Abstract
Objective
To assess whether use of folic acid vitamin supplements reduces cardiac and stroke mortality in hemodialysis patients. Further, we examined whether consumption of folic acid from vitamin supplements greater than 1000 μg compared to standard 1000 μg, and 1000 μg compared to either lower dose or no consumption were associated with reduced cardiac and stroke mortality risk.
Design
Secondary analysis of data from the Hemodialysis (HEMO) Study, a randomized clinical trial examining dialysis treatment regimens over three years follow-up. Participants: One thousand eight hundred and forty-six hemodialysis patients previously participating in the HEMO study.
Interventions
None
Main Outcome Measure
Cardiac and stroke mortality.
Results
From time-dependent Cox proportional hazard regression models, folic acid consumption from vitamin supplements, above or below the standard 1000 μg dose was not associated with decrease or increase in cardiac mortality (P = 0.53 above vs. standard dose and P = 0.46, below vs. standard dose). There was also no association between folic acid consumption and mortality from stroke (P = 0.27, above vs. standard dose and P = 0.64, below vs. standard dose).
Conclusion
Consumption of higher than the standard 1000 μg prescribed dose of folic acid was not beneficial in reducing cardiac or stroke mortality in hemodialysis patients. Similarly, consumption of lower than standard dose was not associated with an increase in either cardiac or stroke mortality.
Keywords: Folic acid, cardiac, stroke, mortality, hemodialysis
Introduction
Mortality rates due to cardiovascular disease (CVD) are approximately 15 times higher among patients with chronic kidney disease (CKD) than in the general population 1-3 CVD accounts for almost half of all deaths in hemodialysis patients 1-3. Reducing cardiac risk factors for hemodialysis patients is therefore of major importance for them. In CKD, risk factors for CVD morbidity and mortality in CKD include unmodifiable risk factors such as older age, white race, male gender, as well as modifiable risk factors such as higher levels of low density lipoprotein and lower levels of high density lipoproteins, dyslipidemias including elevated serum lipoprotein(a) and apolipoprotein(a) isoforms and lipoprotein remnants, hypertension, diabetes mellitus, inflammation, infection, and anemia and other hemodynamic and metabolic abnormalities 3. Elevated circulating levels of homocysteine (a sulfur containing amino acid that is a vascular toxin) is also an established risk factor for CVD in CKD patients 3.
More than 90% of patients with CKD have mild to moderately elevated levels of homocysteine (in the range of 20-80 umol/L) 4-10. The precise mechanisms for these elevated levels in CKD are not clear but include deficiencies of folic acid, vitamins B6 and B12 due to low intake, loss of these vitamins during hemodialysis, disorders in their metabolism, and the presence of uremic toxins 8-10. Administration of folic acid, vitamins B6 and B12 reduces serum homocysteine levels in patients with normal renal function, but the effects on mortality due to CVD are variable 11-24. High doses of folic acid (5-60 mg/day), vitamin B6 (up to 100 mg/day), and vitamin B12 (up to 1 mg/day) can reduce homocysteine levels in CKD patients, including those on hemodialysis. While homocysteine levels can be reduced by 30-50%, there is little benefit of folic acid doses greater than 15 mg/d and less than 10% of them achieve levels in the normal range 25-35.
Since the 4 existing randomized trials of the effects of folic acid on CVD mortality in patients with CKD have been inconclusive and few hemodialysis patients were included in these CKD studies, we conducted a secondary analysis using data from the Hemodialysis (HEMO) Study to assess the relationship between the use of folic acid containing vitamin supplements and cardiac and stroke mortality in patients on hemodialysis 36-39. The HEMO study was a large randomized clinical trial sponsored by the National Institutes of Health (NIH), conducted from 1995 through 2001 40. First, we assessed the association between use of folic acid vitamin supplements and cardiac and stroke mortality. Second, we investigated whether higher or lower doses of folic acid compared to the standard 1000 μg dose recommended clinically were associated with lower cardiac and stroke mortality 41-42.
Methods
HEMO Study Design and Population
The design, methods, and primary outcomes of the HEMO Study are described elsewhere 40. This prospective, multi-center, randomized clinical trial, which was sponsored by the National Institutes of Health’s National Institute of Diabetes, Digestive and Kidney Disease, compared effects of two dialysis doses and two dialysis membrane fluxes on morbidity and mortality in hemodialysis patients. Between 1995 and 2001, 1846 CKD patients of all races between the ages of 18-80 who underwent thrice weekly in-center maintenance hemodialysis therapy at 15 clinical center sites across the country participated in the trial. The mean years of follow-up were 2.84 years and the annual mortality rate was 16.6% 40.
