Abstract
The classic Hering-Breuer inflation reflex (HBIR) is a widely-held tenet for understanding the lung volume-related vagal control of respiratory rhythm. Recent evidence, however, has revealed that the fictive HBIR elicited by electrical vagal stimulation in rats is not static but may be attenuated centrally by two forms of nonassociative learning (habituation and desensitization) that continually mitigate the reflex effects with exponential adaptations like a differentiator or high-pass filter. Desensitization is analogous to habituation but exhibits an explicit short-term memory (STM) in the form of a rebound response with exponential decay during recovery from stimulation. To investigate whether such learning and memory effects are lung volume-related and use-dependent (practice-makes-perfect), we compared the time-dependent changes in inspiratory and expiratory durations (TI, TE) during and after 1-min or 8-min unilateral lung inflation or high-frequency/low-intensity vagal stimulation in anesthetized, uni- or bi-vagotomized rats. Unilateral lung inflation and vagal stimulation both elicited abrupt shortening of TI and lengthening of TE (HBIR effects) and gradual habituation and desensitization throughout the 1- or 8-min test period, followed by rebound responses in TI and TE with exponential recovery (STM effects) in the post-test period. In both cases, the STM time constants for TI and TE were significantly longer with the 8-min test than the 1-min test (17-45 sec vs. 4-11 sec, p<0.01). We conclude that the HBIR and its central habituation and desensitization are mediated peripherally by lung volume-related vagal afferents, and that the STM of desensitization is use-dependent. The translational implications of these findings are discussed.
Keywords: Nonassociative learning, habituation, desensitization, Hering-Breuer reflex, respiratory control
Introduction
In 1868, E. Hering and J. Breuer reported that inflation of the lungs in vagi-intact animals elicited an abrupt inspiration-terminating and expiration-lengthening reflex whereas deflation elicited the opposite effect (Breuer, 1970; Hering, 1970). For more than a century thereafter, the Hering-Breuer inflation-deflation reflex (HBIR, HBDR) has been widely held as a tenet for understanding the vagal proprioceptive control of respiratory rhythm following abrupt increases or decreases of lung volume (Widdicombe, 2006). In contrast, little attention has been paid to the complex compensatory responses that might ensue when lung inflation or deflation is prolonged while arterial blood gas tensions and pH are maintained constant (Stanley et al., 1975; Grippi et al., 1985).
Recently, systematic studies of the central adaptation of the fictive HBIR modulation of respiratory frequency (f) and expiratory duration (TE) during electrical vagal stimulation in rats revealed that a component of the adaptation indeed satisfies many of the classic criteria of habituation (Poon et al., 2000a; Siniaia et al., 2000). In neurophysiological terms, habituation refers to a progressive depression of the central nervous system's responsiveness to a continued or repeated stimulus, often with a latent short-term memory (STM) during recovery from the stimulus (Thompson & Spencer, 1966; Christoffersen, 1997). Habituation is use-dependent (practice-makes-perfect): a longer stimulus results in greater habituation in an exponentially decaying manner. It is the most prevalent and well studied form of nonassociative learning (use-dependent plasticity of the CNS that is induced by a single stimulus alone), and is distinguished from associative learning (e.g., Pavlovian conditioning) which requires co-activation with other stimuli (Kandel, 1978; Poon & Siniaia, 2000).
Importantly, the study of Siniaia et al. (2000) also identified a second component of the central adaptation of the fictive HBIR, termed ‘desensitization’, as a novel form of nonassociative learning during vagal stimulation. In essence, desensitization is similar to habituation but with an explicit (instead of implicit) STM in the form of a rebound response with exponential decay during recovery from the vagal input (Fig. 1). Unlike habituation, desensitization of the HBIR is abolished after NMDA (N-methyl-D-aspartate) receptor blockade or lesioning of the Kölliker-Fuse nucleus or medial parabrachial nucleus (Poon et al., 2000a; Siniaia et al., 2000; MacDonald et al., 2007), which constitute the traditional ‘pneumotaxic center’ in the rat dorsolateral pons (Song & Poon, 2004; Song et al., 2006). Interestingly, it has been pointed out (Poon & Siniaia, 2000; Poon et al., 2000a; Poon & Young, 2006; MacDonald et al., 2007) that the decrementing adaptation patterns of habituation and desensitization bear close resemblance to those of a neural differentiator that is gated or non-gated to the input (with implicit or explicit STM), suggesting a high-pass filtering mechanism for preferential transmission of phasic over tonic afferent inputs to the CNS (Fig. 1).
Figure 1.
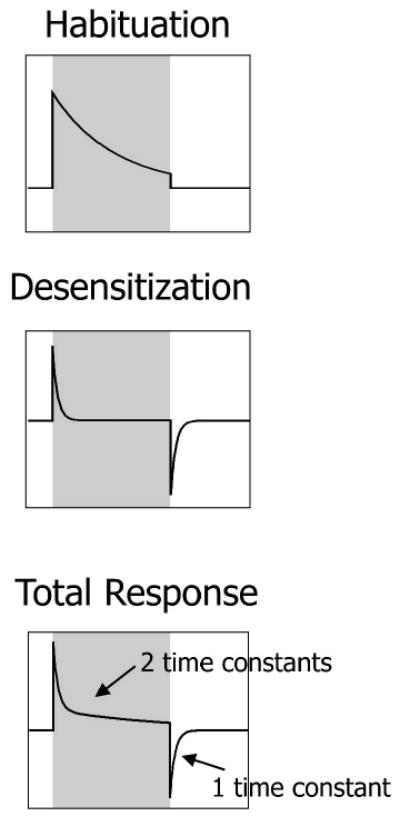
Habituation and desensitization as gated and non-gated neural differentiators. Ordinate indicates response; abscissa indicates time. The initial response to a sustained or repetitive stimulus (shaded region) decays exponentially over time due to habituation, desensitization or both. (A) Habituation is monophasic with abrupt termination of response at end of stimulation. Short-term memory (STM) effect of the habituation is directly gated to the stimulus and is not observable once the stimulus ceases. (B) Desensitization is biphasic. A rebound occurs at the end of stimulation and it exponentially returns to the baseline with an explicit short-term memory (STM) that is not gated to the stimulus. (C) The sum of habituation and desensitization has two time constants during adaptation and a single time constant during STM. The response patterns for habituation and desensitization resemble a differentiator or high-pass filter. Adapted from Poon & Young (2006).
