Summary
Wounds in fetal skin heal without scar, however the mechanism is unknown. We have identified a novel group of protein and nucleotides-positive particles in fetal and adult mouse blood and in human blood, and termed them “Dot cells”. Freshly isolated Dot cells regenerate wounds with less scarring and can be cultured without feeder layers.
Because the morphology of Dot cells has never been described, in this study we describe the specific characterizations of Dot cells, including their growth pattern in vitro, and their expressions of stem cell markers using FACS analyses and immunofluorescent histology. Our data indicates that cultured Dot cells express stem cell surface markers and embryonic stem cell transcription markers, such as Oct4, Nanog and Sox-2. In addition, Dot cells express VASA, the germ plasm specific marker.
To confirm if Dot cells maintain their wound regenerative activity after in vitro expansion, in vitro cultured Dot cells were transplanted to wounded mice. Dot cells from albino mice maintain their wound regenerative activities after intravenous transplantation to black-background diabetic mice. Also, Dot cells regenerate both the epithelial and dermal cells in the wounds of wild-type mice. The regenerated hair follicles, smooth muscle, and dermal tissues express transiently to VASA.
Our data demonstrate that Dot cells are newly identified organisms located in the blood and bone marrow of mammals. They express germ cell, embryonic stem cell and adult stem cell markers. Dot cells maintain their regenerative function after in vitro expansion.
Keywords: Stem cells, wound healing, VASA, tissue regeneration, germ plasm-like Dot cells
Introduction
The primordial germ cells (PGCs) are derived from germ plasm, the cytoplasmic substances of the oocytes, in lower species (1–3). Germ plasm is morphologically distinct from the somatic cytoplasm. The components of germ plasm is composed of aggregates of mitochondrial derivatives and electron dense bodies or “nuage”, which are crucial for PGC formation (4–7). It is now known that the electron dense granules in germ plasm are enriched in RNAs that are stored in a translationally repressed state until needed for PGC development (8). Although germ plasm is not the original source for PGCs in mammals, it could be very important for developing a fetus because the crucial materials needed for cloning a sheep are the germ plasm of an oocyte and the nucleus of a somatic cell.
Bone marrow (BM) and blood are the important postnatal stem cell reservoirs for tissue regeneration (9, 10). Unfractionated BM stem cells can regenerate muscle, neurons, hepatocytes, smooth muscle cells and other tissues (11–14). The successful bone marrow transplantations suggest that the regenerative function of bone marrow is due to the presence of the stem cells. Several types of bone marrow stem cells have been found: 1) hematopoietic stem cells (HSCs) that express c-kit(+), lin(−) and sca1(+), which differentiate into hematopoietic tissues (15–17), and 2) mesenchymal stem cells (MSCs) that differentiate towards osteogenic, adipogenic, myogenic, and chondrogenic lineages (18, 19). Recently, a group of very small embryonic like (VSEL) cells was identified (20, 21). VSEL cells are about 3–5 µm, with large nuclei and little cytoplasmic components; however, VSEL cells do not grow in the absence of feeder layer cells. On the feeder layer cells, these VSEL cells differentiate to mesenchymal-like cells within the first two or three days after culture. In addition, a group of newly isolated monkey embryonic stem cells expressing VASA, a germ plasm marker, has been reported (22).
Until now, scientists have had difficulties in maintaining the morphology and surface marker expression of BM stem cells during their in vitro expansion. Isolated BM stem cells can only been driven down to separate mesenchymal cellular lineages, but not to ectoderm- or endoderm-derived lineages, by applying various chemicals and growth factors in vitro. In culturing, BM stem cells change to mesenchymal-like cellular morphology, which do not maintain the multi-lineage regenerative function after transplantation in vivo. Due to the unsuccessful in vitro expansion of BM stem cells, only freshly isolated bone marrow cells are used for transplantation (23). In addition, the mechanism of stem cell self-renewal is another unknown question. The self-renewal of BM stem cells has only been detected in vivo after freshly isolated BM cell transplantation (23). Although attempts to prospectively isolate BM stem cells have been made for years, the results are still unclear and controversial (24, 25).
We isolated a group of E-cadherin-positive small dot-shaped particles from mouse blood, BM, and from human blood. Initially, we thought that these particles were small eukaryotic cells and therefore named them “Dot cells”(26). The ratio of Dot cells in fetal mouse blood is more than twenty times higher than in the adult blood. Freshly isolated Dot cells regenerate skin wounds and reduce scar formation in postnatal mice after transplantation via tail vein. Freshly isolated Dot cells strongly express E-cadherin, integrin β1/CD29, CXCR4, CD34, CD13 (low) and Sca1 (low) (26).
Here we provide new evidence that Dot cells have a unique rapid self-renewal pattern in culture. They form colonies and spheroids, similar to that described for embryonic stem cells. Dot cells also express embryonic stem cell transcription markers, such as Oct4, nanog and Sox-2. In addition, Dot cells express germ plasm marker VASA. In vitro expanded Dot cells maintain their wound regenerative activity and do not induce host rejection after transplantation to different strains in mice. These data also provide evidence that Dot cells may have therapeutic regenerative potential.
Experimental Procedures
Animals and materials
Four-week-old male, 8 to 10 week old female Balb/C mice and diabetic (B6.Cg-m +/+ Leprdb/J, Jackson lab) mice were bred or maintained in the Stanford Animal Care Laboratory. Mice received food and water ad libitum. All procedures with animals were conducted in accordance with Stanford University Administrative Panel on laboratory Animal Care (APLAC)-approved protocols according to NIH guidelines. VASA, E-cadherin, integrin β1, Oct3/4, Nanog, Sox-2 and c-kit antibodies were from Santa Cruz biotechnology (Santa Cruz, CA). CD34 and CD90 antibodies were from Abcam. CD45, Sca-1 and c-kit antibodies were from BD Pharmingen (San Diego, CA). Alexa Fluor goat anti-rabbit IgG was from Molecular Probes (Eugene, OR). Anti-rabbit IgG-conjugated magnetic beads and columns were from Miltenyi Biotech Inc. (Auburn, CA). Other chemicals were from Sigma Chemical Co., (St. Louis, MI).
Dot cell isolation and in vitro expansion
Blood collected from 4-week old Balb/C or GFP transgenic mice through cardiac puncture was diluted with PBS before passing through a cell strainer and followed by incubation in red blood cell lysis buffer. Magnetic bead cell sorting was followed per manufacturer’s instructions. Sorted E-cadherin positive cells were cultured on collagen coated plates in α-MEM containing 20% FBS and antibiotics in a 5% CO2 humid incubator at 37°C. After cell colonies reached sub-confluence, cells were trypsinized and passaged to fresh collagen coated plates.
