Abstract
Previous work has shown remarkable differences in the pressure-flow relations of the pulmonary circulation between birds and mammals. For example several studies suggest that the avian pulmonary blood vessels behave like rigid tubes, very different from the situation in mammalian lung. We therefore speculated that birds would develop high pulmonary artery pressures when the cardiac output was substantially increased during heavy exercise, for example during flight. However because of the technical difficulties of measuring pulmonary artery pressures in flight, the metabolic rate and cardiac output in anesthetized chickens were increased by infusing 2,4 Dinitrophenol (DNP) and the mean pressure was measured by means of a catheter in the pulmonary artery. Although the pulmonary artery pressure rose steadily as cardiac output increased, it remained below the high levels predicted from the previous studies for similar changes in pulmonary blood flow. Furthermore the increase in pressure was less than in mammals where recruitment and distension of pulmonary capillaries are known to occur. The reasons for this unexpected result are not clear.
Keywords: Pulmonary vascular resistance, pulmonary capillaries, recruitment, distension, pulmonary hypertension, oxygen consumption
1. Introduction
It is remarkable that the evolutionary lines that produced the birds and mammals, and that diverged some 300 million years ago, both resulted in highly efficient lungs but of radically different structure. The pulmonary circulation is of particular interest because of the different environment of the pulmonary capillaries. Several pieces of evidence indicate that the pressure-flow relations in the pulmonary circulation of birds and mammals are very different. For example when Powell et al. (1985) occluded the pulmonary artery to the left lung in anesthetized ducks, they found that the pulmonary vascular resistance more than doubled on the average. Similar results were reported in chickens (Wideman, 2001). These findings suggest that the pulmonary blood vessels behave like rigid tubes. By contrast the same intervention in human and dog lungs causes a marked fall in pulmonary vascular resistance of the unoccluded lung because of recruitment and distension of pulmonary capillaries (Widimsky and Kasalicky, 1964; Harris et al., 1968; Permpikul et al., 2000).
Other experiments suggest that the avian pulmonary circulation behaves like a system of rigid tubes. In perfused in situ preparations, the pulmonary vascular resistance of the lungs of chickens remained essentially constant in spite of large alterations in pulmonary arterial and venous pressures and flow rates (West et al., 2007). Furthermore, morphometric studies of the caliber of pulmonary capillaries in chickens showed only small changes when the capillary transmural pressure was altered over a large range (Watson et al., 2008). A particularly interesting feature of the latter studies was that when the pressure outside the pulmonary capillaries was raised 35 cmH2O above the pressure inside, little change in caliber of the vessels was seen whereas in mammalian lungs under the same conditions, the capillaries collapse completely. We have suggested that this rigidity of the capillaries in both expansion and compression can be explained by the support provided by the surrounding epithelial bridges of the air capillaries (Watson et al., 2007; Watson et al., 2008).
These results raise the intriguing question of what happens to the pulmonary artery pressure in birds during heavy exercise such as flight, accompanied by a proportionately large increase in cardiac output. Flying is very energetic and some birds have higher maximal oxygen consumptions in relation to body weight than any mammals (Tucker, 1972). In addition the aerobic scope of some birds exceeds that of any mammals (Butler, 1991). The pulmonary artery pressure in flying birds has apparently never been measured and indeed this would be a formidable undertaking.
Apart from flight, raising the cardiac output in birds is difficult. Some studies have been made by training chickens or ducks to run on a treadmill (Kiley and Fedde, 1983; Brackenbury and el-Sayed 1985), but the technical problems including measuring pulmonary artery pressure under these conditions are great. In the present study we increased the metabolic rate of anesthetized chickens by the intravenous infusion of 2,4 Dinitrophenol (DNP), a drug that uncouples oxidative phosphorylation and thus causes a substantial increase in cardiac output similar to that occurring during intense exercise. This experimental approach has previously been successfully used to raise the cardiac output in ducks in order to study the effects of increased metabolism on pulmonary gas exchange (Geiser et al., 1984). We placed a catheter in the pulmonary artery to measure the phasic and mean pressure, and cardiac output was determined from the Fick principle. Our hypothesis was that there would be a large rise in pulmonary artery pressure with increasing cardiac output, and that the rise in pressure would be greater than in mammals for a comparable increase in cardiac output. We believe that this is the first time that the relationship between the pulmonary artery pressure and cardiac output in birds has been measured, and certainly under conditions of greatly increased cardiac output.
