Abstract
The MerTK receptor tyrosine kinase is an important negative regulator of dendritic cell function and is required to prevent B cell autoimmunity in vivo. It is not currently known however, if any causal relationship exists between these two aspects of MerTK function. We sought to determine if dendritic cells from mice lacking MerTK (mertk−/− mice) have characteristics that may aid in the development of B cell autoimmunity. Specifically, we found that mertk−/− mice contain an elevated number of splenic dendritic cells, and this population contains an elevated proportion of cells secreting the critical B cell pro-survival factor, B cell activating factor (BAFF). Elevated numbers of BAFF-secreting cells were also detected among mertk−/− bone marrow-derived dendritic cell (BMDC) populations. This was observed in both resting BMDC, and BMDC stimulated with lipopolysaccharide (LPS) or treated with exogenous apoptotic cells. We also found that dendritic cells in general have a pro-survival effect on resting B cells in co-culture. However, despite containing more BAFF-secreting cells, mertk−/− BMDC were not superior to C57BL/6 or baff-deficient BMDC at promoting B cell survival. Furthermore, using decoy receptors, we show that dendritic cells may promote B cell survival and autoimmunity through a BAFF-and APRIL-independent mechanism.
Keywords: MerTK, BAFF, Dendritic Cell, Autoantibody, B cell Survival
INTRODUCTION
Systemic Lupus Erythematosus (SLE) is an autoimmune disease in which a breakdown of tolerance mechanisms permits self-reactive B cell clones to produce autoantibodies against a variety of self antigens normally found in the nuclei of intact cells [1]. Numerous single-gene perturbation mouse models have demonstrated that a wide variety of regulatory mechanisms exist in both non-B cells and B cells that are necessary to maintain B cell tolerance to nuclear antigens [2, 3]. One such regulatory mechanism involves the members of the TAM (Tyro3, Axl, MerTK) family of receptor tyrosine kinases. Mice lacking all three of these receptors (TAM mice) develop splenomegaly, produce autoantibodies, and exhibit spontaneous lymphocyte activation, demonstrating that the TAM family is involved in immune homeostasis and tolerance [4]. Immune dysfunction is also found in mice solely deficient in the mertk gene (mertk−/− or mertkkd mice). These animals have increased cytokine production [4, 5], and develop autoantibodies to several nuclear antigens targeted in SLE including: dsDNA, ssDNA, chromatin, and Sm [4, 6–8]. Autoimmunity in mertk−/− mice is likely to be primarily driven by B cell extrinsic mechanisms since MerTK is expressed in macrophage, dendritic cells (DC), natural killer (NK) cells, NK-T cells, and non-hematopoietic cell types, but is notably absent from non-malignant B and T cells. [9–12]. This makes mertk−/− mice a valuable model for examining how B-cell extrinsic mechanisms can facilitate self-reactive B cells to bypass tolerance safeguards.
The TAM family members play a role in the recognition and phagocytosis of apoptotic cells [8, 13]. Our lab has demonstrated that these receptor TKs are used in different combinations during the phagocytosis of apoptotic cells depending on the tissue and cell type involved [13]. Unlike macrophage and retinal pigmented epithelial cells, DC do not require MerTK for the phagacytosis of apoptotic cells [9, 12, 13]. Instead, MerTK is used by DC to transduce inhibitory signals in response to encounters with apoptotic cells. Apoptotic cells render DC refractory to LPS-induced maturation and inefficient at antigen presentation to T cells [12, 14–16]. Mechanistically, this inhibition proceeds through PI3Kδ and culminates in impaired activation of the transcription factor NF-κB [12]. Mertk−/− DC lack this inhibitory response [12].
Dysregulated DC may contribute to the B cell autoreactivity observed in mertk−/− mice. One mechanism by which DC have the potential to modify B cell behavior is through their production of the cytokines BAFF (also known as BLyS, TALL-1, and CD257) and APRIL (A PRoliferation-Inducing Ligand). BAFF is a type II transmembrane protein that contains a C-terminal cytokine domain which is released upon proteolytic clevage at an extracellular site [17, 18]. Functionally, BAFF and APRIL play a critical role during the middle and later stages of B cell life history, ranging from the late transitional stages to plasma cells. Perhaps most important is BAFF’s ability to regulate the size and repertoire of the mature B cell pool by delivering a necessary pro-survival signal to resting B cells [19–21]. Baff-deficient mice have severely reduced numbers of mature splenic B cells [22, 23], whereas mice carrying a baff transgene display elevated B cell numbers and signs of autoimmunity [24–26]. Furthermore BCR-transgenic mouse models have been used to demonstrate that limiting BAFF availability is a mechanism by which the immune system excludes autoreactive B cell clones from the mature B cell repertoire [27, 28]. In vitro, recombinant BAFF and APRIL promote B cell survival by activating several pro-survival signaling pathways and down-regulating pro-apoptotic pathways [29–33].
In contrast to the numerous studies that have focused on BAFF’s effect on B cell physiology, there is a relative paucity of evidence concerning BAFF production by DC and other cell types. A small number of studies have shown that DC-derived BAFF can enhance B cell proliferation [34], plasmablast differentiation [35], Ig class switching [36], and Ig secretion [36, 37]. However, direct evidence that DC promote B cell survival, either in vitro or in vivo, via a BAFF-dependent mechanism is lacking. Furthermore, bone marrow chimera studies involving baff-null mice have demonstrated that systemic BAFF levels and the maintenance of a normal B cell compartment require BAFF derived from radiation-resistant (stromal), but not radiation-sensitive (bone marrow derived), cell populations [38]. Thus the influence that DC-derived BAFF has on B cell survival in vitro and in vivo is unclear. Also ambiguous is what effect, if any, the over-production of BAFF by dysregulated DC may have on the survival of nearby B cells. Intriguingly, splenic DC from TAM mice were recently shown to express elevated levels of baff mRNA [39]. However, it is not known whether or not DC lacking individual members of the TAM family has a similar BAFF expression profile. More importantly, the consequence of BAFF over-expression by TAM DC in terms of B cell physiology was not investigated.
Given the following observations: 1) mertk−/− mice make autoantibodies, 2) excess BAFF promotes B cell autoimmunity, 3) DC normally express BAFF, and 4) MerTK functions as a negative regulator of DC activation, we set out to examine the possibility that MerTK regulates BAFF production by DCs and therefore influences interactions between DC-B cells. In agreement with TAM DC [39] we found that DC lacking only MerTK have an enhanced capacity to secrete BAFF, both at rest and in response to LPS or apoptotic cells. A novel B cell survival assay was then designed to study the biological significance of DC-derived BAFF. Unexpectedly, excess BAFF production by mertk−/− DC did not translate into an enhanced ability to augment B cell survival in vitro. In fact, utilizing baff-deficient animals and decoy receptors, we found that DC support of B cell survival occurs through a BAFF- and April-independent mechanism. These results indicate that although MerTK regulates DC BAFF production, this may not be a critical contributing factor in its role in preventing B cell autoimmunity in vivo.
MATERIALS AND METHODS
Mice
C57BL/6 mice were purchased from The Jackson Laboratory and then bred in-house. Mertk−/− mice (known previously as merkd or mertkkd are essentially null mice that do not express the protein)have been described previously [5, 13]. The animals used in these experiments were backcrossed to C57BL/6 for 6 generations. Mice bearing a homozygous deletion of the baff gene (baff−/− mice) (also backcrossed to C57BL/6, n=8) were produced by Dr. Martin Scott and provided by Dr. Susan Kalled (Biogen Idec) [23]. All mice were kept under pathogen-free conditions in our UNC Division of Laboratory Animal Medicine facilities and in accordance with the Institutional Animal Care and Use Committee approved protocols.
Autoantibody ELISA assays
For the anti-dsDNA ELISA, calf thymus dsDNA (Promega) was pre-treated with S1 nuclease (Promega) to remove contaminating ssDNA. Maxisorp plates (NUNC) were then filled with 10 μg of S1-treated dsDNA in 100 μl water, which was evaporated overnight in a 37° oven. Plates were blocked for 2 hours at room temperature with 4% fetal bovine serum. After washing, sample sera (diluted 1:100) and standards were added in triplicate and incubated for 2 hours at room temperature. After washing, anti-mouse IgGHRP (R&D Systems) was added and incubated for 1 hour at room temperature. ABTS (Sigma) was used as a substrate and absorbance at 405 nm was measured on a microplate reader (Bio-Tek Instruments). The standard curve consisted of sera pooled from multiple 6 month old MRL/lpr mice. A 1:100 dilution of this serum was arbitrarily designated as 100 Units/ml, 2-fold serial dilutions were then made down to 1:6400 or 1.56 Units/ml. This was the limit of detection for the assay. A semi-logarithmic plot was used to derive the standard curve equation (y=mLn(x)+b). The anti-nucleosome assay was performed as described previously [40].
Ex vivo splenic dendritic cells
DC were enriched from spleens using anti-CD11c microbeads (Miltenyi Biotech) according to the manufacturer’s instructions. Recovered cells were typically 75–95% CD11c+, as assessed by flow cytometry.
Bone marrow-derived dendritic cell (BMDC) culture
To generate DC in vitro, femurs were collected from 2–3 month old mice [41]. Following a brief ethanol wash and PBS rinse, femurs were flushed with RPMI 1640 (Gibco) to extract marrow which was then dissociated with gently pipetting. After osmotic red blood cell lysis, remaining cells were washed in PBS, suspended in media and counted. 2×106 bone marrow cells were plated in 4 ml of media in 6-well ultra low cluster plates (Corning). BMDC media consisted of RPMI 1640 containing 10% FBS (Atlanta Biologicals), 50 U/ml penicillin, 50 μg/ml streptomycin, 1 mM sodium pyruvate, 0.1 mM non-essential amino acids, 0.05 mM β-Me (all from Gibco), 10 ng/ml GM-CSF and 10 ng/ml IL-4 (both from Peprotech). Cells were maintained at 37°C with 5% CO2. On day 1 of culture non-adherent cells were removed, diluted approximately 2-fold with fresh media containing 20 ng/ml GM-CSF and 20 ng/ml IL-4, and plated in 2 new 6-well ultra low cluster plates. Cultures were fed on day 4 by adding 2 ml of fresh media containing 30 ng/ml GM-CSF and 30 ng/ml IL-4. On day 7 cultures were fed again by removing 2 ml of old media and replacing with 2 ml of fresh media containing 30 ng/ml GM-CSF and 30 ng/ml IL-4. BMDC culture was harvested for experiments on day 8 at which time CD11c+ cells comprised 85–95% of the culture.
