Abstract
Rationale
Evidence links longevity to dietary restriction (DR). A decrease in body temperature (Tb) is thought to contribute to enhanced longevity because lower Tb reduces oxidative metabolism and oxidative stress. It is as yet unclear how DR decreases Tb.
Objective
Here, we test the hypothesis that prolonged DR decreases Tb by sensitizing adenosine A1 receptors (A1AR) and adenosine-induced cooling.
Methods and results
Sprague–Dawley rats were dietary restricted using an every-other-day feeding protocol. Rats were fed every other day for 27 days and then administered the A1AR agonist, N6-cyclohexyladenosine (CHA; 0.5 mg/kg, i.p.). Respiratory rate (RR) and subcutaneous Tb measured using IPTT-300 transponders were monitored every day and after drug administration. DR animals displayed lower RR on day 20 and lower Tb on day 22 compared to animals fed ad libitum and displayed a larger response to CHA. In all cases, RR declined before Tb. Contrary to previous reports, a higher dose of CHA (5 mg/kg, i.p.) was lethal in both dietary groups. We next tested the hypothesis that sensitization to the effects of CHA was due to increased surface expression of A1AR within the hypothalamus. We report that the abundance of A1AR in the membrane fraction increases in hypothalamus, but not cortex of DR rats.
Conclusion
These results suggest that every-other-day feeding lowers Tb via sensitization of thermoregulatory effects of endogenous adenosine by increasing surface expression of A1AR.
Discussion
Evidence that diet can modulate purinergic signaling has implications for the treatment of stroke, brain injury, epilepsy, and aging.
Keywords: Caloric restriction, Every-other-day feeding, Intermittent fasting, Intermittent feeding, Thermoregulation, Sensitization, Metabolic suppression
Introduction
Dietary restriction (DR), defined by a decrease in food intake, lowers core body temperature, enhances longevity, and attenuates progression of neurodegenerative diseases in animal models (Contestabile 2009). These effects have been suggested to be through a reduction in metabolic demand associated with a decrease in body temperature (Tb; Ungvari et al. 2008). A recent study found that increasing temperature of the hypothalamus in mice decreased core Tb and increased life span (Conti et al. 2006). However, a mechanistic link between metabolism or nutrient homeostasis and temperature is lacking (Tabarean et al. 2009).
It has also been shown that stimulation of the adenosine A1 receptor (A1AR) within the hypothalamus decreases Tb (Shintani et al. 2005). Although there are many studies showing that A1ARs play a role in thermoregulation (Steiner et al. 2002; Swoap et al. 2007; Tamura et al. 2005), there are no studies to our knowledge that have linked alterations in purinergic signaling to DR-induced cooling.
In this study, we test the hypothesis that DR imposed by every-other-day feeding (EODF) lowers Tb by sensitizing the response to A1AR stimulation. Moreover, we test the hypothesis that sensitization is associated with increased A1AR surface expression within the hypothalamus. Sensitization to cooling effects of A1AR stimulation via increased A1AR surface expression may account for the chronic decrease in Tb following DR and contribute to increased longevity in DR animals.
Materials and methods
Experimental animals
All procedures were approved by UAF's Animal Care and Use Committee. Male Sprague–Dawley rats (90 days old; Simonson Laboratories, Gilroy, CA, USA) were housed two per cage at an ambient temperature of 20°C, on 12:12 L:D, and fed either ad libitum (AL) or a dietary-restricted diet. IPTT-300 transponders (BioMedic, Seaford, DE, USA) used to monitor Tb were implanted on day 1. DR was started on day 2. Dietary-restricted animals were fed every other day (AL for 24 h periods), and food was removed on alternate days. Body temperature (Tb) and respiratory rate (RR), monitored by visual inspection, were monitored daily between 11:00 a.m. and 1:00 p.m. just prior to feeding if food was added. Body weight was measured every fourth day after collecting Tb and RR. Drug was delivered on day 29 after 27 days of DR. On day 29, five animals from each dietary group were injected intraperitoneal with either N6-cyclohexyladenosine (CHA; 0.5 or 5.0 mg/kg; Sigma-Aldrich, St. Louis, MO, USA) or vehicle (0.01 M phosphate buffer pH 7.0 for 0.5 mg/kg and DMSO, 1% for 5.0 mg/kg). Tb and RR were monitored every 30 min commencing just prior to drug injection and for 4 h after drug injection. Tb and RR were recorded again 24 h after drug injection. DR was continued for 48 h after drug testing. Animals were anesthetized with isoflurane, and brain tissue was rapidly dissected and frozen in liquid nitrogen then stored at −80°C until use.
Cell fractionation for western blot
In order to insure that changes in receptor levels were not influenced by the introduction of CHA, only tissue from DR and AL animals treated with vehicle were used in the western blot experiments. Approximately 30 µg of frontal cortex or whole hypothalamus were homogenized and fractionated as described previously (Raval et al. 2003). Briefly, tissue was suspended in ten times volume of freshly made ice-cold homogenization buffer [4 mM ATP, 100 mM KCl, 10 mM imidazole, 2 mM EGTA, 1 mM MgCl2, 20% glycerol, 17 µg/mL (1 mM) PMSF, 20 µg/mL soybean trypsin inhibitor, 25 µg/mL leupeptin, and 25 µg/mL aprotinin] with 0.05% Triton X-100. The suspended tissue was homogenized in an all-glass homogenizer (ten to 15 strokes) then centrifuged (1,000×g, 4°C) for 10 min. The resulting supernatant (soluble and cytosolic fraction) was removed and centrifuged at 16,000×g and 4°C for 15 min and stored at −80°C until use. The initial pellet (particulate and membrane fraction) was re-suspended in 250 µL of the same lysis buffer containing 1% Triton X-100 and extracted on ice for 60 min. The extracted membrane fraction was then centrifuged (16,000×g, 4°C) for 15 min to remove debris and stored at −80°C until use.
