Abstract
T helper (Th) 17 cells have recently been implicated in psoriasis pathogenesis, but mechanisms of how these cells traffic into inflamed skin are unknown. By immunostaining for interleukin (IL)-17A and IL-22, we show numerous cells present in psoriasis lesions that produce these cytokines. We next found that Th17 cytokines (IL-17A, IL-22, and tumor necrosis factor (TNF)-α) markedly increased the expression of CC chemokine ligand (CCL) 20, a CC chemokine receptor (CCR)6 ligand, in human keratinocyte monolayer and raft cultures in a dose- and time-dependent manner. Lastly, we showed in mice that subcutaneous injection with recombinant IL-17A, IL-22, or TNF-α led to the upregulation of both CCL20 and CCR6 expression in skin as well as cutaneous T-cell infiltration. Taken together, these data show that Th17 cytokines stimulate CCL20 production in vitro and in vivo, and thus provide a potential explanation of how CCR6-positive Th17 cells maintain their continual presence in psoriasis through a positive chemotactic feedback loop.
INTRODUCTION
Interleukin (IL)-23 is a heterodimeric protein consisting of p19 and p40 subunits that is overexpressed in psoriasis lesional skin, as shown by increased p19 and p40 transcripts (Lee et al., 2004; Chan et al., 2006; Zaba et al., 2007) and by the presence of elevated numbers of p19-positive and p40-positive dendritic cells detected by immunohistochemistry (IHC) in the dermis of the affected skin (Lee et al., 2004; Piskin et al., 2006; Wilson et al., 2007). IL-23 is also expressed by keratinocytes (KC) in lesional psoriatic skin (Piskin et al., 2006), although transcripts and protein appear less abundant when compared with dendritic cells. By contrast, most recent reports show no increased expression of the IL-23-related protein, IL-12, in psoriasis; that is, transcripts for the IL-12-specific subunit p35 are not increased in psoriatic plaques (Lee et al., 2004; Chan et al., 2006; Zaba et al., 2007). Furthermore, large-scale genomic studies have identified IL-23R, specific for IL-23 signaling, as a psoriasis susceptibility gene, whereas no psoriasis association was found for IL-12Rβ1, the signaling receptor for IL-12 (Capon et al., 2007; Cargill et al., 2007).
IL-23 promotes survival and proliferation of T helper (Th) 17 cells, whereas IL-12 promotes the development of Th1 cells (Blauvelt, 2007; Fitch et al., 2007). In psoriasis, a large body of literature supports the presence of interferon (IFN)-γ in psoriasis (Austin et al., 1999; Zhou et al., 2003; Lew et al., 2004), which has long been regarded as a prototypic Th1 cell-derived cytokine. Of recent relevance, a distinct subset of human Th17 cells have also been shown to produce IFN-γ (Annunziato et al., 2007; Acosta-Rodriguez et al., 2007a, b; Wilson et al., 2007), so that the presence of IFN-γ and downstream IFN-γ-regulated genes in psoriasis, as well as other human diseases, can no longer be equated with the presence of Th1 cells and with a “Th1 disease” state. Recently, transcripts for Th17 cell-specific cytokines, notably IL-17A and IL-22, have been documented at high levels in psoriasis (Chan et al., 2006; Wolk et al., 2006; Wilson et al., 2007; Zaba et al., 2007) and Th17 cells have been identified by flow cytometry in lesions of psoriasis (Lowes et al., 2008). Quantification of Th17 cytokine-producing cells within psoriatic tissue by IHC has not been published to date.
Little is known about how Th17 cells traffic into skin. Recently, several groups have characterized the chemokine receptor expression pattern on human Th17 cells, and found that Th17 cells, secreting IL-17A, but not IFN-γ, express CC chemokine receptors (CCR) 6 and CCR4, whereas CCR6 and CXC chemokine receptor 3 positivity identified both Th17 T cells that secreted both IL-17A and IFN-γ and Th1 cells that secrete IFN-γ (Annunziato et al., 2007; Acosta-Rodriguez et al., 2007a, b). Consistently, differentiation of T cells into a Th17 phenotype resulted in a preferential induction of CCR6 expression and CCR6 was expressed on all IL-17-producing cells (Singh et al., 2008). A recent report by Hirota et al. (2007) confirmed that Th17 T cells predominantly express CCR6, whereas Th1 cells do not. This group also reported that CCR6 was critical for leukocyte migration into inflamed joints in a mouse model rheumatoid arthritis (Hirota et al., 2007). By contrast, Lim et al. (2008) reported that Th17, Th1, Th2, and regulatory T cells exhibit significant overlap in chemokine receptor expression patterns and this overlap allows for co-migration of different T-cell subsets to the site of tissue inflammation.
The primary chemokine ligands for CCR6, CC chemokine ligand (CCL) 20 and β-defensin 2, are upregulated in psoriasis (Homey et al., 2000; Ong et al., 2002; Wilson et al., 2007). In skin, these two chemotactic molecules are produced predominantly by KC and CCL20 is a key stimulus for chemoattracting both CCR6-positive immature dendritic cells (Le Borgne et al., 2006) and T cells (Varona et al., 2005; Paradis et al., 2007) from blood into inflamed cutaneous tissue. Currently, little is known about whether Th17 cytokines affect CCL20 production in KC. In this report, we found that IL-17A, IL-22, and tumor necrosis factor (TNF)-α stimulated CCL20 expression by KC. These results suggest a mechanism by which Th17 cells can promote their continued presence in psoriatic tissue, by stimulating a positive chemotactic feedback loop that involves KC-derived CCL20 and CCR6 expressed on Th17 cell surfaces.
