Abstract
The contraction of adult mammalian ventricular cardiomyocytes is triggered by the influx of Ca2+ ions through sarcolemmal L-type Ca2+ channels (LCCs). However, the gating properties of unitary LCCs under physiologic conditions have remained elusive. Towards this end, we investigated the voltage-dependence of the gating kinetics of unitary LCCs, with a physiologic concentration of Ca2+ ions permeating the channel. Unitary LCC currents were recorded with 2 mM external Ca2+ ions (in the absence of LCC agonists), using cell-attached patches on K-depolarized adult rat ventricular myocytes. The voltage-dependence of the peak probability of channel opening (Po vs. Vm) displayed a maximum value of 0.3, a midpoint of -12 mV, and a slope factor of 8.5. The maximum value for Po of the unitary LCC was significantly higher than previously assumed, under physiologic conditions. We also found that the mean open dwell time of the unitary LCC increased two-fold with depolarization, ranging from 0.53 +/- 0.02 msec at -30 mV to 1.08 +/- 0.03 msec at 0 mV. The increase in mean LCC open time with depolarization counterbalanced the decrease in the single LCC current amplitude; the latter due to the decrease in driving force for Ca2+ ion entry. Thus, the average amount of Ca2+ ions entering through an individual LCC opening (∼ 300 to 400 ions) remained relatively constant over this range of potentials. These novel results establish the voltage-dependence of unitary LCC gating kinetics using a physiologic Ca2+ ion concentration. Moreover, they provide insight into local Ca2+-induced Ca2+ release and a more accurate basis for mathematical modeling of excitation-contraction coupling in cardiac myocytes.
Keywords: unitary L-type Ca2+ channels, gating kinetics, excitation-contraction coupling, cardiac myocytes, single Ca2+ currents
Introduction
In adult ventricular cardiomyocytes Ca2+ release from the SR is controlled by the local influx of Ca2+ ions through individual sarcolemmal L-type Ca2+ channels (LCCs) [1]. Much of what is known about the unitary LCC currents has been derived from experiments in which their properties (i.e., conductance and gating) have been significantly altered by a high concentration of divalent ions to increase the signal-to-noise ratio of the patch recording, and/or the addition of a Ca2+ channel agonist to increase channel open time and activity [2-11]. Thus, the unitary properties of LCCs recorded with a physiologic Ca2+ ion concentration have remained largely unknown. A detailed knowledge of the properties of unitary LCCs is necessary not only for an appreciation of the gating mechanisms of LCCs, but also for a basic understanding of how LCC currents trigger Ca2+ release from the sarcoplasmic reticulum [12-16]. We have previously shown that unitary LCC currents can be reliably recorded and analyzed over a range of concentrations of Ca2+ ions, as well as Ba2+ ions, using cell-attached patches on K-depolarized adult rat ventricular myocytes [17-20]. In the present study we focus on the voltage-dependence of the gating kinetics of LCC currents recorded with 2 mM Ca2+ ions, in the absence of LCC agonists. The novel results underscore the importance of studying LCC properties under more physiologic conditions, and may lead to a better understanding of local Ca2+ signaling in heart cells.
Methods
Myocyte Preparation
Ventricular myocytes were isolated from male Sprague-Dawley rats (250 to 300 g; 2 to 3 months old) using a two-step digestion process [see [17] for details] in accordance with NIH guidelines for the care and use of animals.
Single Channel Recording and Analysis
Cell-attached patch clamp recording of unitary L-type Ca2+ channels was performed as previously described [17]. The myocytes were superfused with a high potassium depolarizing solution (HiK) (containing (in mM): 120 K-Aspartate; 25 KCl; 10 HEPES; 10 Glucose; 2 MgCl2; 1 CaCl2; 2 EGTA; 6 KOH, (pH 7.2, 290 mOsm) to depolarize the cells to near 0 mV so that Vm was equal to –Vpatch. The pipette solution contained 2 mM CaCl2, 10 mM CsCl and 5 mM 4-aminopyridine to block K currents, 10 mM Hepes, and TEA-OH to pH 7.4. Currents were recorded with an Axopatch 200B patch clamp and PClamp software via a Digidata 1200A signal acquisition system (Molecular Devices, Sunnyvale, CA, USA). Data was filtered at 2 kHz (-3dB, 4-pole Bessel) and digitized at 10 kHz sampling rate. Voltage steps were applied (at 1/sec) from a holding potential of −50 mV. The protocol was repeated 100 - 200 times. All experiments were performed at room temperature (22.5 to 23.5°C).
