Abstract
Extracellular nucleotides (e.g. ATP, UTP, ADP) are released by activated endothelium, leukocytes and platelets within the injured vasculature and bind specific cell-surface type-2 purinergic (P2) receptors. This process drives vascular inflammation and thrombosis within grafted organs. Importantly, there are also vascular ectonucleotidases i.e. ectoenzymes that hydrolyze extracellular nucleotides in the blood to generate nucleosides (viz. adenosine). Endothelial cell NTPDase1/CD39 has been shown to critically modulate levels of circulating nucleotides. This process tends to limit the activation of platelet and leukocyte expressed P2 receptors and also generates adenosine to reverse inflammatory events. This vascular protective CD39 activity is rapidly inhibited by oxidative reactions, such as is observed with liver ischemia reperfusion injury. In this review, we chiefly address the impact of these signaling cascades following liver transplantation. Interestingly, the hepatic vasculature, hepatocytes and all non-parenchymal cell types express several components co-ordinating the purinergic signaling response. With hepatic and vascular dysfunction, we note heightened P2- expression and alterations in ectonucleotidase expression and function that may predispose to progression of disease. In addition to documented impacts upon the vasculature during engraftment, extracellular nucleotides also have direct influences upon liver function and bile flow (both under physiological and pathological states). We have recently shown that alterations in purinergic signaling mediated by altered CD39 expression have major impacts upon hepatic metabolism, repair mechanisms, regeneration and associated immune responses. Future clinical applications in transplantation might involve new therapeutic modalities using soluble recombinant forms of CD39, altering expression of this ectonucleotidase by drugs and/or using small molecules to inhibit deleterious P2-mediated signaling while augmenting beneficial adenosine-mediated effects within the transplanted liver.
Keywords: Transplantation, Liver, Purinergic Receptors, Bile, CD39, ecto-ATPase, Endothelium, Immunology, Metabolism, NTPDase, Platelet, Vasculature, Review
2. INTRODUCTION
Purinergic signaling comprises release of extracellular nucleotides, stimulation of purinergic receptors and the modulation of nucleotide levels by ectoenzymes. Over the past decade, many advances have been made in the study of purinergic receptors and the regulation of extracellular nucleotide concentrations (1). It is now generally accepted that extracellular nucleotides (e.g. ATP, UTP, ADP), and the derivative nucleosides (e.g. adenosine from ATP), are released in a regulated manner by cells to provide the primary components for purinergic responses (2). Specific nucleotide/nucleoside mediators bind defined purinergic receptors that comprise the second requirement for this intricate signaling network. Almost all cells express cell-surface type-2 purinergic (P2) receptors for nucleotides and adenosine or type-1 purinergic (P1) receptors. There are seven ionotropic (P2X), at least eight metabotropic (P2Y) and four adenosine receptor subtypes that have been identified and characterized to date (3–7). Hepatic parenchymal and non-parenchymal cells express multiple P2X and P2Y receptor subtypes (see later). These receptors operate in both auto- and paracrine loops and are thought to play a complex, important role in the regulation of vascular and immune cell-mediated responses. For example ATP stimulation of endothelium and lymphocytes largely induces pro-inflammatory responses, such as the release of interleukin-1 and interleukin-6. Exposure of dendritic cells (DC) to extracellular ATP induces migration and drives differentiation to promote cellular immune responses (8). Depending upon the receptor subtype, the cell types and signaling pathway involved, these receptors trigger and mediate short-term (acute) processes that affect metabolism, adhesion, activation or migration. Activation of select receptors in a repetitive manner also has a profound impact upon long-term reactions, including cell proliferation, differentiation and apoptosis, as seen in several chronic inflammatory states.
The third, and final, component of purinergic signaling systems comprises ectonucleotidases (9). These ectoenzymes hydrolyze extracellular nucleotides to generate nucleosides that in turn activate adenosine receptors, often with opposing effects as compared to those mediated by P2-receptors. Within the past decade, ectonucleotidases belonging to several enzyme families have been discovered, cloned and further characterized by pharmacological means. Modulated, distinct ectonucleotidase expression may regulate nucleotide-mediated signaling in the vasculature in both temporal and spatial manners. Vascular endothelial and accessory cell activation with associated proliferative responses within grafts may have important consequences for platelet activation, thrombogenesis, angiogenesis, vascular remodeling and the metabolic milieu of the vasculature, in response to inflammatory stress and/or immune reactions (9).
This review considers important P2-receptors and major ectonucleotidases of the vascular endothelium and liver, and relates the expression and functions of these to vascular injury, thromboregulatory issues, rejection patterns, metabolic disturbances and disordered regeneration seen in certain hepatic grafts.
3. OVERVIEW: PRINCIPLE AND PHYSIOLOGY
3.1. Hepatic purinergic signaling
States of biological stress may lead to alteration of release or uptake of nucleotides or may alternatively decrease enzymatic function of ectonucleotidases (9). Besides being implicated in thrombosis, inflammation and vascular injury, purinergic signaling regulates important hepatic processes such as bile secretion, glycogen metabolism and responses to insulin. Hence, the liver is of major importance for purine homeostasis and for the regulation of nucleotide synthesis. This highly metabolically active organ provides the bulk of all purine precursor substrates for the peripheral tissues (10, 11). Salvage of nucleotides in bile was recognized over 40 years ago. This process is associated with both systemic nucleotide homeostasis and the potential for local intercellular signaling within the canalicular networks and biliary systems (12).
Within the liver, ATPase activity has been shown to be associated with the vasculature (NTPDase1), periportal fibroblasts (NTPDase2) and bile canaliculus (NTPDase8). Other ATPases such as NTPDase3 and NTPDase5 have been found in hepatic tissue, but their functional relevance is still unclear.
After hepatic ischemia and reperfusion injury, vascular NTPDase activity is lost and deletion of NTPDase1 in mice leads to significantly increased injury and decreased survival. Furthermore, post-transplant biochemical activity of NTPDase1 is lost initially postoperatively and is reestablished within days to weeks later in the surviving, potentially accommodated grafts (13).
In the following sections, we focus on the expression and function of purinergic P2 receptors and ectonucleotidases within hepatic tissue (Table 1) (14). We describe the known implications of purinergic signaling in processes such as vascular injury, modulation of bile and metabolism e.g. of blood glucose and tissue nucleotide levels. We also briefly allude to diseases that are impacted upon by perturbations in nucleotide flux e.g. liver fibrosis and tumor formation.
Table 1.
Expression and function of P2-receptors and ectonucleotidases by cellular components of the liver
| Cellular component | Expression | Function | References |
|---|---|---|---|
| Hepatocytes | P2Y1, P2Y2, P2Y4, P2Y6, P2Y13, NTPDase8 (apical membrane) |
Glycogen metabolism Insulin resistance |
108–111 |
| Cholangiocytes | P2Y1, P2Y2, P2Y4, P2Y6, P2X2, P2X3, P2X4, P2X6 | Bile (anion) secretion Nucleotide salvage Canalicular contraction Interaction with hepatocytes Adenosine resorption from bile |
115, 117 |
| Endothelial cells | P2Y1, P2Y2, P2Y6, P2X4, P2X7 NTPDase1 |
NO release Secretion of prostaglandins E2 |
96–102 |
| Vascular smooth muscle cells | P2Y1, P2Y2, P2Y6, P2X4, P2X7 | Portal vein contraction | 48, 50, 164, 174 |
| Hepatic stellate cells | P2Y2, P2Y4, P2Y6, NTPDase2 | Secretion of Prostaglandin F2 and D2 Cell contraction |
60, 83–85 |
| Portal fibroblasts | NTPDase2 | Hepatic fibrosis | 48, 92, 93, 95 |
| Kupffer cell/macrophages | P2Y1, P2Y2, P2Y4, P2Y6, P2X1, P2X4, P2X7, NTPDase1 |
Killing of intracellular pathogens Secretion of prostaglandin E2 Interleukin 6 |
120–134 |
| Liver associated lymphocytes: NK, NKT, T cells, B cells |
P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y14 P2X1, P2X2, P2X4, P2X7, NTPDase1 |
Modulate Concanavalin A mediated hepatitis | 135–145 |
| Neutrophils | P2Y2 | Chemotaxis | 174 |
3.2. Nucleotide release
Extracellular nucleotides and nucleosides are released in a regulated manner from cells by a variety of mechanisms. Such pathways include exocytosis of ATP/UTP-containing vesicles, facilitated diffusion via connexin-43 hemichannels, by putative ABC transporters or potentially by poorly understood electrodiffusional movements through ATP/nucleotide channels (2, 15–21). Under pathophysiological conditions, the release of nucleotides and the expression of purinergic receptors is increased markedly in injured or stressed cells (17).
Extracellular ATP entering the liver by the portal vein is rapidly metabolized after a single passage through the liver (22). Therefore, metabolic stimulation by extracellular nucleotides might expected to be more pronounced in the periportal region. In addition, ATP can be released by hepatocytes into different extracellular compartments: via basolateral, sinusoidal or apical exocrine routes. Secretion of ATP into the bile is mediated by an increase in cholangiocyte cell volume, which stimulates nucleotide release by vesicular exocytosis (23, 24). Canalicular nucleotide and nucleoside levels are further regulated and controlled by the presence of a canalicular Na+-dependent nucleoside transporter that removes adenosine from the bile (25). Salvage of nucleotides in bile may be crucial in the maintenance of appropriate nucleotide/nucleoside concentrations within hepatocytes or within the entire organism (11).
3.3. Purinergic receptors
Over fifteen P2-receptors of different specificities transmit signals from extracellular nucleotides, triggering and modulating vascular and immune cell activation processes, metabolism, nitric oxide (NO) release, adhesion, migration, proliferation and apoptosis (26, 27). Since the original cloning of the P2Y2R subtype, at least eight P2YR and seven P2XR subtypes have been cloned and functionally identified.
There are two main families of nucleotide receptors: P2X are "rapid" ligand-gated ion channels permeable for Na+, K+ and also Ca2+ (subtypes P2X1-7) (28) and P2Y are the "slow" metabotropic receptors (P2Y1, 2, 4, 6, 11–14). P2Y receptors are 7-transmembrane Gq- or Gi-protein linked and initiate signal transduction coupled to activation of phospholipase C, or to inhibition of adenylate cyclase, respectively (29).
There are also four known P1 cell surface receptors for nucleosides, chiefly adenosine (A1, A2a, A2b and A3) that are classified according to their affinities for adenosine and variant coupling to adenylate cyclase (30, 31).
Depending upon the repertoire of receptors and signaling components, P2R influence cellular activation, proliferation and the induction of apoptosis. For example, in the vascular system, extracellular nucleotides and nucleosides can influence platelet activation, thrombosis, inflammatory processes, cardiac function and vasomotor responses, (32–35). ATP and ADP appear to regulate hemostasis through the activation of platelet P2 receptors. ADP is a major platelet recruiting factor originating from platelet dense granules, released upon activation whereas ATP derived from the same sources is considered a competitive antagonist of ADP for platelet P2Y1 receptors (17, 36, 37). Platelet P2Y12 is perhaps the best known purinergic receptor and several drugs (thienopyridine agents e.g. clopidogrel) are used in clinical practice to target ADP-mediated cardiovascular thrombosis (38).
ATP (and UTP) also stimulate endothelial P2Y receptors to release prostacyclin (PGI2) and nitric oxide (NO); two vasodilators and inhibitors of platelet aggregation (39–43). This latter protective action of ATP may limit the extent of intravascular platelet aggregation and to help localize thrombus formation to areas of vascular damage (41, 44, 45). The receptors P2Y1 and P2Y2 on vascular endothelial cells, smooth muscle cells and monocytes are also important receptors in the mediation of vascular inflammation (46–48).
In addition, ATP also stimulates P2X receptors to cause plasma membrane permeabilization, induction of apoptosis, organic anion transport, and stimulation of Ca2+ mobilization (33, 49). The active form of ATP that causes membrane permeabilization is a fully ionized tetrabasic ion (or ATP4) that interacts with the unique multimeric P2X7 receptors expressed on endothelium and monocyte-macrophages (50). This nucleotide is a relative inhibitor of NTPDases, as these are divalent cation dependent enzymes (51). Such P2X7 receptors are also stimulated by the nonhydrolyzable ATP analog ATP-γ-S but are unresponsive to UTP (35, 52). The major effect of P2X7 receptors is the induction of cellular activation and apoptosis (52, 53).
Pathways of nucleotide-mediated signaling are further complicated by P2-receptor desensitization phenomena. Thus, adenine nucleotides may also directly limit ADP-mediated reactivity of platelets (33, 36, 54, 55). Like other members of the G protein coupled receptor family, some P2Y receptors readily undergo agonist-induced desensitization (56). This may involve phosphorylation of the receptor by multiple protein kinases (27). For unclear reasons, P2X- and P2Y-type receptors differ widely with respect to desensitization rates: rapidly desensitizing receptors and/or channels (P2Y1, P2Y2, P2X1 and P2X3) contrast with slow desensitizing receptors such as the P2Y6, P2X2 and P2X7 receptors (the last has proven to be very important in inflammatory reactions); P2X4 is intermediate in this regard (57).