Western blot
Protein concentration was determined using the Bio-Rad protein assay kit (Bio-Rad, Hercules, CA, USA). Twenty-five micrograms of protein was separated on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels, transferred to nitrocellulose membrane, and blocked with 5% milk in Tris-buffered saline (TBS; 10 mM Tris–HCl, pH 7.5, and 150 mM NaCl) for 1 h at room temperature with gentle agitation. Membranes were then incubated overnight with rabbit anti-A1AR (1:1,000, Cat#PC158, Calbiochem, San Diego, CA, USA) in TBST+5% milk overnight at 4°C with gentle agitation. The membrane was washed three times over 30 min in 5% milk-TBST (TBS and 0.1% Tween 20) at room temperature with gentle agitation, then incubated in secondary antibody (Goat anti-rabbit, 1:10,000, Santa Cruz Biotechnology) for 1 h at room temperature. The bands were visualized with enhanced chemiluminescence (Supersignal West Pico Substrate, Pierce, Rockford, IL, USA) then digitally imaged using an AutoImager 3400 (Alpha Innotech Corporation, San Leandro, CA, USA). The membrane was then stripped by incubation with TBS (pH 2.0; 10 mM Tris–HCl and 150 mM NaCl) for 30 min and re-probed with rabbit anti-Na+/K+ATPase β-1 (1:5,000, Cat#06-170 Upstate, Lake Placid, NY, USA) to assess the purity of the membrane fractions. After visualization, the membrane was stripped and re-probed in the same manner with mouse anti-β-actin (1:5,000 product# A5316, Sigma) as a loading control using Goat anti-mouse HRP (1:10,000, Bio-Rad) as the secondary antibody.
Data analysis
Digitized images of chemiluminescent bands were analyzed using ImageQuant 5.2. Total A1AR content was calculated as the summed densities of the cytosolic and membrane fractions and normalized as a percent of the mean of the control (AL) group on each gel. Surface expression of A1AR was assessed from the ratio of density of the particulate A1AR bands to the density of the cytosolic A1AR bands.
Statistics
To assess the effect of diet on Tb and RR, data was analyzed by analysis of variance (ANOVA) with repeated measures over time followed by two-way ANOVAs and Tukey post hoc comparisons (SAS, v. 9.1). Baseline Tb and RR prior to drug treatment are shown and were analyzed as raw data. Tb and RR after CHA or vehicle injection is shown as raw data but was analyzed as percent of pre-injection values to normalize for differences in Tb and RR between DR and AL groups at the time of drug injection. Data are shown as mean±SEM. Densities of immunoreactive bands were expressed as the ratio of cytosolic to membrane fractions normalized to the average of the AL group for each blot. Western blot results for DR and AL groups were compared by t tests (Excel, v. 2007).
Results
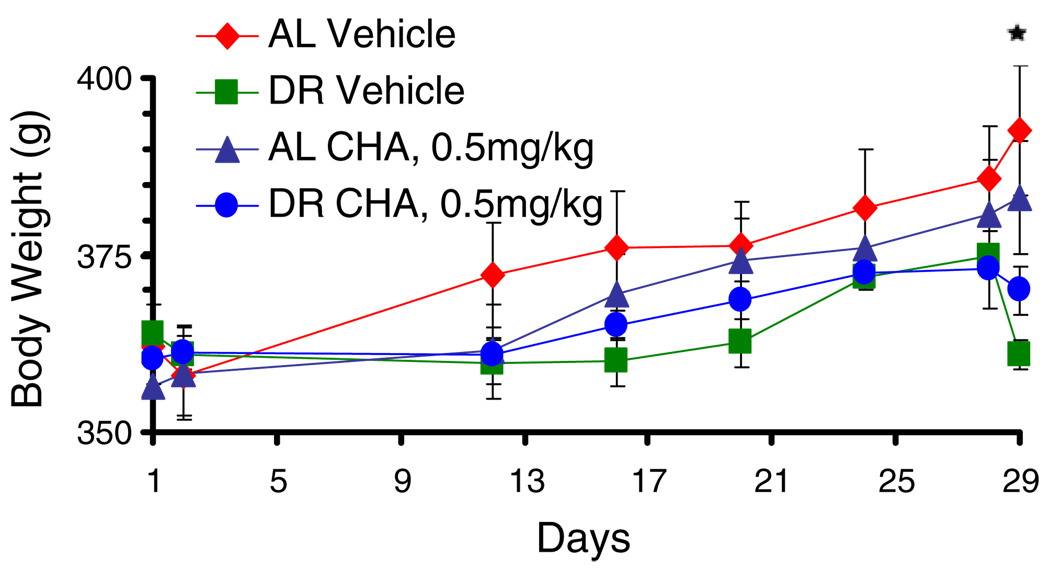
Body weight, monitored every 4 days until the day of injection, increased in both DR and AL-fed rats until the day of injection (p<0.0001, n=20, main effect of time). By the day of injection, however, DR rats weighed significantly less than the AL rats (p<0.0001, diet×time; p<0.05, n=10,10, DR vs. AL on day 29, Tukey test; Fig. 1). No difference was noted in weight gain between groups of DR and AL-fed animals that were subsequently tested with CHA or vehicle.
Fig. 1.
