Abstract
Previous MRI studies with participants prior to manifest Huntington disease have been conducted in small single-site samples. The current study reports data from a systematic multi-national study during the prodromal period of Huntington disease and examines whether various brain structures make unique predictions about the proximity to manifest disease. MRI scans were acquired from 657 participants enrolled at one of 32 PREDICT-HD research sites. Only prodromal Huntington disease participants (those not meeting motor criteria for diagnosis) were included and subgrouped by estimated diagnosis proximity (Near, Mid, and Far) based upon a formula incorporating age and CAG repeat length. Results show volumes of all three subgroups differed significantly from Controls for total brain tissue, cerebral spinal fluid, white matter, cortical gray matter, thalamus, caudate, and putamen. Total striatal volume demonstrated the largest differences between Controls and all three prodromal subgroups. Cerebral white matter offered additional independent power in the prediction of estimated proximity to diagnosis. In conclusion, this large cross-sectional study shows that changes in brain volume are detectable years to decades prior to estimated motor diagnosis of Huntington disease. This suggests that a clinical trial of a putative neuroprotective agent could begin as much as 15 years prior to estimated motor diagnosis in a cohort of persons at risk for but not meeting clinical motor diagnostic criteria for Huntington disease, and that neuroimaging (striatal and white matter volumes) may be among the best predictors of diagnosis proximity.
Keywords: Huntington disease, MRI, clinical trials, observational study
1. Introduction
Huntington disease (HD) is an autosomal dominant neurodegenerative disease caused by a polyglutamine expansion within the N-terminus of the huntingtin protein [14]. HD is characterized by loss of movement control, cognitive ability, and emotional regulation and results in death approximately 15–20 years following motor diagnosis [12, 35]. Overall loss of brain mass is substantial, on the order of 30% by the time of death [38]. The unique value of HD as a model for the development of neuroprotective therapies for neurodegenerative disorders hinges on the ability to know, with certainty and years prior to diagnosis, which individuals will develop the disease. PREDICT-HD is a long-term observational study of a large population of participants who are known to carry the HD gene mutation, but who were not yet diagnosed with the disease at the time of entry into the study. The goals of PREDICT-HD are to identify measurable and sensitive neuropsychological, clinical, or imaging changes that emerge prior to the somewhat coarse endpoint of disease diagnosis. It is reasonable to think that neuroprotective therapies will have more benefit the earlier they are provided, but demonstration of the benefits will require the development of sensitive clinical and imaging markers of disease progression in the prodromal stage of HD.
Previous MRI studies have documented striatal volume decrements long before diagnosis [4-5, 29], as well as a steady progression of volume decrement as diagnosis approaches [3, 28]. In addition, imaging studies have identified changes in the cerebral cortex that also occur either prior to the diagnosis or early in the course of the disease [17, 24, 26-27, 29, 32, 37]. However, most of these studies have been relatively small, single site investigations.
The purpose of the current study was to cross-sectionally examine baseline imaging measures in a multi-national sample of prodromal HD participants [27-28]. The utility of imaging for early disease assessment as a possible biomarker for clinical trial design was a primary focus. All participants had structural MRI scans at 1.5 Tesla, followed by automated volumetric analysis of both cortical and subcortical brain regions.
2. Materials and Methods
2.1. Participants
Participants were recruited from the PREDICT-HD study, an ongoing longitudinal observational study conducted at 32 sites in the United States, Canada, Australia, Germany, Spain, and the United Kingdom. All aspects of the study were approved by the Institutional Review Board at each participating institution, and all aspects of the study are in compliance with national legislation and the Declaration of Helsinki. Participants underwent informed consent procedures and signed consents for both participation and to allow de-identified research data to be sent to collaborative institutions for analyses. All participants underwent detailed motor, cognitive, psychiatric, and functional evaluations annually as previously described [27-28]. At-risk participants have previously been described as “presymptomatic,” “preclinical,” “prediagnosed,” “premanifest,” “pre-HD,” or “prodromal HD.” We will use the term “prodrome” to describe the phase prior to the manifestation of the movement disorder clinically diagnosable as HD.
Only participants considered to be in the prodromal phase of disease according to the diagnostic confidence level of the Unified Huntington Disease Rating Scale UHDRS [1] were included in the current paper. Diagnostic confidence level is a scale from 0 (normal) to 4 (operationally defined as the unequivocal presence of an otherwise unexplained movement disorder in a participant at risk for HD). Participants with ratings less than 4 were considered. MRI scans from 657 participants were processed. Of these participants, 491 carried the HD gene mutation and 166 carried only normal alleles; the latter were used as the comparison group.
2.2. Proximity to Diagnosis
Estimated years to diagnosis was calculated using a CAG-and-age based predictive model developed from a worldwide sample of 2,913 [20] and validated with nearly 100 prospectively diagnosed patients from the PREDICT-HD study [21, 27-28]. Participants were considered “Far” from diagnosis if estimated proximity to clinical diagnosis was greater than 15 years, “Mid” to diagnosis if their estimated proximity was 9–15 years, and “Near” to diagnosis if estimated proximity was less than 9 years. Table 1 displays the demographic and clinical characteristics of the sample divided into groups according to diagnostic proximity. Gender was similar across groups (from 61% to 68% female), although age was significantly younger in the far from diagnosis group. As expected, CAG lengths were significantly different across all groups, with those closer to estimated diagnosis having longer repeat lengths.
