Abstract
High-risk neuroblastoma is a childhood malignancy with a poor prognosis. Gradual improvements in survival have correlated with therapeutic intensity, and the ability to harvest, process and store autologous hematopoietic stem cells has allowed for dose intensification beyond marrow tolerance. The use of high-dose chemotherapy with autologous hematopoietic stem cell rescue in consolidation has resulted in improvements in survival, although further advances are still needed. Newer approaches to SCT and supportive care, most notably the transition to PBSC, have resulted in further improvement in survival and decreases in treatment-related mortality. Research into experimental approaches to hematopoietic SCT is ongoing.
Keywords: neuroblastoma, stem cell transplantation, tandem transplant
Introduction
Neuroblastoma is the second most common solid malignancy of childhood (after CNS tumors). While neuroblastoma has a broad spectrum of clinical presentations and behavior, high-risk neuroblastoma is still a significant clinical challenge and remains one of the most difficult pediatric tumors.1 Some progress in treating high-risk neuroblastoma has correlated with escalation of therapeutic intensity,2 although even with a CR achieved with maximal intensity induction therapy, long-term event-free survival remains less than 40–50%. In order to achieve dose escalation beyond marrow tolerance, harvest and storage of hematopoietic stem cells (HSC) capable of reestablishing trilinear hematopoiesis is required. Several recent studies comparing high-dose chemotherapy with stem cell rescue to maintenance chemotherapy have shown improved event-free survival (EFS) using this modality, although outcomes still remain relatively poor.3,4 Further intensification of consolidation therapy, through the use of tandem auto-logous SCT, has been facilitated by the ability to collect adequate peripheral blood HSC, and early studies suggest that this is a feasible approach which may improve outcome in this deadly disease.5,6
This review of SCT for high-risk neuroblastoma will cover several topics, including (1) a brief overview of autologous SCT, (2) the state of clinical experience using autologous SCT for high-risk neuroblastoma, (3) the challenges and approaches to HSC collection, processing and administration in these young patients and (4) experimental SCT for high-risk neuroblastoma.
Autologous SCT: overview
The harvest and storage of a patient's own HSC followed by reinfusion of those HSC to the patient following high-dose chemotherapy (HDC) is commonly referred to as autologous hematopoietic SCT, or stem cell rescue. The HDC regimen administered is generally beyond the tolerance of the patient's marrow (myeloablative), meaning that no hematopoietic recovery can occur without the stored HSC. There are, however, submyeloablative HDC regimens in which HSC rescue is not absolutely required for engraftment, but is used to speed recovery, decrease toxicity and the interval between courses of chemotherapy.7,8 There are certain criteria which may indicate the appropriate setting in which to use autologous SCT for malignancies. These include (1) a chemo-responsive tumor with good initial response to induction chemotherapy, but a poor 3-or 5-year EFS, (2) the use of an HDC regimen that may be dose escalated safely past marrow tolerance, (3) an HDC regimen that utilizes multiple agents active against the disease, including, ideally, agents different than those used in the induction regimen and (4) the use of optimal supportive care. Thus, high-risk neuroblastoma represents a malignancy likely to benefit from autologous SCT, as it meets the first three design criteria listed above. Specifically, it is a chemotherapy-sensitive disease in which most patients can achieve a CR or very good partial remission with induction chemotherapy, but in which a high CR rate does not translate into a high EFS rate. While 80–85% of patients have chemotherapy-responsive disease, <20% are long-term survivors with conventional chemotherapy. A principal difficulty in employing autologous SCT in these patients lies in the challenges inherent in harvesting adequate HSC from small children. Despite the difficulties, the improvements in EFS demonstrated in phase III studies have made autologous SCT as consolidation therapy the standard of care for patients with high-risk neuroblastoma.
Autologous SCT for neuroblastoma
The use of autologous SCT for high-risk neuroblastoma has been under investigation for over two decades. Several early single-arm or retrospective studies indicated that autologous transplant might improve the EFS of patients with high-risk neuroblastoma, although none of the studies were randomized, and their results may have been influenced by selection bias.9–11 In 1997, a large EBMT retrospective analysis of 1070 autologous transplant for high-risk neuroblastoma noted that 2-year survival among the group of patients who had reached an SCT procedure was 49%. In many of these procedures, the stem source was bone marrow. Most relapses occurred within the first 18 months following transplant, although relapses were found as late as 7 years from transplant. There were no survivors among the group of the 48 patients who relapsed and underwent a second SCT.12
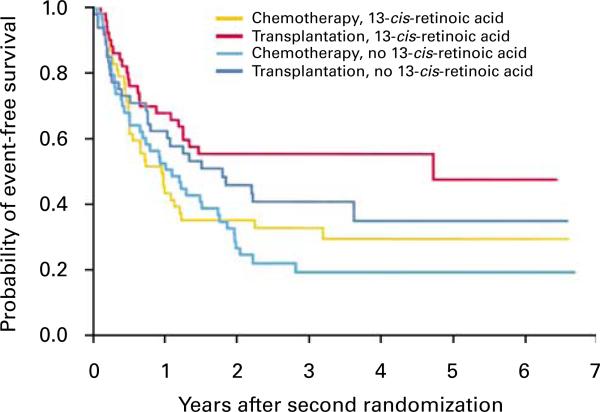
The largest randomized, phase III trial of autologous SCT for high-risk neuroblastoma was the Children's Cancer Group 3891 study. It employed a 2 × 2 factorial design in which patients were randomized to a consolidation regimen with autologous SCT (supported by purged bone marrow, as discussed below) versus continuation chemotherapy. Following consolidation, patients were then randomized to biologic therapy with isotretinoin (a maturational agent) versus no further therapy.13 The study found that event-free survival among patients with high-risk neuroblastoma was significantly better with high-dose chemotherapy and radiotherapy followed by transplantation of purged autologous bone marrow than with chemotherapy alone. They also noted that treatment with isotretinoin further improved the outcome among patients without progressive disease (see Figure 1). In the initial report, the authors estimated a 3.7-year EFS from diagnosis in the best group as being 38%. This represented a remarkable step forward in the treatment of high-risk neuroblastoma, and helped establish autologous transplant followed by 6 months of oral isotretinoin therapy as the standard of care for these patients. Despite its important results, this study was subject to the challenges of a 2 × 2 design and a complex treatment plan: of 579 eligible patients, 379 underwent the first randomization, and 258 patients underwent the second. This reduced the population of patients being studied to approximately 50 patients in each of the four treatment groups.
