Abstract
Speech motor control emerges in the neurophysiologic context of widely distributed, powerful coordinative mechanisms, including those mediating respiratory function. It is unknown, however, whether developing children are able to exploit the capabilities of neural circuits controlling homeostasis for the production of speech and voice. Speech and rest breathing were investigated in eleven 15-month-old children using inductance plethysmography (Respitrace). Rib cage and abdominal kinematics were studied using a time-varying correlational index of thoracoabdominal coupling (i.e., reflecting the synchrony of movement of the rib cage and abdomen) as well as simple classification of the moment-to-moment kinematic relationship of these two functional components (i.e., concurrent expansion or compression, or oppositional movement). Results revealed markedly different patterns of movement for rest breathing and speech breathing, although within types of vocalization (nonspeech vocalization, babbling, true word production) no differences were apparent. Whereas rest breathing was characterized by tight coupling of rib cage and abdominal movement (average correlation coefficients usually exceeded .90), speech breathing exhibited weak coupling (the correlation coefficient ranged widely, but averaged about .60). Furthermore, speech production by these toddlers included the occurrence of both rib cage and abdominal paradoxing, which are observed infrequently in adult speakers. These results fail to support the suggestion that speech emerges from the extant coordinative organization of rest breathing. Rather, even in its earliest stages breathing for speech and voice exhibits kinematic properties distinct from those of other observed behaviors.
Keywords: speech development, respiration, motor control, kinematics, babbling
The neurophysiologic development of speech has frequently been described as deriving its coordinative framework from existing motor control mechanisms (e.g., Grillner, 1982). A wide range of candidate coordinative mechanisms has been discussed with respect to speech production, some depending on well-known patterns of oromotor organization (e.g., chewing) and others relying on related organizational structures in other motor systems (e.g., locomotion; Grillner, 1982). The paucity of physiologic observations of speech development renders arguments regarding the validity of these mechanisms moot. Sufficient empirical support has yet to be built. The coordinative organization for speech may arise from extant motor control mechanisms, including those of respiration (Feldman & Smith, 1995; Smith, Ellenberger, Ballanyi, Richter, & Feldman, 1991; von Euler, 1982), mastication (Davis & MacNeilage, 1995), and nonspeech vocalization (Chiao, Larson, Yajima, & Ko, 1994; Larson, Yajima, & Ko, 1994). Alternatively speech motor control mechanisms may develop autonomously, independent of numerous separate, but related, behaviors.
The functional components of the respiratory system afford a unique opportunity for empirical evaluation of models of speech development. In addition to being easily and noninvasively accessible, this system can be simply, accurately, and completely modeled by the dynamic changes in abdominal and rib cage volumes (Hixon, Goldman, & Mead, 1973; Hoit, Hixon, Watson, & Morgan, 1990). By quantifying the manner in which these two functional components are altered to achieve changing behavioral goals (e.g., gas exchange, modulation of alveolar pressure, abdominal fixation), changing control structures, strategies, and mechanisms can be observed. For example, one plausible hypothesis is that cortical inputs for speech production bypass the relatively sophisticated sensorimotor integration afforded by the brainstem-level respiratory pattern generator underlying rest breathing (Feldman & Smith, 1995). It can be reasoned that, because the behavioral targets for speech and homeostatic breathing are so different, and because the mechanisms involved are comparatively simple compared to other speech processes, speech motor control must rely on a distinct coordinative mechanism. Observations of respiratory dynamics may reveal the presence or absence of these control-system redundancies during speech development. This question of shared control structures has been the focus of previous investigations of development of speech motor control (Moore & Ruark, 1996; Ruark & Moore, 1997).
Speech as a Successor to Earlier Emerging Behaviors
The notion that speech develops as a successor to earlier developing mechanisms (e.g., homeostatic ventilation) has the appeal of parsimony and evolutionary precedent (MacNeilage & Davis, 2000). Adaptation and exploitation of fully functioning mechanisms enable the child to apply established skills to the external demands of emergent speech communication. It would seem most efficient during motor learning to constrain the many degrees of freedom across speech subsystems by employing fully formed, intact coordinative systems (Bernstein, 1967). The intersystem coordination required for speech production (e.g., among respiratory, phonatory, and articulatory systems) might lead the toddler to recruit whatever organizational units are present, thereby reducing the relatively large error anticipated during motor learning. Development of new motor behaviors, including speech, may invoke the adaptation and refinement of established coordinative relationships to achieve new behavioral goals.
This idea has motivated several models of speech production that explicitly incorporate the motor organization of established central pattern generators (CPGs; Grillner, 1982; Wolff, 1991). These models rely on discrete brainstem nuclei, empirically observed in animals (Feldman & Smith, 1995), as the essential coordinative mechanism underlying rhythmic behaviors such as walking, chewing, and respiration. With respect to speech development specifically, Fawcus has stated this argument most strongly: “The harnessing of basic movements by the CNS is the essential problem in…the normal development of speech” (Fawcus, 1969, p. 558). Darley, Aronson, and Brown (1975), drawing on their extraordinary wealth of clinical intuition, elaborated this idea: “As the baby progresses to soft and then to solid food … jaw movements are modified for chewing. … The motor control of these nutritional movements must be adapted for speech production…. It is readily apparent that the pursing of the lips for sucking may be adapted to produce the phoneme /oo/ … opening of the jaws is necessary for the phoneme /o/ …” (p. 65). Consistent with these models of speech development, speech breathing and rest breathing would also be understood as sharing a common neural network. Grillner (1982) has suggested that centrally patterned muscle synergies are fractionated into smaller, tightly constrained units, which in turn may be independently controlled, perhaps by cortical centers. These functional units are putatively derived from parts of the CPGs for respiration, mastication, and swallowing (Grillner, 1982), each of which can constrain speech subsystems in a way that simplifies the control problems for speech development. An important immediate goal would then be to compare the coordinative characteristics of these behaviors with developing speech.
The many similarities of babbling and speech motivate the conceptualization of speech as a successor to babbling; similarly, babbling can be seen as succeeding other closely related, earlier emerging, centrally patterned behaviors. Rhythmic babbling may employ control mechanisms that underlie rhythmic patterns of respiration, phonation, and supralaryngeal articulatory movement (e.g., mandibular movement; Davis & MacNeilage, 1995; MacNeilage & Davis, 2000). Mature speech may reflect the neurophysiologic basis of these structures in the subtle rhythms that appear across languages (e.g., English has alternating strong-weak syllable patterns; Spanish is syllable-timed; Kent, Mitchell, & Sancier, 1991). These linguistic universals of phonologic development and rhythm lend further support to the idea that the neurophysiologic basis of emergent speech may be derived from the shaping and melding of endogenous control mechanisms.
The interaction of these CPGs during speech development can be applied to speech production by the super-imposition of rhythmic vocalization (including rhythmic jaw movement) on the rhythmic pattern of rest breathing. A child may exploit these patterned movements as she or he begins to produce speech by establishing a locus of control, presumably cortical, to coordinate these rhythms. The resulting behavior would be the product of the endogenous rhythms underlying the precursor behaviors. Support for this idea is derived from observations of mutual entrainment of biologic oscillators, which is widely observed across motor behavior (Kelso, Holt, Rubin, & Kugler, 1981).
The Emergence of Speech Independent of Other Motor Processes
Alternative representations of speech development are economically less appealing, even though these suggestions have better empirical support. These alternatives require that speech emerge as a behavior that is entirely distinct and separate from others. Support for this view is found in comparative studies of speech and nonspeech movements by adults (Moore, 1993; Moore, Smith, & Ringel, 1988; Wohlert & Goffman, 1994) and from studies of mandibular movement in 15-month-olds (Moore & Ruark, 1996) and lip movement in 2-year-olds (Ruark & Moore, 1997). Speech production has been shown in these studies to be sufficiently different from earlier emerging behaviors that it has become increasingly difficult to hypothesize any benefit to speech from extant mechanisms.
