Abstract
Adherence and growth rates of human aortic endothelial cells (HAEC) on plasma polymerized poly(vinylacetic acid) films were measured as functions of the surface density of —COOH groups and plasma deposited film thickness. Pulsed plasma polymerization was employed to produce films containing 3.6 to 9% —COOH groups, expressed as a percent of total carbon content. Endothelial cells exhibited increased cell adherence and proliferation with increasing —COOH surface densities. Additionally, and unexpectedly, cell growth was also dependent on the film thicknesses, which ranged from 25 to 200 nm. The results indicate that optimization of the functional group surface density and film thickness could produce significant enhancements in initial adhesion and subsequent growth of the HAEC cells.
Keywords: surface modification, plasma polymerization, film chemistry, film thickness, endothelial cells, carboxylic acids
Introduction
Arterial endothelium, which consists of an ultra thin lining (monolayer) of endothelial cells, is known to play a pivotal role in maintaining vascular functions, as manifested in a variety of ways. For instance, endothelium serves as a natural barrier to prevent platelet adhesion and thrombosis. It is also involved in relaxation of underlying smooth muscle cells in response to biomechanical forces exerted on arterial walls. Disruption of the endothelium, such as that encountered in vascular stent implantation, can lead to thrombosis, inflammation, and restenosis. Although drug eluting stents (DES) are now employed to minimize restenosis, there are increasingly reports of late-stent thrombosis and restenosis associated with the use of DES. It is believed that these late term effects are partially due to the slow growth of the endothelial cells to regenerate the protective endothelium monolayer on the stent materials.1 To overcome this limitation, surface modifications have been applied to provide both improved cell adhesion and accelerated cell growth on biomaterials.2 Despite significant advances in this field, further improvements in the surface treatments of biomaterials are very much needed, particularly for endothelial cells, which are known to exhibit relatively poor adhesion and slow growth on biomaterials.
In light of needs such as those noted above, surface physical and chemical properties have been examined extensively to improve the overall biocompatibility of materials. In particular, a variety of surface functional groups have been evaluated to improve cell adhesion and growth. Examples of such studies include amines (—NH2),3,4 imines (=NH),5 hydroxyls (—OH),6–8 esters (—COOC—),9,10 and carboxylic acids (—COOH).11–13 A number of such studies have included comparisons of various surface functional groups on cell adhesion and proliferation.14–18 For instance, several studies have shown that the presence of surface —COOH groups promote both improved cell attachment and growth compared to unmodified controls.14,16,17 Additionally, to some extent, the —COOH surface improvement has also been reported in comparison with other functional groups, such as thiol (—SH), alcohol, esters, and hydrocarbons.15,18 In contrast to these studies, other reports have indicated that —COOH functionalized surfaces reduce cell adhesion and proliferation, relative to the control surfaces, as reported in studies involving smooth muscle cells,11 endothelial cells,15 and fibroblasts.19
A variety of innovative approaches have been employed to modify solid surfaces in these studies of cell-surface interactions. Examples of techniques employed for this purpose include self-assembled monolayers (SAM's),14–19 photoinitiated grafting,20 grafting,11 radio frequency glow discharge (RFGD), also identified as plasma enhanced chemical vapor deposition (PECVD) or plasma polymerization,3,6,7,12,13,21 and Ar plasma treatment.22 The present study utilized the RFGD approach to deposit polymeric films containing —COOH groups, as obtained by the plasma polymerization of vinylacetic acid. Adhesion and growth of human aortic endothelial cells (HAEC) were studied on these functionalized surfaces. A distinguishing feature of the present work, relative to prior studies of this type, is that cell adhesion and proliferation were examined as functions of both surface density of the —COOH groups and thickness of the plasma deposited polymer films. For this purpose, a pulsed plasma discharge was employed, in addition to the conventional continuous-wave (CW) operational mode. As shown previously, with a variety of monomers, variation of the plasma duty cycle during the polymer formation provides an unusually convenient and exact method to control film composition.23–27 In the present work, the compositional control of interest was the extent of retention of monomer —COOH groups in the resultant films. Additionally, under a given pulsed plasma condition, the film deposition rate varies linearly with deposition time, thus providing a convenient control of film thickness.27
Materials and Methods
Deposition of poly(vinylacetic acid) film by RFGD plasma polymerization
Vinylacetic acid (CH2=CH—CH2—COOH) (abbreviated as VAA) was purchased from Sigma-Aldrich, St. Louis, MO and had a stated purity of 97%. The monomer was repeatedly freeze-thawed to remove any dissolved gases before use. Monomer vapor was subjected to RFGD, at room temperature, in a bell-shaped reactor chamber, as described elsewhere.25 After substrates were placed inside the reactor, the system was evacuated to a background pressure of 4 mtorr. Monomer vapor was introduced into the chamber and an RF plasma glow discharge ignited. Three different power input conditions were employed, namely, pulsed discharges at duty cycles of 2/30 and 10/30 (time on/time off, ms), plus runs using the CW operational mode. All samples were prepared using a 150 W peak power input. Although all runs were carried out at a 150 W peak power, it is important to note that the average power input differs significantly in contrasting the pulsed and CW depositions. The average power is computed from the plasma duty cycle (ratio of on time to the sum of the on plus off time) multiplied by the peak power. Thus the average power inputs were 9.4 and 37.5 W for the 2/30 and 10/30 runs, respectively, compared to the 150 W for the CW experiments. Monomer pressure of 160 mtorr was employed for the 2/30 duty cycle polymerizations and 40 mtorr for the 10/30 duty cycle and CW runs. Deposition times were adjusted to deposit ∼100 nm thick films for each set of plasma conditions employed.
In the second set of experiments involving film thickness variation, a single duty cycle (2/30) pulsed plasma was employed to polymerize VAA, and the deposition time was varied accordingly to obtain different film thicknesses ranging from 25 to 200 nm. In all experiments, standard polystyrene tissue culture well-plates (TCPS) were used as substrate for the plasma deposited films, as well as the control samples in the cell culture studies. Polished silicon wafers were used as substrates for x-ray photoelectron spectroscopy (XPS), atomic force microscopy (AFM), and water contact angle measurements. All silicon wafers were treated with acetone, methanol and hexane to clean the wafer surface before use.