Body weight (grams) increases over days. Asterisk p<0.05, n=10,10, dietary restriction vs ad libitum, Tukey test
Dietary restriction decreases resting body temperature and respiratory rate
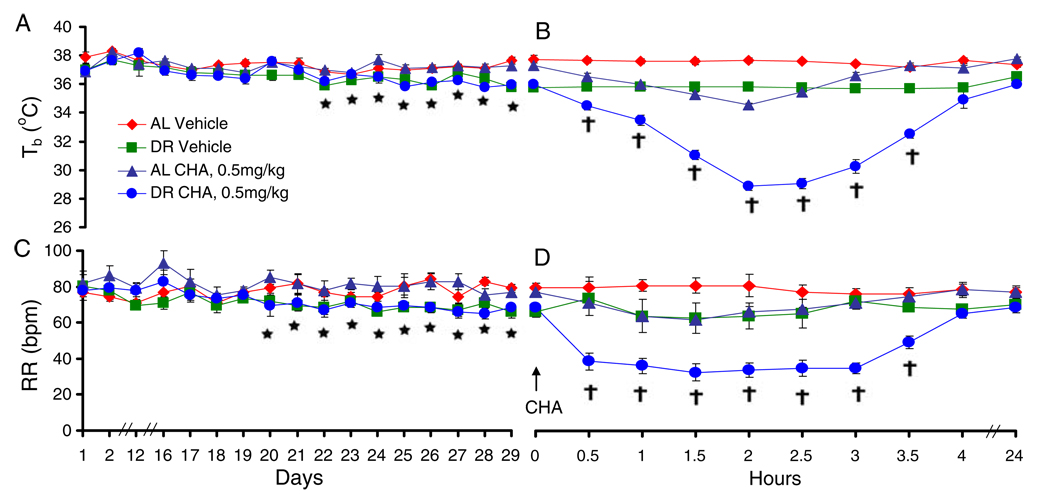
As expected, DR decreased resting Tb and RR. Three-way ANOVAs showed main effects of diet on Tb (p<0.0001) and RR (p<0.001). Interestingly, the diet-induced decrease in resting RR occurred 2 days prior to the diet-induced decrease in resting Tb. RR was consistently different between groups beginning on day 20 (p<0.05 Tukey tests; Fig. 2c). By contrast, resting Tb was consistently different between dietary groups beginning on day 22 through to the day of drug testing (p<0.05 Tukey tests; Fig. 2a).
Fig. 2.
Resting Tb is shown over days (a) and every 30 min for 4 h and at 24 h after N6-cyclohexyladenosine (CHA) administration (b). Similarly, resting respiratory rate is shown over days (c) and every 30 min for 4 and at 24 h after CHA (d). Asterisk p<0.05, n=10,10; dietary restriction (DR) vs ad libitum (AL), Tukey test; dagger p<0.05, n=5,5; CHA DR vs CHA AL, Tukey test
Dietary restriction sensitizes rats to the effects of CHA on body temperature and respiratory rate
The A1AR agonist CHA produced a significant decrease in Tb in both AL and DR groups (Fig. 2b; p<0.0001, n=5,5, diet×drug×time for three-way ANOVA; p<0.0001 drug×time in AL group; and p<0.0001 drug×time in DR group in subsequent two-way ANOVAs). Post hoc analysis showed that in animals fed AL, CHA induced a decrease in Tb that was different from vehicle-treated animals between 0.5 and 2.5 h after drug injection (p<0.05 CHA vs vehicle at 0.5 to 2.5 h, Tukey test). In animals fed a restricted diet, CHA induced a decrease in Tb that was different from vehicle-treated animals between 0.5 and 3.5 h after drug injection (p<0.05 CHA vs vehicle at 0.5 to 3.5 h Tukey test).
The effect of CHA on Tb was greater in DR rats than in AL rats (p<0.0001, n=5,5 diet×time where DR animals treated with CHA were compared to AL animals treated with CHA, and vehicle-treated animals were not included in the two-way ANOVA). Post hoc comparisons showed a difference between AL and DR groups treated with CHA at 0.5 to 3.5 h after drug injection; p<0.05 Tukey tests, Fig. 1b). These results demonstrate that prolonged DR sensitizes rats to the cooling effects of CHA.
In addition to decreasing Tb, CHA also induced a significant decrease in RR in both dietary groups (Fig. 1d; p<0.05, n=5,5, diet×drug×time for three-way ANOVA; p<0.01 drug×time in AL group; and p<0.0001 drug×time in DR group in subsequent two-way ANOVAs). Post hoc analysis showed that in animals fed AL, CHA induced a decrease in RR that was different from vehicle-treated animals between 1.0 and 1.5 h after drug injection (p<0.05 CHA vs vehicle at 1.0 to 1.5 h, Tukey test). Likewise, in animals fed a restricted diet, CHA induced a decrease in RR that was different from vehicle-treated animals between 0.5 and 3.5 h after drug injection (p<0.05 CHA vs vehicle at 0.5 to 3.5 h, Tukey test). These data suggested that the effects of CHA on RR were enhanced in DR animals.
DR-induced decrease in Tb is thought to enhance longevity, in part, by decreasing metabolism. RR declines with metabolic rate in studies of metabolic suppression during onset of torpor (Elvert and Heldmaier 2005) and is thus an indirect indicator of metabolic suppression. We therefore asked if DR sensitized animals to the effects of CHA on RR. The CHA-induced decrease in RR was indeed greater in the DR group than in the AL group out to 3.5 h after drug administration (Fig. 2d). This was evident from a two-way ANOVA comparing the effects of CHA in DR and AL-fed animals that excluded vehicle-treated animals (p<0.0001, n=5,5 diet×time). Post hoc comparisons showed a difference between AL and DR groups treated with CHA between 0.5 and 3.5 h after drug injection (p<0.05 Tukey tests). These results demonstrate that prolonged DR sensitizes rats to the effects of CHA on RR.
