Abstract
Conjugation of the E. faecalis plasmid pCF10 is triggered in response to peptide sex pheromone cCF10 produced by potential recipients. Regulation of this response is complex and multi-layered and includes a small regulatory RNA, Anti-Q that participates in a termination/antitermination decision controlling transcription of the conjugation structural genes. In this study, the secondary structure of the Anti-Q transcript and its sites of interaction with its target, Qs, were determined. The primary site of interaction occurred at a centrally-located loop whose sequence showed high variability in analogous molecules on other pheromone responsive plasmids. This loop, designated the specificity loop, was demonstrated to be important but not sufficient for distinguishing between Qs molecules from pCF10 and another pheromone-responsive plasmid pAD1. A loop 5’ from the specificity loop which carries a U-turn motif played no demonstrable role in Anti-Q-Qs interaction or regulation of the termination/antitermination decision. These results provide direct evidence for a critical role of Anti-Q/Qs interactions in posttranscriptional regulation of pCF10 transfer functions.
Keywords: Antisense RNA, Conjugation, Plasmid, Transcription attenuation, RNA structure
1. Introduction
The pCF10 pheromone-inducible conjugative system (Bae et al., 2000, 2004; Bae and Dunny, 2001) is highly regulated with a number of checkpoints. Pheromone import by responsive donor cells triggers the cytoplasmic molecular switch, PrgX, increasing the initiation and processivity of transcription of the prgQ operon (Refer to Fig. 1. for genetic map of the pCF10 conjugation regulatory region). In general, the transcripts from the 5’ and 3’ end of the prgQ operon are involved in regulatory and conjugative functions, respectively (Nakayama et al., 1994). The major 5’ transcript, Qs, is constitutively expressed and encodes a functional peptide, the iCF10 inhibitor (Nakayama et al., 1994). The function of iCF10 is to suppress the expression of the conjugative machinery at low levels of the pheromone, cCF10, as a competitive inhibitor (Hedberg et al., 1996). Upon induction with cCF10, Qs levels increase and antitermination of Qs transcripts leads to formation of a longer mRNA species, QL (Chung and Dunny, 1995). The downstream genes of the prgQ operon encode the conjugation proteins including aggregation substance (PrgB), surface exclusion protein (PrgA), etc. The small regulatory RNA Anti-Q is proposed to regulate the termination/antitermination decision at the Qs terminator IRS1 by suppressing readthrough into the conjugative structural genes.
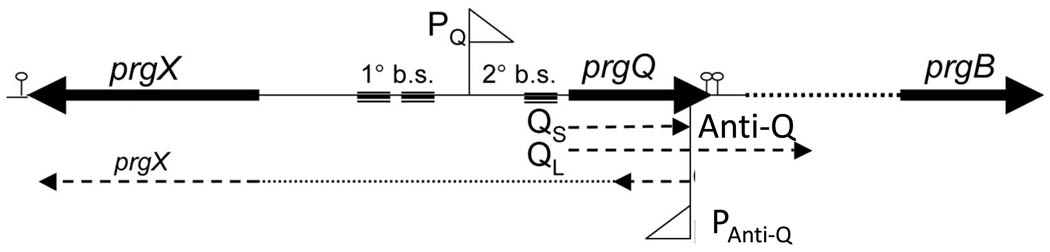
Fig. 1. Genetic map of the pCF10 conjugation regulatory region.
The triangular arrows above and below the line represent the prgQ (PQ) and Anti-Q (PAnti-Q) promoters, respectively. PQ directs the transcription of the Qs transcript which is terminated at the IRS1 terminator, the first lollipop structure to the right of prgQ, in the absence of cCF10. cCF10 induction leads to readthrough of terminators IRS1 and IRS2, production of QL and eventually the conjugation structural genes. Transcription from PAnti-Q generates both the regulatory Anti-Q RNA and the prgX mRNA which encodes the negative transcriptional regulator of the system. Anti-Q is processed from this larger transcript by an unknown mechanism. The hatched boxes labeled 1° b.s. and 2° b.s. indicate the primary and secondary binding sites for PrgX which control transcription from PQ.
Regulatory, or antisense, RNAs were first identified in studies of mobile genetic elements like plasmids, transposons, and bacteriophage, but have been more recently found to be ubiquitous on bacterial chromosomes as well (Brantl, 2002a; Brantl, 2007; Fozo et al., 2008; Gerdes et al., 1997; Gottesman, 2005; Wagner et al., 2002; Weaver, 2007). In general, regulatory RNAs are not translated, although exceptions exist (Benito et al., 2000; Novick, 2003). These molecules regulate gene expression by several different mechanisms including RNA folding interference, translational repression or activation, premature transcriptional termination, and target RNA degradation. Regulation by the majority of antisense RNAs involves complex formation by complementary nucleotide pairing with their target RNAs. These RNAs have evolved several structural characteristics, which allow an accelerated pairing with the target RNA within a short time-frame. The rate-limiting step in antisense-target RNA complex formation is usually an initial base-pairing interaction between two loops, or a loop and a single-stranded RNA (Franch and Gerdes, 2000). The presence of a common structural motif, the U turn motif, was revealed upon comparative sequence analysis of antisense RNAs. Consisting of a conserved 5’-YUNR-3’ sequence, the U turn motif was first identified as a structural element found on the anticodon loops of tRNAs (Ashraf et al., 1999; Quigley and Rich, 1976), where they facilitate rapid codon–anticodon interaction. The U-turn motif is considered to be a general binding rate accelerator that promotes initial RNA–RNA pairing in some, but not all, RNA-regulated systems.
According to the current model of Anti-Q-mediated transcription attenuation (Bae et al., 2004), when Anti-Q is in excess it interacts with its complementary region in the nascent prgQ transcript and promotes the formation of a terminator structure producing Qs RNA and preventing expression of the downstream conjugative machinery. When Qs is in excess, unpaired prgQ RNA favors an anti-terminator structure allowing transcription of the downstream conjugation structural genes. Anti-Q is a cis-acting antisense RNA (Brantl, 2007), being transcribed from the opposite strand of its target. However, Anti-Q is unusual in that it is part of a longer transcript that includes the prgX gene encoding the transcriptional repressor of prgQ. Anti-Q RNA is processed from the full length Anti-Q-prgX mRNA and is positively regulated by PrgX by an unknown mechanism.
In this manuscript molecular details of the interaction between Anti-Q and Qs are reported. Structural analysis of Anti-Q revealed that it formed a cloverleaf like structure with three stem-loops. Loop I, the 5’ most loop, contains a 5’-YUNR-3’ sequence that could provide a U-turn motif for Anti-Q-Qs interaction, but both in vitro and in vivo results indicated that it is not essential for Anti-Q function. In contrast, structural analysis of Anti-Q-Qs complexes suggested that critical contacts existed at Anti-Q loops II and III. Phylogenetic analysis suggested that, while the majority of the Anti-Q sequence was conserved among related pheromone-responsive conjugative plasmids, considerable variation was observed in loop II, suggesting that it was important for interaction specificity. While mutational analysis confirmed that loop II was important for determining specificity, it was not sufficient. The implications of these results for the overall mechanism of conjugation control in pheromone-responsive plasmids are discussed.
2. Materials and Methods
2.1 Bacterial strains, media and culture conditions
E. coli strain, DH5α (Invitrogen) was used for constructing the templates for generating in vitro transcripts. It was routinely cultured in Luria Bertani (LB) (Sambrook et al., 1989) broth at 37°C. E. faecalis strain OG1X (pAD1) (Clewell et al., 1982) was used as a source of pAD1 DNA. E. faecalis strain OG1Sp (Fixen et al., 2007) was used for β-galactosidase assays. E. faecalis strains were cultured in Todd Hewitt broth (DIFCO) and M9-YE media (Dunny et al., 1985). Erythromycin (Sigma) was used at concentration of 300µg/ml.