Apoptotic cells
Apoptotic cells were prepared by dissociating mouse thymi in RPMI 1640 and then irradiating (600 Rads) in a calibrated 137Cs gamma irradiator. Irradiated thymocytes were incubated at 37°C for 12 hours before using in experiments. Apoptosis was confirmed by Annexin V and propidium iodide staining by flow cytometry.
Flow cytometry
Before staining cells were treated with Fc-block (anti-CD16/CD32) from either BD Biosciences or Caltag. Cells were then stained with monoclonal antibodies to the following surface markers where applicable: I-AFITC, CD3FITC, CD11cPE, CD11cPE-Cy7, CD19PE-Cy5, CD80FITC, CD86FITc. Polyclonal anti-MerTK, and anti-goat IgGPE (both from R&D systems) were also used. All antibodies were diluted in 1% fetal bovine serum (Atlanta Biologicals). All washes were done with phosphate buffered saline (Gibco).
BAFF real time RT-PCR
Total RNA was isolated from spleen tissue, ex vivo splenic DC, or cultured BMDC with TRIZOL reagent (Invitrogen). Further purification, as well as DNase digestion, was carried out with RNeasy columns (Qiagen). Total RNA was quanitated and then converted to cDNA using Superscript II reverse transcriptase with random hexamer primers (Invitrogen). cDNA was used as template in each real-time PCR reaction, which also included 2× Master Mix (Applied Biosystems) and the following primer/probe sets: baff fwd-primer (5′CCC AAAACACTGCCCA ACA3′), baff rev-primer (5′CTCATCTCCTTCTTCCAG CCTC3′), baff TaqMan probe (5′TTCCTGCTACTCGGCTGGCATCG3′) or endogenous control HPRT fwd-primer (5′GCA AACTTTGCTTTCCCTGG3′), HPRT rev-primer (5′TTCGAGA GGTCCTTTTCA CCA3′), HPRT TaqMan probe (5′AAGCTTGCAACCTTAACC ATTTTGGGGCT3′) (all from Applied Biosystems). 96-well reaction plates were run on either the ABI 7700 or ABI 7500 Real Time PCR machine in the Expression Profiling and SNP Genotyping Core Facility of the UNC Neuroscience Center. Relative quanitation of baff expression was made using the comparative 2−ΔΔCt method, with hprt serving as the endogenous control. This method was validated for equal efficiency of baff and hprt amplification by a cDNA titration experiment. For the time course measurements of baff expression in the spleen a total of 5 reactions plates were run. A pool of spleen cDNA from 1-month old B6 mice was made and served as the calibrator sample on each reaction plate. However, each data point in the spleen time course represents 5 mice whose baff expression was measured independently (unpooled).
BAFF ELISA and ELISPOT assay
The matched pair of monoclonal anti-murine BAFF antibodies 5A8 (capture) and biotinylated 1C9 (detection) from Apotech were used for both the ELISA and ELISPOT assays. ELISA measuring serum BAFF was carried out according to manufacturer’s instructions in Maxisorp plates (Nunc). Recombinant murine BAFF (Apotech) was used to generate a standard curve ranging from 0.75 to 48 ng/ml. Mouse sera were diluted 1:2 and pre-cleared with Protein G (Amersham) before analysis. For BAFF ELISPOT sterile 96-well Multiscreen plates (Millipore) were coated at 4°C overnight with 5 μg/ml 5A8 antibody, then washed with PBS and blocked at room temperature for 2 hours with 1% BSA (Sigma). BMDC were plated at 1×105/well in BMDC media with or without the indicated concentration of LPS (O11:B4, UltraPure, Invivogen), or with apoptotic cells. In some experiments apoptotic cells were also pre-incubated with 100nM recombinant murine Gas6 (R&D Systems) and washed three times in PBS before adding to BMDC. After 3 days plates were washed with PBS, followed by PBS-tween, then 2 μg/ml biotinylated 1C9 antibody was added and plates incubated overnight at 4°C. After washing with PBS-tween strepavidin-HRP (BD Biosciences) was added and plates incubated overnight at 4°C. Plates were developed with 3-amino-9-ethyl carbazole and counted on an ELISPOT plate reader (CTL).
B cell survival assays
Resting B cells were isolated from mouse spleens by negative selection using a B cell Isolation kit (Miltenyi Biotec). B cell purity was assessed by flow cytometry to determine CD19 expression and was routinely >95%. B cell survival was assessed on B cell only cultures or B cell:BMDC cocultures. In some assays B cell survival was enhanced by recombinant BAFF (Peprotech). This effect could be blocked using soluble BAFFR-Fc or TACI-Fc decoy receptors, but not a control-Fc reagent (kindly provided by ZymoGenetics).
The B cell:BMDC co-culture was carried out in 24-well Ultra Low Cluster plates (Corning) in the RPMI-based media described for culturing BMDC. Co-cultures consisted of 5×105 total cells comprised of varying numbers of B cells and BMDC depending the ratio. For example, a B cell:BMDC ratio of 4:1 consisted of 4×105 B cells and 1×105 BMDC, while a 16:1 ratio consisted of 4.71×105 B cells and .29×105 BMDC. In some experiments, BMDC were stimulated for 20 hours prior to adding to co-culture. For LPS and IFNγ stimulations, upon harvest, BMDC were prepared for coculture by washing 3 times in PBS (Invitrogen). Alternatively, when BMDC were treated with fresh apoptotic cells, Lympholyte-M (Cedarlane) was used to separate apoptotic cells from BMDC prior to using in coculture. In some experiments TACI-Fc, a BAFF/APRIL decoy receptor, or a control-Fc reagent were added to coculutre wells at a final concentration of 10 μg/ml. Cocultures were incubated at 37° in 5% CO2 for 3 days. B cell viability was measured as either the percent of VAD-FMK− B cells or the absolute number of VAD-FMK− B cells. To determine the absolute number of viable B cells remaining, first the B cells in each coculture well (distinguished from BMDC by size and morphology), were counted on a hemocytometer. The percent of viable B cells was then assessed by staining harvested cells with VAD-FMKFITC (Promega) and CD19PE-Cy5, followed by analysis on a Cyan flow cytometer (Dako Cytomation). The absolute number of viable B cells was determined by the following formula:
Statistics
Statistical tests were conducted using JMPIN software (SAS Institute). The non-parametric Wilcoxon Rank-Sum and Kruskal-Wallis tests were used. A p-value of <0.05 was considered significant.
RESULTS
Elevated anti-nucleosome and anti-dsDNA autoantibody
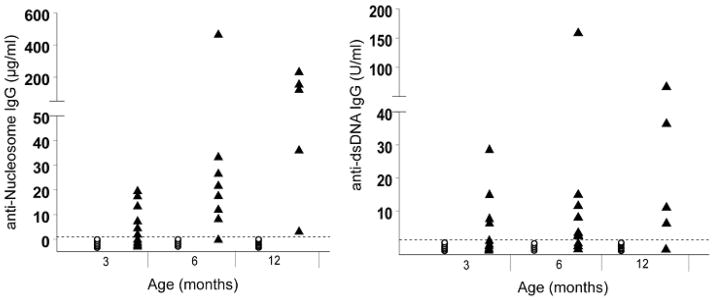
As mertk−/− mice age they develop progressively elevated titers of autoantibodies to ssDNA and chromatin [6]. The presence of anti-dsDNA antibodies has also been documented in mertk−/− mice. However these studies were done at a single time point with a qualitative assay [8]. To further characterize the pattern of anti-dsDNA, we examined serum from C57BL/6 and mertk−/− mice at multiple time points using a semi-quantitative assay. We also examined the pattern of anti-nucleosome antibodies in this same group of animals. Similar to published findings of other autoantibodies, anti-dsDNA and anti-nucleosome IgG levels were elevated between 3–6 months of age and steadily increased thereafter (Figure 1).
Figure 1. Anti-nucleosome and Anti-dsDNA Antibodies Increase in mertk−/− Mice with Age.
Levels of anti-nucleosome (A) and anti-dsDNA (B) antibodies were measured by ELISA in a cohort of C57BL/6 (○) and mertk−/− (▲) mice. The dotted line represents the limit of detection in each assay. These limits are 15 ng/ml and 1.56 Units/ml for the anti-nucleosome and anti-dsDNA assays, respectively. Statistical significance was determined by Wilcoxon Rank Sum test at each time point (* p<0.05, ** p<0.001, *** p<0.005).