Contrary to expectations that metabolic suppression results from a reduction in Tb, we found that a decrease in RR preceded the decline in Tb. This was true for resting RR during 27 days of DR and for CHA-induced decreases in RR. In support of this interpretation, a one-way ANOVA with repeated measures over time showed that in DR animals, RR reached a minimum within 0.5 h after CHA, while Tb continued to decline for 2 h after CHA (p<0.05, Tukey test). Similarly, the small decrease in Tb in the AL fed rats was preceded by a decrease in RR. In the AL group, a one-way ANOVA showed that RR reached a minimum at 1 and 1.5 h after CHA. By contrast, Tb did not reach a minimum until 2 h after CHA.
Effects of the higher 5 mg/kg dose of CHA were compromised by side effects and subsequent death. All ten animals were administered this dose in one test session, and subsequent use of this high dose was abandoned.
Dietary restriction increases surface expression of A1AR in hypothalamus
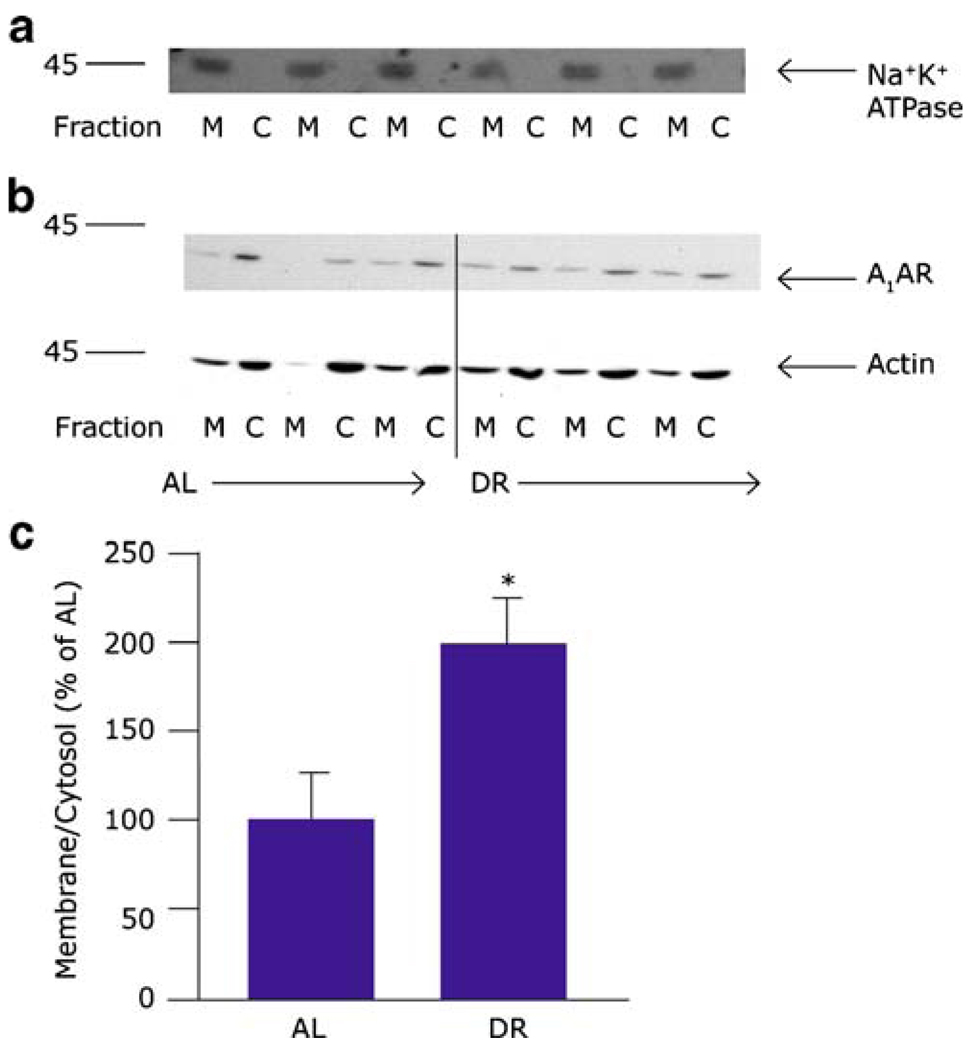
We next asked if surface expression of A1AR increased in DR animals as a potential mechanism of sensitization. The relative concentrations of A1AR were determined in the cytosolic and membrane fractions of vehicle-treated AL or DR rat cortex and hypothalamus by western blot. In cortex, the relative density of total A1AR protein, calculated from the sum of band densities in the cytosolic and membrane fractions, was not different between the DR and AL animals (p>0.05, n=9,10, t test). Similarly, the relative proportion of A1AR in the cytosolic and membrane fractions was not different between the AL and DR groups (p>0.05, n=9,10, t test). When the total expression of A1AR was determined in hypothalamus, again by summing density of bands in the cytosolic and membrane fractions, there was no significant difference between the AL and DR animals (p>0.05, n=8,10, t test). However, the ratio of densities of A1AR in cytosolic and membrane fractions of the AL animals was significantly different than the ratio in DR animals (p<0.05, n=8,10, t test, Fig. 3). This shift in the relative densities of the A1AR from the cytosol to the membrane suggests that A1AR are mobilized from a nonfunctional cytosolic pool to a functional surface pool.