3.4. Ectonucleotidases
Ectonucleotidases consist of families of nucleotide metabolizing enzymes that are expressed on the plasma membrane and have externally orientated active sites (Figure 1). These ectoenzymes operate in concert or consecutively and metabolize nucleotides to the respective nucleoside analogs. They have the potential to decrease extracellular concentrations of nucleotides and to generate nucleosides. The relative contribution of the distinct enzymes to the modulation of purinergic signaling may depend on the availability and preference of substrates and on cell and tissue distribution.
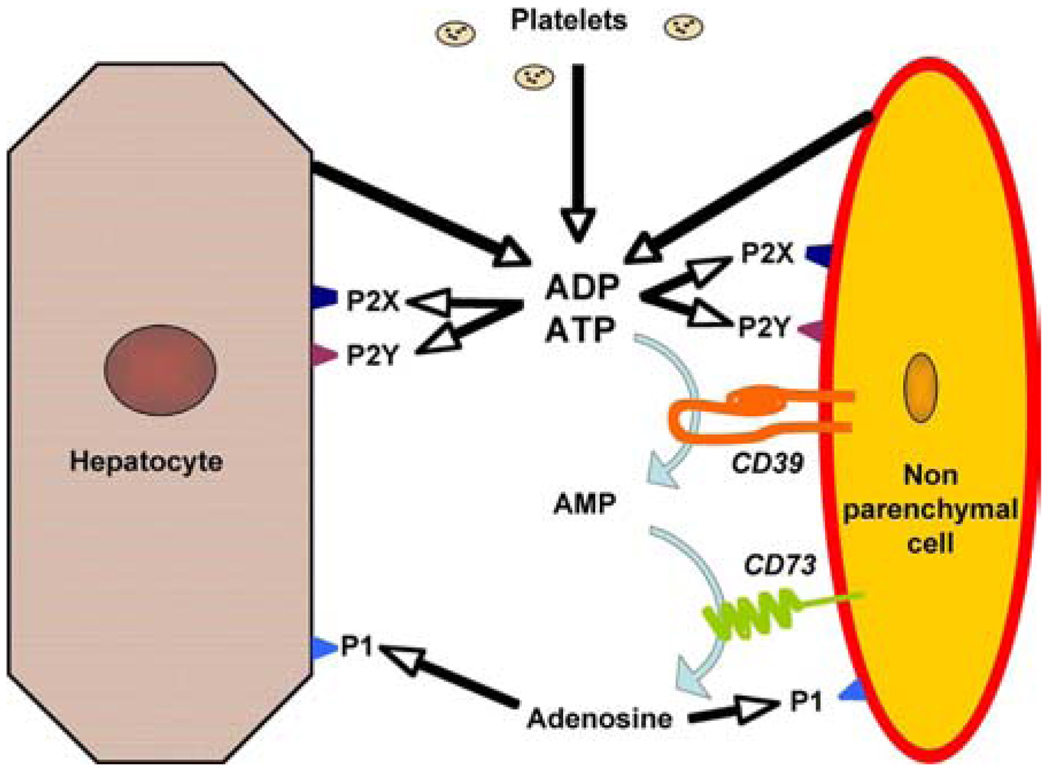
Figure 1.
Principles of purinergic signaling in the hepatic microenvironment. Nucleotides are released from parenchymal cells, non-parenchymal cells and platelets. These nucleotides then activate P2 receptors or are hydrolyzed by NTPDases and 5’nucleotidase to adenosine, which activates P1 receptors.
Ectonucleotidases modulate P2-receptor-mediated signaling. Alterations in extracellular nucleotide levels can increase or decrease P2 activity or lead to P2 receptor desensitization (58). Desensitization of P2 receptors is dose dependent. In vitro the desensitization of P2-mediated anion secretion responses requires 10-min preincubations with the P2Y2 receptor agonist UTP. Recovery from UTP or ATP-induced desensitization can either be rapid (<10 min) at preincubation concentrations of approximately 2 µM or requires progressively longer time periods at higher concentrations. Furthermore, generation of extracellular adenosine not only abrogates nucleotide-mediated effects but also activates adenosine receptors, often with opposing (patho-) physiological effects.
Ectonucleotidases also produce the key molecules for purine salvage and consequent replenishment of ATP stores within multiple cell types (59). Indeed, although nucleotides do not appear to be taken up by cells, dephosphorylated nucleoside derivatives interact with several specific transporters to enable intracellular uptake via membrane passage (11, 60).
In the liver functional ATPases were shown as early as 1960 to be associated with canalicular plasma membranes in association with alkaline phosphatases (61). This canalicular ecto-ATPase activity was subsequently shown to be modulated by bile duct ligation and to be distinct from the classical Na+K+-ATPase-pumps that are preferentially expressed on the basolateral membranes (11). The initially cloned putative ecto-ATPase was not a true ATPase but rather shared significant sequence homology with several canalicular glycoproteins such as C-Cam105 (11).
There are four major families of ectonucleotidases in the liver microenvironment namely CD39/NTPDases (ecto-nucleoside triphosphate diphosphohydrolases), nucleotide pyrophosphatase/phosphodiesterase (NPP)-type ecto-phosphodiesterases, alkaline phosphatases and ecto-5’-nucleotidases/CD73 (62).
3.4.1. Ecto- nucleoside triphosphate diphosphohydrolases (NTPDases of the CD39 family)
It is now known that there are three ectonucleotidases largely responsible for the bulk of ATPase activity in the liver: NTPDase1, which is present on Kupffer and vascular endothelial cells, NTPDase2, which is expressed by portal fibroblasts and activated hepatic stellate cells and NTPDase8 that seems to be the major ATPase of the hepatic canaliculus. Other hepatic NTPDases such as NTPDase3 on stellate cells and NTPDase5 have been described on liver cells, however, functional data is still lacking.
3.4.2. Nucleotide pyrophosphatase/phosphodiesterases (NPP)
NPP hydrolyze pyrophosphate or phosphodiester bonds of nucleotides, metabolizing ATP to AMP and diphosphates. The family of NPPs consists of seven members (NPP1-7). NPP1 is expressed in hepatocytes and localizes basolateral membrane (63–65). The expression of NPP1 is associated by hepatocellular growth. It is absent during the fetal period and decreased during liver regeneration following 70% partial hepatectomy (66). This observation underscores the relevance of ATP for hepatocellular proliferation (67). NPP3 is the major NPP isoenzyme at the apical membrane of both hepatocytes and cholangiocytes (64, 65).
3.4.3. Alkaline phosphatases (ALP)
Alkaline phosphatases are hydrolases responsible for removing phosphate groups in the 5- and 3- positions from many types of molecules, including nucleotides, proteins, and alkaloids. As the name suggests, alkaline phosphatases are most effective in an alkaline environment. Biliary and canalicular expression of ALP may provide a defense mechanism modulating endotoxin toxicity, not addressed here in detail (68))
3.4.4. Ecto-5’-nucleotidase / CD73
Ecto-5’-nucleotidase (CD73; EC 3.1.3.5) terminates the dephosphorylation cascade of nucleotides to adenosine (69). This enzyme has been detected in the canalicular plasma membrane, in the connective tissue of the portal triads and central veins, and HSC (11, 70). Ecto-5’-nucleotidase is co-expressed with NTPDase8 on the canalicular membrane, thereby regulating biliary nucleotide and nucleoside levels (71).
3.5. Distribution and functions
3.5.1. Hepatocytes
3.5.1.1. Purinergic receptors
Select P2X and P2Y purinergic receptors are expressed by hepatocytes (Figure 1) (72, 73). Of the P2Y receptor family, mRNA species of P2Y1, P2Y2, P2Y4, P2Y6 and P2Y13 have been observed. Of these, only the P2Y1, P2Y2 and P2Y13 receptors appear to be of functional relevance for the hepatocyte (74, 75). P2X receptors have been noted in association with the apical membrane as shown below.
Extracellular nucleotides have several effects on isolated hepatocytes such as repetitive changes of transients in cytosolic free calcium concentration ([Ca (2+)]i) directly in response to ATP or UTP, or in response to ADP after conversion to ATP (76–79). On the basis of the pharmacological profile of the nucleotide-induced Ca2+-responses in human hepatocytes, it is believed that extracellular ATP and UTP increase [Ca2+]i by activating P2Y2 and possibly P2Y4 receptors coupled to the Ca2+-phosphatidylinositol signaling cascade (80). The major metabolic effects of ATP include modulation of glycogen metabolism by glycogenolysis and inactivation of glycogen synthesis (81). For example, it has been demonstrated that activation of P2Y1 receptors in rat hepatocytes substantially stimulates glycogen phosphorylase (82).
3.5.1.2. Ectonucleotidases
The major ectonucleotidases activity of hepatocytes lies within the bile canalicular domain and is comparable to what is described for the cholangiocyte, as discussed below.
3.5.2. Cholangiocytes / bile ducts
3.5.2.1. Purinergic receptors
Cholangiocytes express mRNA of distinct P2Y receptor family members P2Y1, P2Y2, P2Y4 and P2Y6 (83). Microperfusion of intrahepatic bile ducts by apyrase and the P2 receptor inhibitor suramin also reveals the presence of functional apical P2 receptors (60). Similar experiments using the perfusion of ATP-γ-S show net alkalization of bile ducts (83).
The apical purinergic receptor agonist preference is consistent with the P2Y2 subtype and this receptor has been shown to be a marker of the apical cilia (60, 84). On the basolateral membrane the function of P2Y receptors appears to be more complex. The kinetics of ATP degradation in apical and basolateral compartments are distinct, suggesting that there are domain-specific signaling pathways that contribute to purinergic responses in polarized cholangiocytes (60, 83). Reverse transcriptase PCR from cultured normal rat cholangiocytes shows transcripts for P2X receptors P2X2, P2X3, P2X4, and P2X6. In lysates of cholangiocytes, P2X4 protein can be also detected, and immunohistochemical staining of intact rat liver reveals P2X4 protein within intrahepatic bile ducts. Of P2X receptors, P2X4 is chiefly involved in regulation of bile secretion in vitro (85). Hepatocytes and bile ductular cells have been shown to interact and communicate via ATP release in a paracrine manner and nucleotides may be involved in the regulation of canalicular contraction and bile secretion. ATP/UTP within the cholangiole stimulates anion and fluid secretion. The binding of nucleotides to apical P2Y2-receptors may facilitate the coordination of bile formation as a consequence of the paracrine hepatobiliary coupling. (85)
3.5.2.2. Ectonucleotidases
Functional ATPases were previously shown to be associated with bile canalicular plasma membranes by histochemical techniques (12). The corresponding enzyme was originally identified as c-CAM105, but this turned out to be incorrect (86–88). Recent studies have revealed that the canalicular ecto-ATPase corresponds to NTPDase8 (89).
Human and rat NTPDase8 cDNA has been cloned and the genes are located on chromosome loci 9q34 and 3p13, respectively (71, 89). NTPDase8 resembles the chicken ecto-ATPase cloned from oviduct and liver (90, 91). Major ATPase and ADPase activity was detected in the bile canaliculi by immunohistochemistry after incubation with nucleotides. It has been proposed that NTPDase8 is the canalicular ecto-ATPase and that it is responsible for a major proportion of canalicular NTPDase activity (71).
3.5.3. Vasculature
3.5.3.1.Purinergic receptors
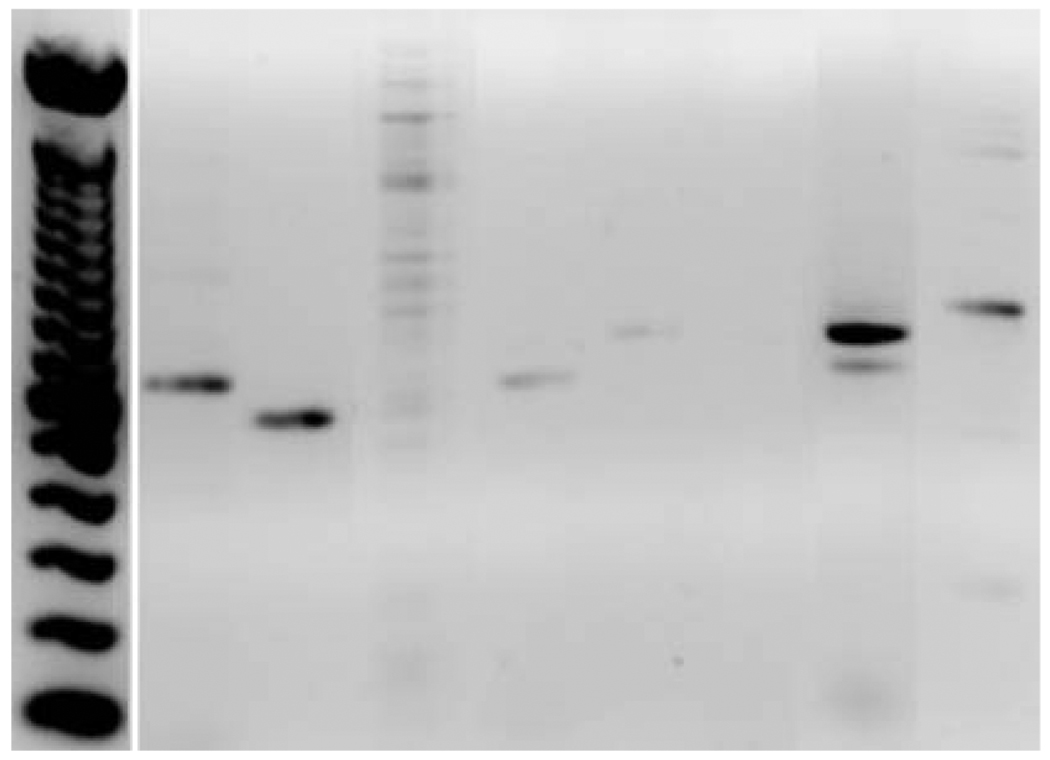
Functional expression profiles of P2Y1 and P2Y2 receptors have been described for the hepatic artery and portal vein (92, 93). These endothelial cells of the hepatic artery and portal vein mediate nitric oxide (NO) release by P2Y mediated mechanisms (92–94)., We have detected mRNA for P2Y1, P2Y2, P2Y6, P2X4 and P2X7 receptors in sinusoidal endothelial cells (Figure 2). In response to nucleotides, sinusoidal endothelial cells secrete Prostaglandin E2 (PGE2) in a P2Y dependent manner (95).