Characterization of plasma deposited poly(vinylacetic acid) films
The VAA polymeric films were characterized by FTIR spectroscopy, XPS, AFM and water contact angle measurements. The FTIR spectral analyzes were carried out using a Bruker Vector-22 FTIR spectrophotometer operated at 4 cm−1 resolution on polymer films deposited on KBr disks. XPS spectra were obtained using a Perkin–Elmer PSI 5000 series instrument equipped with a monochromator and a 8.95 eV pass energy. A neutralizer was used these measurements since the samples were nonconductive. The high resolution XPS spectra were analyzed using Casa XPS software. The binding energy of the carbon atoms not directly bonded to any heteroatoms were centered at 284.6 eV. Surface roughness of the deposited films was determined using an AFM-SPM Nanoscope from Veeco. A phosphorus (n) doped silicon tip (RTESP from Veeco Probes) was used to scan the surfaces under tapping mode operation. A Rame-Hart sessile drop goniometer was used to measure the static water contact angle of the polymeric films. Film thickness measurements were obtained using a Tencor Alpha step 200 profilometer. A metal tipped pen was employed to scratch a thin line in the polymer films deposited on polished silicon wafers. The thickness of the films reported is an average of three measurements taken for each sample.
Cell preparation
HAEC were purchased from Cascade Biologics, OR. The cells were cultured with Medium 199 (Invitrogen Corp., CA) supplemented with 5% Fetal Bovine Serum (Hyclone, UT), Endothelial Growth Supplement (Cascade Biologics, OR), and 1% penicillin-streptomycin (Invitrogen, CA) at 37°C. Cells between passage 5 and 10 were used for all the experiments.
Cell adhesion and proliferation
All poly-VAA treated substrates employed in this study were subjected to overnight vacuum oven exposure at 40°C before the spectral analyzes and cell culture studies. This vacuum oven treatment was carried out to eliminate any monomer molecules or oligomers which may have been incorporated in the films during the plasma polymerization depositions. This was taken as a precautionary step to minimize the cytotoxicity of the plasma deposited poly-VAA films. Substrates were washed twice with sterile PBS before cell seeding.
For all cell adhesion and proliferation studies, cells were seeded at a density of 5 × 103 cells/cm2 on the poly-VAA coated 12-well tissue culture plates (TCPS) as well as the untreated TCPS controls. Cells were incubated for 6 h or 3 days for adhesion or proliferation on poly-VAA with different —COOH surface densities. In cell growth studies on poly-VAA with 9% —COOH, but different film thickness (25–200 nm), cells were incubated up to 7 days. The cell culture media (same as in cell preparation) were changed every 48 h. At the predetermined time, cells grown on the substrates were lysed with 1% Triton X-100 (MP Biomedicals, OH). The total cell DNA was analyzed using the PicoGreen dsDNA kit (Invitrogen, CA) following the manufacturer's instructions. The cell numbers were obtained by calculation based on the cell DNA, using a calibration curve of Total Cell DNA versus known numbers of cells.
Immunostaining of HAECs
HAECs were seeded at 5 × 103 cells/cm2 on poly-VAA treated and untreated 35 mm polystyrene petri dishes. After culturing for 24 h, cells were fixed with 4% cold formaldehyde for 30 min and treated with 0.02% Triton X-100 5 minutes for membrane permeability before staining. Rabbit anti-human von Willebrand Factor (vWF) IgG and, bovine anti-rabbit IgG-FITC (Santa Cruz) were used as the primary and secondary antibody to stain the cells. After staining, samples were mounted with UltraCruz™ Mounting Medium (Santa Cruz) containing 1.5 μg/mL DAPI for DNA counter-staining. The cells were observed with the Zeiss fluorescent microscope at the objective of 20 ×.
Quantitative study of fibronectin adsorption as a function of polymer film thickness
Fibronectin PBS solution (33 μg/mL, 2 mL/well) was added to 12-well TCPS coated with 100-nm thick poly-VAA with different —COOH surface density (3.6, 6.2 or 9%), or poly-VAA with 9% —COOH but varying film thickness of 25, 50, 100, and 200 nm. Untreated TCPS plates were used as the control. The samples were incubated for 2 h at 37°C, after which the fibronectin solutions were discarded. To detach the adsorbed fibronectin, 400 μL of 1% SDS solution was added to each well and incubated for 1 h with constant shaking. The fibronectin concentration was measured using the BCA protein assay (Pierce, IL) following the manufacturer's instructions.
Statistical analysis
Data were obtained at least in triplicates and presented as mean ± standard error of the mean. One-way ANOVA at a significance level of p < 0.05 was performed using StatView 5.0 software (SAS Institute).
Results
Characterization of the polymer films
FTIR spectra of the plasma polymerized VAA films, although relatively qualitative in nature, show progressive changes in film composition with variations in the RF duty cycles employed during the film deposition process. Figure 1 shows a plot of FTIR transmission spectra of the poly-VAA films deposited at pulsed discharges at 2/30 and 10/30 on/off ratios (in ms) and under CW conditions, reading from top to bottom. These spectra reveal a progressive increase in the retention of the monomer's —COOH content with decreasing RF duty cycle (plasma on time/plasma off time) employed during the deposition. This increase can be easily noted by comparing the relative intensities of the C=O stretching frequency for —COOH (1706 cm−1) and the characteristic H-bonded —OH stretch for COOH (broad region from 3300 cm−1 to 2500 cm−1). Additionally, there is a progressive increase in the intensity of the C—O stretching vibration (1100 cm-1) with decreasing RF duty cycle, which is consistent with increasing amounts of —COOH in the films. These spectra reveal that the extent of C=O retention is proportional to —OH retention in the film which would be consistent with the increasing presence of intact —COOH functional groups. It can also be noted that the intensities of the C—H (∼2900 cm−1) absorptions, relative to the C—O containing moieties, increase with increasing RF duty cycle. This is consistent with the decreased retention of —COOH functionality, as the plasma duty cycle is increased. FTIR spectra were also obtained (not shown here) for the films of varying thicknesses. As expected, the absorption intensities of the C=O and C—O peaks increased as the thickness of the films increased, but the overall spectral features were the same in all films.