RESULTS AND DISCUSSION
IL-17A-positive and IL-22-positive cells are present in psoriasis lesional skin
Transcript levels for IL-17A and IL-22 are increased in psoriasis plaques (Chan et al., 2006; Wolk et al., 2006; Wilson et al., 2007; Zaba et al., 2007), however, quantification of Th17 cells within lesions of psoriasis by IHC had not been previously published. We found abundant IL-17A-positive cells (Figure 1, Table 1) and many (although fewer) IL-22-positive cells (Figure 1, Table 2) in skin affected by psoriasis. We determined optimal IHC staining conditions for these cytokines using formalin-fixed (for IL-17A IHC) and fresh-frozen (for IL-22 IHC) tissue sections (data not shown). In these experiments, IL-17A-positive cells were more numerous than IL-22-positive cells, although this could simply be a feature of differing antibody affinities for each of these cytokines. By comparison, CD3 positive T cells were on average 2.4 times more abundant than IL-17A-positive cells and 5.7 times more abundant than IL-22-positive cells (Tables 1 and 2). Single IL-17A-positive or IL-22-positive immunoreactive cells were present throughout the dermal papillae and upper dermis. No positive cells were found in more superficial regions of the epidermis. The cells were small and mostly round, consistent with the morphology of T cells. Only rare IL-17A- or IL-22-positive cells were detected in tissue sections obtained from normal skin of healthy individuals (Figure 1).
Figure 1. IL-17A-positive and IL-22-positive cells are abundant in psoriasis lesional skin.
Formalin-fixed paraffin-embedded tissue sections obtained from seven psoriasis patients and three healthy individuals were examined by IHC. Tissue was stained with either anti-CD3 or anti-IL-17A antibodies. In six separate individuals with psoriasis, lesional skin was biopsied and snap frozen. Fresh-frozen sections were stained with either anti-CD3 or anti-IL-22 antibodies. Increased numbers of IL-17A- and IL-22-expressing cells (brown or purple in color) were present in lesional skin when compared with healthy skin. Representative photos are shown. Bar = 10 μm in figures labeled × 40, 20 μm in figures labeled × 20, and 40 μm in figures labeled × 10.
Table 1.
IL-17A-positive cells are abundant in formalin-fixed psoriasis tissue
| CASE No. | CD3 positive (cells per HPF in 10 h.p.f.) | IL-17A positive (cells per HPF in 10 h.p.f.) | CD3 positive/IL-17A positive |
|---|---|---|---|
| 1 | 39.6 | 15.5 | 2.6 |
| 2 | 43.8 | 24.9 | 1.8 |
| 3 | 79.3 | 29.4 | 2.7 |
| 4 | 89.9 | 43.6 | 2.1 |
| 5 | 99 | 49.8 | 2.0 |
| 6 | 86.1 | 27.2 | 3.2 |
| 7 | 103.6 | 43.3 | 2.4 |
| Mean | 77.3 | 33.4 | 2.4 |
HPF, high-power field.
Table 2.
IL-22-positive cells are present in fresh-frozen lesional psoriasis tissue
| CASE No. | CD3 positive (cells per HPF in 10 h.p.f.) | IL-22 positive (cells per HPF in 10 h.p.f.) | CD3 positive/IL-22 positive |
|---|---|---|---|
| 8 | 31.8 | 5.5 | 5.8 |
| 9 | 47.3 | 11.7 | 4.0 |
| 10 | 29.6 | 6.5 | 4.6 |
| 11 | 69.8 | 11.1 | 6.3 |
| 12 | 95.1 | 11.7 | 8.1 |
| 13 | 44.3 | 8.3 | 5.3 |
| Mean | 53.0 | 9.1 | 5.7 |
HPF, high-power field.
The demonstration of numerous IL-17A-positive cells and less abundant IL-22-positive cells localizing to psoriasis lesions is consistent with the high levels of transcripts for these Th17 cytokines detected by previous RT-PCR studies (Chan et al., 2006; Wolk et al., 2006; Wilson et al., 2007; Zaba et al., 2007). These data are also consistent with recent flow cytometry studies on T-cell populations isolated from psoriatic skin explants, where IL-17A+ T cells were identified in less than 10% of T cells (Kryczek et al., 2008; Lowes et al., 2008). Because of the time tissue manipulation and ex vivo culture conditions required to quantify IL-17A+ cells in psoriatic lesions by flow cytometry, we believe these methods would be predicted to underestimate the actual number of IL-17A+ cells in psoriatic tissue when compared with IHC. We doubt that our IL-17A staining is due to extracellular-bound IL-17A, as keratinocytes express IL-17 receptors, and these cells did not stain positively in our experiments (Figure 1). A limitation of our study, however, is that we cannot definitively conclude that our IL-17A+ and IL-22+ cells represent Th17 cells. It will be important to perform IHC double-labeling experiments on psoriatic tissue to identify these cells as Th17 cells.
