Summary
Ever since T cell exhaustion was initially characterized and thoroughly analyzed in the murine LCMV model, such a functional impairment has been validated in other chronic viral infections such as HIV, HCV, and HBV. In tumor immunology, it has always been postulated that tumor-reactive T cells could also become functionally exhausted due to the high tumor-antigen load and accompanying inhibitory mechanisms. However, the empirical evidences for this hypothesis have not been as extensive as in chronic infection perhaps because much of the focus on T cell dysfunction in tumor immunology has been, and appropriately so, on breaking or bypassing immune tolerance and anergy to tumor/self antigens. Based on recent reports, it is becoming clear that T cell exhaustion also plays a critical role in the impairment of antitumor immunity. In this review, we will comparatively evaluate the T cell responses in cancer and chronic infection, and the therapeutic strategies and interventions for both diseases.
Introduction
The immune system is evolutionarily programmed to respond to a variety of foreign pathogens. Therefore it is not surprising that a significant part of our current understanding of T cell immunity comes from acute and chronic viral infections. Analyses using acute viral models have led to the elucidation of immunological T cell memory, a cardinal property of adaptive immunity, as re-exposure to the same pathogen results in more rapid and robust T cell responses [1-6]. On the other hand, in chronic infections, the persistence of viral antigens results in dysfunctional T cell responses. Therefore, therapeutic vaccines have been designed in hopes of boosting the overall immune response against chronic viral infections, such as HIV [7-9], HBV [10,11], and HCV [12-14]. However, the results were not as promising as initially envisioned, indicating that during chronic viral infections, there exists an intricate network of regulatory mechanisms that are suppressing the necessary immune responses required for pathogen clearance.
Because the important discoveries on immunological memory and functional exhaustion of T cells have been made in acute and chronic viral models [1-6,15-19], they serve as practical models for studying T cell responses in cancer. Tumor immunology has made significant progresses in the past decade, and various modalities of cancer immunotherapy have been used to determine the extent to which anti-tumor responses, in particular the T cell effector function, could be generated. However, the tumor microenvironment, like the immunological milieu of chronic infection, contains a multitude of suppressive mechanisms that allow tumors to escape immune surveillance. Consequently, various treatment methods in tumor immunotherapy have been met with outcomes similar to those seen in chronic infections.
This is a brief review of the features of responding T cells in cancer and chronic viral infection. We will look at the extent to which responding tumor-reactive and chronic viral-specific T cells are similar to and different from each other. In addition, we will discuss current immunotherapeutic treatments for chronic infection and cancer, and future treatment strategies to perhaps overcome immunological barriers that limit the success of tumor and antiviral immunotherapy.
Responding T cells in Chronic Viral Infection
In chronic viral infection, where antigen and/or inflammation persist, virus-specific T cells exhibit various levels of exhaustion. CD8+ T cell exhaustion was first analyzed in chronic LCMV infection of mice [16] and could be described in several stages: partial exhaustion I & II, full exhaustion, and deletion [15,20,21], in which the hierarchical loss of effector cytokines, IL-2, TNF-α, and IFN-γ, and ex vivo cytotoxicity were well-demonstrated. Antigen-independent proliferation was also diminished in exhausted CD8+ T cells, as they were poorly responsive to IL-7 and IL-15 [5]. As for virus-specific CD4+ T cells in chronic LCMV infection, these cells, like their CD8+ T cell brethren, lost the capacity to produce IL-2 and TNF-α immediately post-infection and were unresponsive to rechallenge with antigen [22]. In addition, they exhibited increased production of immunosuppressive IL-10 in the spleen and more significantly in the liver [22]. It has been well-documented that CD4+ T cell help is important for maintaining the functionality of CD8+ T cells during chronic infections [23,24]. Interestingly, there does not seem to be deletion of virus-specific CD4+ T cells, albeit inactivated, during chronic LCMV infection [22], hence a potential for therapeutic restoration of their helper function, which may then increase the CTL response. Last but not the least, T regulatory cells during chronic infection minimize tissue damage, but at the same time, aid the establishment of viral persistence [25].
An extensive genome-wide array analysis has been performed on exhausted viral-specific CD8+ T cells in chronic LCMV infection, compared to effector and memory CD8+ T cells in acute LCMV infection [18]. One of the more pronounced results from the analysis was the overexpression of mRNA for inhibitory cell-surface molecules. It had been determined that PD-1 was highly expressed during chronic LCMV infection and capable of regulating CD8+ T cell exhaustion [19]. This array analysis also showed that PD-1 was one of the most over-expressed inhibitory receptors by exhausted CD8+ T cells. Other highly-expressed inhibitory receptors mentioned in this array analysis were 2B4, CTLA-4, and LAG-3. Neither CTLA-4 nor LAG-3 blockade in vivo improved virus-specific T cell responses in chronic LCMV infection [26], but LAG-3, and not CTLA4, blockade, showed synergy with PD-1 blockade [26]. Furthermore, when compared to genome profiles of memory cells, exhausted CD8+ T cells exhibited decreased expression of cytokine receptors, IL-4Rα, IL-7Rα, and IL-2Rβ, and their unresponsiveness to IL-7 and IL-15 may be explained by deficiencies in cytokine signaling molecules, Jak1 and Stat5b [18]. The gene array analysis also showed that exhausted CD8+ T cells expressed a distinct set of transcription factors, exhibited altered gene expression for chemotaxis, adhesion and migration, and displayed dramatic deficiencies in metabolism and energy [18]. Finally, certain anergy-associated genes, such as Egr-2, Egr-3, and grail, were not selectively expressed in exhausted CD8+ T cells, suggesting that anergy and exhaustion were distinct processes in chronic LCMV infection [18].