2. Material and Methods
2.1 Animal Preparation
The animal protocol for this experiment was approved by the Animal Care Committees of the University of California San Diego. White leghorn chickens (Gallus gallus domesticus) weighing between 1.3 and 1.7 kg were anesthetized with intramuscular ketamine/xylazine (33 mg per kg body mass) and a tracheostomy tube was inserted to ensure airway patency. A PE 60 polythene catheter was introduced into the right jugular vein and passed into the pulmonary artery via fluoroscopic control with the aid of a thin flexible guide wire. The position of the catheter was checked by injecting radio-opaque dye and also from the pulmonary artery pressure tracing (Figure 1). The bird was ventilated with a constant volume pump (Harvard Apparatus Co.) with an initial tidal volume of about 15 mL and frequency about 15 min−1. The O2 and CO2 concentrations of the inspired and expired gas were continuously measured by a respiratory mass spectrometer (Perkin Elmer 1100 Medical Gas Analyzer) and the tidal volume was adjusted to give an end-tidal PCO2 between 30 and 35 mmHg. Catheters were inserted into a wing artery and vein. Body temperature was monitored by a thermistor in the cloaca. Blood drawn from the pulmonary and systemic arteries was analyzed using blood-gas electrodes (Instrumentation Laboratory Gem Premier 3000) and the blood oxygen concentrations were measured with an Instrumentation Laboratory 682 Co-Oximeter. The only critical blood measurements for the calculation of cardiac output were the oxygen concentrations in pulmonary and systemic arterial blood, and the Co-Oximeter analyzer was previously calibrated using fully saturated and fully desaturated chicken blood to ensure that the device was linear. Expired gas was collected in a large syringe and the O2 and CO2 concentrations of mixed expired gas were measured with the mass spectrometer. Knowing the oxygen consumption and the oxygen concentrations of the mixed venous and arterial blood, cardiac output was calculated by the Fick principle.
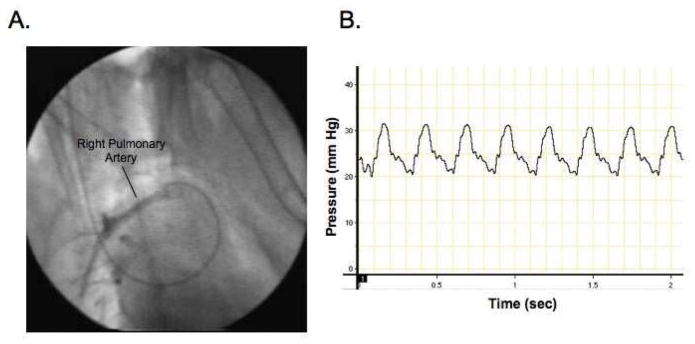
Fig. 1.
Fig. 1A. Fluoroscopic image showing the catheter in the right pulmonary artery Radio-opque dye has been injected.
Fig. 1B. Pressure tracing confirming the position of the catheter in the right pulmonary artery
2.2 Experiment Protocol
Two baseline measurements of mean pulmonary pressure and cardiac output were made after the bird had assumed a steady-state and the mean values are shown in Table 1. Then an intravenous infusion of 7.5 mg of 2,4 DNP was given slowly over a period of 4 min. This dose was based on the previous report by Geiser et al. (1984) and pilot experiments in our laboratory. At the beginning of the infusion the systemic blood pressure sometimes began to fall but then recovered. In addition the bird sometimes made respiratory efforts as previously described by Geiser et al. (1984) who successfully used 2,4 DNP to increase metabolism in anesthetized ducks. During the infusion the ventilation was increased to maintain an end-tidal PCO2 of 30 – 35 mmHg. After the infusion of DNP was completed, a few minutes was allowed for a steady-state to be established based on the expired O2 and CO2 values, and the blood and gas samples were taken. Both pulmonary arterial and systemic arterial samples were analyzed after each dose of DNP and the measurements included PO2, PCO2, pH, oxygen concentration, oxygen saturation, hemoglobin, hematocrit, and base excess. We found that the birds usually developed a metabolic acidosis but this was successfully corrected by injecting 1 mEq of sodium bicarbonate in saline for each 3 mEq fall in measured base excess. In five animals, four infusions of 7.5 mg DNP were given before the animal died and in two animals there were five infusions, while in two animals only three infusions were possible. Death resulted from circulatory failure with a sudden fall in systemic arterial pressure. At this time the muscles of the bird became rigid, presumably as a result of ATP depletion. After death the chest was opened and the position of the right heart catheter in the pulmonary artery was verified.
Table 1.