IL-17A, IL-22, and TNF-α increase CCL20 mRNA and protein expression by normal human KC in vitro
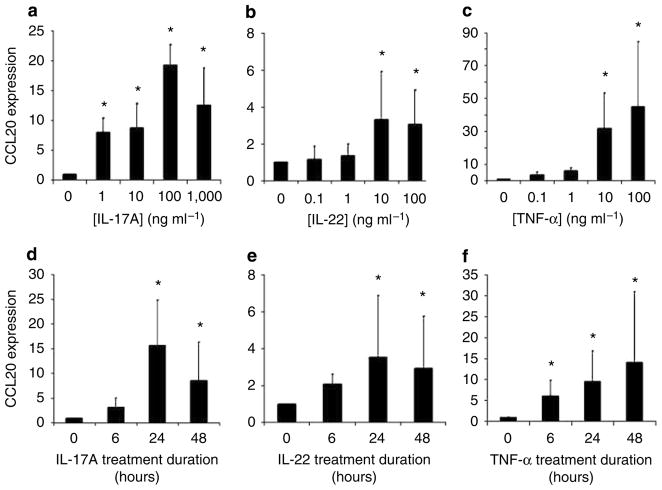
Using normal human KC cultured as monolayers on plastic dishes, we found that IL-17A, IL-22, and TNF-α markedly upregulated CCL20 mRNA at 24 hours in a dose-dependent manner (Figure 2a–c). Optimal cytokine stimulation dosage was found to be 100 ng ml−1 for both IL-17A and TNF-α (19- and 45-fold increases, respectively, normalized to GAPDH expression and compared with no cytokine stimulation), whereas 10 ng ml−1 was optimal for IL-22 (17-fold increase) (Figure 2a–c). In contrast, neither TGF-β1 nor IFN-γ increased CCL20 expression by KC (data not shown). Using these optimal doses, we also found Th17 cytokines upregulate CCL20 mRNA expression in a time-dependent manner (Figure 2d–f). The various cytokines each had somewhat different time course patterns, with IL-17A and IL-22 showing maximal CCL20 mRNA expression at 24 hours post treatment, and TNF-α at 48 hours. Dose- and time-dependent increases in CCL20 protein were also demonstrated by ELISA using cell-free supernatants of KC cultures under the same cytokine-stimulated conditions described above (Figure 3a–f).
Figure 2. IL-17A, IL-22, and TNF-α increase CCL20 mRNA expression by normal human KC in a dose- and time-dependent manner in vitro.
Cells were treated with the indicated concentrations of (a) IL-17A, (b) IL-22, or (c) TNF-α for 24 hours. Cells were treated with the optimal cytokine concentrations of (d) IL-17A, (e) IL-22, or (f) TNF-α for 6, 24, or 48 hours. mRNA was harvested and CCL20 transcripts were quantified by real-time RT-PCR analyses for all experiments. All experiments were performed in triplicate at least three separate times and the average mRNA increase and SD is reported. Significant differences were detected using the Mann–Whitney unpaired two-tailed t-test (*Indicates significance, P<0.05).
Figure 3. IL-17A, IL-22, and TNF-α increase CCL20 protein expression by normal human KC in a dose- and time-dependent manner in vitro.
Cells were treated with the indicated concentrations of (a) IL-17A, (b) IL-22, or (c) TNF-α for 24 hours. Cells were treated with the optimal cytokine concentrations of (d) IL-17A, (e) IL-22, or (f) TNF-α for 6, 24, or 48 hours. Cell-free supernatants were harvested and CCL20 protein levels were quantified by ELISA for all experiments. All experiments were performed in triplicate at least three separate times and the average protein levels and SD are reported. Significant differences were detected using the Mann–Whitney unpaired two-tailed t-test (*Indicates significance, P<0.05).
IL-17A, IL-22, and TNF-α increase CCL20 mRNA and protein expression by reconstructed human epidermis (RHE)
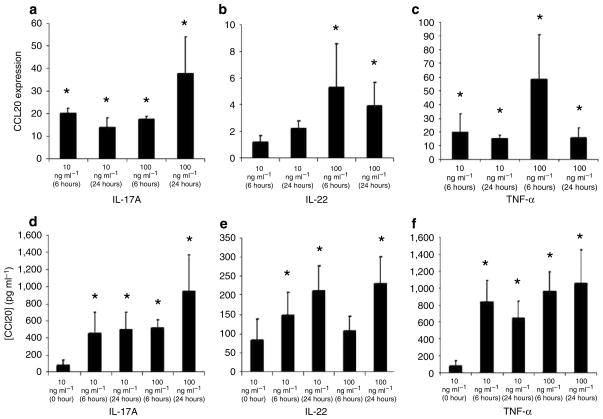
KC grown as monolayers on plastic dishes differentially respond to certain cytokine signals when compared with KC grown on dermal substrates at air–liquid interfaces, which allows for KC stratification and terminal differentiation (Poumay et al., 2004; Farley et al., 2006, 2008). These KC cultures are called as reconstructed human epidermis (RHE) because they resemble normal human epidermis, and may be a more physiologic model for studying KC biology when compared with KC grown in monolayers on plastic. To study CCL20 mRNA and protein expression following stimulation with Th17 cytokines using this model, RHE were treated with 10 or 100 ng ml−1 of IL-17A, IL-22, or TNF-α for either 6 or 24 hours. IL-17A, IL-22, and TNF-α stimulated CCL20 mRNA and protein production in a dose- and time-dependent manner (Figure 4a–f).