With respect to respiratory control of speech and nonspeech behaviors, distinct coordinative mechanisms may be suggested by the gross differences in the physiologic goals of each. The competition of these behaviors entails task prioritization and functional compromise. During speech breathing blood gas levels and extracellular pH likely depart significantly from homeostatic levels, with speakers hyperventilating during normal speech (Bunn & Mead, 1971). This divergence from homeostasis might seem to entail different control systems in meeting the distinct goals of these competing behaviors. Von Euler (1982) noted that the goal of breathing during speech production is distinct from that of homeostasis (i.e., achievement of target alveolar pressure versus maintenance of blood gas levels). This deviation from homeostasis is easily tolerated under normal speaking conditions, but is obviously limiting during conditions of increased metabolic need (e.g., aerobic exercise; Bunn & Mead, 1971).
The competitive relationship of speech and homeostatic demands is also elucidated by the observation that ventilation demands may supersede those of speech during vigorous exercise; speech phrasing decreases in length with increased exertion (Bunn & Mead, 1971; von Euler, 1982). Depending on external and internal demands, then, one of these two control systems can be seen to dominate the coordinative organization for breathing. Finally, the observable musculoskeletal actions characteristic of adult speech breathing and rest breathing are measurably distinct (Estenne, Zocchi, Ward, & Macklem, 1990; Hixon, Goldman, & Mead, 1973; Hoit, Plassman, Lansing, & Hixon, 1988). Muscle activation patterns underlying speech breathing are quite unlike those observed during rest breathing. Active expiratory effort characterizes speech breathing, whereas the expiratory phase of rest breathing is primarily passive.
In summary, models of speech development can be seen to differ widely in their reliance on extant motor behaviors. Mature speech and nonspeech oromotor behaviors exhibit distinct control modes and timing patterns, although their essential properties include shared anatomic and organizational structures. The empirical problem is one of distilling the generalized, behavior-dependent control structures from the observed movement dynamics of variable individual events. Detection of these control structures will allow us to track and describe the emergence of the control mechanisms for speech. Finally, the developmental process incorporating rhythmic, patterned movements with the contextual and external demands of speech will be revealed.
Respiratory Kinematics in Speech and Rest Breathing
The respiratory system is most accessible in infants and adults through observations of rib cage and abdominal motion. Respiratory kinematics in adults have been quantified most reliably for rest breathing, which has been shown to be dominated by synchronized displacement of the rib cage and abdomen (Fugl-Meyer, 1974; Hixon, 1973; Hixon et al., 1973; Konno & Mead, 1967; Sharp, Goldberg, Druz, & Danon, 1975). During rest breathing in adults these two functional components appear coupled, exhibiting synchronous expansion and compression consequential to diaphragmatic activation patterns. Inspiration is correlated with volumetric increases in both the rib cage and the abdomen; expiration is correlated with decreases in each (Hixon, 1973). These two components do not contribute equally to air volume exchange during rest breathing, however. Rest breathing is most commonly characterized by greater relative displacement of the rib cage than the abdomen, although equal contributions by each component or a predominance of abdominal displacement can also be observed (Hixon, 1973; Hixon et al., 1973; Hoit & Hixon, 1986; Sharp et al., 1975). Because the respiratory system is open (i.e., with respect to the atmosphere) during rest breathing, either subcomponent (the rib cage or the abdomen) can be used independently to modulate alveolar pressure and airflow (Hixon, 1973; Konno & Mead, 1967).
Speech breathing kinematics have been characterized as significantly more variable than patterns associated with resting respiration in adults (Hixon et al., 1973) and in children less that 3 years old (Boliek, Hixon, Watson, & Morgan, 1996, 1997). Generation of subglottal pressure for speech production is achieved by a net compression of the lungs, which can be accomplished by a range of rib cage and abdominal contributions. The changing relaxation pressures of the system at different lung volumes dictate how target pressures can be achieved most efficiently (Hixon, 1973), although a range of combined forces can be employed to modulate alveolar pressure. The effects of trade-offs among such factors as elastic forces, muscle efficiency, latency of aerodynamic response to muscle activation, and motor control complexity are unknown. Estenne and colleagues (1990) have shown in adults that speech breathing, like rest breathing, exhibits a predominance of rib cage displacement compared to the relatively smaller displacement of the abdomen. Stathopoulos, Hoit, Hixon, Watson, and Solomon (1991) similarly have shown that abdominal volumes are relatively lower and rib cage volumes are higher during speech production than during rest breathing. This finding is in agreement with earlier observations (Hixon, 1973) that abdominal muscles are more active during speech production than during rest.
One explanation for these differing configurations is that activation of abdominal muscles during speech breathing enhances the effects of diaphragm activity, promoting the rapid inspiratory phases that are characteristic of speech (Sharp et al., 1975). Hoit and colleagues (1988) observed abdominal muscle activity during speech and rest breathing, showing that abdominal muscle activity increases during speech breathing compared to rest breathing. Again, the explanation for differences in abdominal activity may be that more forceful activity is required during speech to keep “constituent parts in favorable mechanical circumstances to meet the inspiratory and expiratory requirements of the speech breathing cycle” (Hoit et al., 1988). Speech and rest breathing are further distinguished by the modulation of expiratory effort seen during speech to underlie patterns of stress and intonation (Murdoch, Chenery, Stokes, & Hardcastle, 1991).
Paradoxical movement of the rib cage and abdomen also distinguishes speech breathing from rest breathing. Rib cage or abdominal paradoxing is characterized by the uncoupling of rib cage and abdominal movement to the extent that these components appear to work in opposition. This normally occurring oppositional movement is a well-documented component of speech breathing (Hixon, 1973; Hodge & Rochet, 1989; Hoit, 1994; Hoit & Hixon, 1986), changing in its frequency of occurrence during postnatal development and even during sleep stages. It occurs most frequently during REM (rapid eye movement) sleep (e.g., Goldman, Williams, Soo Hoo, Trang, & Gaultier, 1995). Abdominal paradoxing during speech (i.e., abdominal volume increasing during expiration or decreasing during inspiration) is more common than rib cage paradoxing (i.e., rib cage volume increasing during expiration or decreasing during inspiration) and is seen most often at high lung volumes (Hodge & Rochet, 1989; Hoit & Hixon, 1986). Estenne and colleagues (1990) have suggested that abdominal paradoxing in speech breathing “prevents pressure from dissipating and prevents shortening of the diaphragm so therefore optimizes inspiratory muscle function” (p. 2081). There is general agreement that paradoxing occurs during speech, but its frequency of occurrence is uncertain.
Boliek and colleagues investigated breathing during a wide variety of behaviors on the part of 40 infants between two age ranges: 5 weeks to 1 year (1996) and 1 year to 3 years (1997). These investigators demonstrated clear differences between breathing patterns associated with vocalization and those associated with rest breathing (e.g., initiation of vocalization at lung volumes that exceeded the predicted end-inspiratory level for tidal breathing) and showed a remarkable degree of variability across behavior types. The patterns observed included paradoxical movement of the abdomen and rib cage, which was observed about 20% of the time in children 1 year old or younger. These investigators speculated that infants experiment with a wide range of spatiotemporal patterns of breathing movements during vocalization.
Methodologic Considerations in Studying Respiratory Kinematics in Toddlers
One major obstacle in studying the development of speech and nonspeech breathing in toddlers is maintaining instrumental calibration (Boliek et al., 1996, 1997). This problem has usually been overcome by frequent recalibration and experimental control of recording conditions and experimental tasks. However, the calibration procedures and movement restraint techniques themselves may prove to be too intrusive to allow observation of naturally occurring behavior. Alternatively, for observations of very young children in the early developmental periods of speech, it may be possible and desirable to use uncalibrated abdominal and rib cage signals.
Essential elements of speech breathing include the timing of expiratory effort with respect to speech onset and the modulation of expiratory airflow and pressure (Boliek et al., 1996, 1997; Netsell, Lotz, Peters, & Schulte, 1994). Quantification of the coordinative framework underlying speech has typically required carefully controlled aerodynamic measures, including direct measures of airflow and pressure. Alternatively for populations that will not tolerate a face mask, or cannot remain sufficiently still (e.g., young children), the relative roles of thoracic and abdominal muscle groups can be described and categorized with time-series analyses of their kinematic signals (e.g., concurrent downward slopes in both abdominal and rib cage circumference signals can be interpreted as giving rise to expiratory airflow).