Elevated numbers of splenic dendritic cells in mertk−/− mice
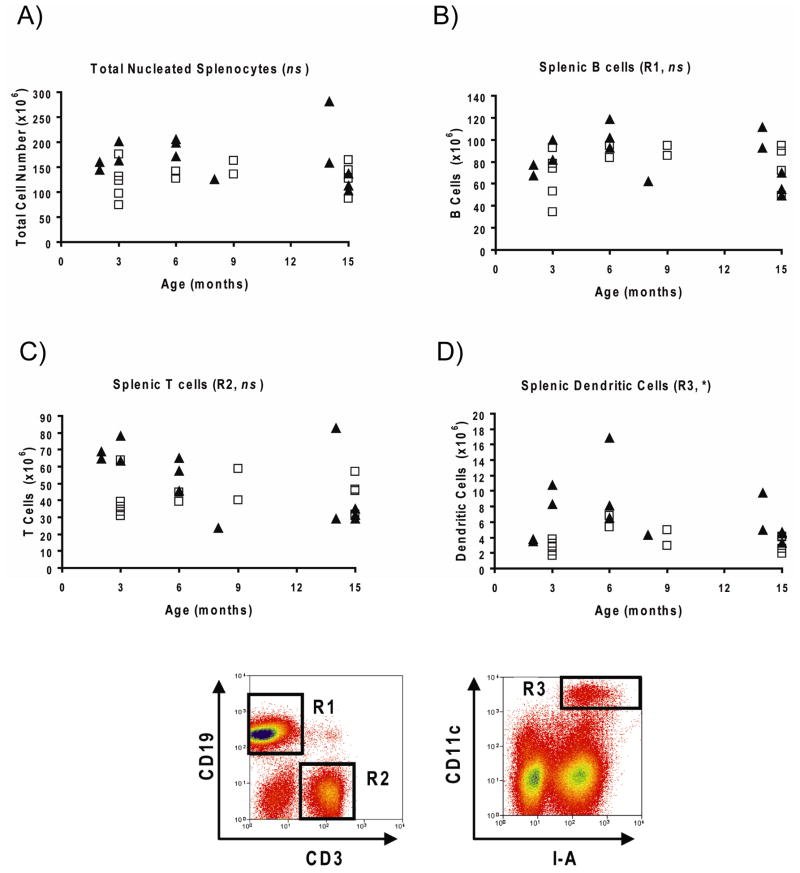
Autoimmunity in mouse models is frequently associated with enlarged lymphocyte compartments. Therefore, we next examined whether there were any perturbations in the composition of splenocyte populations in mertk−/− mice. Surprisingly, the total number of nucleated splenocytes, as well as the number of B and T cells, were not significantly elevated in mertk−/− mice at any age tested (Figure 2, A–C). In contrast, CD11c+ I-A+ splenic DC (spDC) were significantly elevated in mertk−/− mice compared to C57BL/6 counterparts (Figure 2D). Similar trends were seen when comparing the percentages of these cell types in the spleens of C57BL/6 and mertk−/− mice: B and T cells were not different, while spDC were elevated in mertk−/− mice (data not shown).
Figure 2. Spleens of mertk−/− Mice Contain Elevated Numbers of Dendritic Cells.
Shown are the numbers of total nucleated splenocytes (A), splenic B cells (B), splenic T cells (C), and splenic dendritic cells (D) from C57BL/6 (□) and mertk−/− (▲) mice of various ages. Sample flow cytometry plots showing the gating scheme used to measure the various cell types are also shown (R1=B cells, R2=T cells, R3=Dendritic Cells). Total numbers of a specific population for a given mouse were calculated by multiplying the total number of nucleated splenocytes by the percentage of those cells falling within the assigned gate. Statistical significance of differences between cell populations from C57BL/6 and mertk−/− mice was determined by the Wilcoxon Rank Sum test, the results of which are shown to the right of each graph title (ns, not significant, * p<0.05).
BAFF is elevated in spleens but not serum of mertk mice
Mertk−/− mice have a defect in the clearance of apoptotic cells [6, 8]. Given that the antigens targeted by autoantibodies in mertk−/− mice are known to be on the surface of apoptotic cells, and thus exposed to the immune system [42–45], the simplest explanation for autoimmunity in these mice would be that autoantibodies result from an overabundance of self antigen due to impaired clearance of apoptotic cells. However, Cohen et al. found that injection of apoptotic cells into young mertk−/ − mice failed to accelerate the appearance of autoantibodies [6]. This result argues against the premise that elevated antigen load alone is sufficient to drive autoimmunity. This led us to question what other age-related changes take place in vivo that might correlate with the timing of autoantibodies in mertk−/− mice. We first turned our attention towards the cytokine BAFF. When overexpressed in vivo, BAFF is known to promote autoantibody production and autoimmune disease. Furthermore, our lab has shown dysregulated expression of another member of the TNF superfamily, TNF-α, in mertk−/− mice and macrophages [5].
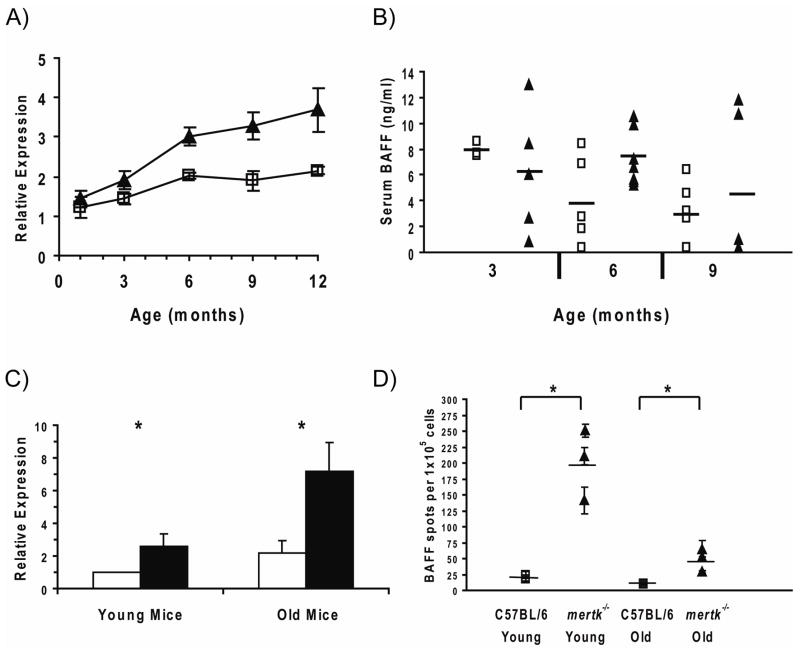
Initially we set out to determine if age-related changes in baff mRNA or protein levels could be detected in mertk−/− mice. An analysis of baff mRNA expression in spleen tissue from mice of varying ages revealed a 2–3 fold increase in spleens from mertk−/− compared to C57BL/6 mice beginning at 6 months of age (Figure 3A). However, serum levels of BAFF protein were not significantly elevated in mertk−/− mice at any age tested (Figure 3B). When considered in light of the aforementioned bone marrow chimera studies [38], this data suggests that stromal cell populations likely produce normal levels of BAFF in mertk−/− mice. However it does not preclude the possibility that a bone marrow-derived cell type (which does not measurably contribute to serum BAFF [38]) may be overexpressing BAFF enough to cause elevated mRNA levels in the spleens of mertk−/− mice.
Figure 3. Splenic DC from mertk−/− Mice Produce Excess BAFF.
A) Real time PCR analysis of baff mRNA expression in the spleen. Each data point represents the mean expression (± sem) in the spleen of five C57BL/6 (□) or mertk−/− (▲) mice from the indicated age group. B) ELISA analysis of serum BAFF levels in C57BL/6 (□) or mertk−/− (▲) mice (n = 3–7 mice per group. Horizontal lines depict the mean of each group. C) Real time PCR analysis of baff mRNA expression in splenic DC (spDC) isolated from C57BL/6 (open bars) or mertk−/− (filled bars) mice. Each bar represents the mean value (± sem) of pooled data from spDC isolated from 4–5 different mice. D) ELISPOT analysis of ex vivo BAFF secretion by spDC isolated from young and old C57BL/6 (□) and mertk−/− (▲) mice. Each data point represents the number of BAFF spots counted (mean ± sem) from four wells containing spDC from a single donor mouse. Horizontal lines represent the mean number of BAFF spots for the 3 donor mice in each group. In both (C) and (D) “Young” mice were 2–3 months of age, while “Old” mice were 10–14 months of age. Statistical significance was determined using the Wilcoxon Rank Sum test ( p<0.05).
p<0.05).
BAFF expression in splenic DC from mertk−/− mice
The elevated numbers of DC and elevated baff mRNA transcript levels in the spleens from mertk−/− mice led us to investigate if mertk−/− spDC produce excess BAFF. In agreement with recent findings from TAM spDC [39], we found elevated baff mRNA levels in preparations of spDC taken from mertk−/− mice compared to those from C57BL/6 mice (Figure 3C). The elevated baff mRNA was predominantly found in spDC from older mertk−/− mice. Furthermore, when ex vivo spDC were examined for BAFF production by ELISPOT, we found that a higher frequency of spDC from mertk−/− mice produced BAFF in culture compared to spDC from C57BL/6 mice (Figure 3D). Unlike baff mRNA expression, elevated BAFF protein production was most pronounced in the spDC from younger mertk−/− mice. However, the trend of increased numbers of spDC producing BAFF also held for the older mertk−/− mice when compared to older C57BL/6 counterparts. Importantly, this was not due to a general increase in the activation state of these spDC, as surface expression of I-A was similar on spDC taken from both C57BL/6 and mertk−/− mice (data not shown). These results demonstrate that, in addition to a larger overall spDC pool in mertk−/− mice, this population contains a higher frequency of BAFF-producing cells.
If spDC production of BAFF is a contributing factor to B cell autoimmunity in mertk−/− mice this effect likely occurs locally, rather than globally, since we did not find that systemic BAFF levels, or B cell numbers, were elevated in mertk−/− mice. To address this further, we compared the activation state of splenic B cells taken from C57BL/6 and mertk−/− mice, as well as their expression of BAFFR. Splenic B cells displayed equivalent levels of CD80, CD86, MHC class II, and BAFFR in C57BL/6 and mertk−/− mice (data not shown). This indicates that elevated BAFF production by spDC does not cause a global change in B cell physiology in mertk−/− mice.