FIGURE 1.
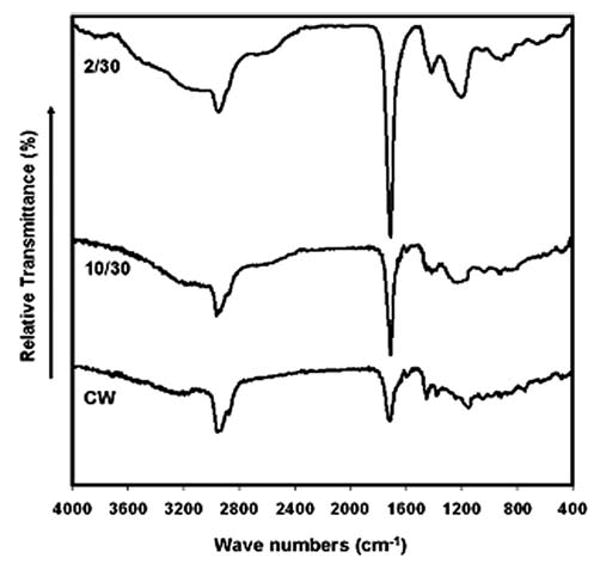
FTIR spectra for poly-VAA films deposited under pulsed (2/30 and 10/30) and CW plasma conditions.
High resolution C(1s) XPS spectra are shown in Figure 2, along with accompanying peak assignments. The peaks centered at 284.6 eV represents C—C and C—H groups, that is, carbons not bonded directly to any oxygen atoms. The other peaks were fitted using the following assignments: a β-shifted carbon bonded to carboxylic acid (C—COOH) at 285.3 eV, alcohol/ether (C—OH/C—O—C) at 286.3 eV, carbonyl (C=O) at 287.5 eV and carboxylic acid (COOH) at 288.9 eV. These peak assignments are in accord with many prior analyzes of this type.28 Clearly, there is a progressive decrease in the number of carbon atoms present as —COOH as the plasma on time is increased in the order of duty cycles 2/30, 10/30 to CW mode. Table I provides a quantitative measure of the percent surface carbon functionalities obtained from integration of the deconvoluted XPS high resolution C(1s) peaks. As these data show, there is a steady decrease in the surface density of —COOH functionality from ∼9% to ∼3.6%, expressed as a percent of total surface carbons, as the deposition condition switched from pulsed plasma (duty cycle, 2/30) to CW plasma operating mode. The progressive increase in the peak at 284.6 eV, that is, an increase in the C—C and C—H groups compared to other functional groups, is indicative of the increase in polymer cross-linking with increasing average power input as the plasma duty cycle is increased. It should be noted explicitly that films containing up to 20% —COOH can be obtained from the VAA monomer using even lower average power inputs than those reported here.29 However, we observed that films containing in excess of the 9% —COOH were relatively unstable in the cell culture media, no doubt reflecting the lower degree of film cross-linking as the average power is decreased. For this reason, we limited the present study to films having a maximum —COOH surface density of 9%.
FIGURE 2.
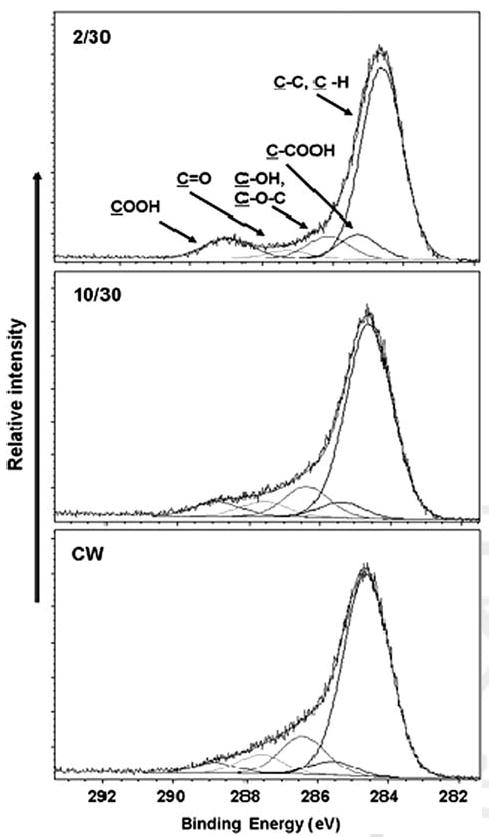
High resolution C(1s) X-ray photoelectron spectra for poly-VAA films deposited under pulsed (2/30 and 10/30) and CW plasma conditions.
TABLE I. Percent Surface Functional Groups of the Plasma Polymerized Vinylacetic Acid Films Deposited Under Pulsed (2/30 and 10/30) and CW Plasma Conditions.
| Plasma Conditions | Avg. Power Input (W) | O/C Ratio | C—C, C—H 284.6 eV | C—COOH 285.3 eV | C—OH, C—O—C 286.3 eV | C=O 287.5 eV | COOH 288.9 eV |
|---|---|---|---|---|---|---|---|
| 2/30 | 9.4 | 0.24 | 70.2 | 9.0 | 8.5 | 3.3 | 9.0 |
| 10/30 | 37.5 | 0.22 | 71.2 | 6.2 | 10.8 | 5.6 | 6.2 |
| CW | 150 | 0.19 | 72.6 | 3.6 | 13.6 | 6.6 | 3.6 |
High resolution C(1s) XPS spectra were also obtained for a series of films ranging in thickness from 25 to 200 nm, all deposited using the 2 ms on: 30 ms off pulsed plasma. Each of these films exhibited the same XPS spectrum as that shown in the top spectrum of Figure 2, thus revealing no measurable changes in the polymer composition with increasing film thickness.