Figure 2.
P2 receptor expression by hepatic sinusoidal endothelial cells. High levels of mRNA for P2Y1, P2Y2, P2X4 and P2X7 are noted. The expression of P2Y6 is weak and no signals for P2Y4, P2X1 and P2X2 are noted.
On vascular smooth muscle cells, P2Y1, P2Y2, P2Y6, P2X1, P2X3, P2X4, P2X5 and P2X7 have been described (96–101). Vasoconstriction is the major effect on arterial smooth muscle cells in response to ATP and UDP and is mediated by P2X and P2Y6 receptors (96, 97). In the portal vein, vascular responses seem to be mediated by P2X and P2Y2 receptors (98, 99, 102).
3.5.3.2. Ectonucleotidases
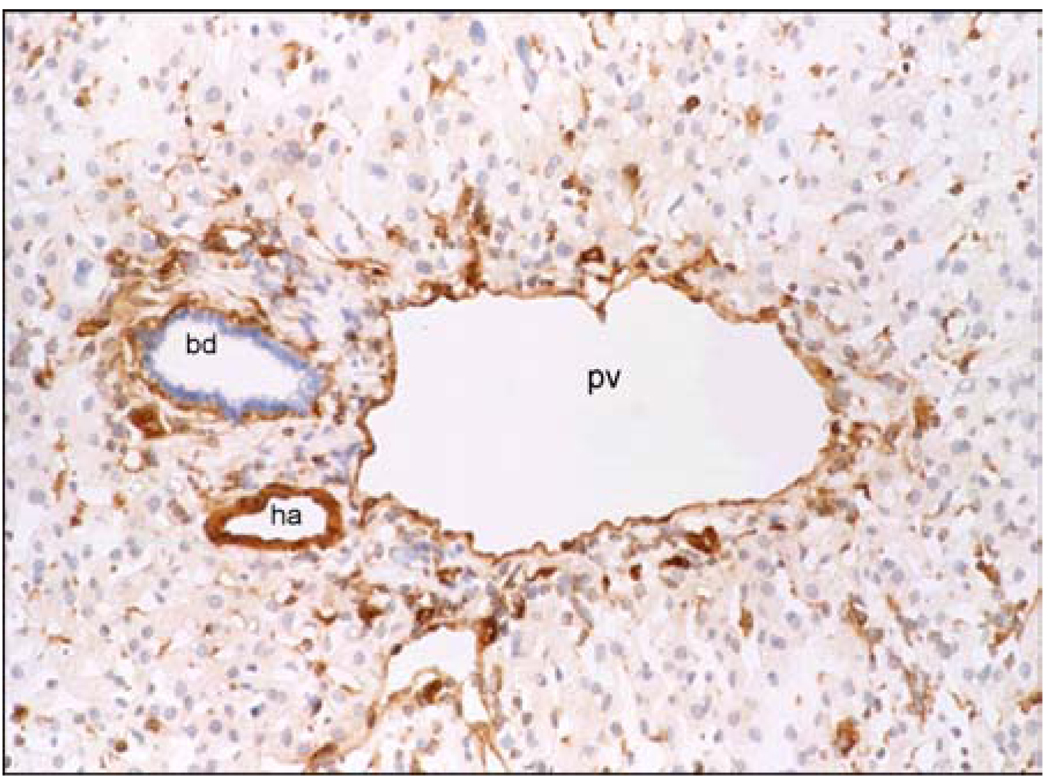
In the liver NTPDase1 can be detected immunohistochemically on endothelial cells of muscularized vessels and Kupffer cells (Figure 3) (103). The NTPDase/CD39 family includes genes that share considerable sequence homology inclusive of five conserved regions (102). CD39 was originally characterized as a cell activation marker, identified on B cells, subsets of activated NK-cells and T-lymphocytes (104–106). We and others have since demonstrated that CD39 is an NTPDase (EC 3.6.1.5) that hydrolyzes both extracellular ATP and ADP to AMP (50, 105, 107). Members of this family of ectoenzymes are all membrane-attached ab initio, with external-oriented active sites. Select members such as NTPDase5 and NTPDase6 are solubilized after proteolytic degradation. Quiescent sinusoidal endothelial cells do not express CD39. However, under specific conditions of activation e.g. proliferation after partial hepatectomy, expression of CD39 on sinusoidal endothelial cells is notably upregulated and is of substantial functional relevance (Beldi et al, manuscript submitted). In all human and murine specimens examined to date, neither canalicular nor biliary expression of NTPDase1 has been observed, whereas strong expression on Kupffer cells and endothelial cells of muscularized vessels is found (Figure 3) (108).
Figure 3.
Immunohistochemical patterns of expression of CD39 in donor human liver. Endothelial cells of the portal vein (pv), hepatic artery (ha) and Kupffer cells strongly express CD39. Quiescent sinusoidal endothelial cells and bile duct (bd) epithelia do not stain for CD39.
Interactions between hepatic NTPDases and P2 may exist, as NTPDases may modulate hepatocyte or bile duct epithelial P2-receptor activity. However, there is no current data to support this hypothesis. Other evidence suggests that certain P2-receptors expressed in the gastrointestinal tract can undergo desensitization in the Cd39-null mouse (58).
3.5.4. Stellate cells / portal fibroblasts
3.5.4.1. Purinergic receptors
Quiescent hepatic stellate cells (HSC) can function as pericytes in the liver and express the P2Y subtypes P2Y2 and P2Y4 that are activated by ATP and UTP. Activated HSC in turn express the P2Y subtype P2Y6 (108). Early reports have shown secretion of prostaglandins F2 and D2 after stimulation with ATP (109). Recent studies revealed that P2Y receptors link extracellular ATP to inositol triphosphate-mediated cytosolic calcium signals resulting in localized calcium signaling and cell contraction (110, 111).
Portal fibroblasts show functional P2Y activity and express NTPDase2. NTPDase2 (or CD39L1/Cd39L1) was originally cloned from rat brain and chicken smooth muscle and was characterized later from human/mouse sources (112–114). This protein shares 40% sequence homology with rat NTPDase1 but has a 32-fold preference for hydrolysis of ATP over ADP (leading to sustained accumulation of ADP). Functional and molecular expression of NTPDase2 has been shown in portal fibroblasts, near the basolateral membranes of bile duct epithelia (115). This distribution may represent a previously unrecognized mechanism for regulation of nucleotide signaling in bile ducts and other epithelia. Under basal conditions portal fibroblasts hydrolyze ATP to AMP and thereby inhibit activation of basolateral P2Y receptor expression by bile duct epithelia. After injury, such as following bile duct ligation, portal fibroblasts transform into portal myofibroblasts. Loss of NTPDase2 might predispose to stimulation of proliferation of bile duct epithelia (116). Decreased levels of NTPDase2 expression in human biliary cirrhosis, as well as in models of bile duct ligation in the rat, have been observed. Expression of NTPDase 2 also shifts from the portal area to the areas with bridging fibrous bands in cirrhosis with hepatitis C (117).
3.5.4.2. Ectonucleotidases
Hepatic stellate cells express CD39 mRNA both under quiescent and activated conditions (108). Upon activation HSC also express NTPDase2 (108, 115). The functional relevance of NTPDase2 on HSC remains unclear. NTPDase 2 is also expressed on portal fibroblasts as discussed below. In vivo, NTPDase2 is not expressed on hepatocytes (118). However, certain wild-type mouse hepatoma cells also contain both constitutive and inducible ecto-ATPase activities with unclear cellular localization (118). NTPDase2 was also found to be expressed on mouse hepatocytes in vitro after dioxin (2,3,7,8-tetrachlorodibenzo-p-dioxin) treatment, but this remains controversial (119).
3.5.5. Kupffer cells
3.5.5.1.Purinergic receptors
There are limited data concerning the expression and function of P2 receptors on Kupffer cells. Peritoneal macrophages typically express mRNA for P2Y1, P2Y2, P2Y6 and possibly P2Y4 as well as P2X1, P2X4 and P2X7 receptors (120–122). P2X7 activation is associated with Interleukin-1 secretion and killing of Mycobacteria, Chlamydia and fungi in monocyte-macrophages (52, 123–128). Polymorphisms of P2X7 have been associated with decreased killing of Mycobacteria (129–131). Furthermore secretion of interleukin-6 possibly by stimulation of P2X4 receptors activates innate immunity (132). In one report, liver injury by ATP was attenuated by administration of gadolinium chloride, which depletes Kupffer cells; Suramin a P2 receptor antagonist has comparable effect. (133). Prostaglandin E2 is released after P2Y receptor activation in non-parenchymal cells (95).
3.5.5.1. Ectonucleotidases
NTPDase1/CD39 is expressed on Kupffer cells, however its function remains unclear. In theory, NTPDase1 would modulate P2 receptor function on Kupffer cells and mimic effects on macrophage function with respect to cytokine production (132, 134).
3.5.6. Liver associated lymphocytes
3.5.6.1.Purinergic receptors
Natural killer (NK) and natural killer T (NKT) cells as well as B cells express a variety of P2 receptors. Lymphocytes express P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12 and P2Y14 as well as P2X1, P2X2, P2X4 and P2X7 (135–143). In the liver activation of P2X7 by released nucleotides has been shown to mediate, at least in part, the injury noted in Concanavalin A dependent autoimmune hepatitis (144).
3.5.6.2. Ectonucleotidases
NTPDase1/CD39 is expressed on subsets of liver lymphocytes such as NK, NKT and B cells. We have recently shown, that NTPDase1 is expressed on regulatory T cells (145).
4. IMPACT OF PURINERGIC SIGNALING
4.1.Hepatic homeostasis in health
4.1.1. Bile flow
Bile acid secretion is disturbed after liver transplantation, thereby possibly exacerbating hepatic ischemia and reperfusion injury (146, 147). Extracellular nucleotides are released into the canaliculus and modulate bile secretion. The biliary epithelium and hepatocytes constitutively release ATP into the bile (11). In biliary epithelium ATP is stored in vesicles and is released in response to cell swelling (23); concentrations of canalicular adenine nucleotides in bile samples are estimated to be 5±0.9µM (148, 149). Extracellular nucleotides entering the bile ducts are potent stimuli for secretion of bile fluid including anions, and canalicular contraction, which is in part explained by interaction and communication between hepatocytes and bile duct cells via local ATP release (25, 149–151). These effects are mediated and coordinated by apical P2Y2 receptors and NTPDase8 (71, 83, 89).
4.1.2. Nucleotide synthesis and salvage
Canalicular localization of NTPDase8 in tandem with ecto-5’-ectonucleotidase suggests involvement in the regulation of nucleoside salvage and consequently nucleotide homeostasis and purine salvage (71). Ultimately, the generation of extracellular adenosine from dephosphorylated ATP not only activates adenosine receptors but also produces the key molecule for purine salvage and consequent replenishment of ATP within many cell types (11, 59). In the canaliculus Na+ dependent nucleoside transporters might further aid in concentrating nucleotides within the bile (152). Nucleoside transporters are of major importance to organs and cells incapable of de novo nucleotide synthesis such as the brain, muscle, intestinal mucosa and bone marrow (10, 25).
Erythrocytes also have these nucleoside transporters and may facilitate the transport of purines between hepatocytes and dependent tissues (153). As the liver appears to be a major source of purines for these tissues, curtailment of nucleotide loss into the bile may be important to maintain appropriate nucleotide/nucleoside concentrations within hepatocytes (11). Thus, dephosphorylation of nucleotides by ectonucleotidases may be critical for appropriate systemic purine homeostasis (25).