The identification of single LCC opening and closing transitions using a 50% amplitude threshold was performed as described in detail previously [17-19]. The presence of a single active Ca2+ channel in a given patch was assessed by the absence of overlapping currents recorded upon depolarization to 0 mV; only single channel patches were used for analysis. Grouped data are reported as means ± standard error (SE). Testing for statistical significance was accomplished using an analysis of variance (ANOVA). Data analysis and non-linear curve fitting were performed using Origin v7.5 (OriginLab Corp,.Northampton, MA, USA)
All chemicals used in the cell isolation procedure were purchased from Mallinckrodt Chemicals Co. (Paris, KY, USA), except for HEPES (ICN Biochemicals Inc., Aurora, OH, USA), MgSO4 (Mallinckrodt Baker Inc., Phillipsburg, NJ, USA), and enzymes [17]. Chemicals used for electrophysiological recordings were purchased from Sigma Chemical Co.,USA, except for Sucrose (ICN) and NaOH (Mallinckrodt). Pentobarbital (Sigma) was dissolved 30 mg/ml in a 10% ethanolic aqueous solution.
Results
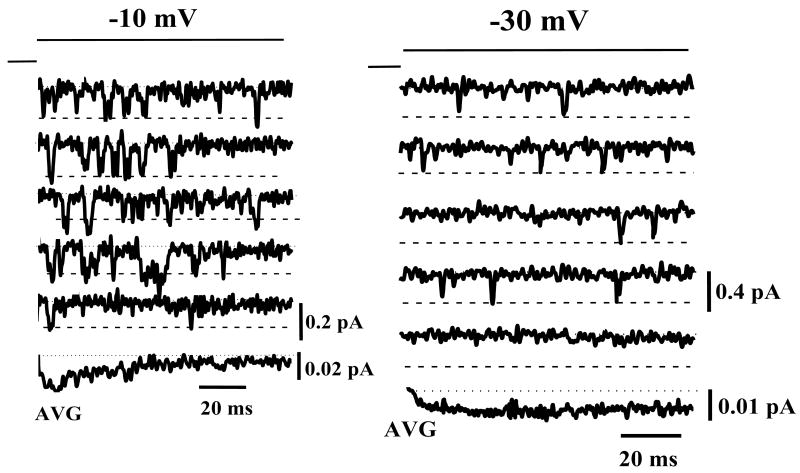
Figure 1 shows representative unitary LCC currents, recorded with a 2 mM external Ca2+ ion concentration, in the absence of channel agonists. [Figure 1 near here] At depolarized potentials (-10 mV, column A) the unitary LCC activates rapidly and channel re-openings are frequent. It is also apparent that LCC openings are usually clustered near the beginning of the voltage step. At more negative potentials (-30 mV, column B) LCC openings are infrequent and randomly interspersed throughout the duration of the voltage step. The ensemble average current at -10 mV (bottom trace) is correspondingly greater that that at -30 mV (despite the smaller single channel amplitude at the depolarized potential) and displays a characteristic early peak followed by a time-dependent decay. In contrast, at -30 mV the ensemble average is nearly time-invariant.
Figure 1. Examples of unitary LCC currents recorded using a 2 mM external Ca2+ concentration.
Representative traces are shown at -10 mV (left column) and at -30 mV (right column), elicited from a holding potential of -50 mV. The bottom trace in each column is the ensemble average current of 200 individual traces. Note the difference in current calibrations for -10 mV and -30 mV.
An assessment of the degree of unitary LCC activation is provided by measurement of the peak probability of opening (Po), as calculated using the ensemble average current. [Figure 2 near here] Figure 2 shows the relationship between peak Po of the unitary LCC (obtained using 2 mM Ca2+) and the test potential (Vm). The data points are the averaged peak Po at each test potential (n = 5 cells). For each experiment the peak Po was calculated from the ensemble average, using the following equation:
Figure 2. The voltage-dependence of unitary L-type Ca2+ channel peak Po, recorded with 2 mM Ca2+.