Elevated BAFF expression in bone marrow-derived DC
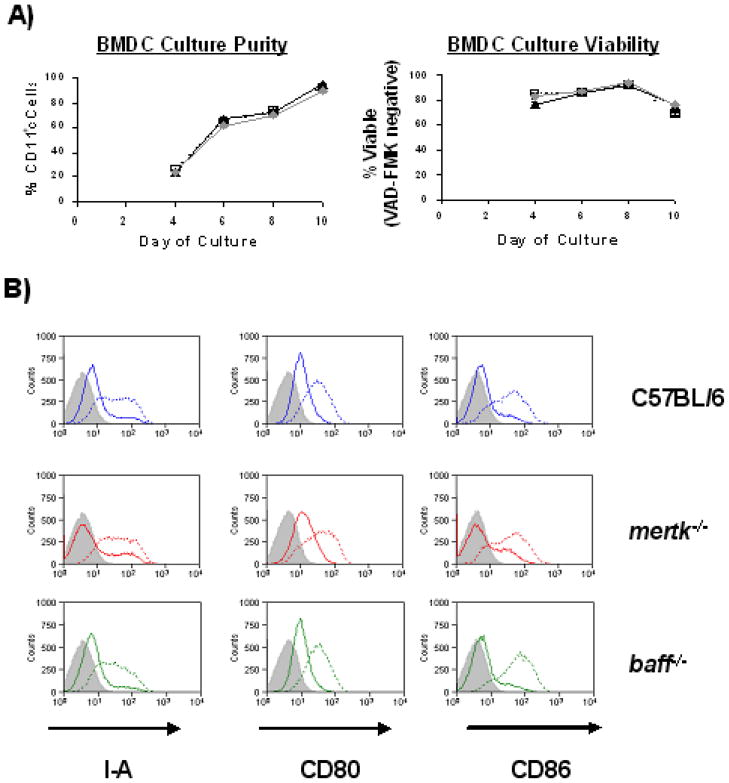
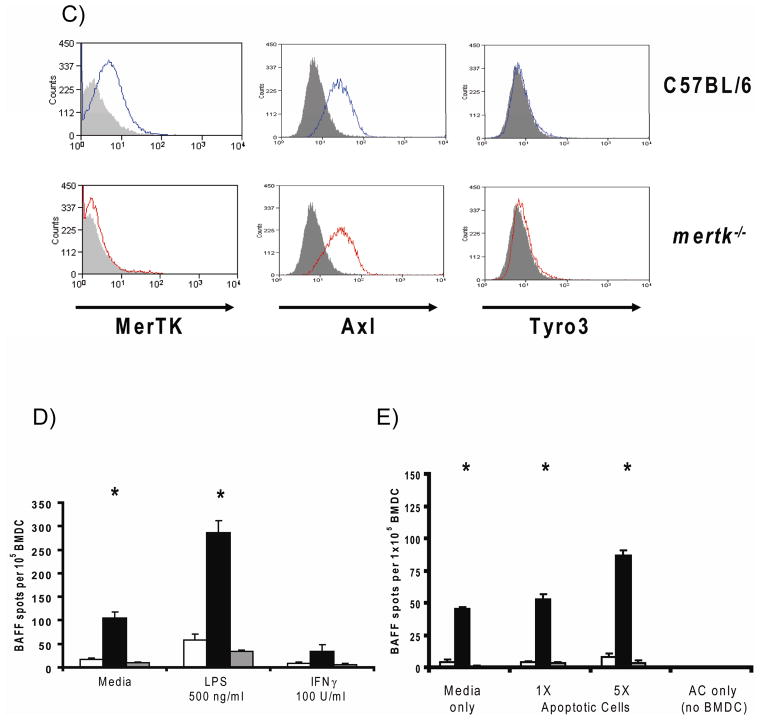
Since baff expression was elevated in spDC from older mertk−/− mice we examined whether the same was true of bone marrow-derived DC (BMDC) which could be used as a more obtainable source of DC for further experimentation. In some experiments BMDC were also generated from baff-deficient (baff−/−) mice as a negative control. We first compared the efficiency of our BMDC culture method by tracking the differentiation of DC from the various mouse strains. As shown in Figure 4A, C57BL/6, mertk−/−, and baff−/− BMDC cultures generated viable CD11c+ cells with similar efficiencies. It was also important to determine whether the various strains of BMDC possessed similar levels of activation markers in the immature and mature state. The surface expression of I-A and the costimulatory molecules CD80 and CD86 was not different among the three genotypes of BMDC, nor was it different after LPS-induced maturation (Figure 4B). Thus, both mertk−/− and baff−/− BMDC differentiate similarly and have a similar maturation status to WT BMDC. In terms of their expression of TAM family members, C57BL/6 BMDC were MerTK+, Axl+, Tyro3−, which is consistent with a previous report [39]. More importantly, the lack of MerTK does not alter the expression level of Axl on mertk−/− BMDC (Figure 4C).
Figure 4. BMDC Cultures Generated from mertk−/− Mice Spontaneously Produce BAFF in vitro.
A) Bone Marrow-Derived Dendritic Cell (BMDC) cultures from C57BL/6 (□, dotted line), mertk−/− (▲, solid line), and baff−/− ( , solid gray line) mice were compared in their ability to generate CD11c+ cells (left) and the viability of CD11c+-gated cells (right). Culture conditions were as described in Materials and Methods section with the exception that volumes and starting cell numbers were scaled down to accommodate 24-well Ultra Low Cluster plates. Each data point represents the mean (± sem) measurement from three culture wells. Data is representative of three experiments. B) C57BL/6, mertk−/− and baff−/− BMDC were cultured in media alone (solid line) or with 500 ng/ml LPS (dotted line) for 2 days, after which time surface expression of I-A, CD80 and CD86 was analyzed by flow cytometry. Appropriate isotype control staining is also shown (gray fill). Data is representative of 2–4 experiments per genotype. C) C57BL/6 and mertk−/− BMDC were stained with antibodies against MerTK, Axl, or Tyro3 (solid lines) and the appropriate isotype control (gray fill). All histograms are gated on CD11c+ events. D and E) The number of BAFF-producing cells within C57BL/6 (open bars), mertk−/− (black bars) and baff−/− (gray bars) BMDC cultures responding to LPS (500 ng/ml) or IFNγ (100U/ml) (D), or apoptotic cells (E) was measured by ELISPOT. In (E) BMDC were treated with an equal number (1X) or 5-fold excess (5X) of apoptotic cells (AC). Data from wells containing only apoptotic cells is also shown. Each bar represents the mean (± sem) of three ELISPOT culture wells. Data is representative of three independent experiments. Statistical significance within each treatment group was determined by the Kruskal-Wallis test (* p<0.05).
, solid gray line) mice were compared in their ability to generate CD11c+ cells (left) and the viability of CD11c+-gated cells (right). Culture conditions were as described in Materials and Methods section with the exception that volumes and starting cell numbers were scaled down to accommodate 24-well Ultra Low Cluster plates. Each data point represents the mean (± sem) measurement from three culture wells. Data is representative of three experiments. B) C57BL/6, mertk−/− and baff−/− BMDC were cultured in media alone (solid line) or with 500 ng/ml LPS (dotted line) for 2 days, after which time surface expression of I-A, CD80 and CD86 was analyzed by flow cytometry. Appropriate isotype control staining is also shown (gray fill). Data is representative of 2–4 experiments per genotype. C) C57BL/6 and mertk−/− BMDC were stained with antibodies against MerTK, Axl, or Tyro3 (solid lines) and the appropriate isotype control (gray fill). All histograms are gated on CD11c+ events. D and E) The number of BAFF-producing cells within C57BL/6 (open bars), mertk−/− (black bars) and baff−/− (gray bars) BMDC cultures responding to LPS (500 ng/ml) or IFNγ (100U/ml) (D), or apoptotic cells (E) was measured by ELISPOT. In (E) BMDC were treated with an equal number (1X) or 5-fold excess (5X) of apoptotic cells (AC). Data from wells containing only apoptotic cells is also shown. Each bar represents the mean (± sem) of three ELISPOT culture wells. Data is representative of three independent experiments. Statistical significance within each treatment group was determined by the Kruskal-Wallis test (* p<0.05).
Previous reports have demonstrated that DC can be induced to produce BAFF by stimulation with LPS or IFNγ [18, 36]. Therefore, we decided to compare the BAFF-induction response of mertk−/− and C57BL/6 BMDC by ELISPOT analysis. In the resting state, BMDC populations lacking mertk contained more BAFF-producing cells than C57BL/6 BMDC (Figure 4D). LPS, but not IFNγ, stimulation led to a further increase in the number of mertk−/− BMDC that produced BAFF. Interestingly, neither of these stimuli induced BAFF production by C57BL/6 BMDC. While this finding differs from the aforementioned studies using human DC, it is consistent with previous results using mouse BMDC stimulated with LPS [46].
MerTK functions as a recognition molecule for apoptotic cells by DC. Although MerTK is not required for the phagocytosis of apoptotic cells by DC [9, 12, 13], it does alter DC physiology in response to apoptotic cells by making them refractory to LPS-induced NF-κB activation [12]. Since others have shown that apoptotic cells can induce BAFF surface expression on DC [47] we next wanted to see how apoptotic cells affect BAFF production by C57BL/6 and mertk−/− BMDC via ELISPOT analysis. Once again, in the resting state a proportion of mertk−/− BMDC spontaneously released BAFF (Figure 4E). This fraction could be nearly doubled by addition of a 5-fold excess of apoptotic cells to the culture. Apoptotic cells did not induce BAFF production by C57BL/6 BMDC and did not contribute to background signal in this ELISPOT assay. These results demonstrate that MerTK is an important negative regulator of BAFF production in a subpopulation of dendritic cells.