Figure 4. IL-17A, IL-22, and TNF-α increase CCL20 mRNA and protein expression by RHE in a dose- and time-dependent manner.
Stratified KC in RHE were treated with the indicated concentrations of (a) IL-17A, (b) IL-22, or (c) TNF-α for 6 or 24 hours. Total RNA was harvested and CCL20 transcripts were quantified by real-time RT-PCR analyses. RHE were treated with the optimal cytokine concentrations of (d) IL-17A, (e) IL-22, or (f) TNF-α for 6 or 24 hours. Cell-free supernatants were harvested and CCL20 protein levels were quantified by ELISA. All experiments were performed in triplicate at least three separate times and the average protein levels and SD are reported. Significant differences were detected using the Mann–Whitney unpaired two-tailed t-test (*Indicates significance, P<0.05).
In both monolayers of KC and RHE, Th17 cytokines induced the expression of CCL20 mRNA and protein (Figures 2–4). In order of potency, all of the in vitro KC experiments suggest that TNF-α is the most potent Th17 cytokine that induces CCL20 expression, with IL-17A showing the next highest effects followed by IL-22. It is interesting to speculate that TNF-α-blocking agents for psoriasis may work at least in part by blocking TNF-α-induced upregulation of CCL20. These findings are consistent with previous reports indicating that IL-17A induces CCL20 expression by KC (Homey et al., 2000), airway epithelial cells (Kao et al., 2005), and synoviocytes (Hirota et al., 2007). In addition, Kao et al. (2004) reported that IL-17A stimulated β-defensin 2, another CCR6 ligand, in airway epithelium. Importantly, we extend the KC results of (Homey et al., (2000) by (1) showing that additional Th17 cytokines can stimulate CCL20 production (Figures 2–4), by (2) reporting that RHE, in addition to monolayers of KC, can be stimulated by Th17 cytokines to produce CCL20 (Figure 4), and by (3) showing that injection of these cytokines induces the upregulation of CCL20 and CCR6 as well as T-cell infiltration in vivo (see Figure 5 below).
Figure 5. CCL20 and CCR6 upregulation and T-cell infiltration in murine skin injected with Th17 cytokines.
(a–c) Balb/c mouse ears were injected with 500 ng of IL-17A, IL-22, TNF-α, or PBS daily for 5 days. (a and c) CCL20 and CCR6 mRNA expression levels in injected ears were determined by real-time RT-PCR. Significant differences were detected using the Mann–Whitney unpaired two-tailed t-test (*Indicates significance, P<0.05). (b) CCL20 protein expression was measured by ELISA. Significant differences were detected using the unpaired t-test with Welch’s correction (*Indicates significance, P<0.05). (a–c) Data from 10 mice in each treatment group are shown. (d) Representative histologic photos showing CD3 positive T cells in murine skin injected with Th17 cytokines. Bar= 40 μm.
CCL20 and CCR6 upregulation and T-cell infiltration in murine skin injected with Th17 cytokines
Ears of Balb/c mice were injected with 500 ng of recombinant murine IL-17A, IL-22, TNF-α, or PBS daily for 5 days. Total RNA or total protein were extracted from each ear and CCL20 and CCR6 mRNA expression was assessed by real-time RT-PCR, whereas CCL20 protein expression was assessed by ELISA. CCL20 mRNA was markedly upregulated by all of the Th17 cytokines, with TNF-α causing the greatest increase (mean of 55-fold compared with PBS-injected ears), and IL-17A and IL-22 causing 3-fold and 2.5-fold elevations, respectively (Figure 5a). Both TNF-α and IL-17A injections led to concomitant increases in CCL20 protein levels in cytokine-injected ears (Figure 5b), whereas IL-22-injected ears showed no clear elevations in CCL20 protein expression in these experiments. Similarly, CCR6 mRNA was elevated by TNF-α, IL-17A, and IL-22 by 4-, 2-, and 2.5-fold, respectively, when compared with PBS-injected ears (Figure 5c). Lastly, we showed that Th17 cytokine injections induced CD3-positive T-cell infiltration within murine skin (Figure 5d). Only rare T cells were observed in PBS-injected skin (Figure 5d).
CCR6–CCL20 interactions have been studied in the context of dendritic cell and T-cell trafficking in other murine diseases. Specifically, immature CCR6-positive dendritic cells were recruited to sites of inflamed epithelial tissue in mice following trauma (Le Borgne et al., 2006). CCR6 was also shown to be critical in T-cell trafficking into skin in murine models of graft-versus-host disease (Varona et al., 2005) and contact hypersensitivity (Paradis et al., 2007). Recently, CCR6–CCL20 interactions were shown to be critical in mediating leukocyte migration into inflamed joints in a mouse model of rheumatoid arthritis; anti-CCR6 monoclonal antibodies inhibited Th17 T-cell trafficking and early disease development in this model (Hirota et al., 2007). In short-term 5-day experiments, we show for the first time that Th17 cytokines induce CCL20 and CCR6 in vivo, with influx of T cells into skin. It is noteworthy that we did not observe keratinocyte hyperproliferation following 5 days of IL-22 injections (Figure 5d). This is not surprising, given that IL-22-mediated keratinocyte hyperproliferation in vivo takes longer to occur (approximately 2 weeks) (Ma et al., 2008). Our results do not exclude other chemokine–chemokine receptor interactions in mediating Th17 cell chemotaxis (for example, involvement of CCR4). In fact, prominent redundancy in leukocyte-trafficking pathways has been well documented. Thus, elucidation of additional mechanisms by which Th17 cells may enter the skin requires further study.
