of H+ efflux were observed in the als3 and als5 mutants. The highest H+ efflux was observed 17 min after 50 μM Al exposure in the Al-sensitive mutants (als3 and als5) and after 22 min in the wild type and alr104 mutant (Fig. 5).
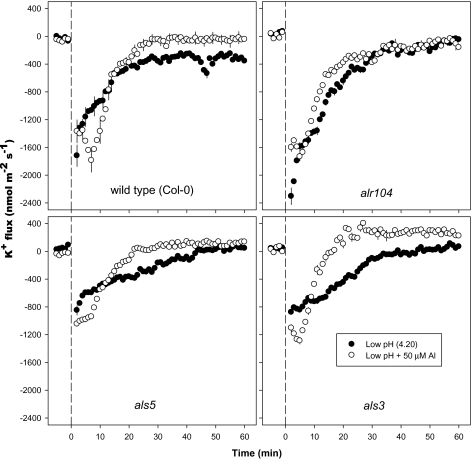
K+ flux:
The low-pH treatment induced K+ efflux from the DEZ, which decreased gradually over time in all genotypes (Fig. 6). Among the mutants, als3 and als5 had lower levels of K+ efflux than the wild type and alr104. During the first 30 min of the low-pH treatment, K+ efflux was higher in the alr104 mutant than in the wild type, but the opposite occurred between 30 min and 60 min after the start of the treatment.
Fig. 6.
Effect of low pH and combined low pH plus 50 μM Al3+ on K+ fluxes measured at the distal elongation zone of 4- to 5-day-old Arabidopsis thaliana roots. The low-pH and combined low-pH/Al3+ treatments were imposed at time=0; the data recorded in the first 5 min before time=0 represent K+ fluxes at pH 5.5. Negative K+ flux values indicate efflux, and positive values indicate influx. Error bars are ±SE (n=10–12 seedlings). Arabidopsis seedlings were conditioned in basal salt medium (BSM; 0.1 mM CaCl2+1 mM KCl+0.2 mM MgCl2, pH 5.5) for 20 min before treatments were imposed in unbuffered BSM.
The combined low-pH/50 μM Al treatment generally induced lower levels of K+ efflux in the DEZ than the low-pH treatment alone after ∼10–15 min in all genotypes, except for the alr104 mutant which showed little difference between K+ flux in the low-pH and low-pH/Al treatments (Fig. 6). Interestingly, under the combined low-pH/50 μM Al treatment, the K+ flux changed from efflux to influx in the als3 and als5 mutants after 12 and 20 min, respectively, whereas the wild type and alr104 mutant maintained K+ efflux for the entire 60 min period.
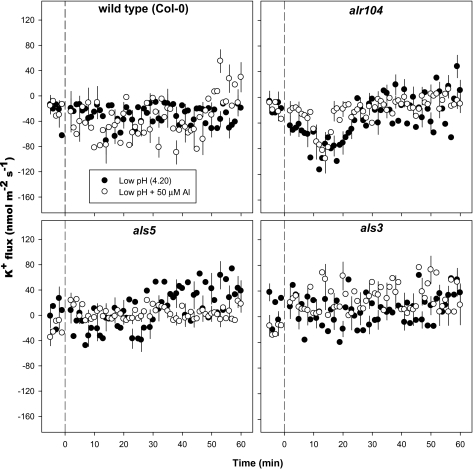
In the MZ, no distinct differences in K+ flux were observed between the low-pH and combined low-pH/50 μM Al treatments for all genotypes tested (Fig. 7). There was a tendency toward K+ efflux for the wild type and alr104, whereas the net fluxes oscillated around zero for the two Al-sensitive mutants.
Fig. 7.
Effect of low pH and combined low pH plus 50 μM Al3+ on K+ fluxes measured at the mature zone of 4- to 5-day-old Arabidopsis thaliana roots. The low-pH and combined low-pH/Al3+ treatments were imposed at time=0; the data recorded in the first 5 min before time=0 represent K+ fluxes at pH 5.5. Negative K+ flux values indicate efflux and positive values indicate influx. Error bars are ±SE (n=10–12 seedlings). Arabidopsis seedlings were conditioned in basal salt medium (BSM; 0.1 mM CaCl2+1 mM KCl+0.2 mM MgCl2, pH 5.5) for 20 min before treatments were imposed in unbuffered BSM.