Functional exhaustion of T cells was not only observed in chronic LCMV infection but also has been confirmed in other chronic mouse models and human chronic infections. In HIV infection, persistent antigen load has shown to be a major cause for impairment of the ability of HIV viral-specific CD8+ T cells to generate multiple effector cytokines and upregulation of PD-1 [27-29]. In vitro blockade of the PD-1/PD-L1 pathway has shown to improve the effector function of not only HIV [27-29]- but also HCV [30-32]- and HBV [33]-specific CD8+ T cells, which also had upregulated levels of PD-1 during infection. Furthermore, IL-10 production was all increased in HIV [34,35], HCV [36-38], and HBV [39] infections, indicating that like chronic LCMV infection, the IL-10/IL-10R pathway plays a key regulatory role in viral persistence. Another inhibitory molecule that has been garnering attention is the Tim-3 receptor, a member of the T cell Ig and mucin family of proteins with galectin-9 as its ligand. In CD8+ and CD4+ T cells of HIV-infected individuals, Tim-3 was significantly elevated in both T cell types [40]. Similar to PD-1+ CD8+ T cells, Tim-3+ CD8+ T cells correlated positively with viral load and inversely with the number of CD4+ T cells during progressive HIV infection [40]. More interestingly, in HIV infection, Tim-3+ T cells were identified as a functionally exhausted population distinct from PD-1+ T cells, and Tim-3 blockade restored T cell effector function [40]. Subsequent findings of Tim-3 as a regulator of T cell exhaustion have also been made very recently in HCV [41] and HBV [42].
Responding T cells in Cancer
Cancer and chronic infection have been often paired together due to their ability to establish high antigen and immunosuppressive environment. However, a fundamental difference between the two pathogeneses is that viral antigens in general are exogenous and quite immunogenic since no central tolerance is involved, whereas tumor antigens are self-molecules that are weakly immunogenic due to the deletion of high avidity T cells during the thymic selection process. Moreover, high avidity cells that have escaped are inactivated by peripheral tolerance mechanisms. Because of the poor immunogenicity of tumor antigens and the low functional frequency of tumor-reactive T cells, one of the initial methods to overcome these hurdles has been to adoptively transfer in vitro stimulated and expanded tumor-reactive T cells and observe their antitumor responses. It has been shown that tumor-reactive CD8+ cells with central memory qualities confer better antitumor immunity than their effector memory counterparts [43]. In addition, IL-2 treated tumor-reactive CD8+ T cells, albeit highly cytolytic, were shorter lived and were less efficacious in vivo than their IL-15-treated counterparts, partly due to their lack of terminal effector differentiation [44]. Interestingly, induction of Wnt-β-catenin signaling prevented tumor-reactive CD8+ T cells from differentiating into effector cells, but rather promoted the development of self-renewing multipotent CD8+ memory stem cells [45], which exhibited superior proliferative and antitumor properties than both central and effector memory T cells.
Tumor-reactive T cells in high tumor antigen load have shown to respond in an analogous fashion as viral-specific T cells in chronic infection. First, their phenotypic (upregulation of inhibitory molecules and downregulation of cytokine receptors) and functional (loss of production of effector cytokines) profiles resemble those of exhausted T cells from chronic infection. For instance, in a retroviral-induced murine CML model, CML-specific CD8+ T cells displayed upregulation of PD-1 and decreased production of IFN-γ, TNF-α, and IL-2 [46]. Tumor infiltrate lymphocytes (TIL) from human metastatic-melanoma lesions also exhibited similar phenotypic expression and functional impairment. Both CD8+, in particular MART-1-specific, and CD4+ TILs had significantly higher expression levels of PD-1 than peripheral blood T cells and those from normal tissues [46,47]. Phenotypic analysis revealed that compared to T cells from normal tissues and blood, a large proportion of CD8+ TILs were CTLA-4+, which was mainly expressed by PD1+ CD8+ TILs [47]. Furthermore, CD25 and IL-7Rα were lacking in PD1+ CD8+ TILs, indicating that these cells were unable to proliferate, produce effector cytokines, and differentiate into memory cells [47]. CD4+ PD-1 TILs also shared similar phenotypic expression, as they upregulated CTLA-4 and lacked CD25 [47]. Lastly, the impairment of effector function of PD-1+ CD8+ TILs was evident by significant reduction of IFN-γ-production, compared to that of PD-1- CD8 TILs [47]. Another study involving human metastatic melanoma has shown that PD-1 was highly expressed in NY-ESO-1-specific CD8+ TILs, and that PD-1 blockade enhanced the frequency of cytokine-producing cells [48]. Besides PD-1 and CTLA-4, LAG-3 has shown to be expressed in a substantial number of CD8+ TILs in cancer patients and tumor-bearing mice [49,50]. As mentioned previously, Tim-3 has been shown to be upregulated on exhausted T cells in several chronic infections. In tumor settings, it has yet to be determined the extent to which Tim-3 is expressed in TILs and regulates the T cell effector function.