Wound creation and cell transplantation
For diabetic mouse wound healing, mice were evenly distributed into two groups according to their body weights. Three mice were used in each group. One 1.5 cm × 1.5 cm open wound was created on the dorsal skin of each diabetic mouse. Although the cutting surface was measured as 1.5 cm × 1.5 cm, the wound surface was immediately stretched to about 5 cm2 due to the gravitation. The body weight of the mouse for the Dot cell treated and control groups was evenly distributed. One and a half million cultured Dot cells from passage-2 were then immediately transplanted through tail vein to each wounded diabetic mouse. The control group received saline through tail-vein injection. Wound surface of each mouse was measured every day or every other day until the wound was closed by the double-blind method. Animals were sacrificed on 25-day post-wound when all wounds were healed. For wild-type mouse wound healing studies, 8 to 10 week-old Balb/C mice were anesthetized. Two 0.6 cm diameter excisional wounds were made with a biopsy punch on the dorsal skin. One million cultured GFP-labeled Dot cells in 100 µl normal saline were then injected with 26-gauge needle through the tail-vein of each wounded mouse.
Immunofluorescent staining
For fluorescent studies, cultured Dot cells were fixed, washed, blocked and then reacted with different antibodies at dilutions ranging between 1:50 and 1:200 in blocking buffer over night at 4°C. Cells were then washed and reacted with a fluorescent conjugated secondary antibody for 1 hour. After washing, cells were counterstained with DAPI and photographed by confocal microscopy (Leica DM IRE2). For tissue studies, wounds were collected from 1-day to 7-day post-wounding, fixed and then embedded in OCT for frozen section. Sections were blocked and reacted with anti-VASA (1:100 dilution) overnight at 4°C. After washing, sections were counterstained with DAPI and photographed by confocal microscopy (Leica DM IRE2) or standard fluorescent microscopy (Axioplan 2 microscope, Zeiss). All control groups were treated without primary antibody. The scanned confocal images were further analyzed with Volocity software for 3 dimensional and 360-degree rotation movie images.
Fluorescent cell sorting (FACS)
Dot cells from either freshly sorted or collected from culture plates were washed with PBS and blocked with 1% normal horse serum (NHS) for one hour. Then, cells were labeled with different antibodies in the dilution from 1:50 or 1:100 in 100 µl PBS with 1% NHS for 30 min and followed with washes in PBS with 1% NHS and reacted with secondary antibody conjugated with either FITC (fluorescien isothiocyanate) or PE for 30 min on ice. After washing, the ratio of positive labeling was analyzed using the LSR FACS machines at the Stanford Shared FACS Facility. Flow cytometry data was acquired with CellQuest software (BD Biosciences) and analyzed with FlowJo software (FlowJo, Palo Alto, CA). Isotope antibody labeled cells were used for non-specific labeling control. Three separate experiments were performed to confirm the reproducible results.
GFP transfection
Ten µg of lentiLox 3.7 (pLL3.7) vector, together with 6.5 µg of CMVΔR8.74 plasmid and 3.5 µg VSV.G plasmid, were transfected to 5 × 106 293T cells in 100 mm plate using calcium precipitation method. Medium was changed with Iscove’s medium containing 10% FBS 16 hours after transfection. After 24 hours, medium was collected from 293T cells and infected to the cultured Dot cells, together with 6 µg/ml Polybrene. After overnight infection, medium was replaced with fresh medium (20% FBS in α-MEM). Cells were collected 48 hours after the infection. The infection efficiency was analyzed by fluorescent microscopy; about 70% of cells were GFP positive.
Statistical analysis
The sizes of wound surface on each mouse on day 0 were not same due to the skin stretch by the difference of the body weights. Therefore, the wound surface on each post-wounding day was calculated as the ratio of day 0, which was represented as 1. The ratio of the wound surface in each group was calculated as mean ± SD (N=3). The differences between two groups at the same time period were analysis using 2-tailed Standard t-test by the software Microsoft Excel 2004. The p-value ≤ 0.05 was considered significant (*).
Results
Dot cells have a unique self-renewal pattern in vitro
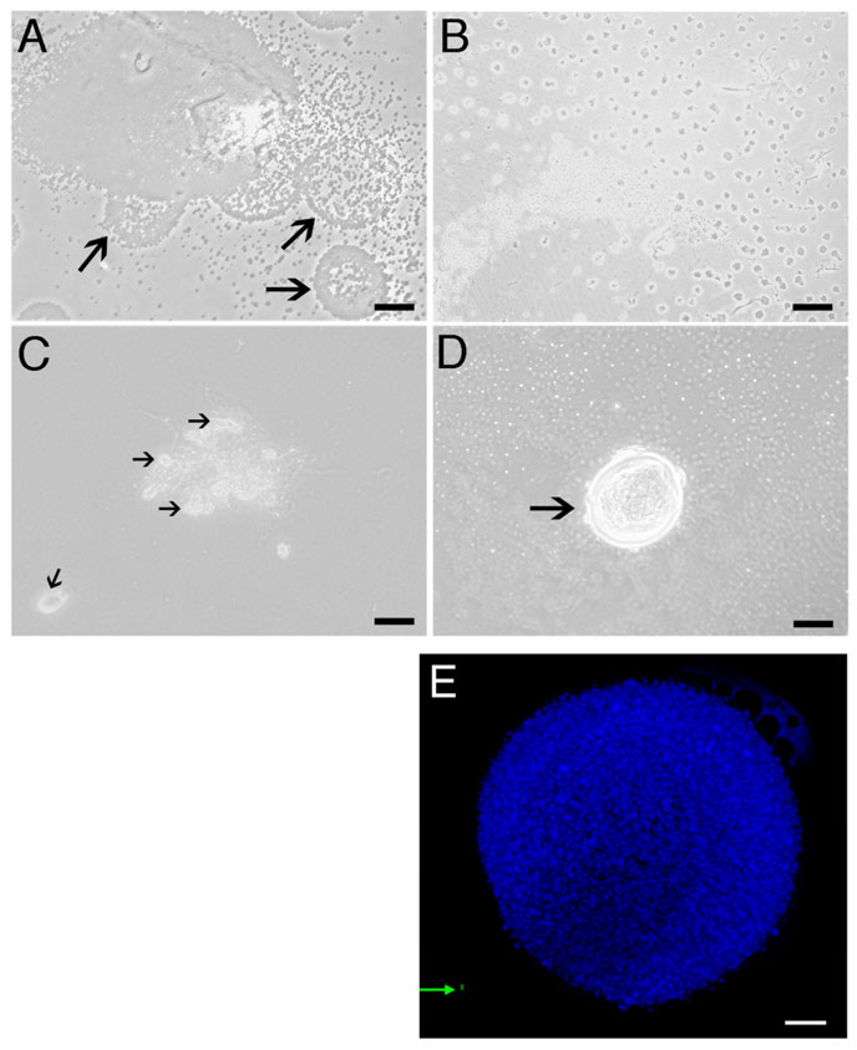
Dot cells were sorted using anti-E-cadherin antibody and cultured on collagen-coated plates. Although few eukaryotic cells were initially present with the Dot cells, only Dot cells survived after one passage. Blood-derived Dot cells have a unique self-renewal pattern in vitro. Before Dot cells reach confluence, multiple newly produced Dot cells were released from the large colony and rapidly spread in the culture plate (Fig. 1A). These circular masses of Dot cells migrated away from the colony and scattered on the culture plate, observed under lower magnification (Fig. 1B). The self-renewal pattern of Dot cells suggests that their replication is not through mitotic division. When Dot cells become confluent, large spheroids formed on top of the Dot cell monolayer, which were releasing grouped Dot cells (Fig. 1C). The large spheroids composed of Dot cells have similar morphology to that described for embryonic stem cells. Figure 1D shows a large spheroid on top of the confluent Dot cell monolayer. A shell-like structure comprised the outer layer of this spheroid. Multiple un-attached (white dots) and attached (dark dots) Dot cells were observed in the background. Due to the small size of Dot cells, it is difficult to distinguish the detailed morphology of the cultured spheroids. Confocal microscopy shows a 3-dimensional image of a cultured spheroid using high magnification (Fig. 1E). The spheroid was composed of numerous small bodies of DAPI-positive nuclear material.