The purpose of the present study was threefold. First, we investigated whether the habituation and desensitization of the vagal modulation of f and TE in rats also applied to the control of inspiratory duration (TI). Second, to obviate the possible pitfalls of vagal stimulation we examined whether similar differentiator effects could be elicited by sustained lung inflation. To ensure constant chemical drive during lung inflation, we developed a unilateral lung inflation technique (Guz et al., 1966) for independent ventilation of the inflated and non-inflated lungs in anesthetized rats. Finally, and most importantly, we tested whether the STM effects of desensitization were dependent on the duration of inflation or vagal stimulation per se, i.e., whether the memory exhibited use-dependent properties as in nonassociative learning (Castellucci et al., 1978). Our results confirm the presence of prominent habituation and desensitization differentiator effects in the CNS that continually mitigate the HBIR modulation of TI and TE with use-dependent STM in anesthetized rats. These findings provide a new perspective for understanding the complex learning and memory mechanisms that serve to offset the HBIR during acute or chronic lung inflation in health and in disease states.
Methods
All experimental protocols had been reviewed and approved by the MIT Committee on Animal Care in accordance with published guidelines.
Animal preparation
Experiments were performed on 14 adult male Sprague-Dawley rats (290-400g). The animal was anesthetized with urethane (1.5 g/kg i.p.) and supplemental doses (0.15-0.3 g/kg i.v.) were administered periodically where necessary as indicated by changes in blood pressure, heart rate and respiratory rate. A femoral arterial and vein were cannulated for blood pressure monitoring and drug administration, respectively. The animals were tracheotomized and injected with atropine (0.05 mg/kg s.c.) to minimize tracheal secretions. Subsequently, the animal was paralyzed with pancuronium bromide (Sigma; initial dose 1 mg/kg i.v., supplemented every hour at 40-50% of the initial dosage) and ventilated (CWE, Model AVNS-1 servo-ventilator) with O2-enriched medical-grade air (40% O2). The ventilation rate was continually adjusted by the servo-ventilator in order to maintain the end-tidal CO2 level constant at 4.5%-5.0% as measured by an infrared CO2 analyzer (CWE, Capstar-100). A temperature controlled heating pad (CWE, TC-831) was used to regulate the rectal temperature at 37.5±0.2°C.
Electrophysiology
To monitor respiratory output, a phrenic nerve was dissected at the C5 level via a ventral approach and mounted on a custom-made silver-wire bipolar recording electrode (0.01″ OD). Phrenic recordings were sampled at 10,000 samples/sec (Labview, National Instruments), along with airway pressure and CO2. Phrenic activity was amplified and low-pass filtered (Axon Instruments, CyberAmp 380) and the resulting raw signal was time-averaged with a leaky integrator (Paynter filter, time constant ∼15 ms). Both the raw (Phr) and integrated phrenic nerve activity (∫Phr) were monitored on a Tektronix digital oscilloscope and recorded digitally (National Instruments, AT-MIO-16E-1) on a computer. Integrated phrenic data were analyzed using custom-made Matlab programs to detect peak slopes indicating the start and end of inspiration. Inspiratory and expiratory durations (TI, TE) were calculated as the time intervals between the onset and the peak of ∫Phr within the same cycle and between cycles, respectively.
Electrical vagal stimulation
In vagal stimulation experiments, both vagi were sectioned at the cervical level. A bipolar silver-wire electrode (FHC, 0.125mm OD) connected to a voltage pulse generator (A.M.P.I., Master 8) through a stimulus-isolation unit (A.M.P.I., ISO-Flex) was used to stimulate the central end of a vagus nerve. The stimulation threshold for each animal was defined as the lowest stimulus current that produced a discernible reflex inhibition of phrenic activity over a 5-sec interval. Stimulus pulses with currents between 1.5-2x threshold, or roughly 15-70 μA, were applied repetitively (80 Hz, 0.1 ms pulse duration) to the vagus nerve to evoke HBIR, as described previously (Siniaia et al., 2000). All exposed nerves were protected from dehydration by immersion in warm paraffin mineral oil pools.
Constant electrical vagal stimulation was applied for either 1 or 8 min once in separate animals. Each stimulation session was preceded by a 1-min baseline period and followed by a post-stimulation recovery period of up to 5 min.
Unilateral lung inflation
In unilateral lung inflation experiments, only the left vagus nerve was cut at the cervical level. A custom-made polyethylene tube (0.048″ OD) with an epoxy occlusion cuff at the tip, (0.9-1.0″ OD) was inserted into the right bronchus such that the right lung was sealed off from the trachea by the cuff (Fig. 2). This tube permitted the vagal-intact lung to be inflated with a pneumatic pump (Harvard Apparatus, Neurophore BH-2) while the vagotomized lung was independently ventilated to maintain constant end-tidal CO2 levels (4.5-5%). Typically, a pump pressure of 34-80cm H2O was needed to elicit the HBIR when only one lung was inflated as lower pressures (<20 cmH2O) were found to be unstable. The actual inflation pressure at the lung could not be measured but was bound to be lower because of possible leaks around the cuff, which varied from animal to animal. Lung inflation was maintained for 1 or 8 min. An example of an 8-min unilateral lung inflation test (with an airway pressure of ∼40 cmH20) is illustrated in Fig. 3. The inflation pressure showed an initial overshoot that stabilized within the first 2-3 breaths, and remained stable throughout the remaining duration of the inflation. At the offset of inflation the airway pressure dissipated promptly without any disturbances. In animals where the lung inflation was unstable, the corresponding data were discarded.
Figure 2.
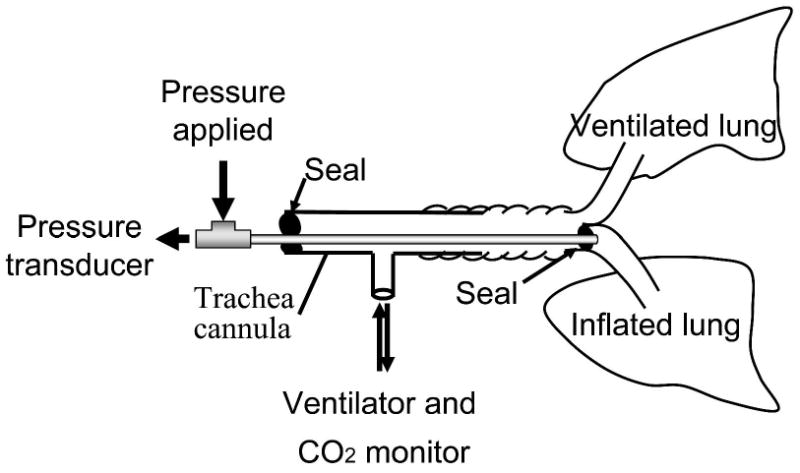
Experimental setup for unilateral lung inflation in rats. The right lung is sealed from the ventilator and is inflated. The left lung is ventilated to control end-tidal CO2.