The present investigation compared relative kinematics of the abdomen and rib cage in toddlers to provide a detailed description of timing and relative displacement of the respiratory components in developing speech. Of particular interest was whether children, like adults, exhibit task-specific movement patterns for speech breathing. This investigation also assessed the use of time-series analysis as a quantitative technique that avoids the limitations usually associated with calibration of respiratory kinematic signals. Relative changes in circumference of the two functional components of the respiratory system (i.e., abdomen and rib cage) over very brief periods (about one second) were compared to provide a dynamic index of their changing coordinative interaction.
Method
Subjects
Subjects were 11 15-month-old children (7 girls, 4 boys) who were participants in a longitudinal study of speech development. Subjects were free of known neurologic deficit, passed otoscopic and tympanometric screening (when tolerated by each child; tympanometry failed for four children, who were asymptomatic for middle ear pathology by parental report), and were developing normally according to parental report of achievement of gross motor, fine motor, cognitive, speech, and language milestones.
Experimental Protocol
Rest breathing and speech breathing were monitored in children using a Respitrace system (Ambulatory Monitoring, Inc.), a commercially available respiratory plethysmograph. This system transduces the circumferences of the rib cage and the abdomen using two elasticized bands, “respibands,” as transducers. Respibands were placed either on bare skin or over very light clothing (e.g., a T-shirt) for each subject and were secured only by the elasticity of the bands themselves. An experimental assistant monitored the transducer position constantly, repositioning it as necessary to maintain transducer sensitivity and isolation of the abdomen and rib cage signals. Small changes in the position of each respiband were easily accommodated by signal processing and by the analytic procedures described below. The rib cage respiband was placed around the rib cage, underneath the arms, as high as possible; the abdominal transducer was centered vertically on the umbilicus and placed posteriorly so that it overlapped the ribs as little as possible, if at all. The transducers did not overlap. Output signals from the transducers were low-pass filtered using analog filters with a cut-off of 30 Hz, then recorded using an FM instrumentation recorder (frequency response: DC-1250 Hz; S/N > 50 dB) for subsequent digitization. Speech audio signals were obtained using a wireless lapel microphone worn by the subject and were recorded on a separate AM channel of the same instrumentation recorder. Subjects were seated in a highchair with the adjustable tray positioned as close as possible to the child without applying pressure on the child or on the Respitrace bands. This positioning minimized unnecessary movements by the child. All stimuli and objects of interest were directly in front of the subject; this minimized reaching and extraneous leaning movements. One investigator was seated next to the child and described the child's activities online. This continuous description included a gloss of all utterances (i.e., vocalizations, babbling, and true words) produced, identification of rest breathing periods, and alerts with respect to extraneous movement by the child. Postural changes and reaching movements by the child usually yielded artifact in the Respitrace channels, necessitating exclusion of affected periods from the analysis. Spontaneous and imitative utterances were elicited using a variety of toys, books, and games. Each child's caregiver was present to reduce any anxiety the child might experience and to provide assistance in eliciting vocalizations. Speech utterances were recorded over a period of approximately 20 minutes. Rest breathing data, which were observed throughout the session, were identified as uninterrupted periods of rest breathing of at least 10 seconds duration constituting at least three respiratory cycles.
Sampling Procedures
The recorded data were transcribed from the original FM tape, noting periods of rest breathing, speech, and artifactual movement. Speech samples contaminated by concurrent movement, crying, laughter, chewing, or any other artifact were omitted from the analysis. Sampling criteria required further that the audio signal for each speech sample be free of acoustic artifact (i.e., audio signals were not contaminated by simultaneous utterances by the experimenter or the parent). Samples were collected and categorized as rest breathing (including at least three contiguous cycles), babbling, vocalization (single phonemes ≥ 1 s duration), and speech (true words). Classification of babbling on the basis of the acoustic signal alone is particularly difficult in children at this stage (Oller, 1978). Accordingly, the online gloss and subsequent transcription relied heavily on context. Utterances that were clearly referential or explicit requests, for example, were classified as speech. Utterances that were recognized by the parent as a part of the child's meaningful speech repertoire also were classified as speech. Conversely, utterances that were nonreferential and not requests were classified as babbling. Multisyllabic utterances were parsed into individual syllables before analysis; syllable order for each token was coded as an additional sample descriptor. These classifications provided a detailed description of the behaviors sampled, although all data obtained from speech, babbling, and vocalization utterances were subsequently combined into a single category, “speech,” for statistical analysis. Each sample was coded for subject identity, behavior type (rest breathing, speech, nonspeech, and vocalizations), position of each syllable in the utterance sequence (e.g., second of three syllables), and total number of syllables in the sample. All tokens meeting sampling criteria within each subject's session were included in the data corpus.
Sampling criteria for parsing target behaviors were intended to maximize the number of samples acquired while maintaining the homogeneity of each sample by minimizing the inclusion of nontarget behaviors (e.g., speech breathing samples did not include leading or trailing rest breathing). Rest-breathing samples included 3 to 7 breathing cycles and were analyzed as a single behavior. Speech samples were identified using only the audio channel and were demarcated by acoustic onset and offset. The boundaries of speech, babbling, and vocalization samples were identified by simultaneously viewing the digitized sample and listening to the original audio recording, which ensured accurate identification of the beginning and end of each utterance. Multiple sequential utterances, operationally defined as utterances separated by more than 500 ms, were digitized separately. Reliability for the method used to identify speech and rest samples was confirmed by reanalysis of 10% of the data by the same investigator. Correlations of the raw counts of each kinematic category between the two separate analyses ranged from .96 to 1.
Digitization and Signal Processing
Respitrace (two channels) and audio (one channel) waveforms from all acceptable samples were filtered for anti-aliasing (flp = 30 Hz) and digitized at 66.7 samples per second per channel. Following digitization, the audio waveforms were full-wave rectified and integrated to facilitate identification of speech onset and offset during the analysis.
Analysis
Time-series analyses were used to identify the changing within-cycle motion of the rib cage and abdomen during rest breathing and the other target tasks. The analyses were designed to provide two indices of respiratory function: (1) a dynamic index reflecting the coupling of rib cage and abdominal movements, and (2) a four-way classification scheme reflecting moment-by-moment changes in the relative direction of movement of each of the two components (i.e., both expanding, both compressing, abdominal compression with rib cage expansion, or abdominal expansion with rib cage compression). Using custom routines written for Matlab (Mathworks, 1999), a moving rectangular window, one second in length, was used to compute the simple correlation of the abdomen and rib cage signals over the course of each observation. The window was advanced one point (15 ms) for each correlation computation, yielding a function (“rmoving”) made up of the coefficients derived from each computation of the correlation. The 1-s window width, selected empirically from a range of 100 ms to 2 s, was judged to provide the most appropriate temporal resolution. Window sizes larger than one second were overly smoothed and were insufficiently sensitive to detect changes within speech events, each of which was less than one second in duration. Smaller window sizes were overly sensitive, with the resultant function emphasizing very brief (e.g., less than 100 ms) or transient relationships between the two signals. Speech or rest breathing segments were isolated from surrounding events. Portions of the rmoving function associated with rest or speech were isolated using computer-assisted identification of speech onset and offset, or inspiratory onsets. Rmoving values within each segment were transformed using the Fisher Z transform and were averaged. This average coefficient provided an index of coupling for each observation.
