Abstract
Objective
Research on the relationship of depression to mortality has yielded mixed results. Limitations of previous studies include mostly one-time assessment of depression, short follow-ups, and failure to model changes in depression appropriately. We attempted to use a joint modeling approach to examining the association between longitudinal changes in depressive symptoms and mortality.
Methods
Data were obtained from the Florida Retirement Study, a prospective cohort study of community-dwelling oldest old individuals. At baseline, 879 people (M age = 80.6, 65.8% women) had comprehensive psychosocial assessment, including the Center of Epidemiological Studies–Depression Scale (CES-D). They were then assessed annually up to 11 years. Longitudinal changes of CES-D, modeled by a joint modeling approach of repeated measures and survival data, were used to predict mortality at follow-up (15 years after baseline), while adjusting for five classes of covariates.
Results
Total mortality rate was 69.9%. CES-D at baseline was not predictive of mortality at 15-year follow-up after adjusting for baseline covariates. The joint modeling revealed that an annual increase of one point in CES-D scores over the years was associated with a 57% higher risk of mortality (HR = 1.57, p<.001) at follow-up. Compared to those whose CES-D scores were stable over time, subjects with increasing CED-D scores over time had a 70% increase in mortality risk, p<.001, and their median survival time was 4 years shorter.
Conclusion
Although baseline CES-D was not predictive of mortality, the increase in depressive symptoms over time was associated with higher mortality. It is important to assess longitudinal changes in depression.
Keywords: Depression, Mortality, Longitudinal Study, Joint Modeling, Elderly, Community Sample
Depression is a common psychiatric condition that is prevalent in both younger and older populations (1). It is a disabling as well as deadly disorder. Depression may negatively affect health and mortality via multiple mechanisms (2-6), such as decreased heart rate variability, chronic activation of the Hypothalamus-Pituitary-Adrenal system, sedentary life style, reduced ability of self-care, and treatment non-compliance (7). These may be coupled with medical comorbidities such as coronary heart disease (CHD), which place them at a higher risk of mortality. Previous research has shown that depression is a risk factor for developing CHD (8, 9), and is also associated with increased mortality among patients after their first episode of myocardial infarction (6, 10). Furthermore, depression is correlated with other personality factors such as neuroticism that may independently contribute to poor health and mortality (11). In addition, there is a high risk of suicide among severely depressed patients.
Among studies of community samples, available evidence from two meta-analyses suggests that depression contributes to an increased risk of mortality (4, 12). However, methodological limitations and inconsistent findings across studies have weakened the strength of the depression-mortality relationship (4, 9, 13). Wulsin et al. (4) reviewed 57 studies published before 1996 on the relationship between depression and mortality. 12 studies of community samples were judged to be better quality studies, out of which 4 showed positive associations, 5 reported negative findings (i.e., no significant association between depression and mortality, after controlling for covariates), and 3 had “mixed” findings (significant findings only in subgroups of samples). Schulz et al. (5) conducted a follow up review for studies published between 1997 and 2001, and similarly, they found that among 22 community studies, 9 reported positive findings, 7 had negative results, and 6 showed mixed results. Since 2001, there have been at least another 14 studies published on the depression-mortality association in community samples, based on our literature search. Six of them reported positive results (14-19), 5 showed negative findings (20-24), and 3 had mixed findings (25-27). In summary, at least half of the studies reported negative or mixed findings regarding the association between depression and mortality.
A number of methodological limitations may have contributed to inconsistent findings in these studies. First, very few studies had depression assessed twice or more (16, 25, 28-30). Such designs may be inadequate to capture the changing nature of depression, especially in older populations (1). Changes in depressive symptoms may represent different risks for mortality (16). Progressive depression may reflect deterioration in health status, life satisfaction, and daily functioning (31, 32), which may not be captured by a single assessment. Therefore, multiple assessments of depression over many years are needed to examine the changes of depression and its association with increased mortality. Second, most studies had relatively short follow-up periods to ascertain mortality, which captured a small percentage of deaths and produced potentially truncated and biased samples. Few studies had follow-up periods of more than ten years (17, 20, 23, 33-35) with mortality rates more than 50% (18, 20, 35, 36). Third, many studies did not have comprehensive covariate adjustment in their analysis. Wulsin et al. identified important covariates that need to be controlled in this area of study, including age, sex, severity of physical illness, level of functioning, smoking, alcohol use, and socio-economic status (4). If certain covariates were controlled for in these studies, the significant relationship between depression and mortality may be eliminated (22). Therefore, it is necessary to examine the link between depression and mortality risks in large community samples that utilize multiple depression assessments over a long period of time, while controlling comprehensively for possible confounding factors. Such a study would also require application of appropriate statistical methodology necessary to effectively take advantage of the longitudinal design.
In previous studies, statistical methods used to analyze longitudinal data often failed to take advantage of the longitudinal nature of the data structure that can reflect changes in depressive symptoms. Simply calculating a cumulative average of depression scores over several assessments effectively eliminates the opportunity of examining changes in depression (29). Some studies attempted to describe changes in depression by categorizing differences between two assessments of depression into chronic, remitting, or none. It is well known among statisticians that two time points are inadequate to examine change (37). One study (16) characterized the clinical course of depression by fitting individual linear regressions and using the intercepts and slopes to predict mortality in Cox regression. Although this was a good approach to study change, the two-stage nature of individual linear regression and survival analysis contains computational pitfalls that limit the statistical power of modeling change/survival and lead to biases in parameter estimation (37, 38).
Recent methodological advances have made it possible to model longitudinal changes via multiple repeated measures and survival data simultaneously, as opposed to running in two separate stages, yielding better parameter estimations and more precise hypothesis testing (39, 40). This technique was first developed in the clinical trial field in an attempt to link changes in biomarkers to treatment outcomes (41). It combines the advantages of modeling intra-individual change trajectory in a growth curve analysis with the power of predicting time-to-event outcomes in a traditional survival analysis, which tends to focus on inter-individual differences. In the joint modeling approach, the longitudinal trajectory of the variable of interest serves as the predictor for the time-to-event outcome variable, which is different from being the dependent variable in hierarchical linear modeling or mixed modeling. This new joint modeling approach has been successfully applied to investigating the relationships between longitudinal changes in cognitive functioning and the onset of Alzheimer's disease or mortality (42, 43), but, to our knowledge, it has not been used in the study of depression and mortality. Modeling the longitudinal course of depression has also practical implications. Depression tends to be chronic and recurring in elderly (1), and persistent depression is associated with poor treatment adherence (7), leading to worsened health status. However, it is largely unknown about how the longitudinal course of depression affects mortality. The present study was an attempt to use the new joint modeling approach to examine the association between changes in depressive symptoms, vs. baseline depression, and mortality. We studied a sample of community dwelling elderly individuals with annual assessment of depressive symptoms and multiple covariates, and followed them up to 15 years. This gave us a chance to examine the progression and change trajectory of depressive symptoms and its impact on mortality risks.
Methods
Study Design
Data for this research were obtained from the Florida Retirement Study, a panel study which focuses on late-life adaptation of community dwelling elderly persons (44, 45). It is an ongoing longitudinal investigation of individuals who, in 1990 (N = 1000), were living in one of three independent living retirement communities located on the west coast of Florida. These individuals were living independently in their own homes and no meal or personal care services were offered by the communities.
A total of 3,905 households were screened to identify eligible individuals for the Florida Retirement Study. They were randomly selected from residential listings provided by the management of the three retirement communities. Eligibility criteria included: (a) living in one of three specified retirement communities in January 1990; (b) aged 72 years or older at baseline; (c) living in Florida at least nine months out of the year; and (d) reporting that they were “sufficiently healthy” to complete a ninety minute face-to-face interview (44). Selected households were contacted by telephone to determine if a member of the household met eligibility criteria. Of the 3,905 households contacted, 48.9% (n = 1,909) did not meet eligibility criteria (25.8% due to age younger than 72 years, 15.5% due to part-time residency, and 7.6% due to self-report of poor health). Additionally, 13.4% of households contacted refused participation and 14.5% of households could not be reached after a series of five attempts by the research staff. As such, 1,000 respondents completed an in-home, face-to-face interview, and were assessed in terms of his or her demographic, psychosocial, behavioral, cognitive, functional, and health status. Face-to-face interviews conducted by trained interviewers were conducted annually for all study respondents. Mortality was ascertained by the Social Security Death Index, based on participants' social security numbers, as of 12/31/2005, up to 15 years from the baseline. The study was approved by the Case Western Reserve University Institutional Review Board.