Table 1.
Demographic Information
| Controls | Far from Diagnosis | Midway to Diagnosis | Near Diagnosis | Statistic p-value | Contrasts | |
|---|---|---|---|---|---|---|
| N | 166 | 180 | 184 | 127 | – | – |
| Gender (% Female) | 65 | 67 | 68 | 61 | Chi-sq = 2.30 p = 0.51 | ns |
| Age Mean (SD) | 44.09 (11.67) | 37.53 (8.45) | 43.95 (10.11) | 45.68 (9.98) | F = 21.35 p < 0.0001 | Far<Controls, Mid, Near |
| CAG Mean (SD) | 20.03 (3.36) | 41.06 (1.56) | 42.34 (2.14) | 43.97 (2.85) | F = 3188.65 p < 0.0001 | Controls<Far<Mid<Near |
| UHDRS Motor Total Mean (SD) | 2.91 (3.51) | 3.69 (4.14) | 4.65 (4.87) | 8.13 (7.13) | F = 30.29 p < 0.0001 | Controls, Far<Mid<Near |
| UHDRS Psychiatric Mean (SD) | 4.62 (7.43) | 8.09 (10.36) | 9.78 (15.50) | 8.46 (12.91) | F = 5.72 p = 0.0007 | Controls<Far, Mid, Near |
| Symbol Digit Total Mean (SD) | 55.72 (8.50) | 56.49 (12.54) | 52.21 (10.48) | 45.56 (10.72) | F = 30.22 p < 0.0001 | Controls, Far>Mid>Near |
| Stroop Word Mean (SD) | 103.43 (14.18) | 103.65 (18.83) | 98.76 (17.24) | 92.66 (15.89) | F = 13.37 p < 0.0001 | Controls, Far>Mid>Near |
| Stroop Color Mean (SD) | 83.03 (12.28) | 82.00 (14.64) | 78.66 (13.26) | 74.19 (13.31) | F = 12.32 p < 0.0001 | Controls, Far>Mid>Near |
| Stroop Interference Mean (SD) | 47.76 (9.95) | 49.02 (10.62) | 45.57 (10.24) | 41.45 (9.38) | F = 15.32 p < 0.0001 | Controls, Far>Mid>Near |
| Verbal Fluency Mean (SD) | 44.41 (12.34) | 43.18 (10.95) | 42.02 (11.86) | 41.10 (13.31) | F = 2.06 p = 0.10 | ns |
2.3. Motor Function
Movement disorder specialists examined the participants and rated the presence and severity of 15 individual motor signs (e.g., finger tapping, chorea, dysarthria) using the UHDRS [1]. The sum of motor signs can range from 0 to 124 with higher scores indicating more impaired motor functioning.
2.4. MRI Measures
All scans were obtained using a standard multi-modal protocol that included an axial 3D volumetric spoiled gradient echo series (~1×1×1.5 mm voxels) and a dual echo proton density/T2 (~1×1×3 mm voxels) series. Thirty sites used General Electric 1.5 Tesla scanners, and two sites used Siemens 1.5 Tesla scanners.
Scans were processed through an automated procedure implemented in BRAINS [23] reoriented by stepwise coregistration to a set of template images, centered on the anterior commissure, and resampled to 1 mm resolution. The T1 image was the final T1 image, and all images were intensity normalized and inhomogeneity corrected.
A discriminant tissue classification was then performed [13] and a brain mask was created using an artificial neural network (ANN) [30]. Measures of gray matter, white matter, and CSF volumes were then completed using the standard Talairach method [2]. ANNs were applied to each scan to measure subcortical structures, including caudate, putamen, and thalamus. Volumes of caudate and putamen were summed to create a measure of total striatum.
Results of this procedure were visually inspected, and greater than 90% of the scans passed all stages successfully. The most common reason for failure was poor co-registration of the multiple modes. No known variable that was the subject of this report, including gender and HD gene-expansion status, significantly predicted scan failures.
2.5. Statistics
We report observed volumes in cubic centimeters (cc). However, to control for differences in overall head size, all measures were analyzed as a proportion of intracranial volume (ICV). Each brain measure was treated as the outcome measure, with fixed predictor variables defined as prognostic group (Control, Far, Mid, or Near). Between groups, pair-wise contrasts were considered statistically significant at a conservative Bonferroni-corrected observed p < 0.008, and alternative Tukey-Kramer within-contrast adjustment methods yielded identical decisions. All analyses were adjusted for inter-scanner variation (as a random effect), gender, and age. Age was treated as a quadratic effect, as both linear and squared terms are significant for many, but not all, structures. Estimation and inference was via the residual (restricted) maximum likelihood method, and the Satterthwaite approximation was used for estimating degrees-of-freedom on all F tests. Effect sizes for contrasts with the Control group used the square root of (residual error variance + inter-scanner variance) as an estimate of mean within-group standard deviation. The relationship of neuroimaging measures with estimated proximity to motor diagnosis was examined further by fitting non-linear models, adjusting for age, gender and ICV [28]. Pearson correlations were conducted to determine the association among imaging and clinical measures.