In addition to the magnitude of each coefficient on the correlation function, each rmoving point was categorized according to the slope direction of each Respitrace signal at that point. The slope of each signal was categorized as upward, downward, or flat within the middle 200 ms of the 1-s rmoving window. Again, the choice of a 200-ms window reflected an empirically determined decision regarding the sensitivity of the measure to small fluctuations in each signal. Table 1 includes four possible respiratory movement combinations as defined by the average slopes of the waveforms in each 1-s window: (1) Coupled Inspiration–both waveforms positive-going; (2) Coupled Expiration–both negative-going; (3) AB↑– oppositional movement with a negative-going rib cage signal and a positive-going abdominal signal; (4) RC↑– oppositional movement with positive-going rib cage signal and a negative-going abdominal signal). For the oppositional movement categories, no inference of airflow direction was attempted, as the transducers yielded uncalibrated values and airflow was not monitored. An additional category, annotated as Unspecified, consisted of those samples during which the average slope of either signal was nearly zero (i.e., one or both of the signals was flat, such as might occur at the peaks and troughs of the waveforms). Operationally, Unspecified points were defined as those occurrences for which the slope of the 200-ms analysis window was less than 30.3% of the standard deviation of the derivative of the entire signal for that channel. The inclusion of this category greatly reduced false indications of oppositional movement, which arose when the slopes of each signal were near zero. Slight differences or asynchronies at those points can yield opposite slope directions, which were not judged to be true instances of paradoxing. Occurrences of samples in each of these five categories are included in graphs of the rmoving function. This graphic representation provided a three-dimensional composite that showed (1) strength of coupling between abdominal and rib cage movement (i.e., the magnitude of rmoving) and (2) the relative kinematics (i.e., the symbol used to plot rmoving) over (3) time.
Table 1.
Categorization of all combinations of rib cage and abdominal slopes.
| Net slope of rib cage circumference signal | Net slope of abdominal circumference signal | ||
|---|---|---|---|
| Increasing | Decreasing | “Flat” | |
| Increasing | Coupled inspiratory movement | Oppositional movement with RC↑ | Unspecified |
| Decreasing | Oppositional movement with AB↑ | Coupled expiratory movement | Unspecified |
| “Flat” | Unspecified | Unspecified | Unspecified |
In Figures 1 and 2 symbols on the rmoving waveform reflect categorization of changing thoracoabdominal coordination, especially highlighting occurrences of oppositional movement. Coupled inspiration was inferred from observation of concurrently rising signals (i.e., expansion of each subsystem) and was plotted with light gray dots; coupled expiration was inferred from concurrent decreases in both signals (i.e., compression of each subsystem resulting in expiration) and was plotted with dark gray dots. Oppositional movements were classified into two types: AB↑, which was defined as decreasing rib cage circumference concurrent with increasing abdominal circumference (plotted with +s), and RC↑, which was defined as increasing rib cage circumference concurrent with decreasing abdominal circumference (plotted with ×s). (AB↑ and RC↑ are nonstandard symbols referring to oppositional movements of the rib cage and abdomen. Their use was necessitated by methodologic limitations imposed by the use of uncalibrated signals. Because the direction of airflow [i.e., inspiratory or expiratory] could be inferred only during vocalization [i.e., expiratory], it was not possible to distinguish rib cage paradoxing [i.e., expansion of the rib cage during expiration, or compression of the rib cage during inspiration] from abdominal paradoxing [i.e., expansion of the abdomen during expiration, or compression of the abdomen during inspiration] during rest breathing. The present description simply annotated the expansive component during an oppositional event [e.g., RC↑ indicated expansive rib cage displacement with compressive abdominal displacement]. Ribcage and abdominal paradoxing could be identified during vocalization, however, because no vocalized event was observed to rely on inspiratory flow.)
Figure 1.
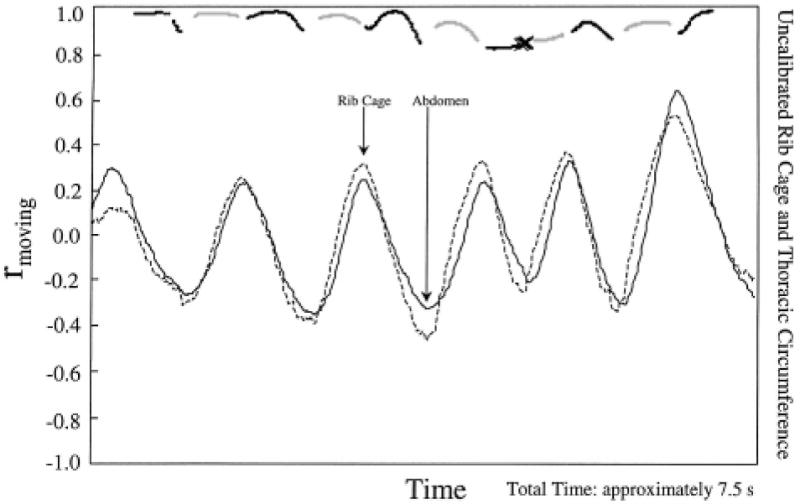
Results of windowed correlational analysis during rest breathing by Subject A. The tight coupling and synchrony of abdominal and rib cage signals (lower two traces) is reflected by uniformly high values (i.e., approximately 1.0) for rmoving (top trace) and the infrequent occurrence of RC arrow up oppositional events (indicated by × symbols in the rmoving trace). The light dots, dark dots, ×, and + symbols represent, respectively, coupled inspiration, coupled expiration, RC↑, and AB↑ in this figure and in Figure 2.
Figure 2.
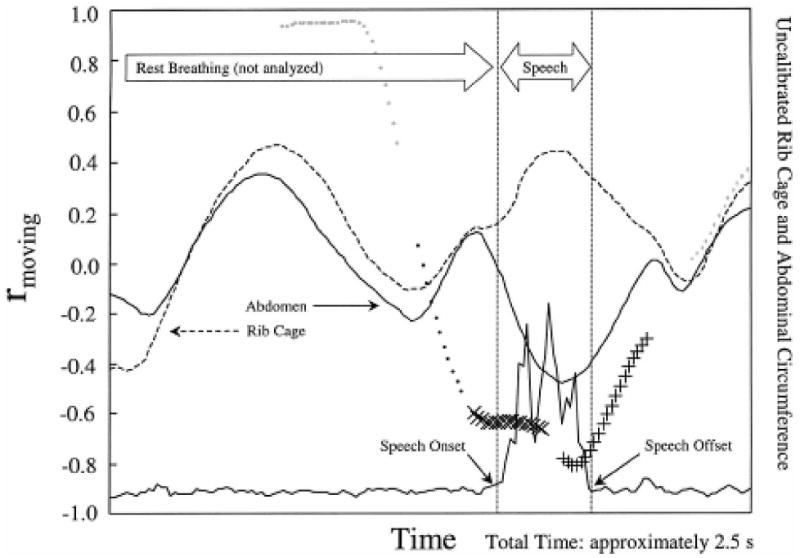
Results of the windowed correlational analysis of the utterance /εnt/ by Subject C revealed RC↑ (× symbols), then AB↑ (+ symbols), oppositional movement during speech (portion between dotted lines). The entire graph comprises approximately 2.5 s.
Type 5 (i.e., unspecified) points were not plotted, appearing as gaps in the rmoving function where the slope of at least one of the signals was nearly flat.
The coupling strength and the relative movement of the rib cage and abdomen for rest breathing and speech behaviors were compared using each of these measurements. The composite function of rmoving and categorization of kinematics facilitated qualitative and quantitative evaluation of the dynamics between these components. Quantitative differences among behaviors were tested by statistical analysis of rmoving and of the proportionate occurrences of kinematic categories. The averaged Fisher Z transforms of the correlation coefficients and the proportionate frequencies of kinematic configurations from each interval analyzed were subjected to descriptive and inferential statistical analyses (one-way analysis of variance: ANOVA).
Results
This investigation was designed to describe and contrast the coupling and relative dynamics of the rib cage and abdomen in 15-month-old children during rest breathing and speech breathing. A total of 339 samples were obtained for a range of behaviors from these 11 children. Table 2 summarizes the complete data set. These signals were evaluated with relatively high temporal resolution (i.e., sample rate = 66.7 Hz) to observe transient relative changes in thoracoabdominal motion and rmoving values. Figures 1 and 2 illustrate the nature of these measures in rest breathing and in speech respectively.
Table 2.