2.2 Production of in vitro transcripts
Plasmid pXPCAT (Bae et al., 2000) was used as a PCR template for generating the pCF10 Anti-Q and Qs in vitro transcription templates used in this study. Genomic DNA from OG1X (pAD1) was used as a PCR template for generating mD and Qs-pAD1 in vitro transcription templates. The likely starting points for these RNAs (for mD, −121 base pairs from the start codon of ORF-TTS1; for Qs-pAD1, −36bp from the start codon of iad) were based on sequence provided by Pontius and Clewell, 1992 and alignment with the pCF10 homologs. Genomic DNA was extracted using the Masterpure™ gram positive DNA purification kit from Epicentre Biotechnologies as per manufacturer’s instructions. Primers used for all constructs are listed in Table 1. PCR was performed using PCR supermix Hi fidelity (Invitrogen) according to manufacturer’s protocol.
Table 1.
Primers for in vitro Transcription
| Construct | Primers (5’→3’) | |
|---|---|---|
| Anti-Q | 5’Anti-Q-T7 | 5’-TGTAATACGACTCACTATAGGGGTTGTAA CTAATGTTGCAACAAACGA -3’ |
| 3’Anti-Q | 5’-GAACCGACTGCCATAGGACGGCA -3’ | |
| Qso/t | 5’Qs-T7 | 5’- TGTAATACGACTCACTATAGGGATAGGAGGG GTGTAAATGAA-3’ |
| 3’Qso/t | 5’-GAACTGTATACATTCCCCGC -3’ | |
| Qs | 5’Qs-T7 | 5’- TGTAATACGACTCACTATAGGGATAGGAGGGG TGTAAATGAA-3’ |
| 3’Qs | 5’- AAAAGCGGGGAACATATACATGAA-3’ | |
| Anti-Q-U | 5’Anti-QU- T7 | 5’-TGTAATACGACTCACTATAGGGGTTGTAA CCAATGTTGCAACAAACGA -3’ |
| 3’Anti-Q | 5’-GAACCGACTGCCATAGGACGGCA -3’ | |
| Qso/t-U | 5’Qs-T7 | 5’- TGTAATACGACTCACTATAGGGATAGGAGGGG TGTAAATGAA-3’ |
| 3’Qso/t-U MP | 5’-TGTTGCAACATTGGTTACAACGTATA -3’ | |
| 5’Qso/t-U OP | 5’-TACGTTGTAACCAATGTTGCA-3’ | |
| 3’Qso/t | 5’-GAACTGTATACATTCCCCGC -3’ | |
| Anti-Q-pAD1 | 5’Anti-Q-T7 | 5’-TGTAATACGACTCACTATAGGGGTT GTAACTAATGTTGCAACAAACGA -3’ |
| 3’Anti-Q-pAD1 MP | 5’-CAATTCATCTGTACCCATGAACTCT -3’ | |
| 5’Anti-Q-pAD1 OP | 5’-CAATTCATCTGTACCCATGAACTCT -3’ | |
| 3’Anti-Q | 5’-CATGAATTGTTTAACTGC -3’ | |
| Qs-pAD1 | 5’Qs-pAD1-T7 | 5’- TGTAATACGACTCACTATAGGGTGTAAGTTAA GTTTAAATAAG-3’ |
| 3’Qso/t | 5’-GAACTGTATACATTCCCCGC -3’ | |
| mD | 5’Anti-Q-T7 | 5’-TGTAATACGACTCACTATAGGGGTTGTAACTAA TGTTGCAACAAACGA -3’ |
| 3’Anti-Q | 5’-GAACCGACTGCCATAGGACGGCA -3’ |
MP: Mutagenic Primer, OP: Overlapping primer, and EP: End Primer
The wild type Anti-Q and Qs templates for in vitro transcription were generated using end primers to fuse the T7 promoter sequence at the 5’ end. For generating mutants, either an end primer was used to introduce the mutation (for Anti-Q-U mutant) or the mutant was generated using a three step site-specific mutagenic PCR approach (for Qso/t-U and Anti-Q-pAD1) as previously described (Shokeen et al., 2008). The primers (Table 1) were used at a final concentration of 200nM. All PCR products were sequenced at the University of South Dakota DNA sequencing core facility to confirm the expected DNA sequence.
In vitro transcripts were synthesized using T7 polymerase (Ambion Megashortscript kit) as per manufacturer’s instructions with the following modifications. Approximately 300nM amounts of purified PCR product (using Promega Wizard SV Gel and PCR Clean-Up Kit) was used as a template for transcription reactions. The transcription reactions were incubated in a cabinet incubator for 4 hours at 37°C. The remaining DNA template was digested using DNaseI as per manufacturer’s protocol. The transcripts were resolved on a 5% polyacrylamide denaturing (7M Urea) gel. The RNA bands were visualized and excised by placing a Fluor-coated TLC plate (Ambion) under the gel and exposing the gel to a handheld UV lamp (shortwave UV light 254nm). The excised bands were soaked in crush soak solution (10mM Tris.HCl (pH 7.5 at 23°C), 200 mM NaCl, 1 mM EDTA (Winkler, et al., 2002). The RNA was recovered from the crush-soak solution by precipitation with ethanol.
The transcripts were end labeled as per the protocol described by Greenfield et al., 2001. The position of transcripts was visualized by exposing the gel to X-ray film and then the band excised from the appropriate position on the gel and extracted as above.
2.3 Generation of in vivo constructs
To make pBK2, the PstI-EcoRI fragment was digested from p043lacZ (Kozlowicz et al., 2004) and was inserted into a similarly digested pCI3340 backbone (Hayes et al., 1990). An XhoI site was also engineered at prgQ −173 (Kozlowicz, B. K., 2005 Ph.D. Dissertation). This fragment contains the prgX-Q region with a lacZ fusion downstream of IRS1.
pBK2–24, with the U-turn mutation, was generated using the Quikchange™ site-directed mutagenesis technique (Stratagene) using Pfu Ultra II polymerase (Stratagene) and a pBK2 template with primer pair YUNR U9➔C Forward (5'- GTTCTCGTTTGTTGCAACATTGGTTACAACGT-3’) and YUNR U9➔ C Reverse (5’- AATGTTGCAACAAACGAGAACCGAGTAGAGTTC -3’). The XhoI-XbaI fragment containing the mutation was gel purified and cloned into a similarly digested pBK2 backbone.
2.4 Gel Shift Assay
End labeled target RNA was incubated with 10 to 20 molar excess of the appropriate unlabelled binding RNA in TMN binding buffer (Greenfield et al., 2000) at 37°C for 30 min for complex formation. The reactions were stopped by diluting with an equal volume of FD loading buffer (Greenfield et al., 2000) on ice. For positive controls, incubating RNAs were “fully duplexed” by gradually increasing the temperature to 65°C and bringing the temperature back to 25°C in a span of 30 min. Reactions were run on 5% denaturing polyacrylamide gels as described by Greenfield et al., 2000. The gels were dried and exposed to a storage phosphor screen (GE Healthcare) overnight and imaged using a Typhoon 9410 scanner (GE Healthcare). A time course study was carried out to calculate the rates of RNA interaction (Greenfield et al., 2000). The band volumes were calculated to determine the density of each band using Imagequant software (GE Healthcare). These band volumes were used to calculate the percentage of unshifted RNA. The binding rate constants for binding of the RNAs were determined as per Persson et al., 1988, using the exponential slope for each sample. This rate of interaction was calculated as a second-order binding rate constant which serves as a measure of the kinetics of the interaction of these RNAs.