Participants
879 participants were administered the Center for Epidemiological Studies – Depression Scale (CES-D) at baseline or had at least one assessment of CES-D at a later time. Therefore, they constituted the sample of the present study. At Year 11 from the baseline, 486 participants had died during the course of the study, 209 participated in the final interview, 58 were lost at follow-up, and 126 refused to participate or were otherwise unavailable to participate. There were an additional 128 deaths by the end of 2005, making a total of 614 mortality cases (69.9%). At baseline, 65.8% of the sample were female. The mean age at baseline was 80.64 ± 4.80 years, with a range of 72 to 104 years. The average number of years of education was 13.60 ± 2.55. The sample was 100% Caucasian. Median annual income was about $25,000. In the sample, 45.5% were married, and 48.9% were widowed, with the remaining 5.6% divorced or never married. In this sample, the mean number of follow-up interview participation was 6.0 (SD = 3.6, Median = 6.0). Each annual follow-up face-to-face interview included a number of different measures, but the CES-D was always completed every time. The participation rate for each follow-up year ranged from 77.02% to 23.21% from the baseline, and 75.84% to 96.90% from each proceeding year. In other words, the attrition rate was in average about 10 to 15% each year from the proceeding year. Table 1 describes the characteristics of the sample in terms of demographic, social, health, behavioral, and functional characteristics.
Table 1.
Descriptive statistics of demographic, health behavior, chronic illnesses, functional, and cognitive variables in the sample at baseline.
| Mean ± SD or % of Sample at Baseline | |
|---|---|
| Mortality cases | 614/879 (69.9%) |
| Age (year) at baseline | 80.6 ± 4.8 |
| Gender: Female | 65.8% |
| Ethnicity: White | 100% |
| Education (# years) | 13.60 ± 2.55 |
| Marital status: Married | 45.5% |
| Marital status: Widowed | 48.9% |
| Annual Income (Median) | $25,000 |
| Drinks alcohol regularly | 22% |
| Smoking Cigarettes | 5.3% |
| Exercise regularly | 50.3% |
| Body Mass Index (BMI) | 24.08 ± 3.80 |
| History of Hypertension | 34% |
| History of Coronary Heart Disease | 27.5% |
| History of Diabetes Mellitus | 7.8% |
| History of Cancer | 8.8% |
| History of Stroke | 4.6% |
| History of Hyperlipidemia | 24.5% |
| Activity of Daily Living (ADL) | 5.30 ± 1.50 |
| Instrumental ADL (IADL) | 3.78 ± 1.73 |
| Cognitive impairment (moderate or marked) | 3.3% |
| CES-D, Baseline | 18.27 ± 5.50 |
Measures
Demographic Information: Participants were asked in interview questions about their age, sex, number of years of education, annual income, marital status, and living arrangement. Their responses were coded into categorical variables.
CES-D (46): This is a commonly used depression assessment tool in epidemiological studies, and it has been validated in the elderly population (47). The original scale consisted of 20 items, but a short version of the scale with 10 items (48) was used in the present study. The short version has been used in several studies on depression and mortality (22, 26, 29, 49), and it has been shown to be highly correlated with the full 20-item scale (48). Most somatic symptoms of depression that can be caused by physical illnesses were excluded from the short version of the CES-D. Sample items include “I felt lonely”, “I had crying spells”, and “I felt depressed”. The scale asked an individual to rate how often he or she had felt each symptom during the past week. A 5-point Likert scale was adopted for each item: 1 = Never/Rarely, 2 = Some/little, 3 = Occasionally, 4 = Most of the time, and 5 = All of the time. The range of possible scores was 10 to 50. The coefficient alpha of the scale at baseline was 0.84.
Health Behavior: Interview questions were used to assess smoking status (yes or no), alcohol consumption (none, occasional, or regular), and whether exercise was performed at least three times a week and at least 20 minutes each time (yes or no) (50).
Chronic Illnesses: The Older Americans Resource Study (OARS) Illness Index (51) was used to measure chronic illness. The OARS Chronic Illness Index was selected for inclusion based on its widespread use in the field and documented psychometric properties among elderly respondents (52). Respondents were asked whether they had been diagnosed by a physician with one or more of 25 chronic illnesses over the past year. For each illness condition reported by the respondent, a code of (1) was given and a code of (0) for those conditions not mentioned. For the purpose of the present study, major chronic illnesses included hypertension, coronary heart disease, diabetes mellitus, stroke, cancer, and hyperlipidemia.
Body Mass Index (BMI): Individuals were weighed in street clothes. Height without shoes was assessed with a standard ruler. BMI was computed as weight in kilograms divided by height in meters squared.
Global self-rated health was measured by a one-item question with five options: 1 = excellent, 2 = good, 3 = fair, 4 = poor, and 5 = very poor (53).
Physical functioning was measured at baseline by the OARS Activities of Daily Living (ADL) and Instrumental Activities of Daily Living (IADL) Index (54, 55). To assess IADL levels, respondents were asked a series of three questions reflecting difficulty with carrying out the following instrumental activities of daily living: (1) doing your own housework, (2) preparing your own meals, and (3) shopping for groceries. Coding and response categories for the three-item index are (1) “never or no trouble performing” to (4) “always have trouble performing”. An IADL index was created by summing across the three items. ADL functioning was assessed through the use of six questions asking the respondent to what extent they have trouble with personal tasks such as (1) washing and bathing, (2) dressing and putting on shoes, (3) getting to or using the toilet, (4) getting in/out of bed unassisted, (5) eating without assistance, and (6) getting in/out of a chair. The same procedure used to create the IADL index was utilized to create the ADL index.
Cognitive impairment was assessed using the Short Portable Mental Status Questionnaire (SPMSQ) (56) at baseline. Respondents were asked a series of ten questions (e.g., “what day of the week is it”, “what year is it”, “what is the name of the President”). Incorrect responses are coded as (1) and correct responses as (0). Incorrect responses (errors) were counted, with higher scores indicating greater cognitive impairment. Responses were classified into four categories: no impairment (no error made), mild impairment (1 or 2 errors made), moderate impairment (3 or 4 errors made), and marked impairment (5 or more errors made).
Statistical Analysis
We first conducted traditional survival analysis using Cox proportional hazard regression to test whether the baseline CES-D score was predictive of mortality, with and without controlling for baseline covariates in separate models. Functional forms of the covariates were assessed by checking the martingale residual plots. Kaplan-Meier analysis was used to compute median time of survival after baseline. We then used the joint modeling approach (39) to model the trajectory of CES-D score over time, and the effect of CES-D trajectory on mortality simultaneously. Again, the analysis was run with and without controlling for baseline covariates in separate models. The joint modeling used a Weibull function, which is a parametric approach as opposed to the semi-parametric Cox regression, to model the relationship between the repeated measures and mortality data. To model the trajectory of CES-D scores, linear and quadratic functions were used. The interaction effects between the intercept (baseline) and slopes (linear or quadratic) on mortality were also tested. They were to be dropped if they were not statistically significant. If the change of CES-D was significantly associated with mortality, further exploratory analysis would be conducted to examine how the trajectory of CES-D changes affect mortality. To do that, a 2-stage OLS growth curve analysis was conducted to model the individual change trajectory. For each subject, an individual linear regression was fitted to CES-D scores against time, and the estimated slope and intercept were computed and saved in the dataset. The linear slope of CES-D for each individual reflects the average annual change in CES-D scores over time, with a positive sign representing an increase in scores and a negative sign representing a decrease in scores. Then the sample was divided into sub-groups based on an individual's linear slope. The sub-groups were compared by using Cox regression analysis in terms of risks of mortality, while controlling for covariates.