Prior studies by other workers have demonstrated that surface roughness can effect cellular behavior on surfaces.30–32 In particular, changes in the surface roughness in the micron range have been shown to effect cell attachment and morphology.30–32 For that reason, it was important that we examine the surface roughness of the films employed in the present study. The surface roughness of plasma deposited VAA films, deposited at conditions of 2/30 and 10/30 duty cycles and CW mode, having film thicknesses 100 nm, as well as the films of different thicknesses (25, 100, 200 nm) deposited at the 2/30 duty cycle, were examined by AFM. The mean roughness values (RMS) shown in Table II are an average of three different regions on each sample. As tabulated in Table II, relatively small changes, of the order of 0.15 nm, were observed in the root mean square roughness of the surfaces employed in this study. Little change in surface roughness was noted with variation of the film thickness produced at the constant 2/30 duty cycle. Surface roughness does not vary more than ±0.1 nm as the thickness of plasma polymerized VAA film increases from 25 to 200 nm, as shown in Table II. Given the fact that that the surface roughness of these modified materials is less than one nanometer and not significantly different from each other (our surface roughness is ca. 0.4–0.6 nm in Table II), we can assume that the variations in cell adhesion and proliferation observed in the present study are not due to any roughness variation effects of the plasma polymerized films.
TABLE II. AFM Mean Roughness Values for Plasma Polymerized VAA Films Obtained for Different —COOH Surface Densities and Film Thicknesses.
| Plasma Condition (nm) | Avg. Power Input (W) | % COOH Retention | Roughness (RMS) |
|---|---|---|---|
| CW, 100 | 150 | 3.6 | 0.64 ± 0.1 |
| 10/30, 100 | 37.5 | 6.2 | 0.51 ± 0.1 |
| 2/30, 25 | 9.4 | 8.9 | 0.50 ± 0.01 |
| 2/30, 100 | 9.4 | 9.0 | 0.49 ± 0.01 |
| 2/30, 200 | 9.4 | 8.8 | 0.37 ± 0.01 |
The water contact angle goniometer measurements obtained are summarized in Table III. The contact angles shown represent the average of at least three measurements of each film. The measurements show a slight decrease in water contact angle with decreasing plasma on time (decreasing duty cycle). Lower contact angles clearly indicate the increased retention of polar groups, such as —COOH, on the surfaces as the plasma duty cycle is decreased, in accord with the spectroscopic data provided in Figures 1 and 2. The actual wettabilities, encompassing contact angles ranging from ∼40° to 60°, are in the range reported to be ideal for enhanced cell adhesion on polymer surfaces.16,34
TABLE III. Sessile Drop Water Contact Angles for Plasma Polymerized VAA Films Having Different —COOH Surface Densities and Film Thicknesses.
| Plasma Conditions (Duty Cycle, Thickness) (nm) | Water Contact Angle After Deposition (°) |
|---|---|
| 2/30, 100 | 38 ± 2 |
| 10/30, 100 | 48 ± 3 |
| CW, 100 | 60 ± 1 |
| 2/30, 25 | 39 ± 1 |
| 2/30, 50 | 38 ± 2 |
| 2/30, 200 | 39 ± 2 |
Cell adhesion and proliferation as a function of —COOH surface densities on poly-VAA films of identical thickness
The HAEC adhesion and proliferation studies were performed on 100-nm thick poly-VAA film coated TCPS, with the –COOH surface densities of 3.6, 6.2 or 9%. As shown in Figure 3, after incubation for 6 h, the HAECs had significantly higher adherence (∼180%) on poly-VAA with 9% —COOH surface density compared to untreated TCPS (p < 0.05). In contrast, poly-VAA with low —COOH concentration (3.6% and 6.2%) did not show significant differences of cell adhesion relative to the untreated TCPS. Cell proliferation results obtained after three days of culture, were similar to those of the adhesion study. Significantly higher amounts (∼140%) of HAECs were present on poly-VAA films with the 9% —COOH groups relative to the uncoated TCPS controls. In common with the initial adherence results, cell growth on poly-VAA with 3.6 or 6.2% —COOH was less than that observed for the untreated TCPS. Combined, these data strongly indicate a cell sensitivity to the —COOH surface density, in this case indicating that 9% —COOH coverage clearly provides a more effective surface than the other surfaces tested in this work.
FIGURE 3.
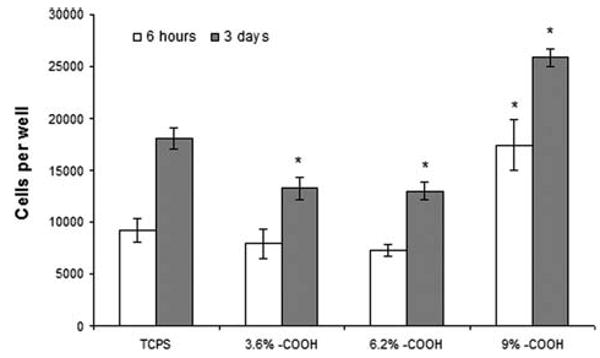
HAEC adhesion (6 hours after seeding) and proliferation (3 days after seeding) on poly-VAA film of 3.6, 6.2 or 9% —COOH surface densities. N = 4, *: p < 0.05 compared to the TCPS of the same time point.
Cell adhesion and proliferation as a function of film thickness on poly-VAA films having constant surface density of —COOH groups
In these experiments, cell adhesion (6 hours incubation) and proliferation (1, 3, 7 days incubation) on poly-VAA films, all having the same 9% —COOH surface density but different film thicknesses (25, 50, 100, and 200 nm), were measured and compared to that observed using the uncoated TCPS controls. An interesting finding was significantly higher HAEC adhesion on the 100-nm thick poly-VAA films than on the untreated TCPS (Fig. 4). In contrast, poly-VAA films of the other three thicknesses showed relatively small differences in cell adhesion compared to untreated TCPS plates. After one day of incubation, cell proliferation on the 100-nm thick films was significantly higher than on any of the other samples. However, as shown in Figure 4, with increased incubation of 3 and 7 days, relatively small differences were observed in cell proliferation among the – COOH samples, although the overall cell growth of these samples remained measurably higher than the untreated controls.
FIGURE 4.