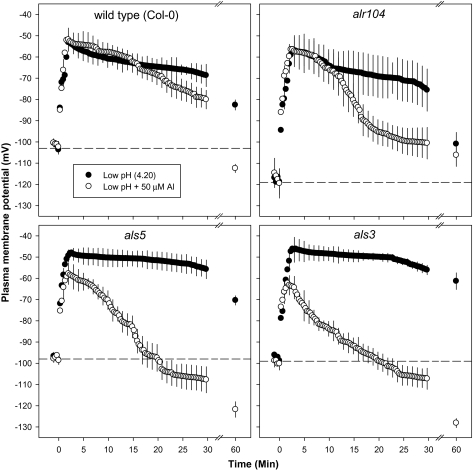
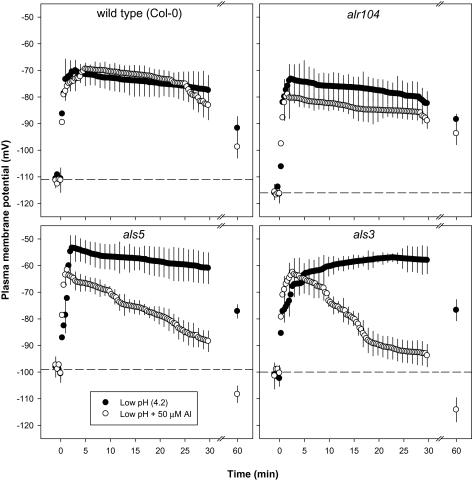
Plasma membrane potential (Em):
The resting Em (at pH 5.5) of the alr104 mutant was more negative than the Em of the wild type and the Al-sensitive mutants (als3 and als5) in both root zones (Figs 8, 9). There was no difference in the resting potential observed between the wild type and Al-sensitive mutants (als3 and als5) in the DEZ (Fig. 8). However, in the MZ, the wild type had a more negative resting potential than the sensitive mutants (als3 and als5; Fig. 9).
Fig. 8.
Effect of low pH and combined low pH/Al treatments imposed at time=0 on the plasma membrane potential in the distal elongation zone of 4- to 5-day-old Arabidopsis thaliana roots. Error bars indicate ±SE (n=8–10 seedlings). The horizontal dotted lines represent the resting plasma membrane potential of the respective genotypes at pH 5.5. Arabidopsis seedlings were conditioned in basal salt medium (BSM; 0.1 mM CaCl2+1 mM KCl+0.2 mM MgCl2, pH 5.5) for 20 min before treatments were imposed in unbuffered BSM.
Fig. 9.
Effect of low pH and combined low pH/Al treatments imposed at time=0 on the plasma membrane potential in the mature zone of 4- to 5-day-old Arabidopsis thaliana roots. Error bars indicate ±SE (n=8–10 seedlings). The horizontal dotted lines represent the resting plasma membrane potential of the respective genotypes at pH 5.5. Arabidopsis seedlings were conditioned in basal salt medium (BSM; 0.1 mM CaCl2+1 mM KCl+0.2 mM MgCl2, pH 5.5) for 20 min before treatments were imposed in unbuffered BSM.
The low-pH treatment depolarized the plasma membrane in both root zones and in all genotypes, but to different extents. Low pH induced more depolarization in the als3 and als5 mutants than in the wild type and alr104 mutant (Figs 8, 9).
In the DEZ of the Al-sensitive mutants (Fig. 8), the initial depolarization of the plasma membrane was less in the combined low-pH/50 μM Al treatment than in the low-pH treatment. Em depolarization in the DEZ lasted for 30 min in the wild type and in alr104. In the Al-sensitive mutants (als3 and als5), Em depolarization was maintained for the 60 min measuring period in the low-pH treatment, whereas the low-pH/50 μM Al treatment hyperpolarized the plasma membrane after 20 min in both Al-sensitive mutants. After 60 min of low-pH/Al treatment, Em was still depolarized in alr104, but became hyperpolarized in the other three genotypes.
In the MZ of wild type and alr104 mutant roots, the low-pH/Al treatment did not induce a significant difference in the depolarization pattern when compared with the low-pH treatment for up to 60 min (Fig. 9). In contrast, in the Al-sensitive mutants, the low-pH/Al treatment depolarized the plasma membrane to a lesser extent than the low-pH treatment. After 60 min of low-pH/Al treatment, plasma membrane hyperpolarization was observed in the Al-sensitive mutants, whereas the wild type and alr104 maintained depolarized states.
Discussion
Arabidopsis mutants differed in their responses to low-pH and Al stresses: als5 grew better under the low-pH treatment (Fig. 2) and poorly in the Al treatment (Fig. 3A), whereas als3 was sensitive and alr104 was tolerant to both stresses (Figs 2, 3A). These results agree with those of Ikka et al. (2007), who classified 260 A. thaliana strains for Al and low-pH tolerance based on the results of QTL analysis.