2.5 Secondary structure determination using Pb (II) probing
Pb (II) probing analysis of end-labelled in vitro transcripts was carried out as previously described by Greenfield et al., 2001. Pb(II) cleaves any single stranded RNA with no sequence specificity. Reactions were run alongside a ladder produced by partial hydrolysis of Anti-Q RNA with Alkaline hydrolysis buffer (Ambion) and a commercially available size standard marker (Decade Marker, Ambion) to determine sites of cleavage. Fragment sizes are 4 nt larger than coordinates of Anti-Q in Fig. 1 because 4 nt were added to the 5’ end of the in vitro constructs by the T7 promoter fusion primer.
To determine sites of interaction, Pb(II) cleavage was performed on end-labeled Anti-Q or relevant Anti-Q mutants incubated with 10 molar excess unlabelled Qs or Qso/t in TMN buffer for 30 minutes. Because protection of Loop II from Pb(II) cleavage was the most prominent change upon complex formation, an interaction rate was determined based on a time course of Loop II protection. The binding rate constants for binding of the RNAs were carried out as per Persson et al. (1988).
2.6 β-Galactosidase Activity Assay
β-Galactosidase activity was assayed as described by Miller, 1972, with the following modifications: cells were cultured overnight in M9-YE with selective antibiotics. The overnight culture was diluted 1:5 in fresh medium with or without 50ng/ml cCF10 and grown for 90 minutes to mid-log phase. The cells were pelleted and resuspended in assay buffer (Z buffer-0.06M Na2HPO4.7H2O, 0.04M NaH2PO4.H2O, 0.01M KCl, 0.001M MgSO4, 0.05M β-mercaptoethanol, pH to 7.0.) to eliminate error due to the effects of different carbon sources in the growth medium on the β-galactosidase enzyme activity.
3. Results
3.1. Secondary structure of Anti-Q
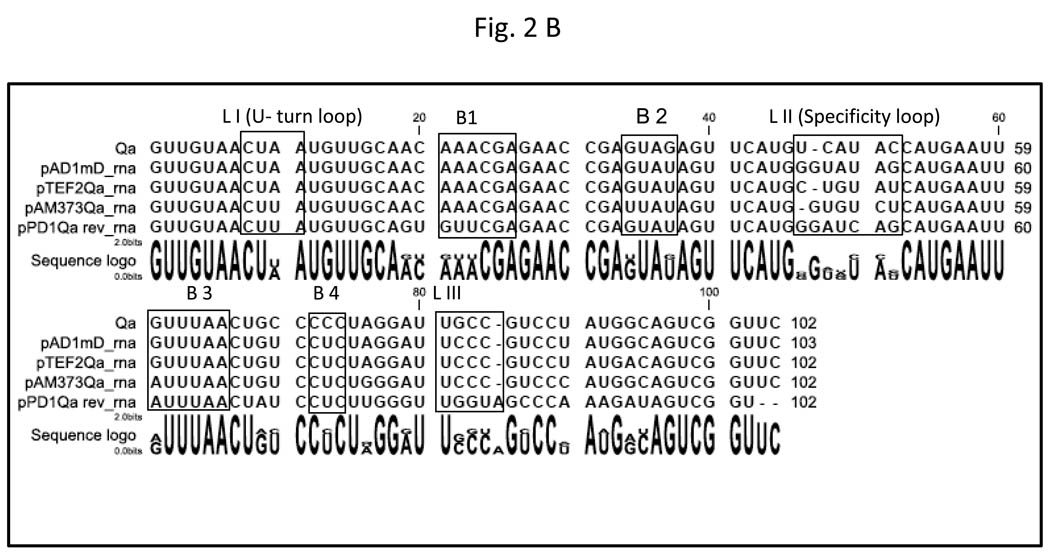
In order to evaluate the mechanism by which Anti-Q interacts with nascent prgQ transcripts, it was important first to determine the secondary structure of Anti-Q RNA. Binding of antisense and target RNAs is usually initiated at single stranded regions such as loops and bulges. The computer predicted structure of Anti-Q RNA, as shown in Fig. 2A, suggested a compact clover-leaf structure with three major stem-loops and several bulges. Two potentially important structures were identified in Loop I and Loop II. Loop I contains a potential “U–turn motif” that has been demonstrated to accelerate the interaction between complementary RNAs in other RNA regulated systems (Franch et al., 1999). Loop I is highly conserved in Anti-Q homologs identified on other pheromone responsive plasmids (Fig. 2B). Loop III and the four bulges are fairly conserved with mostly single nucleotide variability except in the case of pPD1 which appears to be more divergent. In contrast, Loop II is highly variable with each molecule showing multiple base substitutions. Based on this bioinformatic analysis, it was hypothesized that Loop II provided the specificity for Anti-Q-Qs interaction while Loop I was important for controlling the rate of the interaction.
Fig. 2. Secondary structure of Anti-Q RNA.
A. The RNA secondary structure of Anti-Q was predicted using Sfold analysis (http://sfold.wadsworth.org/). Various stem loops (SL1-3), isolated stems (S1 and S2); and bulges (B1-4) were predicted and are as marked. Loop I harbors the U-turn motif, 5’-CUAA-3’ corresponding to the 5’-YUNR-3’ consensus. Loop II was designated as the specificity loop. Pb(II) cleavage sites are indicated with arrows. The ‘✳’ symbol denotes Pb (II) hypersensitive sites. The circled U residue in Loop I was mutated to a C in the YUNR mutant. In the Anti-Q-pAD1 mutant the entire 5’-UCAUAC-3’ sequence of Loop II was replaced with the 5’-GGUACGA-3’ sequence of the corresponding Loop II of the pAD1 Anti-Q homolog, mD (Pontius and Clewell, 1992; Tomita and Clewell, 2000). B. Sequence comparison of Anti-Q with pAD1 mD and other putative related regulatory RNAs. The sequence of mD RNA was as previously published (Pontius and Clewell, 1992; Tomita and Clewell, 2000). Other sequences were derived by examination of analogous regions of other sequenced pheromone responsive plasmids pTEF2 (Paulsen et al., 2003), pAM373 (De Boever et al., 2000), and pPD1 (Nakayama et al., 1995). Alignment was performed using CLC Combined Workbench under default settings. Stem loops and bulges are represented by labeled boxes.