Figure 1.
The unique morphology and replication pattern of Dot cells. Dot cells were sorted using E-cadherin antibody from adult mouse blood and then cultured in αMEM with 20% FBS. Dot cells have an unusual self-renewal pattern. The newly replicated Dot cells group as circular shape (arrows), and are released from the colony (1A). These grouped Dot cells migrate away from the colony and spread in a scattered pattern throughout the plate (1B). When Dot cells reach confluence, large spheroids forms on top of the monolayer (1C). This spheroid constantly releases grouped Dot cells (arrows) that migrate away. A large spheroid is located on top of the Dot cell monolayer with a shell-like structure at the outer layer of this spheroid (arrow in 1D). Using 100× oil lens plus 1.5× zoom, 1E shows a merged confocal image of a cultured spheroid. Bars in 1A, 1C and 1D = 40 µm; bar in 1B = 160 µm; bar in 1E = 10 µm.
Dot cells express embryonic stem cell markers
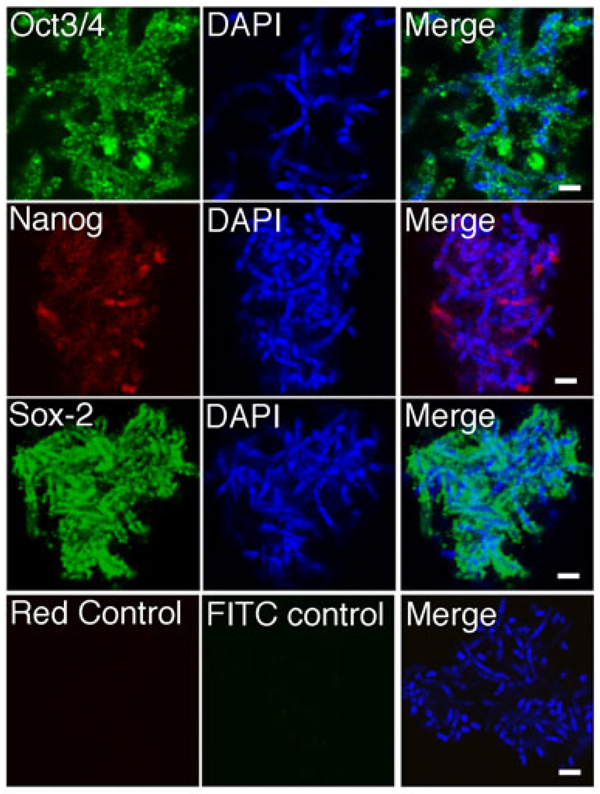
Specific transcription factors have been used to reprogram somatic cells to multipotent stem cells (27, 28). Expression of Oct4, Nanog and Sox-2 in cultured Dot cells was examined. Figure 2 shows that Dot cells express Oct4, Nanog, and Sox-2. However, the expression of Sox-2 was not directly adjacent to the DAPI stains, suggesting Sox-2 is located on the surface of Dot cells. Nanog and Oct4 were expressed close to the DAPI stains, suggesting that these two molecules are located inside Dot cells and close to the nuclear material. The different locations of these factors in Dot cells suggest that these factors may function differently in the Dot cell-derived eukaryotic cells. The DAPI staining showed that some Dot cells were in the rod shape, instead of the dot shape. The rod shape is similar to that of the chromosomes. To clarify that Dot cells are not chromosomes, we stained chromosomes of differentiated cells using karyotyping method, and also obtained the images of chromosomes in a differentiated cells during mitotic division. Supplementary data 1A shows the morphology of the chromosomes obtained from karyotyping method. Supplementary data 1B shows the chromosomes of DAPI stains in a differentiated cell during mitotic division. Both images indicate that the morphology of chromosomes is different from the DAPI stains of Dot cells in Figure 2. Also, the size of each Dot cells is smaller than a single chromosome. These data suggest that Dot cells, although they are not eukaryotic cells, express embryonic stem cell markers. Although the detailed information on the mechanism of the stem cell formation is still unclear, one stem cell may be formed by one Dot cell aggregate. We also, noticed that only aggregated Dot cells, but not the Dot cells in the colonies or spheroids (as these in Fig. 1), express the embryonic transcription markers.
Figure 2.
Dot cells express embryonic stem cell markers. Cultured Dot cells were examined by immunofluorescent methods for the expression of Oct3/4, Nanog and Sox-2. The images were cropped from 4000× confocal microscopy images. Bars = 2 µm.
Cultured Dot cells change the expression of surface markers
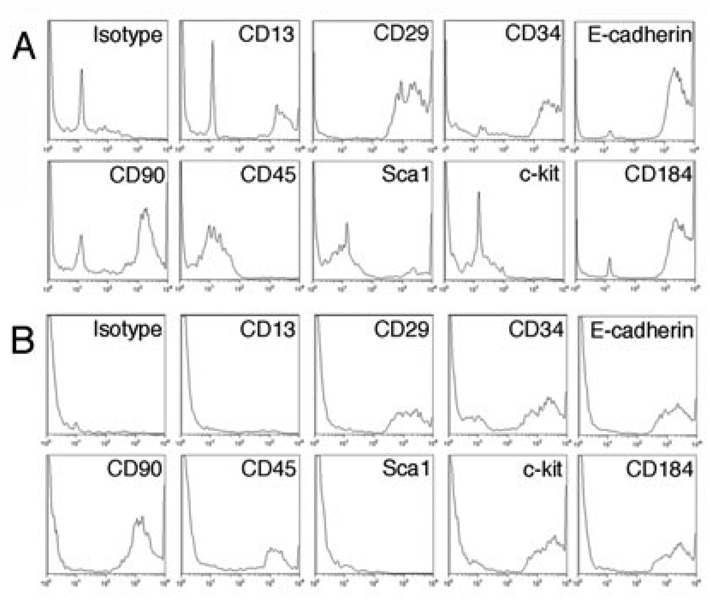
We have previously reported that Dot cells freshly isolated from blood express E-cadherin, CXCR4, integrin β1, CD34 but low to CD13 and Sca1(26). They do not express CD45 and c-kit. Dot cells expanded in culture were collected and their surface marker expression was examined using FACS. Figure 3 shows the FACS analysis of surface markers on in vitro expanded Dot cells. FACS data of freshly isolated Dot cells (26) were used for comparison. After one month in culture, the fraction of Dot cells that expresses E-cadherin, integrin β1 and CD184 was significantly reduced, and the fraction that expresses CD90 and CD34 remained the same. Interestingly, a significant number of Dot cells started to express c-kit and CD45, but none expressed Sca1. The surface marker change suggests that the differential stage of Dot cells in long-term culture is different from freshly isolated Dot cells. About 13% of freshly isolated Dot cells express CD13, while none of the cultured cells express CD13, a marker for the differentiated cells, suggesting that freshly isolated Dot cells are more mature than the culture-expanded Dot cells.