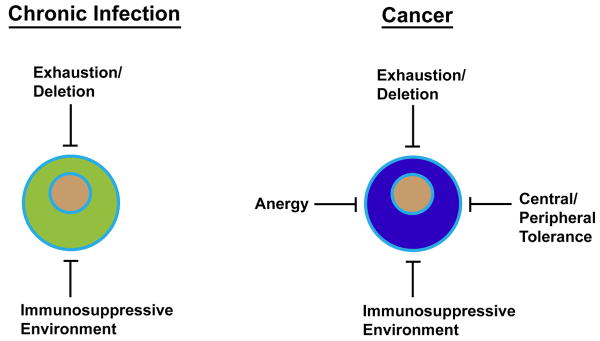
In chronic viral infection, high antigen load is the major driving force in T cell dysfunction through functional exhaustion, but in cancer, anergy also influences the impairment of T cell function (Figure 1). First, tumor cells themselves are poor APCs as they are incapable of expressing costimulatory molecules to provide the second signal, rendering TILs anergic. Immature myeloid-derived dendritic cells (MDC) [51], plasmacytoid DCs (PDC) [52], myeloid-derived suppressor cells (MDSC) [53] and tumor-associated macrophages (TAM) [54] have also shown to be potent inducers of T-cell anergy. It has been suggested that induction of antigen-specific T cell anergy is an early event in the course of tumor progression and significantly occurs before the immunosuppression generally seen in advanced tumor burdens [55]. On the other hand, another study has demonstrated that highly immunogenic tumor growth created antigen overload, causing functional exhaustion and rapid elimination of tumor-reactive T cells [56]. Therefore, from a temporal standpoint, T cell anergy may be dominant early on, but T cell exhaustion likely plays critical roles in the later stages of tumor progression (Figure 2). As stated previously, the gene expression profile of T cell exhaustion has shown to be distinct from anergy in chronic LCMV infection [18]. Thus, a similar analysis at different time points of tumor progression will reveal the extent to which TILs are anergized and/or exhausted at each pathogenic stage, and the results may have important therapeutic implications. For example, if TILs predominantly show the molecular signature of functional exhaustion in advanced tumor burdens, immunotherapeutic modalities that have shown success in chronic viral infections could provide similar therapeutic efficacy in cancer patients particularly in the later phases of their illnesses.
Figure 1.
Comparison of T cell dysfunction between chronic infection and cancer. In chronic infection, T cell dysfunction mainly occurs through functional exhaustion driven by high antigen load. In addition, there is an increased level of IL-10- and Treg-mediated immunosuppression of T cells. In cancer, functional exhaustion and immunosuppressive environment also negatively influence antitumor T cell responses, but there are additional factors that contribute to T cell dysfunction. Since most tumor antigens are endogenous, tumor-reactive T cells are inherently influenced by central and peripheral tolerance mechanisms. Anergy also plays a major part in T cell impairment in cancer. For example, tumor cells lack costimulatory molecules and are unable to provide the second signal to TILs during direct priming, and various antigen presenting cells in the tumor microenvironment have shown to induce T cell anergy.
Figure 2.
Comparison of T cell dynamics between chronic infection and cancer. In chronic infection, antigen load primarily drives T cells to hierarchical exhaustion and ultimately deletion. In cancer, tumor/self-reactive T cells are initially kept in check by central and peripheral tolerance. Anergy is believed to occur immediately in tumor pathogenesis perhaps as early as in in situ cancer, whereas exhaustion/deletion most likely affects T cell function in more invasive cancer stages. One of the main purposes of tumor immunotherapy is to break immune tolerance and anergy. Treg depletion and CTLA-4 blockade can unleash tumor-reactive T cells for a potent antitumor response, but exhaustion/deletion may ultimately limit the treatment efficacy. Therefore, the therapeutic strategies used in chronic infection to rescue T cells from exhaustion, such as PD-1 or PD-1 plus LAG-3 blockade, also should be considered in tumor immunotherapy.
Therapeutic Interventions for Cancer and Chronic Viral Infection
For chronic viral infection, therapeutic interventions aim to counter the effects of the immunosuppressive environment and high antigen load. One approach for boosting T cell responses during chronic infection is therapeutic vaccination (e.g., recombinant vaccinia vaccine, DNA vaccine, peptide vaccine, DC vaccines, etc…), which is to modulate host immune responses in an antigen specific manner by providing a better stimulus for virus-specific T cells. For the most part, the effectiveness of therapeutic vaccines for HIV, HBV, and HCV, as stated previously, has not been as strong as initially expected. Therefore, therapeutic vaccination in combination another immune-based modality may prove to be a more effective strategy to achieve additive or synergistic efficacy. For instance, the combination of LCMV GP33-encoding vaccinia vaccine and anti-PD-L1 blocking antibody significantly improved viral-specific CD8+ T cell immunity and consequently decreased viral load in chronic LCMV infection, compared to either modality alone [57]. Similar enhancement of antiviral T cell responses was seen upon neutralization of IL-10, followed by administration of DNA vaccine encoding LCMV antigen [58].
Just as in chronic infection, therapeutic vaccines have been developed against cancer to increase the effector function of endogenous tumor-reactive T cells. To increase the activation of these T cells, cancer vaccines, in numerous tumor-bearing hosts, have been paired with modalities that break intrinsic inhibitory elements and/or counter the immunosuppressive tumor microenvironment. For instance, a combinatorial treatment, using HER-2/neu-targeted vaccine cells that are retrovirally-transduced to secret GM-CSF (GVAX) for enhancing DC recruitment/cross-priming and cyclophosphamide to deplete T regulatory cells, has resulted in increased activation of high avidity CD8+ T cells [59]. Similarly, GVAX using irradiated tumor cells combined with CTLA-4 [60] or PD-1 [61] blockade significantly potentiated tumor-reactive T cells compared to either the vaccine or the antibody treatment alone. Besides GM-CSF, Fms-like tyrosine kinase 3 ligand (Flt3L), which supports the survival, proliferation, and differentiation of hematopoietic progenitors, and induces and chemoattracts DCs, has also exhibited similar synergy with anti-CTLA-4 antibody when it was retrovirally-transduced into tumor cells used for vaccination [62]. Other cancer vaccines utilizing vaccinia virus, peptides, DNA, and dendritic cells, all of which have been used for chronic viral infection, have also shown promise in enhancing antitumor immunity and generating better T cell responses especially when some were combined with CTLA-4 blockade [63,64] or 4-1BB stimulation [65].