Fig. 3.
Blot (a) demonstrates fraction purity using Na+/K+ ATPase as a marker for the membrane fraction. Blot (b) shows one representative blot of hypothalamus where density of A1AR bands in the cytosolic fractions is decreased and density of A1AR bands in the membrane fractions is increased in rats fed every other day. Graph in (c) shows that normalized ratios of membrane to cytosolic band densities in hypothalamus from rats fed a restricted diet are significantly higher than for rats fed ad libitum (asterisk p<0.05, n=5 per group)
We next asked if the effect of DR on A1AR surface expression or CHA-induced lowering of body temperature depended on a change in food intake or body weight. In the DR group, all five animals tested gained less weight than the AL-fed animals. The body weight gain in the DR group ranged from 7 to 14 g, and the drug-induced decrease in body temperature in this group ranged from 6.6°C to 7.1°C. The body weight gain in the AL group ranged from 15 to 44 g, and the drug-induced decrease in body temperature ranged from 2.4°C to 2.8°C, (n=5 per group). These results show that despite a variable weight gain in the AL-fed animals, weight gain had little influence on drug-induced lowering of body temperature. The small range of body weight gain and drug-induced change in body temperature in the DR animals made it difficult to assess how weight gain influenced other dependent variables.
Discussion
Here, we show for the first time that DR-induced modification of thermoregulation is associated with changes in components of the purinergic neuromodulatory system. DR imposed by EODF sensitizes rats to the cooling and metabolic depressant effects of CHA, an A1AR agonist. We also report that DR is associated with altered surface expression of A1AR in hypothalamus, but not cortex. Taken together, these data suggest that DR sensitizes A1AR through an increase in surface expression in thermoregulatory regions of the hypothalamus and in this way contributes to the decline in Tb and RR in animals subjected to DR.
Here, we used a well-characterized paradigm of DR, described in the literature as EODF or intermittent fasting, to test the hypothesis that a decrease in Tb is associated with sensitization to the cooling effects of an A1AR agonist. The EODF paradigm, like other DR paradigms, decreases Tb (Fraifeld and Kaplanski 1997) typically within 4 weeks of onset (Wan et al. 2004) and increases longevity (Goodrick et al. 1983). Effects of EODF on Tb (Fraifeld and Kaplanski 1997) are similar to the effects observed with several other paradigms of DR that are associated with increased longevity (Goodrick et al. 1983) and decreased metabolism (McCarter and McGee 1989; McCarter and Palmer 1992; Valle et al. 2005).
The amount of food consumed by rodents fed every other day varies with strain. Food intake has been reported to be 30% less than rats fed AL (Wan et al. 2004). However, in C57BL/6 mice, increased food intake on feeding days offsets absence of food intake on fasting days such that overall intake is not decreased (Anson et al. 2003). Individual food intake was not monitored in the present study to avoid the potential effects of stress associated with individual housing. Body weight gain in rats fed every other day in the present study differed only slightly from rats fed AL, suggesting that food intake was decreased but was not dramatically different from AL-fed rats.
When compared with 40% caloric restriction, EODF produces slightly different decreases in heart rate, blood pressure, and blood glucose (Mager et al. 2006). It remains to be determined if all DR paradigms enhance the cooling effects of CHA and if sensitization of A1AR is a common mechanism for altering thermoregulation in all DR paradigms or if a decrease in food intake is necessary for the observed effect. Stress and other aspects of EODF that are not related to decreased food intake may contribute to lowered resting Tb and sensitization to CHA.
A negative energy balance could compromise thermogenic capacity and contribute to lower resting Tb. Others (Fraifeld and Kaplanski 1997) show that lipopolysaccharide induces similar maximal Tb in rats fed every other day suggesting that in their study, thermogenic capacity was not compromised. Compromised thermogenic capacity due to a negative energy balance is unlikely since body weight gain in DR rats differed so slightly from AL-fed animals.
Restricted feeding schedules, which limit food availability to a single meal each day, induce and entrain circadian rhythms in food-anticipatory activities in rodents (Verwey and Amir 2009). Animals in the DR group of rats may have entrained to feeding which occurred at the same time every other day. Anticipation of food on feeding days would have been expected to increase activity and Tb for 2 to 3 h prior to feeding in the DR group (Fuller et al. 2008). Since Tb and RR were measured just before feeding, food entrainment would have increased Tb and RR in the DR group, but not in the AL-fed animals. While a food entrainment-induced increase on Tb and RR in the DR group can not be ruled out, it was not sufficient to override a significant decrease in resting Tb and RR in this group of animals. Similarly, since RR and Tb were monitored during the middle of the light period, activity and resting Tb should have been at a minimum in AL animals. This assumed minimum in RR and Tb in the AL-fed animals was still higher than resting RR and Tb in the DR animals. Thus, while food entrainment in the DR animals and circadian rhythms in the AL animals may have influenced RR and Tb, it is unlikely that the observed differences between these two groups in resting values or CHA-induced decreases in Tb or RR are artifacts of these influences.
In the present study, DR induced a decrease in resting RR, an indicator of oxidative metabolism prior to a decrease in resting Tb. Moreover, the CHA-induced decrease in RR occurred prior to the CHA-induced decrease in Tb. A decrease in respiration that precedes a decrease in Tb mimics what is observed in hibernating animals during entrance into torpor (Drew et al. 2007; Elvert and Heldmaier 2005; Karpovich et al. 2009) and suggests that inhibition of metabolism precedes the decline in Tb rather than vice-versa as is often suggested for DR (Conti 2008).