For the joint modeling, the longitudinal model applied to the CES-D score Y of patient j assessed at Year i has the following form
where α0j and α1j are random effects representing the intercept and slope of CES-D score of patient j, which were assumed a multivariate normal distribution. Subjects with only one measurement contributed to the estimation of the mean intercept. The linearity assumption was made so that we could relate quantifiable characteristics of the CES-D longitudinal trajectory to mortality. We considered the Weibull survival model for the mortality
where rtr−1 with r > 0 is the baseline hazard; Zj consists of other risk factors at baseline. The parameters γ0 and γ1 represent of the effects of the CES-D trajectory (intercept and slope) on mortality. The final joint model would control for baseline covariates. Univariate analysis for each baseline covariate was run first to screen non-significant predictors. A predictor with a p-value greater than 0.1 in the univariate analysis was excluded from the final multivariate analysis. Because this new modeling approach demands considerable computational power, controlling for longitudinal changes in covariates is not possible at this time. Furthermore, controlling for longitudinal changes in covariates may lead to time-dependent confounding effects that complicate the interpretation of the results. In addition, it is common to get non-converged solution to the modeling if too many covariates are included in the equation (42). Five classes of covariates were controlled for in the present study. 1) Demographic characteristics, including age, sex, number of years of education, marital status, annual income, and living situation (e.g., living alone or living with someone else). 2) Health behavior variables, including smoking, alcohol consumption, and exercise. 3) General health status, such as global self-rated health and BMI. 4) Chronic illnesses, including heart disease, stroke, cancer, diabetes mellitus (DM), hypertension, cancer, and hyperlipidemia. These covariates were coded as yes/no dichotomous variables in the data analysis. 5) Functional impairment variables, including ADL, IADL, and cognitive impairment measured by the SPMSQ. These covariates were chosen because of their potential association with both depression and mortality, as elaborated by Wulsin et al (4).
Subjects who were lost to follow-up due to reasons other than death were assumed to be ignorable dropouts (57) and they were included in the analysis. Subjects who refused to participate in the study didn't provide any data, and were excluded from the analysis. The CES-D was added to the study protocol after the baseline interview started. Therefore, not everyone had a chance to respond to the CES-D at baseline. This situation might introduce bias. However, this was not a systematic bias because the missing data was not associated with any other factors. All data analyses, except the joint modeling, were conducted using SPSS version 14. The joint modeling was conducted by using Proc NLMIXED in SAS version 9.1.
Results
Traditional Cox Regression
We first conducted traditional Cox proportional hazard regression using the baseline CES-D to predict mortality. In a univariate analysis, baseline CES-D significantly predicted mortality (HR = 1.02, p = .012, 95% CI = 1.01 – 1.03). After adjusting for age and sex, baseline CES-D was still a significant predictor of mortality (HR = 1.03, p < .001, 95% CI = 1.01 – 1.04). However, in the multivariate analysis, after controlling for all five classes of covariates (demographics, health behavior, general health status, chronic illness, and functional impairment), baseline CES-D was no longer predictive of mortality (HR = .99, p > .20, 95% CI = .97 – 1.01). At baseline, male gender, older age, lower income, history of hypertension and diabetes, lower BMI, less exercise, poorer self-rated health, marked cognitive impairment, and worse instrumental ADL significantly predicted increased mortality in the multivariate model. Table 2 shows the results of univariate as well as multivariate Cox regression for each predictor. For categorical variables, it also shows mortality rate in each group and median number of months of survival after baseline.
Table 2.
Results of Cox regression using baseline variables to predict mortality at 15-year follow-up.
| No. (%) of Deaths | Median # Months of Survival | Univariate HR (95% CI) | Adjusted HR1 (95% CI) | |
|---|---|---|---|---|
| Age (years) | ||||
| 70 – 75 | 67 (51.1) | 170.17 | 1.0 | 1.0 |
| 76 – 80 | 240 (64.5) | 134.90 | 1.44 (1.10–1.89) | 1.41 (1.05 – 1.90)* |
| 81 – 85 | 210 (77.8) | 100.07 | 2.17 (1.65–2.87) | 2.03 (1.49 – 2.76)*** |
| ≥ 86 | 97 (91.5) | 54.03 | 4.58 (3.34–6.27) | 3.61 (2.51 – 5.19)*** |
| Sex | ||||
| Women | 378 (65.3) | 127.70 | 1.0 | 1.0 |
| Men | 236 (78.7) | 101.77 | 1.45 (1.23–1.70) | 2.00 (1.63–2.46)*** |
| Education | ||||
| ≤ 12 years | 256 (71.5) | 113.03 | 1.0 | 1.0 |
| > 12 years | 358 (68.7) | 123.00 | .92 (.78–1.08) | .96 (.80 – 1.15) |
| Marital Status | ||||
| Married | 264 (66.0) | 123.47 | 1.0 | 1.0 |
| Widowed | 315 (73.3) | 111.73 | 1.24 (1.05–1.46) | 1.29 (.80 – 2.06) |
| Divorced/Separated | 9 (56.3) | 137.97 | .79 (.41–1.53) | .85 (.36 – 2.00) |
| Never Married | 26 (78.8) | 122.30 | 1.24 (.83–1.85) | 1.28 (.69 – 2.39) |
| Living Alone? | ||||
| Yes | 345 (73.1) | 112.67 | 1.0 | 1.0 |
| No | 269 (66.1) | 123.73 | .82 (.70–.96) | .98 (.62 – 1.56) |
| Annual Income | ||||
| ≤ $19,999 | 231 (73.3) | 113.03 | 1.0 | 1.0 |
| $20,000 - $29,999 | 172 (69.9) | 114.47 | .91 (.74 – 1.10) | .95 (.76 – 1.18) |
| $30,000 - $39,999 | 82 (63.1) | 129.77 | .77 (.60 - .98) | .74 (.57–.98)* |
| ≥ $40,000 | 78 (63.9) | 127.67 | .78 (.60 – 1.01) | .74 (.55–.99)* |
| Hypertension | ||||
| Absent | 393 (67.4) | 118.10 | 1.0 | 1.0 |
| Present | 221 (74.7) | 119.00 | 1.15 (.98–1.36) | 1.24 (1.02 – 1.49)* |
| Coronary Heart Disease | ||||
| Absent | 430 (67.5) | 123.47 | 1.0 | 1.0 |
| Present | 184 (76.0) | 98.73 | 1.36 (1.14–1.61) | 1.16 (.94 – 1.44) |
| Diabetes Mellitus | ||||
| Absent | 553 (68.3) | 122.30 | 1.0 | 1.0 |
| Present | 61 (88.4) | 70.33 | 2.00 (1.53–2.61) | 1.41 (1.04 – 1.91)* |
| Cancer | ||||
| Absent | 557 (69.5) | 120.37 | 1.0 | 1.0 |
| Present | 57 (74.0) | 97.40 | 1.26 (.96–1.66) | 1.33 (.98 – 1.80) |
| Stroke | ||||
| Absent | 580 (69.1) | 120.00 | 1.0 | 1.0 |
| Present | 34 (85.0) | 82.37 | 1.64 (1.16–2.32) | 1.30 (.87 – 1.95) |
| Hyperlipidemia | ||||
| Absent | 479 (72.1) | 114.57 | 1.0 | 1.0 |
| Present | 135 (62.8) | 127.70 | .79 (.66–.96) | .87 (.70 – 1.07) |
| BMI | .96 (.94–.99) | .97 (.95–.99)* | ||
| Smoking Cigarette | ||||
| No | 556 (68.6) | 120.53 | 1.0 | 1.0 |
| Yes | 38 (84.4) | 109.10 | 1.38 (.99–1.91) | 1.35 (.93 – 1.96) |
| Drinking Alcohol | ||||
| None | 259 (71.7) | 105.00 | 1.0 | 1.0 |
| Occasional | 207 (65.1) | 127.67 | .77 (.64–.93) | .87 (.71 – 1.08) |
| Regular | 139 (72.8) | 114.57 | .90 (.74–1.11) | 1.00 (.79 – 1.26) |
| Exercise 3 times a week? | ||||
| No | 331 (76.6) | 97.47 | 1.0 | 1.0 |
| Yes | 274 (62.6) | 137.97 | .64 (.54–.75) | .79 (.65–.96)* |
| Global self-rated health | ||||
| Excellent | 118 (59.3) | 146.17 | 1.0 | 1.0 |
| Good | 326 (70.3) | 120.17 | 1.38 (1.12–1.71) | 1.30 (1.03 – 1.65)* |
| Fair | 129 (77.2) | 90.33 | 1.93 (1.51–2.48) | 1.58 (1.17 – 2.13)** |
| Poor | 38 (86.4) | 49.73 | 3.28 (2.27–4.73) | 1.85 (1.13 – 3.03)* |
| Very Poor | 3 (60.0) | 32.00 | 1.66 (.53–5.22) | .86 (.19 – 3.84) |
| Cognitive impairment | ||||
| None | 481 (68.8) | 122.17 | 1.0 | 1.0 |
| Mild | 108 (71.5) | 107.27 | 1.10 (.90–1.36) | .97 (.77 – 1.23) |
| Moderate | 16 (84.2) | 100.80 | 1.62 (.99–2.67) | 1.16 (.64 – 2.11) |
| Marked | 9 (90.0) | 36.17 | 2.56 (1.32–4.96) | 3.06 (1.39 – 6.76)** |
| ADL | 1.16 (1.11–1.21) | .93 (.84 – 1.02) | ||
| IADL | 1.11 (1.08–1.15) | 1.10 (1.04 – 1.17)*** | ||
| CES-D at Baseline | 1.02 (1.01–1.03) | .99 (.97 – 1.01) |
Note: From multivariate analysis. HR = Hazard Ratio. BMI = Body Mass Index. ADL = Activity of Daily Living. IADL = Instrumental Activity of Daily Living.
p < .05;
p < .01;
p < .001.