One thousand eight hundred and forty-six hemodialysis patients were randomized in the HEMO Study. This secondary analysis included 1790 patients. Forty-eight patients were excluded because they died of CVD in the first 6 months of the follow-up period in the study and an additional 8 were excluded due to missing vitamin supplement data forms during baseline and follow-up.
Use of Vitamin and Mineral Supplements in the HEMO Study
After randomization, all study patients were provided with a free high potency, high folic acid (1000 μg) renal formulated B-complex vitamin with vitamin C, “Nephro-Vite Rx” (R&D Laboratories). Patients were encouraged, but were not required, to use the “Nephro-Vite RX” supplement, and some chose not to do so. In addition, patients were permitted to use over-the-counter or other prescribed vitamin and mineral supplements. Reported intakes of “Nephro-Vite RX”, other physician prescribed vitamin and mineral supplements, and others the patients chose to take on their own were ascertained once at baseline and annually thereafter during follow-up by the HEMO study dietitian during patient interviews. The name, dosage and unit (ie. mg, μg, ml), amount, frequency (day, week, month), and form (tablet, liquid, intravenous) of such vitamin and mineral supplements that were prescribed and consumed by the patient were recorded onto a HEMO Study Diet Prescription and Supplement Documentation Form once at baseline and annually thereafter during follow-up. Nutrient values for each supplement were calculated based on the dosage indicated by the physician prescription, as determined by review of patient’s medical chart, and by the patient’s reported consumption.
Intake of Folic Acid from Vitamin Supplements
In this secondary analysis, intakes of folic acid from all vitamin supplements used by each patient were determined using the data derived from the supplement documentation form at baseline and annually thereafter during follow-up. Imputed values calculated for missing folic acid dosages were estimated using a proportions based median imputation method developed for the use in the HEMO Study that determined the proportions and respective medians of the available dosage data and then randomly assigned imputed values to replace the missing dosages.
Cardiac and Stroke Mortality
The methods used for the collection, classification and validation of causes of death in the HEMO study are published in detail elsewhere 40, 43. Briefly, each death during follow-up was classified by the investigator at the clinical center using a standardized classification system devised by the HEMO study and reported to the Data Coordinating Center. The data that form the basis for the death reports included hospital records, ICD-9 codes, physician accounts, death certificates, and death notification forms to the United States Renal Data System. Causes of death were classified into 24 categories, four of which were cardiac causes including ischemic heart disease, congestive heart failure, arrhythmia and conduction problems, and other heart diseases and conditions. Cerebral vascular (stroke) was also classified as a death outcome. Death classifications were then audited by two members of the study’s Outcome Review Committee. Cause of death was adjudicated with the full Outcome Review Committee if reviewers were in disagreement with the initial classification.
Description of Covariates
The potential confounders in the analyses included baseline age, gender, race, intervention effects (dose of dialysis and membrane flux), total serum cholesterol (measured at each individual laboratory of the clinical centers), and serum albumin (pre-dialysis serum concentrations of albumin were measured in a central laboratory [Spectra East, Rockleigh, NJ] using the nephelomtery method). Additionally, baseline scores for the Index of Co-Existing Disease (ICED) and Index of Disease Severity (IDS) were also considered as potential confounders. The ICED Score is an estimate of the patient’s comorbidity status which is calculated using a composite comorbidity coding system that classifies the presence and severity of diseases and the impact of the diseases on physical function 44. Scores of 0, 1, 2, or 3 represent normal, mild, moderate, or severe disease comorbidity, respectively. Comorbid conditions in the ICED Score include ischemic heart disease, congestive heart failure, arrythmias and conduction problems, other heart disease and conditions, hypertension, cerebral vascular disease, peripheral vascular disease, diabetes mellitus, respiratory disease, musculoskeletal and connective tissue disease, nonvascular nervous system disease, gastrointestinal disease, hepatobiliary disease, urinary tract disease, malignancy, ophthalmologic conditions, hematologic conditions, and anticoagulation therapy. Scores were assigned based on review of comorbidity from the patient’s medical record by a trained data abstractor. IDS scores were the single components of the ICED scores and followed the same scoring system as the ICED 44. The IDS components included congestive heart failure, arrhythmias and conduction problems, cerebral and peripheral vascular disease, ischemic and other heart disease, respiratory disease, nonvascular nervous system disease, gastrointestinal and hepatobiliary disease, and malignancy.