Several mechanisms have been proposed for the increased plant tolerance to Al toxicity (Matsumoto, 2000; Kochian et al., 2004), some of which could also increase tolerance to low-pH stress, such as increased rhizosphere alkalinization. It was found that the als5 and alr104 mutants had higher rhizosphere pH than the wild type and the als3 mutant, reflecting the low-pH tolerances of the former mutants (Fig. 4). In line with the impaired rhizosphere alkalinization mechanism under the combined low-pH/Al treatment, the Al-sensitive mutants (als3 and als5) exhibited lower rhizosphere pHs in both root zones during short-term exposure experiments if compared with the Al-tolerant genotypes (wild type and alr104; Fig. 4). Interestingly, a suppressor mutant of als3 (alt1-1) has enhanced capability for pH adjustment of the rhizosphere (Gabrielson et al., 2006). Therefore, rhizosphere alkalinization appears to be a regulatory mechanism of plant tolerance to low-pH and Al stresses.
Although rhizosphere pH changes are the net result of the dynamics of cation/anion uptake and release (including H+, OH–, and organic acids), the MIFE® technique does not allow separate ion flux measurements; hence, it is difficult to establish which ion fluxes are responsible for a specific pattern of pH changes. However, a close correlation was found between rhizosphere pH changes and changes in H+ flux (r ≥0.93). Thus, H+ flux across the root tissue is likely to be an important contributor to pH changes in the rhizosphere under low-pH and combined low-pH/Al stresses.
The low-pH treatment induced an increase in H+ influx in both root zones for all the genotypes tested (Fig. 5). This H+ influx could be the result of (i) passive entry of H+ from the external media into the root tissue because acidification of the external pH by one unit can increase the H+ electrochemical gradient across the plasma membrane by 60 mV (Babourina et al., 2001; Yamashita et al., 2003) and/or (ii) decreased activity of the H+-ATPase (Kasamo, 1986; Zhao et al., 2008). However, a decrease in H+-ATPase activity under low pH conditions was not supported by Yan et al. (1998, 1992); instead, they reported that re-entry of H+ ions into the root cells was enhanced at low pH. Increased H+ influx into the root tissue would cause intracellular acidification (Gerendas et al., 1990; JB, OB, and ZR, unpublished results), thereby disturbing the cytoplasmic pH. Earlier reports showed that cytoplasmic pH regulatory genes were down-regulated in the low-pH-hypersensitive Arabidopsis stop1 mutant (Iuchi et al., 2007; Sawaki et al., 2009). In the present experiments, higher H+ influx (and greater biomass growth) was observed in the low pH-tolerant mutants (als5 and alr104) compared with the low-pH-sensitive wild type and als3 mutant (Figs 2, 3A). Hence, it is proposed that the low-pH-tolerant mutants (als5 and alr104) have a better cytoplasmic pH regulatory mechanism than the wild type and als3 mutant.
The combined low-pH/Al treatment decreased net H+ influx in the DEZ and increased net H+ efflux in the MZ for all the genotypes tested (Fig. 5). This could result from Al ions either inhibiting H+ influx or inducing H+ efflux, which is consistent with earlier studies on squash roots where Al-treated root apices were not able to alkalinize media to the same extent as in control low-pH media (Ahn et al., 2002). The MIFE technique used in the present study estimates the net H+ flux across the plasma membrane. Hence, it can only be speculated that Al ions, because of their strong affinity for the plasma membrane surface, might have shifted the plasma membrane surface potential towards relatively positive values (Ahn et al., 2001, 2004a). A positively charged plasma membrane surface would impede the uptake of cations, including H+ ions. This might explain the observed inhibition of H+ influx in the DEZ. The H+ influx inhibition by Al ions would also result in measured enhancement of the net H+ efflux in the MZ. Furthermore, Ahn et al. (2004a) reported that Al ions shifted the plasma membrane surface potential towards positive values in an Al-sensitive (ES8) but not in an Al-tolerant wheat genotype (ET8). Similarly, plasma membrane surface potential differences between Arabidopsis genotypes exposed to low-pH/Al treatment in the present study could have been linked to greater H+ influx inhibition and enhanced H+ efflux in the Al-sensitive mutants (als3 and als5) compared with the wild type and the Al-tolerant alr104 mutant.
Similarly to Al-sensitive maize (Calba and Jaillard, 1997) and wheat roots (Kinraide, 1988), enhanced net H+ release from Al-sensitive mutants (als3 and als5) under Al stress would decrease the net H+ influx from the DEZ and increase the net H+ efflux from the MZ (Fig. 5). Protein kinases can regulate H+-ATPase activity across the plasma membrane (Trofimova et al., 1997). Though up-regulation of protein kinases was reported for Al-tolerant genotypes of some plant species (Osawa and Matsumoto, 2001; Shen et al., 2005), protein kinase inhibition by Al ions was observed for the Al-sensitive Arabidopsis stop1 mutant (Sawaki et al., 2009). It is tempting to hypothesize that Al can specifically inhibit the Arabidopsis protein kinase PKS5, a negative regulator of the membrane H+-ATPase, thereby inducing H+ efflux and acidification of the external medium (cf. Fuglsang et al., 2007).
