Abstract
We investigated glutamate-related neuronal dysfunction in the anterior cingulate (AC) early in schizophrenia before and after antipsychotic treatment. A total of 14 minimally treated schizophrenia patients and 10 healthy subjects were studied with single-voxel proton magnetic resonance spectroscopy (1H-MRS) of the AC, frontal white matter and thalamus at 4 T. Concentrations of N-acetylaspartate (NAA), glutamate (Glu), glutamine (Gln) and Gln/Glu ratios were determined and corrected for the partial tissue volume. Patients were treated with antipsychotic medication following a specific algorithm and 1H-MRS was repeated after 1, 6 and 12 months. There were group × region interactions for baseline NAA (P = 0.074) and Gln/Glu (P = 0.028): schizophrenia subjects had lower NAA (P = 0.045) and higher Gln/Glu (P = 0.006) in the AC before treatment. In addition, AC Gln/Glu was inversely related to AC NAA in the schizophrenia (P = 0.0009) but not in the control group (P = 0.92). Following antipsychotic treatment, there were no further changes in NAA, Gln/Glu or any of the other metabolites in any of the regions studied. We conclude that early in the illness, schizophrenia patients already show abnormalities in glutamatergic metabolism and reductions in NAA consistent with glutamate-related excitotoxicity.
Keywords: 1H-MRS, schizophrenia, NAA, glutamate, glutamine, antipsychotic drug
Introduction
It has been postulated that in schizophrenia there is a progressive excitotoxic process involving excessive glutamatergic activity in various cortical and subcortical fields that may account for the deteriorating course of the illness.1 As the disease progresses, it is hypothesized that there is neuronal/dendritic damage without gliosis or widespread neuronal death. This leads to neuronal dysfunction and underlies the deteriorating cognitive and social functional state characteristic of the illness, despite the beneficial symptomatic effects of antipsychotic medications. Most of the evidence for this process is indirect and stems from pharmacological studies with N-methyl-D-aspartate receptor (NMDAR) blockers. Rodents treated acutely with MK801 show a neurotoxic effect in various cortical regions.2 Rats exposed to ketamine have increases in extracellular glutamate in the frontal cortex.3 More prolonged NMDAR blockade with low-dose phencyclidine reduces N-acetylaspartate (NAA, a marker of neuronal viability4). In humans, acute, subanesthetic-dose ketamine induces many of the symptoms and cognitive defects of schizophrenia.5 Using 4T proton magnetic resonance spectroscopy (1H-MRS), our group documented transitory elevations in the anterior cingulate (AC) glutamine (Gln; Rowland et al.6) and in Gln to glutamate (Glu) ratios (Rowland LM, unpublished observation) in healthy subjects exposed to ketamine. However, the relevance of these acute changes to a chronic illness such as schizophrenia is unclear.
Only one 4T 1H-MRS study has examined Glu, its metabolite Gln (synaptic Glu is metabolized to Gln in the glia) and NAA, early in schizophrenia, before treatment.7 They found increased Gln in the AC and thalamus and normal NAA. Another study at 4T in treated elderly schizophrenia found elevated Glu plus Gln and reduced NAA, but only the white matter was examined.8 Finally, elevations in Gln/Glu ratios were found in the cerebrospinal fluid of antipsychotic-naïve young schizophrenics.9 Hence, it is possible that early dysfunction of glutamate–glutamine cycling and/or antipsychotic medication effects predispose to NAA reductions later in the illness.
The primary aim of this study was to use high-field 1H-MRS to examine for evidence of glutamate-related neuronal dysfunction early in schizophrenia. For this purpose, we studied schizophrenia patients early in the illness with minimal previous antipsychotic exposure and healthy volunteers. As a secondary aim, to examine the stability of any neurometabolic findings and potential medication effects, patients were re-scanned at 1, 6 and 12 months following treatment with an explicit antipsychotic drug algorithm. We used the same 1H-MRS technique with which we previously documented transient Gln and Gln/Glu elevation with ketamine exposure in AC.6 We hypothesized reduced NAA and increased Gln and Gln/Glu in AC in the schizophrenia group before treatment, consistent with the NMDAR hypofunction model. We explored other regions (the thalamus and frontal white matter) with previously reported glutamatergic abnormalities.7,8 Finally, on the basis of the available animal data,10 we expected no neurometabolic change with treatment.
Methods
Subjects
Patients were recruited from the Mental Health Center at the University of New Mexico. Inclusion criteria were: (1) Diagnostic and Statistical Manual, 4th Edition Revised (DSM-IV) diagnosis of schizophrenia, schizoaffective or schizophreniform disorder made through consensus by two research psychiatrists (JL and JB) using all available information derived from the Structured Clinical Interview for DSM-IV Patient Version (SCID-P), review of medical and psychiatric records and family informants; and (2) lifetime antipsychotic exposure of <3 weeks. Exclusion criteria were diagnosis of neurological disorder, mental retardation, history of head trauma or substance use disorder not fully remitted. Controls were recruited from the community and were excluded if they had: (1) any DSM-IV axis I disorder, determined by Structured Clinical Interview for DSM-IV Non-Patient Version (SCID-NP); (2) first-degree relatives with schizophrenia or other psychotic disorders; and (3) history of neurological disorder. All subjects gave written informed consent before entry into the study and were paid for their participation. The study was approved by the local Institutional Review Board.