Composition of the complete data set (i.e., number of samples of each behavior type produced by each subject).
| Subject | Babbling samples | True word samples | Vocalization samples | Sum of babbling, speech, and vocalization samples | Rest-breathing samples |
|---|---|---|---|---|---|
| A | 6 | 22 | – | 28 | 3 |
| B | 18 | 5 | 5 | 28 | 5 |
| C | 5 | 32 | 3 | 40 | 3 |
| D | 12 | 12 | 3 | 27 | 6 |
| E | 11 | 34 | 1 | 46 | 5 |
| F | 9 | 3 | – | 12 | 1 |
| G | 3 | 40 | – | 43 | 3 |
| H | 16 | – | – | 16 | 6 |
| I | 4 | 1 | – | 5 | 3 |
| J | 27 | – | – | 27 | 4 |
| K | 14 | 11 | – | 25 | 3 |
| Total | 125 | 160 | 12 | 297 | 42 |
Figure 1 provides an example of the results of these correlational and classification analyses for approximately 7.5 s of rest breathing. Interpretation of the rmoving function is quite straightforward; a correlation of 1 indicated synchronous increase and/or decrease of the two waveforms, and a correlation of −1 indicated that the signals were moving in perfect opposition within the 1-s analysis window. Points plotted with dots indicated that the signals were both increasing or decreasing; points plotted with × or + indicated oppositional movement (i.e., RC↑ and AB↑, respectively). The most obvious relationship in this figure is the coupling of the rib cage and abdominal traces. Coupled inspiration and expiration constituted almost 80% of the points represented in this figure. This stable coupling was also reflected in the results of the correlational analysis, which revealed a consistently high rmoving function (usually exceeding .90; top trace). Occurrences of AB↑ or RC↑ were found to be absent or very infrequent (i.e., together constituting only 1% of samples), as reflected by the absence or small number of + and × points, respectively, in the rmoving function. The remaining 19% of rest-breathing analysis points shown in this figure were Unclassified, indicating that at least one of the two signals failed to exhibit a slope in excess of the criterion and was classified as “flat.” These unspecified points occurred almost exclusively at the peaks and troughs of each respiratory cycle.
Figure 2, which includes a single vocalization preceded by several seconds of rest breathing, illustrates a very different pattern, reflecting the dramatic differences sometimes observed for the utterances sampled. Only those points between speech onset and offset, as indicated on Figure 2, were analyzed for speech production, yielding a comparatively small data set. In sharp contrast to rest breathing, speech production was usually characterized by a sharp drop in the value of rmoving and a greater frequency of occurrence of oppositional movements (i.e., 83% of the kinematic samples shown in Figure 2 were categorized as paradoxical; 17% were unspecified; none showed coupled inspiration or expiration in this utterance). The numeric data sets from which Figures 1 and 2 were derived are shown in Table 3. Included in this table are the raw counts of the frequency of occurrence of each kinematic category, the relative frequency of occurrence for each category in that sample (expressed as percentages), and the mean and standard deviation of the correlation coefficients constituting rmoving for each analyzed section.
Table 3.
Raw data sets obtained for individual samples of rest breathing, shown in Figure 2, and speech breathing, shown in Figure 3. Absolute counts are shown with proportionate distributions (%) of each type. Also tabulated are the mean and standard deviations for rmoving for each behavior.
| Behavior/Subject/Figure | Absolute frequency of occurrence for kinematic categories | Relative frequency (%) of occurrence for kinematic categories | Coupling (rmoving) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | Mean r | SD | |
| Rest/A/2 | 163 | 206 | 0 | 5 | 90 | 35% | 44% | 0% | 1% | 19% | .96 | .05 |
| Speech/C/3 | 0 | 0 | 7 | 13 | 4 | 0% | 0% | 29% | 54% | 17% | −.70 | .07 |
Kinematic categories: 1 = Coupled Inspiration: concurrent rib cage and abdominal expansion
2 = Coupled Expiration: concurrent rib cage and abdominal compression
3 = AB↑: abdominal expansion with oppositional rib cage compression
4 = RC↑: rib cage expansion with oppositional abdominal compression
5 = Unspecified: one or both signals near a slope of zero
Analysis of the complete data set proceeded in several stages, each of which represented successively greater reduction. Initially, frequencies of occurrence of each category type and average rmoving values within each observation were collapsed across repetitions of each task (e.g., repeated observations of rest breathing or of vocalizations) within subjects. Analysis of variance revealed a significant subject effect for coupling strength [F(10) = 2.71, p = .003], as reflected by the averaged Fisher Z transform of the correlation coefficient function (rmoving). No significant subject effect was observed for the frequency of occurrence of coupled inspiration [F(10) = 1.42, p = .141], coupled expiration [F(10) = 1.05, p = .398], or unspecified samples [F(10) = .59; p = .819]. Post hoc pairwise multiple comparisons of rmoving using the Tukey procedure revealed no significant differences between any two subjects. Four of the 11 subjects yielded mean rmoving values in excess of .75; one yielded a mean rmoving of less than .30. There were significant subject effects for AB↑ [F(10) = 3.59, p is less than or equal to .001] and RC↑ [F(10) = 3.18, p < .001]. Post hoc pairwise multiple comparisons using the Tukey procedure revealed significant differences for AB↑ only between Subject A and 6 of the other 10 subjects (p < .05). The mean frequency of occurrence of AB↑ exhibited by Subject A (16.0% across tasks) well exceeded the mean for the remaining 10 subjects (5.5%). Similarly, significant differences between subjects for RC↑ were obtained only between Subject G and 7 of the 10 other subjects (p < .05). The mean frequency of occurrence of RC↑ by Subject G (26.1%) was approximately three times the mean for the remaining subjects (8.4%). These findings suggested that subjects generally exhibited similar patterns of respiratory movement, with varying degrees of coupling between abdomen and rib cage, and that subject-specific differences in patterns of movement could be substantial.
Another consideration was whether different behavior types (i.e., rest breathing, nonspeech vocalization, babbling, and production of true words) exhibited different kinematic patterns or coupling between the two components. Rest breathing was typified by strong coupling (average rmoving = .94), exhibiting coupled inspiration and coupled expiration with rare occurrences of oppositional movement. Paradoxical movements of the rib cage and abdomen constituted only about 3% (i.e., 1.5% and 1.8% for AB↑ and RC↑, respectively) of all rest-breathing kinematic patterns. The three vocalization behaviors, however, exhibited very different patterns of rib cage and abdominal movement. These tasks showed infrequent (about 4% of the time) coupled inspiration, as expected for vocalization that is dominated by expiratory flow. These findings of inspiration during vocalization were usually caused by the short, but unavoidable, extension of the analysis window into the prespeech or postspeech interval. Speech and speech-like tasks exhibited significantly more paradoxing (6.9% and 10.3% for AB↑ and RC↑ respectively, totaling 17.2% of all classifications, five times more than that observed for rest breathing) and much weaker thoracoabdominal coupling (average rmoving = .62) than rest breathing. Inspection of the means in the lowest three rows of Table 4 supported the suggestion that the three utterances types showed minimal differences. Statistical analysis of this task effect and post hoc analysis of pairwise differences among tasks confirmed that task differences were significant only between rest breathing and each of the three utterance types. Analysis of variance revealed a main effect for task for coupled inspiration [F(3) = 102.90, p < .001], RC↑ [F(3) = 5.11, p = .002], unspecified samples [F(3) = 8.61, p < .001], and rmoving [F(3) = 18.69, p < .001]; nonsignificant differences were obtained for task effect for coupled expiration [F(3) = .42, p = .736] and AB arrow up [F(3) = 2.44, p = .064]. Post hoc pairwise multiple comparisons using the Tukey procedure revealed that these differences were associated with statistically significant differences between rest breathing and each of the three remaining utterance conditions (i.e., 12 of 18 pairwise comparisons of rest breathing with utterance types across conditions were significant, with p < .05). Conversely, none of the 18 post hoc comparisons among utterance types was statistically significant. In accordance with this finding, the data set was further analyzed using only two behavior categories: speech (i.e., the combined averaged values for vocalizations, babbling, and production of true words) and rest breathing.
Table 4.