Much of combination tumor immunotherapy have centered on cancer vaccines plus one of the following modalities of blocking inhibitory receptors, activating costimulatory receptors or depleting Tregs. This type of combination treatments arose out of necessity to unleash endogenous tumor/self-reactive T cells from the regulatory checkpoints, thereby potentiating the efficacy of the vaccines. It is interesting that such combination pairings have not been fully explored for adoptive T cell therapy because the addition of an immunomodulating agent may significantly improve its efficacy. One example is the augmented therapeutic efficacy of adoptive T cell therapy when combined with agonistic anti-4-1BB mAb in a dose dependent manner [66]. In light of the negative correlation between prolonged in vitro culture of tumor-reactive T cells and their in vivo function [67,68], the evidence of superior antitumor immunity generated by adoptively-transferred effector cells derived from naïve rather than central memory CD8+ T cells [68], and the need to investigate in vivo priming of adoptively transferred naïve T cells, perhaps now is the time to explore various combination treatments centered around adoptive T cell therapy.
Even though numerous studies on CTLA-4 blockade as a therapeutic modality for cancer has shown promise in enhancing T cell responses, it has had mixed results in chronic infection. In in vitro settings, blockade of the CTLA-4 inhibitory pathway augmented HIV-specific CD4+ T cell function [69] and exhibited synergy with PD-1 blockade in restoring intrahepatic HCV-specific CD8+ T cell exhaustion [70]. However, in chronic LCMV-infected mice, anti-CTLA-4 treatment had no effect on T cell function and viral control in vivo, whereas PD-1 blockade rescued T cells from functional exhaustion and reduced the virus load [19]. Furthermore, in the LCMV murine model, CTLA-4 deficient mice following virus infection showed no significant alteration in regulating viral-specific immunity [71]. In the SIV macaque model, which is a closer in vivo reflection of human HIV disease than murine LCMV infection, CTLA-4 blockade not only was unable to improve viral-specific T cell responses, but also increased viral replication at mucosal sites [72], whereas the treatment with partially humanized mouse anti-human PD-1 Ab enhanced SIV-specific immunity and showed reductions in the viral load [73]. This disparate in vivo efficacy between CTLA-4 and PD-1 blockade in chronic infections compared to cancer possibly suggests that the inductive mechanisms of T cell dysfunction differ between chronic infection and cancer.
Conclusions
The phenotypic, functional, and molecular changes that occur in T cell exhaustion have been extensively analyzed in chronic viral infections, which have served as a practical model for T cell dysfunction during tumor growth and pathogenesis. Some of the key features of exhausted T cells in chronic infection are exhibited in TILs, in particular upregulation of the inhibitory receptor PD-1 and loss of production of effector cytokines. The initial aim of tumor immunotherapy has been to break immunetolerance and anergy, but the efficacy of this strategy may now be limited by T cell exhaustion (Figure 2). The discovery of PD-1 as a major regulator and its blockade as a potent rejuvenator of T cell exhaustion has translated into clinical cancer trials. Recently, a phase I/II clinical trial using anti-PD-1 human mAB MDX-1106 has been conducted in patients with various solid tumors, and the antibody treatment induced clinical responses against renal cell carcinoma and melanoma with well-tolerable side effects [JR Brahmer et al., abstract in ASCO Annual Meeting 2009, No. 3018]. Thus, understanding T cell responses in chronic infections has helped to broaden our view on the functional dynamics of T cells in the tumor microenvironment, and will continue to play a major role in therapeutic advancement of antiviral and tumor immunotherapy.
Acknowledgments
We thank Dr. Barry T. Rouse for helpful comments and suggestions. This work was supported by grants from the National Institutes of Health (to R.A.).
Footnotes
Ethics in Publishing: General Statement: The authors comply with the Ethics in Publishing.
Conflicts of Interest: The authors have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Peter S. Kim, Email: pskim@emory.edu.
Rafi Ahmed, Email: rahmed@emory.edu.
References
- 1.Kaech SM, Ahmed R. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naïve cells. Nat Immunol. 2001;2:415–422. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 3.Kaech SM, Hemby S, Kersh E, Ahmed R. Molecular and functional profiling of memory CD8 T cell differentiation. Cell. 2002;111:837–851. doi: 10.1016/s0092-8674(02)01139-x. [DOI] [PubMed] [Google Scholar]