Joint Modeling of Repeated-Measures and Survival Data
Univariate analysis was conducted first, without any covariates. The model estimated the intercept (α0j in the model), which was the estimated mean baseline CES-D score, as 18.27 ± 5.50, and the linear slope (αij in the model), which was the estimated mean annual change in CES-D score, as .56 ± 2.13. In other words, on average, the participants in the sample had increased CES-D scores of 0.56 points annually, and increased score of 5.6 points at the end of this study (i.e., 0.56 × 10 years). A quadratic term of change in CES-D scores (i.e., acceleration or deceleration of change over time) was also tested in the model, but it turned out to be non-significant, HR = .98, p = .12, therefore it was dropped from the model in further analysis. In this univariate model, both intercept (i.e., baseline CES-D) and linear slope significantly predicted mortality, HR's = 1.03 and 1.49, p's < .01, respectively.
A multivariate model was then fitted to the data, controlling for potential covariates. The results showed that the intercept (i.e., estimated baseline CES-D) became non-significant in predicting mortality, HR = 1.01, p > .50, which is consistent with what was found in the traditional Cox regression. In contrast, the linear slope was still a significant predictor, HR = 1.57, p < .001. This means that an annual increase of 1 point on CES-D was associated with a 57% higher risk of mortality. If an individual had an increased CES-D score of 2 points annually, the risk of mortality would have increased by 2.46 times. The interaction between intercept and slope was not statistically significant, therefore it was dropped from the final model. The baseline covariates included in the final model were similar to those in the traditional Cox regression shown above. Table 3 summarizes the results of the joint modeling.
Table 3.
Results of Multivariate Joint Modeling of Repeated-Measures and Survival Data.
| Predictor | Estimate | SE | Adjusted HR | 95% CI |
|---|---|---|---|---|
| CES-D Intercept | 0.01 | 0.01 | 1.01 | 0.98 – 1.03 |
| CES-D Slope | 0.45 | 0.15 | 1.57 | 1.18 – 2.08** |
| Age (years) | ||||
| 70 – 75 | 1.0 | |||
| 76 – 80 | 0.29 | 0.14 | 1.33 | 1.01 – 1.76* |
| 81 – 85 | 0.63 | 0.15 | 1.87 | 1.40 – 2.50*** |
| ≥ 86 | 1.18 | 0.18 | 3.26 | 2.31 – 4.60*** |
| Sex | ||||
| Women | 1.0 | |||
| Men | 0.64 | 0.10 | 1.89 | 1.54 – 2.31*** |
| Coronary Heart Disease | ||||
| Absent | 1.0 | |||
| Present | 0.14 | 0.10 | 1.15 | 0.95 – 1.40 |
| Diabetes Mellitus | ||||
| Absent | 1.0 | |||
| Present | 0.49 | 0.15 | 1.64 | 1.21 – 2.20** |
| Stroke | ||||
| Absent | 1.0 | |||
| Present | 0.16 | 0.19 | 1.17 | 0.81 – 1.69 |
| Hyperlipidemia | ||||
| Absent | 1.0 | |||
| Present | -0.16 | 0.11 | 0.85 | 0.70 – 1.05 |
| Living Alone? | ||||
| Yes | 1.0 | |||
| No | -0.29 | 0.09 | 0.75 | 0.62 – 0.90 |
| BMI | -0.03 | 0.01 | 0.97 | 0.95 – 0.99** |
| Smoking Cigarette | ||||
| No | 1.0 | |||
| Yes | 0.29 | 0.18 | 1.34 | 0.94 – 1.90 |
| Drinking Alcohol | ||||
| None | 1.0 | |||
| Occasional | -0.11 | 0.10 | 0.89 | 0.73 – 1.09 |
| Regular | 0.00 | 0.11 | 1.00 | 0.80 – 1.25 |
| Exercise 3 times a week? | ||||
| No | 1.0 | |||
| Yes | -0.27 | 0.09 | 0.76 | 0.63 – 0.92** |
| Global self-rated health | ||||
| Excellent | 1.0 | |||
| Good | 0.25 | 0.12 | 1.28 | 1.02 – 1.61* |
| Fair | 0.37 | 0.15 | 1.45 | 1.08 – 1.94* |
| Poor | 0.56 | 0.24 | 1.75 | 1.09 – 2.80* |
| Very Poor | -0.30 | 0.79 | 0.74 | 0.16 – 3.48 |
| Cognitive impairment | ||||
| None | 1.0 | |||
| Mild | -0.12 | 0.11 | 0.89 | 0.71 – 1.11 |
| Moderate | 0.15 | 0.28 | 1.16 | 0.67 – 2.02 |
| Marked | 0.93 | 0.41 | 2.52 | 1.12 – 5.66* |
| ADL | 0.09 | 0.02 | 1.10 | 1.05 – 1.15*** |
| IADL | -0.05 | 0.05 | 0.95 | 0.85 – 1.03 |
Note: p < .05;
Note: p < .01;
Note: p < .001.
BMI = Body Mass Index. ADL = Activity of Daily Living. IADL = Instrumental Activity of Daily Living. SE = Standard Error. HR = Hazard Ratio. CI = Confidence Interval.
A Two-Stage Growth Curve and Survival Analysis
In the 2-stage growth curve analysis, the mean linear slope of CES-D scores was .56 ± 2.13, the same as that from the univariate joint modeling described above. To categorize the change pattern of CES-D scores over time, the whole sample was divided into three groups, based on the tertiles of CES-D linear slopes. The first tertile group represented a marked increase of CES-D scores over time, with a mean slope of 2.32 ± 2.02. The second tertile group represented a slight increase but mostly stable in CES-D scores over time, with a mean slope of .42 ± .23. The third tertile group represented a decrease in CES-D scores over time, with a mean slope of -1.06 ± 1.94. To interpret the results more intuitively, the three groups were named as the “Up”, “Stable”, and “Down” groups, respectively. The “Up” group consisted of subjects with an increase in CES-D scores and progression of depressive symptoms over time. The “Stable” group consisted of subjects whose CES-D scores and depressive symptoms were largely unchanged over time. The “Down” group consisted of subjects with a decrease in CES-D scores and improvement of depressive symptoms over time.
The three groups were used in a traditional multivariate Cox regression to predict mortality, the results of which are shown in Table 4. Compared to the “Stable” group, the “Up” group had HR = 1.70, p <.001. The “Down” group was not significantly different from the “Stable” group. When recoding the reference group to the “Down” group, the “Up” group had HR = 1.40 (95% CI = 1.10 – 1.78), p = .007.
Table 4.