Figure 1.
Results of the CCG 3891 study as initially published.3 EFS among patients who reached both the first and second randomizations. Patients were assigned to BMT + isotretinoin, BMT without isotretinoin, continuation chemotherapy + isotretinoin and continuation chemotherapy without isotretinoin. Follow-up began at the time of the second randomization (34 weeks after diagnosis). EFS was significantly improved in the BMT + isotretinoin group compared to the continuation chemotherapy without isotretinoin group (P=0.02). Figure reproduced with permission from the N Engl J Med.
Further studies of autologous SCT in high-risk neuroblastoma built on the results of the 3891 study, and sought to modify the techniques of autologous SCT to allow for a further increase in dose intensity. Table 1 shows EFS rates at or around 3 years for several major studies. Several trends can be seen in these data. First, some studies have utilized TBI and some have not. The use of TBI in these young children is controversial because of the issue of long-term side effects, and there is no randomized comparison of a TBI-containing versus non-TBI regimen to demonstrate whether it adds efficacy to such consolidation regimens. Based on the COG A3973 study (see below), the effective standard conditioning regimen in the United States at this time is carboplatin, etoposide and melphalan. Carboplatin and etoposide are effective anti-neuroblastoma agents which can be dose escalated beyond marrow tolerance (see above), and melphalan is another active agent which (in effective doses) can only be utilized in the setting of consolidation. In sum, these studies as well as the PBSC studies described below have established the core standard for neuroblastoma treatment in 2007—five or six cycles of effective multiagent induction chemotherapy, surgery, radiotherapy at least to the primary tumor bed and SCT followed by oral isotretinoin.
Table 1.
Results from selected studies of autologous SCT in high-risk neuroblastomaa
| Group | N | Study type | EFS from | EFS | Myeloablative regimen(s) |
|---|---|---|---|---|---|
| EBMT12 | 1070 | Retrospective | Transplant | 2 year (49%) | Various |
| 5 year (33%) | |||||
| CCG 38913 | 539 | Phase III | Estimated from diagnosis | 3.7 year (38%) | CEM/TBI |
| Grupp et al.5 | 97 | Phase II | Diagnosis | 3 year (55%) | |
| No. 1 CECtx | |||||
| No. 2 melphalan/TBI | |||||
| Kletzel et al.7 | 25 | Phase II | Diagnosis | 3 year (57%) | |
| No. 1 CE | |||||
| No. 2 CE | |||||
| No. 3 TCtx | |||||
| Villablanca et al.b | 73 | Phase II | Transplant | 3 year (49%) | CEM |
| 5 year (47%) | |||||
| Kreissman et al.23 | 489 | Phase III | Diagnosis | 2 year (49%) | CEM |
| 3 year (~40%)c |
Abbreviations: C = carboplatin; CCG = Children's Cancer Group; Ctx = cyclophosphamide; E = etoposide; EBMT = European Group for Blood and Marrow Transplantation; EFS = event-free survival; M = melphalan; T = thiotepa; TBI = total body irradiation.
Study populations differed significantly in these six studies. The EBMT analysis included allogeneic transplants and transplants after relapse. Villablanca, et al. included only stage 4 > 1 year post-SCT in the group presented.
Villablanca et al., unpublished data.
The median follow-up on A3973 is 2 years, so this is an estimate.
Challenges to PBSC collection, processing and administration
Progress in the management of SCT patients has reduced the mortality rate in autologous transplant to <5% in many studies. This improvement has been a consequence of advances in infection prophylaxis and supportive care, but perhaps even more important has been the switch from bone marrow to mobilized hematopoietic PBSC to support the hematopoietic recovery of the patient. Compared to autologous marrow, PBSC provide faster hematopoietic recovery and therefore lower rates of infection, quicker resolution of mucositis, earlier discharge from the hospital and less risk of prolonged need for transfusions. As a consequence, the use of autologous bone marrow as a source of HSC has all but disappeared. In our own institution, no patient has received autologous bone marrow since 1996. Despite the impressive advantages of PBSC, the young age and small size of patients with high-risk neuroblastoma present significant challenges to the collection and administration of PBSC in adequate numbers. The following subsections will discuss the collection, vascular access, processing and dosing of PBSC in very small patients.