4.1.3. Metabolism (glucose/fatty acids)
Extracellular nucleotides are involved in the regulation of glycogen, fatty acid metabolism and insulin resistance. Extracellular nucleotides activate glycogen storage and are glycogenolytic (81). Overall glycogen phosphorylase and the generation of inositol triphosphate (IP3) are stimulated. Glycogen synthase is inhibited, adenosine 3': 5’-cyclic monophosphate levels are lowered and activation of phospholipase D is decreased (81, 154–156). In addition, administered ATP appears to facilitate up-regulation of the gluconeogenic enzyme, phosphoenolpyruvate carboxykinase, and down-regulation of the glycolytic enzyme, pyruvate kinase; hence promoting glucose output by the liver (157, 158). Of the P2Y receptors that mainly control metabolism and proliferation, P2Y2 seems to primarily control both glycogen metabolism and proliferation-associated responses such as increased [Ca (2+)](c) and modulation of mitogen-activated protein kinase cascades (82).
Effects of extracellular ATP on fatty acid metabolism indicate inhibition of acetyl-CoA carboxylase activity and fatty acid synthesis with a concomitant decrease of intracellular malonyl-CoA concentrations (159).
Purinergic signaling also impacts upon insulin signaling. Disordered regulation of extracellular nucleotides induces insulin resistance. Cd39 knockout mice show impaired glucose tolerance and sensitivity to insulin. Furthermore, plasma insulin levels are significantly higher when compared to wild-type mice. Hyperinsulinemic euglycemic clamp studies have revealed altered glucose metabolism in the livers of mice null for CD39/NTPDase1. The impaired sensitivity was accompanied by increased susceptibility to activation of c-jun N-terminal kinase /SAP by extracellular ATP in CD39 null mice liver, (Enjyoji at al, submitted).
4.2. Liver disease
4.2.1. Vascular injury
Many processes of vascular injury result in the release of adenine nucleotides that exert a variety of inflammatory effects on endothelial cells, platelets and leukocytes (reviewed in (9, 33, 160–162)). Perturbation of nucleotide signaling in the vasculature can influence vasomotor responses, platelet activation, inflammatory processes and also cardiac function (32).
In the setting of organ transplantation NTPDase1 activity is lost with reperfusion or rejection and up-regulation is seen with graft survival (13). In a model of total hepatic ischemia and reperfusion injury we have shown a significantly decreased survival associated with increased biochemical and structural liver injury in Cd39-null animals as compared to wild-type controls (163). Furthermore, ADP is a major platelet recruiting factor originating from platelet dense granules during activation (17, 36, 37). ATP also stimulates endothelial cell production of prostacyclin (PGI2) and NO; two vasodilators and inhibitors of platelet aggregation (39, 40).
Administration of soluble NTPDases in the blood stream or up regulation of CD39 post-adenoviral infection has been shown to prolong transplanted graft survival and abrogate platelet sequestration within the vasculature. Finally, mutant mice deficient in Cd39 have evidence for a vascular-based thrombotic state, paradoxically associated with marked prolongation of experimental bleeding times and acquired platelet hypofunction (164).
4.2.2. Rejection
Purinergic receptors and ectonucleotidases are expressed on a variety of immune cells. However, data concerning hepatic graft survival are lacking. NTPDase1 deficiency appears to have two opposing impacts on nucleotide-mediated signaling, that is, inhibition via desensitization of some but not all P2 receptors and augmentation of certain other responses via impaired hydrolysis of their ligands (134). Consequently, in Cd39 null mice, defects in dendrititc cell function, antigen presentation, T cell responses to haptens (type IV hypersensitivity reactions) and delayed cellular rejection responses are observed (8, 131, 134). We have noted membrane expression of CD39 by classic T-regulatory (Treg) cells that appears to be of crucial importance in the immunomodulation of skin allograft rejection (145). These T cell subsets have recently been shown to directly influence chronic vascular injury (165).
4.2.3. Regeneration
In the area of split liver and living donor related liver transplantation liver regeneration has gained additional interest in order to maintain and increase regenerative capacities of remaining and transplanted liver lobes. ATP activates proliferation of hepatocytes in vitro and in vivo and modulates growth factor responses possibly via P2Y2 receptor activation (67). The response of hepatocytes is comparable to early signaling events associated with liver regeneration such as transient activation of c-jun N-terminal kinase and immediate early genes. VEGF receptor 2 signaling, which is of importance during liver regeneration, is modulated by the P2Y2 receptor (48). In mice null for CD39 liver regeneration is significantly decreased. Stimulation of cultured liver sinusoidal endothelial cells (LSEC) with UTP and VEGF leads to enhanced VEGF receptor 2 phosphorylation that is abrogated in cultures with Cd39 −/− LSEC (Beldi G, Wu Y, Sun X Imai M, Enjoji K, Zimmermann H, Candinas D, Robson SC, The vascular ectonucleotidase CD39 is permissive for sinusoidal endothelial cell proliferation during liver regeneration Hepatology 44 (4): 190A-191A 8 Suppl. 1 2006).
4.2.4. Tumors
Recurrence of hepatocellular carcinoma is an important problem post-transplantation, especially in patients with larger tumors exceeding standard selection criteria for liver transplantation (166, 167). As shown above, deletion of Cd39 and perturbations in extracellular nucleotides influence hepatocellular proliferation. Curiously, mice null for Cd39 also exhibit an increased incidence of spontaneous and diethyl nitrosamine induced hepatocellular carcinoma (Wu Y, Sun X, Imai M, Sultan B, Csizmadia E, Enjyoji K, Jackson S, Usheva A, Robson SC. Modulation of RAS/ERK signaling by CD39/ENTPD1 during liver regeneration. Hepatology 2005 42, 4, Suppl.1, Abst.1138, page 643A). Although the mechanism is unclear, mitogenic effects of extracellular nucleotides may predispose to the development of malignancy in these livers. There are also documented associations between CD39 and RanBPM, a membrane scaffolding protein that may be responsible for heightened levels of hepatocyte Ras activation observed following genetic deletion of CD39. This, in turn, may predispose to hepatocarcinogenesis (168).
4.2.5. Fibrogenesis and cirrhosis
Liver fibrosis and cirrhosis due to an array of underlying etiologies are the leading cause world wide of end stage liver disease. In patients post liver transplantation with hepatitis B and C, fibrosis has additionally been seen in conditions such as fibrosing cholestatic syndrome (169, 170). The main cellular components that cause hepatic fibrosis, namely portal fibroblasts and HSC, contain a variety of P2 receptors and express NTPDase2. In this system, closely regulated extracellular nucleotides can activate HSC leading to transcription of procollagen-1 (108). The proliferation of bile duct epithelium which is important for fibrogenesis is inhibited by NTPDase2 on portal fibroblasts leading to blockade of P2Y activation (116).
4.2.6. Cachexia
Individuals with liver disease may develop metabolic imbalances with cholestasis, consequent bone marrow suppression, wasting or severe neurological manifestations (including cerebral edema) that may progress to coma and death over days to weeks. Hypothetically, uncompensated failure of hepatic nucleotide synthetic pathways and disordered salvage may contribute, at least in part, to these complications of acute or chronic liver failure.
The cachexia seen in untreated AIDS patients also has a component that may be associated with purine salvage defects and adenosine mediated toxicity. Extracellular adenosine deaminase that degrades extracellular adenosine, has crucial functional and structural associations with CD26. This interaction results in co-stimulatory signals in T cells and thereby modulation of cell-to-cell contacts in the immune response. Interestingly, adenosine deaminase is also displaced from CD26 by HIV envelope proteins and the consequent perturbations in purine metabolism might be linked to or associated with T cell death and/or AIDS wasting (146).
5. CONCLUSIONS AND PERSPECTIVE
We have reviewed the basic principles of purinergic signaling in some depth and have also shown substantial experimental data implicating extracellular nucleotides, signaling by purinergic receptors and modulation by NTPDases of the CD39 family in the regulation of hepatic blood flow, bile production, homeostasis and metabolism. These pathways impact upon vascular and immune responses to transplantation. In addition to graft injury, disordered bile flow and ongoing graft ischemia secondary to vascular rejection may impact upon the outcome of organ transplantation.
Additionally, purinergic receptors and CD39 are not only expressed on endothelial cells and hepatic sinusoidal cells but also at high levels on Treg (145, 171, 172) and NKT cell populations (Beldi et al, submitted). It is particularly important that hepatic metabolic changes can be caused by extracellular nucleotides thought to serve primarily as inflammatory mediators. These new data provide evidence for integration of nucleotide-mediated vascular and immune responses and associated metabolic disturbances e.g. insulin resistance that are so crucial to the clinical outcomes following hepatic allografting.
Our expectation is that therapeutic avenues will develop using soluble CD39/NTPDase1 and/or small molecules to enhance NTPDase ecto-enzymatic functions that may be used together with selective adenosine receptor agonists. Other approaches to inhibit NTPDase activity may be considered to slow progression of fibrosis e.g. in chronic pancreatitis. It is of interest that certain drugs already in clinical practice influence CD39 function and expression. Commonly prescribed lipid lowering therapies that have salutary effects on the vasculature (e.g. HMG-CoA reductase inhibitors) and membrane phospholipids have in parallel important positive effects on endothelial cell NTPDase activity (173).
Studies are planned to address the efficacy of CD39-based therapeutics in the setting of hepatic reperfusion injury and in the appropriate immune modulation of hepatic allograft rejection.
ACKNOWLEDGEMENTS
SCR acknowledges grant support from NIH HL57307, HL63972 and HL076540. GB thanks the Swiss National Research Foundation for grant support. (PASMA-115700, PBBEB-112764). KE thanks the American Heart association (AHA - 0530362N).