Magnetic resonance studies
Studies were completed in a 4T magnetic resonance scanner (Varian, Palo Alto, CA, USA), with whole-body gradients capable of 40 mTm−1 and a rise time of 200 µs. All scans were acquired using a pulse and collect transverse electromagnetic head coil. For voxel placement and tissue segmentation, a T1 three-dimensional magnetization prepared fast low angle shot sequence (inversion time (TI) = 700 ms, time-to-echo (TE) = 5 ms, repetition time (TR) = 9 ms, field of view = 200mm, matrix = 256 × 256) was acquired. We used spectroscopic acquisition and analyses methods developed at the University of Western Ontario, which have been described in detail.11 Briefly spectra were acquired using 1H-stimulated echo acquisition mode (STEAM; TR = 2000 ms, TE = 20 ms, TM = 30 ms, dwell time = 500 µs, 256 water-suppressed and 16 water-unsuppressed averages). Water suppression was achieved using three Chemical Shift Selective water suppression pulses. Spectra were shimmed to achieve full-width half maximum (FWHM) of <13Hz (measured on the unsuppressed water signal from the voxel; spectra with larger FWHM were excluded). Higher-order shims were used for all cases. Spectra were analyzed using curve-fitting software that uses a predefined ‘model set’ of simulated spectra for 13 metabolite as prior knowledge. The prior knowledge used was the same for both patients and controls, and includes models for the macromolecules, which are more prominent in short-echo MRS experiments. In this implementation for macromolecules, chemical shifts, linewidths and phase are fixed and only amplitude is allowed to vary independently during fitting. The line-fitting package returned amplitudes and areas for each metabolite of interest, which were normalized to water-yielding quantification of NAA, Glu, Gln, choline (Cho) and creatine (Cre). Individual metabolite fits were excluded if the s.d. of the fit was >20 for the AC and > 30 for the frontal white matter and thalamus data.
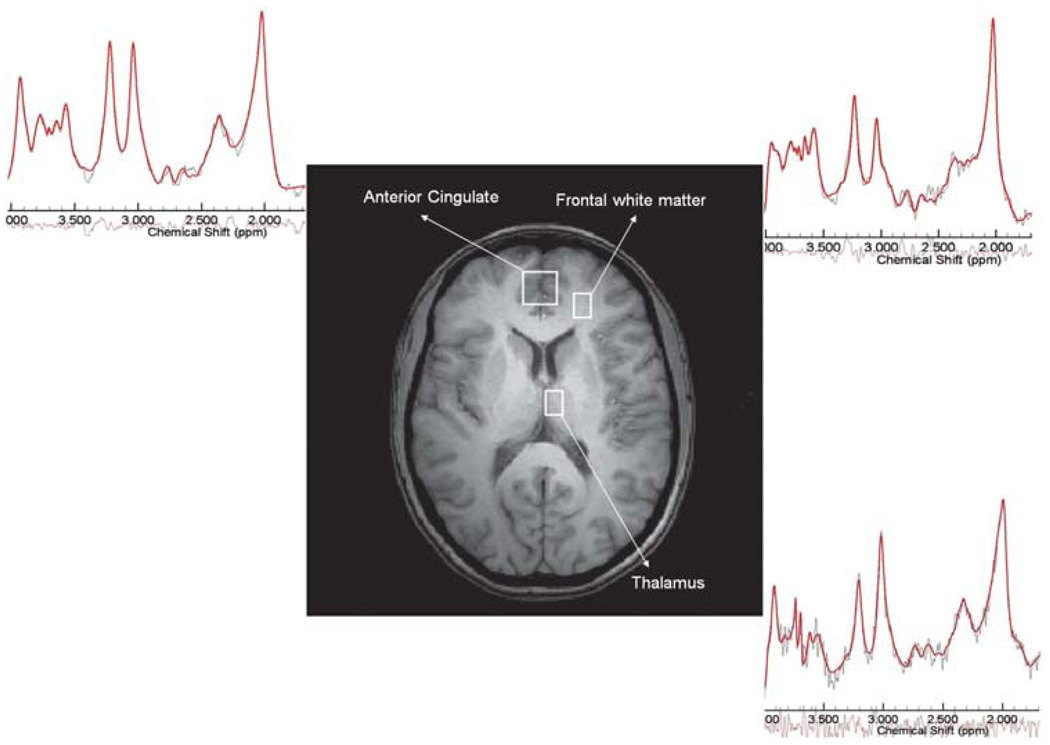
Three spectroscopic regions of interest were acquired in the following order (Figure 1): (1) bilateral AC (20 × 20 × 20 mm3) placed on the axial slice that encompasses the most anterior portion of the corpus callosum; (2) left frontal white matter (15 × 10 × 10 mm3) in the same slice and immediately lateral to the AC voxel so as to encompass only the white matter; and (3) left medial thalamus (15 × 10 × 10 mm3) placed in the most medial and anterior portion of the thalamus on the slice where the dorsal–medial nucleus is most prominent. Voxel size was chosen based on the anatomy to include as much possible of the structure being studied, although minimizing tissue mixing from the surrounding brain structures. This meant that the thalamus and white matter voxels were considerably smaller then the AC voxel. Each spectral acquisition took 12–15 min. For the follow-up scans, the same rules were used. In addition, the MR operator used the initial images illustrating voxel placement relative to the individual anatomical patterns as further guidance.12
Figure 1.
Voxel placement in three regions of interest: the anterior cingulate, left frontal white matter and left thalamus. Representative spectra with the raw (in black) and fitted data (in red) are shown for each region. The color reproduction of the figure is available on the html full text version of the paper.
T1 images were segmented as previously described.13 Partial volume correction was carried out using the following formula:
[Met] is metabolite concentration; Signal(Met) is the signal of the metabolite peak of interest; Signal(H2O) is the signal of the water peak; 2 for the number of water protons; #H(Met) is the number of protons that give rise to the metabolite peak of interest; [H2O(tissue)] is the water concentration in the tissue (that is, H2O in the gray matter is 43.3 m, H2O in the white matter is 35.8 m; Ernst et al.14); [H2O(pure)] is the water concentration in water (55.5 m; Ernst et al.14); and Percent(tissue) is the percentage of tissue (gray or white) volume in the voxel (we assumed that for the AC and thalamus, percentage of tissue was only gray, and for frontal white, only white matter).