Collection of PBSC
An increase in HSC concentration in the peripheral blood (mobilization) can be measured at the hematopoietic nadir following chemotherapy, and can also be induced through the use of hematopoietic growth factors (HGF).14 This mobilization provides the increased concentration of PBSC necessary to obtain adequate numbers for SCT. Following mobilization, HSC can be collected using apheresis. This technology allows for the separation of specific blood components from whole blood continuously, and in real time, by centrifugation. The various blood components can then be either diverted into a collection container for processing and storage, or can be returned to the patient. Even in an effectively mobilized patient, HSC are sufficiently rare and processing of multiple blood volumes is often required, often over more than 1 day, and especially in patients pretreated with multiple courses of chemotherapy.15 A major challenge to achieving an effective apheresis collection in small children is adequate vascular access.
Vascular access for PBSC pheresis
Two ports of vascular access are required for continuous blood processing—one for blood draw, and the other for return. In most adults, these can be achieved using two antecubital lines. However, in a small child, percutaneous antecubital large-bore access is not possible, and conventional Broviac-type catheters can be difficult to use for apheresis, as the lumen tends to collapse under the negative pressure used to draw blood at a rate required for effective pheresis (approximately 1–2 ml/kg/min). Thus, a specially designed apheresis catheter is ideal. It is designed to allow for faster rates of flow by combining a larger lumen size, shorter catheter length and stiffer walls. Although there are several configurations, an apheresis catheter is generally a two-lumen catheter with offset proximal and distal ports, and side holes along the tip of the catheter. This design minimizes the mixing of processed and unprocessed blood, thereby maximizing the efficiency of the collection. The catheters are available for both temporary and tunneled insertion. We have generally used 8 Fr cuffed tunneled catheters (MedComp) in our smaller patients, specifically those who weigh 10 kg or more. Patients smaller than 10 kg may require temporary femoral line placement, as femoral lines are shorter and allow faster collection rates for a given diameter.
Processing of PBSC
Engineering of the PBSC graft is possible to remove or expand desired cell populations. The most researched manipulation in the context of neuroblastoma is the purging of malignant cells prior to the infusion of the HSC product. This is potentially important, as research has suggested that clonogenic tumor cells can be infused with an HSC graft, and that these cells can result in relapse of the malignancy.16
There are two methods to purge an HSC product of tumor cells—either positive selection of HSC to the exclusion of tumor cells, or negative selection designed to specifically remove malignant cells. In terms of positive selection, CD34 selection is the primary technique available to stem cell laboratories. CD34 is an antigen expressed on HSC and progenitors of all hematopoietic lineages. In general, CD34 selection will result in a product that is 60–95% CD34+. Automated processes are available that are capable of selecting the CD34+ cell population away from the 99% of PBSC that are irrelevant for engraftment, including T cells and tumor cells that do not express CD34. One of these technologies, the Isolex 300i device, is FDA approved, and a second, the Miltenyi CliniMACS device, is approved in Europe and may become available in the United States. CD34 selection has been used to purge stem cell products in patients with neuroblastoma, but concerns have been raised that some neuroblastoma cells may express CD34 or surface epitopes cross-reactive with anti-CD34 monoclonal antibodies.17,18 Our data have not confirmed this hypothesis,19 and we, along with others, have used CD34 selection as a purging technique for PBSC products in the clinical setting.20
In terms of negative selection, the most widely used technique in neuroblastoma has been antitumor monoclonal antibodies followed by a magnetic depletion step.21,22 Although the evidence suggests that purging of bone marrow may be important, PBSC are less likely to contain tumor cells than bone marrow, and no study to date has shown that purging itself improves outcome. To address this question, the Children's Oncology Group has recently completed the A3973 trial—a phase III, randomized comparison of purged versus unpurged PBSC given in the context of autologous SCT for high-risk neuroblastoma. Although this study is currently unpublished, data from the A3973 trial have have been presented at American Society of Clinical Oncology (ASCO).23 These preliminary analyses have shown no advantage for patients receiving a purged PBSC product (S Kreissman and W London, unpublished data): the 2-year EFS in the unpurged group was 51%, and in the purged group 47% (P=0.47), with an estimated and preliminary 3-year EFS of approximately 40% overall. This suggests that the central challenge in therapy of neuroblastoma continues to be purging the patient of the tumor.