The low-pH treatment depolarized the Em in all the genotypes tested (Figs 8, 9). This could be the result of a transient increase in H+ influx and/or a decrease in H+-ATPase activity. Under the combined low-pH/Al treatment, Em depolarization was higher in the Al-tolerant genotypes (wild type and alr104) than in the Al-sensitive mutants (als3 and als5) in both root zones (Figs 8, 9). Similar results were observed in wheat (Papernik and Kochian, 1997; Wherrett et al., 2005), indicating that Arabidopsis and wheat might employ similar mechanisms to combat combined low-pH/Al stress. This Al-induced Em depolarization in the Al-tolerant genotypes could be a result of (i) currents caused by the H+ flux across the plasma membrane (see Raven, 1991, and references therein) because higher H+ influx in the DEZ or lower H+ efflux in the MZ of the Al-tolerant genotypes (alr104 and wild type) would cause the Em to depolarize more than in the Al-sensitive mutants (als3 and als5); or (ii) release of organic anions from the Arabidopsis roots upon Al exposure, which would depolarize the Em (Olivetti et al., 1995; Papernik and Kochian, 1997; Kollmeier et al., 2001). Under Al stress, Arabidopsis has been reported to release malate (Hoekenga et al., 2006), citrate (Liu et al., 2009), pyruvate, and succinate (Larsen et al., 1998). Characterization of the AtALMT transporter revealed that Arabidopsis falls into the pattern II category (Ma et al., 2001), requiring 4 h induction to achieve maximum malate release (Kobayashi et al., 2007). For this reason, malate efflux could not have caused the observed Em depolarization in the tolerant genotypes as measured in the present study because the experimental period was only 60 min.
Larsen et al. (1998) reported that the alr104 mutant and the wild type release similar amounts of citrate upon Al exposure. Tricarboxylate citrate3– has a 6- to 8-fold greater ability to chelate Al than bicarboxylate malate2– (Ryan et al., 2001). Citrate efflux occurs through MATE transporters in Arabidopsis (Liu et al., 2009), wheat (Ryan et al., 2009), barley (Furukawa et al., 2007), and sorghum (Magalhaes et al., 2007). The MATE transporters are present in the plasma membrane of epidermal cells along the root apex as well as the MZ (Furukawa et al., 2007; Magalhaes et al., 2007; Ryan et al., 2009) and rapidly (within 20 min) release citrate following Al exposure (Zhao et al., 2003). The release of large amounts of citrate3– upon Al exposure from the Al-tolerant genotypes (alr104 and the wild type) would decrease the intracellular negatively charged citrate thereby maintaining Em depolarization, whereas release of smaller amounts of citrate from Al-sensitive genotypes would diminish Em depolarization (Fig. 8).
Given that K+ transport in plants usually occurs near the electrochemical equilibrium, theoretically any change in Em would affect K+ flux. The low-pH treatment caused immediate depolarization in all the genotypes tested (Fig. 8). This Em depolarization should lead to increased K+ efflux from the roots through K+ channels as shown previously under low-pH conditions (Babourina et al., 2001; Shabala et al., 2006), which was indeed observed in the DEZ of all genotypes in this study (Fig. 6). The combined low-pH/Al treatment caused a smaller initial depolarization in Al-sensitive mutants (both zones) compared with the low-pH treatment (Figs 8, 9). This shift in Em towards less depolarization or even hyperpolarization should decrease K+ efflux or even induce K+ influx, which was observed in the DEZ of all the genotypes tested (Fig. 6). Similar results were reported in soybean suspension cells (Stass and Horst, 1995) and wheat, wherein inhibition of K+ efflux by Al ions was more pronounced in Al-sensitive Scout than Al-tolerant Atlas wheat (Sasaki et al., 1995). Interestingly, Al treatment hyperpolarised the Em in the DEZ of Al-sensitive Arabidopsis mutants (als3 and als5) after 20 min (Fig. 8). K+ influx occurred together with this Em hyperpolarization in the Al-sensitive mutants (Fig. 6), which might have been the result of hyperpolarization-activated K+ inward-rectifying channels (KIRCs) (Maathuis and Sanders, 1995; Lebaudy et al., 2007).
The DEZ-type regulation of K+ flux by Em was not found in the MZ, with no specific pattern of K+ flux changes observed under either low-pH or combined low-pH/Al stress (Fig. 7). Indeed, the MZ has a larger number of K+ transport systems than the root apex (Hanson and Kahn, 1957; Ahn et al., 2004b; Vallejo et al., 2005), but not all K+ transport systems are voltage gated in the MZ of Arabidopsis roots (Lebaudy et al., 2007).