Drug algorithm
After MR scanning, schizophrenia patients were treated with quetiapine (target dose range 100–1200mg QD). If, after a minimum of 2 months of treatment, patients failed to achieve substantial symptomatic improvement or were intolerant as determined by the treating psychiatrist, they were gradually switched to risperidone (dose range 0.25–8mg QD). Again, failures in improvement or tolerability after 2 months lead to a switch in treatment to haloperidol (oral dose range 1–30mg QD and/or haloperidol-depo intramuscular injection 10–300mg monthly—if compliance was an issue). Failure to tolerate or improve on haloperidol for 2 months (or 4 months on haloperidol-depo) led the clinician to start clozapine (dose range 100–900mg QD).
The patients were assessed for psychopathology with the Schedule for Assessment of Positive Symptoms (SAPS; Andreasen15) and the Schedule for Assessment of Negative Symptoms (SANS; Andreasen16), the Simpson–Angus Scale (SAS; Simpson and Angus17) for parkinsonism, the Barnes Akathisia Rating Scale (BARS; Barnes18) for akathisia and the Abnormal Involuntary Movements Scale (AIMS; Guy19) for tardive dyskinesia at regular intervals.
Statistical analyses
Dependent variables of interest were NAA, Glu, Gln, Cho and Cre concentrations in millimoles per liter (mm; as in most clinical MRS studies, this unit is used as an approximation, as T1 and T2 of water and the metabolites were not measured). On the basis of the available literature, the main variables of interest were NAA,20 Gln6,7 and Gln/Glu.9 The overall analyses used PROC MIXED (SAS/STAT User’s guide, Version 8, Cary NC: SAS Institute Inc, 1999), a repeated measures analysis of variance (RM ANOVA), with subject group as the grouping factor and brain region as repeated factors. To protect against type-I errors, only significant interactions or main effects from the RM ANOVA were followed with post hoc tests. To examine progressive changes over time in the schizophrenia group, two approaches were followed using all metabolite data from subjects with more than one scan. An RM ANOVA was carried out with repeated measures of time of scan and brain region. In a second approach we calculated a slope over time for each schizophrenia subject in each region. Metabolite values and their intervals in months were fitted in a regression line as an estimate of change in the metabolite concentration per month of follow-up. RM ANOVA for slopes had the brain region as repeated factor. To further understand significant overall effects, post hoc tests were done with Fisher’s least significance difference method or paired t-tests as appropriate. The relationships between metabolite concentrations and demographic and clinical characteristics were analyzed with Pearson’s or Spearman’s correlations as appropriate. Demographic and clinical characteristics of the sample were compared between groups with t-tests for continuous data, and χ2-tests or Wilcoxon tests for nominal and ordinal data, respectively.
Results
Baseline analyses
Demographic and clinical
In all, 15 patients and 11 controls were enrolled; however, one subject in each group had nonusable MRS data. Hence, 14 patients and 10 controls were effectively studied (see Table 1 for demographic and clinical characteristics). By the end of their clinical follow-up, all patients met diagnosis of schizophrenia. There were no significant differences between the patient and control groups in terms of age, gender, ethnic composition, handedness or socioeconomic status (SES) of the head of household of the family of origin. The schizophrenia group had fewer years of education (t(20) = −3.2, P = 0.006) and worse personal SES (Wilcoxon, P = 0.01; high SES rating means lower SES).
Table 1.
Demographic and clinical characteristics of the subject sample at baseline
| Schizo- phrenia (N = 14) |
Health controls (N = 10) |
|
|---|---|---|
| Age (s.d.) years | 27.2 (8.9) | 28.8 (9.7) |
| Gender (male/female) | 12/2 | 8/2 |
| Ethnicity (Black/White/Hispanic) | 1/7/6 | 0/4/6 |
| Education (s.d.) years | 11.6 (2.5) | 15.4 (2.7)* |
| Socioeconomic status (SES; s.d.) | 4.8 (0.59) | 3.6 (1.1)* |
| SES head of household (s.d.) | 2.5 (1.4) | 3.2 (0.75) |
| Handedness (right/left) | 14/0 | 10/0 |
| Length of illness (s.d.) months | 30.8 (43.6) | N/A |
| Never treated/minimally treated (<3 weeks) |
2/12 | N/A |
| Length of previous treatment (s.d.) in days |
5.2 (6.6) | N/A |
| Positive symptoms (s.d.) | 5.6 (4.1) | N/A |
| Negative symptoms (s.d.) | 12.9 (5.6) | N/A |
Abbreviation: N/A, not applicable.
P < 0.05.
Neurometabolites
The number of voxels discarded at each time point due to FWHM >13Hz are presented in Supplementary Tables 1 and 2. The mean quality of fits for data used is presented as Supplementary data. There were no statistically significant differences in the quality of fits between the groups for any metabolite in any region (for t-tests, all P values were between 0.20 and 0.84).