Mean values with standard errors for 7 chickens
| Units | Baseline | SE | After DNP | SE | P value | |
|---|---|---|---|---|---|---|
| VO2 | mL.min−1 | 12.01 | 0.5 | 48.5 | 2.9 | < 0.001 |
| R | 0.73 | 0.02 | 0.93 | 0.03 | < 0.005 | |
| Arterial O2 concentration | mL.L−1 | 126 | 5 | 94 | 3 | < 0.005 |
| Arterial PO2 | mmHg | 102 | 3.0 | 94 | 3 | < 0.005 |
| Mixed venous O2 concentration | mL.L−1 | 51 | 4 | 23 | 2 | < 0.005 |
| Cardiac output | L.min−1 | 0.17 | 0.02 | 0.69 | 0.05 | < 0.001 |
| Mean PAP | mmHg | 17.2 | 1.3 | 28.9 | 2.8 | < 0.001 |
Note. Baseline measurement before DNP infusions. After DNP: refers to the measurements after the last DNP infusion. R: respiratory exchange ratio. PAP: pulmonary arterial pressure. SE: standard error of mean. P refers to the comparison of baseline and after DNP using paired values.
2.3 Statistics
The effects of an intervention such as infusing DNP on a variable were analyzed using Student’s t test. Significance was accepted at P < 0.05.
3. Results
3.1 Metabolic Rate and Cardiac Output
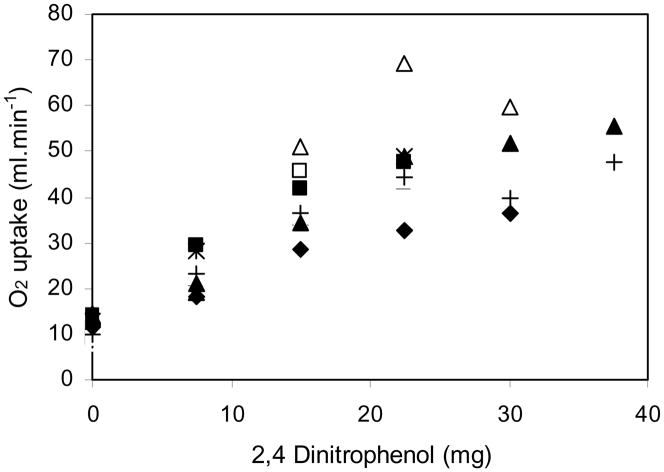
Complete studies were obtained in 7 chickens (Table 1). Figure 2 shows the plot of oxygen consumption against the cumulative DNP dose for every animal, and Table 1 shows some of the variables before the DNP infusion was begun, and after the maximal DNP dose that was tolerated for each animal. Note that the resting O2 consumption averaged 12.0 mL.min−1 and that it increased to a mean of 48.5 mL.min−1, that is by a factor of 4. The increase was similar to that measured in male chickens trained to run at maximum speed on a treadmill (Brackenbury and el-Sayed, 1985). The increase in O2 consumption was accompanied by an increase in CO2 output, the initial respiratory exchange ratio being 0.73 and rising to 0.93. Body temperature rose generally between 2 and 3 °C, which was similar to that found by Geiser et al. (1984) in ducks treated with DNP.
Fig. 2.
Oxygen consumption plotted against the cumulative DNP dose for all animals Note that the DNP infusions resulted in a large increase in oxygen consumption. Different animals are shown by different symbols. R2 = 0.79.