During onset of torpor, the decline of metabolism, heart rate, and RR occur in parallel and before a decline in Tb (Elvert and Heldmaier 2005). Thus, in studies of metabolic suppression, RR and heart rate serve as indirect indicators of metabolic suppression. In the present study, a decrease in RR is interpreted as a decrease in metabolism. This interpretation is made with the caveat that direct effects of adenosine or CHA on respiratory control centers or stress-induced sympathetic responses could influence RR independent of effects on metabolism. Stress-induced effects on resting RR are unlikely since rats were observed daily and would have been expected to habituate to the observer. It is more difficult, however, to discount direct effects on respiratory control centers.
Adenosine plays a neuromodulatory role in thermoregulation (Barros et al. 2006). Recent evidence supports an emerging role for A1AR signaling in thermoregulatory alterations during onset of torpor in hibernating animals (Tamura et al. 2005) as well as in daily torpor in mice (Swoap et al. 2007). Because CHA-induced cooling in DR rats qualitatively resembled torpor in hibernating animals where metabolic suppression precedes a gradual decline in Tb, we asked if a maximal dose of CHA would induce torpor and drive Tb closer to ambient temperature. The high (5.0 mg/kg) dose of CHA proved to be lethal in AL and DR rats. This was unexpected because previous studies showed that 3 mg/kg was well tolerated in rats (Tuovinen and Tarhanen 2004), and 5 mg/kg produced sedation and cooling in mice (Assi 2001). Further deaths were avoided by immediately abandoning use of this dose as soon as side effects and death were noted in the first group of animals tested.
Because sensitization is defined by an altered dose–response curve, we asked if a maximally effective dose of CHA in AL-fed rats would produce an effect similar to a lower dose of CHA in DR rats. Indeed, we tested a higher dose of CHA expecting to see results consistent with a shift in the dose–response curve; however, the higher dose proved lethal in both dietary groups. Inverted U-shaped dose–response curves can cause decreased responsiveness to appear as sensitization when a single dose is tested. Because drug-induced hypothermic effects can follow inverted dose–response curves (Jaszberenyi et al. 2002), the possibility exists that enhanced responsiveness to CHA is due to a rightward shift in the dose–response relationship and not sensitization. Nonetheless, we interpret the current results as evidence for increased responsiveness to CHA because (1) our hypothesis predicted that DR animals would show an increased response to CHA, (2) a decrease in resting Tb supports increased sensitivity to endogenous adenosine, and (3) increased surface expression of A1AR is consistent with sensitization.
A1AR agonists inhibit lipolysis via direct effects on adipocytes in white and brown adipose tissue (WAT and BAT; Viswanadha and Londos 2006). Inhibition of lipolysis in BAT inhibits nonshivering thermogenesis (Asakura 2004) and, therefore, the oxidative metabolism associated with this energy demanding process. Although we suggest that CHA suppresses RR and Tb via effects on central thermoregulatory pathways, we can not rule out a peripheral site of action. Sensitization of A1AR in BAT would increase endogenous adenosine- or CHA-induced inhibition of nonshivering thermogenesis and oxygen consumption and be expected to produce an immediate decline in RR and a subsequent more gradual decrease in Tb.
If glycogen reserves are depleted in DR animals, direct inhibition of lipolysis in WAT could limit fuel availability and impair thermogenesis. Data reported here argues against this interpretation, however, because RR and Tb in the DR group of animals did not vary between days following 24 h fasts and days following 24 h food availability.
Receptor sensitization suggested by the present results may be a common means to modulate purinergic signaling. Sleep deprivation increases adenosine receptor surface expression, which may contribute to sleep drive in humans (Elmenhorst et al. 2007). Although we did not detect a change in total A1AR expression, studies involving transgenic mice indicate that receptor levels limit response to endogenous adenosine as most effects studied were amplified upon increases in receptor level (Yaar et al. 2005). Results suggest that sensitization may result from a change in the distribution of A1AR from the cytosol to the membrane. A1AR interactions with other membrane receptors, enzymes, adaptor and scaffolding proteins, as well as dimerization of A1AR within the membrane play a role in receptor trafficking, internalization, and desensitization (Franco et al. 2005). Knowledge of these mechanisms opens the possibility for further investigation into mechanisms of A1AR sensitization following DR. Moreover, an effect of DR on extracellular levels of adenosine may also play a role in the observed effect. A change in surface expression does not rule out an influence of presynaptic mechanisms where adenosine kinase plays a primary role in regulating extracellular levels of adenosine (Li et al. 2007).
Adenosine is neuroprotective in a variety of brain injury models via multiple mechanisms including a decrease in Tb (Bischofberger et al. 1997; Xu et al. 2006). The present results suggest that diet may be a means to sensitized A1AR responsiveness and in this way facilitate therapeutic effects of endogenous or exogenously applied adenosine. Ketogenic diets, like adenosine, protect the brain from several types of injuries (Prins 2008) and decrease incidence of seizure in intractable epilepsy potentially via purinergic mechanisms (Etherington et al. 2009; Masino and Geiger 2009). EODF leads to intermittent periods of ketosis (Anson et al. 2003), but unlike EODF, ketogenic diets have not been shown to decrease Tb or increase life span.
In summary, we show that DR sensitizes rats to the respiratory depressant and cooling effects of an A1AR agonist. Sensitization of thermoregulatory effects of endogenous adenosine through increased surface expression of A1AR may play a role in lowered body temperature and enhanced longevity associated with DR. Evidence that diet can modulate purinergic signaling has implications for the treatment of brain injury, stroke, epilepsy, and aging.