B cell survival is independent of DC-derived BAFF
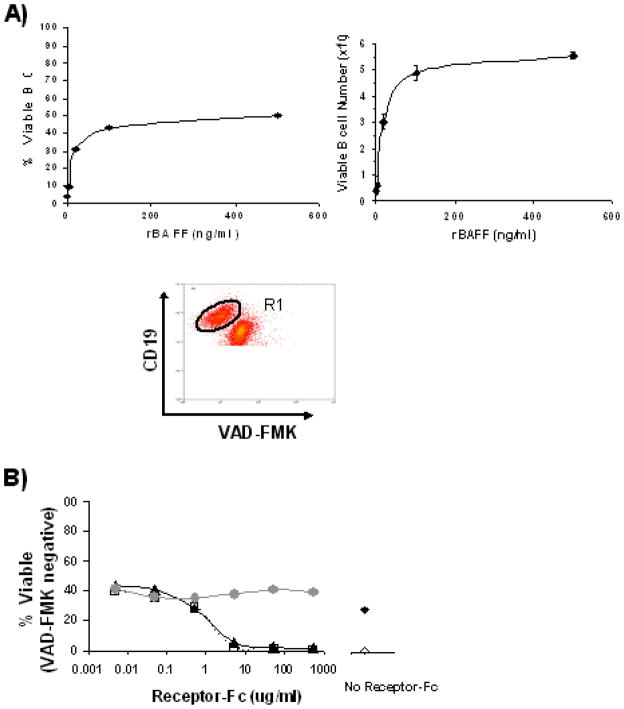
Given that mertkkd DC produce excess BAFF, we wanted to examine what, if any, consequences this had on the outcome of interactions between DC and B cells. One of BAFF’s functions is to provide pro-survival signals for resting B cells [48–51]. We therefore developed an in vitro assay to monitor the survival of resting B cells. The assay involves culturing splenic B cells isolated by negative magnetic selection for 3–4 days and then evaluating their viability using FITC-labeled VAD-FMK. Viability was quantified as either the percentage of B cells (CD19+) that were VAD-FMK−, or in some experiments, the absolute number of VAD-FMK− B cells. First the effect of recombinant BAFF (rBAFF) on B cell survival was evaluated in a titration experiment. In line with previous studies [29, 51] rBAFF elevated both the percentage and absolute number of viable B cells after 4 days in culture (Figure 5A). It appears that a minimum concentration of 100 ng/ml of rBAFF was necessary to sustain maximum viability. Using this concentration we found that the pro-survival benefit of BAFF could be blocked with soluble forms of BAFFR (BAFFR-Fc) or TACI (TACI-Fc, which blocks both BAFF and APRIL) (Figure 5B).
Figure 5. A Novel Assay for Measuring B Cell Survival in Vitro.
A) B cells were isolated from splenocyte populations of C57BL/6 mice by negative magnetic selection. 2×105 B cells were then cultured with rBAFF (500 ng/ml, 100 ng/ml, 20 mg/ml, 4 ng/ml) or media alone for 4 days. Harvested cells were counted and the percent viability among CD19+ events was determined by staining with VAD-FMKFITC. Data (mean ± sem) is plotted as either the percent of VAD-FMK− B cells (left) or the absolute number of viable B cells remaining (right). A sample flow cytometry plot showing how viable B cell populations (VAD-FMK−, gate R1) were identified from within cultured B cells is also shown. B) B cells were cultured in 100 ng/ml rBAFF plus 500, 50, 5, 0.5, 0.05, or 0.005 μg/ml of the soluble decoy receptors BAFFR-Fc (▲) or TACI-Fc (□), or a control-Fc reagent (grey ●). For comparison B cells cultured without decoy Fc reagents in either 100 ng/ml rBAFF (◆), or media alone (◇) are also shown (right). After 3 days the viability was measured by VAD-FMK staining of CD19+ events. Results from one of two independent experiments are shown.
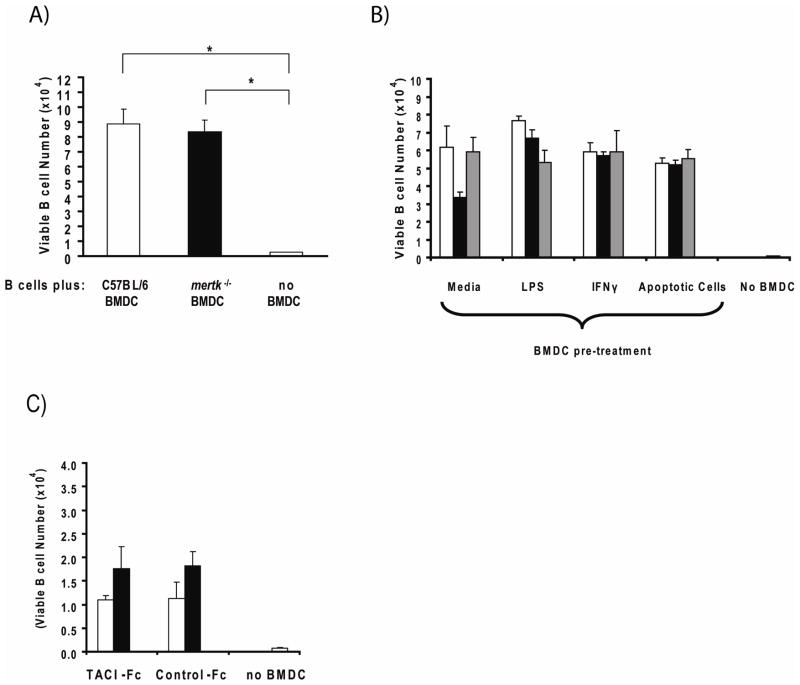
We next set out to determine if BMDC could enhance the survival of resting B cells. Compared to B cells cultured alone, the presence of either C57BL/6 or mertk−/− BMDC in the culture increased the absolute number of VAD-FMK− B cells (Figure 6A). Unexpectedly, culturing B cells with mertk−/− BMDC did not result in greater viability compared to those cultured with C57BL/6 BMDC, although very few viable B cells remained in the absence of BMDCs. Furthermore, fixing BMDC with paraformaldehyde prior to adding to the B cell culture prevented BMDC augmentation of B cell survival, demonstrating that this phenomenon is an active process that requires living BMDC (data not shown). When BMDC were pre-stimulated with LPS or apoptotic cells to increase the number of BAFF-producing cells, mertk−/− BMDC still did not outperform C57BL/6 BMDC (Figure 6B). Thus, despite producing excess BAFF, mertk−/− BMDC do not provide enhanced survival signals to neighboring B cells. In agreement with this, baff−/− BMDC performed similarly to C57BL/6 and mertk−/− BMDC in supporting the survival of B cells (Figure 6B).
Figure 6. BMDC Provide Pro-Survival Signals to Resting B Cells.
A) 4×105 negatively-selected B cells were cultured alone (striped bar) or with 1×105 unstimulated C57BL/6 (open bar) or mertk−/− (black bar) BMDC for 3 days at which time the absolute number viable B cells was determined as described in Materials and Methods. Data bars represent the mean (± sem) of 3 culture wells. Data is representative of two independent experiments. Statistical significance of survival differences between B cells cultured with BMDC versus those cultured alone was determined using the Wilcoxon Rank Sum test (* p<0.05). B) C57BL/6 (open bars), mertk−/− (black bars), and baff−/− (grey bars) BMDC were pre-treated as indicated for 24 hours before washing and culturing with B cells as described above. B cells were also cultured alone (striped bar, “no BMDC”). Each well contained 4×105 B cells and 1×105 BMDC. Data bars represent the mean (± sem) of 3 culture wells. There were no statistically significant differences between BMDC genotypes within each treatment group as determined by the Kruskal-Wallis test. Data is representative of two independent experiments. C) Co-culutres containing resting B cells, untreated C57BL/6 (open bars) or mertk−/− (black bars) BMDC, and 10 μg/ml of either TACI-Fc or Control-Fc soluble decoy receptors were established as described above. B cells were also cultured alone (striped bar, “no BMDC”). After 2 days the number of viable B cells was determined as above. Data bars represent the mean (± sem) of 3 culture wells. There were no statistically significant differences between BMDC genotypes or between the TACI-Fc and control-Fc treated groups as determined by the Wilcoxon Rank Sum test.
These results point towards the existence of a BAFF-independent mechanism by which DC enhance B cell survival. APRIL is another TNF family member that is closely related to BAFF. It too is produced by dendritic cells and causes some, but not all, of the same effects on B cells that BAFF does. The exact role that APRIL plays in B cell survival is unclear since mice lacking APRIL do not display the same deficit in total B cells that baff−/− mice do. Furthermore, while APRIL and BAFF both use the BCMA and TACI receptors, APRIL does not bind to BAFFR, which is the critical receptor for mediating the B cell survival function of BAFF. Nevertheless, it is possible that the ability of BMDC to promote B cell survival independent of BAFF is due to BMDC production of APRIL. To test this we added the soluble form of the TACI receptor (TACI-Fc), or a control-Fc reagent, to our co-culture system. As can be seen in Figure 6C, simultaneous block of APRIL and BAFF function with the TACI-Fc decoy receptor did not reduce the ability of C57BL/6 and mertk−/− BMDC to support B cell survival compared to co-cultures treated with the control-Fc reagent. This result suggests that BMDC promotion of resting B cell survival is independent of both BAFF and APRIL.
DISCUSSION
MerTK is a cell surface receptor tyrosine kinase which functions as an apoptotic cell recognition molecule and a negative regulator of cell activation on dendritic cells [12]. Given that DC have the ability to influence autoreactive B cell responses [52–55] we sought to determine if DC lacking MerTK have characteristics that would be consistent with autoimmunity in mertk−/− mice. In support of this we found that a sub-population of cells spontaneously produced the B cell pro-survival cytokine BAFF exists among spDC and BMDC populations from mertk−/− mice. We were also able to demonstrate that BMDC are able to influence the survival of neighboring B cells. Unexpectedly, however, this phenomenon is not influenced by the absence of mertk or baff expression by DC.
The data presented in this study demonstrates that MerTK has a role in regulating DC physiology in vitro as well as in vivo. We found that mertk−/− mice contain an elevated number of splenic DC. This finding has relevance to autoimmunity because DC made resistant to apoptosis by genetic manipulation can promote autoantibody production in susceptible mouse strains [53]. It is unclear, however, if MerTK is influencing the survival of DC within the spleen [56] or affecting the size of this population in other ways. For example, there may be increased output of DC progenitors from the bone marrow [57], or altered differentiation of blood monocytes or DC precursors into spDC [58–61]. It is interesting that serum from SLE patients has the ability to induce DC differentiation from monocytes in vitro [62]. This was shown to be IFN-α dependent process so it will be informative to determine what the circulating IFN-α levels are in mertk−/− mice.
Despite our findings of a potential role for MerTK in regulating the size of the splenic DC pool, it is also important to point out that splenic DC numbers were not elevated when the mertk−/− allele was moved onto the non-obese diabetic (NOD) genetic background by marker-assisted backcrossing [63]. Our mertk−/− allele is on the C57BL/6 genetic background (n=6). It will be interesting to see if our observations regarding splenic DC numbers persist in mice backcrossed additional generations. The difference between these outcomes may suggest that MerTK’s role in regulating splenic DC populations is dependent upon its interactions with specific alleles (NOD vs. C57BL/6) of other genes.