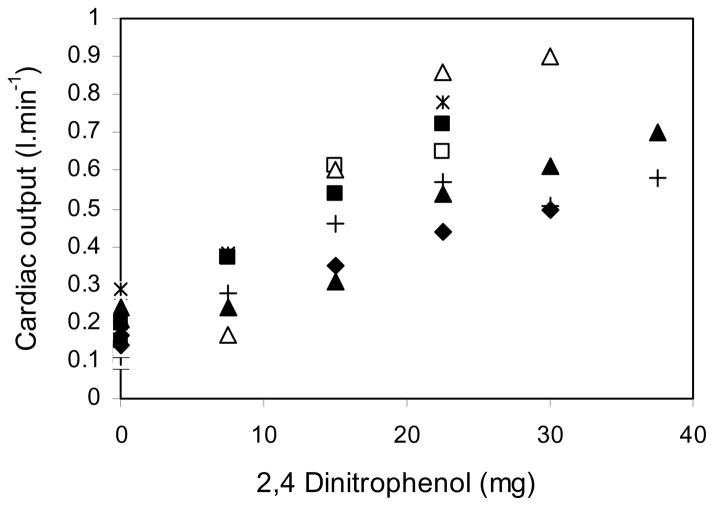
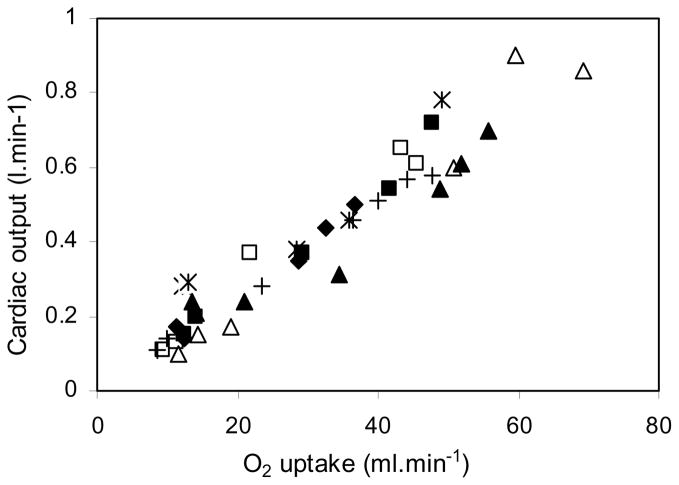
Cardiac output also increased steadily with the cumulative dose of DNP as shown in Figure 3. The mean baseline value was 0.17 L.min−1 and this rose to 0.69 L.min−1 at the end of the DNP infusions, that is a four-fold increase. Most of the rise in cardiac output was the result of an increase in stroke volume because heart rate less than doubled. Figure 4 shows the relationship between cardiac output and oxygen consumption. Both rose by a factor of about 4.
Fig. 3. Cardiac output plotted against the cumulative DNP dose.
The DNP infusion caused a substantial rise in cardiac output. Symbols as in Fig. 1. R2 = 0.73.
Fig. 4. Cardiac output plotted against oxygen consumption.
There is a fairly tight linear correlation. Both increased about four-fold. R2 = 0.92.
3.2 Pulmonary Artery Pressure Response to the Increase in Cardiac Output
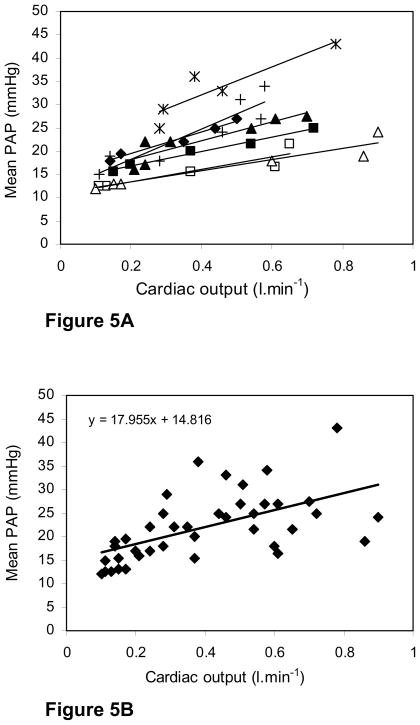
Figure 5A shows the individual data points for all seven chickens with the line of best fit (least squares) for each chicken. Note that the pattern was fairly reproducible although one chicken had substantially higher pulmonary artery pressures than the other 6, for no apparent reason. Figure 5B shows the pooled data for all the animals together with the line of best fit. At rest the mean of the pulmonary artery pressures was 17.2 mmHg which is similar to that in many mammals including man. After the DNP infusion, the mean value was 28.9 mmHg. There was a consistent rise in pulmonary artery pressure with increasing cardiac output with the regression line for all the animals showing a slope of 18.0 mmHg.L −1.min.
Fig. 5.
Fig. 5A. Mean pulmonary artery pressure plotted against cardiac output for all seven chickens The individual lines of best fit (least squares) are shown. In all cases there was a rise in pulmonary artery pressure with increasing cardiac output.
Fig. 5B. Pooled data of the relationship between mean pulmonary artery pressure and cardiac output for all animals with the line at best fit (least squares) The regression equation of this is shown.
4. Discussion
4.1 Comparison of Results with Previous Measurements
It is interesting to compare the data shown in Figures 5A and 5B with previous studies. These include those of Powell et al. (1985) who studied unilateral pulmonary artery occlusion in ducks, previous studies in our laboratory on pressure-flow relations in perfused in situ lungs (West et al., 2007) and measurements of the distensibility of pulmonary capillaries (Watson et al., 2008). It is particularly important to compare the present results with those obtained in ducks because domestication may have altered the pulmonary vasculature of chickens.