3. Results
3.1. Group Comparisons
Mean brain tissue volumes for each group (Control, Far, Mid, Near) as well as analyses of structure ratios (i.e., volumes divided by ICV) adjusted for age and gender are shown in Table 2. Except cerebellum, group differences met our strict criteria for statistical significance for all structures. Planned post-hoc comparisons showed that all prodromal subgroups differed significantly from Controls. Although most subgroups differed from one another in a stepwise fashion for most brain structures, differences between Far and Mid were not significant for cortical gray matter and thalamus.
Table 2.
Observed Mean (SD) Structure Volumes and Statistical Tests between Groups*
| Brain Measure | Control | Far | Mid | Near | F | Degrees of Freedom1 | p | contrasts |
|---|---|---|---|---|---|---|---|---|
| Total Brain Tissue | 1242.3 (133.6) | 1215.5 (130.1) | 1182.4 (121.1) | 1152.6 (129.7) | 73.16 | 3, 641 | <0.0001 | C>F>M>N |
| CSF | 110.30 (47.92) | 110.62 (39.09) | 137.72 (47.94) | 174.99 (61.04) | 73.49 | 3, 641 | <0.0001 | C>F>M>N |
| Cortical Gray Matter | 600.47 (64.79) | 585.32 (61.97) | 576.98 (56.30) | 570.20 (59.60) | 10.32 | 3, 643 | <0.0001 | C>(F,M)>N |
| Cerebral White Matter | 439.48 (59.77) | 428.96 (57.13) | 410.70 (57.60) | 388.33 (64.50) | 50.83 | 3, 644 | <0.0001 | C>F>M>N |
| Cerebellum | 116.52 (13.92) | 116.49 (14.73) | 112.89 (12.17) | 115.90 (13.00) | 3.21 | 3, 650 | 0.02 | ns |
| Thalamus | 11.76 (1.36) | 11.53 (1.32) | 10.90 (1.47) | 10.00 (1.60) | 44.73 | 3, 644 | <0.0001 | C>(F,M)>N |
| Caudate | 7.21 (1.19) | 6.62 (1.15) | 5.72 (1.13) | 4.73 (1.30) | 120.86 | 3, 635 | <0.0001 | C>F>M>N |
| Putamen | 9.81 (1.19) | 9.22 (1.25) | 8.08 (1.29) | 6.99 (1.25) | 148.08 | 3, 641 | <0.0001 | C>F>M>N |
| Total Striatum | 17.03 (2.12) | 15.82 (2.23) | 13.79 (2.23) | 11.71 (2.43) | 166.10 | 3, 634 | <0.0001 | C>F>M>N |
Means and (standard deviations) presented are for unadjusted volumes.
Statistical analyses computed on volumes adjusted for Intracranial Volume (ICV) Ratios, age and gender. Denominator degrees of freedom vary due to Satterthwaite approximations and slight sample size fluctuation from measurement failure. Sample size was 649 for caudate and total striatum, 657 for all other measure
3.2. Effect size calculations
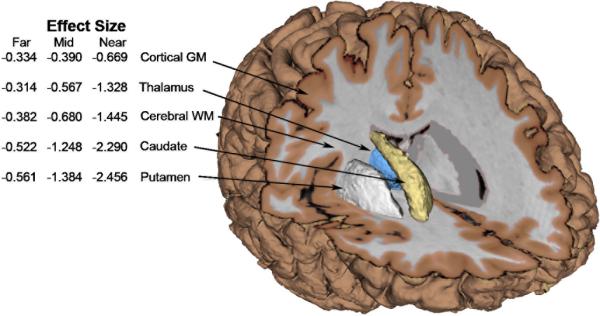
Effect sizes were calculated for each adjusted brain structure to ICV ratio according to the difference between each prodromal subgroup and Controls. With the exception of cerebellum (effect size < 0.14), all effect sizes were moderate to very large, suggesting that several imaging measures provide robust separation from Controls at baseline (see Figure 1). For all prodromal subgroups, effect sizes for total striatum (caudate + putamen) were greater than for other structures, with differences for the Near group at 2.46 SDs below the average for Controls.
Fig. 1.
Observed effect sizes (versus controls) for adjusted ICV ratios. (Within group adjusted standard deviations account for both estimated inter-participant and inter-site-scanner variability)
3.3. Correlations and partial correlations with estimated diagnosis
We calculated correlations of all structure volumes with estimations of diagnosis proximity, while partialling out the demographic variables of age and gender. All brain volumes except cerebellum had a significant association with diagnosis proximity (see Table 3). We then repeated these analyses, adding total striatal volume as a covariate to examine whether associations between structure volumes and estimated diagnosis would remain after the total striatum correlation was controlled. As shown in Table 3, total brain tissue, CSF, and cerebral white matter had significant associations with diagnostic proximity.