Figure 3.
Stem cell surface markers on cultured and freshly isolated Dot cells. The FACS analysis data of the freshly isolated Dot cells (26) was used for the comparison. Dot cells were in culture for more than one month and collected from passage 1. The expression of E-cadherin, integrin β1/CD29 and CD184 on Dot cells was decreased. A significant number of cultured Dot cells expressed c-kit and CD45, which were not detected on freshly sorted Dot cells. Most cultured Dot cells express CD90. N=3.
In vitro expanded Dot cells maintain their regenerative activity
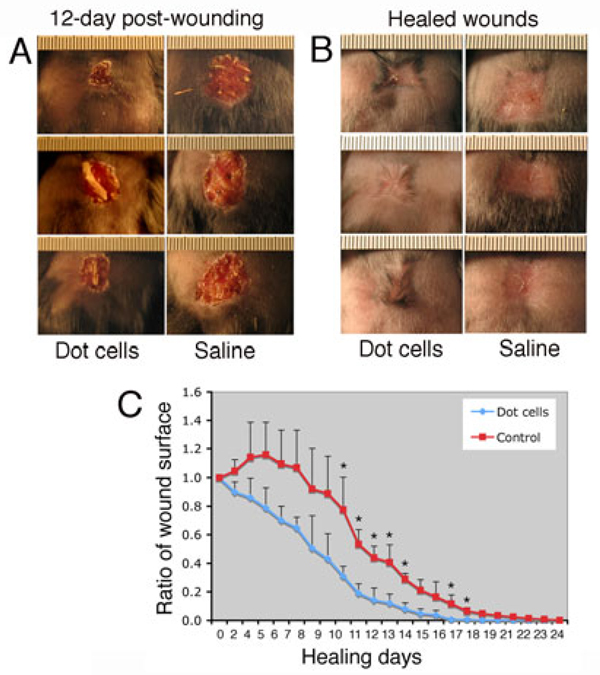
In our previous report, we found that freshly sorted Dot cells regenerate wounds with less scarring (26). Dot cells specifically migrate to the wound beds to regenerate damaged tissues. However, because some eukaryotic cells that also stain positive to E-cadherin were isolated together with Dot cells, it is not clear which is the true regenerative factor during wound healing. To answer this question, cultured Dot cells that show uniform morphology without any eukaryotic cell contamination, were transplanted to wounded adult diabetic mice via tail vein injection. Because the healing speed in diabetic mice is relatively slow, we chose diabetic mice for this experiment to easily examine the regenerative function of Dot cells. Three diabetic mice were used in each group for the wound study. A 1.5 cm square wound was created in the dorsal skin of each diabetic mouse immediately before Dot cell-transplantation. Figure 4A shows 12-day unhealed wounds, and 4B shows healed wounds in both Dot cell-transplanted and control mice. On the 12-day post-wounds, all three Dot cell-transplanted mice showed smaller wound surfaces compared to the control mice, suggesting that accelerated healing occurs in Dot cell-transplanted mice. After wounds were healed, newly grown hair was observed at the scar periphery of Dot cell-transplanted mice but not at the scar periphery for the saline control group. In addition, the scar tissues in the Dot cell-transplanted mice were smaller compared to the control group. Wound area of each diabetic mouse was measured every one or two days until complete healing occurred. Quantitative wound size analysis confirmed that significantly accelerated healing was detected in Dot cell-transplanted diabetic mice by the tenth day after wounding (Fig. 4C). These data indicate that cultural-expanded Dot cells not only reduce scarring, but also increase reepithelialization and hair growth.
Figure 4.
In vitro expanded Dot cells accelerate wound healing in diabetic mice. A 1.5 cm2 open wound was created on the dorsal skin of each diabetic mouse before 1.5 million cultured Dot cells were transplanted through tail-vein injection. The control group received saline. Three mice were used for each group. 12-day (4A) and 24 day (4B) healed wounds on Dot cell-transplanted and control diabetic mice. Newly grown hairs (arrows) surrounded the scar of each Dot cell-transplanted mouse. 4C shows quantitative wound area during the healing. The surfaces of wound areas were measured daily or every another day and the data was calculated. Accelerated healing was observed in Dot cell-transplanted diabetic mice by the tenth day after wounding. *P value = ≤ 0.05 was considered significant.