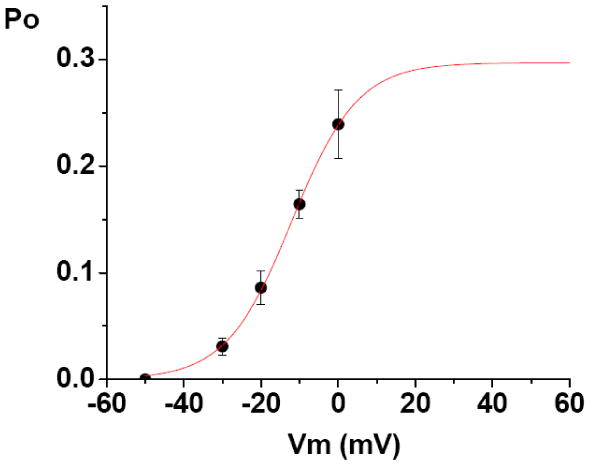
The data points are the averaged peak Po (+/- SE) at each test potential (n = 5 cells). The population means are significantly different at the p< 0.05 level. The Po vs. Vm data was best fit using a Boltzmann equation (red curve), with a maximum value of 0.29 +/- 0.014, midpoint of -12.0 +/- 1.07 mV, and a slope factor of 8.5 +/- 0.5. The Chiˆ2/ (degrees of freedom) of the fit was 0.00001, and the R2 value was 0.99945.
where “I” is the peak ensemble average current; “N” is the number of functional channels in the patch recording; and “i” the unitary current amplitude at the test potential (determined as in Guia, et al., 2001). The Po vs. Vm data was best-fit using a Boltzmann equation, with a maximum value of 0.3, midpoint of -12 mV, and a slope factor of 8.5. The slope factor of the Boltzmann fit can be used to calculate the number of gating charges of channel activation (zg), as follows:
The value obtained for the slope factor indicates that approximately 3 electron charges are transferred across the membrane electric field during channel activation. The present values for the half-activation voltage and slope are comparable to those previously reported for the activation of the macroscopic LCC current [9]. The consequences of the value for maximum Po will be addressed in the Discussion.
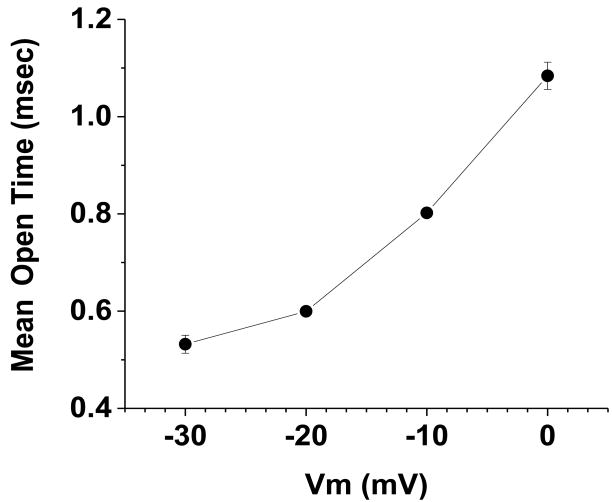
We next examined the average open time of the LCC, as there is scant previous data available for this parameter, recorded using 2 mM Ca2+ ions. The voltage-dependence of the mean open time (MOT) of the LCC, is shown in Figure 3. [Figure 3 near here] The data points are the mean open time at each test potential (n = 5 cells). As can be seen, the relationship between MOT and Vm is voltage-dependent over the range of potentials from -30 mV to 0 mV. The average LCC open time at 0 mV (1.08 +/- 0.03 msec) is two-fold greater than at -30 mV (0.53 +/- 0.02 msec). The increase in LCC open time with depolarization offset the decrease in the single LCC current amplitude; the latter due to the decrease in driving force for Ca2+ ion entry. Thus, the average number of Ca2+ ions entering through an individual LCC opening (∼ 300 to 400 ions) remains relatively constant over the range of potentials from -30 through 0 mV.
Figure 3. The voltage-dependence of the mean open time (MOT) of the LCC, recorded using 2 mM Ca2+ ions.
The data points are the average open time (+/- SE), during the initial 100 msec at each test potential (n = 5 cells). The population means are significantly different at the p< 0.005 level. The total number of events analyzed was 45,019. The standard error bars for the data points at -20 mV and -10 mV are not visible as they are smaller than the size of the symbol.