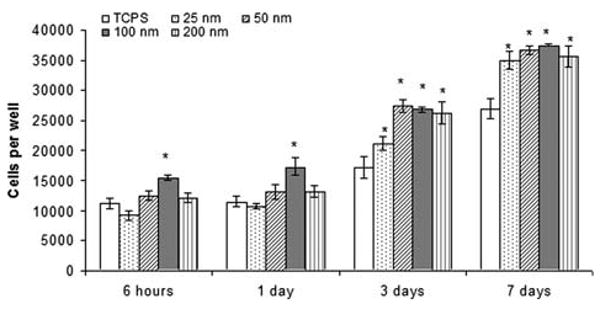
HAEC adhesion (6 hours after seeding) and proliferation (1, 3, 7 days after seeding) on poly-VAA film with the same 9% —COOH surface density but different thickness (25–200 nm). N = 4, *: p < 0.05 compared to TCPS of the same time point.
Immunostaining of von willebrand factor (vWF)
vWF, an important glycoprotein synthesized by vascular endothelial cells, is often used as a vascular endothelial cell marker to examine endothelial cell function.35 The purpose of this study is to see whether ECs grown on poly-VAA still preserve their functionality. As shown in Figure 5, the cells grown on the poly-VAA films with 100 nm thickness had similar VWF expression as the cells grown on untreated TCPS. The cells also showed a normal EC morphology. These results suggested that 100-nm thick poly-VAA is a compatible material for EC to support their growth and maintain the cell function.
FIGURE 5.
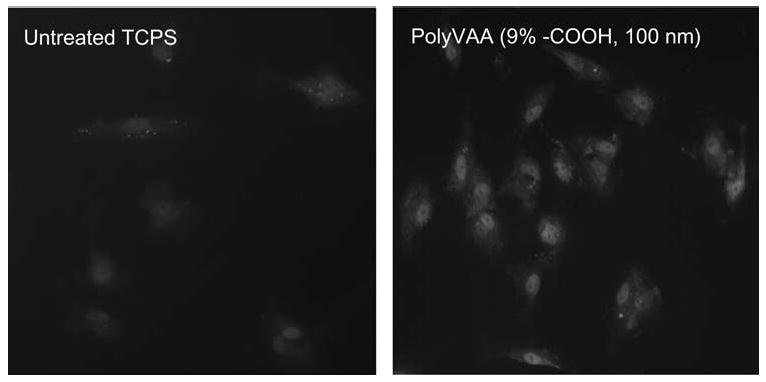
Fluorescence immunostaining von Willebrand Factor (vWF) of HAEC grown on untreated TCPS or 100-nm thick poly-VAA films with 9% —COOH surface density. Magnification: 200×. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Fibronectin adsorption on poly-VAA
Cell responses including adhesion and proliferation are all regulated by cell-extracellular matrix (ECM) interactions. Fibronectin is one of the most important ECM proteins involved in cell adhesion and proliferation by binding to cell intergrins. Since fibronectin is a major ECM protein existing in the cell culture medium containing FBS, and it has been shown to play an important role in cell adhesion and proliferation, we hypothesized that the difference of cell adhesion on poly-VAA might be due to the variance of fibronectin adsorption onto the substrate surface. To explore the mechanisms of enhanced EC adhesion and proliferation on poly-VAA, the passive fibronectin adsorption on these films was studied. As shown in Figure 6(a), poly-VAA, with 9% —COOH, exhibited more than twice as much fibronectin adsorption than the untreaed TCPS, or the poly-VAA surfaces with 3.6% or 6.2% —COOH. Additionally, the thickness of the poly-VAA films also affected the fibronectin adsorption, especially for the 9% —COOH films having thickness ranging from 25 to 200 nm. As shown in Figure 6(b), the 100-nm thick poly-VAA film had the highest fibronectin adsorption, while the uncoated TCPS plates had the least amounts of the protein deposited.
FIGURE 6.
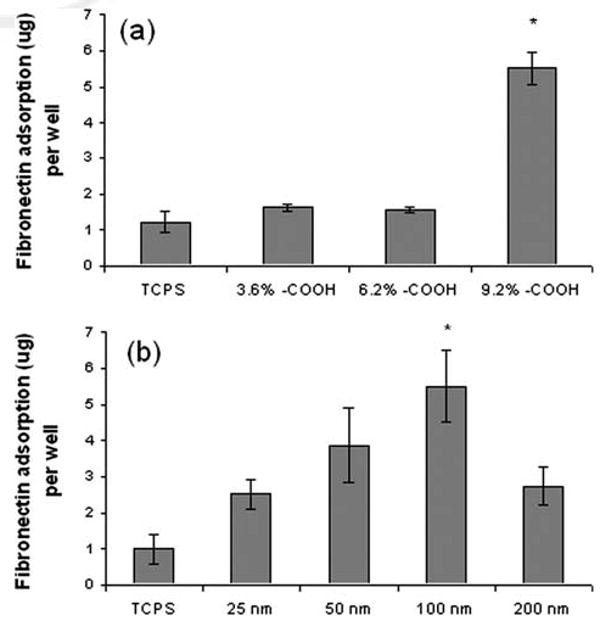
Fibronectin adsorption on poly-VAA film with 3.6, 6.2 or 9% —COOH surface densities (a), or on poly-VAA film (9% —COOH) with the thickness of 25–200 nm. N = 4, *: p < 0.05 compared to untreated TCPS.
Discussion
Given the essential constancy of surface roughness and surface wettabilities, we are able to focus on the effects of surface functional group densities and film thickness with respect to cell growth and proliferation. Our goal was to employ these variables as a possible route to achieve enhanced cell adhesion and growth rates on surfaces, an important objective particularly with respect to the generally slow growth of endothelial cells. In fact, as documented above, both initial adhesion and proliferation of HAEC cells are indeed affected by surface functional group densities and film thickness (Figs. 3 and 4). Immunostaining of vWF (Fig. 5) reveals that cells maintained their phenotypes and functions on poly-VAA films.