Cultured Dot cells accelerate reepithelialization
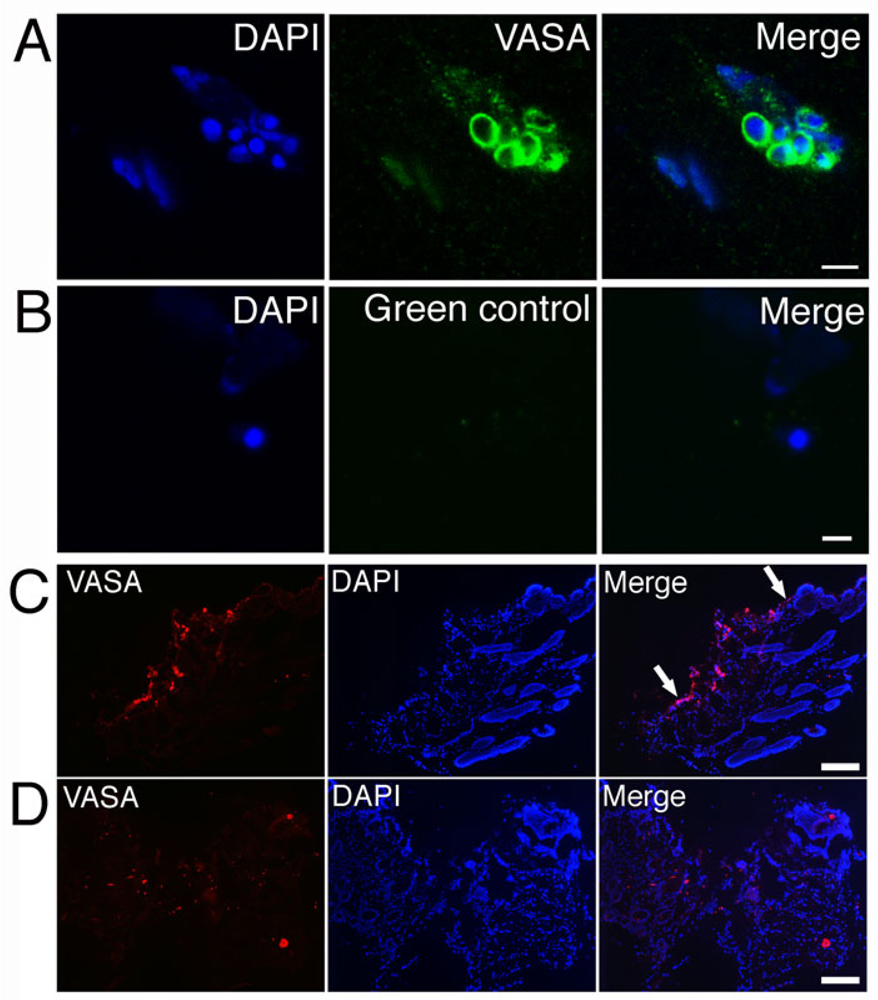
VASA is a RNA binding protein that is specifically expressed in germ plasm to regulate germ cell differentiation (29). The expression of the specific germ plasm marker VASA was tested in cultured Dot cells that were co-cultured with fibroblasts for 10 days. After the fibroblast induction, VASA expression appeared on the surface of Dot cells. Figure 5A shows a group of Dot cells that expressed VASA on their surface. The control section in 5B shows no positive stain. To understand the mechanism of the increased healing by cultured Dot cells, VASA expression was examined on the wound sections. Figure 5C shows a 3-day post-wound section of Dot cell transplanted mouse. Strong VASA-expressing cells were migrating along the epidermal layer. These cells were rebuilding the epithelial layer and hair follicles. However, no VASA-positive cell migration along the epidermis was observed in the control wound section (Figure 5D). Figure 5D also shows that although VASA-positive cells were observed in the 3-day wound bed of the control sections, their number was much less than that of Dot cell-transplanted wounds. This data support the results in Figure 4C, that significant small wound surface was detected on day 10 in Dot cell transplanted mice.
Figure 5.
VASA-expressing Dot cells regenerate epithelial cells. VASA expression on Dot cells was induced after 10 day co-cultured with dermal fibroblasts. 5A shows a group of Dot cells that express VASA on their surface. The control section in 5B shows no positive stain. Figure 5C shows a 3-day post-wound section of Dot cell transplanted mouse with strong VASA-expressing cells along the epidermal layer (between arrows in 5C). 5D shows VASA expression in the saline control mouse wound. Bar in 5A = 5 µm; bar in 5B = 2 µm; bars in 5C and 5D = 200 µm.
Cultural expanded Dot cells regenerate dermis
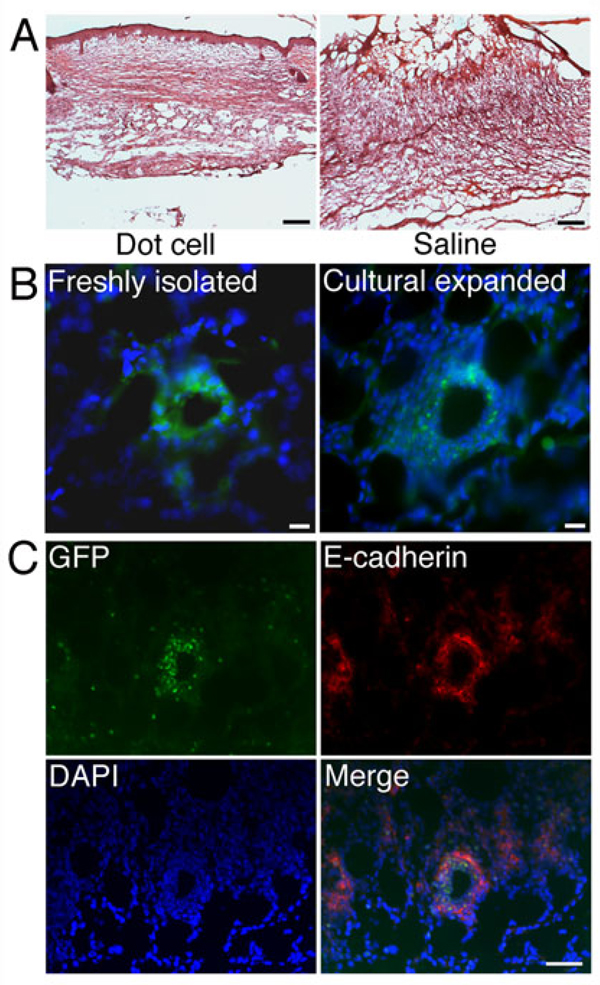
The effects of cultured GFP-labeled Dot cells on postnatal wound repair were examined in Balb/C mice. Figure 6A shows HE stains of 7-day wound sections from in vitro expanded Dot cell-transplanted and the control mice. Wounds in Dot cell-transplanted mice healed with less scarring compared to the saline injected mice. The control wound was filled with fibroid tissue, i.e. the scar. Figure 6B compares the wound regenerative activities of freshly sorted and in vitro expanded Dot cells. The freshly sorted Dot cells represent the Dot cells that were freshly isolated from GFP-transgenic mice and immediately transplanted to wounds. The cultural expanded Dot cells represent the Dot cells that were isolated from GFP-transgenic mice and then cultured and passaged. The GFP expression in the wounds was similar between the freshly sorted and cultural expended Dot cells. Again, lung and liver of cultured GFP-labeled Dot Cell transplanted mice were examined. No GFP positive cells were identified in these organs. The appearance of GFP-expressing cells in the wound indicates that Dot cells migrated to the wounds and differentiated to the dermal cells.
Figure 6.
Cultural expanded Dot cells regenerate dermis. Figure 6A shows HE stains of 7-day wound sections from in vitro expanded Dot cell-transplanted- (Dot cells) and the control (saline) mice. Figure 6B shows that GFP expression in the dermal regenerated areas has similar pattern between cultured Dot cells and freshly isolated Dot cells on 5 day wound sections. Figure 6C shows that both GFP and E-cadherin are expressed in the dermis. GFP and E-cadherin located in the area where the regeneration is not fully recovered. Bars in 6A = 150 µm; bars in 6B = 20 µm; bar in 6C = 50 µm.
We have noticed that cultured GFP-mouse-derived-Dot cells did not express GFP unless they were induced by the co-presence of the differentiated cells. This observation indicates that cultural expanded Dot cells do not express GFP at the time when they are transplanted. The supplementary data 2 shows a mixed population of GFP-mouse-derived-Dot cells and differentiated cells. The differentiated cells express GFP. However, only the Dot cells that attached to the differentiated cells or Dot cells that initiated the differentiation express GFP (arrows). The GFP expression in the Dot cells that initiate the differentiation was stronger compared to the fully differentiated cells.