Discussion
A detailed knowledge of the properties of unitary LCC currents, recorded with a physiologic Ca2+ concentration is essential not only for an understanding of the basic mechanisms of gating and permeation, but also for accurate parameterization of computational models of LCCs and the role of LCCs in local control of Ca2+- induced Ca2+ release in heart cells [21]. The present results, which revise our understanding of the voltage-dependent kinetics of unitary LCCs using a physiologic concentration of permeating Ca2+ ions, provides further evidence that LCC gating [18], as well as conductance [17,19] are significantly altered by the concentration of the permeant ion.
Although of critical importance in understanding cardiac E-C coupling, the unitary properties of LCCs using a physiologic Ca2+ ion concentration have been unclear. The scarcity of physiologic LCC data is related to the technical difficulties in recording and analyzing LCC currents with an external concentration of 2 mM Ca2+ ions, as the unitary currents are extremely small in amplitude (a few tenths of a picoampere). However, we have previously reported that the unitary cardiac LCC (slope) conductance, recorded using 2 mM Ca ions (in the absence of LCC agonists) is 3.0 pS [17]. That value compares closely with the LCC conductance in chick ciliary ganglion neurons (2.6 pS, recorded with a physiologic Ca2+ ion concentration [36]), and with CaV2.2 channels in chick dorsal root ganglion neurons (2.5 pS; [37]).
To circumvent the signal-to-noise problem, the majority of studies of single LCC properties have utilized a high concentration of divalent cations as the charge carrier (typically 70 mM to 110 mM barium ions), and/or the addition of a LCC agonist in order to increase the unitary current amplitude and duration [2-6, 25, 26]. However, elevated divalent concentrations and/or LCC agonists (e.g., BAYK8644 or FPL64176) produce marked changes in the voltage-dependence, kinetics and conductance of the LCC currents [9] so that the physiologic properties of the LCCs are obscured, under those conditions. In a few previous studies LCC kinetics were analyzed using an elevated level of Ca2+ ions as the charge carrier (10 mM – 160 mM), and/or using an LCC agonist [8, 22-24].
In the present study we found that the peak probability of opening (Po), a key unitary LCC parameter that had not previously been measured with a physiologic Ca2+ ion concentration, reached a maximum value of 0.3 at a potential of ∼ +30 mV. That value is significantly higher than reported in previous studies (e.g., 0.03 from [24], using 10 mM Ca2+). Nevertheless, a maximal unitary Po of 0.3 suggests that the combined activity of multiple unitary LCCs (co-localized at the dyadic junction) is necessary to ensure that Ca2+ sparks are triggered at depolarized potentials [27].
In many previous studies unitary Po has been reported using one of the following methods: 1.) with high external Ba2+ ions substituting for Ca2+ ions; 2.) by assuming that Po reaches 1.0 at positive potentials; or 3.) by defining Po as the probability that the channel opens at least once at a given potential (the latter metric is more commonly referred to as channel “availability”). In other LCC studies unitary Po has been calculated as the total open time divided by total recording time during a given step. That calculation yields the average Po over the entire duration of the voltage step (not the peak Po) and is related to the duration of the voltage step used. Thus, when recording with Ca2+ ions, rapid inactivation of the LCC insures that the use of this procedure will result in low values for Po. Clearly, the peak Po (obtained form the ensemble-average as in the present study) provides a more accurate assessment of LCC activity, and is more useful in modeling LCC kinetics and the role of the LCC in triggering Ca2+ release from the SR.
It is widely accepted that the unitary LCC currents locally control the opening of a cluster of neighboring ryanodine receptors (RyRs), thereby allowing the release of Ca2+ ions from the SR, detected as Ca2+ sparks [12-16]. It is also generally held that the probability of generating a Ca2+ spark (Pspark) is related to the amplitude of the single LCC current (i) and to the probability of opening of the LCC (Po) [13,14, 29]. In a recent modeling study [30] an estimate of the Po of the LCC (5-15%) was derived from the findings of 3 previous experimental studies: Rose, et al., 1992; Herzig, et al., 1993 and Handrock, et al., 1998. However, only Rose et al (1992) was conducted using Ca ions as the charge carrier (albeit an elevated concentration of 10 mM); the other 2 papers used 70 mM Ba ions. Thus, the closest comparison with the present study is with Rose, et al. (1992), where the peak ensemble open probability was ∼ 0.03. That value is an order of magnitude lower than the present findings. The present study thus provides compelling evidence that this parameter needs to be re-addressed in future models of local Ca2+-induced Ca2+ release.