Results of kinematic and coupling analyses for each task averaged (with standard deviations in parentheses) across subjects and tokens.
| Task | Frequency of Occurrence | Average rmoving | ||||
|---|---|---|---|---|---|---|
| Coupled Inspiration | Coupled Expiration | AB↑ | RC↑ | Unspecified | ||
| Rest | 32.2% (5.1%) |
38.6% (6.0%) |
1.5% (2.1%) |
1.8% (2.4%) |
26.0% (6.0%) |
0.94 (0.05) |
| Speech Combined | 3.8% (10.2%) |
35.9% (25.6%) |
6.9% (13.2%) |
10.3% (15.3%) |
43.1% (21.4%) |
0.62 (0.42) |
| Babbling | 3.5% (7.7%) |
34.4% (26.6%) |
7.5% (13.1%) |
11.0% (15.8%) |
43.7% (22.8%) |
0.58 (0.47) |
| Speech (true words) | 4.3% (12.0%) |
37.0% (25.2%) |
6.6% (13.6%) |
9.3% (14.5%) |
42.9% (20.6%) |
0.65 (0.38) |
| Vocalization | 2.2% (7.5%) |
37.0% (21.9%) |
5.1% (7.2%) |
15.0% (19.2%) |
40.6% (18.8%) |
0.60 (0.40) |
The average frequency of occurrence for each category was obtained by averaging across the average proportions for each subject.
Differences between speech and rest breathing are presented in Figure 3. These differences were evaluated statistically using this collapsed data set, which combined raw values across subjects and utterance types (i.e., vocalization, babbling, and production of true words). This analysis addressed directly the primary focus of this investigation, which was the determination of whether rest breathing and speech breathing exhibit different respiratory kinematics during early development of speech. One-way analysis of variance for speech versus rest breathing measures revealed main effects for the frequency of occurrence of coupled inspiration [F(1) = 308.80, p < .001], AB↑ [F(1) = 6.68, p = .010], RC↑ [F(1) = 12.65, p < .001], unspecified samples [F(1) = 25.62, p < .001], and for the magnitude of rmoving [F(1) = 54.39, p < .001]. Only the difference in frequency of occurrence of coupled expiration during speech and rest breathing failed to achieve statistical significance [F(1) = .46, p = .497]. Also apparent in Table 4 was the larger variability characteristic of the speech results with respect to rest breathing. Standard deviations ranged from 2.1% to 6.0% for rest breathing and from 10.2% to 25.6% for speech breathing.
Figure 3.
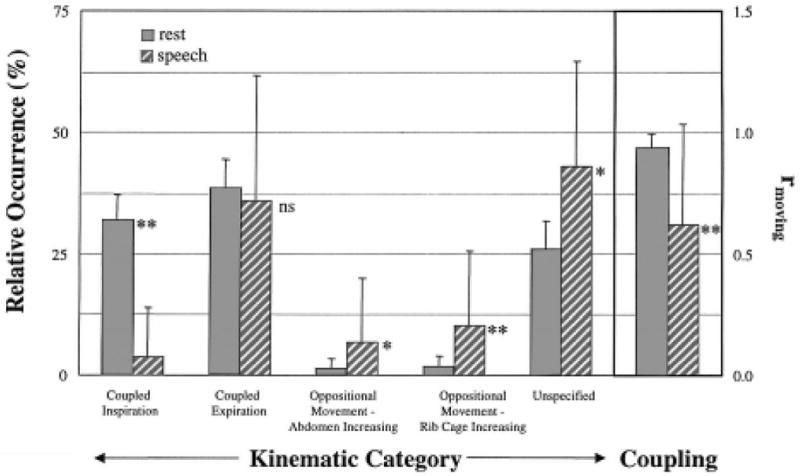
Averaged results, combined across utterance types, for relative occurrence of respiratory kinematic categories (left side) and coupling (i.e., rmoving) during rest breathing and speech production (right side, inside box). Results of post hoc statistical comparisons are indicated beside each pair (* p ≤ .05, ** p ≤ .01, ns: not statistically significant).
Discussion
The present results support the suggestion that speech production by very young children emerges within a distinct coordinative framework. Significant differences in respiratory kinematics were observed between rest breathing and speech breathing for this group of toddlers. These 15-month-old children exhibited kinematic patterns for speech breathing that were clearly distinct from those of rest breathing and were qualitatively similar to the speech breathing patterns of adults. Coordinative differences were particularly supported by two quantitative observations: Coupling of rib cage and abdomen was most rigid and consistent during rest breathing; and oppositional movements of the rib cage and abdomen, though relatively infrequent, were observed almost five times more often during speech than during rest breathing. These findings support the idea that toddlers employ a coordinative organization for speech characterized by significantly greater independence of abdominal and rib cage movement than during rest breathing. These differences did not support a hypothetical common control mechanism for rest breathing (e.g., the respiratory pattern generator) and speech vocalization. More generally, these results were consistent with the representation of speech as being separate and distinct from nonspeech tasks (see Luschei, 1991; Moore & Ruark, 1996; Ruark & Moore, 1997; Weismer & Liss, 1991).
Speech breathing by these toddlers exhibited properties that are similar to those described for mature speech production. For example, abdominal paradoxing is observed, albeit infrequently, in the speech breathing of adults (Hoit, 1994; Hoit et al., 1988) using a variety of measurement techniques (Hodge & Rochet, 1989; Hoit & Hixon, 1986; Murdoch et al., 1991). A similar finding in 15-month-olds might be taken to support the suggestion that, even during the earliest stages of speech development, children (like adults) exhibit task-dependent coordination of the respiratory components. Furthermore, they can exhibit respiratory events (i.e., paradoxing) that also are seen in mature speech. In any case, there is no evidence from the present results for the emergence of a control mechanism that incorporates the well-established patterned movements of rest breathing with the contextual and external demands of speech. Breathing for speech may be seen to emerge as distinct from, and even opposite to, established breathing patterns. Physiologic development of speech production clearly entails the generation of kinematic patterns that are distinct from those of rest breathing. A more exhaustive study of respiratory behaviors is required to determine whether this distinction is unique to vocalization and whether these patterns change with development. We are currently completing a longitudinal study across a wider range of behaviors to evaluate these questions.
The appearance of new kinematic patterns for speech breathing parallels the conclusions reached in prior electromyographic findings regarding development of speech motor control of the mandible (Moore & Ruark, 1996) and lips (Ruark & Moore, 1997). In those studies toddlers exhibited coordinative patterns that, in comparison to all behaviors studied, were most like adult patterns, bearing little resemblance to those of earlier emerging behaviors (e.g., chewing; Green et al., 1997). The essence of this hypothesis is that the demands of speech production are unique among the capabilities of the developing child, such that incorporation or modification of extant patterns is not the most efficient or effective route for development of speech breathing. Finally, the emergence of speech motor control may rely on innate capabilities that gradually approximate mature configurations. Subsequent development may amount to little more than refinement of these patterns, rather than transitions to new ones (cf. Thelen & Ulrich, 1991).
The present data set cannot resolve questions of coordinative organization unequivocally, of course. The suggestion that speech production emerges with a coordinative organization that is independent of existing control structures is attractive given its precedent in speech articulatory systems. However, unlike these other speech subsystems (i.e., the lips and the jaw), competing models can be readily supported with respect to development of speech breathing and the present results. For example, several features of the present data set can be taken to suggest that the observed speech breathing patterns do not represent the earliest emergence of an adult-like pattern, but rather are the consequence of biomechanical limits and comparatively poorly developed control structures. It may be that increased task demands (i.e., airflow regulation) reveal an incompletely integrated system made up of passively linked components. For example, during rest breathing, passive forces enforce synchronous abdominal and rib cage displacement; but with glottal resistance and increased muscle activation, this synchrony is disrupted. The greater mechanical compliance of the infant's respiratory system with respect to the adult's (Sharp, Druz, Balagot, Bandelin, & Danon, 1970) may alone be responsible for the observed uncoupling of respiratory components. Increased musculoskeletal compliance yields greater changes in rib cage dimensions, for example, with comparatively small forces, including those resulting from abdominal or diaphragmatic activity. Therefore, the capacity of the infant to rely on passive forces is substantially less than would be possible for the adult system, which can exploit the passive forces resulting from its greater stiffness. The limitations of plethysmography (i.e., changes in a given circumference can result from a variety of active and passive forces) preclude any conclusions in this regard.