Powell et al. (1985) occluded the pulmonary artery to one lung in five anesthetized ducks and found that the mean pulmonary artery pressure increased from 13.9 to 22.0 mmHg. There was essentially no change in cardiac output (0.73 L.min−1 before occlusion and 0.72 L.min−1 after occlusion) and almost no change in left atrial pressure (6.2 mmHg before occlusion and 6.7 after occlusion). Calculated pulmonary vascular resistance more than doubled, rising from 10.6 to 24.1 mmHg.min.L−1. All these are mean values from five animals. Comparable results were reported by Wideman (2001) in chickens. Additional studies from our laboratory showing a rapid essentially linear increase in pulmonary artery pressure with pulmonary flow rate in perfused chicken lungs, and minimal distensibility of pulmonary capillaries over a wide range of capillary transmural pressures are consistent with these earlier measurements. By contrast Figure 5B shows a slower rise in mean pulmonary artery pressure with cardiac output. For example doubling the cardiac output from its mean resting value from 0.17 L.min−1 and using the regression equation of Figure 5B gives an increase in mean pressure from 17.9 to 20.9, that is only 3 mmHg or 17 %. Recall that doubling the blood flow through one lung in the experiments of Powell et al. (1985) caused pulmonary artery pressure to increase by nearly 60 percent.
Pulmonary vascular resistance (PVR) can be estimated from Figure 5B and Table 1, and it shows a considerable fall as cardiac output increases. PVR is calculated from (pulmonary artery pressure – wedge pressure) divided by flow. Pulmonary artery wedge pressure is difficult to measure in chickens but resting values of about 7 mmHg have been reported (Chapman and Wideman, 2001). Assuming this does not change with flow, PVR fell from (17.7−7)/0.17, or 63 mmHg.L−1.min, to (28.9−7)/0.69, or 32 mmHg.L−1.min. In other words it almost halved. If the wedge pressure rose with increasing blood flow which is likely, the fall in PVR would be greater. This marked fall in PVR with increasing flow was not seen in the previous studies (Powell et al., 1985; West et al., 2007; Watson et al., 2008).
An assumption in these studies is that the DNP infusion did not affect the properties of the pulmonary blood vessels. This seems reasonable in view of our earlier studies showing that the pulmonary capillaries in chickens behave as essentially rigid tubes with almost no change in caliber over a large range of capillary transmural pressures (Watson et al., 2008). In fact the capillaries appear to be supported strongly by the surrounding epithelial bridges. To obtain further information on this, the lung was fixed by tracheal instillation of glutaraldehyde at the end of one of the DNP infusions, and the histology was compared with that of a normal chicken with the lung fixed in the same way. The results are shown in Figure 6, and no differences in the appearances of the capillaries or small pulmonary blood vessels can be seen
Fig. 6. Comparison of the histology of lungs in a normal chicken (A and B) and a chicken after DNP infusions (C and D).
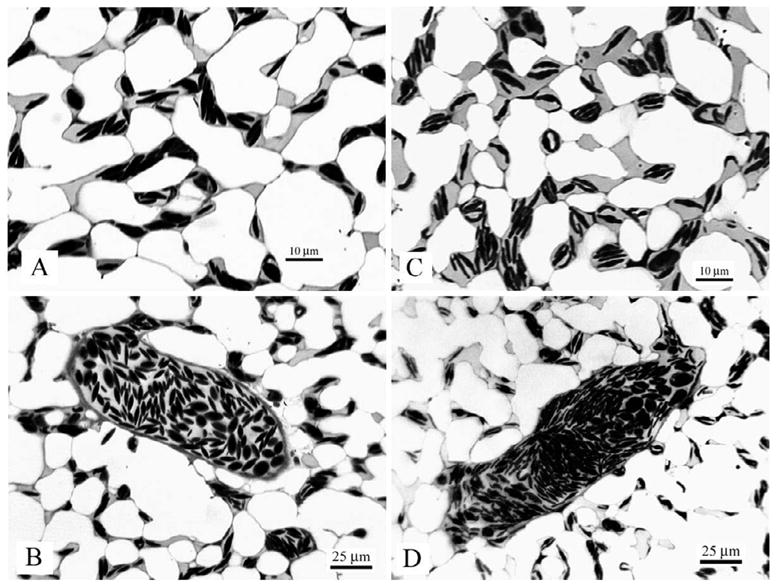
The lungs were prepared by intra-tracheal instillation of glutaraldehyde. No differences are seen.