Another basic parameter of the unitary LCC current that had not been previously measured with a physiologic Ca2+ concentration is the open dwell time. The open time of the LCC is a major determinant of the probability of opening of the LCC during depolarization, and of the local Ca2+ influx and resulting elevation in the free Ca2+ ion concentration in the dyadic junctional space. In previous studies analyzing LCC-RyR communication it has been assumed that the physiologic mean LCC open time is brief (∼ 0.2 msec) and voltage-independent [14, 30-33]. The present finding of a significant increase of the LCC open time with depolarization may well have important ramifications for the modeling of local Ca2+ signaling [34, 35].
The present unitary LCC data also shed insight into the explanation of macroscopic gain, under physiologic conditions. Importantly, these findings demonstrate that the increase in mean open time with depolarization counterbalances the corresponding decrease in unitary LCC current amplitude (Guia, et al., 2001), so that, on average, Ca2+ ion influx per opening is relatively constant across a range of potentials. However, it has been proposed that the single LCC current amplitude (iCa) plays a more important role than single channel open time in triggering sparks [14]. Yet the local Ca2+ transient produced by the LCC opening is critically dependent on local buffering, a parameter that can only be estimated, at present. Thus, with strong depolarization longer-duration openings may result in larger than expected local Ca2+ transients (or sparklets), at least under certain buffer conditions. Therefore, both iCa and open time of the LCC are critical parameters in determining the voltage-dependence of activation of RyRs in a couplon. Notwithstanding, the main reason for the decline in the macroscopic gain curve with depolarization is the voltage-dependent increase in Po of the LCC, a significant part of which derives from the increase in mean open time.
Although the present study is a significant step towards a more physiologic understanding of LCC behavior, several limitations must be acknowledged. First, the present measurements were done at room temperature, which has the advantage of slowing the gating kinetics and thereby improving single channel resolution. Secondly, the measurements presumably sampled only surface sarcolemmal LCCs, not the predominately T-tubular population of LCCs which are largely responsible for E-C coupling. However, at present there is no evidence available that would lead us to speculate that the kinetic properties of those two LCC populations are different.
Increases in unitary LCC open time and Po are physiologic mechanisms for up-regulation of ICa by beta-adrenergic receptor stimulation [7, 11]. Therefore, the present results also provide a strong motivation for re-examining the effects of those regulatory agents on unitary LCC kinetics using a physiologic Ca2+ concentration. Moreover, a significant remodeling in unitary LCC kinetics has been demonstrated in failing human ventricular myocytes [10], which may contribute to the contractile dysfunction. Thus, it will be critical to investigate unitary LCC behavior in the failing heart under more physiologic conditions to better understand, and more accurately model, the altered LCC-RyR communication.
Acknowledgments
This work was supported by intramural grants from the National Institute on Aging (EGL and MDS) and grants from the National Heart Lung and Blood Institute (WJL and EAS). Dr. Josephson was a recipient of a National Research Council Senior Research Associate Award. The author thanks Dr. Christian Soeller for many helpful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bers DM. Cardiac E-C Coupling. Kluwer Publishers; the Netherlands: 2001. Chapter 8. Excitation-contraction coupling and cardiac contractile force. [Google Scholar]
- 2.Reuter H, Stevens CF, Tsien RW, Yellen G. Properties of single calcium channels in cardiac cell culture. Nature. 1982;297:501–504. doi: 10.1038/297501a0. [DOI] [PubMed] [Google Scholar]