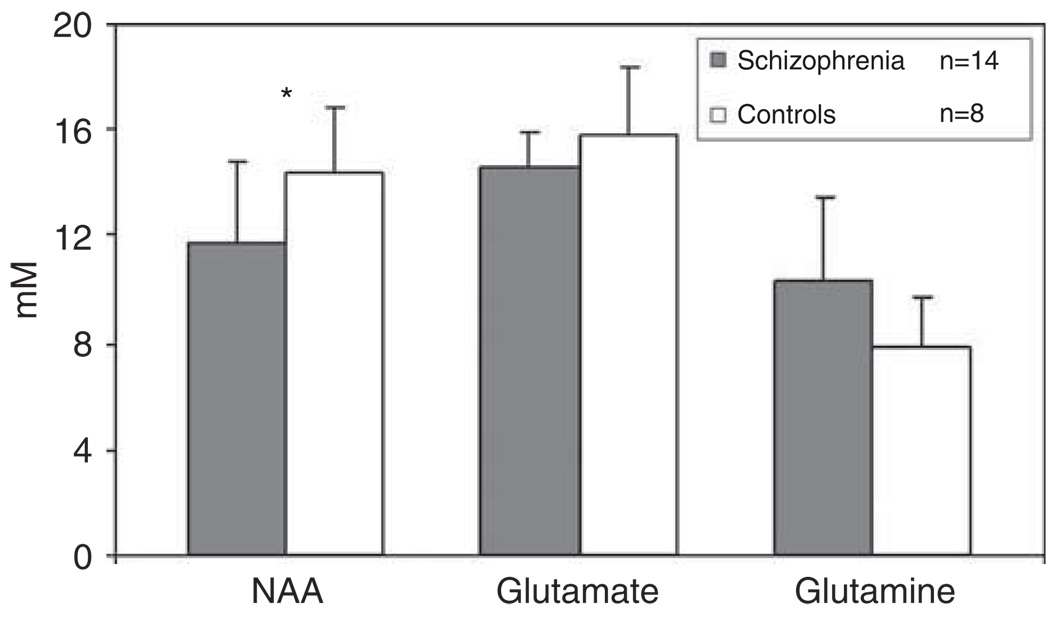
Metabolite values at baseline are presented in Figure 2. For NAA, repeated measures ANOVA found a marginally significant group × region interaction (F(2, 35) = 2.81, P = 0.073), a region main effect (F(2, 35) = 8.25, P = 0.001) and no group main effect (F(1, 23) = 0.43, P = 0.52). Follow-up of the interaction confirmed lower NAA in the schizophrenia group, only in the AC (t(1, 20) = −2.16, P = 0.045; Figure 2; partial-volume corrected data were also lower in schizophrenia: 10.24 ± 2.8 vs 13.1 ± 1.94; t(1, 20) = −2.8, P = 0.01). The group × region interactions were not significant for Glu, Gln, Cho or Cre (all P values between 0.13 and 0.42). Similarly, the group main effects (after removing the interaction from the model) were nonsignificant for Glu, Gln, Cho or Cre (all P values between 0.14 and 0.96). However, the ratio of Gln/Glu had a significant group × region interaction (F(2, 35) = 3.99, P = 0.027), with marginally significant region (F(2, 35) = 2.59, P = 0.09) and nonsignificant group (F(1, 23) = 1.57, P = 0.22) effects. Follow-up of the group × region interaction documented higher Gln/Glu in AC in the schizophrenia group (t(1, 20) = 3.15, P = 0.006; Figure 3). In addition, inclusion of years of education and personal SES (both worse in patients than controls) into the repeated measures ANOVA, only minimally affected the group × region interactions for NAA (P = 0.087) and for Gln/Glu (P = 0.083). Finally, AC Gln/Glu was inversely related to AC NAA in the schizophrenia (Pearson’s r(14) = −0.78, P = 0.0009), but not in the control group (Pearson’s r(8) = −0.044, P = 0.92; Figure 4; partial-volume corrected data were also correlated in schizophrenia (r(14) = −0.77, P = 0.001), but not in controls (r(8) = 0.08, P = 0.83).
Figure 2.
Neurometabolite levels (means ± s.d.) in the anterior cingulate in minimally treated early schizophrenia patients and healthy controls at baseline. For N-acetylaspartate (NAA), t(1, 20) = −2.16, *P = 0.045.
Figure 3.
Glutamine–glutamate ratios (means ± s.d.) in the anterior cingulate in minimally treated early schizophrenia patients and healthy controls at baseline. For Gln/Glu, t(1, 20) = 3.15, *P = 0.006.
Figure 4.
Inverse correlation between anterior cingulate N-acetylaspartate (NAA) and glutamine (Gln)/glutamate (Glu) ratio in schizophrenia at baseline, but not in healthy controls. Pearson’s r(14) = −0.78, P = 0.0009 in schizophrenia. Pearson’s r(8) = −0.044, P = 0.92 in control group.
In the schizophrenia group NAA and Gln/Glu in the AC were not related to duration of illness (r(13) = −0.09, P = 0.76 and r(13) = 0.065, P = 0.83, respectively), SAPS global (r(14) = −0.19, P = 0.51 and r(14) = 0.27, P = 0.34, respectively) or SANS global (r(14) = 0.23, P = 0.42 and r(14) = −0.11, P = 0.71, respectively).
Longitudinal analyses (schizophrenia subjects)
After baseline assessments, patients were followed up for a mean of 7.3 months, s.d. = 5.0. Ten patients returned for the 1-month follow-up, eight for the 6-month and seven for the 12-month follow-up (attrition at each time point was due to unwillingness to continue treatment in the context of the study). Twelve patients were initially treated with quetiapine (one remained on risperidone and one on haloperidol). At the time of their last scan, patients were on the following medications: four on quetiapine, four on risperidone, three on haloperidol (two depo, one oral), two on aripiprazole and none on clozapine (aripiprazole became available during the study implementation and was allowed in these two subjects for clinical reasons). Supplementary Table 3 presents the number of patients taking each antipsychotic medication at each time interval.