Table 3.
Pearson partial correlation coefficients of structure volumes with diagnostic proximity
| Brain region | Partial correlation (controlling for age, gender, and intracranial volume) | Partial correlation (controlling for age, gender, intracranial volume and total striatum) |
|---|---|---|
| Total Brain Tissue | -0.399 (<0.0001) | -0.138 (0.0022) |
| CSF | 0.400 (<0.0001) | 0.140 (0.0020) |
| Cortical Gray Matter | -0.134 (0.0031) | -0.002 (0.9745) |
| Cerebral White Matter | -0.358 (<0.0001) | -0.175 (0.0001) |
| Cerebellum | 0.025 (0.5898) | 0.074 (0.1012) |
| Thalamus | -0.349 (<0.0001) | -0.039 (0.3889) |
| Caudate | -0.544 (<0.0001) | ---- |
| Putamen | -0.554 (<0.0001) | ---- |
| Total Striatum | -0.586 (<0.0001) | ---- |
3.4. Multiple regression predicting proximity of motor diagnosis
Regression analyses were conducted to determine whether any of the brain volume measures would contribute to a prediction of estimated diagnosis after total striatum was forced into a model on step one. A stepwise multiple regression predicting estimated diagnosis within five years (controlling for demographic variables) revealed a significant effect (F = 106.90; df = 4,484; p < 0.0001) in which only white matter volume made a significant contribution (t = 3.90; p = 0.0001; ΔR2 = 0.02) to the prediction of diagnosis proximity after total striatal volume was entered.
3.5. Non-linear models
We fit spline models, adjusting for age, gender and ICV, to examine potential non-linearity in the associations of striatal and white matter volume with estimated diagnosis proximity. For each measure, estimated time to motor diagnosis had a highly significant non-linear p value (striatum: p < 0.0001, adj. R2 = 0.34; white matter: p < 0.0001, adj. R2 = 0.12) and the plots show evidence of a transition point between 15 and 20 years to estimated diagnosis (see Figures 2A and 2B).
Fig. 2A.
Spline plots of striatum volume as a percentage of intracranial volume by years to estimated motor diagnosis, adjusted for age and gender.
Fig. 2B.
Spline plots of white matter volume as a percentage of intracranial volume by years to estimated motor diagnosis, adjusted for age and gender.
3.6 Correlations with clinical phenotype
We calculated correlations of total striatal volume and cerebral white matter with clinical measures from the UHDRS to validate the association among brain imaging and clinical phenotype measures, adjusting for age, gender and ICV. The pearson partial correlation coefficients for total striatum were significant for UHDRS motor total (r = -0.403; p < 0.0001), Symbol Digit total (r = 0.383, p < 0.0001), Stroop Word (r = 0.272, p < 0.0001), Stroop Color (r = 0.243, p < 0.0001), Stroop Interference (r = 0.232, p < 0.0001), and Verbal Fluency (r = 0.124, p = 0.0076), but not for UHDRS psychiatric total (r = -0.058, p = 0.2122). Correlations with white matter were also significant for UHDRS motor total (r = -0.282; p < 0.0001), Symbol Digit total (r = 0.314, p < 0.0001), Stroop Word (r = 0.150, p = 0.0012), Stroop Color (r = 0.154, p = 0.0008), and Stroop Interference (r = 0.193, p < 0.0001) but not for Verbal Fluency (r = 0.096, p = 0.0388) or UHDRS psychiatric total (r = -0.009, p = 0.8530).
4. Discussion
This cross-sectional study of over 600 gene-positive participants in the prodromal stages of HD shows clearly that the volume of all brain structures (except cerebellum), including total brain, cerebral white matter, cortical gray matter, caudate, putamen, and thalamus, is less than that of Controls in all three prodromal subgroups (Near, Mid, and Far from estimated time of diagnosis). Our confidence in these results is enhanced by 1) the large sample size, 2) the step-wise reduction in brain volumes seen in the subgroups, 3) the conservative Bonferroni-corrected alpha level used in the statistical analysis, and 4) control of all comparisons for scanner variation, age, gender, and intracranial volume. However, significant differences between the Control group and the Far from diagnosis group also raise the possibility of a developmental effect of the abnormal HD gene. Further comparison of participants 15–30 years prior to expected diagnosis with age-matched Controls would help to resolve this question.
Our findings are consistent with the hypothesis that selective brain degeneration begins decades before diagnosable HD and progresses over the prodromal period [28-29]. It is important to note that a subsample of the current study (n=261; 40%) was reported in our previous publication [28]. There are several additions to this study other than the larger sample size, including a) the examination of additional brain regions, b) control of scanner variation, c) clinical correlates, d) control of age, gender and intracranial volumes, and d) mutliple regression to determine whether the striatum and white matter findings are redundant or additive.