Dosing of PBSC
As discussed above, CD34 is a marker of most HSC and progenitors of the hematopoietic lineages. Importantly, the number of CD34+ cells infused to a patient correlates with the likelihood of successful engraftment,24,25 and the number of CD34+ cells can be enumerated by flow cytometry. Below a threshold of approximately 1 × 106 CD34+ cells per kg, engraftment becomes unreliable,26 and most centers attempt to achieve minimum doses of 2 × 106 to 2.5 106 CD34+ cells per kg. Doses greater than 5 × 106 CD34+ cells per kg probably do not provide any incremental benefit, and can be challenging to collect in patients who have been treated with multiple courses of chemotherapy. Because of the availability of therapies that might require PBSC support for recurrent disease, collection of a ‘backup’ aliquot of PBSC at the time of initial collection is recommended for patients with good HSC mobilization.25
The ideal time to initiate apheresis is both critical and difficult to predict in patients who have received chemotherapy. Many centers set the start date 1–3 days after the ANC reaches 1000 per μl after the nadir, with concomitant evidence of platelet recovery. Some centers have access to a quantitative assay for CD34+ cells in the peripheral blood. Detection of <5 CD34+ cells per μl is predictive of a poor PBSC collection, while >10–20 correlates with collecting >2.5 × 106 CD34+ cells per kg in a single procedure.27,28
Experimental SCT for high-risk neuroblastoma
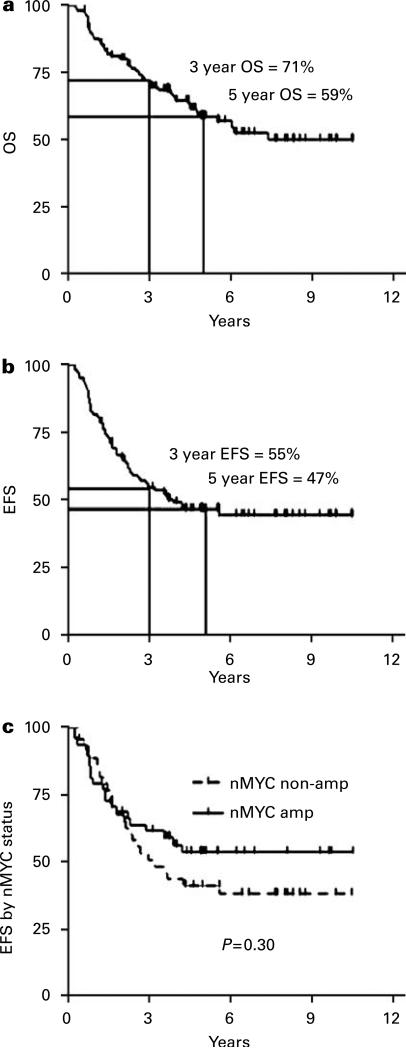
The more rapid recovery afforded by PBSC has allowed the autologous SCT concept to be extended to sequential cycles. This approach (commonly known as ‘tandem transplant’) builds on the hypothesis that improved outcomes correlate with dose intensity. Tandem transplant allows for even greater dose intensity in consolidation with the potential to introduce different active agents at each of the transplants. Initially, this approach used bone marrow as the HSC source, but was complicated by unacceptable treatment-related mortality (TRM).29 Following the switch from bone marrow to PBSC as the HSC source, several groups have tested the tandem transplant approach with more promising results.6,7,30 The largest of these studies was conducted over 6 years at four cooperating institutions. It showed a 3-year EFS of 55% in a sequentially treated group of 97 patients (most recent update shown in Figure 2). The study was designed around early collection of PBSC, the use of CD34 selection as a purging method and two myeloablative consolidation regimens containing distinct agents (first carboplatin/etoposide/cyclophosphamide, then melphalan/TBI). TRM in this study was 6%, and included one patient who died of EBV lymphoproliferative disease (EBV-LPD). Although EBV-LPD is exceedingly uncommon following autologous SCT, this study had three cases among 97 patients. This suggests that the combination of CD34 selection and tandem transplant is more immunosuppressive than autologous SCT using unpurged PBSC.20,31 A second study found similar results using three sequential HDC procedures.7: EFS at 3 years was 57%. A total of 19 of the 25 patients completed the second autologous SCT, 17 went onto the third and 1 late TRM was observed.
Figure 2.
Kaplan–Meier curves showing the overall and event-free survival for patients undergoing tandem autologous SCT for high-risk neuroblastoma. The patients were conditioned with carboplatin/etoposide/cyclophosphamide for the first transplant, then melphalan/TBI for the second. (a) Overall survival (OS) from diagnosis, (b) event-free survival (EFS) from diagnosis and (c) EFS from diagnosis stratified by nMYC amplification.
In addition to increasing the dose intensity through tandem transplant, some groups have tried novel radiopharmaceutical agents either alone or as part of a myeloablative consolidation regimen. Metaiodobenzylguanidine (MIBG) is a compound selectively concentrated in >90% of neuroblastomas. The conjugation of this compound to 131I has been used as an imaging modality for neuroblastoma. It can also be used at higher doses to deliver targeted radiation to neuroblastoma. High-dose 131I-MIBG as a single agent has an excellent response rate in relapsed neuroblastoma patients with MIBG-avid tumors, with an overall complete plus partial response rate on 36% in a recent phase II report.32 The principal toxicity of high-dose MIBG therapy is hematologic, and up to 40% of these patients can require PBSC support32,33 supporting that there may be value in collecting and cryopreserving an extra aliquot of PBSC at the time of the initial pheresis procedure.25 Extending this concept, several studies have combined therapeutic doses of 131I-MIBG with HDC as a consolidation therapy, with preliminary evidence of efficacy in high-risk patients.34,35 Another approach, studied by several groups including the group at Memorial Sloan Kettering, has been an immunotherapy approach using antibodies that target neuroblastoma, most commonly targeting the neuroblastoma surface antigen GD2. These antibodies have been delivered as radionuclide immunoconjugates,36 as fusion proteins containing a cytokine moiety,37 and as unmodified38 or chimerized murine monoclonal antibody.39 The latter monoclonal, ch14.18, is currently being tested in the setting of post-SCT minimal residual disease in the Children's Oncology Group phase III randomized trial ANBL0032.