Consistent with work by Boliek and colleagues (1996, 1997), no significant differences were found among different types of utterances. Kinematic patterns for production of true words were indistinguishable from those of babbling or even simple vocalizations. This finding may be taken to suggest that these children had not yet developed motor control capabilities beyond those necessary for the most rudimentary utterances. In fact, the speech patterns that are typical of most 15-month-olds lack the utterance length, vocal intensity variation, and fundamental frequency modulation that would reflect more efficient, more precise speech motor control. Comparable data for very short utterances by adults are not currently available.
Similarly, the control mechanisms underlying observations of more frequent paradoxing during speech or vocalization cannot be inferred from the present results. A number of possible explanations can be considered, however. These differences between speech and rest breathing may arise from underspecification of the motor output governing abdominal and rib cage compression during speech (i.e., control of only part of the system allows unspecified components to vary freely). Alternatively paradoxing may arise from higher order motor organization in which greater degrees of freedom permit greater control flexibility and independence among effectors. Finally, it may be that differences in lung volume give rise to different kinematic patterns for a given control structure. At higher lung volumes, differences in passive forces arising from differences in rib cage and abdominal compliance may yield patterns of movement that are not observed at lower volumes. No empirical data (e.g., electromyographic recording of chest wall muscles, airflow, subglottal pressure) exist to resolve the differences among these models; each bears further investigation.
With respect to underspecification of motor output, paradoxing may be a consequence of the failure to balance the compliance of elements (i.e., abdominal, diaphragmatic, and rib cage expansive/compressive forces) in this hydrostatically coupled system. Uncompensated muscular activity in one system will result in an equal and opposite mechanical reaction in the opposing system. For example, compressive action by abdominal muscles in the absence of compressive force in the rib cage will yield expiration with thoracic expansion (i.e., rib cage paradoxing) for which the time course and extent may be modulated by glottal resistance. Respiratory mechanics are such that the child could generate net expiratory force by producing sufficient driving force in either system, allowing the opposite subsystem to expand passively until it is eventually stabilized by passive mechanical resistance. Indeed, Figure 2 appears to be consistent with precisely this type of mechanism. The initial decrease in abdominal volume was accompanied by expansion of the rib cage (i.e., rib cage paradoxing, assuming that vocalization occurred with expiratory airflow). Rapid rib cage expansion would have engaged sufficiently large passive recoil forces that the utterance may have proceeded by exploiting these elastic forces, switching compressive activity to the rib cage and allowing the abdomen to expand (i.e., abdominal paradoxing during expiratory vocalization). Throughout this very brief utterance, control of alveolar pressure may have been mediated by expiratory effort of abdominal and rib cage muscles successively.
The motion observed during speech and other vocalizations is in sharp contrast to that observed during rest breathing. Inspiration during rest breathing is generated primarily by the contractile forces of the diaphragm drawing downward on the base of the lungs and the lower rib cage. With the system open to the atmosphere by way of the oral and/or nasal cavities, this action exerts compressive forces on the abdomen, which, without muscular opposition, expands passively. The inspired air concomitantly increases the rib cage diameter such that the motion of the two systems is balanced by these active and passive forces. Similarly, during expiration relaxation of the diaphragm allows the abdomen to compress upward while escaping air decreases the rib cage circumference. During speech these dynamics change, as glottal closure necessitates the generation and maintenance of increased alveolar pressure. The present results do not resolve the question of how these changing conditions are addressed.
Other Considerations
Several details of the present findings require further explication. No statistically significant differences were found among the three utterance types (see Table 4). This finding allowed the consolidation of all vocalizations in comparison with rest breathing, which was a statistical advantage, and supported the suggestion that no developmental trend from earlier- to later-emerging utterance types was apparent. This finding provides an extension of earlier cross-sectional and longitudinal findings by Boliek and colleagues, who demonstrated similar respiratory behaviors for cries, whimpers, grunts, and syllables in children less than one year old (1996) and in children 15-36 months old (1997), and showed that utterance type did not covary with any of the breathing parameters measured. Another finding of nonsignificant difference was for the frequency of occurrence of coupled expiration during speech and rest breathing (see Figure 3). This finding was somewhat puzzling, given the greater frequency of paradoxing observed during speech and the finding of only 35%-40% coupled expiration for either task. Inspection of the data in Table 4 revealed that speech was characterized primarily by coupled expiration, Unspecified coupling (i.e., at least one signal exhibited a fat slope), and paradoxing. During speech production, when children are presumed to be generating expiratory effort nearly all of the time, only about 40% of that effort is produced by concurrent decreases observed in both systems. The rest of the time, these children were generating paradoxical movements or movement in only one system. These results do not coincide with the observations in preschool children of air pressure, flow, and airway resistance by Netsell and his colleagues (1994). These researchers predicted a developmental shift away from the use of expiratory force toward the adult pattern of combined expiratory and inspiratory force. Finally, another obvious difference among these tasks was the variability of the respiratory patterns, which during speech tasks was two to eight times greater than that observed during rest (Table 4). Unlike rest breathing, which is characterized by its stability, the variability associated with speech production by these children was consistent with its well-known “ubiquitous variability” (MacNeilage, 1970) across observational domains.
Acknowledgments
This work was supported by a research grant (DC00822) from the National Institute on Deafness and Other Communication Disorders, National Institutes of Health, the University of Pittsburgh, Pittsburgh, PA, and the University of Washington, Seattle. The authors would like to acknowledge the valued contributions to this work by Donald Moore, Jacki Ruark, Anne Smith, and Malcolm McNeil.
Contributor Information
Christopher A. Moore, University of Washington, Seattle
Tammy J. Caulfield, University of Pittsburgh
Jordan R. Green, University of Wisconsin, Madison
References
- Bernstein N. The coordination and regulation of movements. London: Pergamon; 1967. [Google Scholar]
- Boliek CA, Hixon TJ, Watson PJ, Morgan WJ. Vocalization and breathing during the first year of life. Journal of Voice. 1996;10:1–22. doi: 10.1016/s0892-1997(96)80015-4. [DOI] [PubMed] [Google Scholar]
- Boliek CA, Hixon TJ, Watson PJ, Morgan WJ. Vocalization and breathing during the second and third years of life. Journal of Voice. 1997;11:373–390. doi: 10.1016/s0892-1997(97)80033-1. [DOI] [PubMed] [Google Scholar]
- Bunn JC, Mead J. Control of ventilation during speech. Journal of Applied Physiology. 1971;31:870–872. doi: 10.1152/jappl.1971.31.6.870. [DOI] [PubMed] [Google Scholar]
- Chiao GZ, Larson CR, Yajima Y, Ko P. Neuronal activity in nucleus ambiguus during deglutition and vocalization in conscious monkeys. Experimental Brain Research. 1994;100:29–38. doi: 10.1007/BF00227276. [DOI] [PubMed] [Google Scholar]
- Darley FL, Aronson AE, Brown JR. Motor speech disorders. Philadelphia: W. B. Saunders; 1975. [Google Scholar]