Our finding of white matter differences in the prodromal period of HD are striking and noted even in the Far from estimated diagnosis group. Although total striatal volume offers the largest differences between normal comparison participants and all subgroups of prodromal HD, cerebral white matter offers additional independent predictive power. Though a couple of studies have documented white matter integrity abnormalities in prodromal HD participants using Diffusion Tensor Imaging (DTI), both of these studies report the abnormalities to be localized to white matter within the striatum [31, 34]. The current findings suggest a more widespread, global rather than regional decrement in white matter tissue. Further, our findings mirror previous studies in participants with HD early in the course of the disease (within the first 5 years) have shown that decrements in white matter volume are directly correlated with cognitive deficits [9]. Although the relationship between white matter volume and cognitive function was much stronger than the relationship between striatal volume and cognitive function in the previous study, our findings suggest an important role for both the striatum and white matter in cogntive performance in the prodrome of HD. Further study is needed to determine whether the reduction of white matter volume in the prodromal stages of HD or the striatal volume reductions now well known reflect the neural underpinnings of the subtle cognitive deficits seen in these participants [22, 36].
In regard to why a widespread deficit in cerebral white matter volume may occur, some groups have hypothesized that the pathogenesis of HD begins with an effect on myelination [7-8]. The theory postulates that mutant gene product (huntingtin) has a direct effect on myelination such that once myelinated neuronal circuits become functional, myelin breakdown begins which causes an excitotoxic process with failure of afferent transmissions causing the underlying neuron to be over-stimulated by its efferent feedback. Support for this theory comes, in part, from two post-mortem studies of HD brain obtained prior to motor diagnosis (prodrome) that show significantly elevated densities of oligodendrocytes (the glial cells that produce myelin), presumably increased as a physiologic attempt of the olidgodendrocytes to repair and remyelinate [11, 25]. Though this theory may well be valid in regard to degeneration of white matter near to and after the motor diagnosis of the disease, it may not explain well why deficits in white matter volume are seen decades from diagnosis, unless an extremely slow and long degenerative process is invoked. An alternative theory is that the deficit in white matter volume is a manifestation of abnormal development—instead of decreased volume from a degenerative process; the lower volume is a reflection of an abnormality in the development of white matter. It is interesting to note that the two post-mortem studies of HD brains prior to motor diagnosis showing increased oligodendrocyte density propose in their papers that the etiology of this finding is more likely in abnormal brain morphogenesis rather than a degenerative process [11, 25]. They postulate that the studies show that as disease progresses, oligodendrocytes density remains stable while neurons decline in number and astrocytes increase in number, supporting the notion that the increase in oligodendroglia may be due to abnormal brain development rather than a response to neuron (or myelin) degeneration.
The caudate and putaminal atrophy seen in this study is consistent with the many reports of selective striatal atrophy in HD [3-6, 28], and with previous reports of cortical and extra-striatal subcortical atrophy in HD [16-18, 32, 37]. By contrast, even in our very large dataset, there was no significant change in cerebellum in any group, indicating that atrophy does not involve the entire brain uniformly. The loss of thalamic volume is consistent with other imaging papers that reported abnormalities in the thalamus in both diagnosed [15] as well as prodromal stages of HD [10]. In our study, total cerebral gray matter volume did not explain any additional variance in the estimated proximity of diagnosis above that attributable to striatal volume. Previous reports evaluating the cortex in prodromal HD participants have been inconsistent, with three studies (one in early HD participants [33] and two in prodromal HD participants [26, 32]) showing widespread cortical thinning, and four studies using voxel-based morphometry (both cross sectional [18, 24, 37] and longitudinal studies [19]) showing very little or only regionally specific gray matter loss in prodromal or early HD. Finally, a recent study reported selective cortical gyral thickening along with sulcal thinning in prodromal HD participants compared to Controls [26]. Varying methods of image processing, varying sample size, and varying levels of proximity to onset may explain inconsistent findings between studies [17]. Volume measures of total cerebral cortex are likely to be relatively insensitive measures as the cortex is divided into functionally distinct areas. Future studies evaluating finer measures of cortical morphology such as thickness, surface area, and sulcal/gyral shape are needed to better understand the course of cortical changes both before and after the motor manifestation of disease.
The selection process for imaging markers suitable for use in clinical trials must consider both empirical properties and practical implementation factors. Imaging measures must be reliable, valid, and show robust differential measures in the studied clinical groups. Imaging should also impose as little burden as possible on participants and must be reasonable in cost. Scan acquisition for the measures presented in this paper required less than 30 minutes per participant, and the vast majority of participants tolerated the scanning with no difficulty. Processing methods were automated, with only final human visual check of each result. Over 90% of scans analyzed in this multi-site, international imaging study passed all stages successfully. These findings are encouraging for the design and implementation of future clinical trials. Despite suggestions that imaging is too costly and time-intensive for clinical trials, our findings suggest that imaging can be an efficient way to gather critical information and that automation can expedite the process.