Two types of follow-up analyses were undertaken. Repeated measures ANOVA comparing metabolite values (NAA, Gln/Glu, Glu, Gln, Cho and Cre) for each scanning visit across the three regions in the schizophrenia group showed no visit × region interaction (all P’s between 0.29 and 0.99). Hence, the interaction was removed from the model to examine potential main effects of time. However, the time main effects were nonsignificant for NAA (F(3, 25) = 0.08, P = 0.97) and Gln/Glu (F(3, 25) = 0.55, P = 0.65), or for the other metabolites (all P values between 0.18 and 0.79). Finally, analyses of slopes for NAA, Gln/Glu or the other metabolites had nonsignificant region × time interactions or time main effects (data not shown).
Discussion
Young schizophrenia patients with minimal previous antipsychotic exposure had reduced NAA and increased ratio of Gln/Glu in the AC, but not in the adjacent frontal white matter or thalamus. In the patient group, these metabolic abnormalities were inversely correlated to each other. Group differences in NAA and Gln/Glu were not related to the subject’s socioeconomic or educational levels, or to baseline symptom ratings in the patients.
Few other 1H-MRS studies have measured Glu, Gln or both combined, in schizophrenia/healthy volunteer comparisons. These were mainly cross-sectional in design and used the single-voxel 1H-MRS at high field strengths and short TE. Our study is most comparable with the investigation by Theberge et al.7 because we implemented their STEAM single-voxel sequence in a similar Varian 4 T scanner. They found increased Gln and normal NAA in the AC and thalamus in antipsychotic-naive schizophrenia (mean age = 21 years; mean illness duration = 20 months). An important difference is that we examined a larger AC voxel (8 cm3 compared with 1.5 cm3; Theberge et al.7) that encompassed bilateral AC; this could have resulted in higher signal to noise, and better sensitivity to detect NAA reductions. In addition, our patients were somewhat older (mean age = 27) and perhaps glutamate-related NAA reductions had progressed further. Finally, most of our patients had some, although minimal, antipsychotic exposure. However, several studies in animals have not found effects of antipsychotics on NAA10,21,22 or on Glu or Gln10 in these regions.
We failed to document AC Gln elevations but found increased Gln/Glu. This is consistent with a recent investigation of spinal cerebrospinal fluid in drugnaïve schizophrenia patients.9 In this study Glu and Gln, measured with high-performance liquid chromatography, were normal (Gln was 468.6 ± 146 µm in patients vs 405.6 ± 108 µm in controls; Glu was 4.5 ± 1.77 µm in patients vs 4.73 ± 1.29 mm in controls). However, the Gln/Glu ratio was significantly higher in the patient group. Interestingly, in our study of NMDAR blockade with ketamine in healthy volunteers, in addition to the reported elevation of Gln,6 we also found marginally increased Gln/Glu in AC (8-cm3 voxel) using the same technique as in the current study (ratios mean increase = 0.12, s.d. = 0.19; t(8) = 1.83, P = 0.1). Gln is the principal metabolite of synaptic Glu23 and increased turnover of glutamatergic neurotransmission could potentially result in a greater shift toward Gln with corresponding Glu reduction. Consistently, somatosensory activation in rats resulted in increased Gln and reduced Glu assessed with 1H-MRS at 11.7T, suggestive of ‘…augmented Glu release from glutamatergic neurons and subsequent uptake by high-affinity Glu transporters on the surrounding glial cells and conversion of Glu into Gln by Gln synthetase.’24 Hence, in clinical 1H-MRS studies, Gln/Glu ratio may be a more sensitive measure of glutamatergic release than Gln or Glu concentrations. However, 1H-MRS only provides static metabolite measures. Future studies of glutamate–glutamine cycle in schizophrenia with dynamic 13C-MRS would be informative.
A few other studies have examined glutamatergic metabolism and NAA in different disease stages in schizophrenia. Tibbo et al.25 at 3T (TE = 20), found increased Glu plus Gln and normal NAA in AC in adolescents at risk for schizophrenia (age = 16). Tebartz et al.26 at 2T (TE = 30), found increased Glu and normal NAA in a dorsolateral prefrontal and medial temporal voxels in acutely ill, medicated, schizophrenia subjects (age = 28 years; illness duration = 5.3 years). In chronically ill patients (age = 42 years; length of illness = 15 years), Theberge et al.27 found elevated Gln in the thalamus but Glu plus Gln reduction in AC; NAA was normal in both regions. Finally, Chang et al.8 studied bilateral white matter prefrontal, temporal and occipital regions (total of 6 voxels) at 4T (TE = 30ms) in older schizophrenia (age = 66 years; illness duration = 43 years). They found elevated Glu plus Gln in bilateral prefrontal and left occipital white matter and reduced NAA in bilateral prefrontal and temporal regions. Hence, most of these short TE studies report elevations in glutamatergic indices with normal NAA. Inconsistencies across this literature are likely due to differences in populations, regions of interest studied, as well as spectroscopic technique.
In contrast to the few investigations of glutamate, many studies have measured NAA in schizophrenia/healthy control comparisons, mostly in large single voxels at 1.5 T and longer TEs. These were recently summarized in a meta-analysis (64 studies: 1256 patients, 1209 healthy controls; Steen et al.20). Important findings included consistent NAA reductions in schizophrenia in combined gray and white matter tissue in prefrontal and medial temporal regions, but also found in the AC, thalamus and cerebellum. There was no evidence of lower NAA in chronic compared with early schizophrenia, but the great majority of studies included chronically ill patients (only four ‘first episode’ schizophrenia studies are included in this analysis). Our results are consistent with this broad literature and suggest that at short TE (20 ms), AC NAA reductions are apparent early in the illness.