Abbreviations
- P1
type 1 purinergic (adenosine) receptor
- P2
type 2 purinergic receptor
- ATPase
ATP diphosphohydrolase
- DC
dendritic cell
- EC
endothelial cell
- NTPDase
ecto-nucleoside triphosphate diphosphohydrolase
- ALP
alkaline phosphatases
- IL
interleukin
- NPP
nucleotide pyrophosphatase/phosphodiesterase
- HSC
hepatic stellate cell
- NK
natural killer cell
- NKT
natural killer T cell
- Treg
T regulatory cells
- VEGF
Vascular endothelial growth factor
- LSEC
liver sinusoidal endothelial cell
REFERENCES
- 1.Burnstock G. Purinergic signalling. Br J Pharmacol. 2006;147 Suppl 1:S172–S181. doi: 10.1038/sj.bjp.0706429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eltzschig HK, Eckle T, Mager A, Kuper N, Karcher C, Weissmuller T, Boengler K, Schulz R, Robson SC, Colgan SP. ATP release from activated neutrophils occurs via connexin 43 and modulates adenosine-dependent endothelial cell function. Circ Res. 2006;99:1100–1108. doi: 10.1161/01.RES.0000250174.31269.70. [DOI] [PubMed] [Google Scholar]
- 3.Boeynaems JM, Communi D, Gonzalez NS, Robaye B. Overview of the P2 receptors. Semin Thromb Hemost. 2005;31:139–149. doi: 10.1055/s-2005-869519. [DOI] [PubMed] [Google Scholar]
- 4.Communi D, Gonzalez NS, Detheux M, Brezillon S, Lannoy V, Parmentier M, Boeynaems JM. Identification of a novel human ADP receptor coupled to G (i) J Biol Chem. 2001;276:41479–41485. doi: 10.1074/jbc.M105912200. [DOI] [PubMed] [Google Scholar]
- 5.Duhant X, Schandene L, Bruyns C, Gonzalez NS, Goldman M, Boeynaems JM, Communi D. Extracellular adenine nucleotides inhibit the activation of human CD4+ T lymphocytes. J Immunol. 2002;169:15–21. doi: 10.4049/jimmunol.169.1.15. [DOI] [PubMed] [Google Scholar]
- 6.Marteau F, Gonzalez NS, Communi D, Goldman M, Boeynaems JM, Communi D. Thrombospondin-1 and indoleamine 2,3-dioxygenase are major targets of extracellular ATP in human dendritic cells. Blood. 2005;106:3860–3866. doi: 10.1182/blood-2005-05-1843. [DOI] [PubMed] [Google Scholar]
- 7.Marteau F, Le Poul E, Communi D, Communi D, Labouret C, Savi P, Boeynaems JM, Gonzalez NS. Pharmacological characterization of the human P2Y13 receptor. Mol Pharmacol. 2003;64:104–112. doi: 10.1124/mol.64.1.104. [DOI] [PubMed] [Google Scholar]
- 8.Mizumoto N, Kumamoto T, Robson SC, Sevigny J, Matsue H, Enjyoji K, Takashima A. CD39 is the dominant Langerhans cell-associated ecto-NTPDase: modulatory roles in inflammation and immune responsiveness. Nat Med. 2002;8:358–365. doi: 10.1038/nm0402-358. [DOI] [PubMed] [Google Scholar]
- 9.Robson SC, Wu Y, Sun X, Knosalla C, Dwyer K, Enjyoji K. Ectonucleotidases of CD39 family modulate vascular inflammation and thrombosis in transplantation. Semin Thromb Hemost. 2005;31:217–233. doi: 10.1055/s-2005-869527. [DOI] [PubMed] [Google Scholar]
- 10.Lajtha LG, Vane JR. Dependence of bone marrow cells on the liver for purine supply. Nature. 1958;182:191–192. doi: 10.1038/182191a0. [DOI] [PubMed] [Google Scholar]
- 11.Che M, Gatmaitan Z, Arias IM. Ectonucleotidases, purine nucleoside transporter, and function of the bile canalicular plasma membrane of the hepatocyte. Faseb J. 1997;11:101–108. doi: 10.1096/fasebj.11.2.9039951. [DOI] [PubMed] [Google Scholar]
- 12.Novikoff AB, Essner E. The liver cell. Some new approaches to its study. Am J Med. 1960;29:102–131. doi: 10.1016/0002-9343(60)90011-5. [DOI] [PubMed] [Google Scholar]
- 13.Imai M, Takigami K, Guckelberger O, Lin Y, Sevigny J, Kaczmarek E, Goepfert C, Enjyoji K, Bach FH, Rosenberg RD, Robson SC. CD39/vascular ATP diphosphohydrolase modulates xenograft survival. Transplant Proc. 2000;32:969. doi: 10.1016/s0041-1345(00)01065-4. [DOI] [PubMed] [Google Scholar]
- 14.Burnstock G, Knight GE. Cellular distribution and functions of P2 receptor subtypes in different systems. Elsevier. 2004 doi: 10.1016/S0074-7696(04)40002-3. [DOI] [PubMed] [Google Scholar]
- 15.Bodin P, Burnstock G. Evidence that release of adenosine triphosphate from endothelial cells during increased shear stress is vesicular. J Cardiovasc Pharmacol. 2001;38:900–908. doi: 10.1097/00005344-200112000-00012. [DOI] [PubMed] [Google Scholar]
- 16.Carabelli V, Carra I, Carbone E. Localized secretion of ATP and opioids revealed through single Ca2+ channel modulation in bovine chromaffin cells. Neuron. 1998;20:1255–1268. doi: 10.1016/s0896-6273(00)80505-x. [DOI] [PubMed] [Google Scholar]
- 17.Luthje J. Origin, metabolism and function of extracellular adenine nucleotides in the blood. Klin Wochenschr. 1989;67:317–327. doi: 10.1007/BF01741386. [DOI] [PubMed] [Google Scholar]
- 18.Abraham EH, Prat AG, Gerweck L, Seneveratne T, Arceci RJ, Kramer R, Guidotti G, Cantiello HF. The multidrug resistance (mdr1) gene product functions as an ATP channel. Proc Natl Acad Sci U S A. 1993;90:312–316. doi: 10.1073/pnas.90.1.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grierson JP, Meldolesi J. Shear stress-induced [Ca2+]i transients and oscillations in mouse fibroblasts are mediated by endogenously released ATP. J Biol Chem. 1995;270:4451–4456. doi: 10.1074/jbc.270.9.4451. [DOI] [PubMed] [Google Scholar]
- 20.Tsujimoto Y. Apoptosis and necrosis: intracellular ATP level as a determinant for cell death modes. Cell Death Differ. 1997;4:429–434. doi: 10.1038/sj.cdd.4400262. [DOI] [PubMed] [Google Scholar]
- 21.Abbracchio MP, Ceruti S, Bolego C, Puglisi L, Burnstock G, Cattabeni F. Trophic roles of P2 purinoceptors in central nervous system astroglial cells. Ciba Found Symp. 1996;198:142–147. doi: 10.1002/9780470514900.ch8. discussion 147–8. [DOI] [PubMed] [Google Scholar]
- 22.Haussinger D, Stehle T, Gerok W, Tran-Thi TA, Decker K. Hepatocyte heterogeneity in response to extracellular ATP. Eur J Biochem. 1987;169:645–650. doi: 10.1111/j.1432-1033.1987.tb13656.x. [DOI] [PubMed] [Google Scholar]
- 23.Gatof D, Kilic G, Fitz JG. Vesicular exocytosis contributes to volume-sensitive ATP release in biliary cells. Am J Physiol Gastrointest Liver Physiol. 2004;286:G538–G546. doi: 10.1152/ajpgi.00355.2003. [DOI] [PubMed] [Google Scholar]
- 24.Feranchak AP, Fitz JG, Roman RM. Volume-sensitive purinergic signaling in human hepatocytes. J Hepatol. 2000;33:174–182. doi: 10.1016/s0168-8278(00)80357-8. [DOI] [PubMed] [Google Scholar]
- 25.Che M, Nishida T, Gatmaitan Z, Arias IM. A nucleoside transporter is functionally linked to ectonucleotidases in rat liver canalicular membrane. J Biol Chem. 1992;267:9684–9688. [PubMed] [Google Scholar]
- 26.Harden TK, Lazarowski ER, Boucher RC. Release, metabolism and interconversion of adenine and uridine nucleotides: implications for G protein-coupled P2 receptor agonist selectivity. Trends in Pharmacological Sciences. 1997;18:43–46. [PubMed] [Google Scholar]
- 27.Weisman GA, Erb L, Garrad RC, Theiss PM, Santiago-Perez LI, Flores RV, Santos-Berrios C, Mendez Y, Gonzalez FA. P2Y nucleotide receptors in the immune system: Signaling by a P2Y (2) receptor in U937 monocytes. Drug Development Research. 1998;45:222–228. [Google Scholar]
- 28.Buell G, Collo G, Rassendren F. P2X RECEPTORS - AN EMERGING CHANNEL FAMILY [Review] European Journal of Neuroscience. 1996;8:2221–2228. doi: 10.1111/j.1460-9568.1996.tb00745.x. [DOI] [PubMed] [Google Scholar]
- 29.Fredholm BB, Abbracchio MP, Burnstock G, Daly JW, Harden TK, Jacobsen KA, Leff P, Williams M. Nomenclature and classification of purinoreceptors. Pharmacol Rev. 1994;46:143–152. [PMC free article] [PubMed] [Google Scholar]
- 30.Adair TH. Growth regulation of the vascular system: an emerging role for adenosine. Am J Physiol Regul Integr Comp Physiol. 2005;289:R283–R296. doi: 10.1152/ajpregu.00840.2004. [DOI] [PubMed] [Google Scholar]
- 31.Linden J. Molecular approach to adenosine receptors: receptor-mediated mechanisms of tissue protection. Annu Rev Pharmacol Toxicol. 2001;41:775–787. doi: 10.1146/annurev.pharmtox.41.1.775. [DOI] [PubMed] [Google Scholar]
- 32.Boeynaems JMJD. Pearson: P2 purinoceptors on vascular endothelial cells: physiological significance and transduction mechanisms. Trends Pharmacol Sci. 1990;11:34–37. doi: 10.1016/0165-6147(90)90039-b. [DOI] [PubMed] [Google Scholar]
- 33.Dubyak GRC. el-Moatassim: Signal transduction via P2-purinergic receptors for extracellular ATP and other nucleotides. Am J Physiol. 1993;265:C577–C606. doi: 10.1152/ajpcell.1993.265.3.C577. [DOI] [PubMed] [Google Scholar]
- 34.Brake AJ, Julius D. Signaling by extracellular nucleotides. Annu Rev Cell Dev Biol. 1996;12:519–541. doi: 10.1146/annurev.cellbio.12.1.519. [DOI] [PubMed] [Google Scholar]
- 35.Franceschi C, Abbracchio MP, Barbieri D, Ceruti S, Ferrari D, Iliou JP, Rounds S, Schubert P, Schulzelohoff E, Rassendren FA, Staub M, Volonte C, Wakade AR, Burnstock G. Purines and cell death. Drug Development Research. 1996;39:442–449. [Google Scholar]
- 36.Fijnheer R, Boomgaard MN, van den Eertwegh AJ, Homburg CH, Gouwerok CW, Veldman HA, Roos D, de Korte D. Stored platelets release nucleotides as inhibitors of platelet function. Thromb Haemost. 1992;68:595–599. [PubMed] [Google Scholar]
- 37.Hechler B, Leon C, Vial C, Vigne P, Frelin C, Cazenave JP, Gachet C. The P2Y1 receptor is necessary for adenosine 5'-diphosphate-induced platelet aggregation. Blood. 1998;92:152–159. [PubMed] [Google Scholar]
- 38.Sharis PJ, Cannon CP, Loscalzo J. THE ANTIPLATELET EFFECTS OF TICLOPIDINE AND CLOPIDOGREL [Review] Annals of Internal Medicine. 1998;129:394–405. doi: 10.7326/0003-4819-129-5-199809010-00009. [DOI] [PubMed] [Google Scholar]
- 39.Yang S, Cheek DJ, Westfall DP, Buxton IL. Purinergic axis in cardiac blood vessels. Agonist-mediated release of ATP from cardiac endothelial cells. Circ Res. 1994;74:401–407. doi: 10.1161/01.res.74.3.401. [DOI] [PubMed] [Google Scholar]
- 40.Denlinger LC, Fisette PL, Garis KA, Kwon G, Vazquez-Torres A, Simon AD, Nguyen B, Proctor RA, Bertics PJ, Corbett JA. Regulation of inducible nitric oxide synthase expression by macrophage purinoreceptors and calcium. J Biol Chem. 1996;271:337–342. doi: 10.1074/jbc.271.1.337. [DOI] [PubMed] [Google Scholar]
- 41.Marcus AJ, Safier LB. Thromboregulation: multicellular modulation of platelet reactivity in hemostasis and thrombosis. [Review] Faseb Journal. 1993;7:516–522. doi: 10.1096/fasebj.7.6.8472890. [DOI] [PubMed] [Google Scholar]
- 42.Juul B, Plesner L, Aalkjaer C. Effects of ATP and related nucleotides on the tone of isolated rat mesenteric resistance arteries. Journal of Pharmacology & Experimental Therapeutics. 1993;264:1234–1240. [PubMed] [Google Scholar]
- 43.Motte S, Communi D, Pirotton S, Boeynaems JM. Involvement of multiple receptors in the actions of extracellular ATP: the example of vascular endothelial cells. [Review] International Journal of Biochemistry & Cell Biology. 1995;27:1–7. doi: 10.1016/1357-2725(94)00059-x. [DOI] [PubMed] [Google Scholar]
- 44.Cote YP, Picher M, St JP, Beliveau R, Potier M, Beaudoin AR. Identification and localization of ATPdiphosphohydrolase (apyrase) in bovine aorta: relevance to vascular tone and platelet aggregation. Biochimica et Biophysica Acta. 1991;1078:187–191. doi: 10.1016/0167-4838(91)99008-g. [DOI] [PubMed] [Google Scholar]
- 45.Cote YP, Filep JG, Battistini B, Gauvreau J, Sirois P, Beaudoin AR. Characterization of ATP-diphosphohydrolase activities in the intima and media of the bovine aorta: evidence for a regulatory role in platelet activation in vitro. Biochimica et Biophysica Acta. 1992;1139:133–142. doi: 10.1016/0925-4439(92)90092-2. [DOI] [PubMed] [Google Scholar]