4.2 Comparison between Chickens and Mammals
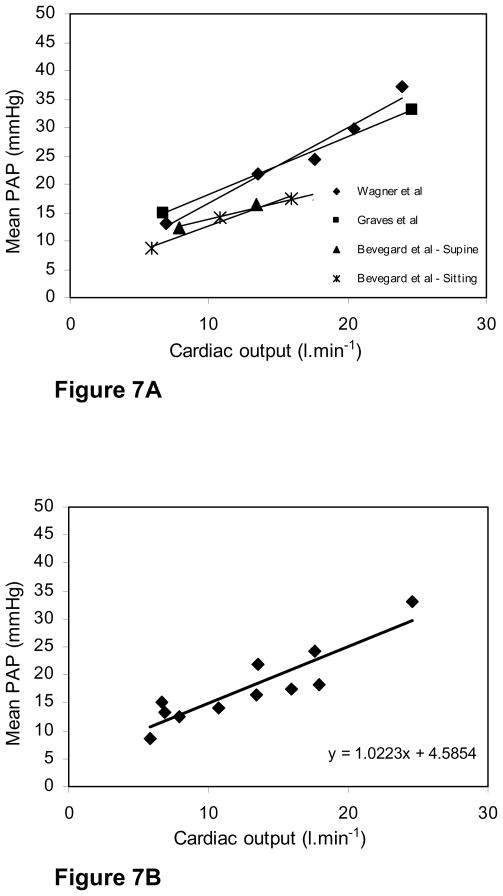
It is interesting to compare our results in chickens with pressure-flow studies in mammals because it is well known that mammalian capillaries undergo marked recruitment and distension as flow rises whereas our previous measurements of capillary morphology in chickens shows that this alters little. Simultaneous measurements of mean pulmonary artery pressure and cardiac output during exercise are available in humans, and Figure 7 shows the results of two sets of studies in normal humans where high levels of cardiac output were obtained by using a bicycle ergometer (Wagner et al., 1986; Groves et al., 1987). In a third study, measurements were made in both the supine and sitting positions with the subjects pushing against resistances with their legs but the maximal exercise levels were lower (Bevegard et al., 1960). There have been a number of earlier studies but in most of these the levels of exercise were very low and therefore the increases in cardiac output were small (Hickam and Cargill, 1948; Riley et al., 1948; Dexter et al., 1951; Donald et al., 1955; Granath et al., 1964).
Fig. 7. Fig. 7A. Increases in mean pulmonary artery pressure with oxygen consumption during exercise in three human studies (15–17).
In two of these, high levels of exercise were obtained, while in the third, measurements were made of subjects in both the sitting and supine positions but the levels of exercise were lower.
Fig. 7B. Pooled data from the three human studies with the line of best fit (least squares) R2 = 0.81.
Figure 7 shows that in the study by Wagner et al. (1986) the cardiac output increased from 6.9 to 23.9 L.min−1 and this was accompanied by an increase in mean pulmonary artery pressure from 13.2 to 37.2 mmHg. Similar results were found in the study by Groves et al. (1987) where the cardiac output increased from 6.7 to 24.6 L.min−1 with the mean pulmonary artery pressure rising from 15.0 to 33. By contrast in the study by Bevegard et al. (1960) the maximal cardiac outputs were much less being about 18 L.min−1 in the supine position and 16 L.min−1 in the sitting posture. Maximal pulmonary artery pressures were correspondingly lower.
To compare the relative rates of rise of pulmonary artery pressure with increase in cardiac output in chickens and humans, the results shown in Figures 5 and 7 need to be scaled. One way to do this is to calculate the rise in pressure for a four-fold increase in cardiac output from its resting value. Table 1 gives the mean resting values for cardiac output and mean pressure for the chicken as 0.17 L.min−1 and 17.2 mmHg respectively. At a four-fold increase of cardiac output, that is 0.68 L.min−1, the regression equation gives a mean pressure of 27.0 mmHg. This is an increase of 9.8 mmHg. The corresponding calculation for humans using the regression equation of Figure 7B and a resting cardiac output of 6.8 L.min−1, gives an increase in mean pressure of from 11.5 to 32.11 mmHg. This is an increase of 20.9 mmHg. Thus the rise in pressure for a scaled increase in cardiac output is larger in humans than in chickens.