Compared with other genotypes, the highest shoot K+ concentration (Fig. 3B) and lowest K+ efflux (Fig. 6) observed for als3 under low-pH stress independently supports the observation of Koyama et al. (2001) that K+ transport is altered at low pH values in Arabidopsis. The higher shoot K+ concentration in the Al-sensitive mutants (als3 and als5 mutants) could be linked to decreased K+ efflux or enhanced K+ influx in the DEZ (Fig. 6), indicating a disturbance in K+ homeostasis. This shift in K+ flux towards influx might be due to a direct or indirect effect of ALS3 and ALS mutations. ALS3 is a plasma membrane-localized ABC transporter-like protein (Larsen et al., 2005). Although it shares high similarity with other plant ABC transporters, it lacks the ATP-binding cassette. It has been proposed that ALS3 is involved in translocation of Al from Al-sensitive tissues (Larsen et al., 2005). This conclusion is based on its high expression in the phloem. However, 24 h exposure to Al had no effect on ALS3 expression in the phloem, but shifted ALS3 expression from external to internal cells (Larsen et al., 2005). In the current study, an immediate difference between the wild type and als3 mutants in ion fluxes and Em was observed after exposure to Al. It indicates that ALS3 functioning may be linked to maintenance of Em depolarization, K+ efflux, H+ influx, and, in the longer term, to K+ homeostasis. These findings are consistent with physiological studies on plants with alt1-1 mutation (a suppressor of als3 mutation), which demonstrated that alt1-1 mutation increased Al resistance by pH adjustment rather than Al exclusion (Gabrielson et al., 2006).
In summary, the enhanced ability of the als5 and alr104 mutants to alkalinize the rhizosphere and take up H+ from a low-pH environment is responsible for the low-pH tolerance in these mutants. Higher tolerance to combined low-pH/Al stress in the wild type and alr104 mutant coincided with a higher resting Em and continuous Em depolarization, higher K+ efflux, and higher H+ influx, which are linked to the plant's ability to make the rhizosphere less acidic. Low-pH tolerance (als5 mutant) was associated with higher H+ uptake under low-pH stress; however, this ability was abolished by exposure to Al. Therefore, the mechanisms that underlie plant tolerance to acidic and Al stresses appear to be different.
Acknowledgments
JB is a recipient of the Endeavour International Postgraduate Research Scholarship and University Postgraduate Award. Additional support was provided by the Australian Research Council.
References
- Ahn SJ, Rengel Z, Matsumoto H. Aluminum-induced plasma membrane surface potential and H+-ATPase activity in near-isogenic wheat lines differing in tolerance to aluminum. New Phytologist. 2004a;162:71–79. [Google Scholar]
- Ahn SJ, Shin R, Schachtman DP. Expression of KT/KUP genes in Arabidopsis and the role of root hairs in K+uptake. Plant Physiology. 2004b;134:1135–1145. doi: 10.1104/pp.103.034660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn SJ, Sivaguru M, Chung GC, Rengel Z, Matsumoto H. Aluminium-induced growth inhibition is associated with impaired efflux and influx of H+across the plasma membrane in root apices of squash (Cucurbita pepo) Journal of Experimental Botany. 2002;53:1959–1966. doi: 10.1093/jxb/erf049. [DOI] [PubMed] [Google Scholar]
- Ahn SJ, Sivaguru M, Osawa H, Chung GC, Matsumoto H. Aluminum inhibits the H+-ATPase activity by permanently altering the plasma membrane surface potentials in squash roots. Plant Physiology. 2001;126:1381–1390. doi: 10.1104/pp.126.4.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon DI, Johnson CM. Influence of hydrogen ion concentration on the growth of higher plants under controlled conditions. Plant Physiology. 1942;17:525–539. doi: 10.1104/pp.17.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babourina O, Hawkins B, Lew RR, Newman I, Shabala S. K+transport by Arabidopsis root hairs at low pH. Australian Journal of Plant Physiology. 2001;28:635–641. [Google Scholar]
- Calba H, Jaillard B. Effect of aluminium on ion uptake and H+release by maize. New Phytologist. 1997;137:607–616. [Google Scholar]
- Cuin TA, Shabala S. Exogenously supplied compatible solutes rapidly ameliorate NaCl-induced potassium efflux from barley roots. Plant and Cell Physiology. 2005;46:1924–1933. doi: 10.1093/pcp/pci205. [DOI] [PubMed] [Google Scholar]