Figure 3.
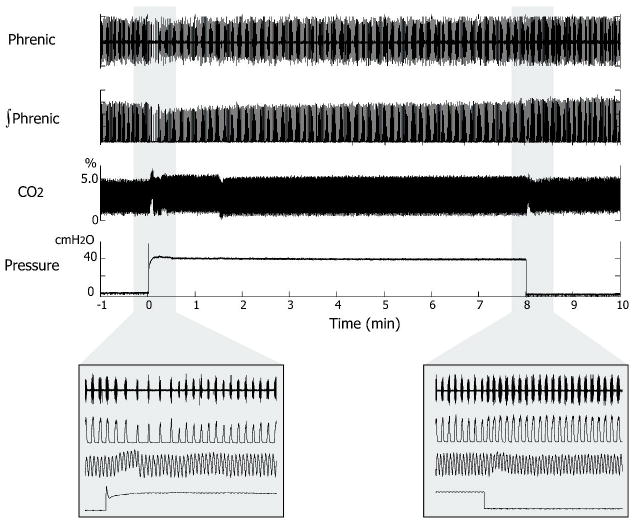
Recordings in an 8-min unilateral lung inflation trial. From top to bottom: raw and integrated phrenic discharge, airway CO2 level, and airway pressure. The expanded views (insets) show the same at the beginning and end of lung inflation. Changes in the CO2 trace at the onset and offset of lung inflation were pressure artifacts. Note the slowing of the respiratory rhythm at the onset of inflation reflecting the HBIR and its rebound acceleration at the offset indicating STM.
Data analysis
Computed TI and TE for each animal were normalized (with respect to the control values) and averaged over several breaths within specific moving time windows with bin sizes varying from 3-5 sec (for periods of fast transients) to 10-20 sec (close to steady state), as with previous studies (Poon et al., 2000a; Siniaia et al., 2000). The binned data were then averaged for each test group. The average data were tested against the baseline using two-way ANOVA with repeated measures for statistical significance of the stimulated response.
Exponential curve fittings of the averaged data were done using nonlinear least squares regression in OriginPro (OriginLab, Northampton, MA). Equations of the form “y = y0 + Anexp(-t/τn) + An+1exp(-t/τn+1) + …” were used to fit the data. The curve-fitting results were presented as means ± SE. The appropriate number of exponential terms to be fitted was determined by using the F test (p<0.01). Comparison of time constants between the 8-min and 1-min groups was performed using two-tailed Student's t test (p<0.01).
Results
Vagal stimulation vs. lung inflation
Figure 4a shows the time course of the responses in TI and TE to 8-min vagal stimulation (n=5). Vagal stimulation elicited an abrupt HBIR-like shortening of TI and lengthening of TE. Thereafter, both TI and TE returned gradually towards the baseline throughout the 8-min stimulation period. Exponential curve fitting (Table 1) indicated that TI increased towards baseline with two time constants (τ1 = 13.0 sec, τ2 = 440.3 sec) while TE decreased towards baseline with two time constants (τ1 = 5.1 sec, τ2 = 82.5 sec). At the end of stimulation, both TI and TE rebounded in an opposition direction to that at the beginning of stimulation. Thereafter, TI and TE gradually returned to baseline with a single time constant (τ3 = 16.5 sec for TI and 21.8 sec for TE).
Figure 4.
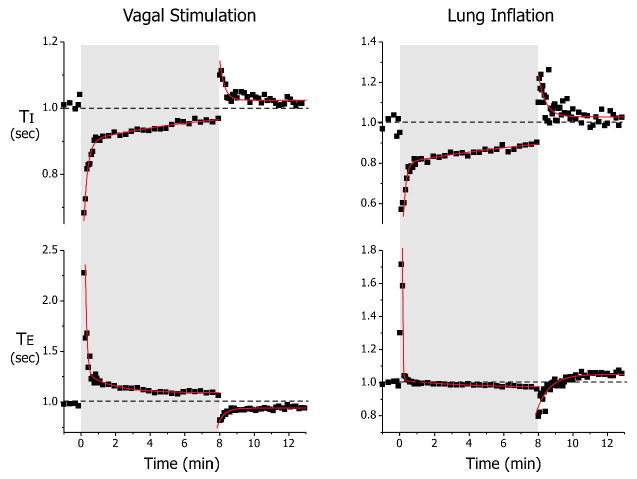
Normalized TI and TE responses to 8-min electrical vagal stimulation (n=5) and unilateral lung inflation (n=3). Data are average values for each group (error bars not shown). The group data during the test and recovery phases for each experiment were all highly significantly different from the corresponding baseline values (p ≪ 0.01, 2-way ANOVA with repeated measures) except the recovery from 8-min lung inflation, which was marginal (p=0.04). Solid lines indicate exponential curve fits. The appropriate number of exponential functions to be fitted was determined by F-test (p<0.01). Corresponding group data and curve fits for the 1-min tests demonstrated similar significance levels (not shown).
Table 1.
Curve fitting results in the 1-min and 8-min electrical vagal stimulation and unilateral lung inflation tests.
| Vagal Stimulation | Lung Inflation | |||||
|---|---|---|---|---|---|---|
| 8min (n=5) | 1min (n=3) | 8min (n=3) | 1min (n=3) | |||
| TI | Adaptation | A1 | -0.52 ±0.08 | -0.24 ±0.01 | -0.36 ±0.09 | -0.17 ±0.08 |
| τ1 | 13.0 ±1.7 | 13.2 ±1.9 | 9.7 ±2.0 | 25.7 ±29.1* | ||
| A2 | -0.10 ±0.01 | -0.20 ±0.01 | ||||
| τ2 | 440.3 ±55.9 | 742.0 ±74.6 | ||||
| STM | A3 | 0.12 ±0.02 | 0.09 ±0.01 | 0.21 ±0.04 | 0.32 ±0.07 | |
| τ3 | 16.5±4.0 | 4.35 ±1.19 | 16.8 ±4.2 | 9.2 ±2.7 | ||
| TE | Adaptation | A1 | 1.02 ±0.28 | 2.8 ±0.4 | 0.56 ±0.01 | 0.59 ±0.08 |
| τ1 | 5.1 ±1.5 | 2.6 ±0.4 | 1.5 ±0.2 | 3.2 ±0.8 | ||
| A2 | 0.20 ±0.04 | 0.05 ±0.01 | ||||
| τ2 | 82.5 ±23.9 | 56.0 ±15.0 | ||||
| STM | A3 | -0.12 ±0.01 | -0.15 ±0.01 | -0.20 ±0.01 | -0.25 ±0.04 | |
| τ3 | 21.8 ±3.4 | 8.15 ±1.3 | 44.8 ±7.2 | 11.3 ±2.3 | ||
Equations of the form “y = y0 + Anexp(-t/τn) + An+1exp(-t/τn+1) + …” were used to fit the data. A1 and A2 are the magnitudes (normalized) of the fast and slow adaptation components; τ1 and τ2 are corresponding time constants (in sec). A3 and τ3 (in sec) are the magnitude and time constant of STM.