However, our results may be limited by the fact that our imaging measures were correlated with predicted, not actual, proximity to diagnosis. Although the accuracy of the formula for predicting the motor diagnosis has recently been shown in a prospective validation study [21], it is unknown whether the addition of imaging measures can improve the prediction of motor diagnosis. Since it is possible that clinical trials may use motor diagnosis as a clinical endpoint for assessing treatment effectiveness in prodromal HD, further research is warranted. For such trials to be efficient, it will be necessary to predict accurately which participants have the greatest probability of receiving a motor diagnosis during the period of the clinical trial. Based on the current findings, striatal volume and white matter volume together may provide markers for the selection of prodromal HD participants who are best suited for clinical trials of putative neuroprotective therapies. Although the error variations in the scatter plots appear large, our results suggest that we can identify those at such reduced risk for imminent diagnosis that their inclusion in a clinical trial can contribute very little. It is probable that dramatic sample size benefits could be realized if only participants above the median risk were enrolled. More research is needed to determine whether a combination of “risk measures” might be developed to assist with the selction of enriched samples for clincial trials.
Future directions for this research include longitudinal study to address the utility of these imaging measures as biomarkers (and eventual surrogate markers) for clinical trials. Longitudinal studies will be necessary to determine whether striatal and/or white matter volumes have predictive value for onset of manifest motor HD and other clinical endpoints. Inter-site measurement variability reduction for the strength of imaging biomarkers will continue by prospective acquisition, quality control, and post-processing medical imaging method improvements.
In summary, this cross-sectional study has provided a key insight into the pathogenesis of HD, confirming in a large sample population that reduction in striatal volume and white matter volume is identifiable up to 15 years before estimated time of disease diagnosis. This suggests clinical trials of neuroprotective agents might be applicable to gene positive patients at a much earlier time than previously suspected, and that imaging might be used as a tool to select participants appropriate for such studies.
Supplementary Material
Acknowledgements
We thank the PREDICT-HD sites, the study participants, and the National Research Roster for Huntington Disease Patients and Families. This research is supported by the National Institutes for Health, National Institute of Neurological Disorders and Stroke (NS40068) and CHDI Foundation, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Huntington Study Group Unified Huntington's Disease Rating Scale: reliability and consistency. Mov. Disord. 1996;11:136–142. doi: 10.1002/mds.870110204. [DOI] [PubMed] [Google Scholar]
- 2.Andreasen NC, Rajarethinam R, Cizadlo T, Arndt S, Swayze VW, 2nd, Flashman LA, O'Leary DS, Ehrhardt JC, Yuh WT. Automatic atlas-based volume estimation of human brain regions from MR images. J. Comput. Assist. Tomogr. 1996;20:98–106. doi: 10.1097/00004728-199601000-00018. [DOI] [PubMed] [Google Scholar]
- 3.Aylward EH. Change in MRI striatal volumes as a biomarker in preclinical Huntington's disease. Brain Res. Bull. 2007;72:152–158. doi: 10.1016/j.brainresbull.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 4.Aylward EH, Brandt J, Codori AM, Mangus RS, Barta PE, Harris GJ. Reduced basal ganglia volume associated with the gene for Huntington's disease in asymptomatic at-risk persons. Neurology. 1994;44:823–828. doi: 10.1212/wnl.44.5.823. [DOI] [PubMed] [Google Scholar]
- 5.Aylward EH, Codori AM, Barta PE, Pearlson GD, Harris GJ, Brandt J. Basal ganglia volume and proximity to onset in presymptomatic Huntington disease. Arch. Neurol. 1996;53:1293–1296. doi: 10.1001/archneur.1996.00550120105023. [DOI] [PubMed] [Google Scholar]
- 6.Aylward EH, Codori AM, Rosenblatt A, Sherr M, Brandt J, Stine OC, Barta PE, Pearlson GD, Ross CA. Rate of caudate atrophy in presymptomatic and symptomatic stages of Huntington's disease. Mov. Disord. 2000;15:552–560. doi: 10.1002/1531-8257(200005)15:3<552::AID-MDS1020>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 7.Bartzokis G, Lu PH, Tishler TA, Fong SM, Oluwadara B, Finn JP, Huang D, Bordelon Y, Mintz J, Perlman S. Myelin breakdown and iron changes in Huntington's disease: pathogenesis and treatment implications. Neurochem. Res. 2007;32:1655–1664. doi: 10.1007/s11064-007-9352-7. [DOI] [PubMed] [Google Scholar]