It is interesting to compare the present results with prior reports involving cell cultures on —COOH functionalized surfaces. For example, Haddow et al.13 showed there was little or no significant variation in cell attachment on surfaces having different concentrations of —COOH for keratinocytes when compared to a polyhydroxybutyrate control sample. However, an improved keratinocyte adhesion to plasma polymerized acrylic acid surfaces was reported for a surface having a low —COOH concentration (∼2%); a result comparable to that observed with —COOH terminated SAMs.36 Both the —COOH plasma modified and the —COOH terminated SAM surfaces exhibited significantly higher cell attachment and proliferation than the control TCPS surfaces employed. Cooper et al.37 also observed an increased number of attached fibroblasts on —COOH terminated SAMs than on —CH3 terminated ones but, unfortunately, did not report values for a TCPS surface. In contrast, other workers11,12 reported a negative effect of carboxylic acid groups on smooth muscle cell attachments. Tidwell et al.15 also reported a decreased number of endothelial cell attachments to —COOH SAM modified surfaces than on TCPS plates. Thus, prior reports reveal both unchanged as well as increased or decreased cell adhesion and growth on —COOH modified surfaces.
The magnitude of enhanced cell adhesion and proliferation observed in the present study is quite significant. Additionally, the influence of film thickness on endothelial cell adhesion was unexpected. This aspect has not been considered in quantitative fashion in previous studies of this type. Possibly, the enhanced HAEC growth observed in the present work, compared to the decreased growth reported by Tidwell et al.15 using —COOH terminated SAMs, can be rationalized in terms of this thickness effect. In the SAMs work, the actual width of the —COOH containing portion of the surface would have been much smaller than the smallest film thickness (25 nm) employed in the present study.
There are many factors or mechanisms that might account for the film thickness effects on cell growth observed in the present study. Our studies can eliminate film compositional and surface roughness effects since, within our experimental capabilities, these variables did not change with thickness. It is well-known that cell adhesion and proliferation are affected by the adsorbed extracellular matrix proteins from culture media containing serum. Our results showed that adsorption of fibronectin, a major ECM protein in culture media containing serum, was drastically increased on 100-nm thick poly-VAA with high —COOH density (9%). This increased surface bound fibronectin might account for the increased EC adhesion and subsequent EC proliferation observed in this study. It should be noted explicitly that the variation in fibronectin adsorptions among the various surfaces cannot be rationalized in terms of surface wettabilities in that the highest fibronectin adsorption is observed on the —COOH film having the highest —COOH content. It is generally accepted that protein adsorption depends on the surface wettability;16,33 with adsorption being more pronounced on hydrophobic surfaces than on the hydrophilic ones. However, in the current study, the poly-VAA with 9% —COOH is the least hydrophobic surface among the three different —COOH surfaces, with the water contact angle of ∼38°. Also, differing amounts of fibronectin adsorption were observed on the 9% —COOH films of differing thickness, but essentially identical water contact angles [Fig. 6(b)] Future studies of ECM production from seeded cells are certainly needed to further investigate the molecular mechanisms responsible for cellular responses toward the thickness change of the substrate surface.
Another potential mechanism for this cell growth is the mechanical stiffness of the substrates, as recent interesting reports show that cells adhere and grow differently on substrates of varied mechanical stiffness.38–41 It is now well established that cells sense the mechanical stiffness of both the ECM and the neighboring cells, and, in some cases, vary their phenotypes.42,43 In regard to the mechanical properties of plasma polymers, recent studies have shown that plasma polymer films become softer as the peak power input is decreased.44,45 For example, film hardness has been shown to vary from 1.7 to 3.3 GPa with increasing plasma duty cycle.44,45 Additionally, increased film softness has been observed for thicker films deposited at the same average power inputs.44 In our study, significantly higher numbers of cells adhered on the lower duty cycle, less cross-linked (2/30) films with 9% —COOH surface density, which would be a softer surface than the other films tested. Nevertheless, it is also clear that the cells preferred the 100-nm thick films even though the 200 nm films would be equal or even softer surfaces. Since different cell lines have unique preferences with respect to surface stiffness;46 it is possible that the poly-VAA films of a certain thickness exhibit an optimum surface hardness which maximizes the endothelial cell response. Obviously, this is an interesting question which requires further, more detailed studies in the future.
Overall, the present study has aptly demonstrated that RFGD treatment can be successfully employed to increase significantly the affinity of adherence and proliferation of vascular endothelial cells on a modified surface. The availability of improved EC adherence and proliferation is of considerable interest in a variety of biomaterials applications, including particularly vascular grafts and stents. In the present case, the enhanced cell growth is shown to be a function of both the surface chemistry and the thickness of the plasma deposited films. It will be of considerable interest to investigate if such variables are also influential with various other cell lines. We anticipate that continued studies of this type will help provide important new insights into the mechanisms and dynamics involved in the initial adherence and subsequent proliferation of cells on surface modified substrates.
Conclusion
The adherence and proliferation of endothelial cells were found to depend on the —COOH surface density and film thickness of substrates coated with plasma polymerized poly(vinyl acetic acid). The number of attached and proliferated cells for the 9% maximum attainable concentration of —COOH groups and optimum film thickness was significantly higher than that obtained in normal tissue culture polystyrene (TCPS) well plates. In conclusion, for endothelial cell cultures, it was possible to adjust the —COOH densities and film thickness to generate surfaces that provided significantly higher cell adhesion and proliferation than observed on untreated control substrates.
Acknowledgments
Contract grant sponsor: NIH; contract grant number: HL082644.