Despite the increase in spDC number seen in both young and old mertk−/− mice autoantibody production in mertk−/− mice does not begin until later in life. This suggests that some other factor exists that precipitates a breakdown in B cell tolerance in older mice. We found elevated levels of baff mRNA in spleen tissue from mertk−/− mice beginning at 6 months of age. However, this increase was not sufficient to cause an increase in circulating systemic BAFF. This is not inconsistent with a role for MerTK in regulating BAFF production by selected cell types of the immune system since detectable levels of systemic BAFF are produced exclusively by non-hematopoetic cell types [38]. Our finding of WT levels of serum BAFF in mertk−/− mice (Figure 3B) is also consistent with their normal B cell numbers (Figure 2B), since this population is expanded when BAFF levels are systemically elevated [64, 65]. Interestingly, SLE patient clinical disease scores and autoantibody levels are significantly correlated with blood leukocyte baff mRNA levels, but not with serum BAFF protein levels [66]. This fits a model in which myeloid-lineage cells, while not able to contribute significantly to the pool of serum BAFF, are still able to influence B cell survival and tolerance locally via the production of BAFF.
We chose to examine DC as a source of BAFF, as opposed to other MerTK-expressing cell types, specifically because of their role in interacting with and activating antigen-specific B cells [35, 67–69]. Recently spDC from mice lacking all three TAM family members were shown to have elevated baff mRNA expression levels [39]. While this demonstrates that as a family, MerTK, Axl, and Tyro3 have a net inhibitory role on BAFF production, we clarify these results by showing that the lack of mertk alone is sufficient to cause elevated baff mRNA in spDC (Figure 3C). Moreover, we were able to confirm this data at the protein level by showing that spDC in mertk−/− mice harbor a population of cells that spontaneously release BAFF protein ex vivo (Figure 3D). Given that mertk−/− mice have a larger DC population comprised of an elevated proportion of BAFF-producing cells, DC may be participants in autoantibody production in mertk−/− mice. However, it is also important to consider other cell types that might be involved. Macrophages for example, also express MerTK, BAFF and APRIL, and populate similar anatomical locations in the spleen as DC [13, 18, 34, 70, 71]. To investigate whether dysregulated BAFF expression by DC specifically is sufficient to drive B cell autoimmunity in mertk−/− mice, we are currently generating a conditional baff−/− mouse. Since there are two non-redundant pools of baff-producing cells (hematopoetic and stromal) [38] our conditional baff−/− mouse will be very useful in delineating the sources of BAFF relevant to protective and pathogenic humoral immune responses.
To further our understanding of how MerTK regulates DC BAFF production we used BMDC cultures. We found that C57BL/6 BMDC produced very little BAFF, even in the presence of LPS or IFNγ. In keeping with MerTK’s role as an inhibitory molecule in DC physiology [12] we found that MerTK is a negative regulator of BMDC BAFF production. However, in contrast to other MerTK-regulated DC activities such as the inhibition of NF-κB activation, the importance of MerTK in controlling BAFF production is apparent even in the absence of exogenous stimuli. The number of BAFF-producing mertk−/− BMDC could be further increased in the presence of LPS. Thus, BAFF secretion is tightly regulated in mouse DC, and MerTK may be a participant in this regulatory mechanism. Importantly, both C57BL/6 and mertk−/− BMDC upregulate MHC class II, CD80 and CD86 to a similar degree in the presence of LPS. This demonstrates that the C57BL/6 BMDC are not refractory to stimulation in general, and that the reduced control over BAFF-production by mertk−/− BMDC is not simply due to a general heightened activation state. It should be mentioned that a previous report demonstrated elevated levels of CD80, CD86, and MHC class II on spDC from TAM mice [4]; however, the triple mutant mice used in that study had a heterogeneous genetic background, as opposed to our C57BL/6-backcrossed mertk−/− mice. Moreover, our results on this issue are consistent with data obtained by Roland Tisch’s group using NOD. mertk−/− mice [16, 63].
Apoptotic cells deliver a negative signal to DC by preventing LPS- or CD40L-induced activation of the NF-κB signaling pathway by a MerTK-dependent mechanism [12]. Surprisingly we found that, rather than conveying an inhibitory effect, apoptotic cells increased the proportion of BAFF-producing mertk−/− BMDC. C57BL/6 DC can be prompted to release BAFF by immune complexes of chromatin and IgG [46]. This response may partly explain our experimental results given that chromatin is exposed on the surface of apoptotic cells [45]. However the lack of a positive BAFF response to apoptotic cells by C57BL/6 BMDC in our experiments suggests that an inhibitory mechanism exists to prevent BAFF release by DC upon encounter with apoptotic cells, but presumably is not involved in inhibiting the response to IgG-bound chromatin. The positive BAFF response by mertkkd BMDC suggests that MerTK may be involved in this proposed inhibitory mechanism such that during an encounter with apoptotic cells BAFF-induction signals are provided to DC unimpeded.
DC are known to capture, recycle, and display antigens in a way that preserves their epitopes for recognition by cognate B cells [67–69, 72]. While this “native antigen presentation” has yet to be demonstrated for material derived from apoptotic cells, DC pre-fed apoptotic cells are efficient at inducing anti-nuclear antibodies upon injection into C57BL/6 mice, suggesting that antigen was made accessible to B cells [52, 54]. Therefore, our finding that apoptotic cells can deliver a BAFF induction signal to DC contributes to the existing literature by raising the possibility that DC which have engulfed apoptotic cells are capable of providing both intact self-antigen and BAFF to autoreactive B cells.
We also found that BMDC have a pro-survival effect on neighboring B cells in culture. Unexpectedly, this involves a BAFF-independent mechanism, perhaps involving APRIL, since culturing B cells with either C57BL/6 BMDC, which produce very little BAFF under all conditions tested, or with baff−/− BMDC resulted in similar numbers of viable B cells compared to those cultured with mertk−/− BMDC which contain a higher frequency of BAFF-producing cells. The augmentation of B cell survival by BMDC may involve other secreted factors (e.g. APRIL) or signals delivered via cell-cell contact. Interestingly, autoantibody production still occurs in lupus-prone New Zealand Mixed 2328 mice bearing a homozygous deletion of the baff gene (NZM.baff−/−) [73], though no one has yet crossed this strain to APRIL-deficient mice. Our in vitro data demonstrating that DC-mediated enhancement of B cell survival is BAFF-independent could offer a mechanistic explanation for the persistent autoimmunity seen in NZM.baff−/− mice.
This is the first study involving DC from baff-deficient mice. Our results illustrate how much there is yet to learn about the importance of DC-derived BAFF and APRIL during in vitro and in vivo interactions with B cells. Although DC-derived BAFF is not necessary to promote B cell survival, it may play an important role in the differentiation of antigen-stimulated B cells into antibody-secreting cells.
In summary, we have found that DC production of the BAFF cytokine is regulated by the receptor tyrosine kinase MerTK. In the absence of MerTK, there is an increase in baff mRNA expression in the spleen and among spDC from mertk−/− mice. Furthermore, BAFF is constitutively produced by a proportion of mertk−/− BMDC at rest, while more BMDC can be recruited into the BAFF-producing pool by treatment with LPS or apoptotic cells. However, this excess BAFF production capacity does not translate into improved viability of neighboring B cells. Thus, the augmentation of resting B cells survival by BMDC in vitro is independent of BAFF and MerTK. It remains to be determined whether MerTK, BAFF and APRIL influence DC-mediated B cell activation, plasma cell differentiation and autoantibody production.
Acknowledgments
We thank Dr. Susan Kalled (Biogen Idec) for review of this manuscript. This work is supported by NIAID AI50736.