- 46.Enjyoji K, Sevigny J, Lin Y, Frenette PS, Christie PD, Esch JSA, Imai M, Edelberg JM, Rayburn H, Lech M, Beeler DL, Csizmadia E, Wagner DD, Robson SC, Rosenberg RD. Targeted disruption of cd39/ATP diphosphohydrolase results in disordered hemostasis and thromboregulation. Nature Medicine. 1999;5:1010–1017. doi: 10.1038/12447. [DOI] [PubMed] [Google Scholar]
- 47.Weisman GA, Griffin K, Santiago-Perez LI, Liu J, Krugh B, Flores RV, Chorna NE, Santos-Berrios C, Vivas-Mejia PE, Garrad RC, Gonzalez FA, Erb L. P2Y (2) receptors regulate multiple signal transduction pathways in monocytic cells. Drug Development Research. 2001;53:186–192. [Google Scholar]
- 48.Seye CI, Yu N, Gonzalez FA, Erb L, Weisman GA. The P2Y2 nucleotide receptor mediates vascular cell adhesion molecule-1 expression through interaction with VEGF receptor-2 (KDR/Flk-1) J Biol Chem. 2004;279:35679–35686. doi: 10.1074/jbc.M401799200. [DOI] [PubMed] [Google Scholar]
- 49.Chow SC, Kass G, Orrenius S. Purines and their roles in apoptosis. Neuropharmacology. 1997;36:1149–1156. doi: 10.1016/s0028-3908(97)00123-8. [DOI] [PubMed] [Google Scholar]
- 50.Kaczmarek E, Koziak K, Sevigny J, Siegel JB, Anrather J, Beaudoin AR, Bach FH, Robson SC. Identification and characterization of CD39/vascular ATP diphosphohydrolase. J Biol Chem. 1996;271:33116–33122. doi: 10.1074/jbc.271.51.33116. [DOI] [PubMed] [Google Scholar]
- 51.Frassetto SS, Dias RD, Sarkis JJ. Characterization of an ATP diphosphohydrolase activity (APYRASE, EC 3.6.1.5) in rat blood platelets. Molecular & Cellular Biochemistry. 1993;129:47–55. doi: 10.1007/BF00926575. [DOI] [PubMed] [Google Scholar]
- 52.Ferrari D, Chiozzi P, Falzoni S, Dal Susino M, Melchiorri L, Baricordi OR, Di Virgilio F. Extracellular ATP triggers IL-1 beta release by activating the purinergic P2Z receptor of human macrophages. J Immunol. 1997;159:1451–1458. [PubMed] [Google Scholar]
- 53.VonAlbertini M, Palmetshofer A, Kaczmarek E, Koziak K, Stroka D, Grey ST, Stuhlmeier KM, Robson SC. Extracellular atp and adp activate transcription factor nf-kappa-b and induce endothelial cell apoptosis. Biochemical and Biophysical Research Communications. 1998;248:822–829. doi: 10.1006/bbrc.1998.9055. [DOI] [PubMed] [Google Scholar]
- 54.Hourani SMO, Hall DA. Receptors for ADP on human blood platelets. TIPS. 1994;15:103–107. doi: 10.1016/0165-6147(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 55.Lages B, Weiss HJ. Enhanced Increases in Cytosolic Ca2+ in ADP-Stimulated Platelets From Patients With Delta-Storage Pool Deficiency - a Possible Indicator of Interactions Between Granule-Bound ADP and the Membrane ADP Receptor. Thrombosis & Haemostasis. 1997;77:376–382. [PubMed] [Google Scholar]
- 56.Enjyoji K, Sevigny J, Lin Y, Frenette PS, Christie PD, Esch JSA, Imai M, Edelberg JM, Rayburn H, Lech M, Beeler DL, Csizmadia E, Wagner DD, Robson SC, Rosenberg RD. Targeted disruption of cd39/ATP diphosphohydrolase results in disordered hemostasis and thromboregulation. Nature Medicine. 1999;5:1010–1017. doi: 10.1038/12447. [DOI] [PubMed] [Google Scholar]
- 57.Koshimizu T, Tomic M, Koshimizu M, Stojilkovic SS. Identification of Amino Acid Residues Contributing to Desensitization of the P2x (2) Receptor Channel. Journal of Biological Chemistry. 1998;273:12853–12857. doi: 10.1074/jbc.273.21.12853. [DOI] [PubMed] [Google Scholar]
- 58.Clarke LL, Harline MC, Otero MA, Glover GG, Garrad RC, Krugh B, Walker NM, Gonzalez FA, Turner JT, Weisman GA. Desensitization of P2Y2 receptor-activated transepithelial anion secretion. Am J Physiol. 1999;276:C777–C787. doi: 10.1152/ajpcell.1999.276.4.C777. [DOI] [PubMed] [Google Scholar]
- 59.Plesner L. Ecto-ATPases: identities and functions. Int Rev Cytol. 1995;158:141–214. doi: 10.1016/s0074-7696(08)62487-0. [DOI] [PubMed] [Google Scholar]
- 60.Salter KD, Fitz JG, Roman RM. Domain-specific purinergic signaling in polarized rat cholangiocytes. Am J Physiol Gastrointest Liver Physiol. 2000;278:G492–G500. doi: 10.1152/ajpgi.2000.278.3.G492. [DOI] [PubMed] [Google Scholar]
- 61.Turchini JP. [Application of technics for the demonstration of adenosine triphosphatase and adenosine-5-phosphatase activities in the liver of normal and cold-exposed mice.] Ann Histochim. 1960;5:31–44. [PubMed] [Google Scholar]
- 62.Stefan C, Jansen S, Bollen M. NPP-type ectophosphodiesterases: unity in diversity. Trends Biochem Sci. 2005;30:542–550. doi: 10.1016/j.tibs.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 63.Goding JW, Terkeltaub R, Maurice M, Deterre P, Sali A, Belli SI. Ectophosphodiesterase/pyrophosphatase of lymphocytes and non-lymphoid cells: structure and function of the PC-1 family. Immunol Rev. 1998;161:11–26. doi: 10.1111/j.1600-065x.1998.tb01568.x. [DOI] [PubMed] [Google Scholar]
- 64.Yano Y, Hayashi Y, Sano K, Nagano H, Nakaji M, Seo Y, Ninomiya T, Yoon S, Yokozaki H, Kasuga M. Expression and localization of ecto-nucleotide pyrophosphatase/phosphodiesterase I-1 (E-NPP1/PC-1) and −3 (E-NPP3/CD203c/PD-Ibeta/B10/gp130 (RB13-6)) in inflammatory and neoplastic bile duct diseases. Cancer Lett. 2004;207:139–147. doi: 10.1016/j.canlet.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 65.Scott LJ, Delautier D, Meerson NR, Trugnan G, Goding JW, Maurice M. Biochemical and molecular identification of distinct forms of alkaline phosphodiesterase I expressed on the apical and basolateral plasma membrane surfaces of rat hepatocytes. Hepatology. 1997;25:995–1002. doi: 10.1002/hep.510250434. [DOI] [PubMed] [Google Scholar]
- 66.Stefan C, Stalmans W, Bollen M. Growth-related expression of the ectonucleotide pyrophosphatase PC-1 in rat liver. Hepatology. 1998;28:1497–1503. doi: 10.1002/hep.510280608. [DOI] [PubMed] [Google Scholar]
- 67.Thevananther S, Sun H, Li D, Arjunan V, Awad SS, Wyllie S, Zimmerman TL, Goss JA, Karpen SJ. Extracellular ATP activates c-jun N-terminal kinase signaling and cell cycle progression in hepatocytes. Hepatology. 2004;39:393–402. doi: 10.1002/hep.20075. [DOI] [PubMed] [Google Scholar]
- 68.Beumer C, Wulferink M, Raaben W, Fiechter D, Brands R, Seinen W. Calf intestinal alkaline phosphatase, a novel therapeutic drug for lipopolysaccharide (LPS)-mediated diseases, attenuates LPS toxicity in mice and piglets. J Pharmacol Exp Ther. 2003;307:737–744. doi: 10.1124/jpet.103.056606. [DOI] [PubMed] [Google Scholar]
- 69.Zimmermann H. 5'-Nucleotidase: molecular structure and functional aspects. Biochem J. 1992;285(Pt 2):345–365. doi: 10.1042/bj2850345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schmid TC, Loffing J, Le Hir M, Kaissling B. Distribution of ecto-5'-nucleotidase in the rat liver: effect of anaemia. Histochemistry. 1994;101:439–447. doi: 10.1007/BF00269494. [DOI] [PubMed] [Google Scholar]
- 71.Fausther M, Lecka J, Kukulski F, Levesque SA, Pelletier J, Zimmermann H, Dranoff JA, Sevigny J. Cloning, purification and identification of the liver canalicular ecto-ATPase as NTPDase8. Am J Physiol Gastrointest Liver Physiol. 2006 doi: 10.1152/ajpgi.00293.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Keppens S, De Wulf H. P2-purinergic control of liver glycogenolysis. Biochem J. 1985;231:797–799. doi: 10.1042/bj2310797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Keppens S, De Wulf H. Some P2 purinergic agonists increase cytosolic calcium but not inositol 1,4,5-trisphosphate in isolated rat hepatocytes. Biochim Biophys Acta. 1995;1269:316–322. doi: 10.1016/0167-4889(95)00132-7. [DOI] [PubMed] [Google Scholar]
- 74.Jacquet S, Malaval C, Martinez LO, Sak K, Rolland C, Perez C, Nauze M, Champagne E, Terce F, Gachet C, Perret B, Collet X, Boeynaems JM, Barbaras R. The nucleotide receptor P2Y13 is a key regulator of hepatic high-density lipoprotein (HDL) endocytosis. Cell Mol Life Sci. 2005;62:2508–2515. doi: 10.1007/s00018-005-5194-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dixon CJ, Woods NM, Webb TE, Green AK. Evidence that rat hepatocytes co-express functional P2Y1 and P2Y2 receptors. Br J Pharmacol. 2000;129:764–770. doi: 10.1038/sj.bjp.0703103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dixon CJ. Evidence that 2-methylthioATP and 2-methylthioADP are both agonists at the rat hepatocyte P2Y (1) receptor. Br J Pharmacol. 2000;130:664–668. doi: 10.1038/sj.bjp.0703350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dixon CJ, Hall JF, Boarder MR. ADP stimulation of inositol phosphates in hepatocytes: role of conversion to ATP and stimulation of P2Y2 receptors. Br J Pharmacol. 2003;138:272–278. doi: 10.1038/sj.bjp.0705016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Capiod T. ATP-activated cation currents in single guinea-pig hepatocytes. J Physiol. 1998;507(Pt 3):795–805. doi: 10.1111/j.1469-7793.1998.795bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Glavy JS, Wu SM, Wang PJ, Orr GA, Wolkoff AW. Down-regulation by extracellular ATP of rat hepatocyte organic anion transport is mediated by serine phosphorylation of oatp1. J Biol Chem. 2000;275:1479–1484. doi: 10.1074/jbc.275.2.1479. [DOI] [PubMed] [Google Scholar]
- 80.Schofl C, Ponczek M, Mader T, Waring M, Benecke H, von zur Muhlen A, Mix H, Cornberg M, Boker KH, Manns MP, Wagner S. Regulation of cytosolic free calcium concentration by extracellular nucleotides in human hepatocytes. Am J Physiol. 1999;276:G164–G172. doi: 10.1152/ajpgi.1999.276.1.G164. [DOI] [PubMed] [Google Scholar]
- 81.Keppens S, Vandekerckhove A, De Wulf H. Extracellular ATP and UTP exert similar effects on rat isolated hepatocytes. Br J Pharmacol. 1992;105:475–479. doi: 10.1111/j.1476-5381.1992.tb14278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dixon CJ, Hall JF, Webb TE, Boarder MR. Regulation of rat hepatocyte function by P2Y receptors: focus on control of glycogen phosphorylase and cyclic AMP by 2-methylthioadenosine 5'-diphosphate. J Pharmacol Exp Ther. 2004;311:334–341. doi: 10.1124/jpet.104.067744. [DOI] [PubMed] [Google Scholar]
- 83.Dranoff JA, Masyuk AI, Kruglov EA, LaRusso NF, Nathanson MH. Polarized expression and function of P2Y ATP receptors in rat bile duct epithelia. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1059–G1067. doi: 10.1152/ajpgi.2001.281.4.G1059. [DOI] [PubMed] [Google Scholar]
- 84.Huang BQ, Masyuk TV, Muff MA, Tietz PS, Masyuk AI, Larusso NF. Isolation and characterization of cholangiocyte primary cilia. Am J Physiol Gastrointest Liver Physiol. 2006;291:G500–G509. doi: 10.1152/ajpgi.00064.2006. [DOI] [PubMed] [Google Scholar]
- 85.Doctor RB, Matzakos T, McWilliams R, Johnson S, Feranchak AP, Fitz JG. Purinergic regulation of cholangiocyte secretion: identification of a novel role for P2X receptors. Am J Physiol Gastrointest Liver Physiol. 2005;288:G779–G786. doi: 10.1152/ajpgi.00325.2004. [DOI] [PubMed] [Google Scholar]
- 86.Lin SH, Guidotti G. Cloning and expression of a cDNA coding for a rat liver plasma membrane ecto-ATPase. The primary structure of the ecto-ATPase is similar to that of the human biliary glycoprotein I. J Biol Chem. 1989;264:14408–14414. [PubMed] [Google Scholar]
- 87.Lin SH, Culic O, Flanagan D, Hixson DC. Immunochemical characterization of two isoforms of rat liver ecto-ATPase that show an immunological and structural identity with a glycoprotein cell-adhesion molecule with Mr 105,000. Biochem J. 1991;278(Pt 1):155–161. doi: 10.1042/bj2780155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Knowles AF. The rat liver ecto-ATPase/C-CAM cDNA detects induction of carcinoembryonic antigen but not the mercurial-insensitive ecto-ATPase in human hepatoma Li-7A cells treated by epidermal growth factor and cholera toxin. Biochem Biophys Res Commun. 1995;207:529–535. doi: 10.1006/bbrc.1995.1220. [DOI] [PubMed] [Google Scholar]