Results of 2-Stage Growth Curve and Survival Analysis, and Characteristics of Samples in the Three Groups.
| Groups | Stable (N = 253) |
Down (N = 253) |
Up (N = 253) |
|---|---|---|---|
| Adjusted HR (95% CI) | 1.0 | 1.22 (.95 – 1.56) |
1.70*** (1.35 – 2.15) |
| Median # Months of Survival | 150.93 | 127.27 | 102.43 |
| CES-D Slope | .42 ± .23a | -1.06 ± 1.94a | 2.32 ± 2.02a |
| No. (%) of Deaths | 154 (60.9) | 162 (64.0) | 194 (76.7) |
| Age (years) at Death | 89.52 ± 4.68 | 88.71 ± 5.04 | 88.23 ± 5.37* |
| Baseline Characteristics | |||
| Age (years) at Baseline | 79.49 ± 4.46 | 80.34 ± 4.57* | 81.00 ± 4.42*** |
| % Women | 62.1 | 64.4 | 70.8 |
| Baseline CES-D | 16.87 ± 4.70 | 20.08 ± 5.84b | 17.23 ± 4.95 |
| Chronic Illnesses | |||
| Hypertension | 34.4% | 30.8% | 35.6% |
| Coronary Heart Disease | 24.1% | 26.9% | 29.2% |
| Diabetes Mellitus | 6.3% | 7.1% | 9.5% |
| Cancer | 7.1% | 8.7% | 9.1% |
| Stroke | 1.6% | 5.1% | 5.1% |
| Hyperlipidemia | 28.9% | 23.7% | 23.3% |
Note: 120 participants had only one assessment of CES-D, therefore no slope was computed.
p < .001, all three groups were significantly different from each other.
p < .001, compared to either “Stable” or “Up” groups in terms of baseline CES-D scores.
p < .05, compared to “Stable” group;
p < .01, compared to “Stable” group;
p < .001, compared to “Stable” group.
Table 4 also shows characteristics of the three groups at baseline. As noted, the HR for the “Down” group was higher, but it was not statistically different from the “Stable” group. Interestingly, the “Down” group had significantly higher baseline CES-D scores than either the “Stable” or “Up” group. Starting from a relatively high CES-D baseline may account for the observation that the decreasing trend of CES-D scores did not confer a significantly higher risk of mortality, compared to the “Stable” group. However, an attempt to test the interaction between baseline CES-D and CES-D change groups did not yield statistically significant results. Further analysis of the baseline characteristics among the three groups revealed that the “Up” group were significantly older and had more women. Naturally, one would ask whether the “Up” group had more health problems that might lead to a higher mortality rate. However, Table 4 shows that the three groups were not significantly different in chronic illnesses at baseline, although the “Up” group tended to have a slightly higher percentage of sick people.
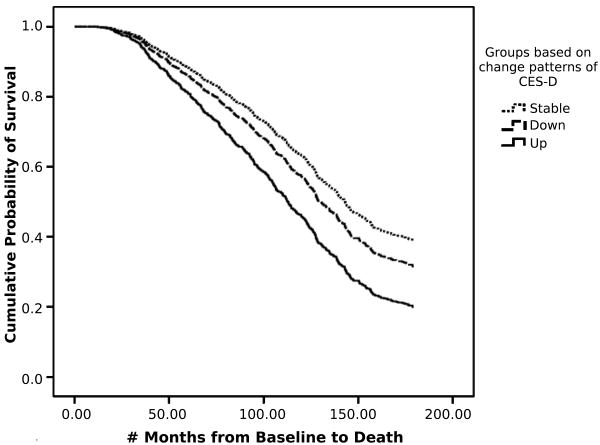
Figure 1 shows the Kaplan-Meier survival curves for the three groups. Compared to those in the “Stable” group, individuals in the “Up” group had a 70% higher risk of mortality. Compared to those in the “Down” group, individuals in the “Up” group had a 40% higher risk of mortality. The “Stable” group had the longest survival with a median of 150.93 months. On average, individuals in the “Up” group (Median months of survival = 102.43) lived four years less than those in the “Stable” group (Median months of survival = 150.93), while individuals in the “Down” group (Median months of survival = 127.27) lived two years less than those in the “Stable” group.
Figure 1.
Kaplan-Meier Survival Curves for the three groups based on change pattern of CES-D scores: “Stable” (the upper curve), “Down” (the middle curve) and “Up” (the lower curve). Reference group for the Hazard Ratios (HR) is the “Stable” group. For the “Up” group, HR = 1.70, p < .001. For the “Down” group, HR = 1.22, p > .10.
Discussion
The present study utilized a new statistical methodology, joint modeling of repeated-measures and survival data, to examine changes in depressive symptoms over time and their associations with mortality in a large sample of elderly community-dwelling individuals. All participants were assessed annually, up to 11 years from baseline. The results show that baseline depressive symptoms were not significantly predictive of mortality. However, the linear change rate of CES-D scores was a significant predictor of mortality even after adjusting for all five classes of covariates. The HR was 1.57 in the joint modeling, suggesting that an average annual increase of one point on the CES-D conferred a 57% higher risk of mortality. Further exploratory analysis demonstrated that individuals with marked increases in depressive symptoms over time suffered a 70% higher risk of mortality (adjusted HR = 1.70) than those who remained stable or had a slight increase in depressive symptoms. On average, the increased depressive symptom group (“Up” group) died four years earlier than the stable or slight increase in depressive symptom group (“Stable” group). Individuals who had relatively higher CES-D scores at baseline but had a decreasing trend over time did not statistically differ from those with stable or slight increase in CES-D scores in mortality, although their HR was 1.22.
Previous studies on the association between depression and mortality in community samples produced mixed findings, perhaps partly due to methodological limitations. The present study attempted to overcome these problems. First, most studies used one-time assessment of depression at baseline to predict mortality at follow-up. Only two studies had twice measured depression (25, 28), and only three studies had data on more than two waves of depression assessment (16, 29, 30). In contrast, our study had up to 11 annual assessments of depressive symptoms. This gave a better picture of how one's depressive symptoms change over time, reflecting the chronic, waxing and waning nature of depression. Second, many studies had relatively short follow-up periods to ascertain mortality. Only a few studies had follow-up periods of more than ten years (17, 20, 23, 33-35), or had sample mortality rate more than 50% (18, 20, 35, 36). Our study had up to 15 years follow-up. With a longer follow-up, we were able to capture more events of mortality (almost 70%) and therefore increase the power of our statistical analysis. Third, statistical methods in previous studies were often inadequate in reflecting the changes in depressive symptoms over time. Our study utilized the most recent methodological advances to jointly model longitudinal multiple repeated measures and survival data simultaneously (39, 40). To our knowledge, it is the first time that the joint modeling approach is used in the study of depression and mortality. In addition, the type and number of covariates controlled for in previous studies were inconsistent, as shown in both Wulsin et al. (4) and Schultz et al. (5). The number of covariates controlled for ranged from 2 (i.e., age and sex) to 20. The present study adjusted for five classes of potentially confounding factors, including all of which Wulsin et al. (4) have identified, as well as marital status, living arrangement, self-reported health status, and cognitive impairment. In the present study, the baseline CES-D became non-significant in predicting mortality after adjusting for covariates. This is consistent with what several researchers have speculated, that is, depending on the type of covariates used, a study may or may not find a significant association between depression and mortality (4, 5, 12, 22). Although not all covariates in our study were significant in the final model, some covariates did contribute to the prediction of mortality. The single item global self-rated physical health question predicted mortality in multiple studies (58, 59). Although not directly using the same measure, one study did show that depression became a non-significant predictor of mortality after controlling for self-reported health variables (22). Cognitive impairment also predicted mortality in some of the previous studies (42). Consequently, it is important to control for these variables in order to demonstrate the independent association between depression and mortality.