- 8.Bartzokis G, Lu PH, Tishler TA, Perlman S. In vivo assessment of iron in Huntington's disease and other age-related degenerative brain diseases. In: Sigel A, Sigel H, Sigel RKO, editors. Metal Ions in life sciences. Wiley; Chichester: 2006. pp. 151–177. [Google Scholar]
- 9.Beglinger LJ, Nopoulos PC, Jorge RE, Langbehn DR, Mikos AE, Moser DJ, Duff K, Robinson RG, Paulsen JS. White matter volume and cognitive dysfunction in early Huntington's disease. Cogn. Behav. Neurol. 2005;18:102–107. doi: 10.1097/01.wnn.0000152205.79033.73. [DOI] [PubMed] [Google Scholar]
- 10.Feigin A, Tang C, Ma Y, Mattis P, Zgaljardic D, Guttman M, Paulsen JS, Dhawan V, Eidelberg D. Thalamic metabolism and symptom onset in preclinical Huntington's disease. Brain. 2007;130:2858–2867. doi: 10.1093/brain/awm217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomez-Tortosa E, MacDonald ME, Friend JC, Taylor SA, Weiler LJ, Cupples LA, Srinidhi J, Gusella JF, Bird ED, Vonsattel JP, Myers RH. Quantitative neuropathological changes in presymptomatic Huntington's disease. Ann. Neurol. 2001;49:29–34. [PubMed] [Google Scholar]
- 12.Gusella JF, MacDonald ME. Trinucleotide instability: a repeating theme in human inherited disorders. Annu. Rev. Med. 1996;47:201–209. doi: 10.1146/annurev.med.47.1.201. [DOI] [PubMed] [Google Scholar]
- 13.Harris G, Andreasen NC, Cizadlo T, Bailey JM, Bockholt HJ, Magnotta VA, Arndt S. Improving tissue classification in MRI: a three-dimensional multispectral discriminant analysis method with automated training class selection. J. Comput. Assist. Tomogr. 1999;23:144–154. doi: 10.1097/00004728-199901000-00030. [DOI] [PubMed] [Google Scholar]
- 14.Huntington's Disease Collaborative Research Group A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. Cell. 1993;72:971–983. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- 15.Jernigan TL, Salmon DP, Butters N, Hesselink JR. Cerebral Structure on MRI, part II: Specific Changes in Alzheimers and Huntingtons Diseases. Biol. Psychiatry. 1991;29:68–81. doi: 10.1016/0006-3223(91)90211-4. [DOI] [PubMed] [Google Scholar]
- 16.Kassubek J, Bernhard Landwehrmeyer G, Ecker D, Juengling FD, Muche R, Schuller S, Weindl A, Peinemann A. Global cerebral atrophy in early stages of Huntington's disease: quantitative MRI study. Neuroreport. 2004;15:363–365. doi: 10.1097/00001756-200402090-00030. [DOI] [PubMed] [Google Scholar]
- 17.Kassubek J, Gaus W, Landwehrmeyer GB. Evidence for more widespread cerebral pathology in early HD: an MRI-based morphometric analysis. Neurology. 2004;62:523–524. doi: 10.1212/wnl.62.3.523-a. author reply 524. [DOI] [PubMed] [Google Scholar]
- 18.Kassubek J, Juengling FD, Kioschies T, Henkel K, Karitzky J, Kramer B, Ecker D, Andrich J, Saft C, Kraus P, Aschoff AJ, Ludolph AC, Landwehrmeyer GB. Topography of cerebral atrophy in early Huntington's disease: a voxel based morphometric MRI study. J. Neurol. Neurosurg. Psychiatry. 2004;75:213–220. [PMC free article] [PubMed] [Google Scholar]
- 19.Kipps CM, Duggins AJ, Mahant N, Gomes L, Ashburner J, McCusker EA. Progression of structural neuropathology in preclinical Huntington's disease: a tensor based morphometry study. J. Neurol. Neurosurg. Psychiatry. 2005;76:650–655. doi: 10.1136/jnnp.2004.047993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Langbehn DR, Brinkman RR, Falush D, Paulsen JS, Hayden MR. A new model for prediction of the age of onset and penetrance for Huntington's disease based on CAG length. Clin. Genet. 2004;65:267–277. doi: 10.1111/j.1399-0004.2004.00241.x. [DOI] [PubMed] [Google Scholar]
- 21.Langbehn DR, Hayden MR, Paulsen JS. CAG-repeat length and the age of onset in Huntington disease (HD): A review and validation study of statistical approaches. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2009 doi: 10.1002/ajmg.b.30992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lemiere J, Decruyenaere M, Evers-Kiebooms G, Vandenbussche E, Dom R. Longitudinal study evaluating neuropsychological changes in so-called asymptomatic carriers of the Huntington's disease mutation after 1 year. Acta. Neurol. Scand. 2002;106:131–141. doi: 10.1034/j.1600-0404.2002.01192.x. [DOI] [PubMed] [Google Scholar]
- 23.Magnotta VA, Harris G, Andreasen NC, O'Leary DS, Yuh WT, Heckel D. Structural MR image processing using the BRAINS2 toolbox. Comput. Med. Imaging Graph. 2002;26:251–264. doi: 10.1016/s0895-6111(02)00011-3. [DOI] [PubMed] [Google Scholar]