- 89.Bigonnesse F, Levesque SA, Kukulski F, Lecka J, Robson SC, Fernandes MJ, Sevigny J. Cloning and characterization of mouse nucleoside triphosphate diphosphohydrolase-8. Biochemistry. 2004;43:5511–5519. doi: 10.1021/bi0362222. [DOI] [PubMed] [Google Scholar]
- 90.Nagy AK, Knowles AF, Nagami GT. Molecular cloning of the chicken oviduct ecto-ATP-diphosphohydrolase. J Biol Chem. 1998;273:16043–16049. doi: 10.1074/jbc.273.26.16043. [DOI] [PubMed] [Google Scholar]
- 91.Knowles AF, Nagy AK, Strobel RS, Wu-Weis M. Purification, characterization, cloning, and expression of the chicken liver ecto-ATP-diphosphohydrolase. Eur J Biochem. 2002;269:2373–2382. doi: 10.1046/j.1432-1033.2002.02898.x. [DOI] [PubMed] [Google Scholar]
- 92.Minamiyama Y, Takemura S, Kawada N, Inoue M. Role of nitric oxide in extracellular nucleotide-induced contractile status of assorted vessels including parts of the portal vasculature. J Hepatol. 1998;28:314–319. doi: 10.1016/0168-8278(88)80019-9. [DOI] [PubMed] [Google Scholar]
- 93.Malmsjo M, Edvinsson L, Erlinge D. P2X receptors counteract the vasodilatory effects of endothelium derived hyperpolarising factor. Eur J Pharmacol. 2000;390:173–180. doi: 10.1016/s0014-2999(00)00010-8. [DOI] [PubMed] [Google Scholar]
- 94.Wilkinson GF, McKechnie K, Dainty IA, Boarder MR. P2Y purinoceptor and nucleotide receptor-induced relaxation of precontracted bovine aortic collateral artery rings: differential sensitivity to suramin and indomethacin. J Pharmacol Exp Ther. 1994;268:881–887. [PubMed] [Google Scholar]
- 95.Hashimoto N, Watanabe T, Shiratori Y, Ikeda Y, Kato H, Han K, Yamada H, Toda G, Kurokawa K. Prostanoid secretion by rat hepatic sinusoidal endothelial cells and its regulation by exogenous adenosine triphosphate. Hepatology. 1995;21:1713–1718. [PubMed] [Google Scholar]
- 96.Phillips JK, McLean AJ, Hill CE. Receptors involved in nerve-mediated vasoconstriction in small arteries of the rat hepatic mesentery. Br J Pharmacol. 1998;124:1403–1412. doi: 10.1038/sj.bjp.0701976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vial C, Evans RJ. P2X (1) receptor-deficient mice establish the native P2X receptor and a P2Y6-like receptor in arteries. Mol Pharmacol. 2002;62:1438–1445. doi: 10.1124/mol.62.6.1438. [DOI] [PubMed] [Google Scholar]
- 98.Pacaud P, Gregoire G, Loirand G. Release of Ca2+ from intracellular store in smooth muscle cells of rat portal vein by ATP-induced Ca2+ entry. Br J Pharmacol. 1994;113:457–462. doi: 10.1111/j.1476-5381.1994.tb17011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Orre M, Pennefather JN, Story ME, Haynes JM. The effects of P2 purinoceptor agonists on the isolated portal vein of the guinea pig. Eur J Pharmacol. 1996;316:229–236. doi: 10.1016/s0014-2999(96)00687-5. [DOI] [PubMed] [Google Scholar]
- 100.Ishizaki M, Iizuka Y, Suzuki-Kusaba M, Kimura T, Satoh S. Nonadrenergic contractile response of guinea pig portal vein to electrical field stimulation mimics response to UTP but not to ATP. J Cardiovasc Pharmacol. 1997;29:360–366. doi: 10.1097/00005344-199703000-00009. [DOI] [PubMed] [Google Scholar]
- 101.Mironneau J, Coussin F, Morel JL, Barbot C, Jeyakumar LH, Fleischer S, Mironneau C. Calcium signalling through nucleotide receptor P2X1 in rat portal vein myocytes. J Physiol. 2001;536:339–350. doi: 10.1111/j.1469-7793.2001.0339c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Handa M, Guidotti G. Purification and cloning of a soluble ATP-diphosphohydrolase (apyrase) from potato tubers (Solanum tuberosum) Biochem Biophys Res Commun. 1996;218:916–923. doi: 10.1006/bbrc.1996.0162. [DOI] [PubMed] [Google Scholar]
- 103.Sevigny J, Robson SC, Waelkens E, Csizmadia E, Smith RN, Lemmens R. Identification and characterization of a novel hepatic canalicular ATP diphosphohydrolase. J Biol Chem. 2000;275:5640–5647. doi: 10.1074/jbc.275.8.5640. [DOI] [PubMed] [Google Scholar]
- 104.Kansas GS, Wood GS, Tedder TF. Expression, distribution, and biochemistry of human CD39. Role in activation-associated homotypic adhesion of lymphocytes. J Immunol. 1991;146:2235–2244. [PubMed] [Google Scholar]
- 105.Marcus AJ, Broekman MJ, Drosopoulos JH, Islam N, Alyonycheva TN, Safier LB, Hajjar KA, Posnett DN, Schoenborn MA, Schooley KA, Gayle RB, Maliszewski CR. The endothelial cell ecto-ADPase responsible for inhibition of platelet function is CD39. J Clin Invest. 1997;99:1351–1360. doi: 10.1172/JCI119294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Favaloro EJ. Differential expression of surface antigens on activated endothelium. Immunol Cell Biol. 1993;(Pt 6):571–581. doi: 10.1038/icb.1993.63. [DOI] [PubMed] [Google Scholar]
- 107.Wang TF, Guidotti G. CD39 is an ecto-(Ca2+,Mg2+)-apyrase. J Biol Chem. 1996;271:9898–9901. [PubMed] [Google Scholar]
- 108.Dranoff JA, Ogawa M, Kruglov EA, Gaca MD, Sevigny J, Robson SC, Wells RG. Expression of P2Y nucleotide receptors and ectonucleotidases in quiescent and activated rat hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2004;287:G417–G424. doi: 10.1152/ajpgi.00294.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Athari A, Hanecke K, Jungermann K. Prostaglandin F2 alpha and D2 release from primary Ito cell cultures after stimulation with noradrenaline and ATP but not adenosine. Hepatology. 1994;20:142–148. doi: 10.1016/0270-9139(94)90146-5. [DOI] [PubMed] [Google Scholar]
- 110.Takemura S, Kawada N, Hirohashi K, Kinoshita H, Inoue M. Nucleotide receptors in hepatic stellate cells of the rat. FEBS Lett. 1994;354:53–56. doi: 10.1016/0014-5793(94)01090-0. [DOI] [PubMed] [Google Scholar]
- 111.Kruglov E, Correa P, Arora G, Yu J, Nathanson MH, Dranoff JA. Molecular Basis for Calcium Signaling in Hepatic Stellate Cells. Am J Physiol Gastrointest Liver Physiol. 2007 doi: 10.1152/ajpgi.00401.2006. [DOI] [PubMed] [Google Scholar]
- 112.Chadwick BP, Frischauf AM. Cloning and mapping of a human and mouse gene with homology to ecto-ATPase genes. Mamm Genome. 1997;8:668–672. doi: 10.1007/s003359900534. [DOI] [PubMed] [Google Scholar]
- 113.Smith TM, Carl SA, Kirley TL. Immunological detection of ecto-ATPase in chicken and rat tissues: characterization, distribution, and a cautionary note. Biochem Mol Biol Int. 1998;45:1057–1066. doi: 10.1002/iub.7510450523. [DOI] [PubMed] [Google Scholar]
- 114.Smith TM, Kirley TL. Cloning, sequencing, and expression of a human brain ecto-apyrase related to both the ecto-ATPases and CD39 ecto-apyrases1. Biochim Biophys Acta. 1998;1386:65–78. doi: 10.1016/s0167-4838(98)00063-6. [DOI] [PubMed] [Google Scholar]
- 115.Dranoff JA, Kruglov EA, Robson SC, Braun N, Zimmermann H, Sevigny J. The ecto-nucleoside triphosphate diphosphohydrolase NTPDase2/CD39L1 is expressed in a novel functional compartment within the liver. Hepatology. 2002;36:1135–1144. doi: 10.1053/jhep.2002.36823. [DOI] [PubMed] [Google Scholar]
- 116.Jhandier MN, Kruglov EA, Lavoie EG, Sevigny J, Dranoff JA. Portal fibroblasts regulate the proliferation of bile duct epithelia via expression of NTPDase2. J Biol Chem. 2005;280:22986–22992. doi: 10.1074/jbc.M412371200. [DOI] [PubMed] [Google Scholar]
- 117.Dranoff JA, Kruglov EA, Toure J, Braun N, Zimmermann H, Jain D, Knowles AF, Sevigny J. Ectonucleotidase NTPDase2 is selectively down-regulated in biliary cirrhosis. J Investig Med. 2004;52:475–482. doi: 10.1136/jim-52-07-42. [DOI] [PubMed] [Google Scholar]
- 118.Knowles AF, Chiang WC. Enzymatic and transcriptional regulation of human ecto-ATPase/E-NTPDase 2. Arch Biochem Biophys. 2003;418:217–227. doi: 10.1016/j.abb.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 119.Wood E, Johan Broekman M, Kirley TL, Diani-Moore S, Tickner M, Drosopoulos JH, Islam N, Park JI, Marcus AJ, Rifkind AB. Cell-type specificity of ectonucleotidase expression and upregulation by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Arch Biochem Biophys. 2002;407:49–62. doi: 10.1016/s0003-9861(02)00465-4. [DOI] [PubMed] [Google Scholar]
- 120.del Rey A, Renigunta V, Dalpke AH, Leipziger J, Matos JE, Robaye B, Zuzarte M, Kavelaars A, Hanley PJ. Knock-out mice reveal the contributions of P2Y and P2X receptors to nucleotide-induced Ca2+ signaling in macrophages. J Biol Chem. 2006;281:35147–35155. doi: 10.1074/jbc.M607713200. [DOI] [PubMed] [Google Scholar]
- 121.da Cruz CM, Ventura AL, Schachter J, Costa-Junior HM, da Silva Souza HA, Gomes FR, Coutinho-Silva R, Ojcius DM, Persechini PM. Activation of ERK1/2 by extracellular nucleotides in macrophages is mediated by multiple P2 receptors independently of P2X7-associated pore or channel formation. Br J Pharmacol. 2006;147:324–334. doi: 10.1038/sj.bjp.0706559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bowler JW, Bailey RJ, North RA, Surprenant A. P2X4, P2Y1 and P2Y2 receptors on rat alveolar macrophages. Br J Pharmacol. 2003;140:567–575. doi: 10.1038/sj.bjp.0705459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Stober CB, Lammas DA, Li CM, Kumararatne DS, Lightman SL, McArdle CA. ATP-mediated killing of Mycobacterium bovis bacille Calmette-Guerin within human macrophages is calcium dependent and associated with the acidification of mycobacteria-containing phagosomes. J Immunol. 2001;166:6276–6286. doi: 10.4049/jimmunol.166.10.6276. [DOI] [PubMed] [Google Scholar]
- 124.Li CM, Campbell SJ, Kumararatne DS, Hill AV, Lammas DA. Response heterogeneity of human macrophages to ATP is associated with P2X7 receptor expression but not to polymorphisms in the P2RX7 promoter. FEBS Lett. 2002;531:127–131. doi: 10.1016/s0014-5793(02)03424-5. [DOI] [PubMed] [Google Scholar]
- 125.Koshlukova SE, Araujo MW, Baev D, Edgerton M. Released ATP is an extracellular cytotoxic mediator in salivary histatin 5-induced killing of Candida albicans. Infect Immun. 2000;68:6848–6856. doi: 10.1128/iai.68.12.6848-6856.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Coutinho-Silva R, Perfettini JL, Persechini PM, Dautry-Varsat A, Ojcius DM. Modulation of P2Z/P2X (7) receptor activity in macrophages infected with Chlamydia psittaci. Am J Physiol Cell Physiol. 2001;280:C81–C89. doi: 10.1152/ajpcell.2001.280.1.C81. [DOI] [PubMed] [Google Scholar]
- 127.Fairbairn IP, Stober CB, Kumararatne DS, Lammas DA. ATP-mediated killing of intracellular mycobacteria by macrophages is a P2X (7)-dependent process inducing bacterial death by phagosome-lysosome fusion. J Immunol. 2001;167:3300–3307. doi: 10.4049/jimmunol.167.6.3300. [DOI] [PubMed] [Google Scholar]
- 128.Kusner DJ, Barton JA. ATP stimulates human macrophages to kill intracellular virulent Mycobacterium tuberculosis via calcium-dependent phagosome-lysosome fusion. J Immunol. 2001;167:3308–3315. doi: 10.4049/jimmunol.167.6.3308. [DOI] [PubMed] [Google Scholar]
- 129.Shemon AN, Sluyter R, Fernando SL, Clarke AL, Dao-Ung LP, Skarratt KK, Saunders BM, Tan KS, Gu BJ, Fuller SJ, Britton WJ, Petrou S, Wiley JS. A Thr357 to Ser polymorphism in homozygous and compound heterozygous subjects causes absent or reduced P2X7 function and impairs ATP-induced mycobacterial killing by macrophages. J Biol Chem. 2006;281:2079–2086. doi: 10.1074/jbc.M507816200. [DOI] [PubMed] [Google Scholar]