- 4.Wherry EJ, Teichgräber V, Becker TC, Masopust D, Kaech SM, Antia R, von Andrian UH, Ahmed R. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 5.Wherry EJ, Ahmed R. Memory CD8 T-cell differentiation during viral infection. J Virol. 2004;78:5535–5545. doi: 10.1128/JVI.78.11.5535-5545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarkar S, Kalia V, Haining WN, Konieczny BT, Subramaniam S, Ahmed R. Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates. J Exp Med. 2008;205:625–640. doi: 10.1084/jem.20071641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oxenius A, Price DA, Günthard HF, Dawson SJ, Fagard C, Perrin L, Fischer M, Weber R, Plana M, García F, et al. Stimulation of HIV-specific cellular immunity by structured treatment interruption fails to enhance viral control in chronic HIV infection. Proc Natl Acad Sci U S A. 2002;99:13747–13752. doi: 10.1073/pnas.202372199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Markowitz M, Jin X, Hurley A, Simon V, Ramratnam B, Louie M, Deschenes GR, Ramanathan M, Jr, Barsoum S, Vanderhoeven J, et al. Discontinuation of antiretroviral therapy commenced early during the course of human immunodeficiency virus type 1 infection, with or without adjunctive vaccination. J Infect Dis. 2002;186:634–643. doi: 10.1086/342559. [DOI] [PubMed] [Google Scholar]
- 9.Lindenburg CE, Stolte I, Langendam MW, Miedema F, Williams IG, Colebunders R, Weber JN, Fisher M, Coutinho RA. Long-term follow-up: no effect of therapeutic vaccination with HIV-1 p17/p24:Ty virus-like particles on HIV-1 disease progression. Vaccine. 2002;20:2343–2347. doi: 10.1016/s0264-410x(02)00102-0. [DOI] [PubMed] [Google Scholar]
- 10.Michel ML, P S, Brechot C, Tiollais P. Immunotherapy of chronic hepatitis B by anti HBV vaccine: from present to future. Vaccine. 2001;19:2395–2399. doi: 10.1016/s0264-410x(00)00461-8. [DOI] [PubMed] [Google Scholar]
- 11.Mancini-Bourgine M, Fontaine H, Scott-Algara D, Pol S, Bréchot C, Michel ML. Induction or expansion of T-cell responses by a hepatitis B DNA vaccine administered to chronic HBV carriers. Hepatology. 2004;40:874–882. doi: 10.1002/hep.20408. [DOI] [PubMed] [Google Scholar]
- 12.Hoofnagle JH. Course and outcome of hepatitis C. Hepatology. 2002;36:S21–29. doi: 10.1053/jhep.2002.36227. [DOI] [PubMed] [Google Scholar]
- 13.Stauber RE, S V. Novel approaches for therapy of chronic hepatitis C. J Clin Virol. 2006;36:87–94. doi: 10.1016/j.jcv.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Leroux-Roels G, Batens AH, Desombere I, Van Den Steen B, Vander Stichele C, Maertens G, Hulstaert F. Immunogenicity and tolerability of intradermal administration of an HCV E1-based vaccine candidate in healthy volunteers and patients with resolved or ongoing chronic HCV infection. Hum Vaccin. 2005;1 doi: 10.4161/hv.1.2.1554. [DOI] [PubMed] [Google Scholar]
- 15.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77:4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DJ, Suresh M, Altman JD, Ahmed R. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;75:5099–5107. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wherry EJ, Barber DL, Kaech SM, Blattman JN, Ahmed R. Antigen-independent memory CD8 T cells do not develop during chronic viral infection. Proc Natl Acad Sci U S A. 2004;101:16004–16009. doi: 10.1073/pnas.0407192101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ** 18.Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL, Ahmed R. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]; The authors performed a very thorough comparative analysis of the gene-expression profiles of exhausted LCMV-specific CD8+ T cells from chronic infection and functional LCMV-specific effector and memory CD8+ T cells after acute infection. Their gene profiling data showed an extensive amount of molecular features that distinctly differentiated exhausted CD8+ T cells from the other cell types.
- 19.Barber DL, W E, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 20.Fuller MJ, Zajac AJ. Ablation of CD8 and CD4 T cell responses by high viral loads. J Immunol. 2003;170:477–486. doi: 10.4049/jimmunol.170.1.477. [DOI] [PubMed] [Google Scholar]
- 21.van der Most RG, Murali-Krishna K, Lanier JG, Wherry EJ, Puglielli MT, Blattman JN, Sette A, Ahmed R. Changing immunodominance patterns in antiviral CD8 T-cell responses after loss of epitope presentation or chronic antigenic stimulation. Virology. 2003;315:93–102. doi: 10.1016/j.virol.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 22.Brooks DG, Teyton L, Oldstone MB, McGavern DB. Intrinsic functional dysregulation of CD4 T cells occurs rapidly following persistent viral infection. J Virol. 2005:10514–10527. doi: 10.1128/JVI.79.16.10514-10527.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matloubian M, Concepcion RJ, Ahmed R. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J Virol. 1994;68:8056–8063. doi: 10.1128/jvi.68.12.8056-8063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Battegay M, Moskophidis D, Rahemtulla A, Hengartner H, Mak TW, Zinkernagel RM. Enhanced establishment of a virus carrier state in adult CD4+ T-cell-deficient mice. J Virol. 1994;68:4700–4704. doi: 10.1128/jvi.68.7.4700-4704.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keynan Y, Card CM, McLaren PJ, Dawood MR, Kasper K, Fowke KR. The role of regulatory T cells in chronic and acute viral infections. Clin Infect Dis. 2008;46:1046–1052. doi: 10.1086/529379. [DOI] [PubMed] [Google Scholar]
- * 26.Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrated that coexpression of multiple inhibitory receptors resulted in a greater degree of T cell exhaustion and viral infection. Their coregulation of CD8+ T cell exhaustion was found to be distinct and nonredundant, as co-blockade of PD-1 and LAG-3 synergistically improved T cells responses and reduced viral load.