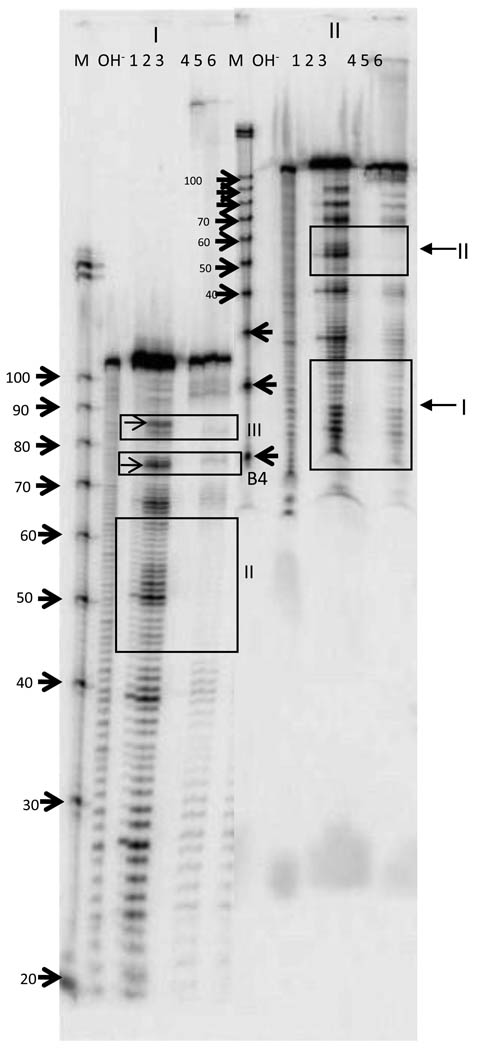
To confirm the predicted structure of Anti-Q RNA, secondary structural analysis of in vitro produced Anti-Q RNA was performed by partial Pb(II) cleavage. The cleavage pattern of Anti-Q is shown in Fig 3 and Fig. S1 and the sites of Pb(II) cleavage are mapped to the predicted Anti-Q structure in Fig. 2A. As predicted, Loop I and Loop II provided prominent cleavage clusters at nucleotides 9–12 and 46–51, respectively, centrally located between regions of relative insensitivity identifying the stems. In each case, the 5’ nucleotide of the loop appeared to be hypersensitive to cleavage. In addition, cleavage was observed at nts 80 – 83, the predicted position of Loop III. The structures carrying these single stranded loops were designated SL (stem loop) 1, 2, and 3 respectively. Further lead cleavages occurred between the SL1 and SL2 at nts 21–26 and nts 34–37 at predicted single strand bulges, B1 and B2. Two more bulges were observed between SL2 and SL3 at nts 60– 65 and nts 71– 73 and these bulges were named B3 and B4, respectively.
Fig. 3. Secondary structure analysis of Anti-Q using Pb(II) probing.
Anti-Q RNA transcript was produced in vitro and end labeled. 32P- labeled RNA was subjected to partial cleavage with Pb(II) and fractionated on 10–12% denaturing polyacrylamide gels. Autoradiograph is shown with the concentration of Pb(II) in mM amounts indicated above each lane. A hydroxyl ladder and a size standard marker, Decade Marker from Ambion (10, 20, 30, 40, 50, 60, 70, 80, 90, 100 nucleotides) were also resolved on the same gel. The position of bands of the size standard are marked by the arrows on the left, but the actual ladder is not shown in this image. As mentioned in Materials and Methods, in vitro transcription adds 4 nt to the 5’ end of the Anti-Q transcript relative to that shown in Fig. 1 so the positions of cleavage sites are modified accordingly. The positions of different structures identified corresponding to the proposed structure of Anti-Q in Fig. 1 are as indicated. In both SL1 and SL2, the first nucleotide in the loop appears to be more accessible than the others; these bands are indicated by arrows on the right side of the gel. Longer runs showing better resolution of the 3’ end structures are shown in Figure S1.
Thus, the Pb(II) probing data supported the predicted secondary structure of Anti-Q. In particular, Pb(II) cleavage data demonstrated that the putative U- turn motif and specificity loop were exposed on single stranded loops that could provide the primary sites of interaction with nascent Qs.
3.2. Analysis of the Anti-Q/Qs binding mechanism
To further investigate Anti-Q-Qs interaction, complex formation was initially evaluated using gel shift assays. RNA-RNA interactions controlling plasmid functions frequently must occur within a “window of opportunity” in which the target is in the proper conformation to allow interaction with the regulator (Brantl and Wagner, 1998). Once transcription of the target has proceeded beyond this point, interaction is no longer efficient. Since the putative function of Anti-Q is to control the balance between terminator and antiterminator structures, it was possible that full length Qs might not interact efficiently with Anti-Q since the terminator was already formed. Therefore, a shorter transcript without the Qs terminator region was constructed and designated Qso/t. Gel shift experiments with both labeled Anti-Q and each unlabeled Qs construct and labeled full length Qs and Qso/t with unlabeled Anti-Q were performed. In head to head comparisons, a greater proportion of complex was consistently seen with Qso/t rather than full length Qs (data not shown). The proportion of Anti-Q RNA in complex after thirty minutes of incubation with Qs was measured as a ratio of shifted Anti-Q RNA to the total amount of Anti-Q RNA. On average, 27±5 % of the labeled Anti-Q was shifted with Qso/t while only 18±3 % of the labeled Anti-Q was shifted in the presence of 20 molar excess of Qs. Although this difference was not statistically significant due to high variability between individual experiments, Qso/t was used routinely for further experiments because it consistently shifted more Anti-Q. The rate of interaction of Anti-Q and Qso/t as determined by gel shift was 3 ± 0.8 × 103M−1s−1. This is very slow compared to RNA interaction in other antisense RNA regulated systems where second order binding rate constants between 1.0 × 106M−1s−1 and 5.0 × 106M−1s−1 are observed (Franch et al., 1999). Furthermore, even after prolonged incubation, complete shifting of Anti-Q was not observed (data not shown). This suggested that the gel shift approach, while clearly showing that the Anti-Q and Qs RNAs interact, may not be optimal for measuring interaction rates. These results also suggested the neither Qs nor Qso/t are the optimal interacting partner with Anti-Q. However, six other truncated Qs products, from Qs266 lacking the entire 3’ side of the antiterminator structure to Qs367 lacking only half of the 3’ side of the terminator structure, showed no significant difference from Qso/t in their interaction with Anti-Q (data not shown). Further work will be required to determine whether other nascent Qs products interact more strongly with Anti-Q.
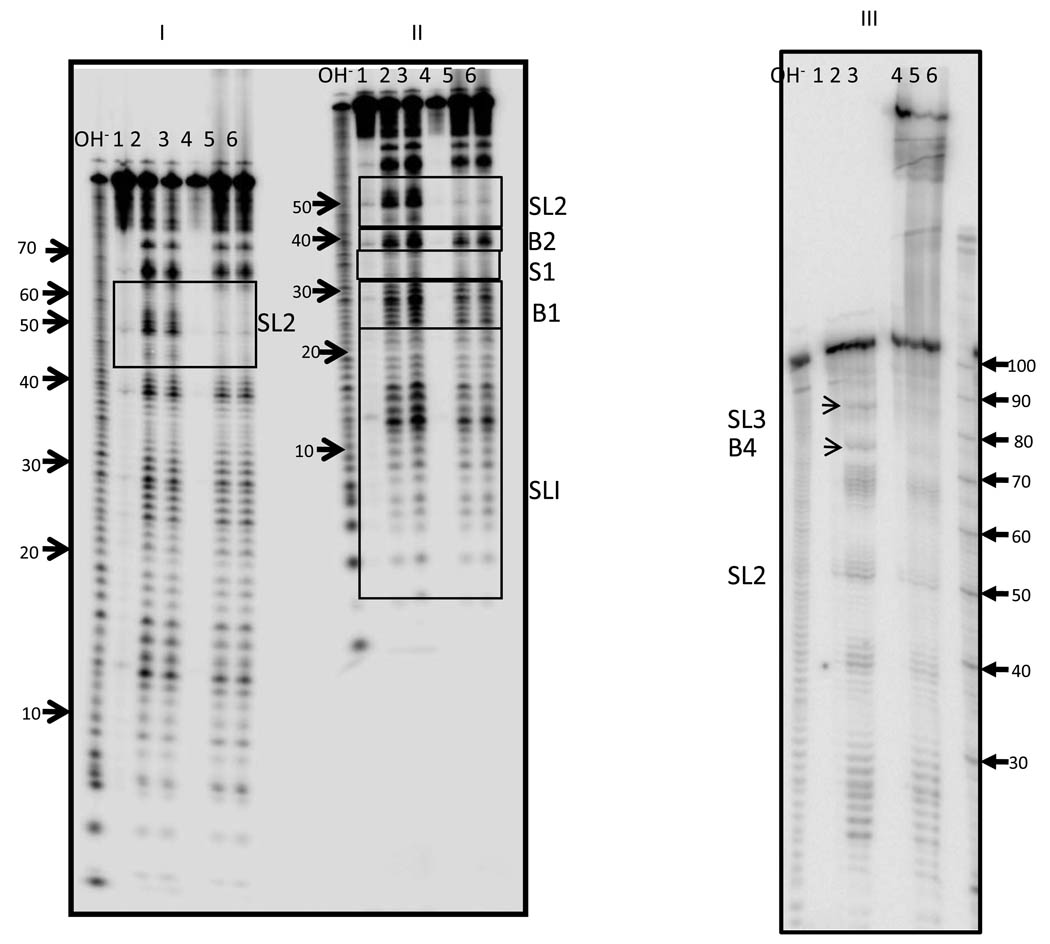
To more accurately measure the interaction of Anti-Q and Qs, interaction points in the Anti-Q-Qso/t complex were determined by identifying regions of protection from Pb(II) cleavage in the Anti-Q structure in complex. While results shown in Fig. 4 show Qso/t-mediated protection at the specificity loop (II in Fig. 4), no protection was observed at the Loop I U-turn motif (I). Protection was also observed in some bases of Loop III (III) and bulge 4 (B4). A similar pattern of protection was observed when labeled Anti-Q was incubated with full length Qs (data not shown). The rate of interaction of the two RNAs was determined, based on the kinetics of specificity loop protection, to be 3.6 ± 0.14 × 106M−1s−1 . This rate of binding is similar to that of other antisense RNA bimolecular interactions.