Finally, as an alternative to autologous SCT, some groups have studied allogeneic SCT in an attempt to harness an immunotherapeutic effect. A graft-versus-malignancy effect has been well described in allogeneic transplant for liquid tumors, but has not yet been convincingly demonstrated in the setting of solid tumors.40 Although initial studies of conventional allogeneic SCT for high-risk neuroblastoma failed to show clear benefit,12,41 the advent of nonmyeloablative conditioning regimens has provided hope that reduced intensity conditioning will reduce TRM and allow for the detection of a therapeutic benefit. As a result, institutions are beginning to explore the possibility of an allogeneic effect in neuroblastoma. At this point, this is still an investigational and unusual application of allogeneic transplant, with 38 such cases reported to the EBMT from 1991 to 2002.42 Some recent case reports have provided preliminary evidence for a graft-versus-tumor effect in neuroblastoma. A 2003 case report described a patient who underwent allogeneic SCT after a relapse. Although the patient received further chemotherapy after the allogeneic transplant and response could not be correlated to GVHD, the patient did enter a CR sustained for at least 4 years.43 In a more recent report, development of GVHD correlated temporally with disease response in a patient who had undergone a reduced-intensity allogeneic bone marrow transplant.44 In a similar regard, the group at Columbia has been testing reduced intensity allogeneic cord blood transplants in patients with a wide range of diagnoses, including neuroblastoma.45,46
The data continue to show improving outcomes with increasing intensity of consolidation regimens assisted by PBSC-based autologous SCT rescue. This therapeutic modality remains the standard of therapy for patients undergoing their primary treatment for neuroblastoma. Other modalities, including radionuclide therapy and especially allogeneic SCT, should only be undertaken in the context of a clinical trial which targets patients appropriate for such experimental therapies. One such group is patients who have recurred after intensive therapy, where there is no established curative option. Another group to target in such trials has been termed ‘ultrahigh-risk’. This group includes patients who have failed the test of chemosensitivity and still have significant disease burdens, with either stable disease after induction or other indicators of nonchemosensitive disease. These include significant residual disease at metastatic sites at end induction: residual bone marrow infiltration with tumor detected by microscopy immunocytology,47 or presence of multiple sites of skeletal disease as demonstrated by 123I-MIBG scintigraphy.48,49 These patients have a poor outcome after SCT: Katzenstein et al.48 report 0% EFS in patients with three or more sites of active disease still present prior to SCT. These data suggest that this group of ultrahigh-risk patients are candidates for experimental therapies.
Conclusion
In 2007, the standard therapy for high-risk neuroblastoma is based on a backbone of multicycle induction, PBSC collection early in induction, testing of the PBSC product for neuroblastoma contamination, as complete surgical resection as possible without organ removal, autologous SCT, (although there is no clearly superior combination of consolidation agents) and local radiotherapy before or after the autologous SCT. The next phase III COG trial will test single versus tandem transplant as consolidation therapy. Having reached an effective limit in terms of chemotherapeutic intensity with tandem transplant, future trials will need to focus on targeted therapies and/or immunotherapy in the hope of improving outcomes for children afflicted with this cruel disease.