Our findings suggest a novel paradigm for human Th17 disease. Human Th17 cells are able to maintain their own presence in peripheral inflamed tissue by secreting cytokines that stimulate epithelial cells to produce the chemokine CCL20, which in turn induces chemotaxis of additional Th17 cells. This biologic paradigm fits with clinical disease features of psoriasis. Clinical features of skin affected by psoriasis (for example, erythema, induration, and scaling) are remarkably stable when untreated. Persistent presence of Th17 cells within the dermis driven by a continual chemotactic gradient coming from epidermal KC would manifest as phenotypically stable skin. Breaking this cycle would require the death of Th17 cells. The experimental biologic agents ustekinumab and ABT 874, which are monoclonal antibodies directed against the p40 subunit of IL-23, lead to dramatic clinical improvement in psoriasis (Krueger et al., 2007; Kimball et al., 2008; Leonardi et al., 2008; Papp et al., 2008). We postulate that these agents are clinically effective in psoriasis because they block IL-23, the cytokine signal needed by Th17 cells to survive. Death of Th17 cells would stop Th17 cytokine production, and thus break the chemotactic gradient present in psoriasis. Future mechanism of action studies using psoriatic skin obtained from individuals receiving anti-p40 monoclonal antibody therapy are necessary to test this hypothesis. Our findings also suggest the possible therapeutic use of agents designed to block chemokine–chemokine receptor interactions in patients with psoriasis.
MATERIALS AND METHODS
IHC
The OHSU Institutional Review Board approved all of the described studies, and the study was conducted according to the Declaration of Helsinki Principles. For archival tissue, written informed consent was waived as the tissue specimens were anonymized. Formalin-fixed, paraffin-embedded lesional skin obtained from seven individuals with psoriasis and three healthy individuals were retrieved from the OHSU dermatopathology archives maintained by Dr Clifton White. Sections were de-paraffinized and hydrated through heating and washing sections in a graded alcohol series. The sections were incubated in antigen-unmasking solution (Vector Laboratories, Burlingame, CA) at 95 °C for 20 minutes, and then exposed to either goat anti-human IL-17A polyclonal Abs (R&D Systems, Minneapolis, MN) or rabbit anti-human CD3 (Thermo Fisher Scientific, Waltham, MA) monoclonal Abs overnight at 4 °C. Samples were washed and incubated for 1 hour with biotinylated anti-goat or anti-rabbit secondary Abs (Vector Laboratories), followed by 1 hour incubation with streptavidin-conjugated horseradish peroxidase (Vectastain Elite ABC kit, Vector Laboratories), developed using Vector VIP substrate kit for peroxidases (Vector Laboratories), and counter-stained with hematoxylin (Vector Laboratories).
For fresh tissue, patients first gave their written informed consent. Four-mm punch biopsies of lesional skin from six individuals with psoriasis were obtained and frozen immediately in OCT compound. Frozen sections were cut, fixed with methanol for 10 minutes, and then incubated with either rabbit anti-human IL-22 polyclonal Abs (Capralogics, Hardwick, MA) or CD3 mAbs overnight at 4 °C. Samples were washed and incubated for 1 hour with biotinylated anti-rabbit secondary Abs (Vector Laboratories) and developed and counter-stained as above.
Frozen mouse skin sections were cut and fixed in methanol for 10 minutes and incubated overnight with rat anti-mouse CD3 monoclonal Abs (Biolegend, San Diego, CA). Sections were then incubated for 1 hour with biotinylated goat anti-rat secondary Abs (Jackson ImmunoResearch Laboratories, West Grove, PA), for 45 minutes with streptavidin-conjugated horseradish peroxidase (Vectastain Elite ABC kit, Vector Laboratories), and developed and counter-stained as above.
All IHC images were captured using a microscope equipped with Imagepro software. For each antibody, the number of positive cells was counted in 10 separate high-powered (×40) fields and then divided by 10 to obtain the average number of positive cells per high-powered field.
Normal human KC cultures
Primary normal human KC (Cascade Biologics, Portland, OR) were cultured in Keratinocyte Growth Media containing bovine pituitary extract, human epidermal growth factor, insulin, hydrocortisone, gentamicin, and amphotericin B. Cytokine stimulation experiments were initiated on 60% confluent KC cultured between passages 2–5 in 6 cm plastic dishes at 37 °C in the presence of 0.05% heat-inactivated bovine serum albumin and in the absence of hydrocortisone. KC were cultured either alone or with one of the following cytokines: 1, 10, 100, or 1,000 ng ml−1 of IL-17A; 0.1, 1, 10, or 100 ng ml−1 of IL-22, TNF-α, or IFN-γ; or 0.002, 0.02, 0.2, or 2 ng ml−1 of TGF-β1 (R&D Systems). At indicated time points following cytokine exposure, conditioned culture media and cells were collected for CCL20 protein and mRNA quantification, respectively.