Our finding that changes in depressive symptoms over time significantly predicted mortality is consistent with the few previous studies that have attempted to assess longitudinal changes in depression. Pulska et al. (28) conducted two assessments for depression, five years apart, using a DSM-III semi-structured interview with 813 elderly Finnish people, and classified the sample into three groups: 1) recurrent depression, 2) recovered from depression, and 3) no depression. They showed that compared to the no depression group, the recurrent depression group had a relative risk of 1.5 in mortality, whereas the recovered from depression group was not statistically different. Anstey and Luszcz (25) conducted a similar study using two CES-D assessments with two years apart, and classified the sample into four groups: never depressed, remitted depression, chronic depression, and incident depression where one was not depressed initially but became depressed later. The incident depression and chronic depression groups had elevated risks of mortality relative to the never depressed group (RR's = 2.10 and 1.52, respectively). Interestingly, the remitted depression group was not statistically different from the never depressed group in mortality risk. Penninx et al. (30) studied an American sample of 3701 elderly people with three assessments of CES-D in six years, and created three groups, never depressed, newly depressed, and chronically depressed. They demonstrated that the newly depressed group, similar to the “Up” group in our study, had 31% higher risks of mortality than the never depressed group, and that the chronically depressed group, similar to the “Stable” group in our study, did not have statistically higher mortality risks. Unfortunately, their study did not have a group that showed remitted depression or decreasing depressive symptoms. The work of Geerlings et al. (16) was similar to our study in that they also used two-stage growth curve and survival analysis to consider the change in depression and its association with mortality, with eight assessments of CES-D in three years and follow-up at 6.5 years. However, their classifications of change groups of depression were not based on the linear slopes of individual regression. Rather, six groups were created based on an increase of five points on CES-D between any two time points. Compared to the no depression group, only the chronic depression group had elevated mortality risks, adjusted RR = 2.11. In their two-stage analysis, both intercept (baseline CES-D) and slope (linear change rate of CES-D) were significant predictors of mortality. Compared to our study, they did not control cognitive impairment, health behaviors such as smoking, alcohol intake, and exercise, and global self-rated health. They had a smaller sample size (N = 484), shorter follow-up period (6.5 years), and fewer assessments of CES-D. More importantly, the two-stage analysis yielded less precise estimates of the risks of mortality from changes in depression.
The present findings may have clinical implications. Increases in depression over time conveyed a significantly, both statistically and clinically, higher risk of mortality, whereas individuals with decreasing trend of depression over time, although having started from a higher baseline, enjoyed a lower mortality risk and two more years of life. This perhaps suggests that if we can screen and treat depression in its early stages, and if we can stop the progression of depressive symptoms, we may be able to reduce the excess mortality in this high risk population among the elderly. Several recent randomized controlled clinical trials have shown evidence to support this claim (60-63). One study showed a higher percentage of patients achieving reduction in depressive symptoms in the intervention group compared to the usual care group (60). Based on the findings of the present study and several previous studies, future research should perhaps prioritize interventions for the chronically depressed patients who have worsening symptoms over time, who are at higher risk of mortality. Our results suggest that early screening, repeated assessment and treatment of depression should focus on stopping the progression of depression, thus preventing the excess mortality that depression confers on the elderly.
In spite of the advantages of the present study, several limitations should be noted. First, assessment of depression was based on self-report measures and not a clinical or diagnostic interview. Therefore, we do not know how many participants in the study were actually clinically depressed. However, the CES-D has been widely used in the literature. It is easy and cheap to administer in large epidemiological studies and it has been shown to be sensitive in detecting depression (47, 48). Second, we did not have information on the specific causes of death for participants who died in the course of the study. Thus, we were not able to analyze the association of depression with specific diagnostic categories such as cardiac illness. We could have obtained death certificates to ascertain the cause of death, but some authors have argued against the use of death certificates (64-66). Third, the CES-D items used in the study were based on five anchor points, instead of the four-point Likert scale that are most commonly used, making univariate findings difficult to compare with other studies. Other studies have used a cut-off point of 8 on the CES-D 10-item scale to define clinical depression. Because we do not know how the 5-point Likert scale performs in detecting depression, a cut-off point was not used in our study. Nevertheless, because we focused on the longitudinal changes in CES-D scores, clinical categorization of depression for each subject was not necessary. Fourth, the sample was all white, relatively healthy, elderly living in retirement communities, and they had relatively higher socio-economic status. The nature of the sample may potentially limit the generalizability of the findings. However, the characteristics of the sample make the results more relevant to primary care physicians who see well elderly patients on a daily basis. Fifth, due to space limitations, we did not report any moderation analysis to explore potential subsamples in which the depression-mortality association may be different. We did test the moderation effect of sex, but there was no significant difference between men and women in this sample.
Finally, most of the data on covariates were also based on self-report or interview information, and we did not collect data on laboratory tests or biomarker data, such as blood pressure, blood cholesterol level, and C-reactive protein, which have been shown to predict mortality in many studies. We recognize that the list of covariates controlled for is still not complete. Future studies should try to test potential psychophysiological (5, 67) and behavioral mediators (31) underlying the link between depression and mortality, pinpointing the optimal entry for intervention. Furthermore, with the advances in the joint modeling approach, future studies should be able to model changes in depression as well as changes in covariates, and explore the associations among changes in these variables (68) and mortality. Because the joint modeling is a relatively new methodology, its limitations have not been fully understood. In the present study, a linear function form was assumed for the CES-D trajectories so that the characteristics of the trajectories such as “intercept” and “slope” could be related to the outcome. However, a linear trend may not be adequate to describe the time course of the CES-D scores. Model misspecification may occur if the CES-D trajectories were not linear over time, potentially leading to biased results. To relate the CES-D trajectories to the outcome, a nonparametrics model may be more appropriate. However, it would be more difficult to find suitable characteristics of the model to reflect clinically meaningful concepts. Therefore it would be difficult to relate the CES-D trajectories to mortality. Future research in the joint modeling methodology should overcome these issues.
In summary, we found that an annual increase of one point in CES-D score conferred a 57% higher risk of mortality. Compared to those with stable CES-D scores, people with increased depressive symptoms over time had a 70% higher risk of mortality. On average, they died more than 4 years earlier. If a person's CES-D score declined over time, the risk of mortality was not statistically different from those with stable CES-D scores, and on average, they gained almost two more years of life compared to those with increased depressive symptoms. Baseline CES-D was not predictive of mortality, but change in depressive symptoms over time was a significant predictor of mortality, even after adjusting for five classes of covariates. Therefore, in longitudinal studies, it is very important to examine change trajectories of depressive symptoms and their effects on mortality.