E-cadherin is the epithelial cell marker. Under physiological condition, E-cadherin is mainly expressed on the epithelial cells. Because Dot cells express E-cadherin, the co-localization of GFP and E-cadherin was examined in the wounds of Dot cell transplanted mice. Figure 6C shows that both GFP and E-cadherin were expressed on the dermal cells. GFP and E-cadherin co-localized in the areas where the regeneration was occurring. These data provide evidence that in vitro expanded Dot cells migrate specifically to the wound beds to regenerate damaged dermal tissue.
In vitro expanded Dot cells show multipotent regeneration
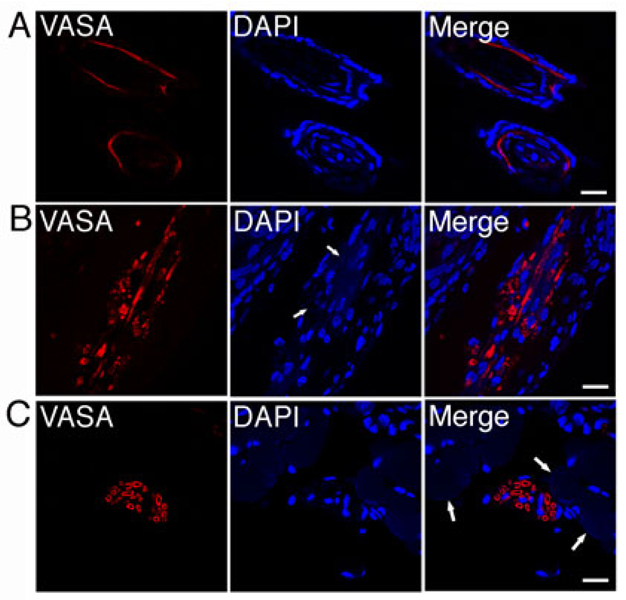
The wound regenerative function of Dot cells was examined using confocal microscopy on 3-day and 5-day wound sections. Circular-shaped VASA expression, frequently in grouped patterns, and less often in scattered patterns, was observed in the wound areas. VASA was expressed in hair follicles, dermal tissues and smooth muscle, representing ectoderm and mesoderm lineages. In two differentiated hair follicles (Figure 7A), VASA was expressed in the inner sheath area of the hair follicles. This data is consistent with our GFP-labeled Dot cell transplantation data in Figure 8A. In Figure 7B, VASA was expressed in the dermal wound area near, but not related to, the cell nuclei. Faint DAPI stains were detected in the areas where VASA was positive. This data suggests that the faint DAPI stains are Dot cell RNAs that undergo degradation during the tissue regeneration. VASA stains also expressed in a circular pattern, suggesting the contents inside each circular pattern are the degraded Dot cells. Figure 7C shows VASA expression in smooth muscle tissue. VASA was expressed in the area surrounded by smooth muscle fibers. Weak DAPI stains were colocalized with VASA stains, again, suggesting the weak DAPI stained materials are RNAs that have undergone the degradation. Only a few nuclei appeared in these large VASA positive areas, suggesting that few Dot cells undergo nuclear programming to become final nuclei of this smooth muscle tissue, instead most Dot cells are degraded together with the transiently expressed VASA protein. The large VASA positive areas suggest that transplanted Dot cells fuse into large spheroids for tissue regeneration. These data also suggest that the expression of VASA is initiated when the Dot cell-spheroids initiate the tissue regeneration. Both VASA and RNA are degraded when the tissue regeneration is completed. Figure 8A shows that the newly formed hair follicles are GFP positive. GFP is expressed in almost every cell in the undifferentiated hair follicles, but only expressed in the cells at the inner sheath area of the differentiated hair follicles.
Figure 7.
Dot cells regenerate epithelial and dermal cells. One million Dot cells were transplanted via tail vein into skin-wounded adult mice. Wounds were stained using rabbit anti-VASA antibody and followed with fluorescent-labeled secondary antibody. VASA expression was examined. Confocal images indicate that the expression of VASA is found in hair follicles (7A), in the dermal wound area near by, but not related to, cell nuclei (arrows in 7B), and in the area that was surrounded by smooth muscle fibers (arrows in 7C). Bars = 20 µm.
Figure 8.
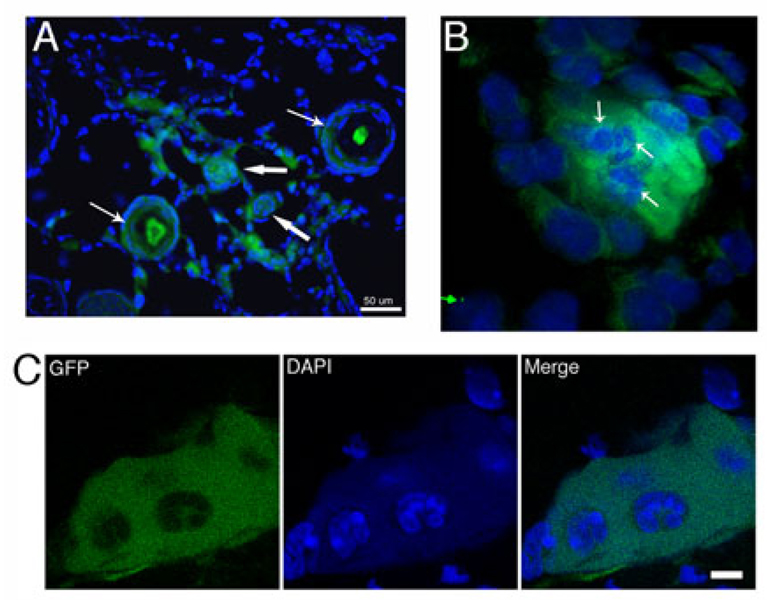
Differentiated cells are released from Dot cell spheroids in vivo. Figure 8A shows that both hair follicles and dermal cells express GFP. The GFP was expressed in whole follicle cells in the undifferentiated hair follicles (thick arrows), but only expressed in the cells at the inner sheath area of the differentiated hair follicles (thin arrows). A merged image shows a GFP spheroid with DAPI stained nuclei embedded inside (arrows in 8B). Figure 8C shows the confocal images of a 5-day wound section after GFP-labeled Dot cell-transplantation. Bar in 8A = 50 µm. Image in 8B was taken by 100 × oil lens and 2× zoom. Bar in 8C = 10 µm.
Differentiated cells are regenerated from Dot cell spheroids in vivo
The mechanism of Dot cells tissue regeneration was examined. One million GFP labeled Dot cells were transplanted to each wounded mouse through tail vein injection. We found that transplanted Dot cells formed spheroids in vivo. Large cells were embedded inside or on the surface of these spheroids. Figure 8B shows a spheroid from the 3-dimensional Movie 1. Large nuclei are embedded in this GFP-expressing spheroid, suggesting they are eukaryotic cells formed inside the spheroid. Figure 8C shows a confocal image of a GFP positive spheroid. GFP image show a large GFP-positive spheroid that has hollows inside. DAPI image shows that the area around these large nuclei stains positively for DAPI, however much weaker compared to the DAPI stains of the nuclei. The merged image suggests that these nuclei are embedded inside the GFP spheroid. The weak DAPI stain is evenly distributed, which is different from the DAPI stains of the spheroid in Figure 1E. The in vitro spheroid in Figure 1E contains numerous small DAPI particles. The differences of the DAPI stains between the in vitro and in vivo spheroids suggest that under in vivo condition, the nuclear material of Dot cells in the spheroid fuse and reprogram and from that, the eukaryotic cells are derived. This suggestion is supported by the different sizes of the nuclear particles inside the in vitro spheroid in Figure 1E and the in vivo spheroid.