Acknowledgements
This research was supported by NS041069-06 (National Institute of Neurological Disorders and Stroke, National Institute of Mental Health), by US Army Research Office (W911NF-05-1-0280), and by a Flint Hills undergraduate research award. The authors would like to acknowledge Dr. Ron Barry for assistance with statistical analysis.
References
- Anson RM, Guo Z, de Cabo R, Iyun T, Rios M, Hagepanos A, Ingram DK, Lane MA, Mattson MP. Intermittent fasting dissociates beneficial effects of dietary restriction on glucose metabolism and neuronal resistance to injury from calorie intake. Proc Natl Acad Sci U S A. 2003;100:6216–6220. doi: 10.1073/pnas.1035720100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakura H. Fetal and neonatal thermoregulation. J Nippon Med Sch. 2004;71:360–370. doi: 10.1272/jnms.71.360. [DOI] [PubMed] [Google Scholar]
- Assi AA. N6-cyclohexyladenosine and 3-(2-carboxypiperazine-4-yl)-1-propenyl-1-phosphonic acid enhance the effect of antiepileptic drugs against induced seizures in mice. J Pharm Pharm Sci. 2001;4:42–51. [PubMed] [Google Scholar]
- Barros RC, Branco LG, Carnio EC. Respiratory and body temperature modulation by adenosine A1 receptors in the anteroventral preoptic region during normoxia and hypoxia. Respir Physiol Neurobiol. 2006;153:115–125. doi: 10.1016/j.resp.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Bischofberger N, Jacobson KA, von Lubitz DK. Adenosine A1 receptor agonists as clinically viable agents for treatment of ischemic brain disorders. Ann N Y Acad Sci. 1997;825:23–29. doi: 10.1111/j.1749-6632.1997.tb48411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contestabile A. Benefits of caloric restriction on brain aging and related pathological states: understanding mechanisms to devise novel therapies. Curr Med Chem. 2009;16:350–361. doi: 10.2174/092986709787002637. [DOI] [PubMed] [Google Scholar]
- Conti B. Considerations on temperature, longevity and aging. Cell Mol Life Sci. 2008;65:1626–1630. doi: 10.1007/s00018-008-7536-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti B, Sanchez-Alavez M, Winsky-Sommerer R, Morale MC, Lucero J, Brownell S, Fabre V, Huitron-Resendiz S, Henriksen S, Zorrilla EP, de Lecea L, Bartfai T. Transgenic mice with a reduced core body temperature have an increased life span. Science. 2006;314:825–828. doi: 10.1126/science.1132191. [DOI] [PubMed] [Google Scholar]
- Drew KL, Buck CL, Barnes BM, Christian SL, Rasley BT, Harris MB. Central nervous system regulation of mammalian hibernation: implications for metabolic suppression and ischemia tolerance. J Neurochem. 2007;102:1713–1726. doi: 10.1111/j.1471-4159.2007.04675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmenhorst D, Meyer PT, Winz OH, Matusch A, Ermert J, Coenen HH, Basheer R, Haas HL, Zilles K, Bauer A. Sleep deprivation increases A1 adenosine receptor binding in the human brain: a positron emission tomography study. J Neurosci. 2007;27:2410–2415. doi: 10.1523/JNEUROSCI.5066-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elvert R, Heldmaier G. Cardiorespiratory and metabolic reactions during entrance into torpor in dormice, Glis glis. J Exp Biol. 2005;208:1373–1383. doi: 10.1242/jeb.01546. [DOI] [PubMed] [Google Scholar]
- Etherington LA, Patterson GE, Meechan L, Boison D, Irving AJ, Dale N, Frenguelli BG. Astrocytic adenosine kinase regulates basal synaptic adenosine levels and seizure activity but not activity-dependent adenosine release in the hippocampus. Neuropharmacology. 2009;56:429–437. doi: 10.1016/j.neuropharm.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraifeld V, Kaplanski J. Dietary restriction modifies fever response in aging rats. Arch Gerontol Geriatr. 1997;24:133–140. doi: 10.1016/s0167-4943(96)00746-7. [DOI] [PubMed] [Google Scholar]
- Franco R, Ciruela F, Casado V, Cortes A, Canela EI, Mallol J, Agnati LF, Ferre S, Fuxe K, Lluis C. Partners for adenosine A1 receptors. J Mol Neurosci. 2005;26:221–232. doi: 10.1385/JMN:26:2-3:221. [DOI] [PubMed] [Google Scholar]
- Fuller PM, Lu J, Saper CB. Differential rescue of light- and food-entrainable circadian rhythms. Science. 2008;320:1074–1077. doi: 10.1126/science.1153277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrick CL, Ingram DK, Reynolds MA, Freeman JR, Cider NL. Effects of intermittent feeding upon growth, activity, and lifespan in rats allowed voluntary exercise. Exp Aging Res. 1983;9:203–209. doi: 10.1080/03610738308258453. [DOI] [PubMed] [Google Scholar]
- Jaszberenyi M, Bujdoso E, Kiss E, Pataki I, Telegdy G. The role of NPY in the mediation of orexin-induced hypothermia. Regul Pept. 2002;104:55–59. doi: 10.1016/s0167-0115(01)00339-1. [DOI] [PubMed] [Google Scholar]