References
- 1.Maris JM, Hogarty MD, Bagatell R, Cohn SL. Neuroblastoma. Lancet. 2007;369:2106–2120. doi: 10.1016/S0140-6736(07)60983-0. [DOI] [PubMed] [Google Scholar]
- 2.Cheung NV, Heller G. Chemotherapy dose intensity correlates strongly with response, median survival, and median progression-free survival in metastatic neuroblastoma. J Clin Oncol. 1991;9:1050–1058. doi: 10.1200/JCO.1991.9.6.1050. [DOI] [PubMed] [Google Scholar]
- 3.Matthay KK, Villablanca JG, Seeger RC, Stram DO, Harris RE, Ramsay NK, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. N Engl J Med. 1999;341:1165–1173. doi: 10.1056/NEJM199910143411601. [DOI] [PubMed] [Google Scholar]
- 4.Berthold F, Boos J, Burdach S, Erttmann R, Henze G, Hermann J, et al. Myeloablative megatherapy with autologous stem-cell rescue versus oral maintenance chemotherapy as consolidation treatment in patients with high-risk neuroblastoma: a randomised controlled trial. Lancet Oncol. 2005;6:649–658. doi: 10.1016/S1470-2045(05)70291-6. [DOI] [PubMed] [Google Scholar]
- 5.George RE, Li S, Medeiros-Nancarrow C, Neuberg D, Marcus K, Shamberger RC, et al. High-risk neuroblastoma treated with tandem autologous peripheral-blood stem cell-supported transplantation: long-term survival update. J Clin Oncol. 2006;24:2891–2896. doi: 10.1200/JCO.2006.05.6986. [DOI] [PubMed] [Google Scholar]
- 6.Grupp SA, Stern JW, Bunin N, Nancarrow C, Ross AA, Mogul M, et al. Tandem high-dose therapy in rapid sequence for children with high-risk neuroblastoma. J Clin Oncol. 2000;18:2567–2575. doi: 10.1200/JCO.2000.18.13.2567. [DOI] [PubMed] [Google Scholar]
- 7.Kletzel M, Katzenstein HM, Haut PR, Yu AL, Morgan E, Reynolds M, et al. Treatment of high-risk neuroblastoma with triple-tandem high-dose therapy and stem-cell rescue: results of the Chicago Pilot II Study. J Clin Oncol. 2002;20:2284–2292. doi: 10.1200/JCO.2002.06.060. [DOI] [PubMed] [Google Scholar]
- 8.Kreissman SG, Rackoff W, Lee M, Breitfeld PP. High dose cyclophosphamide with carboplatin: a tolerable regimen suitable for dose intensification in children with solid tumors. J Pediatr Hematol Oncol. 1997;19:309–312. doi: 10.1097/00043426-199707000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Matthay KK. Impact of myeloablative therapy with bone marrow transplantation in advanced neuroblastoma. Bone Marrow Transplant. 1996;18(Suppl 3):S21–S24. [PubMed] [Google Scholar]
- 10.Matthay KK, O'Leary MC, Ramsay NK, Villablanca J, Reynolds CP, Atkinson JB, et al. Role of myeloablative therapy in improved outcome for high risk neuroblastoma: review of recent Children's Cancer Group results. Eur J Cancer. 1995;31A:572–575. doi: 10.1016/0959-8049(95)00015-b. [DOI] [PubMed] [Google Scholar]
- 11.Dini G, Philip T, Hartmann O, Pinkerton R, Chauvin F, Garaventa A, et al. Bone marrow transplantation for neuroblastoma: a review of 509 cases. EBMT Group. Bone Marrow Transplant. 1989;4(Suppl 4):42–46. [PubMed] [Google Scholar]
- 12.Philip T, Ladenstein R, Lasset C, Hartmann O, Zucker JM, Pinkerton R, et al. 1070 myeloablative megatherapy procedures followed by stem cell rescue for neuroblastoma: 17 years of European experience and conclusions. European Group for Blood and Marrow Transplant Registry Solid Tumour Working Party. Eur J Cancer. 1997;33:2130–2135. doi: 10.1016/s0959-8049(97)00324-9. [DOI] [PubMed] [Google Scholar]
- 13.Matthay KK, Villablanca JG, Seeger RC, Stram DO, Harris RE, Ramsay NK, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children's Cancer Group. N Engl J Med. 1999;341:1165–1173. doi: 10.1056/NEJM199910143411601. [DOI] [PubMed] [Google Scholar]
- 14.Gazitt Y. Comparison between granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor in the mobilization of peripheral blood stem cells. Curr Opin Hematol. 2002;9:190–198. doi: 10.1097/00062752-200205000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Rowley SD, Donaldson G, Lilleby K, Bensinger WI, Appelbaum FR. Experiences of donors enrolled in a randomized study of allogeneic bone marrow or peripheral blood stem cell transplantation. Blood. 2001;97:2541–2548. doi: 10.1182/blood.v97.9.2541. [DOI] [PubMed] [Google Scholar]
- 16.Rill DR, Santana VM, Roberts WM, Nilson T, Bowman LC, Krance RA, et al. Direct demonstration that autologous bone marrow transplantation for solid tumors can return a multiplicity of tumorigenic cells. Blood. 1994;84:380–383. [PubMed] [Google Scholar]
- 17.Hafer R, Voigt A, Gruhn B, Zintl F. Neuroblastoma cells can express the hematopoietic progenitor cell antigen CD34 as detected at surface protein and mRNA level. J Neuroimmunol. 1999;96:201–206. doi: 10.1016/s0165-5728(99)00030-2. [DOI] [PubMed] [Google Scholar]