The two distinct time constants of the TI and TE adaptations to the vagal input suggested the existence of multiple learning processes (habituation and/or desensitization), whereas the post-stimulation STM indicated the existence of desensitization as defined in Fig. 1. Because the curve fitting procedure had limited resolution for closely-spaced exponential terms, we cannot rule out the possibility of additional learning and memory components which had similar time constants as the ones detected.
As with vagal stimulation, 8-min unilateral lung inflation (n=3) elicited similar biphasic responses in TI and TE (Fig. 4b). The TI response during unilateral lung inflation comprised two time constants (τ1 = 9.7 sec, τ2 = 742.0 sec) and the TE response also comprised two time constants (τ1 = 1.5 sec, τ2 = 56.0 sec). The post-inflation recovery of TI and TE occurred at a rate comparable to that in the vagal stimulation test, with a time constant τ3 = 16.8 sec for TI and 44.8 sec for TE (Table 1).
The very fast time constant adaptation component of TE probably reflected in part the rapid airway pressure overshoot at the beginning of inflation (Fig. 3). The adaptation of TE eventually converged to slightly below the baseline while its recovery rose slightly above the baseline, indicating possible residual effects of lung inflation. Despite these limitations, the overall adaptation patterns for TI and TE were qualitatively similar to those of vagal stimulation.
8-min vs. 1-min tests
The responses in TI and TE to 1-min vagal stimulation and unilateral lung inflation showed similar (albeit shorter) biphasic adaptation patterns as in the 8-min tests. Exponential curve fitting for the adaptation phase (Table 1) yielded only the fast time constant term in this case as 1 min was not long enough for the long time constant term to be reliably resolved. In the 1-min unilateral lung inflation test, the adaptation time constant for TI also did not reach significance level because of variability of TI within the relatively short test duration.
In contrast, curve fitting for the recovery phase of the 1-min vagal stimulation and unilateral lung inflation tests yielded highly significant STM exponential terms (Table 1). In Fig. 5, the STM time constants for TI and TE are seen to be significantly longer for the 8-min tests than the corresponding 1-min tests, suggesting that the memory stayed longer following a longer HBIR challenge (Fig. 5). This use-dependent STM effect of HBIR was consistently detected in both the vagal stimulation and unilateral lung inflation tests.
Figure 5.
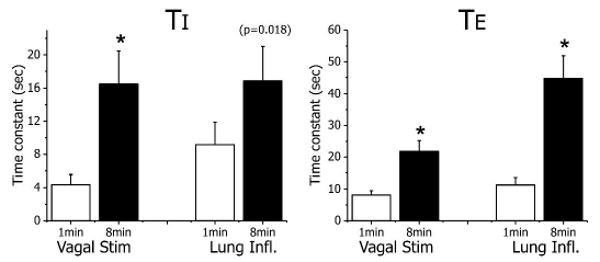
Comparison of STM time constants for 1-min and 8-min tests. Use-dependent STM of HBIR desensitization is revealed by the significantly longer time constants in the 8-min tests than the corresponding 1-min tests for both vagal stimulation and unilateral lung inflation (* indicates p<0.01, two-tailed Student's t test).
Discussion
Critique of preparations
Early studies used electrical vagal stimulation or lung inflation in dogs, cats or rabbits for studying the central adaptation effects of the HBIR (Bartoli et al., 1973; Stanley et al., 1975; D'Angelo, 1977; Finkler & Iscoe, 1984; Grippi et al., 1985; Younes & Polacheck, 1985). Both approaches have drawbacks. Vagal stimulation may activate not only slowly adapting receptor (SAR) fibers but potentially also those from rapidly adapting receptors (RARs). In the rabbit, vagal stimulation after dorsolateral pontine lesion has been shown to lengthen (rather than shorten) inspiration (Takano & Kato, 2003), an effect that is consistent with the activation of RAR or pulmonary C fibers in this species (Davies & Roumy, 1982; Sibuya et al., 1993). Lung inflation provides a more physiologic stimulus presumably more specific for SAR (though not exclusive of RAR) activation, but may be confounded by the adaptation of the SARs themselves especially when it is maintained over an extended period. Also, stability of chemical drive and of lung volume during inflation are more difficult to maintain accurately for quantitative analysis of the learning and memory effects in accordance with Fig. 1.
As a precaution, we therefore used both vagal stimulation and unilateral lung inflation to characterize the learning and memory processes associated with the HBIR in the rat. The latter has been established as an animal model of choice in recent years and much information has been accumulated in the literature regarding the central and peripheral neural organization of the HBIR in this mammalian species (Tsubone, 1986; Bonham & McCrimmon, 1990; Bergren & Peterson, 1993; Hayashi et al., 1996; Seifert & Trippenbach, 1998; Ezure & Tanaka, 2004; Wang et al., 2008). In particular, recent studies have shown that RAR fibers are rare in the rat (Bergren & Peterson, 1993) and their target relay neurons in the nucleus tractus solitarius (NTS) are inhibited by a subset of GABAergic neurons (“inhibitory pump cells”) that receive SAR inputs, such that high-frequency low-intensity vagal stimulation preferentially activates SAR instead of RAR pathways in the NTS (Ezure, 2004). These unique neuroanatomic characteristics of the rat make it an ideal animal model for studying the central integration of SAR inputs resulting from high-frequency low-intensity vagal stimulation or lung inflation. In the present study, both methods of inducing HBIR did not provoke any augmented or prolonged inspiratory activity, which are hallmarks of RAR afferent activation during inflation (Davies & Roumy, 1982; Sant'Ambrogio & Widdicombe, 2001). The consistency of the results with both methods is reassuring, in that RAR afferent activations (if any) are unlikely to produce similar adaptation effects in both cases.