- 3.Cavalié A, Ochi R, Pelzer D, Trautwein W. Elementary currents through Ca2+ channels in guinea pig myocytes. Pflügers Arch. 1983;398:284–297. doi: 10.1007/BF00657238. [DOI] [PubMed] [Google Scholar]
- 4.Hess P, Lansman JB, Tsien TW. Calcium channel selectivity for divalent and monovalent cations: Voltage and concentration dependence of single channel current in ventricular heart cells. J Gen Physiol. 1986;88:293–319. doi: 10.1085/jgp.88.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caffrey JM, Josephson IR, Brown AM. Calcium channels of Amphibian stomach and mammalian aorta smooth muscle cells. Biophys J. 1986;49:1237–1242. doi: 10.1016/S0006-3495(86)83753-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McDonald TF, Cavalié A, Trautwein W, Pelzer D. Voltage-dependent properties of macroscopic and elementary calcium channel currents in guinea pig ventricular myocytes. Pflugers Arch. 1986;406:437–448. doi: 10.1007/BF00583365. [DOI] [PubMed] [Google Scholar]
- 7.Yue DT, Herzig S, Marban E. Beta-adrenergic stimulation of calcium channels occurs by potentiation of high-activity gating modes. Proc Natl Acad Sci U S A. 1990 Jan;87(2):753–7. doi: 10.1073/pnas.87.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Imredy J, Yue D. Mechanism of Ca2+-sensitive inactivation of L-type Ca2+ channels. Neuron. 1994;12:1301–1318. doi: 10.1016/0896-6273(94)90446-4. [DOI] [PubMed] [Google Scholar]
- 9.McDonald T, Pelzer S, Trautwein W, Pelzer D. Regulation and modulation of calcium channels in cardiac, skeletal, and smooth muscle cells. Physiol Rev. 1994;74:365–507. doi: 10.1152/physrev.1994.74.2.365. [DOI] [PubMed] [Google Scholar]
- 10.Schröder F, Handrock R, Beuckelmann DJ, Hirt S, Hullin R, Priebe L, Schwinger RH, Weil J, Herzig S. Increased availability and open probability of single L-type calcium channels from failing compared with nonfailing human ventricle. Circulation. 1998 Sep 8;98(10):969–76. doi: 10.1161/01.cir.98.10.969. [DOI] [PubMed] [Google Scholar]
- 11.Schröder F, Herzig S. Effects of β2 -adrenergic stimulation on single-channel gating of rat cardiac L-type Ca2+ channels. Am J Physiol. 1999;276:H834–H843. doi: 10.1152/ajpheart.1999.276.3.H834. [DOI] [PubMed] [Google Scholar]
- 12.Cannell MB, Cheng H, Lederer WJ. The control of calcium release in heart muscle. Science. 1995;268:1045–1050. doi: 10.1126/science.7754384. [DOI] [PubMed] [Google Scholar]
- 13.Lopez-Lopez JR, Shacklock PS, Balke CW, Wier WG. Local calcium transients triggered by single L-type calcium channel currents in cardiac cells. Science. 1995;268:1042–1045. doi: 10.1126/science.7754383. [DOI] [PubMed] [Google Scholar]
- 14.Santana LF, Cheng H, Gomez AM, Cannell MB, Lederer WJ. Relation between the sarcolemmal Ca2+ current and Ca2+ sparks and local control theories for cardiac excitation-contraction coupling. Circ Res. 1996;78:166–171. doi: 10.1161/01.res.78.1.166. [DOI] [PubMed] [Google Scholar]
- 15.Shorofsky SR, Izu L, Wier WG, Balke CW. Ca2+ sparks triggered by patch depolarization in rat heart cells. Circ Res. 1998;82:424–429. doi: 10.1161/01.res.82.4.424. [DOI] [PubMed] [Google Scholar]
- 16.Wang SQ, Song LS, Lakatta EG, Cheng H. Ca2+ signalling between single L-type Ca2+ channels and ryanodine receptors in heart cells. Nature. 2001;410(6828):592–6. doi: 10.1038/35069083. [DOI] [PubMed] [Google Scholar]
- 17.Guia A, Stern MD, Lakatta EG, Josephson IR. Ion concentration-dependence of rat cardiac unitary L-type calcium channel conductance. Biophys J. 2001;80:2742–2750. doi: 10.1016/S0006-3495(01)76242-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Josephson IR, Guia A, Lakatta EG, Stern MD. Modulation of the gating of unitary cardiac L-type Ca2+ channels by conditioning voltage and divalent ions. Biophys J. 2002a;83:2575–2586. doi: 10.1016/S0006-3495(02)75268-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Josephson IR, Guia A, Lakatta EG, Stern MD. Modulation of the conductance of unitary cardiac L-type Ca2+ channels by conditioning voltage and divalent ions. Biophys J. 2002b;83:2587–2594. doi: 10.1016/S0006-3495(02)75269-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Josephson IR, Guia A, Lakatta E, Lederer WJ, Stern M. Ca2+ - dependent components of inactivation of unitary cardiac L-type Ca channels. J Physiol. 2010;558(1):213–223. doi: 10.1113/jphysiol.2009.178343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stern MD. Theory of local control of cardiac excitation-contraction coupling. Biophys J. 1992;63(2):497–517. doi: 10.1016/S0006-3495(92)81615-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yue DT, Backx PH, Imready JP. Calcium-sensitive inactivation in the gating of single calcium channels. Science. 1990;250:1735–1738. doi: 10.1126/science.2176745. [DOI] [PubMed] [Google Scholar]