Fig. 4. Structural analysis of complex formed by Anti-Q and Qso/t interactions.
Anti-Q RNA transcript was produced in vitro and end labeled. 32P- labeled Anti-Q RNA was allowed to interact with 10 molar excess of unlabeled Qso/t transcript at 37°C for 30 minutes and the complex was subjected to partial cleavage with Pb(II). Digestion reactions were run on 10–12% denaturing polyacrylamide gels. End labeled Anti-Q RNA was also subjected to partial Pb(II) cleavage and resolved on the same gel. The autoradiograph of the cleavage pattern at different run lengths is shown in panels, I and II. A hydroxyl ladder (lane OH−) and a size standard marker (lane M) (10, 20, 30, 40, 50, 60, 70, 80, 90, 100 nucleotides) were also resolved on the same gel. Marker bands are indicated by arrows with their sizes. Due to tightness of space, arrows for the fastest three migrating marker bands are on the right in panel II, and are 10, 20 and 30 nucleotides respectively. Lane 1, Anti-Q- 0mM Pb(II); Lane 2, Anti-Q- 40mM Pb(II); Lane 3, Anti-Q- 120mM Pb(II); Lane 4, Anti-Q-Qso/t- 0mM, Lane 5, Anti-Q-Qso/t- 40mM; and Lane 6, Anti-Q-Qso/t- 120mM. The positions of Loop I (I), Loop II (II), Bulge 4 (B4), and Loop III (III) are boxed and labeled. Note that while all bands of Loop II appear to be protected in complex, the protection at Loop III and bulge 4 is more subtle with prominent bands protected (arrows) above and below fainter bands, respectively.
3.3. Effect of mutation of the U turn motif of Anti-Q on Anti-Q-Qso/t complex formation
The lack of interaction at the U-turn motif was somewhat surprising and worthy of further investigation. To better evaluate the role of Loop I in the interaction between Anti-Q and Qs, an Anti-Q mutant with a defective U-turn motif was constructed. In this mutant, the invariant U at nt 9 of the U-turn motif (see Fig. 2A) was mutated to C. A complementary change was also made in the Qso/t so that the effect of the U-turn structure could be examined without affecting complementarity. To assess the possibility that the interaction at the U-turn motif might be transient yet required for interaction at the other loops, interaction was assessed by gel shift and Pb(II) cleavage structural analysis. No difference in interaction was observed in gel shift experiments (data not shown) and, as shown in Fig. 5, protection was still observed at Loop II and III and bulge 4 in the U-turn loop mutant. To assess the possibility that, while not required for interaction, the U-turn motif might be required for optimal interaction rates, the rate of protection of Loop II was determined. The rates of interaction of the Anti-Q-U-turn mutant with Qso/t U-turn mutant and wild-type Qso/t were determined to be 4.4 ± 0.21 × 106M−1s−1 and 4.4 ± 0.98 × 106M−1s−1 respectively, not significantly different from the rate of interaction of the wild-type Anti-Q with Qso/t, suggesting that the U-turn motif was not important for this interaction.
Fig. 5. Secondary structural analysis of Anti-Q-Qso/t-U turn mutant interactions.
Anti-Q-Umut RNA transcript was produced in vitro and end labeled. 32P- labeled Anti-Q-Umut RNA was allowed to interact with 10 molar excess of unlabeled Qso/t-U transcript at 37°C for 30 minutes and the complex was subjected to partial digestion with Pb(II). Digestion reactions were run on 10–12% denaturing polyacrylamide gels. End labeled Anti-Q-Umut RNA was also subjected to partial Pb(II) cleavage and resolved on the same gel. The autoradiograph of the cleavage pattern at different run lengths is shown in panels, I, II and III. A hydroxyl ladder and a size standard marker (10, 20, 30, 40, 50, 60, 70, 80, 90, 100 nucleotides) were also resolved on the same gel. Lane 1, Anti-Q-U- 0mM Pb(II); Lane 2, Anti-Q-U- 40mM Pb(II); Lane 3, Anti-Q-U- 120mM Pb(II); Lane 4, Anti-Q-U-Qso/t-U- 0mM, Lane 5, Anti-Q-U-Qso/t-U- 40mM; and Lane 6, Anti-Q-U-Qso/t-U- 120mM. The positions of Loop I, Loop II, Bulge 4, and Loop III are as indicated. While protection of Loop II is clearly apparent in panels I and II, B4 and Loop III bands are obscured by heavy exposure. A longer run more clearly showing the prominent B4 and Loop III cleavages in uncomplexed Anti-Q and their absence in complex is shown in panel III.
These in vitro results suggested that the U-turn motif was not important for interaction of Anti-Q with Qs under these conditions and might, therefore, not be required to ensure proper termination of Qs at the T1 terminator. To evaluate the role of the U-turn motif in termination, the U-to-C mutation was introduced in pBK2 (Kozlowicz, B. K., 2005 Ph.D. Dissertation), a pheromone response reporter plasmid with the lacZ gene fused downstream of the IRS1 terminator. Since Anti-Q and Qs are transcribed from opposite strands of the same DNA in this construct, the mutant plasmid, designation pBK2–24, retains complementarity of the interacting RNAs but disrupts the U-turn motif. The U-turn motif mutation resulted in a slight increase in uninduced levels of readthrough into the lacZ (170 ± 1.41 MU in the mutant vs. 108 ± 36.76 MU in the wild type) and no effect in pheromone-induced cells (734 ± 72.12 MU in the mutant vs. 677 ± 94.75 MU in the wild type). Therefore, both in vitro and in vivo results support the conclusion that the U-turn motif present in Loop I is not required for Anti-Q-mediated regulation of termination/antitermination of Qs transcription.