Use-dependent habituation and desensitization of rat HBIR
Current understanding of nonassociative learning and memory and their cellular basis (Kandel, 1978) is built upon extensive studies conducted in past decades with primarily invertebrate models – most notably the habituation and sensitization of the Aplysia gill withdrawal reflex (Pinsker et al., 1970). Although nonassociative learning has been implicated in a variety of behavioral and brain functions and neurological disorders in humans (Braff et al., 1995; Poon & Young, 2006), extension of the results from invertebrate animal studies to humans has been hampered by the lack of a pertinent mammalian model that is amenable to systematic investigation at multiple levels of organization. We suggest that the habituation and desensitization of the HBIR in the rat could provide such a mammalian model.
Stanley and coworkers (Stanley et al., 1975) were the first to demonstrate an adaptation of the HBIR in dogs during prolonged lung inflation, an effect which they ascribed to a central “habituation” mechanism since similar adaptation was not seen in pulmonary stretch receptor single-fiber afferent discharge. Such central adaptation effects have been confirmed by other investigators using similar single-fiber recordings during lung inflation or no-inflation in cats (Finkler & Iscoe, 1984; Zhou et al., 1989). However, in none of those studies were such adaptation effects verified against established criteria of habituation (Thompson & Spencer, 1966; Groves & Thompson, 1970; Thompson et al., 1973). Subsequently, Grippi et al. (1985) reported that the central adaptation of HBIR under constant chemical drive in dogs was followed by rebound changes in TI and TE during recovery, whereas Younes & Polacheck (1985) found similar rebound effects following vagal stimulation in cats. In both studies, consistency of the adaptation and rebound effects resulting from vagal stimulation or lung inflation was not verified in the same animal model. Nevertheless, the anomalous rebound responses reported in both cases appeared to contradict the notion of habituation proposed by Stanley et al. (1975). Instead, the notion of leaky integrators (converse of differentiators) mediating the central processing of vagal control of TI and TE was proposed (Younes & Polacheck, 1985).
In an attempt to resolve this dilemma, Siniaia et al. (2000) showed that the central adaptation of the vagal modulation of the respiratory frequency in rats was comprised of two separate components that were distinguishable functionally, structurally and pharmacologically. One component was shown to conform with several established properties of habituation, such as inverse dependence on stimulus intensity or frequency, latent STM, dishabituation and habituation of dishabituation. The desensitization component was shown to account for the rebound response during recovery, which was abolished after NMDA receptor blockade or lesioning of the pneumotaxic center. The NMDA receptor-independent habituation and NMDA receptor-dependent desensitization effects in f were ascribable in part to similar effects in TE (Poon et al., 2000a). However, questions remained as to whether these effects were specific to vagal stimulation and not lung inflation or specific to TE and not TI. Indirect evidence of such habituation and desensitization effects during lung inflation was inferred in a recent study in anesthetized rats (MacDonald et al., 2007), in which entrainment of the respiratory rhythm to a ventilator was transiently disrupted after abrupt application of positive end-expiratory pressure (PEEP) but the entrainment resumed after a brief adaptation period. The adaptation to PEEP was weakened (but not abolished) after electrolytic lesioning of the pneumotaxic center, in agreement with the postulated pontine desensitization and extra-pontine habituation of the HBIR.
The present results confirm the notions of habituation and desensitization of the HBIR, by demonstrating that similar differentiator effects for both TE and TI could be consistently produced by either vagal stimulation or unilateral lung inflation in the same animal model. The decrementing adaptation patterns of both TE and TI suggest that the habituation and desensitization of HBIR were use-dependent. More important, our results show that the STMs for both TI and TE during recovery were also use-dependent: the longer the stimulation or inflation, the longer the ensuing desensitization memory. (Corresponding use-dependent effects for habituation STM were not measurable because the memory was latent.) These findings provide strong evidence that the central adaptation of the HBIR elicited by high-frequency and low-intensity vagal stimulation or lung inflation in rats indeed represented nonassociative learning and memory.
Such use-dependent habituation and desensitization in the rat HBIR provide an important link between the respiratory neuroscience field and behavioral neuroscience field for understanding the mechanisms of nonassociative learning and memory in the mammalian CNS. Because such nonassociative learning and memory behaviors were expressed in both TI and TE in this study, their cellular correlates likely reside in central relays of vagal volume-related afferent signals to the rhythm generator(s) that control TI and TE [presumably via vagally-activated postinspiratory inhibition of inspiratory pacemakers in the preBötzinger complex (Wittmeier et al., 2008)]. In particular, the localization of the desensitization component to the pneumotaxic center in previous studies (Siniaia et al., 2000; MacDonald et al., 2007) paves the way for systematic investigations of its neural correlates in this brainstem structure in future. It is also highly likely that the habituation effects may be mediated at least in part by activity-dependent adaptation of certain NTS “pump cells” where SAR afferents terminate. In support of this hypothesis, it has been shown that many second-order NTS neurons exhibit NMDA receptor-independent short-term depression in vitro (Zhou et al., 1997; Poon et al., 2000b), a putative cellular mechanism of habituation in invertebrates (Kandel, 1978). In addition, the majority of second-order neurons in the medial NTS also exhibit NMDA receptor-independent intrinsic long-term depression (i.e., depression of neuronal excitability) (Bantikyan et al., 2008), which may further contribute to the habituation effects. Similar differentiator effects have also been demonstrated in the peripheral chemoreflex control of TE during hypoxia or carotid sinus nerve stimulation (Young et al., 2003; Song & Poon, 2008), suggesting that these forms of nonassociative learning are prevalent in the respiratory system. To our knowledge, this is the first mammalian model of habituation and desensitization in a brain system with tractable central peripheral neural organization and well-defined physiologic function.
Translational implications
The HBIR is pronounced in human neonates and its strength decreases during the first year of life (Rabbette et al., 1994). In adults, the HBIR has been generally thought to be rather weak particularly in the awake state, except at lung volumes well above functional residual capacity (Widdicombe, 1961; Guz et al., 1964; Guz et al., 1970; Clark & von Euler, 1972; Hamilton et al., 1988; Hamilton et al., 1990). However, human subjects do exhibit normal SAR discharge during eupneic breathing (Guz & Trenchard, 1971). Studies have shown that abrupt airway occlusion at end-inspiration prolongs TE (Gautier et al., 1981; Tryfon et al., 2001) whereas occlusion at end-expiration shortens TI (Polacheck et al., 1980), indicating significant phasic volume-related feedback modulation of the eupneic rhythm in humans. Similar phasic modulations of TI and TE have also been demonstrated in awake subjects during the first breath of assisted ventilation or PEEP before significant changes in chemical drive or conscious perception begin to develop (BuSha et al., 2002; Haberthur & Guttmann, 2005). Indeed, mechanically ventilated patients (while awake or under anesthesia or non-REM sleep) often entrain their spontaneous respiratory rhythm to the ventilator cycles (Graves et al., 1986; Simon et al., 1999), an effect that is contingent on phasic volume-related feedback (Muzzin et al., 1989).