- 27.Day CL, Kaufmann DE, Kiepiela P. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 28.Petrovas C, Casazza JP, Brenchley JM, Price DA, Gostick E, Adams WC, Precopio ML, Schacker T, Roederer M, Douek DC, et al. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med. 2006;203:2281–2292. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, Boulassel MR, Delwart E, Sepulveda H, Balderas R, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 30.Urbani S, Amadei B, Tola D, Massari M, Schivazappa S, Missale G, Ferrari C. PD-1 expression in acute hepatitis C virus (HCV) infection is associated with HCV-specific CD8 exhaustion. J Virol. 2006;80:11398–11403. doi: 10.1128/JVI.01177-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Penna A, Pilli M, Zerbini A, Orlandini A, Mezzadri S, Sacchelli L, Missale G, Ferrari C. Dysfunction and functional restoration of HCV-specific CD8 responses in chronic hepatitis C virus infection. Hepatology. 2007;45:588–601. doi: 10.1002/hep.21541. [DOI] [PubMed] [Google Scholar]
- 32.Radziewicz H, Ibegbu CC, Fernandez ML, Workowski KA, Obideen K, Wehbi M, Hanson HL, Steinberg JP, Masopust D, Wherry EJ, et al. Liver-infiltrating lymphocytes in chronic human hepatitis C virus infection display an exhausted phenotype with high levels of PD-1 and low levels of CD127 expression. J Virol. 2007;81:2545–2553. doi: 10.1128/JVI.02021-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boni C, Fisicaro P, Valdatta C, Amadei B, Di Vincenzo P, Giuberti T, Laccabue D, Zerbini A, Cavalli A, Missale G, Bertoletti A, Ferrari C. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J Virol. 2007;81:4215–4225. doi: 10.1128/JVI.02844-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clerici M, Wynn TA, Berzofsky JA, Blatt SP, Hendrix CW, Sher A, Coffman RL, Shearer GM. Role of interleukin-10 in T helper cell dysfunction in asymptomatic individuals infected with the human immunodeficiency virus. J Clin Invest. 1994;173:1085–1091. doi: 10.1172/JCI117031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ameglio F, Cordiali Fei P, Solmone M, Bonifati C, Prignano G, Giglio A, Caprilli F, Gentili G, Capobianchi MR. Serum IL-10 levels in HIV-positive subjects: correlation with CDC stages. J Biol Regul Homeost Agents. 1994;8:48–52. [PubMed] [Google Scholar]
- 36.Accapezzato D, Francavilla V, Paroli M, Casciaro M, Chircu LV, Cividini A, Abrignani S, Mondelli MU, Barnaba V. Hepatic expansion of a virus-specific regulatory CD8(+) T cell population in chronic hepatitis C virus infection. J Clin Invest. 2004;113:963–972. doi: 10.1172/JCI20515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woitas RP, Petersen U, Moshage D, Brackmann HH, Matz B, Sauerbruch T, Spengler U. HCV-specific cytokine induction in monocytes of patients with different outcomes of hepatitis C. World J Gastroenterol. 2002;8:562–566. doi: 10.3748/wjg.v8.i3.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cacciarelli TV, Martinez OM, Gish RG, Villanueva JC, Krams SM. Immunoregulatory cytokines in chronic hepatitis C virus infection: pre- and posttreatment with interferon alfa. Hepatology. 1996;24 doi: 10.1002/hep.510240102. [DOI] [PubMed] [Google Scholar]
- 39.Rico MA, Quiroga JA, Subirá D, Castanón S, Esteban JM, Pardo M, Carreno V. Hepatitis B virus-specific T-cell proliferation and cytokine secretion in chronic hepatitis B e antibody-positive patients treated with ribavirin and interferon alpha. Hepatology. 2001;33:295–300. doi: 10.1053/jhep.2001.21147. [DOI] [PubMed] [Google Scholar]
- * 40.Jones RB, Ndhlovu LC, Barbour J, Sheth PM, Jha AR, Long BR, Wong JC, Satkunarajah M, Schweneker M, Chapman JM, et al. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J Exp Med. 2008;205:2763–2779. doi: 10.1084/jem.20081398. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors showed that Tim-3 is upregulated in CD8+ T cells from HIV-infected chronic progressors. Tim-3 expressing T cells failed to produce cytokine and proliferate, but Tim-3 blockade restored those functions. 41 and 42 also showed similar findings in HCV and HBV infection, respectively. Tim-3 blockade may have therapeutic potential in tumor immunotherapy.
- * 41.Golden-Mason L, Palmer BE, Kassam N, Townshend-Bulson L, Livingston S, McMahon BJ, Castelblanco N, Kuchroo V, Gretch DR, Rosen HR. Negative immune regulator Tim-3 is overexpressed on T cells in hepatitis C virus infection and its blockade rescues dysfunctional CD4+ and CD8+ T cells. J Virol. 2009;83:9122–9130. doi: 10.1128/JVI.00639-09. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors showed that Tim-3 is upregulated in CD8+ T cells from HIV-infected chronic progressors. Tim-3 expressing T cells failed to produce cytokine and proliferate, but Tim-3 blockade restored those functions. 41 and 42 also showed similar findings in HCV and HBV infection, respectively. Tim-3 blockade may have therapeutic potential in tumor immunotherapy.
- * 42.Ju Y, Hou N, Zhang XN, Zhao D, Liu Y, Wang JJ, Luan F, Shi W, Zhu FL, Sun WS, et al. Blockade of Tim-3 pathway ameliorates interferon-gamma production from hepatic CD8+ T cells in a mouse model of hepatitis B virus infection. Cell Mol Immunol. 2009;6:35–43. doi: 10.1038/cmi.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors showed that Tim-3 is upregulated in CD8+ T cells from HIV-infected chronic progressors. Tim-3 expressing T cells failed to produce cytokine and proliferate, but Tim-3 blockade restored those functions. 41 and 42 also showed similar findings in HCV and HBV infection, respectively. Tim-3 blockade may have therapeutic potential in tumor immunotherapy.
- 43.Klebanoff CA, Gattinoni L, Torabi-Parizi P, Kerstann K, Cardones AR, Finkelstein SE, Palmer DC, Antony PA, Hwang ST, Rosenberg SA, et al. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc Natl Acad Sci U S A. 2005;102:9571–9576. doi: 10.1073/pnas.0503726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mueller K, Schweier O, Pircher H. Efficacy of IL-2- versus IL-15-stimulated CD8 T cells in adoptive immunotherapy. Eur J Immunol. 2008;38:2874–2885. doi: 10.1002/eji.200838426. [DOI] [PubMed] [Google Scholar]
- 45.Gattinoni L, Zhong XS, Palmer DC, Ji Y, Hinrichs CS, Yu Z, Wrzesinski C, Boni A, Cassard L, Garvin LM, et al. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat Med. 2009;15:808–813. doi: 10.1038/nm.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * 46.Mumprecht S, Schürch C, Schwaller J, Solenthaler M, Ochsenbein AF. Programmed death 1 signaling on chronic myeloid leukemia-specific T cells results in T-cell exhaustion and disease progression. Blood. 2009;114:1528–1536. doi: 10.1182/blood-2008-09-179697. [DOI] [PubMed] [Google Scholar]; These three studies (46, 47, and 48) collectively showed that PD-1 is a major mediator of T cell exhaustion/dysfunction in various types of cancer. PD-1 blockade improved T cell function in all three studies, indicating the potential wide use of this therapeutic approach in tumor immunotherapy.