- 24.Muhlau M, Weindl A, Wohlschlager AM, Gaser C, Stadtler M, Valet M, Zimmer C, Kassubek J, Peinemann A. Voxel-based morphometry indicates relative preservation of the limbic prefrontal cortex in early Huntington disease. J. Neural. Transm. 2007;114:367–372. doi: 10.1007/s00702-006-0571-x. [DOI] [PubMed] [Google Scholar]
- 25.Myers RH, Sax DS, Koroshetz WJ, Mastromauro C, Cupples LA, Kiely DK, Pettengill FK, Bird ED. Factors associated with slow progression in Huntington's disease. Arch. Neurol. 1991;48:800–804. doi: 10.1001/archneur.1991.00530200036015. [DOI] [PubMed] [Google Scholar]
- 26.Nopoulos P, Magnotta VA, Mikos A, Paulson H, Andreasen NC, Paulsen JS. Morphology of the cerebral cortex in preclinical Huntington's disease. Am. J. Psychiatry. 2007;164:1428–1434. doi: 10.1176/appi.ajp.2007.06081266. [DOI] [PubMed] [Google Scholar]
- 27.Paulsen JS, Hayden M, Stout JC, Langbehn DR, Aylward E, Ross CA, Guttman M, Nance M, Kieburtz K, Oakes D, Shoulson I, Kayson E, Johnson S, Penziner E. Preparing for preventive clinical trials: the Predict-HD study. Arch. Neurol. 2006;63:883–890. doi: 10.1001/archneur.63.6.883. [DOI] [PubMed] [Google Scholar]
- 28.Paulsen JS, Langbehn DR, Stout JC, Aylward E, Ross CA, Nance M, Guttman M, Johnson S, MacDonald M, Beglinger LJ, Duff K, Kayson E, Biglan K, Shoulson I, Oakes D, Hayden M. Detection of Huntington's disease decades before diagnosis: the Predict-HD study. J. Neurol. Neurosurg. Psychiatry. 2008;79:874–880. doi: 10.1136/jnnp.2007.128728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paulsen JS, Magnotta VA, Mikos AE, Paulson HL, Penziner E, Andreasen NC, Nopoulos PC. Brain structure in preclinical Huntington's disease. Biol. Psychiatry. 2006;59:57–63. doi: 10.1016/j.biopsych.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 30.Powell S, Magnotta VA, Johnson H, Jammalamadaka VK, Pierson R, Andreasen NC. Registration and machine learning-based automated segmentation of subcortical and cerebellar brain structures. Neuroimage. 2008;39:238–247. doi: 10.1016/j.neuroimage.2007.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reading SA, Yassa MA, Bakker A, Dziorny AC, Gourley LM, Yallapragada V, Rosenblatt A, Margolis RL, Aylward EH, Brandt J, Mori S, van Zijl P, Bassett SS, Ross CA. Regional white matter change in pre-symptomatic Huntington's disease: a diffusion tensor imaging study. Psychiatry Res. 2005;140:55–62. doi: 10.1016/j.pscychresns.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 32.Rosas HD, Hevelone ND, Zaleta AK, Greve DN, Salat DH, Fischl B. Regional cortical thinning in preclinical Huntington disease and its relationship to cognition. Neurology. 2005;65:745–747. doi: 10.1212/01.wnl.0000174432.87383.87. [DOI] [PubMed] [Google Scholar]
- 33.Rosas HD, Koroshetz WJ, Chen YI, Skeuse C, Vangel M, Cudkowicz ME, Caplan K, Marek K, Seidman LJ, Makris N, Jenkins BG, Goldstein JM. Evidence for more widespread cerebral pathology in early HD: an MRI-based morphometric analysis. Neurology. 2003;60:1615–1620. doi: 10.1212/01.wnl.0000065888.88988.6e. [DOI] [PubMed] [Google Scholar]
- 34.Rosas HD, Tuch DS, Hevelone ND, Zaleta AK, Vangel M, Hersch SM, Salat DH. Diffusion tensor imaging in presymptomatic and early Huntington's disease: Selective white matter pathology and its relationship to clinical measures. Mov. Disord. 2006;21:1317–1325. doi: 10.1002/mds.20979. [DOI] [PubMed] [Google Scholar]
- 35.Ross CA, Margolis RL, Rosenblatt A, Ranen NG, Becher MW, Aylward EH. Huntington disease and the related disorder, dentatorubral-pallidoluysian atrophy (DRPLA) Medicine. 1997;76:305–338. doi: 10.1097/00005792-199709000-00001. [DOI] [PubMed] [Google Scholar]
- 36.Solomon AC, Stout JC, Johnson SA, Langbehn DR, Aylward EH, Brandt J, Ross CA, Beglinger L, Hayden MR, Kieburtz K, Kayson E, Julian-Baros E, Duff K, Guttman M, Nance M, Oakes D, Shoulson I, Penziner E, Paulsen JS. Verbal episodic memory declines prior to diagnosis in Huntington's disease. Neuropsychologia. 2007;45:1767–1776. doi: 10.1016/j.neuropsychologia.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thieben MJ, Duggins AJ, Good CD, Gomes L, Mahant N, Richards F, McCusker E, Frackowiak RS. The distribution of structural neuropathology in pre-clinical Huntington's disease. Brain. 2002;125:1815–1828. doi: 10.1093/brain/awf179. [DOI] [PubMed] [Google Scholar]
- 38.Vonsattel JP, DiFiglia M. Huntington disease. J. Neuropathol. Exp. Neurol. 1998;57:369–384. doi: 10.1097/00005072-199805000-00001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.