3.4. Effect of mutation of the specificity loop of Anti-Q on the Anti-Q-Qso/t complex formation
The lack of an effect of U-turn motif mutations, either in vitro or in vivo, on the interaction of Anti-Q with Qs indicated that interaction at Loop II may be critical for complex formation. To investigate the role of Loop II in Anti-Q-Qs interaction and its role in determining the specificity of interaction, a construct was generated wherein the specificity loop of the Anti-Q RNA was mutated to incorporate the sequence of the specificity loop of mD RNA, the pAD1 Anti-Q counterpart (Pontius and Clewell, 1992; Tomita and Clewell, 2000). This mutant was named Anti-Q-pAD1. In addition, constructs for in vitro production of mD RNA (Pontius and Clewell, 1992; Tomita and Clewell, 2000) and the Qso/t counterpart of pAD1 were also generated. Gel shift assays were carried out to determine the specificity of binding interactions of Anti-Q, Anti-Q-pAD1, and mD with Qso/t and Qso/t-pAD1. The average percent shifted from three experiments is shown in Table 2. Anti-Q and mD interacted well with their cognate Qs targets but poorly with their non-cognate targets. Complex formation was still observed between Anti-Q-pAD1 RNA and Qso/t, although migration of the complex was altered presumably due to an altered conformation of the complex due to the lack of interaction at Loop II (Fig. S2). Pb(II) probing analysis confirmed that interaction at Loop II was lost in this complex and interaction at Loop I did not appear (data not shown). Anti-Q-pAD1 interacted better with Qso/t-pAD1 than did Anti-Q, but not as well as did mD. This was evident both in the percent shifted and the large amount of partially shifted complex that was not observed with mD (Fig S2). And while Anti-Q-pAD1 interacted with Qso/t, mD interacted only with Qso/t-pAD1. These results, as well as those shown in Fig. 5, suggested that the Loop II sequence is important for determining the specificity of Anti-Q-Qs interaction. Other base differences between Anti-Q and mD, e.g. in B4 and Loop III, are likely to also contribute.
Table 2.
Specificity of binding interactions: Percent shift of labeled Anti-Q/ Anti-Q-pAD1/ mD.
| Anti-Q | Anti-Q-pAD1 | mD | |
|---|---|---|---|
| Qso/t | 42.7 ± 1.27 | 50.9 ± 13.45 | 4.46 ± 4.45 |
| Qso/tpAD1 | 4.61 ± 0.26 | 58.91 ± 2.92 | 75.77 ± 3.26 |
Note: Either the shifted or unshifted counts were used in determining the percent shifted. Partially shifted bands were not included.
4. Discussion
For antisense-mediated regulation of plasmid functions, efficient inhibition frequently relies on a rapid bimolecular interaction between the regulatory RNA and its nascent target in a specific window of opportunity. Binding of the antisense/sense RNAs may initiate by loop-loop or linear region-loop interactions. In general, the interacting loops have a high GC content and comprise 5–8 nucleotides and the stems longer than 10 base pairs must contain bulges to prevent RNase III digestion. These structures allow an efficient binding of the antisense/sense RNAs (Hjalt and Wagner, 1992; 1995). In cis-acting antisense RNAs, a U-turn motif frequently stimulates the interaction between regulator and target (Brantl, 2007). Two examples of antisense-mediated regulation of conjugation have been closely examined. Conjugation is regulated at the level of translation in the E. coli F plasmid, where interaction with the FinP regulatory RNA blocks the ribosome binding site for the key positive regulator, TraJ (Brantl, 2002b; Gubbins et al., 2003). In contrast, conjugation in the E. faecalis plasmid pCF10 is regulated at the level of transcription termination. Based on analyses of cis-acting point mutations and RNA analysis, it was suggested that Anti-Q interacts with the complementary region of the prgQ transcript and promotes the formation of a terminator structure, which in turn prevents the expression of downstream conjugative loci (Bae et. al., 2004).
In order to test the model for Anti-Q function and to further elucidate the interaction between Anti-Q and the nascent prgQ/Qs transcript, the structure of Anti-Q and its relevant points of interaction with Qs were determined. Anti-Q forms a cloverleaf structure whose single stranded regions include only three loops and four bulges. Interaction studies demonstrated that the primary sites of interaction between Anti-Q and a version of Qs lacking the IRS1 terminator stem-loop occurred at Loop II and less prominently at Loop III and bulge 4 (B4). Binding rate analysis using protection of the Loop II nucleotides as an indicator of interaction revealed a binding rate constant of approximately 4 × 106M−1s−1, consistent with that observed in other antisense RNA controlled systems. Phylogenetic analysis indicated that the Loop II sequence was highly divergent between Anti-Q homologs in other pheromone-responsive plasmids, suggesting that this structure might provide specificity for suppression of conjugation. This conclusion is supported by the strength of interaction at this site. However, the observation that substitution of the Anti-Q for the mD Loop II sequence allowed interaction with Qs RNAs from both pCF10 and pAD1 rather than resulting in a specificity switch, suggests that additional nucleotide differences, for instance at Loop III and B4, may be involved in determining specificity.
The Loop I sequence contains a U-turn motif which has been shown in other systems to be important for RNA-RNA interactions. The disruption of inter-loop interactions by mutating U- turn motifs has shown a significant reduction in binding rate constants in other systems (Dix et. al., 1986; Ashraf et. al., 1999, Franch et. al., 1999). Surprisingly, analysis of the Anti-Q-Qs interaction showed no interaction at Loop I. Furthermore, mutation of the Anti-Q U-turn motif had no effect on either the pattern of interaction or the binding rate constant regardless of whether it was interacted with Qs with or without a complementary mutation. Additionally, in vivo analysis in which the U-turn mutation was introduced into the natural Anti-Q-Qs context showed little effect on readthrough of the IRS1 terminator. Although Loop II mutants still interacted with Qs, Loop I contacts still were not observed in this complex. It is possible that Loop I might show interaction with an earlier conformation of Qs than the one used here, this explanation does not account for the in vivo results. It is possible that alterations in the balance of Anti-Q and Qs resulting from separating the locus from the rest of pCF10 may obscure subtle effects of Loop I U-turn motif. However, it should be noted that in this construct the Anti-Q and Qs genes are in their natural cis context, utilize their own promoters and include all known regulators necessary to provide their normal production levels. Northern analysis indicates that the normal ratio of AntiQ:Qs is maintained in this construct; the only observable difference between pBK2 and pCF10 is an approximate doubling of both RNAs consistent with the two-fold difference in copy number between the two replicons (data not shown). Therefore, the effects of cloning, if any, should be minimal. Cumulatively, these data indicate that the U-turn motif of Anti-Q Loop I is not involved in the interaction with nascent Qs that regulates the termination/antitermination decision. Since the detailed secondary structure of Qs was not determined in this study, the possibility that U-turn motifs in that molecule might be involved in the Anti-Q-Qs interaction cannot be ruled out. It is also possible that, rather than controlling the Qs termination/antitermination decision, the Loop I U-turn motif plays a role in the interaction of nascent Anti-Q with full length Qs to control readthrough into the prgX gene thereby controlling post-conjugation shut-down of the pheromone response. This possibility is currently under investigation.
The regulatory interactions occurring in and around the PQ and PAnti-Q promoters are multi-faceted, balancing the need for constitutive production of iCF10 with that for effective termination at IRS1. In addition to the Anti-Q-Qs interaction, they involve interaction of PrgX with its regulatory binding sites and proper processing of the PAnti-Q message to produce appropriate amounts of Anti-Q and PrgX. The details of the interaction between Anti-Q and Qs described in this manuscript provide the groundwork for further studies on this complex regulatory system.