RHE cultures
HEKn-E6/E7 cells were established and propagated as described (Iordanov et al., 2002, 2005). HEKn-E6/E7 were propagated in EpiLife basal KC medium (BKM) (Cascade Biologics) supplemented with a semi-defined human keratinocyte growth supplement (HKGS) (Cascade Biologics), which included bovine pituitary extract, bovine insulin, hydrocortisone, bovine transferrin, and human epidermal growth factor. RHE were established as described (Poumay and Coquette, 2007). Briefly, HEKn-E6/E7 cells were plated at 5 × 105cells per ml on 0.4 μm, 12 mm-diameter polycarbonate membranes (Millipore, Billerica, MA) and allowed to reach confluence in BKM + HKGS. Cells were then exposed to the air–liquid interface by removing the medium above the confluent monolayer. Simultaneously, the cells were exposed from underneath the monolayer to a differentiation medium (BKM + HKGSF supplemented with 1.5mM CaCl2, 50 μg ml−1 ascorbic acid, and 10 ng ml−1 rhKGF (Peprotech, Rocky Hill, NJ)). The differentiation medium was changed every 48 hours for 14 days. RHE cultures were treated with 10 or 100 ng ml−1 of IL-17A, IL-22, or TNF-α, and incubated at 37 °C. At indicated time points following cytokine exposure, conditioned culture media and cells were collected for CCL20 protein and mRNA quantification, respectively.
Real-time RT-PCR
Cells from normal human KC monolayer and RHE cultures were lysed directly in TRIzol, whereas mouse ear tissue was minced first and then placed in TRIzol and total RNA was extracted according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA). Integrity of RNA was determined by the appearance of distinct 28S and 18S rRNA bands when analyzed by electrophoresis on 1% agarose gels. The iScript kit (Bio-Rad, Hercules, CA) was employed for cDNA synthesis and resulting cDNA was analyzed to determine CCL20 mRNA levels relative to GAPDH mRNA levels. cDNA was amplified using SYBR green (Bio-Rad) and the following primers: human CCL20, forward TACTCCACCTCTGCGGCGAATCAGAA, and reverse GTGAAACCTCCAACCCCAGCAAGGTT; human GAPDH, forward GAGTCAACGGATTTGGTCGT, and reverse TTGATTTTG GAGGGATCTCG. Primers were annealed at 60 °C and amplified using My IQ single color RT-PCR detection system (Bio-Rad) for a total of 40 cycles.
ELISA
Secreted CCL20 protein levels in conditioned human KC culture media were quantified using the Quantikine ELISA kit according to the manufacturer’s protocol (R&D Systems). For the murine studies, total protein was extracted from ears by snap-freezing tissue in liquid nitrogen, pulverizing tissue while immersed in liquid nitrogen, and subsequently re-suspending in cell extraction buffer (Invitrogen). Samples underwent three freeze-thaw cycles and were centrifuged at full speed for 15 minutes to fractionate samples and remove protein. Mouse CCL20 ELISA (R&D Systems) was performed according to the manufacturer’s instructions using 150 μg total protein.
Animal studies
The Portland VA Medical Center Institutional Animal Care and Use Committee approved all of the described studies using mice. Wild type Balb/c mice were injected in each ear with either 500 ng of IL-17A, IL-22, or TNF-α in 25 μl of PBS/0.1% of BSA, or with 25 μl of PBS/0.1% BSA alone. Ten animals per cytokine treatment group were injected daily for 5 days. All animals were killed at day 5 and mRNA was extracted from ears using a standard Trizol protocol, followed by further purification using an RNeasy kit (Qiagen, Valencia, CA). cDNA was synthesized using an iScript cDNA synthesis kit (Bio-Rad) and amplified using Taqman primers and fluorescent probes for GAPDH, CCL20, and CCR6 using My IQ single color RT-PCR detection system for a total of 40 cycles.
Acknowledgments
This work was supported by a Veterans Affairs Merit Award (AB) and US National Institutes of Health Grant Nos. 1 R21 AR054495-01A1 (AB) and CA 93718 (MI).