References
- 1.Klippel J. Primer on the Rheumatic Diseases. 12. Atlanta, GA: The Arthritis Foundation; 2001. [Google Scholar]
- 2.Jorgensen TN, Gubbels MR, Kotzin BL. New insights into disease pathogenesis from mouse lupus genetics. Curr Opin Immunol. 2004;16(6):787–93. doi: 10.1016/j.coi.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 3.Kono DH, Theofilopoulos AN. Genetics of SLE in mice. Springer Semin Immunopathol. 2006;28(2):83–96. doi: 10.1007/s00281-006-0030-7. [DOI] [PubMed] [Google Scholar]
- 4.Lu Q, Lemke G. Homeostatic regulation of the immune system by receptor tyrosine kinases of the Tyro 3 family. Science. 2001;293(5528):306–11. doi: 10.1126/science.1061663. [DOI] [PubMed] [Google Scholar]
- 5.Camenisch TD, Koller BH, Earp HS, Matsushima GK. A novel receptor tyrosine kinase, Mer, inhibits TNF-alpha production and lipopolysaccharide-induced endotoxic shock. J Immunol. 1999;162(6):3498–503. [PubMed] [Google Scholar]
- 6.Cohen PL, Caricchio R, Abraham V, Camenisch TD, Jennette JC, Roubey RA, Earp HS, Matsushima G, Reap EA. Delayed apoptotic cell clearance and lupus-like autoimmunity in mice lacking the c-mer membrane tyrosine kinase. J Exp Med. 2002;196(1):135–40. doi: 10.1084/jem.20012094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qian Y, Wang H, Clarke SH. Impaired clearance of apoptotic cells induces the activation of autoreactive anti-Sm marginal zone and B-1 B cells. J Immunol. 2004;172(1):625–35. doi: 10.4049/jimmunol.172.1.625. [DOI] [PubMed] [Google Scholar]
- 8.Scott RS, McMahon EJ, Pop SM, Reap EA, Caricchio R, Cohen PL, Earp HS, Matsushima GK. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature. 2001;411(6834):207–11. doi: 10.1038/35075603. [DOI] [PubMed] [Google Scholar]
- 9.Behrens EM, Gadue P, Gong SY, Garrett S, Stein PL, Cohen PL. The mer receptor tyrosine kinase: expression and function suggest a role in innate immunity. Eur J Immunol. 2003;33(8):2160–7. doi: 10.1002/eji.200324076. [DOI] [PubMed] [Google Scholar]
- 10.Caraux A, Lu Q, Fernandez N, Riou S, Di Santo JP, Raulet DH, Lemke G, Roth C. Natural killer cell differentiation driven by Tyro3 receptor tyrosine kinases. Nat Immunol. 2006;7(7):747–54. doi: 10.1038/ni1353. [DOI] [PubMed] [Google Scholar]
- 11.Graham DK, Bowman GW, Dawson TL, Stanford WL, Earp HS, Snodgrass HR. Cloning and developmental expression analysis of the murine c-mer tyrosine kinase. Oncogene. 1995;10(12):2349–59. [PubMed] [Google Scholar]
- 12.Sen P, Wallet MA, Yi Z, Huang Y, Henderson M, Mathews CE, Earp HS, Matsushima G, Baldwin AS, Jr, Tisch RM. Apoptotic cells induce Mer tyrosine kinase-dependent blockade of NF-kappaB activation in dendritic cells. Blood. 2007;109(2):653–60. doi: 10.1182/blood-2006-04-017368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seitz HM, Camenisch TD, Lemke G, Earp HS, Matsushima GK. Macrophages and dendritic cells use different axl/mertk/tyro3 receptors in clearance of apoptotic cells. J Immunol. 2007;178(9):5635–42. doi: 10.4049/jimmunol.178.9.5635. [DOI] [PubMed] [Google Scholar]
- 14.Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med. 2000;191(3):423–34. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stuart LM, Lucas M, Simpson C, Lamb J, Savill J, Lacy-Hulbert A. Inhibitory effects of apoptotic cell ingestion upon endotoxin-driven myeloid dendritic cell maturation. J Immunol. 2002;168(4):1627–35. doi: 10.4049/jimmunol.168.4.1627. [DOI] [PubMed] [Google Scholar]
- 16.Wallet MA, Sen P, Flores RR, Wang Y, Yi Z, Huang Y, Mathews CE, Earp HS, Matsushima G, Wang B, Tisch R. MerTK is required for apoptotic cell-induced T cell tolerance. J Exp Med. 2008;205(1):219–32. doi: 10.1084/jem.20062293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schneider P, MacKay F, Steiner V, Hofmann K, Bodmer JL, Holler N, Ambrose C, Lawton P, Bixler S, Acha-Orbea H, Valmori D, Romero P, Werner-Favre C, Zubler RH, Browning JL, Tschopp J. BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. J Exp Med. 1999;189(11):1747–56. doi: 10.1084/jem.189.11.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nardelli B, Belvedere O, Roschke V, Moore PA, Olsen HS, Migone TS, Sosnovtseva S, Carrell JA, Feng P, Giri JG, Hilbert DM. Synthesis and release of B-lymphocyte stimulator from myeloid cells. Blood. 2001;97(1):198–204. doi: 10.1182/blood.v97.1.198. [DOI] [PubMed] [Google Scholar]
- 19.Brink R. Regulation of B cell self-tolerance by BAFF. Semin Immunol. 2006;18(5):276–83. doi: 10.1016/j.smim.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 20.Tangye SG, Bryant VL, Cuss AK, Good KL. BAFF, APRIL and human B cell disorders. Semin Immunol. 2006;18(5):305–17. doi: 10.1016/j.smim.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 21.Kalled SL. Impact of the BAFF/BR3 axis on B cell survival, germinal center maintenance and antibody production. Semin Immunol. 2006;18(5):290–6. doi: 10.1016/j.smim.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 22.Gross JA, Dillon SR, Mudri S, Johnston J, Littau A, Roque R, Rixon M, Schou O, Foley KP, Haugen H, McMillen S, Waggie K, Schreckhise RW, Shoemaker K, Vu T, Moore M, Grossman A, Clegg CH. TACI-Ig neutralizes molecules critical for B cell development and autoimmune disease. impaired B cell maturation in mice lacking BLyS. Immunity. 2001;15(2):289–302. doi: 10.1016/s1074-7613(01)00183-2. [DOI] [PubMed] [Google Scholar]
- 23.Schiemann B, Gommerman JL, Vora K, Cachero TG, Shulga-Morskaya S, Dobles M, Frew E, Scott ML. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science. 2001;293(5537):2111–4. doi: 10.1126/science.1061964. [DOI] [PubMed] [Google Scholar]
- 24.Gross JA, Johnston J, Mudri S, Enselman R, Dillon SR, Madden K, Xu W, Parrish-Novak J, Foster D, Lofton-Day C, Moore M, Littau A, Grossman A, Haugen H, Foley K, Blumberg H, Harrison K, Kindsvogel W, Clegg CH. TACI and BCMA are receptors for a TNF homologue implicated in B-cell autoimmune disease. Nature. 2000;404(6781):995–9. doi: 10.1038/35010115. [DOI] [PubMed] [Google Scholar]
- 25.Khare SD, Sarosi I, Xia XZ, McCabe S, Miner K, Solovyev I, Hawkins N, Kelley M, Chang D, Van G, Ross L, Delaney J, Wang L, Lacey D, Boyle WJ, Hsu H. Severe B cell hyperplasia and autoimmune disease in TALL-1 transgenic mice. Proc Natl Acad Sci U S A. 2000;97(7):3370–5. doi: 10.1073/pnas.050580697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mackay F, Woodcock SA, Lawton P, Ambrose C, Baetscher M, Schneider P, Tschopp J, Browning JL. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J Exp Med. 1999;190(11):1697–710. doi: 10.1084/jem.190.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lesley R, Xu Y, Kalled SL, Hess DM, Schwab SR, Shu HB, Cyster JG. Reduced competitiveness of autoantigen-engaged B cells due to increased dependence on BAFF. Immunity. 2004;20(4):441–53. doi: 10.1016/s1074-7613(04)00079-2. [DOI] [PubMed] [Google Scholar]
- 28.Thien M, Phan TG, Gardam S, Amesbury M, Basten A, Mackay F, Brink R. Excess BAFF rescues self-reactive B cells from peripheral deletion and allows them to enter forbidden follicular and marginal zone niches. Immunity. 2004;20(6):785–98. doi: 10.1016/j.immuni.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 29.Do RK, Hatada E, Lee H, Tourigny MR, Hilbert D, Chen-Kiang S. Attenuation of apoptosis underlies B lymphocyte stimulator enhancement of humoral immune response. J Exp Med. 2000;192(7):953–64. doi: 10.1084/jem.192.7.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hatada EN, Do RK, Orlofsky A, Liou HC, Prystowsky M, MacLennan IC, Caamano J, Chen-Kiang S. NF-kappa B1 p50 is required for BLyS attenuation of apoptosis but dispensable for processing of NF-kappa B2 p100 to p52 in quiescent mature B cells. J Immunol. 2003;171(2):761–8. doi: 10.4049/jimmunol.171.2.761. [DOI] [PubMed] [Google Scholar]
- 31.Hsu BL, Harless SM, Lindsley RC, Hilbert DM, Cancro MP. Cutting edge: BLyS enables survival of transitional and mature B cells through distinct mediators. J Immunol. 2002;168(12):5993–6. doi: 10.4049/jimmunol.168.12.5993. [DOI] [PubMed] [Google Scholar]
- 32.Craxton A, Draves KE, Gruppi A, Clark EA. BAFF regulates B cell survival by downregulating the BH3-only family member Bim via the ERK pathway. J Exp Med. 2005;202(10):1363–74. doi: 10.1084/jem.20051283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mecklenbrauker I, Kalled SL, Leitges M, Mackay F, Tarakhovsky A. Regulation of B-cell survival by BAFF-dependent PKCdelta-mediated nuclear signalling. Nature. 2004;431(7007):456–61. doi: 10.1038/nature02955. [DOI] [PubMed] [Google Scholar]
- 34.Craxton A, Magaletti D, Ryan EJ, Clark EA. Macrophage- and dendritic cell--dependent regulation of human B-cell proliferation requires the TNF family ligand BAFF. Blood. 2003;101(11):4464–71. doi: 10.1182/blood-2002-10-3123. [DOI] [PubMed] [Google Scholar]
- 35.Balazs M, Martin F, Zhou T, Kearney J. Blood dendritic cells interact with splenic marginal zone B cells to initiate T-independent immune responses. Immunity. 2002;17(3):341–52. doi: 10.1016/s1074-7613(02)00389-8. [DOI] [PubMed] [Google Scholar]
- 36.Litinskiy MB, Nardelli B, Hilbert DM, He B, Schaffer A, Casali P, Cerutti A. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat Immunol. 2002;3(9):822–9. doi: 10.1038/ni829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hanada T, Yoshida H, Kato S, Tanaka K, Masutani K, Tsukada J, Nomura Y, Mimata H, Kubo M, Yoshimura A. Suppressor of cytokine signaling-1 is essential for suppressing dendritic cell activation and systemic autoimmunity. Immunity. 2003;19(3):437–50. doi: 10.1016/s1074-7613(03)00240-1. [DOI] [PubMed] [Google Scholar]