- 23.Mazzanti M, DeFelice LJ. Ca2+ channel gating during cardiac action potentials. Biophys J. 1990;58:1059–1065. doi: 10.1016/S0006-3495(90)82448-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rose WC, Balke CW, Wier WG, Marban E. Macroscopic and unitary properties of physiological ion flux through L-type Ca2+ channels in guinea-pig heart cells. J Physiol. 1992;456:267–284. doi: 10.1113/jphysiol.1992.sp019336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kokubun S, Reuter H. Dihydropyridine derivatives prolong the open state of Ca2+ channels in cultured cardiac cells. Proc Natl Acad Sci USA. 1984;81:4824–4827. doi: 10.1073/pnas.81.15.4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kunze DL, Rampe D. Characterization of the effects of a new Ca2+ channel activator, FPL 64176, in GH3 cells. Mol Pharmacol. 1992 Oct;42(4):666–70. [PubMed] [Google Scholar]
- 27.Inoue M, Bridge JH. Ca2+ sparks in rabbit ventricular myocytes evoked by action potentials: involvement of clusters of L-type Ca2+ channels. Circ Res. 2003 Mar 21;92(5):532–8. doi: 10.1161/01.RES.0000064175.70693.EC. [DOI] [PubMed] [Google Scholar]
- 28.Schröder F, Handrock R, Beuckelmann DJ, Hirt S, Hullin R, Priebe L, Schwinger RH, Weil J, Herzig S. Increased availability and open probability of single L-type calcium channels from failing compared with nonfailing human ventricle. Circulation. 1998 Sep 8;98(10):969–76. doi: 10.1161/01.cir.98.10.969. [DOI] [PubMed] [Google Scholar]
- 29.Cheng H, Wang SQ. Calcium signaling between sarcolemmal calcium channels and ryanodine receptors in heart cells. Frontiers in Bioscience. 2002;7:d1867–1878. doi: 10.2741/A885. [DOI] [PubMed] [Google Scholar]
- 30.Greenstein JL, Hinch R, Winslow RL. Mechanisms of excitation-contraction coupling in an integrative model of the cardiac ventricular myocyte. Biophys J. 2006 Jan 1;90(1):77–91. doi: 10.1529/biophysj.105.065169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stern MD, Song LS, Cheng H, Sham JS, Yang HT, Boheler KR, Ríos E. Local control models of cardiac excitation-contraction coupling. A possible role for allosteric interactions between ryanodine receptors. J Gen Physiol. 1999 Mar;113(3):469–89. doi: 10.1085/jgp.113.3.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rice JJ, Jafri MS, Winslow RL. Modeling gain and gradedness of Ca2+ release in the functional unit of the cardiac diadic space. Biophys J. 1999;77:1871–1884. doi: 10.1016/s0006-3495(99)77030-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greenstein JL, Winslow RL. An integrative model of the cardiac ventricular myocyte incorporating local control of Ca2+ release. Biophys J. 2002;83:2918–2945. doi: 10.1016/S0006-3495(02)75301-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soeller C, Cannell MB, Soeller C, MB Cannell. Cannell. Numerical simulation of local calcium movements during L-type calcium channel gating in the cardiac diad. Biophys J. 1997;73:97–111. doi: 10.1016/S0006-3495(97)78051-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soeller C, Cannell MB. Analysing cardiac excitation-contraction coupling with mathematical models of local control. Prog Biophys Mol Biol. 2004 Jun-Jul;85(2-3):141–62. doi: 10.1016/j.pbiomolbio.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 36.Church PJ, Stanley EF. Single L-type calcium channel conductance with physiological levels of calcium in chick ciliary ganglion neurons. J Physiol. 1996;496:59–68. doi: 10.1113/jphysiol.1996.sp021665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weber AM, Stanley EF. Single channel conductance of CaV2.2 at physiological [Ca2+] Biophys J. 2009;96(3) 1:222a. [Google Scholar]