- * 47.Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, Rosenberg SA. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537–1544. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]; These three studies (46, 47, and 48) collectively showed that PD-1 is a major mediator of T cell exhaustion/dysfunction in various types of cancer. PD-1 blockade improved T cell function in all three studies, indicating the potential wide use of this therapeutic approach in tumor immunotherapy.
- * 48.Fourcade J, Kudela P, Sun Z, Shen H, Land SR, Lenzner D, Guillaume P, Luescher IF, Sander C, Ferrone S, et al. PD-1 is a regulator of NY-ESO-1-specific CD8+ T cell expansion in melanoma patients. J Immunol. 2009;182:5240–5249. doi: 10.4049/jimmunol.0803245. [DOI] [PMC free article] [PubMed] [Google Scholar]; These three studies (46, 47, and 48) collectively showed that PD-1 is a major mediator of T cell exhaustion/dysfunction in various types of cancer. PD-1 blockade improved T cell function in all three studies, indicating the potential wide use of this therapeutic approach in tumor immunotherapy.
- 49.Gandhi MK, Lambley E, Duraiswamy J, Dua U, Smith C, Elliott S, Gill D, Marlton P, Seymour J, Khanna R. Expression of LAG-3 by tumor-infiltrating lymphocytes is coincident with the suppression of latent membrane antigen-specific CD8+ T-cell function in Hodgkin lymphoma patients. Blood. 2006;108:2280–2289. doi: 10.1182/blood-2006-04-015164. [DOI] [PubMed] [Google Scholar]
- 50.Grosso JF, Kelleher CC, Harris TJ, Maris CH, Hipkiss EL, De Marzo A, Anders R, Netto G, Getnet D, Bruno TC, et al. LAG-3 regulates CD8+ T cell accumulation and effector function in murine self- and tumor-tolerance systems. J Clin Invest. 2007;117:3383–3392. doi: 10.1172/JCI31184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bennaceur K, Chapman J, Brikci-Nigassa L, Sanhadji K, Touraine JL, Portoukalian J. Dendritic cells dysfunction in tumour environment. Cancer Lett. 2008;272:186–196. doi: 10.1016/j.canlet.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 52.Mellor AL, Baban B, Chandler P, Marshall B, Jhaver K, Hansen A, Koni PA, Iwashima M, Munn DH. Cutting edge: induced indoleamine 2,3 dioxygenase expression in dendritic cell subsets suppresses T cell clonal expansion. J Immunol. 2003;171:1652–1655. doi: 10.4049/jimmunol.171.4.1652. [DOI] [PubMed] [Google Scholar]
- 53.Bronte V, Apolloni E, Cabrelle A, Ronca R, Serafini P, Zamboni P, Restifo NP, Zanovello P. Identification of a CD11b(+)/Gr-1(+)/CD31(+) myeloid progenitor capable of activating or suppressing CD8(+) T cells. Blood. 2000;96:3838–3846. [PMC free article] [PubMed] [Google Scholar]
- 54.Murdoch C, Muthana M, Coffelt SB, L CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8:618–631. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- 55.Staveley-O'Carroll K, Sotomayor E, Montgomery J, Borrello I, Hwang L, Fein S, Pardoll D, Levitsky H. Induction of antigen-specific T cell anergy: An early event in the course of tumor progression. Proc Natl Acad Sci U S A. 1998;95:1178–1183. doi: 10.1073/pnas.95.3.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Klein L, Trautman L, Psarras S, Schnell S, Siermann A, Liblau R, von Boehmer H, Khazaie K. Visualizing the course of antigen-specific CD8 and CD4 T cell responses to a growing tumor. Eur J Immunol. 2003;33:806–814. doi: 10.1002/eji.200323800. [DOI] [PubMed] [Google Scholar]
- * 57.Ha SJ, Mueller SN, Wherry EJ, Barber DL, Aubert RD, Sharpe AH, Freeman GJ, Ahmed R. Enhancing therapeutic vaccination by blocking PD-1-mediated inhibitory signals during chronic infection. J Exp Med. 2008;205:543–555. doi: 10.1084/jem.20071949. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors performed a very thorough analysis to show that PD-1 blockade synergistically enhanced therapeutic vaccination, resulting in increased CD8+ T cell responses and reduced viral load. This study showed that blocking negative signals is a potent means to enhance vaccine efficacy.
- * 58.Brooks DG, Lee AM, Elsaesser H, McGavern DB, Oldstone MB. IL-10 blockade facilitates DNA vaccine-induced T cell responses and enhances clearance of persistent virus infection. J Exp Med. 2008;205:533–541. doi: 10.1084/jem.20071948. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors showed that IL-10 blockade increased vaccine-induced T cell responses and improved the clearance of chronic infection. This study demonstrated that neutralizing the immunosuppressive environment is important for vaccine to reach high efficacy.