Contract grant sponsors: UTA Research Area Enhancement (RAE), New Faculty Start-up funds (College of Engineering)
References
- 1.Serruys PW, Daemen J. Are drug-eluting stents associated with a higher rate of late thrombosis than bare metal stents? Late stent thrombosis: A nuisance in both bare metal and drug-eluting stents. Circulation. 2007;115:1433–1439. doi: 10.1161/CIRCULATIONAHA.106.666826. [DOI] [PubMed] [Google Scholar]
- 2.Kasemo B. Biological surface science. Surf Sci. 2002;500:656–677. [Google Scholar]
- 3.Wan Y, Yang J, Yang J, Bei J, Wang S. Cell adhesion on gaseous plasma modified poly-(l-lactide) surface under shear stress field. Biomaterials. 2003;24:3757–3764. doi: 10.1016/s0142-9612(03)00251-5. [DOI] [PubMed] [Google Scholar]
- 4.Gumpenberger T, Heitz J, Bäuerle D, Kahr H, Graz I, Romanin C, Svorcik V, Leisch F. Adhesion and proliferation of human endothelial cells on photochemically modified polytetrafluoroethylene. Biomaterials. 2003;24:5139–5144. doi: 10.1016/s0142-9612(03)00460-5. [DOI] [PubMed] [Google Scholar]
- 5.Lakard S, Herlem G, Propper A, Kastner A, Michel G, Vallès-Villarreal N, Gharbi T, Fahys B. Adhesion and proliferation of cells on new polymers modified biomaterials. Bioelectrochemistry. 2004;62:19–27. doi: 10.1016/j.bioelechem.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell SA, Davidson MR, Emmison N, Bradley RH. Isopropyl alcohol plasma modification of polystyrene surfaces to influence cell attachment behavior. Surf Sci. 2004;561:110–120. [Google Scholar]
- 7.France RM, Short RD, Duval E, Jones FR, Dawson RA, Macneil S. Plasma copolymerization of allyl alcohol/1,7-octadiene: Surface characterization and attachment of human keratinocytes. Chem Mater. 1998;10:1176–1183. [Google Scholar]
- 8.Zhang Z, Menges B, Timmons RB, Knoll W, Foerch R. Surface plasmon resonance studies of protein binding on plasma polymerized di(ethylene glycol) monovinyl ether films. Langmuir. 2003;19:4765–4770. [Google Scholar]
- 9.El Khadali F, Hélary G, Pavon-Djavid G, Migonney V. Modulating fibroblast cell proliferation with functionalized poly(methyl methacrylate) based copolymers: Chemical composition and monomer distribution effect. Biomacromolecules. 2002;3:51–56. doi: 10.1021/bm015563x. [DOI] [PubMed] [Google Scholar]
- 10.Ziegelaar BW, Fitton JH, Clayton AB, Platten ST, Maley MA, Chirila TV. The modulation of corneal keratocyte and epithelial cell responses to poly(2-hydroxyethyl methacrylate) hydrogel surfaces: Phosphorylation decreases collagenase production in vitro. Biomaterials. 1999;20:1979–1988. doi: 10.1016/s0142-9612(99)00019-8. [DOI] [PubMed] [Google Scholar]
- 11.Bisson I, Kosinski M, Ruault S, Gupta B, Hilborn J, Wurm F, Frey P. Acrylic acid grafting and collagen immobilization on poly(ethylene terephthalate)surfaces for adherence and growth of human bladder smooth muscle cells. Biomaterials. 2002;23:3149–3158. doi: 10.1016/s0142-9612(02)00061-3. [DOI] [PubMed] [Google Scholar]
- 12.Gupta B, Plummer C, Bisson I, Frey P, Hilborn J. Plasma-induced graft polymerization of acrylic acid onto poly(ethyleneterephthalate) films: Characterization and human smooth muscle cell growth on grafted films. Biomaterials. 2002;23:863–871. doi: 10.1016/s0142-9612(01)00195-8. [DOI] [PubMed] [Google Scholar]
- 13.Haddow DB, Steele DA, Short RD, Dawson RA, Macneil S. Plasma-polymerized surfaces for culture of human keratinocytes and transfer of cells to an in vitro wound-bed model. J Biomed Mater Res A. 2003;64:80–87. doi: 10.1002/jbm.a.10356. [DOI] [PubMed] [Google Scholar]
- 14.Barbosa JN, Barbosa MA, Aguas AP. Adhesion of human leukocytes to biomaterials: an in vitro study using alkanethiolate monolayers with different chemically functionalized surfaces. J Biomed Mater Res A. 2003;65:429–434. doi: 10.1002/jbm.a.10488. [DOI] [PubMed] [Google Scholar]
- 15.Tidwell CD, Ertel SI, Ratner BD. Endothelial cell growth and protein adsorption on terminally functionalized, self-assembled monolayers of alkanethiolates on gold. Langmuir. 1997;13:3404–3413. [Google Scholar]
- 16.Arima Y, Iwata H. Effect of wettability and surface functional groups on protein adsorption and cell adhesion using well-defined mixed self-assembled monolayers. Biomaterials. 2007;28:3074–3082. doi: 10.1016/j.biomaterials.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 17.Elliott JT, Woodward JT, Umarji A, Mei Y, Tona A. The effect of surface chemistry on the formation of thin films of native fibrillar collagen. Biomaterials. 2007;28:576–585. doi: 10.1016/j.biomaterials.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 18.Faucheux N, Schweiss R, Lützow K, Werner C, Groth T. Self-assembled monolayers with different terminating groups as model substrates for cell adhesion studies. Biomaterials. 2004;25:2721–2730. doi: 10.1016/j.biomaterials.2003.09.069. [DOI] [PubMed] [Google Scholar]
- 19.Mcclary KB, Ugarova T, Grainger DW. Modulating fibroblast adhesion, spreading, and proliferation using self-assembled monolayer films of alkylthiolates on gold. J Biomed Mater Res. 2000;50:428–439. doi: 10.1002/(sici)1097-4636(20000605)50:3<428::aid-jbm18>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 20.Bearinger JP, Castner DG, Healy KE. Biomolecular modification of p(AAm-co-EG/AA) IPNs supports osteoblast adhesion and phenotypic expression. J Biomater Sci Polym Ed. 1998;9:629–652. doi: 10.1163/156856298x00064. [DOI] [PubMed] [Google Scholar]