Abbreviations
- BKM
basal KC medium
- CCL
CC chemokine ligand
- CCR
CC chemokine receptor
- HKGS
human keratinocyte growth supplement
- IFN
interferon
- IHC
immunohistochemistry
- IL
interleukin
- KC
human keratinocytes
- RHE
reconstructed human epidermis
- Th
T helper
- TNF
tumor necrosis factor
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
References
- Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007a;8:942–9. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007b;8:639–46. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, et al. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204:1849–61. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin LM, Ozawa M, Kikuchi T, Walters IB, Krueger JG. The majority of epidermal T cells in Psoriasis vulgaris lesions can produce type 1 cytokines, interferon-gamma, interleukin-2, and tumor necrosis factor-alpha, defining TC1 (cytotoxic T lymphocyte) and TH1 effector populations: a type 1 differentiation bias is also measured in circulating blood T cells in psoriatic patients. J Invest Dermatol. 1999;113:752–9. doi: 10.1046/j.1523-1747.1999.00749.x. [DOI] [PubMed] [Google Scholar]
- Blauvelt A. New concepts in the pathogenesis and treatment of psoriasis: key roles for IL-23, IL-17A and TGF-b1. Expert Rev Dermatol. 2007;2:1–10. [Google Scholar]
- Capon F, Di Meglio P, Szaub J, Prescott NJ, Dunster C, Baumber L, et al. Sequence variants in the genes for the interleukin-23 receptor (IL23R) and its ligand (IL12B) confer protection against psoriasis. Hum Genet. 2007;122:201–6. doi: 10.1007/s00439-007-0397-0. [DOI] [PubMed] [Google Scholar]
- Cargill M, Schrodi SJ, Chang M, Garcia VE, Brandon R, Callis KP, et al. A large-scale genetic association study confirms IL12B and leads to the identification of IL23R as psoriasis-risk genes. Am J Hum Genet. 2007;80:273–90. doi: 10.1086/511051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JR, Blumenschein W, Murphy E, Diveu C, Wiekowski M, Abbondanzo S, et al. IL-23 stimulates epidermal hyperplasia via TNF and IL-20R2-dependent mechanisms with implications for psoriasis pathogenesis. J Exp Med. 2006;203:2577–87. doi: 10.1084/jem.20060244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farley SM, Dotson AD, Purdy DE, Sundholm AJ, Schneider P, Magun BE, et al. Fas ligand elicits a caspase-independent proinflammatory response in human keratinocytes: implications for dermatitis. J Invest Dermatol. 2006;126:2438–51. doi: 10.1038/sj.jid.5700477. [DOI] [PubMed] [Google Scholar]
- Farley SM, Purdy DE, Ryabinina OP, Schneider P, Magun BE, Iordanov MS. Fas ligand-induced proinflammatory transcriptional responses in reconstructed human epidermis: recruitment of the epidermal growth factor receptor and activation of MAP kinases. J Biol Chem. 2008;283:919–28. doi: 10.1074/jbc.M705852200. [DOI] [PubMed] [Google Scholar]
- Fitch E, Harper E, Skorcheva I, Kurtz SE, Blauvelt A. Pathophysiology of psoriasis: recent advances on IL-23 and Th17 cytokines. Curr Rheumatol Rep. 2007;9:461–7. doi: 10.1007/s11926-007-0075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota K, Yoshitomi H, Hashimoto M, Maeda S, Teradaira S, Sugimoto N, et al. Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J Exp Med. 2007;204:2803–12. doi: 10.1084/jem.20071397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homey B, Dieu-Nosjean MC, Wiesenborn A, Massacrier C, Pin JJ, Oldham E, et al. Up-regulation of macrophage inflammatory protein-3 alpha/CCL20 and CC chemokine receptor 6 in psoriasis. J Immunol. 2000;164:6621–32. doi: 10.4049/jimmunol.164.12.6621. [DOI] [PubMed] [Google Scholar]
- Iordanov MS, Choi RJ, Ryabinina OP, Dinh TH, Bright RK, Magun BE. The UV (Ribotoxic) stress response of human keratinocytes involves the unexpected uncoupling of the Ras-extracellular signal-regulated kinase signaling cascade from the activated epidermal growth factor receptor. Mol Cell Biol. 2002;22:5380–94. doi: 10.1128/MCB.22.15.5380-5394.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iordanov MS, Sundholm AJ, Simpson EL, Hanifin JM, Ryabinina OP, Choi RJ, et al. Cell death-induced activation of epidermal growth factor receptor in keratinocytes: implications for restricting epidermal damage in dermatitis. J Invest Dermatol. 2005;125:134–42. doi: 10.1111/j.0022-202X.2005.23804.x. [DOI] [PubMed] [Google Scholar]
- Kao CY, Chen Y, Thai P, Wachi S, Huang F, Kim C, et al. IL-17 markedly up-regulates beta-defensin-2 expression in human airway epithelium via JAK and NF-kappaB signaling pathways. J Immunol. 2004;173:3482–91. doi: 10.4049/jimmunol.173.5.3482. [DOI] [PubMed] [Google Scholar]
- Kao CY, Huang F, Chen Y, Thai P, Wachi S, Kim C, et al. Up-regulation of CC chemokine ligand 20 expression in human airway epithelium by IL-17 through a JAK-independent but MEK/NF-kappaB-dependent signaling pathway. J Immunol. 2005;175:6676–85. doi: 10.4049/jimmunol.175.10.6676. [DOI] [PubMed] [Google Scholar]
- Kimball AB, Gordon KB, Langley RG, Menter A, Chartash EK, Valdes JM. Safety and efficacy of ABT-874, a fully human interleukin 12/23 monoclonal antibody, in the treatment of moderate to severe chronic plaque psoriasis: results of a randomized, placebo-controlled, phase 2 trial. Arch Dermatol. 2008;144:200–7. doi: 10.1001/archdermatol.2007.63. [DOI] [PubMed] [Google Scholar]