We are aware of only four other longitudinal early schizophrenia studies that assessed metabolic changes in the context of antipsychotic drug exposure. Three of these were at 1.5 T and focused on NAA. Choe et al.28 found low frontal NAA/Cre at baseline with no further reductions after treatment with typical and atypical agents (follow-up 1–6 months). Fannon et al.29 reported reduced medial temporal NAA/Cre at baseline, which was no longer statistically different from healthy subjects after 3 months of atypical antipsychotic treatment. Although our preliminary report suggested progressive frontal reductions,30 follow-up of the whole sample did not detect NAA reductions in patients randomized to haloperidol or quetiapine for a mean follow-up of 9 months.31 Finally, Theberge et al.32 detected reductions in thalamic glutamine after 30 months of treatment, but no changes in NAA. Hence, the absence of NAA changes with treatment in this study is generally consistent with this literature. However, longer follow-up periods may be necessary to detect changes in other metabolites like glutamine.
Reduced NAA may result from actual loss of neurons or reductions in their size (soma and/or processes) relative to non-neuronal tissue. The postmortem literature does not support classic neurodegenerative changes in schizophrenia with neuronal loss or gliosis.33 However disease-related reductions in synaptic spines34 could result in decreased NAA. A recent study in a model of early human immunodeficiency virus brain infection in monkeys documented that 1H-MRS-measured NAA decrements were related to reductions in synaptophysin, a marker of synaptic integrity.35 Furthermore, subchronic NMDAR blockade with phencyclidine in rats resulted in a 41% reduction in prefrontal spine synapses36 and of temporal NAA.4 Finally, increased mRNA expression of the astrocytic glutamate transporter37 and elevations of phosphate-activated glutaminase, a neuronal enzyme that converts glutamine to glutamate,38 were both detected in the prefrontal cortex of schizophrenia subjects, consistent with increased turnover of synaptic glutamate. Hence, our findings of increased Gln/Glu correlated with lower NAA early in the illness, and are consistent with a process of glutamate-related dendritic toxicity as suggested by the NMDAR hypofunction model of schizophrenia.
Some study limitations should be considered. First, sample size was small and hence replication of the AC findings in larger groups is necessary. In addition, it is possible that our negative findings for frontal white matter and thalamus represent type-II statistical errors. Likewise, the lack of neurometabolic change with follow-up should be interpreted cautiously because of low power. Second, spatial coverage was restricted to only three regions, thus neurometabolic changes in other locations may have been missed. Proton echo-planar spectroscopic imaging techniques at 4T and short TE with broader spatial coverage are being implemented by our group. Third, at the TE of 20 ms, significant contributions from various macro-molecule (MM) resonances will be present, which may be coupled to each other. We did not measure MM resonances in patients or controls but estimated them using common prior knowledge in our fitting model in which only the amplitude is allowed to vary independently. If MM shapes and contributions (and/or their relationships) differ in schizophrenia from control subjects, this could potentially affect our Gln, Glu and NAA findings. Fourth, the voxels for the thalamus and frontal white matter were smaller than the AC voxel, and consequently had lower signal-to-noise ratio (SNR). This reduced SNR might contribute to reduced reliability of our measures, leading us to miss possible differences in these regions due to type-II error. Fifth, most patients had been treated with antipsychotic medications at baseline. Rodent studies suggest that even acute antipsychotic exposure can modulate glutamatergic response to NMDA blockade.39 Hence, our baseline Gln/Glu findings could still be the results of antipsychotic medication. Sixth, we segmented cerebrospinal fluid and partial-volume corrected the metabolite concentrations, but were unable to segment the gray and white matter tissue in each voxel. As NAA concentration is slightly higher in gray than in white matter,40 differences in voxel tissue proportion between the groups could potentially account for the reduced NAA in the AC in the schizophrenia group. Finally, our use of endogenous water as a concentration reference might be problematic if there were substantial alterations in water MR visibility, perhaps due to water dysregulation. However, this would have likely lead to group differences in the same direction across all metabolites studied. Pfefferbaum et al.41 reported prolonged water T2 in schizophrenia. We minimized this possible effect by using a short echo time of 20ms so that any associated alteration of T2 is unlikely to have a substantial effect. Similarly, our use of a 2000-ms recycle time results in minimal saturation effects. Still, differences in water T1 relaxation in voxels with larger water content (such as the AC in the schizophrenia group) could also confound metabolite concentrations. However, analyses of the metabolite data as Cre ratios produced the same baseline results (see Supplementary data). Future studies measuring T1 and T2 in addition to neurometabolites would be challenging but potentially informative.
In summary, these results suggest that NAA reductions and correlated elevations of Gln/Glu in the AC are present early in schizophrenia. The neurometabolic findings are consistent with reductions in neuropil secondary to glutamate-mediated toxicity as predicted by the NMDAR hypofunction model.1 Direct evidence of such a process supports the study of ‘neuroprotective’ glutamate modulating agents, which may prevent cognitive dysfunction and the poor functional outcomes that are still the norm in schizophrenia.