Acknowledgments
This project is supported by the National Institute on Aging grant #AG-010738 awarded to Dr. Eva Kahana. We would like to acknowledge Jane Brown, PhD, for her editorial support and contribution in preparation of the manuscript.
Acronyms
- CHD
coronary heart disease
- CES-D
Center of Epidemiological Studies–Depression Scale
- OARS
The Older Americans Resource Study
- BMI
Body Mass Index
- ADL
Activities of Daily Living
- IADL
Instrumental Activities of Daily Living
- SPMSQ
Short Portable Mental Status Questionnaire
- HR
Hazard Ratio
- RR
Relative Risk
References
- 1.Blazer DG. Depression in late life: review and commentary. J Gerontol A Biol Sci Med Sci. 2003;58:249–65. doi: 10.1093/gerona/58.3.m249. [DOI] [PubMed] [Google Scholar]
- 2.Pozuelo L, Tesar G, Zhang J, Penn M, Franco K, Jiang W. Depression and heart disease: What do we know, and where are we headed? Cleveland Clinic journal of medicine. 2009;76:59–70. doi: 10.3949/ccjm.75a.08011. [DOI] [PubMed] [Google Scholar]
- 3.Kubzansky LD, Davidson KW, Rozanski A. The clinical impact of negative psychological states: expanding the spectrum of risk for coronary artery disease. Psychosomatic medicine. 2005;67 1:S10–4. doi: 10.1097/01.psy.0000164012.88829.41. [DOI] [PubMed] [Google Scholar]
- 4.Wulsin LR, Vaillant GE, Wells VE. A systematic review of the mortality of depression. Psychosomatic medicine. 1999;61:6–17. doi: 10.1097/00006842-199901000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Schulz R, Drayer RA, Rollman BL. Depression as a risk factor for non-suicide mortality in the elderly. Biol Psychiatry. 2002;52:205–25. doi: 10.1016/s0006-3223(02)01423-3. [DOI] [PubMed] [Google Scholar]
- 6.Barth J, Schumacher M, Herrmann-Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease: a meta-analysis. Psychosomatic medicine. 2004;66:802–13. doi: 10.1097/01.psy.0000146332.53619.b2. [DOI] [PubMed] [Google Scholar]
- 7.Kronish IM, Rieckmann N, Halm EA, Shimbo D, Vorchheimer D, Haas DC, Davidson KW. Persistent depression affects adherence to secondary prevention behaviors after acute coronary syndromes. J Gen Intern Med. 2006;21:1178–83. doi: 10.1111/j.1525-1497.2006.00586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wulsin LR, Singal BM. Do depressive symptoms increase the risk for the onset of coronary disease? A systematic quantitative review. Psychosomatic medicine. 2003;65:201–10. doi: 10.1097/01.psy.0000058371.50240.e3. [DOI] [PubMed] [Google Scholar]
- 9.Frasure-Smith N, Lesperance F. Reflections on depression as a cardiac risk factor. Psychosomatic medicine. 2005;67 1:S19–25. doi: 10.1097/01.psy.0000162253.07959.db. [DOI] [PubMed] [Google Scholar]
- 10.Frasure-Smith N, Lesperance F, Talajic M. Depression following myocardial infarction. Impact on 6-month survival. Jama. 1993;270:1819–25. [PubMed] [Google Scholar]
- 11.Roberts BW, Kuncel NR, Shiner R, Caspi A, Goldberg LR. The power of personality: The comparative validity of personality traits, socioeconomic status, and cognitive ability for predicting important life outcomes. Perspectives on Psychological Science. 2007;2:313–45. doi: 10.1111/j.1745-6916.2007.00047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cuijpers P, Smit F. Excess mortality in depression: a meta-analysis of community studies. J Affect Disord. 2002;72:227–36. doi: 10.1016/s0165-0327(01)00413-x. [DOI] [PubMed] [Google Scholar]
- 13.Davidson KW, Rieckmann N, Rapp MA. Definitions and distinctions among depressive syndromes and symptoms: implications for a better understanding of the depression-cardiovascular disease association. Psychosomatic medicine. 2005;67 1:S6–9. doi: 10.1097/01.psy.0000162257.19266.fc. [DOI] [PubMed] [Google Scholar]
- 14.Adamson JA, Price GM, Breeze E, Bulpitt CJ, Fletcher AE. Are older people dying of depression? Findings from the Medical Research Council trial of the assessment and management of older people in the community. Journal of the American Geriatrics Society. 2005;53:1128–32. doi: 10.1111/j.1532-5415.2005.53355.x. [DOI] [PubMed] [Google Scholar]
- 15.Ganguli M, Dodge HH, Mulsant BH. Rates and predictors of mortality in an aging, rural, community-based cohort: the role of depression. Archives of general psychiatry. 2002;59:1046–52. doi: 10.1001/archpsyc.59.11.1046. [DOI] [PubMed] [Google Scholar]
- 16.Geerlings SW, Beekman AT, Deeg DJ, Twisk JW, Van Tilburg W. Duration and severity of depression predict mortality in older adults in the community. Psychol Med. 2002;32:609–18. doi: 10.1017/s0033291702005585. [DOI] [PubMed] [Google Scholar]
- 17.Gump BB, Matthews KA, Eberly LE, Chang YF. Depressive symptoms and mortality in men: results from the Multiple Risk Factor Intervention Trial. Stroke. 2005;36:98–102. doi: 10.1161/01.STR.0000149626.50127.d0. [DOI] [PubMed] [Google Scholar]
- 18.Luukinen H, Laippala P, Huikuri HV. Depressive symptoms and the risk of sudden cardiac death among the elderly. Eur Heart J. 2003;24:2021–6. doi: 10.1016/j.ehj.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Wulsin LR, Evans JC, Vasan RS, Murabito JM, Kelly-Hayes M, Benjamin EJ. Depressive symptoms, coronary heart disease, and overall mortality in the Framingham Heart Study. Psychosomatic medicine. 2005;67:697–702. doi: 10.1097/01.psy.0000181274.56785.28. [DOI] [PubMed] [Google Scholar]
- 20.Ben-Ezra M, Shmotkin D. Predictors of mortality in the old-old in Israel: the Cross-sectional and Longitudinal Aging Study. Journal of the American Geriatrics Society. 2006;54:906–11. doi: 10.1111/j.1532-5415.2006.00741.x. [DOI] [PubMed] [Google Scholar]
- 21.Blazer DG, Hybels CF, Pieper CF. The association of depression and mortality in elderly persons: a case for multiple, independent pathways. J Gerontol A Biol Sci Med Sci. 2001;56:M505–9. doi: 10.1093/gerona/56.8.m505. [DOI] [PubMed] [Google Scholar]
- 22.Everson-Rose SA, House JS, Mero RP. Depressive symptoms and mortality risk in a national sample: confounding effects of health status. Psychosomatic medicine. 2004;66:823–30. doi: 10.1097/01.psy.0000145903.75432.1f. [DOI] [PubMed] [Google Scholar]
- 23.Fu CC, Lee YM, Chen JD. Association between depressive symptoms and twelve-year mortality among elderly in a rural community in Taiwan. J Formos Med Assoc. 2003;102:234–9. [PubMed] [Google Scholar]
- 24.Hybels CF, Pieper CF, Blazer DG. Sex differences in the relationship between subthreshold depression and mortality in a community sample of older adults. Am J Geriatr Psychiatry. 2002;10:283–91. [PubMed] [Google Scholar]
- 25.Anstey KJ, Luszcz MA. Mortality risk varies according to gender and change in depressive status in very old adults. Psychosomatic medicine. 2002;64:880–8. doi: 10.1097/01.psy.0000028827.64279.60. [DOI] [PubMed] [Google Scholar]
- 26.Takeshita J, Masaki K, Ahmed I, Foley DJ, Li YQ, Chen R, Fujii D, Ross GW, Petrovitch H, White L. Are depressive symptoms a risk factor for mortality in elderly Japanese American men?: the Honolulu-Asia Aging Study. Am J Psychiatry. 2002;159:1127–32. doi: 10.1176/appi.ajp.159.7.1127. [DOI] [PubMed] [Google Scholar]
- 27.Yaffe K, Edwards ER, Covinsky KE, Lui LY, Eng C. Depressive symptoms and risk of mortality in frail, community-living elderly persons. Am J Geriatr Psychiatry. 2003;11:561–7. [PubMed] [Google Scholar]
- 28.Pulska T, Pahkala K, Laippala P, Kivela SL. Follow up study of longstanding depression as predictor of mortality in elderly people living in the community. Bmj. 1999;318:432–3. doi: 10.1136/bmj.318.7181.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ariyo AA, Haan M, Tangen CM, Rutledge JC, Cushman M, Dobs A, Furberg CD. Depressive symptoms and risks of coronary heart disease and mortality in elderly Americans. Cardiovascular Health Study Collaborative Research Group. Circulation. 2000;102:1773–9. doi: 10.1161/01.cir.102.15.1773. [DOI] [PubMed] [Google Scholar]
- 30.Penninx BW, Guralnik JM, Mendes de Leon CF, Pahor M, Visser M, Corti MC, Wallace RB. Cardiovascular events and mortality in newly and chronically depressed persons > 70 years of age. Am J Cardiol. 1998;81:988–94. doi: 10.1016/s0002-9149(98)00077-0. [DOI] [PubMed] [Google Scholar]