- Karpovich SA, Toien O, Buck CL, Barnes BM. Energetics of arousal episodes in hibernating arctic ground squirrels. J Comp Physiol B. 2009;179:691–700. doi: 10.1007/s00360-009-0350-8. [DOI] [PubMed] [Google Scholar]
- Li T, Quan Lan J, Fredholm BB, Simon RP, Boison D. Adenosine dysfunction in astrogliosis: cause for seizure generation? Neuron Glia Biol. 2007;3:353–366. doi: 10.1017/S1740925X0800015X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mager DE, Wan R, Brown M, Cheng A, Wareski P, Abernethy DR, Mattson MP. Caloric restriction and intermittent fasting alter spectral measures of heart rate and blood pressure variability in rats. Faseb J. 2006;20:631–637. doi: 10.1096/fj.05-5263com. [DOI] [PubMed] [Google Scholar]
- Masino SA, Geiger JD. The ketogenic diet and epilepsy: is adenosine the missing link? Epilepsia. 2009;50:332–333. doi: 10.1111/j.1528-1167.2008.01771.x. [DOI] [PubMed] [Google Scholar]
- McCarter RJ, McGee JR. Transient reduction of metabolic rate by food restriction. Am J Physiol. 1989;257:E175–E179. doi: 10.1152/ajpendo.1989.257.2.E175. [DOI] [PubMed] [Google Scholar]
- McCarter RJ, Palmer J. Energy metabolism and aging: a lifelong study of Fischer 344 rats. Am J Physiol. 1992;263:E448–E452. doi: 10.1152/ajpendo.1992.263.3.E448. [DOI] [PubMed] [Google Scholar]
- Prins ML. Cerebral metabolic adaptation and ketone metabolism after brain injury. J Cereb Blood Flow Metab. 2008;28:1–16. doi: 10.1038/sj.jcbfm.9600543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raval AP, Dave KR, Mochly-Rosen D, Sick TJ, Perez-Pinzon MA. Epsilon PKC is required for the induction of tolerance by ischemic and NMDA-mediated preconditioning in the organotypic hippocampal slice. J Neurosci. 2003;23:384–391. doi: 10.1523/JNEUROSCI.23-02-00384.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shintani M, Tamura Y, Monden M, Shiomi H. Characterization of N(6)-cyclohexy ladenosine-induced hypothermia in Syrian hamsters. J Pharmacol Sci. 2005;97:451–454. doi: 10.1254/jphs.sc0040178. [DOI] [PubMed] [Google Scholar]
- Steiner AA, Rocha MJ, Branco LG. A neurochemical mechanism for hypoxia-induced anapyrexia. Am J Physiol Regul Integr Comp Physiol. 2002;283:R1412–R1422. doi: 10.1152/ajpregu.00328.2002. [DOI] [PubMed] [Google Scholar]
- Swoap SJ, Rathvon M, Gutilla M. AMP does not induce torpor. Am J Physiol Regul Integr Comp Physiol. 2007;293:R468–R473. doi: 10.1152/ajpregu.00888.2006. [DOI] [PubMed] [Google Scholar]
- Tabarean I, Morrison B, Marcondes MC, Bartfai T, Conti B. Hypothalamic and dietary control of temperature-mediated longevity. Ageing Res Rev. 2009;9:41–50. doi: 10.1016/j.arr.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura Y, Shintani M, Nakamura A, Monden M, Shiomi H. Phase-specific central regulatory systems of hibernation in Syrian hamsters. Brain Res. 2005;1045:88–96. doi: 10.1016/j.brainres.2005.03.029. [DOI] [PubMed] [Google Scholar]
- Tuovinen K, Tarhanen J. Clearance of cyclopentyladenosine and cyclohexyladenosine in rats following a single subcutaneous dose. Pharmacol Res. 2004;50:329–334. doi: 10.1016/j.phrs.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Parrado-Fernandez C, Csiszar A, de Cabo R. Mechanisms underlying caloric restriction and lifespan regulation: implications for vascular aging. Circ Res. 2008;102:519–528. doi: 10.1161/CIRCRESAHA.107.168369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valle A, Catala-Niell A, Colom B, Garcia-Palmer FJ, Oliver J, Roca P. Sex-related differences in energy balance in response to caloric restriction. Am J Physiol Endocrinol Metab. 2005;289:E15–E22. doi: 10.1152/ajpendo.00553.2004. [DOI] [PubMed] [Google Scholar]
- Verwey M, Amir S. Food-entrainable circadian oscillators in the brain. Eur J Neurosci. 2009;30:1650–1657. doi: 10.1111/j.1460-9568.2009.06960.x. [DOI] [PubMed] [Google Scholar]
- Viswanadha S, Londos C. Optimized conditions for measuring lipolysis in murine primary adipocytes. J Lipid Res. 2006;47:1859–1864. doi: 10.1194/jlr.D600005-JLR200. [DOI] [PubMed] [Google Scholar]
- Wan R, Camandola S, Mattson MP. Dietary supplementation with 2-deoxy-D-glucose improves cardiovascular and neuro-endocrine stress adaptation in rats. Am J Physiol Heart Circ Physiol. 2004;287:H1186–H1193. doi: 10.1152/ajpheart.00932.2003. [DOI] [PubMed] [Google Scholar]
- Xu K, Puchowicz MA, Lust WD, LaManna JC. Adenosine treatment delays postischemic hippocampal CA1 loss after cardiac arrest and resuscitation in rats. Brain Res. 2006;1071:208–217. doi: 10.1016/j.brainres.2005.11.060. [DOI] [PubMed] [Google Scholar]
- Yaar R, Jones MR, Chen JF, Ravid K. Animal models for the study of adenosine receptor function. J Cell Physiol. 2005;202:9–20. doi: 10.1002/jcp.20138. [DOI] [PubMed] [Google Scholar]