Why do adult subjects display weak HBIR response despite apparently normal phasic volume-related modulation of the respiratory rhythm? A new perspective for this paradox in light of the present findings is that adult subjects might habituate and/or desensitize rapidly to static lung inflation above the tidal volume but not to normal phasic volume-related feedbacks, which are preferentially transmitted to the respiratory controller. In other words, the high-pass filtering effects of habituation and desensitization (Fig. 1) might be even more effective in humans than in experimental animals. This hypothesis warrants further investigation in relation to the effects of PEEP on patient-ventilator synchrony during mechanical ventilation or the stability of spontaneous breathing during weaning from mechanical ventilation (Putensen et al., 2006; MacDonald et al., 2007).
Another interesting question is whether patients with chronic elevation or depression of lung volume due to increases or decreases in lung compliance demonstrate abnormal HBIR or phasic volume-related feedback compared with healthy subjects. Previous studies have shown that phasic modulations of TI and TE are attenuated in patients with increased lung compliance (as in chronic obstructive pulmonary disease) and augmented in those with decreased lung compliance (as in restrictive lung disease) (Polacheck et al., 1980; Gautier et al., 1981; Tryfon et al., 2001). It has been hypothesized that a possible mechanism for such disease-dependent effects is central adaptation to continued volume-related afferent traffic (van Lunteren, 2001). In agreement with this hypothesis, the present findings raise the possibility that use-dependent memory of the habituation and desensitization to chronic lung distension might eventually blunt the respiratory sensitivity to phasic volume-related feedbacks, whereas chronic lung restriction might produce the opposite effects. Such potential longterm learning and memory effects of the HBIR in humans deserve further study.
The present findings also have important implications in certain neurological diseases such as Rett syndrome, a neurodevelopmental disorder caused by mutations in the X-linked methyl-CpG binding protein 2 (MECP2) gene (Amir et al., 1999) with breathing abnormalities and mental retardation in females (Hagberg et al., 2002). Recent data show that MECP2 mutant mice experience pronounced apneas in response to vagal stimulation or activation of the Kölliker-Fuse nucleus with decreased habituation and increased sensitization of the fictive HBIR (Poon & Song, 2007; Stettner et al., 2007). The present findings raise the possibility that impaired habituation and desensitization of the HBIR and their use-dependent STM may contribute to the breathing arrhythmias in these mutant animals and perhaps Rett Syndrome patients.
Acknowledgments
CT was supported by a predoctoral fellowship award from the American Heart Association. This work was supported by National Institutes of Health grants HL067966, HL072849 and HL079503.
References
- Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23:185–188. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- Bantikyan A, Song G, Feinberg-Zadek P, Poon CS. Intrinsic and Synaptic Long-Term Depression of NTS Relay of Nociceptin-Sensitive and Capsaicin-Sensitive Cardiopulmonary Afferents Hyperactivity. Pflugers Arch (European Journal of Physiology) 2008 doi: 10.1007/s00424-008-0571-9. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoli A, Bystrzycka E, Guz A, Jain SK, Noble MI, Trenchard D. Studies of the pulmonary vagal control of central respiratory rhythm in the absence of breathing movements. J Physiol. 1973;230:449–465. doi: 10.1113/jphysiol.1973.sp010197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergren DR, Peterson DF. Identification of vagal sensory receptors in the rat lung: are there subtypes of slowly adapting receptors? J Physiol (Lond) 1993;464:681–698. doi: 10.1113/jphysiol.1993.sp019657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonham AC, McCrimmon DR. Neurons in a discrete region of the nucleus tractus solitarius are required for the Breuer-Hering reflex in rat. J Physiol (Lond) 1990;427:261–280. doi: 10.1113/jphysiol.1990.sp018171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braff DL, Swerdlow NR, Geyer MA. Gating and habituation deficits in the schizophrenia disorders. Clinical neuroscience (New York, NY. 1995;3:131–139. [PubMed] [Google Scholar]
- Breuer J. Self-steering of respiration through the nerves vagus (circa 1868) In: Porter R, editor. Breathing: Hering-Breuer Centenary Symposium. Churchill; London: 1970. pp. 365–394. [Google Scholar]
- BuSha BF, Stella MH, Manning HL, Leiter JC. Termination of inspiration by phase-dependent respiratory vagal feedback in awake normal humans. J Appl Physiol. 2002;93:903–910. doi: 10.1152/japplphysiol.00153.2002. [DOI] [PubMed] [Google Scholar]
- Castellucci VF, Carew TJ, Kandel ER. Cellular analysis of long-term habituation of the gill-withdrawal reflex of Aplysia californica. Science. 1978;202:1306–1308. doi: 10.1126/science.214854. [DOI] [PubMed] [Google Scholar]
- Christoffersen GR. Habituation: events in the history of its characterization and linkage to synaptic depression. A new proposed kinetic criterion for its identification. Prog Neurobiol. 1997;53:45–66. doi: 10.1016/s0301-0082(97)00031-2. [DOI] [PubMed] [Google Scholar]
- Clark FJ, von Euler C. On the regulation of depth and rate of breathing. J Physiol. 1972;222:267–295. doi: 10.1113/jphysiol.1972.sp009797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Angelo E. Effects of single breath lung inflation on the pattern of subsequent breaths. Respir Physiol. 1977;31:1–18. doi: 10.1016/0034-5687(77)90061-5. [DOI] [PubMed] [Google Scholar]