Discussion
Our previous data demonstrates that Dot cells are present in the blood and bone marrow of humans, rats and mice (26). We believe that Dot cells are an unidentified group in bone marrow and blood. Dot cells are difficult to identify due to their small size (~ 0.5 – 1.5 µm) and their low ratio in blood (about 0.02% of total blood cells in adult mouse). In addition, Dot cells rapidly differentiate when they are co-present with other differentiated cells. Due to these special characteristics, Dot cells have not been recognized or studied until recently. Here we show that although Dot cells are not truly eukaryotic cells, they have most BM stem cell characteristics. First, unlike other BM isolated stem cells, which differentiate to mesenchymal morphology within the first 2 or 3 days after in vitro culture, Dot cells can grow without feeder cells for up to ten passages, or after storage in liquid nitrogen, and keep their unique morphology without mesenchymal cell-like differentiation. This suggests their strong self-renewal ability. Second, Dot cells express most well-known stem cell markers and embryonic stem cell transcription markers, such as CD34, CD90, CXCR4, Oct4, Nanog, and Sox-2. Third, Dot cells express VASA, the germ plasm RNA-associate protein, suggesting they contain mainly RNA and are more primitive than any BM stem cells. Also, Dot cells regenerate epithelial and mesenchymal cells during wound healing, suggesting their multi-lineage potential. And finally, in vitro expanded Dot cells maintained their strong regenerative activity after transplantation. Their regenerative function is through a process, including Dot cell aggregation, fusion and nuclear programming. Some Dot cells form large spheroids in vitro and in vivo that regenerate eukaryotic cells.
Our in vitro data describe how Dot cells maintain their population by self-renewal. This could explain the self-renewal pattern of Dot cells in their bone marrow-and blood-based niches. In bone marrow, they may stay in an isolated environment to maintain self-renewal and also to release Dot cells in the grouped pattern to blood for tissue regeneration. We have found that freshly sorted Dot cells do not, but cultured Dot cells do, express CD45 and c-kit. This difference could be due to that freshly sorted Dot cells contain some eukaryotic cells. We have found that co-culture with eukaryotic cells induces Dot cell differentiation. The freshly isolated Dot cells are derived from a mixed eukaryotic cellular environment, which can be the reason to explain that freshly isolated Dot cells are more mature than cultured Dot cells. This conclusion is also supported by the FACS data that small portion (~ 13%) of freshly isolated Dot cells expresses CD13, a marker for more differentiated cells (30), while none of the cultural-expanded Dot cells express CD13. We have observed that when eukaryotic cells are isolated together with Dot cells, these cells did not proliferate in long-term culture. This observation suggests that Dot cells release inhibitory factors to inhibit large cell proliferation. However, when there are more eukaryotic cells present, they release some factors that induce Dot cell differentiation. These regulatory factors between Dot cells and differentiated cells will be studied in our future investigation.
In our previous publication (26), we have shown that freshly isolated Dot cells migrate to the wound beds by a mechanism possibly involved in their strong expression to CD184/CXCR4, the receptor for stromal cell-derived factor-1 (SDF-1), an important chemokine for stem cell migration (31). Wounds increase the expression of SDF-1, which in turn, leads to the homing of circulating stem cells (32). We have not observed any GFP-labeled Dot cells that migrate to lung or other organs, which had been reported on other transplanted cells, in our more than one hundred mice that received Dot cell-transplantation between the range from 0.5 to 10 million Dot cells/ 100 µl saline.
Our data demonstrate that in vitro-expanded Dot cells can be transplanted to other mouse strains without signs of rejection. Dot cells from white Balb/C mice show significant increased skin regeneration in black diabetic wounded mice. Also, Dot cells from GFP-transgenic mice show skin cell regeneration in Balb/C mice. These data indicate that Dot cells may have potential for therapeutic uses. Our data show that Dot cells regenerate both epithelial and dermal cells. The cell lineage regeneration of Dot cells is determined by the microenvironment. The regulatory factors in the microenvironment for Dot cell lineage determination may be via physical contact between Dot cells and local cells or due to release of intracellular organelles by the local cells, to which Dot cells then respond. Further investigation will be carried out to identify the regulatory factors for Dot cells differentiation.
We believe that Dot cells are more primitive than other previously reported bone marrow stem cells. Dot cells perform their self-renewal through an unknown replication mechanism. In addition, Dot cells undergo nuclear programming before becoming differentiated cells. Dot cells will be a useful therapeutic modality for clinical treatment of injured tissues. Because cultural-expanded Dot cells have comparable repair effects to freshly sorted Dot cells, the amount of blood needed for isolation of Dot cells in future clinical applications will be reduced. Moreover, because postnatal blood-derived Dot cells have a regenerative function, political and ethical barriers for embryonic stem cell therapy will not be an issue.
Supplementary Material
Acknowledgments
This work was supported by NIH grant RO1-GM087609. The Authors thank Liam C. Macleod and Shaunin S. Baoerjiin for the manuscript editing.