- Degenhardt J, Larsen PB, Howell SH, Kochian LV. Aluminum resistance in the Arabidopsis mutant alr-104 is caused by an aluminum-induced increase in rhizosphere pH. Plant Physiology. 1998;117:19–27. doi: 10.1104/pp.117.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuglsang AT, Guo Y, Cuin TA, et al. Arabidopsis protein kinase PKS5 inhibits the plasma membrane H+-ATPase by preventing interaction with 14-3-3 protein. The Plant Cell. 2007;19:1617–1634. doi: 10.1105/tpc.105.035626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa J, Yamaji N, Wang H, Mitani N, Murata Y, Sato K, Katsuhara M, Takeda K, Ma JF. An aluminum-activated citrate transporter in barley. Plant and Cell Physiology. 2007;48:1081–1091. doi: 10.1093/pcp/pcm091. [DOI] [PubMed] [Google Scholar]
- Gerendas J, Ratcliffe RG, Sattelmacher B. 31P nuclear magnetic resonance evidence for differences in intracellular pH in the roots of maize seedlings grown with nitrate or ammonium. Journal of Plant Physiology. 1990;137:125–128. [Google Scholar]
- Gabrielson KM, Cancel JD, Morua LF, Larsen PB. Identification of dominant mutations that confer increased aluminium tolerance through mutagenesis of the Al-sensitive Arabidopsis mutant, als3-1. Journal of Experimental Botany. 2006;57:943–951. doi: 10.1093/jxb/erj080. [DOI] [PubMed] [Google Scholar]
- Hanson JB, Kahn JS. The kinetics of potassium accumulation by corn roots as a function of cell maturity. Plant Physiology. 1957;32:497–498. doi: 10.1104/pp.32.5.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekenga OA, Maron LG, Pineros MA, et al. AtALMT1, which encodes a malate transporter, is identified as one of several genes critical for aluminum tolerance in Arabidopsis. Proceedings of the National Academy of Sciences, USA. 2006;103:9738–9743. doi: 10.1073/pnas.0602868103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikka T, Kobayashi Y, Iuchi S, Sakurai N, Shibata D, Kobayashi M, Koyama H. Natural variation of Arabidopsis thaliana reveals that aluminum resistance and proton resistance are controlled by different genetic factors. Theoretical and Applied Genetics. 2007;115:709–719. doi: 10.1007/s00122-007-0602-5. [DOI] [PubMed] [Google Scholar]
- Iuchi S, Koyama H, Iuchi A, Kobayashi Y, Kitabayashi S, Kobayashi Y, Ikka T, Hirayama T, Shinozaki K, Kobayashi M. Zinc finger protein STOP1 is critical for proton tolerance in Arabidopsis and coregulates a key gene in aluminum tolerance. Proceedings of the National Academy of Sciences, USA. 2007;104:9900–9905. doi: 10.1073/pnas.0700117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasamo K. Purification and properties of the plasma membrane H+ translocating adenosine triphosphatase of Phaseolus mungo L roots. Plant Physiology. 1986;80:818–824. doi: 10.1104/pp.80.4.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd PS, Llugany M, Poschenrieder C, Gunse B, Barcelo J. The role of root exudates in aluminium resistance and silicon-induced amelioration of aluminium toxicity in three varieties of maize (Zea mays L.) Journal of Experimental Botany. 2001;52:1339–1352. [PubMed] [Google Scholar]
- Kidd PS, Proctor J. Why plants grow poorly on very acid soils: are ecologists missing the obvious? Journal of Experimental Botany. 2001;52:791–799. doi: 10.1093/jexbot/52.357.791. [DOI] [PubMed] [Google Scholar]
- Kinraide TB. Proton extrusion by wheat roots exhibiting severe aluminum toxicity symptoms. Plant Physiology. 1988;88:418–423. doi: 10.1104/pp.88.2.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinraide TB. Toxicity factors in acidic forest soils: attempts to evaluate separately the toxic effects of excessive Al3+and H+and insufficient Ca2+ and Mg2+ upon root elongation. European Journal of Soil Science. 2003;54:323–333. [Google Scholar]
- Kobayashi Y, Hoekenga OA, Itoh H, Nakashima M, Saito S, Shaff JE, Maron LG, Pineros MA, Kochian LV, Koyama H. Characterization of AtALMT1 expression in aluminum-inducible malate release and its role for rhizotoxic stress tolerance in Arabidopsis. Plant Physiology. 2007;145:843–852. doi: 10.1104/pp.107.102335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochian LV, Hoekenga OA, Piñeros MA. How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorus efficiency. Annual Review of Plant Biology. 2004;55:459–493. doi: 10.1146/annurev.arplant.55.031903.141655. [DOI] [PubMed] [Google Scholar]
- Kollmeier M, Dietrich P, Bauer CS, Horst WJ, Hedrich R. Aluminum activates a citrate-permeable anion channel in the aluminum-sensitive zone of the maize root apex. A comparison between an aluminum-sensitive and an aluminum-resistant cultivar. Plant Physiology. 2001;126:397–410. doi: 10.1104/pp.126.1.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama H, Toda T, Hara T. Brief exposure to low-pH stress causes irreversible damage to the growing root in Arabidopsis thaliana: pectin–Ca interaction may play an important role in proton rhizotoxicity. Journal of Experimental Botany. 2001;52:361–368. [PubMed] [Google Scholar]