- Davies A, Roumy M. The effect of transient stimulation of lung irritant receptors on the pattern of breathing in rabbits. J Physiol. 1982;324:389–401. doi: 10.1113/jphysiol.1982.sp014119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezure K. Respiration-related afferents to parabrachial pontine regions. Respir Physiol Neurobiol. 2004;143:167–175. doi: 10.1016/j.resp.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Ezure K, Tanaka I. GABA, in some cases together with glycine, is used as the inhibitory transmitter by pump cells in the Hering-Breuer reflex pathway of the rat. Neuroscience. 2004;127:409–417. doi: 10.1016/j.neuroscience.2004.05.032. [DOI] [PubMed] [Google Scholar]
- Finkler J, Iscoe S. Control of breathing at elevated lung volumes in anesthetized cats. J Appl Physiol. 1984;56:839–844. doi: 10.1152/jappl.1984.56.4.839. [DOI] [PubMed] [Google Scholar]
- Gautier H, Bonora M, Gaudy JH. Breuer-Hering inflation reflex and breathing pattern in anesthetized humans and cats. Journal of applied physiology: respiratory, environmental and exercise physiology. 1981;51:1162–1168. doi: 10.1152/jappl.1981.51.5.1162. [DOI] [PubMed] [Google Scholar]
- Graves C, Glass L, Laporta D, Meloche R, Grassino A. Respiratory phase locking during mechanical ventilation in anesthetized human subjects. Am J Physiol. 1986;250:R902–909. doi: 10.1152/ajpregu.1986.250.5.R902. [DOI] [PubMed] [Google Scholar]
- Grippi MA, Pack AI, Davies RO, Fishman AP. Adaptation to reflex effects of prolonged lung inflation. J Appl Physiol. 1985;58:1360–1371. doi: 10.1152/jappl.1985.58.4.1360. [DOI] [PubMed] [Google Scholar]
- Groves PM, Thompson RF. Habituation: a dual-process theory. Psychol Rev. 1970;77:419–450. doi: 10.1037/h0029810. [DOI] [PubMed] [Google Scholar]
- Guz A, Noble MI, Trenchard D, Cochrane HL, Makey AR. Studies on the vagus nerves in man: Their role in respiratory and circulatory control. Clinical science. 1964;27:293–304. [PubMed] [Google Scholar]
- Guz A, Noble MI, Trenchard D, Smith AJ, Makey AR. The Hering-Breuer inflation reflex in man: studies of unilateral lung inflation and vagus nerve block. Respir Physiol. 1966;1:382–389. doi: 10.1016/0034-5687(66)90005-3. [DOI] [PubMed] [Google Scholar]
- Guz A, Noble MIM, Eisele JH, Trenchard D. The role of vagal inflation reflexes in man and other animals. In: Porter R, editor. Breathing: Hering-Breuer Centenary Symposium A Ciba Foundation Symposium. Churchill; London, UK: 1970. pp. 17–40. [Google Scholar]
- Guz A, Trenchard DW. Pulmonary stretch receptor activity in man: a comparison with dog and cat. The Journal of physiology. 1971;213:329–343. doi: 10.1113/jphysiol.1971.sp009385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberthur C, Guttmann J. Short-term effects of positive end-expiratory pressure on breathing pattern: an interventional study in adult intensive care patients. Crit Care. 2005;9:R407–415. doi: 10.1186/cc3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg B, Hanefeld F, Percy A, Skjeldal O. An update on clinically applicable diagnostic criteria in Rett syndrome. Comments to Rett Syndrome Clinical Criteria Consensus Panel Satellite to European Paediatric Neurology Society Meeting, Baden Baden, Germany, 11 September 2001. Eur J Paediatr Neurol. 2002;6:293–297. doi: 10.1053/ejpn.2002.0612. [DOI] [PubMed] [Google Scholar]
- Hamilton RD, Horner RL, Winning AJ, Guz A. Effect on breathing of raising end-expiratory lung volume in sleeping laryngectomized man. Respiration physiology. 1990;81:87–98. doi: 10.1016/0034-5687(90)90072-7. [DOI] [PubMed] [Google Scholar]
- Hamilton RD, Winning AJ, Horner RL, Guz A. The effect of lung inflation on breathing in man during wakefulness and sleep. Respiration physiology. 1988;73:145–154. doi: 10.1016/0034-5687(88)90062-x. [DOI] [PubMed] [Google Scholar]
- Hayashi F, Coles SK, McCrimmon DR. Respiratory neurons mediating the Breuer-Hering reflex prolongation of expiration in rat. J Neurosci. 1996;16:6526–6536. doi: 10.1523/JNEUROSCI.16-20-06526.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hering E. Self-steering of respiration through the nerves vagus (circa 1868) In: Porter R, editor. Breathing: Hering-Breuer Centenary Symposium. Churchill; London: 1970. pp. 359–364. [Google Scholar]
- Kandel ER. A Cell-Biological Approach to Learning. Society for Neuroscience; Bethesda, MD: 1978. [Google Scholar]
- MacDonald SM, Song G, Poon CS. Nonassociative learning promotes respiratory entrainment to mechanical ventilation. PLoS ONE. 2007;2:e865. doi: 10.1371/journal.pone.0000865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzzin S, Trippenbach T, Baconnier P, Benchetrit G. Entrainment of the respiratory rhythm by periodic lung inflation during vagal cooling. Respiration physiology. 1989;75:157–172. doi: 10.1016/0034-5687(89)90060-1. [DOI] [PubMed] [Google Scholar]
- Pinsker H, Kupfermann I, Castellucci V, Kandel E. Habituation and dishabituation of the gill-withdrawal reflex in Aplysia. Science. 1970;167:1740–1742. doi: 10.1126/science.167.3926.1740. [DOI] [PubMed] [Google Scholar]
- Polacheck J, Strong R, Arens J, Davies C, Metcalf I, Younes M. Phasic vagal influence on inspiratory motor output in anesthetized human subjects. J Appl Physiol. 1980;49:609–619. doi: 10.1152/jappl.1980.49.4.609. [DOI] [PubMed] [Google Scholar]
- Poon CS, Siniaia MS. Plasticity of cardiorespiratory neural processing: classification and computational functions. Respir Physiol. 2000;122:83–109. doi: 10.1016/s0034-5687(00)00152-3. [DOI] [PubMed] [Google Scholar]
- Poon CS, Song G. Habituation, desensitization and sensitization of the Hering Breuer reflex in normal and Mecp2 /y knockout mice. J Physiol. 2007;584:359–360. doi: 10.1113/jphysiol.2007.142711. author reply 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon CS, Young DL. Nonassociative learning as gated neural integrator and differentiator in stimulus-response pathways. Behav Brain Funct. 2006;2:29. doi: 10.1186/1744-9081-2-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon CS, Young DL, Siniaia MS. High-pass filtering of carotid-vagal influences on expiration in rat: role of N-methyl-D-aspartate receptors. Neurosci Lett. 2000a;284:5–8. doi: 10.1016/s0304-3940(00)00993-9. [DOI] [PubMed] [Google Scholar]