Supplementary Material
Fig. S1. Secondary structure analysis of Anti-Q using Pb(II) probing. In vitro transcripts were labeled and processed is in Fig. 3, but fragments were separated for longer times on the gel to better resolve structures at the 3’ end of Anti-Q. Structures are labeled as in Fig. 3.
Fig. S2. Effects of mutations in Qa Loop II on the specificity of interaction. Radiolabelled Qa (lanes 1–5), Qa-pAD1 (lanes 6–10), and mD (lanes 11–15) RNA were incubated with 10 molar excess of Qso/t (2nd and 3rd lane of each set) or pAD1Qs (4th and 5th lane of each set) in binding buffer at 37°C for 30 minutes. The reactions were terminated and samples were run on a 5% denaturing polyacrylamide gel. The unbound and bound Qa are as indicated. For positive controls, the samples were gradually heated to 65°C and gradually cooled back to 37°C. Lane 1, Qa; Lane 2, Qa-Qso/t; Lane 3, Qa-Qso/t- positive control; Lane 4, Qa-QspAD1; Lane 5, Qa-QspAD1- positive control; Lane 6, Qa-pAD1; Lane 7, Qa-pAD1-Qso/t; Lane 8, Qa-pAD1Qso/t-positive control; Lane 9, Qa-pAD1; Lane 10, Qa-pAD1-QspAD1- positive control; Lane 11, mD; Lane 12, mD-Qso/t; Lane 13, mD-Qso/t- positive control; Lane 14, mD-QspAD1; and Lane 15, mD-QspAD1- positive control.
Acknowledgements
We acknowledge the technical assistance of Shirisha Reddy from our laboratory. This work was supported by Public Health Service grant GM55544 and the Division of Basic Biomedical Sciences, Sanford School of Medicine (Weaver) and GM49530 (Dunny).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ashraf SS, Ansari G, Guenther R, Sochacka E, Malkiewicz A, Agris F. The uridine in “U-turn”: contributions to tRNA-ribosomal binding. RNA. 1999;5:503–511. doi: 10.1017/s1355838299981931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae T, Clerc-Bardin S, Dunny GM. Analysis of Expression of prgX, a key negative regulator of the transfer of the Enterococcus faecalis phermone-inducible plasmid pCF10. J. Mol. Biol. 2000;297:861–875. doi: 10.1006/jmbi.2000.3628. [DOI] [PubMed] [Google Scholar]
- Bae T, Dunny GM. Dominant negative mutants of PrgX: Evidence for a role of PrgX dimerization in negative regulation of pheromone-inducible conjugation. Mol. Microbiol. 2001;39:1307–1320. doi: 10.1111/j.1365-2958.2001.02319.x. [DOI] [PubMed] [Google Scholar]
- Bae T, Kozlowicz BK, Dunny GM. Characterization of cis-acting prgQ mutants: evidence for two distinct repression mechanism by Anti-Q RNA and PrgX protein in pheromone-inducible enterococcal plasmid pCF10. Mol. Microbiol. 2004;51:271–281. doi: 10.1046/j.1365-2958.2003.03832.x. [DOI] [PubMed] [Google Scholar]
- Benito Y, Kolb FA, Romby P, Lina G, Etienee J, Vandenesch F. Probing the structure of RNAIII, the Staphylococcus aureus agr regulatory RNA, and identification of the RNA domain involved in repression of protein A expression. RNA. 2000;6:668–679. doi: 10.1017/s1355838200992550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brantl S. Antisense RNAs in plasmids: control of replication and maintenance. Plasmid. 2002a;48:165–173. doi: 10.1016/s0147-619x(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Brantl S. Antisense-RNA regulation and RNA interference. Biochem. Biophys. Acta. 2002b;1575:15–25. doi: 10.1016/s0167-4781(02)00280-4. [DOI] [PubMed] [Google Scholar]
- Brantl S. Regulatory mechanisms employed by cis-encoded antisense RNAs. Curr. Opin. Microbiol. 2007;10:102–109. doi: 10.1016/j.mib.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Brantl S, Wagner EGH. Kissing and RNA stability in antisense control of plasmid replication. Trends Biochem. 1998;23:451–454. doi: 10.1016/s0968-0004(98)01322-x. [DOI] [PubMed] [Google Scholar]
- Chung JW, Dunny GM. Transcriptional analysis of a region of the Enterococcus faecalis plasmid pCF10 involved in positive regulation of conjugative transfer functions. J. Bacteriol. 1995;177:2118–2124. doi: 10.1128/jb.177.8.2118-2124.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell DB, Tomich PK, Gawron-Burke MC, Franke AE, Yagi Y, An FY. Mapping of Streptococcus faecalis plasmids pAD1 and pAD2 and studies relating to transposition of Tn917. J. Bacteriol. 1982;152:1220–1230. doi: 10.1128/jb.152.3.1220-1230.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Boever EH, Clewell DB, Fraser CM. Enterococcus faecalis conjugative plasmid pAM373: complete nucleotide sequence and genetic analysis of sex pheromone response. Mol. Microbiol. 2000;37:1327–1341. doi: 10.1046/j.1365-2958.2000.02072.x. [DOI] [PubMed] [Google Scholar]
- Dunny GM, Zimmerman DL, Tortorello ML. Induction of surface exclusion (entry exclusion) by Streptococcus faecalis sex pheromones: use of monoclonal antibodies to identify an inducible surface antigen involved in the exclusion process. PNAS. 1985;82:8582–8586. doi: 10.1073/pnas.82.24.8582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dix DB, Wittenberg WL, Uhlenbeck OC, Thompson RC. Effect of replacing uridine 33 in yeast tRNAPhe on the reaction with ribosomes. J. Biol. Chem. 1986;261:10112–10118. [PubMed] [Google Scholar]
- Fixen KR, Chandler JR, Le T, Kozlowicz BK, Manias DA, Dunny GM. Analysis of the Amino Acid Sequence Specificity Determinants of the Enterococcal cCF10 Sex Pheromone in Interactions with the Pheromone-Sensing Machinery. J. Bacteriol. 2007;189:1399–1406. doi: 10.1128/JB.01226-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fozo EM, Hemm MR, Storz G. Small toxic proteins and the antisense RNAs that repress them. Microbiol. Mol. Biol. Rev. 2008;72:579–589. doi: 10.1128/MMBR.00025-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franch T, Gerdes K. U- turns and regulatory RNAs. Curr. Opin. Microbiol. 2000;3:159–164. doi: 10.1016/s1369-5274(00)00069-2. [DOI] [PubMed] [Google Scholar]
- Franch T, Petersen M, Wagner EG, Jacobsen JP, Gerdes K. Antisense RNA regulation in prokaryotes: rapid RNA/RNA interaction facilitated by a general U-turn loop structure. J. Mol. Biol. 1999;294:1115–1125. doi: 10.1006/jmbi.1999.3306. [DOI] [PubMed] [Google Scholar]
- Gerdes K, Gultyaev AP, Franch T, Pedersen K, Mikkelsen ND. Antisense RNA- regulated programmed cell death. Ann. Rev. Genet. 1997;31:1–31. doi: 10.1146/annurev.genet.31.1.1. [DOI] [PubMed] [Google Scholar]
- Gottesman S. Micros for microbes: non- coding regulatory RNAs in bacteria. Trends Genet. 2005;21:399–404. doi: 10.1016/j.tig.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Greenfield TJ, Ehli E, Kirshenmann T, Franch T, Gerdes K, Weaver KE. The antisense RNA of the par locus of pAD1 regulates the expression of a 33-amino-acid toxic peptide by an unusual mechanism. Mol. Microbiol. 2000;37:652–660. doi: 10.1046/j.1365-2958.2000.02035.x. [DOI] [PubMed] [Google Scholar]