4.3 Possible Reasons for the Failure for the Pulmonary Artery Pressure to Rise as Much as Expected
Our hypothesis that pulmonary artery pressure would rise faster with increasing cardiac output in chickens compared with humans was not borne out. Possible reasons for this include the following:
4.3.1
The DNP may have affected the structures supporting the pulmonary capillaries. Two recent studies from our laboratory (West, 2009; West et al., 2010) have clarified the structures surrounding the pulmonary capillaries in chicken lung and discussed how these are potentially able to provide the rigidity that we have observed experimentally. The capillaries are flanked by epithelial bridges composed two extremely thin epithelial cells. The junctions of the bridges with the capillary walls show thickening of the epithelial cells and an accumulation of extracellular matrix. We have suggested that the epithelial bridges themselves may provide some rigidity to the capillaries. Alternatively the junctions between the bridges and the walls could provide a mechanism that shares the hoop stress in the capillary wall when the pressure inside them is increased. This could also contribute to the rigidity of the vessels.
It is possible that the DNP infusion affected the mechanical properties of the epithelial bridges or the junctions of the bridges with the capillary walls. Unfortunately we have no experimental evidence in favor or against this.
4.3.2
The DNP infusion may have reduced the tone of the vascular smooth muscles in small arterioles and therefore reduced pulmonary vascular resistance. This seems unlikely because all the studies on the effects of changing blood flow and vascular pressures on pulmonary vascular resistance in chicken lung point to the rigid capillaries as being the main site of resistance (Watson et al, 2008). Also if DNP had any effect we would expect this to be to increase the tone of the vascular smooth muscle in the same way that it tends to increase the tone of skeletal muscle. However we have no experimental evidence for or against this.
4.3.3
The DNP infusion may have increased the permeability of the capillaries resulting in pulmonary edema. Again there is no evidence to support this. As Figure 6 shows, the capillaries appear normal and there is no sign of edema. Furthermore we inspected the lungs of all the animals at the end of the experiments and saw no evidence of edema. Finally if there was some edema this would be expected to increase pulmonary vascular resistance rather than to reduce it.
4.3.4
The DNP infusion may have resulted in a shift of blood flow between the paleo-pulmo and neo-pulmo. There is no evidence to suggest this. Furthermore what studies have been carried out comparing the appearances of pulmonary capillaries in the paleo-pulmo and neo-pulmo suggest that there are no differences between the two regions of the lung.
Unfortunately therefore we are not able to come up with a reasonable explanation of why the pulmonary artery pressure did not increase faster with increasing cardiac output. Nevertheless the results of these experiments are interesting and presumably the underlying mechanism will emerge with further experiments. Certainly measurements on exercising birds, ideally during flight, would be highly desirable although technically very challenging.
Acknowledgments
We thank Jeff Struthers for technical support. The work was supported by NIH grant R01 HL 60968.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bevegard S, Holmgren A, Jonsson B. Effect of body positions on the circulation at rest and during exercise, with special reference to the influence on the stroke volume. Acta Physiol. 1960;49:279–298. doi: 10.1111/j.1748-1716.1960.tb01953.x. [DOI] [PubMed] [Google Scholar]
- Brackenbury JH, el-Sayed MS. Comparison of running energetics in male and female domestic fowl. J Exp Biol. 1985;117:349–355. doi: 10.1242/jeb.117.1.349. [DOI] [PubMed] [Google Scholar]
- Butler PJ. Exercise in birds. J Exp Biol. 1991;160:233–262. [Google Scholar]
- Chapman ME, Wideman RF., Jr Pulmonary wedge pressures confirm pulmonary hypertension in broilers is initiated by an excessive pulmonary arterial (precapillary) resistance. Poul Sci. 2001;80:468–473. doi: 10.1093/ps/80.4.468. [DOI] [PubMed] [Google Scholar]
- Dexter L, Whittenberger JL, Hayes FW, Goodale WT, Gorlin R, Sawyer CG. Effect of exercise on circulatory dynamics of normal individuals. J Appl Physiol. 1951;3:439–453. doi: 10.1152/jappl.1951.3.8.439. [DOI] [PubMed] [Google Scholar]