- Poon CS, Zhou Z, Champagnat J. NMDA receptor activity in utero averts respiratory depression and anomalous long-term depression in newborn mice. J Neurosci. 2000b;20:RC73. doi: 10.1523/JNEUROSCI.20-09-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putensen C, Muders T, Varelmann D, Wrigge H. The impact of spontaneous breathing during mechanical ventilation. Curr Opin Crit Care. 2006;12:13–18. doi: 10.1097/01.ccx.0000198994.37319.60. [DOI] [PubMed] [Google Scholar]
- Rabbette PS, Fletcher ME, Dezateux CA, Soriano-Brucher H, Stocks J. Hering-Breuer reflex and respiratory system compliance in the first year of life: a longitudinal study. J Appl Physiol. 1994;76:650–656. doi: 10.1152/jappl.1994.76.2.650. [DOI] [PubMed] [Google Scholar]
- Sant'Ambrogio G, Widdicombe J. Reflexes from airway rapidly adapting receptors. Respir Physiol. 2001;125:33–45. doi: 10.1016/s0034-5687(00)00203-6. [DOI] [PubMed] [Google Scholar]
- Seifert E, Trippenbach T. Effects of baclofen on the Hering-Breuer inspiratory-inhibitory and deflation reflexes in rats. Am J Physiol. 1998;274:R462–469. doi: 10.1152/ajpregu.1998.274.2.R462. [DOI] [PubMed] [Google Scholar]
- Sibuya M, Kanamaru A, Homma I. Inspiratory prolongation by vagal afferents from pulmonary mechanoreceptors in rabbits. Jpn J Physiol. 1993;43:669–684. doi: 10.2170/jjphysiol.43.669. [DOI] [PubMed] [Google Scholar]
- Simon PM, Zurob AS, Wies WM, Leiter JC, Hubmayr RD. Entrainment of respiration in humans by periodic lung inflations. Effect of state and CO(2) Am J Respir Crit Care Med. 1999;160:950–960. doi: 10.1164/ajrccm.160.3.9712057. [DOI] [PubMed] [Google Scholar]
- Siniaia MS, Young DL, Poon CS. Habituation and desensitization of the Hering-Breuer reflex in rat. J Physiol. 2000;523(Pt 2):479–491. doi: 10.1111/j.1469-7793.2000.t01-1-00479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song G, Poon CS. Functional and structural models of pontine modulation of mechanoreceptor and chemoreceptor reflexes. Respirat Physiol Neurobiol. 2004;143:281–292. doi: 10.1016/j.resp.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Song G, Poon CS. Lateral parabrachial nucleus mediates shortening of expiration during hypoxia. Respir Physiol Neurobiol. 2008 doi: 10.1016/j.resp.2008.10.007. accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song G, Yu Y, Poon CS. Cytoarchitecture of pneumotaxic integration of respiratory and nonrespiratory information in the rat. J Neurosci. 2006;26:300–310. doi: 10.1523/JNEUROSCI.3029-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley NN, Altose MD, Cherniack NS, Fishman AP. Changes in strength of lung inflation reflex during prolonged inflation. J Appl Physiol. 1975;38:474–480. doi: 10.1152/jappl.1975.38.3.474. [DOI] [PubMed] [Google Scholar]
- Stettner GM, Huppke P, Brendel C, Richter DW, Gartner J, Dutschmann M. Breathing dysfunctions associated with impaired control of postinspiratory activity in Mecp2-/y knockout mice. J Physiol. 2007;579:863–876. doi: 10.1113/jphysiol.2006.119966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano K, Kato F. Inspiration-promoting vagal reflex in anaesthetized rabbits after rostral dorsolateral pons lesions. The Journal of physiology. 2003;550:973–983. doi: 10.1113/jphysiol.2003.042200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RF, Groves PM, Teyler TJ, Roemer RA. A dual-process theory of habituation: theory and behavior. In: Peeke HVS, Herz MJ, editors. Habituation. Academic Press; New York: 1973. pp. 239–271. [Google Scholar]
- Thompson RF, Spencer WA. Habituation: a model phenomenon for the study of neuronal substrates of behavior. Psychol Rev. 1966;73:16–43. doi: 10.1037/h0022681. [DOI] [PubMed] [Google Scholar]
- Tryfon S, Kontakiotis T, Mavrofridis E, Patakas D. Hering-Breuer reflex in normal adults and in patients with chronic obstructive pulmonary disease and interstitial fibrosis. Respiration; international review of thoracic diseases. 2001;68:140–144. doi: 10.1159/000050483. [DOI] [PubMed] [Google Scholar]
- Tsubone H. Characteristics of vagal afferent activity in rats: three types of pulmonary receptors responding to collapse, inflation, and deflation of the lung. Exp Neurol. 1986;92:541–552. doi: 10.1016/0014-4886(86)90296-7. [DOI] [PubMed] [Google Scholar]
- van Lunteren E. Attenuation of the Hering-Breuer reflex: yet another adverse consequence of COPD? Respiration. 2001;68:131–132. doi: 10.1159/000050480. [DOI] [PubMed] [Google Scholar]
- Wang H, Zhang H, Song G, Poon CS. Modulation of Hering-Breuer reflex by ventrolateral pons. Adv Exp Med Biol. 2008;605:387–392. doi: 10.1007/978-0-387-73693-8_68. [DOI] [PubMed] [Google Scholar]
- Widdicombe J. Reflexes from the lungs and airways: historical perspective. J Appl Physiol. 2006;101:628–634. doi: 10.1152/japplphysiol.00155.2006. [DOI] [PubMed] [Google Scholar]
- Widdicombe JG. Respiratory reflexes in man and other mammalian species. Clinical science. 1961;21:163–170. [PubMed] [Google Scholar]
- Wittmeier S, Song G, Duffin J, Poon C. Pacemakers handshake synchronization mechanism of mammalian respiratory rhythmogenesis. Proceedings of the National Academy of Sciences of the United States of America; 2008. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younes M, Polacheck J. Central adaptation to inspiratory-inhibiting expiratory-prolonging vagal input. J Appl Physiol. 1985;59:1072–1084. doi: 10.1152/jappl.1985.59.4.1072. [DOI] [PubMed] [Google Scholar]
- Young DL, Eldridge FL, Poon CS. Integration-differentiation and gating of carotid afferent traffic that shapes the respiratory pattern. J Appl Physiol. 2003;94:1213–1229. doi: 10.1152/japplphysiol.00639.2002. [DOI] [PubMed] [Google Scholar]
- Zhou D, St John WM, Bartlett D., Jr Activities of pulmonary stretch receptors during ventilatory cycles without lung inflation. Respir Physiol. 1989;77:187–194. doi: 10.1016/0034-5687(89)90005-4. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Champagnat J, Poon CS. Phasic and long-term depression in brainstem nucleus tractus solitarius neurons: differing roles of AMPA receptor desensitization. J Neurosci. 1997;17:5349–5356. doi: 10.1523/JNEUROSCI.17-14-05349.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]