- Davis BL, MacNeilage PF. The articulatory basis of babbling. Journal of Speech and Hearing Research. 1995;38:1199–1211. doi: 10.1044/jshr.3806.1199. [DOI] [PubMed] [Google Scholar]
- Estenne M, Zocchi L, Ward M, Macklem PT. Chest wall motion and expiratory muscle use during phonation in normal humans. Journal of Applied Physiology. 1990;68:2075–2082. doi: 10.1152/jappl.1990.68.5.2075. [DOI] [PubMed] [Google Scholar]
- Fawcus B. Oropharyngeal function in relation to speech. Developmental Medicine and Child Neurology. 1969;11:556–560. doi: 10.1111/j.1469-8749.1969.tb01484.x. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Smith JC. Neural control of respiratory pattern in mammals: An overview. In: Dempsey JA, Pack AI, editors. Lung Biology in Health and Disease: Regulation of breathing. Vol. 79. New York: Marcel Dekker, Inc.; 1995. pp. 36–69. [Google Scholar]
- Fugl-Meyer AR. Relative respiratory contribution of the rib cage and abdomen in males and females with special regard to posture. Respiration. 1974;31:240–251. doi: 10.1159/000193113. [DOI] [PubMed] [Google Scholar]
- Goldman MD, Williams A, Soo Hoo G, Trang TT, Gaultier C. Asynchronous thoracoabdominal movements in chronic airflow obstruction (CAO). Active expiration during spontaneous breathing in sleep and wakefulness. Advances in Experimental Medicine and Biology. 1995;393:95–100. [PubMed] [Google Scholar]
- Green JR, Moore CA, Ruark JL, Rodda PR, Morvee WT, VanWitzenburg MJ. Development of chewing in children from 12 to 48 months: Longitudinal study of EMG patterns. Journal of Neurophysiology. 1997;77:2704–2716. doi: 10.1152/jn.1997.77.5.2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner S. Possible analogies in the control of innate motor acts and the production of sound in speech. In: Grillner S, Lindblom B, Lubker J, Persson A, editors. Speech motor control. New York: Pergamon Press; 1982. pp. 217–230. [Google Scholar]
- Hixon TJ. Respiratory function in speech. In: Minifie FD, Hixon TJ, Williams F, editors. Normal aspects of speech, hearing, and language. Englewood Cliffs, NJ: Prentice Hall, Inc.; 1973. pp. 73–125. [Google Scholar]
- Hixon TJ, Goldman MD, Mead J. Kinematics of the chest wall during speech production: Volume displacements of the rib cage, abdomen, and lung. Journal of Speech and Hearing Research. 1973;16:78–115. doi: 10.1044/jshr.1601.78. [DOI] [PubMed] [Google Scholar]
- Hodge MM, Rochet AP. Characteristics of speech breathing in young women. Journal of Speech and Hearing Research. 1989;32:466–480. doi: 10.1044/jshr.3203.466. [DOI] [PubMed] [Google Scholar]
- Hoit JD. A critical analysis of speech breathing data from the University of Queensland. Journal of Speech and Hearing Research. 1994;37:572–580. doi: 10.1044/jshr.3703.572. [DOI] [PubMed] [Google Scholar]
- Hoit J, Hixon TJ. Body type and speech breathing. Journal of Speech and Hearing Research. 1986;29:313–324. doi: 10.1044/jshr.2903.313. [DOI] [PubMed] [Google Scholar]
- Hoit J, Hixon TJ, Watson PJ, Morgan WJ. Speech breathing in children and adolescents. Journal of Speech and Hearing Research. 1990;33:51–69. doi: 10.1044/jshr.3301.51. [DOI] [PubMed] [Google Scholar]
- Hoit JD, Plassman BL, Lansing RW, Hixon TJ. Abdominal muscle activity during speech production. Journal of Applied Physiology. 1988;65:2656–2664. doi: 10.1152/jappl.1988.65.6.2656. [DOI] [PubMed] [Google Scholar]
- Kelso JAS, Holt KG, Rubin P, Kugler PN. Patterns of human interlimb coordination emerge from the properties of non-linear, limit cycle oscillatory processes: Theory and data. Journal of Motor Behavior. 1981;13:226–261. [PubMed] [Google Scholar]
- Kent RD, Mitchell PR, Sancier M. Evidence and role of rhythmic organization in early vocal development in human infants. In: Fagard J, Wolff PH, editors. The development of timing control and temporal organization in coordinated action. New York: Elsevier Science Publishers; 1991. pp. 135–139. [Google Scholar]
- Konno K, Mead J. Measurement of the separate volume changes of rib cage and abdomen during breathing. Journal of Applied Physiology. 1967;22:407–422. doi: 10.1152/jappl.1967.22.3.407. [DOI] [PubMed] [Google Scholar]
- Larson CR, Yajima Y, Ko P. Modification in activity of medullary respiratory-related neurons for vocalization and swallowing. Journal of Neurophysiology. 1994;71:2294–2304. doi: 10.1152/jn.1994.71.6.2294. [DOI] [PubMed] [Google Scholar]
- Luschei ES. Development of objective standards of nonspeech oral strength and performance: An advocate's views. In: Moore CA, Yorkston KM, Beukelman DR, editors. Dysarthria and apraxia of speech: Perspectives on management. Baltimore: Paul H. Brookes; 1991. pp. 3–14. [Google Scholar]
- MacNeilage PF. Motor control of serial ordering of speech. Psychological Review. 1970;77:18–196. doi: 10.1037/h0029070. [DOI] [PubMed] [Google Scholar]
- MacNeilage PF, Davis BL. On the origin of internal structure of word forms. Science. 2000;28:527–531. doi: 10.1126/science.288.5465.527. [DOI] [PubMed] [Google Scholar]
- Mathworks, Inc. Matlab [software] Cochituate Place, 24 Prime Parkway, Natick, MA 01760: 1999. [Google Scholar]
- Moore CA. Bilateral symmetry of mandibular muscle activation during speech and non-speech tasks. Journal of Speech and Hearing Research. 1993;36:1145–1157. doi: 10.1044/jshr.3606.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CA, Ruark JL. Does speech emerge from earlier appearing motor behaviors? Journal of Speech and Hearing Research. 1996;39:1034–1047. doi: 10.1044/jshr.3905.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CA, Smith A, Ringel R. Task-specific organization of activity in human jaw muscles. Journal of Speech and Hearing Research. 1988;31:670–680. doi: 10.1044/jshr.3104.670. [DOI] [PubMed] [Google Scholar]
- Murdoch BE, Chenery HJ, Stokes PD, Hardcastle WJ. Respiratory kinematics in speakers with cerebellar disease. Journal of Speech and Hearing Research. 1991;34:768–780. doi: 10.1044/jshr.3404.768. [DOI] [PubMed] [Google Scholar]
- Netsell R, Lotz WK, Peters JE, Schulte L. Developmental patterns of laryngeal and respiratory function for speech production. Journal of Voice. 1994;8:123–131. doi: 10.1016/s0892-1997(05)80304-2. [DOI] [PubMed] [Google Scholar]
- Oller DK. Infant vocalizations and the development of speech. Allied Health and Behavioral Sciences. 1978;1:525–549. [Google Scholar]
- Ruark JL, Moore CA. Coordination of lip muscle activity by two-year-old children during speech and nonspeech tasks. Journal of Speech, Language, and Hearing Research. 1997;40:1373–1385. doi: 10.1044/jslhr.4006.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp JT, Druz WS, Balagot RC, Bandelin VR, Danon J. Total respiratory compliance in infants and children. Journal of Applied Physiology. 1970;29:775–779. doi: 10.1152/jappl.1970.29.6.775. [DOI] [PubMed] [Google Scholar]
- Sharp JT, Goldberg NB, Druz WS, Danon J. Relative contributions of rib cage and abdomen to breathing in normal subjects. Journal of Applied Physiology. 1975;39:608–618. doi: 10.1152/jappl.1975.39.4.608. [DOI] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Botzinger complex: A brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stathopoulos ET, Hoit JD, Hixon TJ, Watson PJ, Solomon NP. Respiratory and laryngeal function during whispering. Journal of Speech and Hearing Research. 1991;34:761–767. doi: 10.1044/jshr.3404.761. [DOI] [PubMed] [Google Scholar]
- Thelen E, Ulrich BD. Hidden skills. Monographs of the Society for Research in Child Development. 1991;56:1991. [PubMed] [Google Scholar]
- von Euler C. Some aspects of speech breathing physiology. In: Grillner S, Lindblom B, Lubker J, Persson A, editors. Speech motor control. New York: Pergamon Press; 1982. pp. 95–103. [Google Scholar]
- Weismer G, Liss JM. Reductionism is a dead-end in speech research: Perspectives on a new direction. In: Moore CA, Yorkston KM, Beukelman DR, editors. Dysarthria and apraxia of speech: Perspectives on management. Baltimore: Paul H. Brookes; 1991. pp. 15–28. [Google Scholar]
- Wohlert A, Goffman L. Human perioral muscle activation patterns. Journal of Speech and Hearing Research. 1994;37:1032–1040. doi: 10.1044/jshr.3705.1032. [DOI] [PubMed] [Google Scholar]
- Wolff PH. Endogenous motor rhythms in young infants. In: Fagard J, Wolff PH, editors. The development of timing control and temporal organization in coordinated action. New York: Elsevier Science Publishers; 1991. [Google Scholar]