- Donald KW, Bishop JM, Cumming G, Wade OL. Effect of exercise on the cardiac output and circulatory dynamics of normal subjects. Clin Sci. 1955;14:37–73. [PubMed] [Google Scholar]
- Geiser J, Gratz RK, Hiramoto T, Scheid P. Effects of increasing metabolism by 2,4-dinitrophenol on respiration and pulmonary gas exchange in the duck. Respir Physiol. 1984;57:1–14. doi: 10.1016/0034-5687(84)90028-8. [DOI] [PubMed] [Google Scholar]
- Granath A, Jonsson B, Strandell T. Circulation in healthy old men, studied by right heart catheterization at rest and during exercise in supine and sitting position. Acta Med Scand. 1964;176:425–446. doi: 10.1111/j.0954-6820.1964.tb00949.x. [DOI] [PubMed] [Google Scholar]
- Groves BM, Reeves J, Sutton JR, Wagner P, Cymerman A, Malconian M, Rock P, Young P, Houston C. Operation Everest II: preservation of cardiac function at great altitude. J Appl Physiol. 1987;63:531–539. doi: 10.1152/jappl.1987.63.2.531. [DOI] [PubMed] [Google Scholar]
- Harris P, Segel N, Bishop JM. The relation between pressure and flow in the pulmonary circulation in normal subjects and in patients with chronic bronchitis and mitral stenosis. Cardiovasc Res. 1968;2:73–83. doi: 10.1093/cvr/2.1.73. [DOI] [PubMed] [Google Scholar]
- Hickam JB, Cargill WH. Effect of exercise on cardiac output and pulmonary artery pressure in normal persons and in patients with cardiovascular disease and pulmonary emphysema. J Clin Invest. 1948;27:10–23. doi: 10.1172/JCI101912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiley JP, Fedde MR. Cardiopulmonary control during exercise in the duck. J Appl Physiol. 1983;55:1574–1581. doi: 10.1152/jappl.1983.55.5.1574. [DOI] [PubMed] [Google Scholar]
- Permpikul C, Wang HY, Kriett J, Konopka RG, Moser KM, Spragg RG. Reperfusion lung injury after unilateral pulmonary artery occlusion. Respirology. 2000;5:133–140. doi: 10.1046/j.1440-1843.2000.00239.x. [DOI] [PubMed] [Google Scholar]
- Powell FL, Hastings RH, Mazzone RW. Pulmonary vascular resistance during unilateral pulmonary artery occlusion in ducks. Am J Physiol. 1985;249:R39–R43. doi: 10.1152/ajpregu.1985.249.1.R39. [DOI] [PubMed] [Google Scholar]
- Riley RL, Himmelstein A, Motley HL, Wiener HM, Cournand A. Studies of pulmonary circulation at rest and during exercise in normal individuals and in patients with chronic pulmonary disease. Am J Physiol. 1948;152:372–382. doi: 10.1152/ajplegacy.1948.152.2.372. [DOI] [PubMed] [Google Scholar]
- Tucker VA. Respiration during flight in birds. Respir Physiol. 1972;14:75–82. doi: 10.1016/0034-5687(72)90018-7. [DOI] [PubMed] [Google Scholar]
- Wagner PD, Gale GE, Moon RE, Torr-Bueno J, Stolp BW, Saltzman HA. Pulmonary gas exchange in humans exercising at sea level and simulated altitude. J Appl Physiol. 1986;61:260–270. doi: 10.1152/jappl.1986.61.1.260. [DOI] [PubMed] [Google Scholar]
- Watson RR, Fu Z, West JB. Morphometry of the extremely thin pulmonary blood-gas barrier in the chicken lung. Am J Physiol Lung Cell Mol Physiol. 2007;292:L769–L777. doi: 10.1152/ajplung.00355.2006. [DOI] [PubMed] [Google Scholar]
- Watson RR, Fu Z, West JB. Minimal distensibility of pulmonary capillaries in avian lungs compared with mammalian lungs. Resp Physiol Neurobiol. 2008;160:208–214. doi: 10.1016/j.resp.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West JB. Comparative physiology of the pulmonary blood-gas barrier: the unique avian solution. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1625–R1634. doi: 10.1152/ajpregu.00459.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West JB, Watson RR, Fu Z. Major differences in the pulmonary circulation between birds and mammals. Resp Physiol Neurobiol. 2007;157:382–390. doi: 10.1016/j.resp.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West JB, Fu Z, Deerinck TJ, Mackey MR, Obayashi JT, Ellisman MH. Structure-Function Studies of Blood and Air Capillaries in Chicken Lung Using 3D Electron Microscopy. Resp Physiol Neuro. 2010;170:202–209. doi: 10.1016/j.resp.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wideman RF., Jr Pathophysiology of heart/lung disorders: pulmonary hypertension syndrome in broiler chickens. World’s Poult Sci J. 2001;57:289–307. [Google Scholar]
- Widimsky J, Kasalicky J. Cardiovascular adaptations to acute pulmonary hypertension. Med Thorac (Basel) 1964;21:369–382. [PubMed] [Google Scholar]