- Krueger GG, Langley RG, Leonardi C, Yeilding N, Guzzo C, Wang Y, et al. A human interleukin-12/23 monoclonal antibody for the treatment of psoriasis. N Engl J Med. 2007;356:580–92. doi: 10.1056/NEJMoa062382. [DOI] [PubMed] [Google Scholar]
- Kryczek I, Bruce AT, Gudjonsson JE, Johnston A, Aphale A, Vatan L, et al. Induction of IL-17+ T cell trafficking and development by IFN-gamma: mechanism and pathological relevance in psoriasis. J Immunol. 2008;181:4733–41. doi: 10.4049/jimmunol.181.7.4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Borgne M, Etchart N, Goubier A, Lira SA, Sirard JC, van Rooijen N, et al. Dendritic cells rapidly recruited into epithelial tissues via CCR6/CCL20 are responsible for CD8+ Tcell crosspriming in vivo. Immunity. 2006;24:191–201. doi: 10.1016/j.immuni.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Lee E, Trepicchio WL, Oestreicher JL, Pittman D, Wang F, Chamian F, et al. Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris. J Exp Med. 2004;199:125–30. doi: 10.1084/jem.20030451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardi CL, Kimball AB, Papp KA, Yeilding N, Guzzo C, Wang Y, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1) Lancet. 2008;371:1665–74. doi: 10.1016/S0140-6736(08)60725-4. [DOI] [PubMed] [Google Scholar]
- Lew W, Bowcock AM, Krueger JG. Psoriasis vulgaris: cutaneous lymphoid tissue supports T-cell activation and “Type 1” inflammatory gene expression. Trends Immunol. 2004;25:295–305. doi: 10.1016/j.it.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Lim HW, Lee J, Hillsamer P, Kim CH. Human Th17 cells share major trafficking receptors with both polarized effector T cells and FOXP3+ regulatory T cells. J Immunol. 2008;180:122–9. doi: 10.4049/jimmunol.180.1.122. [DOI] [PubMed] [Google Scholar]
- Lowes MA, Kikuchi T, Fuentes-Duculan J, Cardinale I, Zaba LC, Haider AS, et al. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol. 2008;128:1207–11. doi: 10.1038/sj.jid.5701213. [DOI] [PubMed] [Google Scholar]
- Ma HL, Liang S, Li J, Napierata L, Brown T, Benoit S, et al. IL-22 is required for Th17 cell-mediated pathology in a mouse model of psoriasis-like skin inflammation. J Clin Invest. 2008;118:597–607. doi: 10.1172/JCI33263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151–60. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- Papp KA, Langley RG, Lebwohl M, Krueger GG, Szapary P, Yeilding N, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2) Lancet. 2008;371:1675–84. doi: 10.1016/S0140-6736(08)60726-6. [DOI] [PubMed] [Google Scholar]
- Paradis TJ, Cole SH, Nelson RT, Gladue RP. Essential role of CCR6 in directing activated T cells to the skin during contact hypersensitivity. J Invest Dermatol. 2007;128:628–33. doi: 10.1038/sj.jid.5701055. [DOI] [PubMed] [Google Scholar]
- Piskin G, Sylva-Steenland RM, Bos JD, Teunissen MB. In vitro and in situ expression of IL-23 by keratinocytes in healthy skin and psoriasis lesions: enhanced expression in psoriatic skin. J Immunol. 2006;176:1908–15. doi: 10.4049/jimmunol.176.3.1908. [DOI] [PubMed] [Google Scholar]
- Poumay Y, Coquette A. Modelling the human epidermis in vitro: tools for basic and applied research. Arch Dermatol Res. 2007;298:361–9. doi: 10.1007/s00403-006-0709-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poumay Y, Dupont F, Marcoux S, Leclercq-Smekens M, Herin M, Coquette A. A simple reconstructed human epidermis: preparation of the culture model and utilization in in vitro studies. Arch Dermatol Res. 2004;296:203–11. doi: 10.1007/s00403-004-0507-y. [DOI] [PubMed] [Google Scholar]
- Singh SP, Zhang HH, Foley JF, Hedrick MN, Farber JM. Human T cells that are able to produce IL-17 express the chemokine receptor CCR6. J Immunol. 2008;180:214–21. doi: 10.4049/jimmunol.180.1.214. [DOI] [PubMed] [Google Scholar]
- Varona R, Cadenas V, Gomez L, Martinez AC, Marquez G. CCR6 regulates CD4+ T-cell-mediated acute graft-versus-host disease responses. Blood. 2005;106:18–26. doi: 10.1182/blood-2004-08-2996. [DOI] [PubMed] [Google Scholar]
- Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950–7. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- Wolk K, Witte E, Wallace E, Docke WD, Kunz S, Asadullah K, et al. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: a potential role in psoriasis. Eur J Immunol. 2006;36:1309–23. doi: 10.1002/eji.200535503. [DOI] [PubMed] [Google Scholar]
- Zaba LC, Cardinale I, Gilleaudeau P, Sullivan-Whalen M, Suarez Farinas M, Fuentes-Duculan J, et al. Amelioration of epidermal hyperplasia by TNF inhibition is associated with reduced Th17 responses. J Exp Med. 2007;204:3183–94. doi: 10.1084/jem.20071094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Krueger JG, Kao MC, Lee E, Du F, Menter A, et al. Novel mechanisms of T-cell and dendritic cell activation revealed by profiling of psoriasis on the 63,100-element oligonucleotide array. Physiol Genomics. 2003;13:69–78. doi: 10.1152/physiolgenomics.00157.2002. [DOI] [PubMed] [Google Scholar]