Supplementary Material
Acknowledgments
This work was supported by the Mental Illness and Neuroscience Discovery Institute (DE-FG03-99ER62764/A002) and by the NIMH R01MH084898 to J Bustillo, MD. Dr Brooks is supported in part by the NIH Grant NS039123. The authors are grateful to Ranee Barrow, Tara Biehl, Heather Hawk, Elma Landgraf, Erica Snider, Catie Snyder, Mariebeth Velasquez and Christina Wolff for their contribution to this study.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Olney JW, Farber NB. Glutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry. 1995;52:998–1007. doi: 10.1001/archpsyc.1995.03950240016004. [DOI] [PubMed] [Google Scholar]
- 2.Farber NB, Wozniak DF, Price MT, Labruyere J, Huss J, St Peter H, et al. Age-specific neurotoxicity in the rat associated with NMDA receptor blockade: potential relevance to schizophrenia. Biol Psychiatry. 1995;38:788–796. doi: 10.1016/0006-3223(95)00046-1. [DOI] [PubMed] [Google Scholar]
- 3.Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway for NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1992;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reynolds LM, Cochran SM, Morris BJ, Pratt JA, Reynolds GP. Chronic phencyclidine administration induces schizophrenia-like changes in N-acetylaspartylglutamate in rat brain. Schizophr Res. 2005;73:147–152. doi: 10.1016/j.schres.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Newcomer JW, Krystal JH. NMDA receptor regulation of memory and behavior in humans. Hippocampus. 2001;11:529–542. doi: 10.1002/hipo.1069. [DOI] [PubMed] [Google Scholar]
- 6.Rowland L, Bustillo J, Mullins P, Jung R, Lenroot R, Landgraf E, et al. The effects of ketamine on anterior cingulate glutamate metabolism in healthy humans: a 4.0T proton MRS study. Am J Psychiatry. 2005;162:394–396. doi: 10.1176/appi.ajp.162.2.394. [DOI] [PubMed] [Google Scholar]
- 7.Theberge J, Bartha R, Drost DJ, Menon RS, Malla A, Takhar J, et al. Glutamate and glutamine measured with 4.0T proton MRS in never-treated patients with schizophrenia and healthy volunteers. Am J Psychiatry. 2002;159:1944–1946. doi: 10.1176/appi.ajp.159.11.1944. [DOI] [PubMed] [Google Scholar]
- 8.Chang L, Friedman J, Ernst T, Zhong K, Tsopelas ND, Davis K. Brain metabolite abnormalities in the white matter of elderly schizophrenic subjects: implication for glial dysfunction. Biol Psychiatry. 2007;62:1396–1404. doi: 10.1016/j.biopsych.2007.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hashimoto K, Engberg G, Shimizu E, Nordin C, Lindström LH, Iyo M. Elevated glutamine/glutamate ratio in cerebrospinal fluid of first episode and drug naïve schizophrenic patients. BMC Psychiatry. 2005;5:6. doi: 10.1186/1471-244X-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bustillo J, Barrow R, Paz R, Tang J, Seraji-Bozorgzad N, Moore G, et al. Long term treatment of rats with haloperidol: lack of an effect on brain N-acetyl aspartate levels. Neuropsychopharmacology. 2006;31:751–756. doi: 10.1038/sj.npp.1300874. [DOI] [PubMed] [Google Scholar]
- 11.Bartha R, Drost DJ, Menon RS, Williamson PC. Comparison of the quantification precision of human short echo time (1)H spectro-scopy at 1.5 and 4.0 Tesla. Magn Reson Med. 2000;44:185–192. doi: 10.1002/1522-2594(200008)44:2<185::aid-mrm4>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 12.Brooks WM, Friedman SD, Stidley CA. Reproducibility of 1H-MRS in vivo. Magn Reson Med. 1999;40:193–197. doi: 10.1002/(sici)1522-2594(199901)41:1<193::aid-mrm27>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 13.Petropoulos H, Sibbitt WL, Jr, Brooks WM. Automated T2 quantitation in neuropsychiatric lupus erythematosus: a marker of active disease. J Magn Reson Imaging. 1999;9:39–43. doi: 10.1002/(sici)1522-2586(199901)9:1<39::aid-jmri5>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 14.Ernst T, Kreis R, Ross BD. Absolute quantitation of water and metabolites in the human brain. 1. Compartments and water. J Magn Reson B. 1993;102:1–8. [Google Scholar]
- 15.Andreasen NC. Scale for the Assessment of Positive Symptoms (SAPS) Iowa City: University of Iowa; 1984. [Google Scholar]
- 16.Andreasen NC. Scale for the Assessment of Negative Symptoms (SANS) Iowa City: University of Iowa; 1984. [Google Scholar]
- 17.Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl. 1970;212:11–19. doi: 10.1111/j.1600-0447.1970.tb02066.x. [DOI] [PubMed] [Google Scholar]
- 18.Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154:672–676. doi: 10.1192/bjp.154.5.672. [DOI] [PubMed] [Google Scholar]
- 19.Guy W. ECDEU Assessment Manual for Psychopharmacology: Publication ADM 76–338. Washington, DC: US Department of Health, Education and Welfare; 1976. pp. 534–537. [Google Scholar]
- 20.Steen RG, Hamer RM, Lieberman JA. Measurement of brain metabolites by 1H magnetic resonance spectroscopy in patients with schizophrenia: a systematic review and meta-analysis. Neuropsychopharmacology. 2005;30:1949–1962. doi: 10.1038/sj.npp.1300850. [DOI] [PubMed] [Google Scholar]
- 21.Lindquist DM, Hawk RM, Karson CN, Komoroski RA. Effects of antipsychotic drugs on metabolite ratios in rat brain in vivo. Magn Reson Med. 2000;43:355–358. doi: 10.1002/(sici)1522-2594(200003)43:3<355::aid-mrm6>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 22.Bustillo J, Wolff C, Myers-Gutierrez A, Dettmer T, Cooper T, Allan A, et al. Treatment of rats with antipsychotic drugs: lack of an effect on N acetyl-aspartate levels. Schizophr Res. 2002;1889:1–9. doi: 10.1016/s0920-9964(02)00528-5. [DOI] [PubMed] [Google Scholar]