- Koyama H, Toda T, Yokota S, Dawair Z, Hara T. Effects of aluminum and pH on root growth and cell viability in Arabidopsis thaliana strain Landsberg in hydroponic culture. Plant and Cell Physiology. 1995;36:201–205. [Google Scholar]
- Larsen PB, Degenhardt J, Tai C-Y, Stenzler LM, Howell SH, Kochian LV. Aluminum-resistant Arabidopsis mutants that exhibit altered patterns of aluminum accumulation and organic acid release from roots. Plant Physiology. 1998;117:9–17. doi: 10.1104/pp.117.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen PB, Geisler MJB, Jones CA, Williams KM, Cancel JD. ALS3 encodes a phloem-localized ABC transporter-like protein that is required for aluminum tolerance in Arabidopsis. The Plant Journal. 2005;41:353–363. doi: 10.1111/j.1365-313X.2004.02306.x. [DOI] [PubMed] [Google Scholar]
- Larsen PB, Tai CY, Kochian LV, Howell SH. Arabidopsis mutants with increased sensitivity to aluminum. Plant Physiology. 1996;110:743–751. doi: 10.1104/pp.110.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazof DB, Holland MJ. Evaluation of the aluminium-induced root growth inhibition in isolation from low pH effects in Glycine max, Pisum sativum and Phaseolus vulgaris. Australian Journal of Plant Physiology. 1999;26:147–157. [Google Scholar]
- Lebaudy A, Véry A-A, Sentenac H. K+ channel activity in plants: genes, regulations and functions. FEBS Letters. 2007;581:2357–2366. doi: 10.1016/j.febslet.2007.03.058. [DOI] [PubMed] [Google Scholar]
- Lindberg S, Yahya A. Effects of pH and mineral nutrition supply on K (+) ((86) Rb (+)) influx and plasma membrane ATPase activity in roots of sugar beets. Journal of Plant Physiology. 1994;144:150–155. [Google Scholar]
- Liu J, Jurandir VM, Jon S, Leon VK. Aluminum-activated citrate and malate transporters from the MATE and ALMT families function independently to confer Arabidopsis aluminum tolerance. The Plant Journal. 2009;57:389–399. doi: 10.1111/j.1365-313X.2008.03696.x. [DOI] [PubMed] [Google Scholar]
- Llugany M, Poschenrieder C, Barceló J. Monitoring of aluminium-induced inhibition of root elongation in four maize cultivars differing in tolerance to aluminium and proton toxicity. Physiologia Plantarum. 1995;93:265–271. [Google Scholar]
- Ma JF, Ryan PR, Delhaize E. Aluminium tolerance in plants and the complexing role of organic acids. Trends in Plant Science. 2001;6:273–278. doi: 10.1016/s1360-1385(01)01961-6. [DOI] [PubMed] [Google Scholar]
- Ma JF, Zheng SJ, Matsumoto H, Hiradate S. Detoxifying aluminium with buckwheat. Nature. 1997;390:569–570. [Google Scholar]
- Maathuis FJM, Sanders D. Contrasting roles in ion transport of two K+-channel types in root cells of Arabidopsis thaliana. Planta. 1995;197:456–464. doi: 10.1007/BF00196667. [DOI] [PubMed] [Google Scholar]
- Magalhaes JV, Liu J, Guimaraes CT, et al. A gene in the multidrug and toxic compound extrusion (MATE) family confers aluminum tolerance in sorghum. Nature Genetics. 2007;39:1156–1161. doi: 10.1038/ng2074. [DOI] [PubMed] [Google Scholar]
- Matsumoto H. Cell biology of aluminum toxicity and tolerance in higher plants. International Review of Cytology. 2000;200:1–46. doi: 10.1016/s0074-7696(00)00001-2. [DOI] [PubMed] [Google Scholar]
- Newman IA. Ion transport in roots: measurement of fluxes using ion-selective microelectrodes to characterize transporter function. Plant, Cell and Environment. 2001;24:1–14. doi: 10.1046/j.1365-3040.2001.00661.x. [DOI] [PubMed] [Google Scholar]
- Olivetti GP, Cumming JR, Etherton B. Membrane-potential depolarization of root cap cells precedes aluminum tolerance in snapbean. Plant Physiology. 1995;109:123–129. [Google Scholar]
- Osawa H, Matsumoto H. Possible involvement of protein phosphorylation in aluminum-responsive malate efflux from wheat root apex. Plant Physiology. 2001;126:411–420. doi: 10.1104/pp.126.1.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papernik LA, Kochian LV. Possible involvement of Al-induced electrical signals in Al tolerance in wheat. Plant Physiology. 1997;115:657–667. doi: 10.1104/pp.115.2.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel AF, Mobin M, Rao IM, Horst WJ. Proton toxicity interferes with the screening of common bean (Phaseolus vulgaris L.) genotypes for aluminium resistance in nutrient solution. Journal of Plant Nutrition and Soil Science. 2005;168:607–616. [Google Scholar]