- 18.Voigt A, Hafer R, Gruhn B, Zintl F. Expression of CD34 and other haematopoietic antigens on neuroblastoma cells: consequences for autologous bone marrow and peripheral blood stem cell transplantation. J Neuroimmunol. 1997;78:117–126. doi: 10.1016/s0165-5728(97)00088-x. [DOI] [PubMed] [Google Scholar]
- 19.Donovan J, Temel J, Zuckerman A, Gribben J, Fang J, Pierson G, et al. CD34 selection as a stem cell purging strategy for neuroblastoma: preclinical and clinical studies. Med Pediatr Oncol. 2000;35:677–682. doi: 10.1002/1096-911x(20001201)35:6<677::aid-mpo42>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 20.Kanold J, Yakouben K, Tchirkov A, Carret AS, Vannier JP, LeGall E, et al. Long-term results of CD34(+) cell transplantation in children with neuroblastoma. Med Pediatr Oncol. 2000;35:1–7. doi: 10.1002/1096-911x(200007)35:1<1::aid-mpo1>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 21.Reynolds CP, Seeger RC, Vo DD, Black AT, Wells J, Ugelstad J. Model system for removing neuroblastoma cells from bone marrow using monoclonal antibodies and magnetic immunobeads. Cancer Res. 1986;46:5882–5886. [PubMed] [Google Scholar]
- 22.Seeger RC, Vo DD, Ugelstad J, Reynolds CP. Removal of neuroblastoma cells from bone marrow with monoclonal antibodies and magnetic immunobeads. Prog Clin Biol Res. 1986;211:285–293. [PubMed] [Google Scholar]
- 23.Kreissman SG, Villablanca JG, Diller L, London WB, Maris JM, Park R, et al. Response and toxicity to a dose-intensive multi-agent chemotherapy induction regimen for high risk neuroblastoma (HR-NB): a Children's Oncology Group (COG A3973) study. J Clin Oncol. 2007;25:9505. [Google Scholar]
- 24.Shpall EJ, Champlin R, Glaspy JA. Effect of CD34+ peripheral blood progenitor cell dose on hematopoietic recovery. Biol Blood Marrow Transplant. 1998;4:84–92. doi: 10.1053/bbmt.1998.v4.pm9763111. [DOI] [PubMed] [Google Scholar]
- 25.Grupp SA, Cohn SL, Wall D, Reynolds CP. Collection, storage, and infusion of stem cells in children with high-risk neuroblastoma: saving for a rainy day. Pediatr Blood Cancer. 2006;46:719–722. doi: 10.1002/pbc.20769. [DOI] [PubMed] [Google Scholar]
- 26.Shpall EJ, Champlin R, Glaspy JA. Effect of CD34+ peripheral blood progenitor cell dose on hematopoietic recovery. Biol Blood Marrow Transplant. 1998;4:84–92. doi: 10.1053/bbmt.1998.v4.pm9763111. [DOI] [PubMed] [Google Scholar]
- 27.Linn YC, Heng KK, Rohimah S, Goh YT. Peripheral blood progenitor cell mobilization in three groups of subjects: a comparison of leukapheresis yield and timing. J Clin Apher. 2000;15:217–223. doi: 10.1002/1098-1101(2000)15:4<217::aid-jca1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 28.Yu J, Leisenring W, Bensinger WI, Holmberg LA, Rowley SD. The predictive value of white cell or CD34+ cell count in the peripheral blood for timing apheresis and maximizing yield. Transfusion. 1999;39:442–450. doi: 10.1046/j.1537-2995.1999.39050442.x. [DOI] [PubMed] [Google Scholar]
- 29.Philip T, Ladenstein R, Zucker JM, Pinkerton R, Bouffet E, Louis D, et al. Double megatherapy and autologous bone marrow transplantation for advanced neuroblastoma: the LMCE2 study. Br J Cancer. 1993;67:119–127. doi: 10.1038/bjc.1993.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grupp SA, Stern JW, Bunin N, Nancarrow C, Adams R, Gorlin JB, et al. Rapid-sequence tandem transplant for children with high-risk neuroblastoma. Med Pediatr Oncol. 2000;35:696–700. doi: 10.1002/1096-911x(20001201)35:6<696::aid-mpo46>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 31.Powell JL, Bunin NJ, Callahan C, Aplenc R, Griffin G, Grupp SA. An unexpectedly high incidence of Epstein–Barr virus lymphoproliferative disease after CD34+ selected autologous peripheral blood stem cell transplant in neuroblastoma. Bone Marrow Transplant. 2004;33:651–657. doi: 10.1038/sj.bmt.1704402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matthay KK, Yanik G, Messina J, Quach A, Huberty J, Cheng SC, et al. Phase II study on the effect of disease sites, age, and prior therapy on response to iodine-131-metaiodobenzylguanidine therapy in refractory neuroblastoma. J Clin Oncol. 2007;25:1054–1060. doi: 10.1200/JCO.2006.09.3484. [DOI] [PubMed] [Google Scholar]
- 33.Matthay KK, DeSantes K, Hasegawa B, Huberty J, Hattner RS, Ablin A, et al. Phase I dose escalation of 131I-metaiodobenzylguanidine with autologous bone marrow support in refractory neuroblastoma. J Clin Oncol. 1998;16:229–236. doi: 10.1200/JCO.1998.16.1.229. [DOI] [PubMed] [Google Scholar]