- Greenfield TJ, Ehli Franch T, Gerdes K, Weaver KE. Antisense RNA of the par post-segregational killing system: structural analysis and mechanism of binding of the antisense RNA, RNA II and its target, RNA I. Mol. Microbiol. 2001;42:527–537. doi: 10.1046/j.1365-2958.2001.02663.x. [DOI] [PubMed] [Google Scholar]
- Gubbins MJ, Arthur DC, Ghetu AF, Glover JNM, Frost LS. Characterizing the structural features of RNA/RNA interactions of the F-plasmid FinOP fertility inhibition system. J. Biol. Chem. 2003;278:27663–27671. doi: 10.1074/jbc.M303186200. [DOI] [PubMed] [Google Scholar]
- Hayes F, Daly C, Fitzgerald GF. Identification of the minimal replicon of Lactococcus lactis subsp. Lactis UC317 plasmid pCI305. Appl. Environ. Microbiol. 1990;56:202–209. doi: 10.1128/aem.56.1.202-209.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedberg PJ, Leonard BA, Ruhfel RE, Dunny GM. Identification and characterization of the genes of Enterococcus faecalis plasmid pCF10 involved in replication and in negative control of pheromone-inducible conjugation. Plasmid. 1996;35:46–57. doi: 10.1006/plas.1996.0005. [DOI] [PubMed] [Google Scholar]
- Hjalt TA, Wagner EG. The effect of loop size in antisense and target RNAs on the efficiency of antisense RNA control. Nucleic Acids Res. 1992;20:6723–6732. doi: 10.1093/nar/20.24.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjalt TA, Wagner EG. Bulged-out nucleotides in an antisense RNA are required for rapid target RNA binding in vitro and inhibition in vivo. Nucleic Acids Res. 1995;34:580–587. doi: 10.1093/nar/23.4.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowicz BK, Bae T, Dunny GM. Enterococcus faecalis pheromone responsive protein PrgX: genetic separation of positive autoregulatory functions from those involved in negative regulation of conjugative plasmid transfer. Mol. Microbiol. 2004;54:520–532. doi: 10.1111/j.1365-2958.2004.04286.x. [DOI] [PubMed] [Google Scholar]
- Kozlowicz BK. Minneapolis: University of Minnesota; 2005. Ph.D. Dissertation. [Google Scholar]
- Miller JH. Experiments in Molecular Genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1972. pp. 352–356. [Google Scholar]
- Nakayama J, Ruhfel RE, Dunny GM, Isogai A, Suzuki A. The prgQ gene of the Enterococcus faecalis tetracycline resistance plasmid, pCF10, encodes a peptide inhibitor, iCF10. J. Bacteriol. 1994;176:2003–2004. doi: 10.1128/jb.176.23.7405-7408.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama J, Yoshida K, Kobayashi H, Isogai A, Clewell DB, Suzuki A. Cloning and characterization of a region of Enterococcus faecalis plasmid pPD1 encoding pheromone inhibitor (ipd), pheromone sensitivity (traC), and pheromone shutdown (traB) genes. J. Bacteriol. 1995;177:5567–5573. doi: 10.1128/jb.177.19.5567-5573.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick RP. Autoinduction and signal transduction in the regulation of staphylococcal virulence. Mol. Microbiol. 2003;48:1429–1449. doi: 10.1046/j.1365-2958.2003.03526.x. [DOI] [PubMed] [Google Scholar]
- Paulsen IT, Banerjei L, Myers GS, Nelson KE, Seshadri R, Read TD, Fouts DE, Eisen JA, et al. Role of mobile DNA in the evolution of vancomycin-resistant Enterococcus faecalis. Science. 2003;299:2071–2074. doi: 10.1126/science.1080613. [DOI] [PubMed] [Google Scholar]
- Persson C, Gerhart E, Wagner EGH, Nordstrom K. Control of replication of plasmid R1: kinetics of in vitro interaction between the antisense RNA, CopA, and its target, CopT. EMBO. J. 1988;7:3279–3288. doi: 10.1002/j.1460-2075.1988.tb03195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontius LT, Clewell DB. Conjugative transfer of Enterococcus faecalis plasmid pAD1: nucleotide sequence and transcriptional fusion analysis of a region involved in positive regulation. J. Bacteriol. 1992;174:3152–3160. doi: 10.1128/jb.174.10.3152-3160.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley GJ, Rich A. Structural domains of transfer RNA molecules. Science. 1976;19:796–806. doi: 10.1126/science.790568. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. 2nd edition. Volume 3. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. Appendix B. [Google Scholar]
- Shokeen S, Patel S, Greenfield TJ, Brinkman C, Weaver KE. Translational regulation by an intramolecular stem-loop is required for intermolecular RNA regulation of the par addiction module. J. Bacteriol. 2008;190:6076–6083. doi: 10.1128/JB.00660-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita H, Clewell DB. A pAD1- encoded small RNA molecule, mD, negatively regulates Enterococcus faecalis pheromone response by enhancing transcription termination. J. Bacteriol. 2000;182:162–1073. doi: 10.1128/jb.182.4.1062-1073.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner EGH, Altuvia S, Romby P. Antisense RNAs in bacteria and genetic elements. Adv. Genet. 2002;46:361–398. doi: 10.1016/s0065-2660(02)46013-0. [DOI] [PubMed] [Google Scholar]
- Weaver KE. Emerging plasmid-encoded antisense RNA regulated systems. Curr. Opin. Microbiol. 2007;10:110–116. doi: 10.1016/j.mib.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Winkler WC, Cohen-Chalamish S, Breaker RR. An mRNA structure that controls gene expression by binding FMN. PNAS. 2002;99:15908–15913. doi: 10.1073/pnas.212628899. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Secondary structure analysis of Anti-Q using Pb(II) probing. In vitro transcripts were labeled and processed is in Fig. 3, but fragments were separated for longer times on the gel to better resolve structures at the 3’ end of Anti-Q. Structures are labeled as in Fig. 3.
Fig. S2. Effects of mutations in Qa Loop II on the specificity of interaction. Radiolabelled Qa (lanes 1–5), Qa-pAD1 (lanes 6–10), and mD (lanes 11–15) RNA were incubated with 10 molar excess of Qso/t (2nd and 3rd lane of each set) or pAD1Qs (4th and 5th lane of each set) in binding buffer at 37°C for 30 minutes. The reactions were terminated and samples were run on a 5% denaturing polyacrylamide gel. The unbound and bound Qa are as indicated. For positive controls, the samples were gradually heated to 65°C and gradually cooled back to 37°C. Lane 1, Qa; Lane 2, Qa-Qso/t; Lane 3, Qa-Qso/t- positive control; Lane 4, Qa-QspAD1; Lane 5, Qa-QspAD1- positive control; Lane 6, Qa-pAD1; Lane 7, Qa-pAD1-Qso/t; Lane 8, Qa-pAD1Qso/t-positive control; Lane 9, Qa-pAD1; Lane 10, Qa-pAD1-QspAD1- positive control; Lane 11, mD; Lane 12, mD-Qso/t; Lane 13, mD-Qso/t- positive control; Lane 14, mD-QspAD1; and Lane 15, mD-QspAD1- positive control.