- 59.Ercolini AM, Ladle BH, Manning EA, Pfannenstiel LW, Armstrong TD, Machiels JP, Bieler JG, Emens LA, Reilly RT, Jaffee EM. Recruitment of latent pools of high-avidity CD8(+) T cells to the antitumor immune response. J Exp Med. 2005;201:1591–1602. doi: 10.1084/jem.20042167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Quezada SA, Peggs KS, Curran MA, Allison JP. CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J Clin Invest. 2006;116:1935–1945. doi: 10.1172/JCI27745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * 61.Li B, VanRoey M, Wang C, Chen TH, Korman A, Jooss K. Anti-programmed death-1 synergizes with granulocyte macrophage colony-stimulating factor--secreting tumor cell immunotherapy providing therapeutic benefit to mice with established tumors. Clin Cancer Res. 2009;15:1623–1634. doi: 10.1158/1078-0432.CCR-08-1825. [DOI] [PubMed] [Google Scholar]; These two studies (61 and 62) demonstrated the effectiveness of blocking negative signals to synergize with cancer vaccines. Thus, a combination therapy for chronic infection or cancer would likely need antibody-mediated blockade of coinhibitory pathways
- * 62.Curran MA, Allison JP. Tumor vaccines expressing flt3 ligand synergize with ctla-4 blockade to reject preimplanted tumors. Cancer Res. 2009;69:7747–7755. doi: 10.1158/0008-5472.CAN-08-3289. [DOI] [PMC free article] [PubMed] [Google Scholar]; These two studies (61 and 62) demonstrated the effectiveness of blocking negative signals to synergize with cancer vaccines. Thus, a combination therapy for chronic infection or cancer would likely need antibody-mediated blockade of coinhibitory pathways
- 63.Espenschied J, Lamont J, Longmate J, Pendas S, Wang Z, Diamond DJ, Ellenhorn JD. CTLA-4 blockade enhances the therapeutic effect of an attenuated poxvirus vaccine targeting p53 in an established murine tumor model. J Immunol. 2003;170:3401–3407. doi: 10.4049/jimmunol.170.6.3401. [DOI] [PubMed] [Google Scholar]
- 64.Gregor PD, Wolchok JD, Ferrone CR, Buchinshky H, Guevara-Patino JA, Perales MA, Mortazavi F, Bacich D, Heston W, Latouche JB, et al. CTLA-4 blockade in combination with xenogeneic DNA vaccines enhances T-cell responses, tumor immunity and autoimmunity to self antigens in animal and cellular model systems. Vaccine. 2004;22:1700–1708. doi: 10.1016/j.vaccine.2003.10.048. [DOI] [PubMed] [Google Scholar]
- 65.Kudo-Saito C, Hodge JW, Kwak H, Kim-Schulze S, Schlom J, Kaufman HL. 4-1BB ligand enhances tumor-specific immunity of poxvirus vaccines. Vaccine. 2006;24:4975–4986. doi: 10.1016/j.vaccine.2006.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li Q, Iuchi T, Jure-Kunkel MN, Chang AE. Adjuvant effect of anti-4-1BB mAb administration in adoptive T cell therapy of cancer. Int J Biol Sci. 2007;3:455–462. doi: 10.7150/ijbs.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gattinoni L, Klebanoff CA, Palmer DC, Wrzesinski C, Kerstann K, Yu Z, Finkelstein SE, Theoret MR, Rosenberg SA, Restifo NP. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J Clin Invest. 2005;115:1616–1626. doi: 10.1172/JCI24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hinrichs CS, Borman ZA, Cassard L, Gattinoni L, Spolski R, Yu Z, Sanchez-Perez L, Muranski P, Kern SJ, Logun C, et al. Adoptively transferred effector cells derived from naive rather than central memory CD8+ T cells mediate superior antitumor immunity. Proc Natl Acad Sci U S A. 2009;106:17469–17474. doi: 10.1073/pnas.0907448106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kaufmann DE, Walker BD. PD-1 and CTLA-4 inhibitory cosignaling pathways in HIV infection and the potential for therapeutic intervention. J Immunol. 2009;182:5891–5897. doi: 10.4049/jimmunol.0803771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nakamoto N, Cho H, Shaked A, Olthoff K, Valiga ME, Kaminski M, Gostick E, Price DA, Freeman GJ, Wherry EJ, et al. Synergistic reversal of intrahepatic HCV-specific CD8 T cell exhaustion by combined PD-1/CTLA-4 blockade. PLoS Pathog. 2009;5:1–13. doi: 10.1371/journal.ppat.1000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Homann D, Dummer W, Wolfe T, Rodrigo E, Theofilopoulos AN, Oldstone MB, von Herrath MG. Lack of intrinsic CTLA-4 expression has minimal effect on regulation of antiviral T-cell immunity. J Virol. 2006;80:270–280. doi: 10.1128/JVI.80.1.270-280.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * 72.Cecchinato V, Tryniszewska E, Ma ZM, Vaccari M, Boasso A, Tsai WP, Petrovas C, Fuchs D, Heraud JM, Venzon D, et al. Immune activation driven by CTLA-4 blockade augments viral replication at mucosal sites in simian immunodeficiency virus infection. J Immunol. 2008;180:5439–5447. doi: 10.4049/jimmunol.180.8.5439. [DOI] [PMC free article] [PubMed] [Google Scholar]; Collectively, these two studies (72 and 73) showed that PD-1, and not CTLA-4, blockade enhanced SIV-specific immunity in vivo. Their data suggest that the predominant mechanism of T cell dysfunction in chronic infections is mediated by PD-1.
- * 73.Velu V, Titanji K, Zhu B, Husain S, Pladevega A, Lai L, Vanderford TH, Chennareddi L, Silvestri G, Freeman GJ, et al. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature. 2009;458:206–210. doi: 10.1038/nature07662. [DOI] [PMC free article] [PubMed] [Google Scholar]; Collectively, these two studies (72 and 73) showed that PD-1, and not CTLA-4, blockade enhanced SIV-specific immunity in vivo. Their data suggest that the predominant mechanism of T cell dysfunction in chronic infections is mediated by PD-1.