- 21.Tang L, Wu Y, Timmons RB. Fibrinogen adsorption and host tissue responses to plasma functionalized surfaces. J Biomed Mater Res. 1998;42:156–163. doi: 10.1002/(sici)1097-4636(199810)42:1<156::aid-jbm19>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 22.Tajima S, Chu JS, Li S, Komvopoulos K. Differential regulation of endothelial cell adhesion, spreading, and cytoskeleton on low-density polyethylene by nanotopography and surface chemistry modification induced by argon plasma treatment. J Biomed Mater Res A. 2008;84:828–836. doi: 10.1002/jbm.a.31539. [DOI] [PubMed] [Google Scholar]
- 23.Rinsch CL, Chen X, Panchalingam V, Eberhart RC, Wang JH, Timmons RB. Pulsed radio frequency plasma polymerization of allyl alcohol: Controlled deposition of surface hydroxyl groups. Langmuir. 1996;12:2995–3002. [Google Scholar]
- 24.Wu YJ, Timmons RB, Jen JS, Molock FE. Non-fouling surfaces produced by gas phase pulsed plasma polymerization of an ultra low molecular weight ethylene oxide containing monomer. Colloids Surf B Biointerfaces. 2000;18:235–248. doi: 10.1016/s0927-7765(99)00150-2. [DOI] [PubMed] [Google Scholar]
- 25.Bhattacharyya D, Pillai K, Chyan OMR, Tang L, Timmons RB. A new class of thin film hydrogels produced by plasma polymerization. Chem Mater. 2007;19:2222–2228. doi: 10.1021/cm0630688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tarducci C, Schofield WCE, Badyal JPS, Brewer SA, Willis C. Monomolecular functionalization of pulsed plasma deposited poly(2-hydroxyethyl methacrylate) surfaces. Chem Mater. 2002;14:2541–2545. [Google Scholar]
- 27.Timmons RB, Griggs AJ. Pulsed plasma polymerizations In: Biedermann H, editor Plasma Polymer Films. London: Imperial College Press; 2004. pp. 217–245. [Google Scholar]
- 28.Beamson G, Briggs D. High-Resolution XPS of Organic Polymers, The Scienta ESCA300 Database. New York: Wiley; 1992. [Google Scholar]
- 29.Qiu H. PhD thesis. University of Texas at Arlington; 2001. Controlled chemical and morphological surface modifications via pulsed plasma polymerizations: Synthesis of ultrahydrophobic surfaces. [Google Scholar]
- 30.Xu C, Yang F, Wang S, Ramakrishna S. In vitro study of human vascular endothelial cell function on materials with various surface roughness. J Biomed Mater Res A. 2004;71:154–161. doi: 10.1002/jbm.a.30143. [DOI] [PubMed] [Google Scholar]
- 31.Miller DC, Thapa A, Haberstroh KM, Webster TJ. Endothelial and vascular smooth muscle cell function on poly(lactic-co-glycolic acid) with nano-structured surface features. Biomaterials. 2004;25:53–61. doi: 10.1016/s0142-9612(03)00471-x. [DOI] [PubMed] [Google Scholar]
- 32.Kunzler TP, Drobek T, Schuler M, Spencer ND. Systematic study of osteoblast and fibroblast response to roughness by means of surface-morphology gradients. Biomaterials. 2007;28:2175–2182. doi: 10.1016/j.biomaterials.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 33.Scotchford CA, Gilmore CP, Cooper E, Leggett GJ, Downes S. Protein adsorption and human osteoblast-like cell attachment and growth on alkylthiol on gold self-assembled monolayers. J Biomed Mater Res. 2002;59:84–99. doi: 10.1002/jbm.1220. [DOI] [PubMed] [Google Scholar]
- 34.Lee JH, Lee JW, Khang G, Lee HB. Interaction of cells on chargeable functional group gradient surfaces. Biomaterials. 1997;18:351–358. doi: 10.1016/s0142-9612(96)00128-7. [DOI] [PubMed] [Google Scholar]
- 35.Wijelath ES, Rahman S, Murray J, Patel Y, Savidge G, Sobel M. Fibronectin promotes VEGF-induced CD34 cell differentiation into endothelial cells. J Vasc Surg. 2004;39:655–660. doi: 10.1016/j.jvs.2003.10.042. [DOI] [PubMed] [Google Scholar]
- 36.Haddow DB, France RM, Short RD, Macneil S, Dawson RA, Leggett GJ, Cooper E. Comparison of proliferation and growth of human keratinocytes on plasma copolymers of acrylic acid/1,7-octadiene and self-assembled monolayers. J Biomed Mater Res. 1999;47:379–387. doi: 10.1002/(sici)1097-4636(19991205)47:3<379::aid-jbm13>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 37.Cooper E, Wiggs R, Hutt DA, Parker L, Leggett GJ, Parker TL. Rates of attachment of fibroblasts to self-assembled monolayers formed by the adsorption of alkylthiols onto gold surfaces. J Mater Chem. 1997;7:435–441. [Google Scholar]
- 38.Pelham RJ, Wang Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. PNAS. 1997;94:13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo WH, Frey MT, Burnham NA, Wang YL. Substrate rigidity regulates the formation and maintenance of tissues. Biophys J. 2006;90:2213–2220. doi: 10.1529/biophysj.105.070144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lo CM, Wang HB, Dembo M, Wang YL. Cell movement is guided by the rigidity of the substrate. Biophys J. 2000;79:144–152. doi: 10.1016/S0006-3495(00)76279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 42.Wells RG. The role of matrix stiffness in regulating cell behavior. Hepatology. 2008;47:1394–1400. doi: 10.1002/hep.22193. [DOI] [PubMed] [Google Scholar]
- 43.Rehfeldt F, Engler AJ, Eckhardt A, Ahmed F, Discher DE. Cell responses to the mechanochemical microenvironment—implications for regenerative medicine and drug delivery. Adv Drug Deliv Rev. 2007;59:1329–1339. doi: 10.1016/j.addr.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cech V, Vanek J, Goruppa AA, Jones FR. RF-power-controlled Young's modulus of plasma-polymerized organosilicon films. J Mater Sci. 2005;40:5099–5102. [Google Scholar]
- 45.Cech V, Inagaki N, Vanek J, Prikryl R, Grycova A, Zemek J. Plasma-polymerized versus polycondensed thin films of vinyltriethoxysilane. Thin Solid Films. 2006;502:181–187. [Google Scholar]
- 46.Levental I, Georges PC, Janmey PA. Soft biological materials and their impact on cell function. Soft Matter. 2007;3:299–306. doi: 10.1039/b610522j. [DOI] [PubMed] [Google Scholar]