References
- 1.Kloc M, Larabell C, Chan AP, Etkin LD. Contribution of METRO pathway localized molecules to the organization of the germ cell lineage. Mech Dev. 1998 Jul;75(1–2):81–93. doi: 10.1016/s0925-4773(98)00086-0. [DOI] [PubMed] [Google Scholar]
- 2.Kobayashi S, Amikura R, Okada M. Localization of mitochondrial large rRNA in germinal granules and the consequent segregation of germ line. Int J Dev Biol. 1994 Jun;38(2):193–199. [PubMed] [Google Scholar]
- 3.Beams HW, Kessel RG. Cytokinesis: a comparative study of cytoplasmic division in animal cells. Am Sci. 1976 May–Jun;64(3):279–290. [PubMed] [Google Scholar]
- 4.Mahowald AP. Germ plasm revisited and illuminated. Science. 1992 Mar 6;255(5049):1216–1217. doi: 10.1126/science.1372132. [DOI] [PubMed] [Google Scholar]
- 5.Eddy EM. Germ plasm and the differentiation of the germ cell line. Int Rev Cytol. 1975;43:229–280. doi: 10.1016/s0074-7696(08)60070-4. [DOI] [PubMed] [Google Scholar]
- 6.Chang CC, Dearden P, Akam M. Germ line development in the grasshopper Schistocerca gregaria: vasa as a marker. Dev Biol. 2002 Dec 1;252(1):100–118. doi: 10.1006/dbio.2002.0840. [DOI] [PubMed] [Google Scholar]
- 7.Hay B, Jan LY, Jan YN. Localization of vasa, a component of Drosophila polar granules, in maternal-effect mutants that alter embryonic anteroposterior polarity. Development. 1990 Jun;109(2):425–433. doi: 10.1242/dev.109.2.425. [DOI] [PubMed] [Google Scholar]
- 8.Tadros W, Lipshitz HD. Setting the stage for development: mRNA translation and stability during oocyte maturation and egg activation in Drosophila. Dev Dyn. 2005 Mar;232(3):593–608. doi: 10.1002/dvdy.20297. [DOI] [PubMed] [Google Scholar]
- 9.Shizuru JA, Negrin RS, Weissman IL. Hematopoietic stem and progenitor cells: clinical and preclinical regeneration of the hematolymphoid system. Annu Rev Med. 2005;56:509–538. doi: 10.1146/annurev.med.54.101601.152334. [DOI] [PubMed] [Google Scholar]
- 10.Wagers AJ, Christensen JL, Weissman IL. Cell fate determination from stem cells. Gene Ther. 2002 May;9(10):606–612. doi: 10.1038/sj.gt.3301717. [DOI] [PubMed] [Google Scholar]
- 11.Ferrari G, Cusella-De Angelis G, Coletta M, Paolucci E, Stornaiuolo A, Cossu G, et al. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1998 Mar 6;279(5356):1528–1530. doi: 10.1126/science.279.5356.1528. [DOI] [PubMed] [Google Scholar]
- 12.Mezey E, Chandross KJ, Harta G, Maki RA, McKercher SR. Turning blood into brain: cells bearing neuronal antigens generated in vivo from bone marrow. Science. 2000 Dec 1;290(5497):1779–1782. doi: 10.1126/science.290.5497.1779. [DOI] [PubMed] [Google Scholar]
- 13.Lagasse E, Connors H, Al-Dhalimy M, Reitsma M, Dohse M, Osborne L, et al. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med. 2000 Nov;6(11):1229–1234. doi: 10.1038/81326. [DOI] [PubMed] [Google Scholar]
- 14.Sata M, Saiura A, Kunisato A, Tojo A, Okada S, Tokuhisa T, et al. Hematopoietic stem cells differentiate into vascular cells that participate in the pathogenesis of atherosclerosis. Nat Med. 2002 Apr;8(4):403–409. doi: 10.1038/nm0402-403. [DOI] [PubMed] [Google Scholar]
- 15.Wagers AJ, Sherwood RI, Christensen JL, Weissman IL. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science. 2002 Sep 27;297(5590):2256–2259. doi: 10.1126/science.1074807. [DOI] [PubMed] [Google Scholar]
- 16.Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004 Apr 8;428(6983):668–673. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- 17.Roybon L, Ma Z, Asztely F, Fosum A, Jacobsen SE, Brundin P, et al. Failure of transdifferentiation of adult hematopoietic stem cells into neurons. Stem Cells. 2006 Jun;24(6):1594–1604. doi: 10.1634/stemcells.2005-0548. [DOI] [PubMed] [Google Scholar]
- 18.Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002 Jul 4;418(6893):41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 19.D'Ippolito G, Diabira S, Howard GA, Menei P, Roos BA, Schiller PC. Marrow-isolated adult multilineage inducible (MIAMI) cells, a unique population of postnatal young and old human cells with extensive expansion and differentiation potential. J Cell Sci. 2004 Jun 15;117(Pt 14):2971–2981. doi: 10.1242/jcs.01103. [DOI] [PubMed] [Google Scholar]
- 20.Kucia M, Reca R, Campbell FR, Zuba-Surma E, Majka M, Ratajczak J, et al. A population of very small embryonic-like (VSEL) CXCR4(+)SSEA-1(+)Oct-4+ stem cells identified in adult bone marrow. Leukemia. 2006 May;20(5):857–869. doi: 10.1038/sj.leu.2404171. [DOI] [PubMed] [Google Scholar]
- 21.Kucia M, Halasa M, Wysoczynski M, Baskiewicz-Masiuk M, Moldenhawer S, Zuba-Surma E, et al. Morphological and molecular characterization of novel population of CXCR4+ SSEA-4+ Oct-4+ very small embryonic-like cells purified from human cord blood: preliminary report. Leukemia. 2007 Feb;21(2):297–303. doi: 10.1038/sj.leu.2404470. [DOI] [PubMed] [Google Scholar]
- 22.Muller T, Fleischmann G, Eildermann K, Matz-Rensing K, Horn PA, Sasaki E, et al. A novel embryonic stem cell line derived from the common marmoset monkey (Callithrix jacchus) exhibiting germ cell-like characteristics. Hum Reprod. 2009 Feb 27; doi: 10.1093/humrep/dep012. [DOI] [PubMed] [Google Scholar]
- 23.Sacco A, Doyonnas R, Kraft P, Vitorovic S, Blau HM. Self-renewal and expansion of single transplanted muscle stem cells. Nature. 2008 Sep 17; doi: 10.1038/nature07384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiavegato A, Bollini S, Pozzobon M, Callegari A, Gasparotto L, Taiani J, et al. Human amniotic fluid-derived stem cells are rejected after transplantation in the myocardium of normal, ischemic, immuno-suppressed or immuno-deficient rat. J Mol Cell Cardiol. 2007 Apr;42(4):746–759. doi: 10.1016/j.yjmcc.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 25.Holden C. Stem cells. Controversial marrow cells coming into their own? Science. 2007 Feb 9;315(5813):760–761. doi: 10.1126/science.315.5813.760. [DOI] [PubMed] [Google Scholar]
- 26.Kong W, Li S, Longaker MT, Lorenz HP. Blood-derived small Dot cells reduce scar in wound healing. Exp Cell Res. 2008 Apr 15;314(7):1529–1539. doi: 10.1016/j.yexcr.2008.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007 Dec 21;318(5858):1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007 Nov 30;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 29.Styhler S, Nakamura A, Swan A, Suter B, Lasko P. vasa is required for GURKEN accumulation in the oocyte, and is involved in oocyte differentiation and germline cyst development. Development. 1998 May;125(9):1569–1578. doi: 10.1242/dev.125.9.1569. [DOI] [PubMed] [Google Scholar]
- 30.Lorand-Metze I, Califani SM, Ribeiro E, Lima CS, Metze K. The prognostic value of maturation-associated phenotypic abnormalities in myelodysplastic syndromes. Leuk Res. 2008 Feb;32(2):211–213. doi: 10.1016/j.leukres.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 31.Mohle R, Bautz F, Rafii S, Moore MA, Brugger W, Kanz L. The chemokine receptor CXCR-4 is expressed on CD34+ hematopoietic progenitors and leukemic cells and mediates transendothelial migration induced by stromal cell-derived factor-1. Blood. 1998 Jun 15;91(12):4523–4530. [PubMed] [Google Scholar]
- 32.Lapidot T, Dar A, Kollet O. How do stem cells find their way home? Blood. 2005 Sep 15;106(6):1901–1910. doi: 10.1182/blood-2005-04-1417. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.