- 23.Magistretti PJ, Pellerin L. Cellular mechanisms of brain energy metabolism and their relevance to functional brain imaging. Phil Trans R Soc Lond. 1999;354:1155–1163. doi: 10.1098/rstb.1999.0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu S, Yang J, Li C, Zhu W, Shen J. Metabolic alterations in focally activated primary somatosensory cortex of alpha-chloralose-anesthetized rats measured by H MRS at 11.7T. Neuroimage. 2005;28:401–409. doi: 10.1016/j.neuroimage.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 25.Tibbo P, Hanstock C, Valiakalayil A, Allen P. 3-T proton MRS investigation of glutamate and glutamine in adolescents at high genetic risk for schizophrenia. Am J Psychiatry. 2004;161:1116–1118. doi: 10.1176/appi.ajp.161.6.1116. [DOI] [PubMed] [Google Scholar]
- 26.Tebartz van Elst L, Valerius G, Buchert M, Thiel T, Rusch N, Bubl E, et al. Increased prefrontal and hippocampal glutamate and concentration in schizophrenia: evidence from a magnetic resonance spectroscopy study. Biol Psychiatry. 2005;58:724–730. doi: 10.1016/j.biopsych.2005.04.041. [DOI] [PubMed] [Google Scholar]
- 27.Theberge J, Al-Semaan Y, Williamson PC, Menon RS, Neufeld RW, Rajakumar N, et al. Glutamate and glutamine in the anterior cingulate and thalamus of medicated patients with chronic schizophrenia and healthy comparison subjects measured with 4.0-T proton MRS. Am J Psychiatry. 2003;160:2231–2233. doi: 10.1176/appi.ajp.160.12.2231. [DOI] [PubMed] [Google Scholar]
- 28.Choe BY, Suh TS, Shinn KS, Lee CW, Lee C, Paik IH. Observation of metabolic changes in chronic schizophrenia after neuroleptic treatment by in vivo hydrogen magnetic resonance spectroscopy. Invest Radiol. 1996;6:345–352. doi: 10.1097/00004424-199606000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Fannon D, Simmons A, Tennakoon L, O’Ceallaiagh S, Sumich A, Doku V, et al. Selective deficit of hippocampal N-acetylaspartate in antipsychotic-naïve patients with schizophrenia. Biol Psychiatry. 2003;54:587–598. doi: 10.1016/s0006-3223(03)00185-9. [DOI] [PubMed] [Google Scholar]
- 30.Bustillo J, Lauriello J, Rowland L, Thomson L, Petropoulus H, Hammond R, et al. Longitudinal follow-up of neurochemical changes during the first year of antipsychotic treatment in schizophrenia patients with minimal previous medication exposure. Schizophr Res. 2002;58:313–321. doi: 10.1016/s0920-9964(02)00210-4. [DOI] [PubMed] [Google Scholar]
- 31.Bustillo J, Rowland L, Jung R, Brooks W, Qualls C, Hammond R, et al. Proton magnetic resonance spectroscopy during initial treatment with antipsychotic medication in schizophrenia. Neuropsychopharmacology. 2008;33:2456–2466. doi: 10.1038/sj.npp.1301631. [DOI] [PubMed] [Google Scholar]
- 32.Theberge J, Williamson KE, Aoyama N, Drost DJ, Manchanda R, Malla AK, et al. Longitudinal grey-matter and glutamatergic losses in first-episode schizophrenia. Br J Psychiatry. 2007;191:325–334. doi: 10.1192/bjp.bp.106.033670. [DOI] [PubMed] [Google Scholar]
- 33.Baldessarini RJ, Hegarty JD, Bird ED, Benes FM. Meta-analysis of postmortem studies of Alzheimer’s disease-like neuropathology in schizophrenia. Am J Psychiatry. 1997;154:861–863. doi: 10.1176/ajp.154.6.861. [DOI] [PubMed] [Google Scholar]
- 34.Glantz L, Lewis D. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- 35.Lentz M, Kim J, Westmoreland S, Greco J, Fuller R, Ratai E, et al. Quantitative neuropathologic correlates of changes in ratio of N-acetylaspartate to creatine in macaque brain. Radiology. 2005;235:461–468. doi: 10.1148/radiol.2352040003. [DOI] [PubMed] [Google Scholar]
- 36.Hajszan T, Leranth C, Roth RH. Subchronic phencyclidine treatment decreases the number of dendritic spine synapses in the rat prefrontal cortex. Biol Psychiatry. 2006;60:639–644. doi: 10.1016/j.biopsych.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 37.Matute C, Melone M, Vallejo-Illarramendi A, Conti F. Increased expression of the astrocytic glutamate transporter GLT-1 in the prefrontal cortex of schizophrenics. Glia. 2005;49:451–455. doi: 10.1002/glia.20119. [DOI] [PubMed] [Google Scholar]
- 38.Gluck MR, Thomas RG, Davis KL, Haroutunian V. Implications for altered glutamate and GABA metabolism in the dorsolateral prefrontal cortex of aged schizophrenic patients. Am J Psychiatry. 2002;159:1165–1173. doi: 10.1176/appi.ajp.159.7.1165. [DOI] [PubMed] [Google Scholar]
- 39.López-Gil X, Babot Z, Amargós-Bosch M, Suñol C, Artigas F, Adell A. Clozapine and haloperidol differently suppress the MK-801-increased glutamatergic and serotonergic transmission in the medial prefrontal cortex of the rat. Neuropsychopharmacology. 2007;32:2087–2097. doi: 10.1038/sj.npp.1301356. [DOI] [PubMed] [Google Scholar]
- 40.Lim KO, Hedehus M, Moseley M, de Crespigny A, Sullivan EV, Pferfferbaum A. Proton magnetic resonance imaging of cortical gray and white matter in schizophrenia. Arch Gen Psychiatry. 1998;55:346–352. doi: 10.1001/archpsyc.55.4.346. [DOI] [PubMed] [Google Scholar]
- 41.Pfefferbaum A, Sullivan EV, Hedehus M, Moseley M, Lim KO. Brain gray and white matter transverse relaxation time in schizophrenia. Psychiatry Res. 1999;91:93–100. doi: 10.1016/s0925-4927(99)00023-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.