- 38.Gorelik L, Gilbride K, Dobles M, Kalled SL, Zandman D, Scott ML. Normal B cell homeostasis requires B cell activation factor production by radiation-resistant cells. J Exp Med. 2003;198(6):937–45. doi: 10.1084/jem.20030789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rothlin CV, Ghosh S, Zuniga EI, Oldstone MB, Lemke G. TAM receptors are pleiotropic inhibitors of the innate immune response. Cell. 2007;131(6):1124–36. doi: 10.1016/j.cell.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 40.Kilmon MA, Wagner NJ, Garland AL, Lin L, Aviszus K, Wysocki LJ, Vilen BJ. Macrophages prevent the differentiation of autoreactive B cells by secreting CD40 ligand and IL-6. Blood. 2007 doi: 10.1182/blood-2006-12-061648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bhattacharyya S, Sen P, Wallet M, Long B, Baldwin AS, Jr, Tisch R. Immunoregulation of dendritic cells by IL-10 is mediated through suppression of the PI3K/Akt pathway and of IkappaB kinase activity. Blood. 2004;104(4):1100–9. doi: 10.1182/blood-2003-12-4302. [DOI] [PubMed] [Google Scholar]
- 42.Casciola-Rosen LA, Anhalt G, Rosen A. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J Exp Med. 1994;179(4):1317–30. doi: 10.1084/jem.179.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cocca BA, Cline AM, Radic MZ. Blebs and apoptotic bodies are B cell autoantigens. J Immunol. 2002;169(1):159–66. doi: 10.4049/jimmunol.169.1.159. [DOI] [PubMed] [Google Scholar]
- 44.Mevorach D, Zhou JL, Song X, Elkon KB. Systemic exposure to irradiated apoptotic cells induces autoantibody production. J Exp Med. 1998;188(2):387–92. doi: 10.1084/jem.188.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Radic M, Marion T, Monestier M. Nucleosomes are exposed at the cell surface in apoptosis. J Immunol. 2004;172(11):6692–700. doi: 10.4049/jimmunol.172.11.6692. [DOI] [PubMed] [Google Scholar]
- 46.Boule MW, Broughton C, Mackay F, Akira S, Marshak-Rothstein A, Rifkin IR. Toll-like receptor 9-dependent and -independent dendritic cell activation by chromatin-immunoglobulin G complexes. J Exp Med. 2004;199(12):1631–40. doi: 10.1084/jem.20031942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Diaz-de-Durana Y, Mantchev GT, Bram RJ, Franco A. TACI-BLyS signaling via B-cell-dendritic cell cooperation is required for naive CD8+ T-cell priming in vivo. Blood. 2006;107(2):594–601. doi: 10.1182/blood-2004-12-4708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Batten M, Groom J, Cachero TG, Qian F, Schneider P, Tschopp J, Browning JL, Mackay F. BAFF mediates survival of peripheral immature B lymphocytes. J Exp Med. 2000;192(10):1453–66. doi: 10.1084/jem.192.10.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oliver PM, Vass T, Kappler J, Marrack P. Loss of the proapoptotic protein, Bim, breaks B cell anergy. J Exp Med. 2006;203(3):731–41. doi: 10.1084/jem.20051407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Patke A, Mecklenbrauker I, Erdjument-Bromage H, Tempst P, Tarakhovsky A. BAFF controls B cell metabolic fitness through a PKC beta-and Akt-dependent mechanism. J Exp Med. 2006;203(11):2551–62. doi: 10.1084/jem.20060990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rolink AG, Tschopp J, Schneider P, Melchers F. BAFF is a survival and maturation factor for mouse B cells. Eur J Immunol. 2002;32(7):2004–10. doi: 10.1002/1521-4141(200207)32:7<2004::AID-IMMU2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 52.Bondanza A, Zimmermann VS, Dell’Antonio G, Dal Cin E, Capobianco A, Sabbadini MG, Manfredi AA, Rovere-Querini P. Cutting edge: dissociation between autoimmune response and clinical disease after vaccination with dendritic cells. J Immunol. 2003;170(1):24–7. doi: 10.4049/jimmunol.170.1.24. [DOI] [PubMed] [Google Scholar]
- 53.Chen M, Wang YH, Wang Y, Huang L, Sandoval H, Liu YJ, Wang J. Dendritic cell apoptosis in the maintenance of immune tolerance. Science. 2006;311(5764):1160–4. doi: 10.1126/science.1122545. [DOI] [PubMed] [Google Scholar]
- 54.Georgiev M, Agle LM, Chu JL, Elkon KB, Ashany D. Mature dendritic cells readily break tolerance in normal mice but do not lead to disease expression. Arthritis Rheum. 2005;52(1):225–38. doi: 10.1002/art.20759. [DOI] [PubMed] [Google Scholar]
- 55.Ma L, Chan KW, Trendell-Smith NJ, Wu A, Tian L, Lam AC, Chan AK, Lo CK, Chik S, Ko KH, To CK, Kam SK, Li XS, Yang CH, Leung SY, Ng MH, Stott DI, MacPherson GG, Huang FP. Systemic autoimmune disease induced by dendritic cells that have captured necrotic but not apoptotic cells in susceptible mouse strains. Eur J Immunol. 2005;35(11):3364–75. doi: 10.1002/eji.200535192. [DOI] [PubMed] [Google Scholar]
- 56.Kamath AT, Henri S, Battye F, Tough DF, Shortman K. Developmental kinetics and lifespan of dendritic cells in mouse lymphoid organs. Blood. 2002;100(5):1734–41. [PubMed] [Google Scholar]
- 57.Fogg DK, Sibon C, Miled C, Jung S, Aucouturier P, Littman DR, Cumano A, Geissmann F. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science. 2006;311(5757):83–7. doi: 10.1126/science.1117729. [DOI] [PubMed] [Google Scholar]
- 58.Ardavin C. Origin, precursors and differentiation of mouse dendritic cells. Nat Rev Immunol. 2003;3(7):582–90. doi: 10.1038/nri1127. [DOI] [PubMed] [Google Scholar]
- 59.Naik SH, Metcalf D, van Nieuwenhuijze A, Wicks I, Wu L, O’Keeffe M, Shortman K. Intrasplenic steady-state dendritic cell precursors that are distinct from monocytes. Nat Immunol. 2006;7(6):663–71. doi: 10.1038/ni1340. [DOI] [PubMed] [Google Scholar]
- 60.Shortman K, Naik SH. Steady-state and inflammatory dendritic-cell development. Nat Rev Immunol. 2007;7(1):19–30. doi: 10.1038/nri1996. [DOI] [PubMed] [Google Scholar]
- 61.O’Keeffe M, Hochrein H, Vremec D, Scott B, Hertzog P, Tatarczuch L, Shortman K. Dendritic cell precursor populations of mouse blood: identification of the murine homologues of human blood plasmacytoid pre-DC2 and CD11c+ DC1 precursors. Blood. 2003;101(4):1453–9. doi: 10.1182/blood-2002-03-0974. [DOI] [PubMed] [Google Scholar]
- 62.Blanco P, Palucka AK, Gill M, Pascual V, Banchereau J. Induction of dendritic cell differentiation by IFN-alpha in systemic lupus erythematosus. Science. 2001;294(5546):1540–3. doi: 10.1126/science.1064890. [DOI] [PubMed] [Google Scholar]
- 63.Tisch R. Personal Communication.
- 64.Moore PA, Belvedere O, Orr A, Pieri K, LaFleur DW, Feng P, Soppet D, Charters M, Gentz R, Parmelee D, Li Y, Galperina O, Giri J, Roschke V, Nardelli B, Carrell J, Sosnovtseva S, Greenfield W, Ruben SM, Olsen HS, Fikes J, Hilbert DM. BLyS: member of the tumor necrosis factor family and B lymphocyte stimulator. Science. 1999;285(5425):260–3. doi: 10.1126/science.285.5425.260. [DOI] [PubMed] [Google Scholar]
- 65.Parry TJ, Riccobene TA, Strawn SJ, Williams R, Daoud R, Carrell J, Sosnovtseva S, Miceli RC, Poortman CM, Sekut L, Li Y, Fikes J, Sung C. Pharmacokinetics and immunological effects of exogenously administered recombinant human B lymphocyte stimulator (BLyS) in mice. J Pharmacol Exp Ther. 2001;296(2):396–404. [PubMed] [Google Scholar]
- 66.Collins CE, Gavin AL, Migone TS, Hilbert DM, Nemazee D, Stohl W. B lymphocyte stimulator (BLyS) isoforms in systemic lupus erythematosus: disease activity correlates better with blood leukocyte BLyS mRNA levels than with plasma BLyS protein levels. Arthritis Res Ther. 2006;8(1):R6. doi: 10.1186/ar1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bergtold A, Desai DD, Gavhane A, Clynes R. Cell surface recycling of internalized antigen permits dendritic cell priming of B cells. Immunity. 2005;23(5):503–14. doi: 10.1016/j.immuni.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 68.Huang NN, Han SB, Hwang IY, Kehrl JH. B cells productively engage soluble antigen-pulsed dendritic cells: visualization of live-cell dynamics of B cell-dendritic cell interactions. J Immunol. 2005;175(11):7125–34. doi: 10.4049/jimmunol.175.11.7125. [DOI] [PubMed] [Google Scholar]
- 69.Qi H, Egen JG, Huang AY, Germain RN. Extrafollicular activation of lymph node B cells by antigen-bearing dendritic cells. Science. 2006;312(5780):1672–6. doi: 10.1126/science.1125703. [DOI] [PubMed] [Google Scholar]
- 70.Mebius RE, Kraal G. Structure and function of the spleen. Nat Rev Immunol. 2005;5(8):606–16. doi: 10.1038/nri1669. [DOI] [PubMed] [Google Scholar]
- 71.Ogden CA, Pound JD, Batth BK, Owens S, Johannessen I, Wood K, Gregory CD. Enhanced apoptotic cell clearance capacity and B cell survival factor production by IL-10-activated macrophages: implications for Burkitt’s lymphoma. J Immunol. 2005;174(5):3015–23. doi: 10.4049/jimmunol.174.5.3015. [DOI] [PubMed] [Google Scholar]
- 72.Wykes M, Pombo A, Jenkins C, MacPherson GG. Dendritic cells interact directly with naive B lymphocytes to transfer antigen and initiate class switching in a primary T-dependent response. J Immunol. 1998;161(3):1313–9. [PubMed] [Google Scholar]
- 73.Jacob CO, Pricop L, Putterman C, Koss MN, Liu Y, Kollaros M, Bixler SA, Ambrose CM, Scott ML, Stohl W. Paucity of clinical disease despite serological autoimmunity and kidney pathology in lupus-prone New Zealand mixed 2328 mice deficient in BAFF. J Immunol. 2006;177(4):2671–80. doi: 10.4049/jimmunol.177.4.2671. [DOI] [PMC free article] [PubMed] [Google Scholar]