- 31.Gerstorf D, Ram N, Rocke C, Lindenberger U, Smith J. Decline in life satisfaction in old age: longitudinal evidence for links to distance-to-death. Psychology and aging. 2008;23:154–68. doi: 10.1037/0882-7974.23.1.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mroczek DK, Spiro A., 3rd Personality change influences mortality in older men. Psychol Sci. 2007;18:371–6. doi: 10.1111/j.1467-9280.2007.01907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barefoot JC, Schroll M. Symptoms of depression, acute myocardial infarction, and total mortality in a community sample. Circulation. 1996;93:1976–80. doi: 10.1161/01.cir.93.11.1976. [DOI] [PubMed] [Google Scholar]
- 34.Mallon L, Broman JE, Hetta J. Relationship between insomnia, depression, and mortality: a 12-year follow-up of older adults in the community. Int Psychogeriatr. 2000;12:295–306. doi: 10.1017/s1041610200006414. [DOI] [PubMed] [Google Scholar]
- 35.Pulska T, Pahkala K, Laippala P, Kivela SL. Survival of elderly Finns suffering from dysthymic disorder: a community study. Soc Psychiatry Psychiatr Epidemiol. 1998;33:319–25. doi: 10.1007/s001270050061. [DOI] [PubMed] [Google Scholar]
- 36.Blazer DG, Hybels CF. What symptoms of depression predict mortality in community-dwelling elders? Journal of the American Geriatrics Society. 2004;52:2052–6. doi: 10.1111/j.1532-5415.2004.52564.x. [DOI] [PubMed] [Google Scholar]
- 37.Rogosa DR, Brandt D, Zimowski M. A growth curve approach to the measurement of change. Psychol Bull. 1982;90:726–46. [Google Scholar]
- 38.Singer JD, Willett JB. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. New York, NY: Oxford University Press; 2003. [Google Scholar]
- 39.Guo X, Carlin BP. Separate and joint modeling of longitudinal and event time data using standard computer packages. The American Statistician. 2004;58:16–24. [Google Scholar]
- 40.Vonesh EF, Greene T, Schluchter MD. Shared parameter models for the joint analysis of longitudinal data and event times. Statistics in medicine. 2006;25:143–63. doi: 10.1002/sim.2249. [DOI] [PubMed] [Google Scholar]
- 41.Hogan JW, Laird NM. Mixture models for the joint distribution of repeated measures and event times. Statistics in medicine. 1997;16:239–57. doi: 10.1002/(sici)1097-0258(19970215)16:3<239::aid-sim483>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 42.Ghisletta P, McArdle JJ, Lindenberger U. Longitudinal cognition-survival relations in old and very old age: 13-year data from the Berlin Aging Study. European Psychologist. 2006;11:204–23. [Google Scholar]
- 43.McArdle JJ, Small BJ, Backman L, Fratiglioni L. Longitudinal models of growth and survival applied to the early detection of Alzheimer's disease. J Geriatr Psychiatry Neurol. 2005;18:234–41. doi: 10.1177/0891988705281879. [DOI] [PubMed] [Google Scholar]
- 44.Borawski EA, Kinney JM, Kahana E. The meaning of older adults' health appraisals: congruence with health status and determinant of mortality. The journals of gerontology. 1996;51:S157–70. doi: 10.1093/geronb/51b.3.s157. [DOI] [PubMed] [Google Scholar]
- 45.Kahana E, Lawrence RH, Kahana B, Kercher K, Wisniewski A, Stoller E, Tobin J, Stange K. Long-term impact of preventive proactivity on quality of life of the old-old. Psychosomatic medicine. 2002;64:382–94. doi: 10.1097/00006842-200205000-00003. [DOI] [PubMed] [Google Scholar]
- 46.Radloff LS. The CES-D Scale: a new self-report depression scale for research in the general population. Applied Psychological Assessment. 1977;1:385–401. [Google Scholar]
- 47.Radloff LS, Terri L. Use of the CES-D with older adults. Clinical Gerontologist. 1986;5:119–36. [Google Scholar]
- 48.Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale) American journal of preventive medicine. 1994;10:77–84. [PubMed] [Google Scholar]
- 49.Schulz R, Beach SR, Ives DG, Martire LM, Ariyo AA, Kop WJ. Association between depression and mortality in older adults: the Cardiovascular Health Study. Arch Intern Med. 2000;160:1761–8. doi: 10.1001/archinte.160.12.1761. [DOI] [PubMed] [Google Scholar]
- 50.Marshall AL, Smith BJ, Bauman AE, Kaur S. Reliability and validity of a brief physical activity assessment for use by family doctors. British journal of sports medicine. 2005;39:294–7. doi: 10.1136/bjsm.2004.013771. discussion -7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.George LK, Fillenbaum GG. OARS methodology. A decade of experience in geriatric assessment. Journal of the American Geriatrics Society. 1985;33:607–15. doi: 10.1111/j.1532-5415.1985.tb06317.x. [DOI] [PubMed] [Google Scholar]
- 52.Mangen DJ, Peterson WA, Sanders R. Introduction. In: Mangen DJ, Peterson WA, editors. Research Instruments in Social Gerontologyvol Vol 1, Clinical and Social Psychology. Minneapolis: University of Minnesota Press; 1982. pp. 3–23. [Google Scholar]
- 53.Davies AR, Ware JE. Measuring health perceptions in the health insurance experiment. Santa Monica, CA: Rand McNally; 1981. [Google Scholar]
- 54.Fillenbaum GG. An examination of the vulnerability hypothesis. International Journal of Aging & Human Development. 1977-1978;;8:155–60. doi: 10.2190/aqp7-vgu8-at6u-vtd3. [DOI] [PubMed] [Google Scholar]
- 55.Fillenbaum GG. Multidimensional functional assessment of older adults: The Duke Older Americans Resources and Services procedures. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 56.Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. Journal of the American Geriatrics Society. 1975;23:433–41. doi: 10.1111/j.1532-5415.1975.tb00927.x. [DOI] [PubMed] [Google Scholar]
- 57.Verbeke G, Molenberghs G, Thijs H, Lesaffre E, Kenward MG. Sensitivity analysis for nonrandom dropout: a local influence approach. Biometrics. 2001;57:7–14. doi: 10.1111/j.0006-341x.2001.00007.x. [DOI] [PubMed] [Google Scholar]
- 58.Idler EL, Kasl SV, Lemke JH. Self-evaluated health and mortality among the elderly in New Haven, Connecticut, and Iowa and Washington Counties, Iowa, 1982-1986. American Journal of Epidemiology. 1990;131:91–103. doi: 10.1093/oxfordjournals.aje.a115489. [DOI] [PubMed] [Google Scholar]
- 59.DeSalvo KB, Bloser N, Reynolds K, He J, Muntner P. Mortality prediction with a single general self-rated health question. A meta-analysis. J Gen Intern Med. 2006;21:267–75. doi: 10.1111/j.1525-1497.2005.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Unutzer J, Katon W, Callahan CM, Williams JW, Jr, Hunkeler E, Harpole L, Hoffing M, Della Penna RD, Noel PH, Lin EH, Arean PA, Hegel MT, Tang L, Belin TR, Oishi S, Langston C. Collaborative care management of late-life depression in the primary care setting: a randomized controlled trial. Jama. 2002;288:2836–45. doi: 10.1001/jama.288.22.2836. [DOI] [PubMed] [Google Scholar]
- 61.Bruce ML, Ten Have TR, Reynolds CF, 3rd, Katz II, Schulberg HC, Mulsant BH, Brown GK, McAvay GJ, Pearson JL, Alexopoulos GS. Reducing suicidal ideation and depressive symptoms in depressed older primary care patients: a randomized controlled trial. Jama. 2004;291:1081–91. doi: 10.1001/jama.291.9.1081. [DOI] [PubMed] [Google Scholar]
- 62.Glassman AH, Bigger JT, Gaffney M, Shapiro PA, Swenson JR. Onset of major depression associated with acute coronary syndromes: relationship of onset, major depressive disorder history, and episode severity to sertraline benefit. Archives of general psychiatry. 2006;63:283–8. doi: 10.1001/archpsyc.63.3.283. [DOI] [PubMed] [Google Scholar]
- 63.Gallo JJ, Bogner HR, Morales KH, Post EP, Lin JY, Bruce ML. The effect of a primary care practice-based depression intervention on mortality in older adults: a randomized trial. Annals of internal medicine. 2007;146:689–98. doi: 10.7326/0003-4819-146-10-200705150-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lloyd-Jones DM, Martin DO, Larson MG, Levy D. Accuracy of death certificates for coding coronary heart disease as the cause of death. Annals of internal medicine. 1998;129:1020–6. doi: 10.7326/0003-4819-129-12-199812150-00005. [DOI] [PubMed] [Google Scholar]
- 65.Saydah SH, Geiss LS, Tierney E, Benjamin SM, Engelgau M, Brancati F. Review of the performance of methods to identify diabetes cases among vital statistics, administrative, and survey data. Annals of epidemiology. 2004;14:507–16. doi: 10.1016/j.annepidem.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 66.Lenfant C, Friedman L, Thom T. Fifty years of death certificates: the Framingham Heart Study. Annals of internal medicine. 1998;129:1066–7. doi: 10.7326/0003-4819-129-12-199812150-00013. [DOI] [PubMed] [Google Scholar]
- 67.Skala JA, Freedland KE, Carney RM. Coronary heart disease and depression: a review of recent mechanistic research. Canadian journal of psychiatry. 2006;51:738–45. doi: 10.1177/070674370605101203. [DOI] [PubMed] [Google Scholar]
- 68.Anstey KJ, von Sanden C, Sargent-Cox K, Luszcz MA. Prevalence and risk factors for depression in a longitudinal, population-based study including individuals in the community and residential care. Am J Geriatr Psychiatry. 2007;15:497–505. doi: 10.1097/JGP.0b013e31802e21d8. [DOI] [PubMed] [Google Scholar]