- Raven JA. Terrestrial rhizophytes and H+currents circulating over at least a millimetre: an obligate relationship? New Phytologist. 1991;117:177–185. [Google Scholar]
- Ryan PR, Delhaize E, Jones DL. Function and mechanism of organic anion exudation from plant roots. Annual Review of Plant Physiology and Plant Molecular Biology. 2001;52:527–560. doi: 10.1146/annurev.arplant.52.1.527. [DOI] [PubMed] [Google Scholar]
- Ryan PR, Raman H, Gupta S, Horst WJ, Delhaize E. A second mechanism for aluminum resistance in wheat relies on the constitutive efflux of citrate from roots. Plant Physiology. 2009;149:340–351. doi: 10.1104/pp.108.129155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M, Kasai M, Yamamoto Y, Matsumoto H. Involvement of plasma membrane potential in the tolerance mechanism of plant roots to aluminum toxicity. Plant and Soil. 1995;171:119–124. [Google Scholar]
- Sawaki Y, Iuchi S, Kobayashi Y, et al. STOP1 regulates multiple genes that protect Arabidopsis from proton and aluminum toxicities. Plant Physiology. 2009;150:281–294. doi: 10.1104/pp.108.134700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabala S, Demidchik V, Shabala L, Cuin TA, Smith SJ, Miller AJ, Davies JM, Newman IA. Extracellular Ca2+ ameliorates NaCl-induced K+ loss from Arabidopsis root and leaf cells by controlling plasma membrane K+-permeable channels. Plant Physiology. 2006;141:1653. doi: 10.1104/pp.106.082388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabala SN, Lew RR. Turgor regulation in osmotically stressed Arabidopsis epidermal root cells. Direct support for the role of inorganic ion uptake as revealed by concurrent flux and cell turgor measurements. Plant Physiology. 2002;129:290–299. doi: 10.1104/pp.020005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, He LF, Sasaki T, et al. Citrate secretion coupled with the modulation of soybean root tip under aluminum stress. Up-regulation of transcription, translation, and threonine-oriented phosphorylation of plasma membrane H+-ATPase. Plant Physiology. 2005;138:287–296. doi: 10.1104/pp.104.058065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen R, Ma J, Kyo M, Iwashita T. Compartmentation of aluminium in leaves of an Al-accumulator, Fagopyrum esculentum Moench. Planta. 2002;215:394–398. doi: 10.1007/s00425-002-0763-z. [DOI] [PubMed] [Google Scholar]
- Stass A, Horst WJ. Effect of aluminium on membrane properties of soybean (Glycine max) cells in suspension culture. Plant and Soil. 1995;171:113–118. [Google Scholar]
- Trofimova MS, Smolenskaya IN, Drabkin AV, Galkin AV, Babakov AV. Does plasma membrane H+-ATPase activation by fusicoccin involve protein kinase? Physiologia Plantarum. 1997;99:221–226. [Google Scholar]
- Vallejo AJ, Peralta ML, Santa-Maria GE. Expression of potassium-transporter coding genes, and kinetics of rubidium uptake, along a longitudinal root axis. Plant, Cell and Environment. 2005;28:850–862. [Google Scholar]
- Wherrett T, Ryan PR, Delhaize E, Shabala S. Effect of aluminium on membrane potential and ion fluxes at the apices of wheat roots. Functional Plant Biology. 2005;32:199–208. doi: 10.1071/FP04210. [DOI] [PubMed] [Google Scholar]
- Yamashita K, Mimura T, Shimazaki K-i. Evidence for nucleotide-dependent passive H+ transport protein in the plasma membrane of barley roots. Plant and Cell Physiology. 2003;44:55–61. doi: 10.1093/pcp/pcg005. [DOI] [PubMed] [Google Scholar]
- Yan F, Feuerle R, Schaffer S, Fortmeier H, Schubert S. Adaptation of active proton pumping and plasmalemma ATPase activity of corn roots to low root medium pH. Plant Physiology. 1998;117:311–319. doi: 10.1104/pp.117.1.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan F, Schubert S, Mengel K. Effect of low root medium pH on net proton release, root respiration, and root growth of corn (Zea mays L.) and broad bean (Vicia faba L.) Plant Physiology. 1992;99:415–421. doi: 10.1104/pp.99.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Barkla B, Marshall J, Pittman J, Hirschi K. The Arabidopsis cax3 mutants display altered salt tolerance, pH sensitivity and reduced plasma membrane H+-ATPase activity. Planta. 2008;227:659–669. doi: 10.1007/s00425-007-0648-2. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Ma JF, Sato K, Takeda K. Differential Al resistance and citrate secretion in barley (Hordeum vulgare L.) Planta. 2003;217:794–800. doi: 10.1007/s00425-003-1043-2. [DOI] [PubMed] [Google Scholar]