- 34.Klingebiel T, Bader P, Bares R, Beck J, Hero B, Jurgens H, et al. Treatment of neuroblastoma stage 4 with 131I-meta-iodo-benzylguanidine, high-dose chemotherapy and immunotherapy. A pilot study. Eur J Cancer. 1998;34:1398–1402. doi: 10.1016/s0959-8049(98)00130-0. [DOI] [PubMed] [Google Scholar]
- 35.Yanik GA, Levine JE, Matthay KK, Sisson JC, Shulkin BL, Shapiro B, et al. Pilot study of iodine-131-metaiodobenzylguanidine in combination with myeloablative chemotherapy and autologous stem-cell support for the treatment of neuroblastoma. J Clin Oncol. 2002;20:2142–2149. doi: 10.1200/JCO.2002.08.124. [DOI] [PubMed] [Google Scholar]
- 36.Cheung NK, Kushner BH, Kramer K. Monoclonal antibody-based therapy of neuroblastoma. Hematol Oncol Clin North Am. 2001;15:853–866. doi: 10.1016/s0889-8588(05)70255-0. [DOI] [PubMed] [Google Scholar]
- 37.Osenga KL, Hank JA, Albertini MR, Gan J, Sternberg AG, Eickhoff J, et al. A phase I clinical trial of the hu14.18-IL2 (EMD 273063) as a treatment for children with refractory or recurrent neuroblastoma and melanoma: a study of the Children's Oncology Group. Clin Cancer Res. 2006;12:1750–1759. doi: 10.1158/1078-0432.CCR-05-2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kushner BH, Kramer K, Cheung N-KV. Phase II trial of the anti-GD2 monoclonal antibody 3F8 and granulocyte-macrophage colony-stimulating factor for neuroblastoma. J Clin Oncol. 2001;19:4189–4194. doi: 10.1200/JCO.2001.19.22.4189. [DOI] [PubMed] [Google Scholar]
- 39.Simon T, Hero B, Faldum A, Handgretinger R, Schrappe M, Niethammer D, et al. Consolidation treatment with chimeric anti-GD2-antibody ch14.18 in children older than 1 year with metastatic neuroblastoma. J Clin Oncol. 2004;22:3549–3557. doi: 10.1200/JCO.2004.08.143. [DOI] [PubMed] [Google Scholar]
- 40.Srinivasan R, Barrett J, Childs R. Allogeneic stem cell transplantation as immunotherapy for nonhematological cancers. Semin Oncol. 2004;31:47–55. doi: 10.1053/j.seminoncol.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 41.Matthay KK, Seeger RC, Reynolds CP, Stram DO, O'Leary MC, Harris RE, et al. Allogeneic versus autologous purged bone marrow transplantation for neuroblastoma: a report from the Childrens Cancer Group. J Clin Oncol. 1994;12:2382–2389. doi: 10.1200/JCO.1994.12.11.2382. [DOI] [PubMed] [Google Scholar]
- 42.Gratwohl A, Baldomero H, Demirer T, Rosti G, Dini G, Ladenstein R, et al. Hematopoetic stem cell transplantation for solid tumors in Europe. Ann Oncol. 2004;15:653–660. doi: 10.1093/annonc/mdh142. [DOI] [PubMed] [Google Scholar]
- 43.Inoue M, Nakano T, Yoneda A, Nishikawa M, Nakayama M, Yumura-Yagi K, et al. Graft-versus-tumor effect in a patient with advanced neuroblastoma who received HLA haploidentical bone marrow transplantation. Bone Marrow Transplant. 2003;32:103–106. doi: 10.1038/sj.bmt.1704070. [DOI] [PubMed] [Google Scholar]
- 44.Marabelle A, Paillard C, Tchirkov A, Halle P, Chassagne J, Demeocq F, et al. Graft-versus-tumour effect in refractory metastatic neuroblastoma. Bone Marrow Transplant. 2007;39:809–810. doi: 10.1038/sj.bmt.1705681. [DOI] [PubMed] [Google Scholar]
- 45.Yamashiro DJ, Lee A, Bhatia M, Glade-Bender J, Qualter E, Militano O, et al. Feasibility of autologous stem cell transplant followed by reduced intensity allogeneic stem cell transplantation for high risk neuroblastoma: a single institution pilot study. Biol Blood Marrow Transplant. 2007;13:68–69. [Google Scholar]
- 46.Toro GD, Bradley M, Satwani P, Morris E, Ven Cvd, Cheung YK, et al. Reduced intensity (RI) allogeneic cord blood hematopoietic cell transplantation (Allo CBHCT) in pediatric patients with malignant and non-malignant diseases. Biol Blood Marrow Transplant. 2006;12:125. [Google Scholar]
- 47.Seeger RC, Reynolds CP, Gallego R, Stram DO, Gerbing RB, Matthay KK. Quantitative tumor cell content of bone marrow and blood as a predictor of outcome in stage IV neuroblastoma: a Children's Cancer Group Study. J Clin Oncol. 2000;18:4067–4076. doi: 10.1200/JCO.2000.18.24.4067. [DOI] [PubMed] [Google Scholar]
- 48.Katzenstein HM, Cohn SL, Shore RM, Bardo DM, Haut PR, Olszewski M, et al. Scintigraphic response by 123I-metaiodobenzylguanidine scan correlates with event-free survival in high-risk neuroblastoma. J Clin Oncol. 2004;22:3909–3915. doi: 10.1200/JCO.2004.07.144. [DOI] [PubMed] [Google Scholar]
- 49.Matthay KK, Edeline V, Lumbroso J, Tanguy ML, Asselain B, Zucker JM, et al. Correlation of early metastatic response by 123I-metaiodobenzylguanidine scintigraphy with overall response and event-free survival in stage IV neuroblastoma. J Clin Oncol. 2003;21:2486–2491. doi: 10.1200/JCO.2003.09.122. [DOI] [PubMed] [Google Scholar]