- 130.Gu BJ, Sluyter R, Skarratt KK, Shemon AN, Dao-Ung LP, Fuller SJ, Barden JA, Clarke AL, Petrou S, Wiley JS. An Arg307 to Gln polymorphism within the ATP-binding site causes loss of function of the human P2X7 receptor. J Biol Chem. 2004;279:31287–31295. doi: 10.1074/jbc.M313902200. [DOI] [PubMed] [Google Scholar]
- 131.Wiley JS, Dao-Ung LP, Li C, Shemon AN, Gu BJ, Smart ML, Fuller SJ, Barden JA, Petrou S, Sluyter R. An Ile-568 to Asn polymorphism prevents normal trafficking and function of the human P2X7 receptor. J Biol Chem. 2003;278:17108–17113. doi: 10.1074/jbc.M212759200. [DOI] [PubMed] [Google Scholar]
- 132.Hanley PJ, Musset B, Renigunta V, Limberg SH, Dalpke AH, Sus R, Heeg KM, Preisig-Muller R, Daut J. Extracellular ATP induces oscillations of intracellular Ca2+ and membrane potential and promotes transcription of IL-6 in macrophages. Proc Natl Acad Sci U S A. 2004;101:9479–9484. doi: 10.1073/pnas.0400733101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cui TX, Iwai M, Hamai M, Minokoshi Y, Shimazu T, Horiuchi M. Aggravation of chemically-induced injury in perfused rat liver by extracellular ATP. Life Sci. 2000;66:2593–2601. doi: 10.1016/s0024-3205(00)00593-2. [DOI] [PubMed] [Google Scholar]
- 134.Warny M, Aboudola S, Robson SC, Sevigny J, Communi D, Soltoff SP, Kelly CP. P2Y (6) nucleotide receptor mediates monocyte interleukin-8 production in response to UDP or lipopolysaccharide. J Biol Chem. 2001;276:26051–26056. doi: 10.1074/jbc.M102568200. [DOI] [PubMed] [Google Scholar]
- 135.Conigrave AD, Fernando KC, Gu B, Tasevski V, Zhang W, Luttrell BM, Wiley JS. P2Y (11) receptor expression by human lymphocytes: evidence for two cAMP-linked purinoceptors. Eur J Pharmacol. 2001;426:157–163. doi: 10.1016/s0014-2999(01)01222-5. [DOI] [PubMed] [Google Scholar]
- 136.Scrivens M, Dickenson JM. Functional expression of the P2Y14 receptor in murine T-lymphocytes. Br J Pharmacol. 2005;146:435–444. doi: 10.1038/sj.bjp.0706322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang L, Jacobsen SE, Bengtsson A, Erlinge D. P2 receptor mRNA expression profiles in human lymphocytes, monocytes and CD34+ stem and progenitor cells. BMC Immunol. 2004;5:16. doi: 10.1186/1471-2172-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Glass R, Townsend-Nicholson A, Townsend-Burnstock G. P2 receptors in the thymus: expression of P2X and P2Y receptors in adult rats, an immunohistochemical and in situ hybridisation study. Cell Tissue Res. 2000;300:295–306. doi: 10.1007/s004410000206. [DOI] [PubMed] [Google Scholar]
- 139.Chused TM, Apasov S, Sitkovsky M. Murine T lymphocytes modulate activity of an ATP-activated P2Z-type purinoceptor during differentiation. J Immunol. 1996;157:1371–1380. [PubMed] [Google Scholar]
- 140.Jamieson GP, Snook MB, Thurlow PJ, Wiley JS. Extracellular ATP causes of loss of L-selectin from human lymphocytes via occupancy of P2Z purinocepters. J Cell Physiol. 1996;166:637–642. doi: 10.1002/(SICI)1097-4652(199603)166:3<637::AID-JCP19>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 141.Wiley JS, Chen JR, Snook MB, Jamieson GP. P2Z-purinoceptor of human lymphocytes: actions of nucleotide agonists and irreversible inhibition by oxidized ATP. Br J Pharmacol. 1994;112:946–950. doi: 10.1111/j.1476-5381.1994.tb13172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Markwardt F, Lohn M, Bohm T, Klapperstuck M. Purinoceptor-operated cationic channels in human B lymphocytes. J Physiol. 1997;498(Pt 1):143–151. doi: 10.1113/jphysiol.1997.sp021847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gargett CE, Cornish JE, Wiley JS. ATP, a partial agonist for the P2Z receptor of human lymphocytes. Br J Pharmacol. 1997;122:911–917. doi: 10.1038/sj.bjp.0701447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kawamura H, Aswad F, Minagawa M, Govindarajan S, Dennert G. P2X7 receptors regulate NKT cells in autoimmune hepatitis. J Immunol. 2006;176:2152–2160. doi: 10.4049/jimmunol.176.4.2152. [DOI] [PubMed] [Google Scholar]
- 145.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, Kuchroo VK, Strom TB, Robson SC. Adenosine generation by CD39 and CD73 on T regulatory cells mediates immune suppression. The Journal of Experimental Medicine. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Cutrin JC, Cantino D, Biasi F, Chiarpotto E, Salizzoni M, Andorno E, Massano G, Lanfranco G, Rizzetto M, Boveris A, Poli G. Reperfusion damage to the bile canaliculi in transplanted human liver. Hepatology. 1996;24:1053–1057. doi: 10.1002/hep.510240512. [DOI] [PubMed] [Google Scholar]
- 147.Hoekstra H, Porte RJ, Tian Y, Jochum W, Stieger B, Moritz W, Slooff MJ, Graf R, Clavien PA. Bile salt toxicity aggravates cold ischemic injury of bile ducts after liver transplantation in Mdr2+/−mice. Hepatology. 2006;43:1022–1031. doi: 10.1002/hep.21169. [DOI] [PubMed] [Google Scholar]
- 148.Feranchak AP, Fitz JG. Adenosine triphosphate release and purinergic regulation of cholangiocyte transport. Semin Liver Dis. 2002;22:251–262. doi: 10.1055/s-2002-34503. [DOI] [PubMed] [Google Scholar]
- 149.Chari RS, Schutz SM, Haebig JE, Shimokura GH, Cotton PB, Fitz JG, Meyers WC. Adenosine nucleotides in bile. Am J Physiol. 1996;270:G246–G252. doi: 10.1152/ajpgi.1996.270.2.G246. [DOI] [PubMed] [Google Scholar]
- 150.Keppler D, Arias IM. Hepatic canalicular membrane. Introduction: transport across the hepatocyte canalicular membrane. Faseb J. 1997;11:15–18. doi: 10.1096/fasebj.11.1.9034161. [DOI] [PubMed] [Google Scholar]
- 151.Schlosser SF, Burgstahler AD, Nathanson MH. Isolated rat hepatocytes can signal to other hepatocytes and bile duct cells by release of nucleotides. Proc Natl Acad Sci U S A. 1996;93:9948–9953. doi: 10.1073/pnas.93.18.9948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zimmermann H. Nucleotides and cd39: principal modulatory players in hemostasis and thrombosis. Nat Med. 1999;5:987–988. doi: 10.1038/12419. [DOI] [PubMed] [Google Scholar]
- 153.Henderson JF, Lepage GA. Purine biosynthesis de novo in mouse tissues and a mouse tumor. J Biol Chem. 1959;234:2364–2368. [PubMed] [Google Scholar]
- 154.Keppens S, Vandekerckhove A, De Wulf H. Characterization of purinoceptors present on human liver plasma membranes. FEBS Lett. 1989;248:137–140. doi: 10.1016/0014-5793(89)80448-x. [DOI] [PubMed] [Google Scholar]
- 155.Coade SB, Pearson JD. Metabolism of adenine nucleotides in human blood. Circ Res. 1989;65:531–537. doi: 10.1161/01.res.65.3.531. [DOI] [PubMed] [Google Scholar]
- 156.Keppens S. The complex interaction of ATP and UTP with isolated hepatocytes. How many receptors? Gen Pharmacol. 1993;24:283–289. doi: 10.1016/0306-3623(93)90304-g. [DOI] [PubMed] [Google Scholar]
- 157.Mahmoud MS, Wang P, Chaudry IH. Administration of ATP-MgCl2 following hemorrhage and resuscitation increases hepatic phosphoenolpyruvate carboxykinase and decreases pyruvate kinase activities. Biochim Biophys Acta. 1997;1336:549–556. doi: 10.1016/s0304-4165(97)00069-x. [DOI] [PubMed] [Google Scholar]
- 158.Ichai C, El-Mir MY, Nogueira V, Piquet MA, Chauvin C, Fontaine E, Leverve XM. Exogenous Mg-ATP induces a large inhibition of pyruvate kinase in intact rat hepatocytes. J Biol Chem. 2001;276:6398–6403. doi: 10.1074/jbc.M004169200. [DOI] [PubMed] [Google Scholar]
- 159.Guzman M, Velasco G, Castro J. Effects of extracellular ATP on hepatic fatty acid metabolism. Am J Physiol. 1996;270:G701–G707. doi: 10.1152/ajpgi.1996.270.4.G701. [DOI] [PubMed] [Google Scholar]
- 160.Kahner BN, Shankar H, Murugappan S, Prasad GL, Kunapuli SP. Nucleotide receptor signaling in platelets. J Thromb Haemost. 2006;4:2317–2326. doi: 10.1111/j.1538-7836.2006.02192.x. [DOI] [PubMed] [Google Scholar]
- 161.Dorsam RT, Kunapuli SP. Central role of the P2Y12 receptor in platelet activation. J Clin Invest. 2004;113:340–345. doi: 10.1172/JCI20986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Headrick JP, Hack B, Ashton KJ. Acute adenosinergic cardioprotection in ischemic-reperfused hearts. Am J Physiol Heart Circ Physiol. 2003;285:H1797–H1818. doi: 10.1152/ajpheart.00407.2003. [DOI] [PubMed] [Google Scholar]
- 163.Robson SC, Sun XF, Guckelberger O, Enjyoji K, Imai M. CD39, the vascular ecto-nucleoside triphosphate diphosphohydrolase-1, is a crucial modulator of hepatic ischemia/reperfusion injury. HEPATOLOGY. 2001;672 340A-340A. [Google Scholar]
- 164.Enjyoji K, Sevigny J, Lin Y, Frenette PS, Christie PD, Esch JS, 2nd, Imai M, Edelberg JM, Rayburn H, Lech M, Beeler DL, Csizmadia E, Wagner DD, Robson SC, Rosenberg RD. Targeted disruption of cd39/ATP diphosphohydrolase results in disordered hemostasis and thromboregulation. Nat Med. 1999;5:1010–1017. doi: 10.1038/12447. [DOI] [PubMed] [Google Scholar]
- 165.Ait-Oufella H, Salomon BL, Potteaux S, Robertson AK, Gourdy P, Zoll J, Merval R, Esposito B, Cohen JL, Fisson S, Flavell RA, Hansson GK, Klatzmann D, Tedgui A, Mallat Z. Natural regulatory T cells control the development of atherosclerosis in mice. Nat Med. 2006;5:5. doi: 10.1038/nm1343. [DOI] [PubMed] [Google Scholar]
- 166.Leung JY, Zhu AX, Gordon FD, Pratt DS, Mithoefer A, Garrigan K, Terella A, Hertl M, Cosimi AB, Chung RT. Liver transplantation outcomes for early-stage hepatocellular carcinoma: results of a multicenter study. Liver Transpl. 2004;10:1343–1354. doi: 10.1002/lt.20311. [DOI] [PubMed] [Google Scholar]
- 167.Yoo HY, Patt CH, Geschwind JF, Thuluvath PJ. The outcome of liver transplantation in patients with hepatocellular carcinoma in the United States between 1988 and 2001: 5-year survival has improved significantly with time. J Clin Oncol. 2003;21:4329–4335. doi: 10.1200/JCO.2003.11.137. [DOI] [PubMed] [Google Scholar]
- 168.Wu Y, Sun X, Kaczmarek E, Dwyer KM, Bianchi E, Usheva A, Robson SC. RanBPM associates with CD39 and modulates ecto-nucleotidase activity. Biochem J. 2006;396:23–30. doi: 10.1042/BJ20051568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Schluger LK, Sheiner PA, Thung SN, Lau JY, Min A, Wolf DC, Fiel I, Zhang D, Gerber MA, Miller CM, Bodenheimer HC., Jr Severe recurrent cholestatic hepatitis C following orthotopic liver transplantation. Hepatology. 1996;23:971–976. doi: 10.1002/hep.510230505. [DOI] [PubMed] [Google Scholar]
- 170.Takahashi T, Ashizawa S, Matsumoto H, Furuta K, Omura T, Sato K, Kakita A. Fibrosing cholestatic hepatitis developing after liver transplantation: case report of a patient with HCV-related cirrhosis. Transplant Proc. 2003;35:392–393. doi: 10.1016/s0041-1345(02)03922-2. [DOI] [PubMed] [Google Scholar]
- 171.Deaglio S, Robson SC. Extracellular nucleotides and nucleosides as autocrine and paracrine regulators within the vasculature. Cambridge University Press; (in Aird, W.C., The Endothelium. in press 2007) [Google Scholar]
- 172.Dwyer KM, Deaglio S, Gao W, Friedman D, Strom TB, Robson SC. CD39 and control of cellular immune responses. Purinergic Signalling. doi: 10.1007/s11302-006-9050-y. (2007. Online epub Feb 1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kaneider NC, Egger P, Dunzendorfer S, Noris P, Balduini CL, Gritti D, Ricevuti G, Wiedermann CJ. Reversal of thrombin-induced deactivation of CD39/ATPDase in endothelial cells by HMG-CoA reductase inhibition: effects on Rho-GTPase and adenosine nucleotide metabolism. Arterioscler Thromb Vasc Biol. 2002;22:894–900. doi: 10.1161/01.atv.0000018305.95943.f7. [DOI] [PubMed] [Google Scholar]
- 174.Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, Nizet V, Insel PA, Junger WG. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006;314:1792–1795. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]