Statistical Analysis
Descriptive statistics on the demographic and clinical characteristics of the population were carried out and the amount of folic acid consumed from vitamin supplements was determined. A P-value < 0.05 was considered statistically significant. Time-dependent Cox proportional hazard models were used to examine the intake of folic acid from vitamin supplements on cardiac and stroke mortality. The total folic acid consumed from vitamin supplements was presented as a continuous variable (for every 100 μg folic acid). Consumption of folic acid from vitamin supplements was also examined categorically; <1000 μg (0-999 μg), 1000 μg (the most commonly prescribed amount which was used as the reference group) and > 1000 μg. For the category of < 1000 μg folic acid, those with no use of folic acid supplements (0 μg) were combined with those who did use folic acid supplements but at dosages less than 1000 μg, since the groups with no intake and less than 1000 μg were small during the study follow-up years (e.g. n=109 [8%] for no intake and n=68 [5%] for those with < 1000 μg during follow-up year 1) and would have been insufficient for analytical purposes if they were treated as individual groups. Confounders listed previously, were entered individually in the time-dependent Cox proportional hazard models. These analyses utilized imputed values for missing folic acid dosages. All calculations were performed using the statistical software package SAS version 9.1 (SAS Institute, INC., Cary, NC).
Results
Table 1 presents the baseline clinical and demographic characteristics of the patients without a cardiac or stroke mortality outcome, with cardiac mortality, and with stroke mortality. Patients who died from cardiac causes and stroke tended to be older (P < 0.0001) and to have coexisting conditions of greater severity than those who did not (P < 0.0001). Cardiac related comorbidities were common; those with cardiac and stroke deaths had more ischemic and other heart disease, congestive heart failure, cerebral and peripheral vascular disease, arrhythmias and conduction problems, and conditions of greater severity than those without these causes of death (P < 0.0001).
Table 1.
Baseline characteristics of hemodialysis patients in the HEMO Study
| Characteristics | Cohort without CVD or stroke mortality event (n=1436) | Cohort with CVD mortality event (n=292) | Cohort with stroke mortality event (n=62) | P† |
|---|---|---|---|---|
| Age (mean±SD years) | 56±14 | 63±11 | 61±14 | <0.0001 |
| Female sex (%) | 57 | 56 | 63 | 0.60 |
| Black race (%) | 64 | 56 | 69 | 0.02 |
| Serum albumin (mean±SD gm/dL)* | 3.9±0.3 | 3.8±0.3 | 3.8±0.3 | 0.07 |
| Total cholesterol (mean±SD mg/dL)** | 172±40 | 178±44 | 176±42 | 0.09 |
| Index of Coexisting Disease (ICED) scores | <0.0001 | |||
| none (%) | 0 | 0 | 0 | |
| mild (%) | 39 | 23 | 28 | |
| moderate (%) | 31 | 35 | 24 | |
| severe (%) | 30 | 42 | 48 | |
| Index of Disease Severity (IDS) score | ||||
| Arrhythmia and conduction problems | <0.0001 | |||
| none (%) | 72 | 57 | 53 | |
| mild (%) | 18 | 26 | 32 | |
| moderate (%) | 7 | 13 | 10 | |
| severe (%) | 3 | 4 | 5 | |
| Congestive heart failure | <0.0001 | |||
| none (%) | 64 | 45 | 52 | |
| mild (%) | 26 | 37 | 30 | |
| moderate (%) | 8 | 16 | 16 | |
| severe (%) | 2 | 2 | 2 | |
| Cerebral and peripheral vascular diseases | <0.0001 | |||
| none (%) | 67 | 50 | 52 | |
| mild (%) | 18 | 23 | 24 | |
| moderate (%) | 11 | 15 | 14 | |
| severe (%) | 4 | 12 | 10 | |
| Cerebral vascular disease | <0.0001 | |||
| none (%) | 83 | 71 | 73 | |
| mild (%) | 4 | 8 | 5 | |
| moderate (%) | 12 | 20 | 19 | |
| severe (%) | 1 | 1 | 3 | |
| Ischemic and other heart disease | <0.0001 | |||
| none (%) | 31 | 13 | 19 | |
| mild (%) | 62 | 72 | 68 | |
| moderate (%) | 6 | 14 | 8 | |
| severe (%) | 1 | 1 | 5 | |
| Respiratory disease | 0.06 | |||
| none (%) | 86 | 81 | 76 | |
| mild (%) | 7 | 11 | 14 | |
| moderate (%) | 6 | 7 | 10 | |
| severe (%) | 1 | 1 | 0 | |
| Malignancy (%) | 5 | 8 | 7 | 0.04 |
| Nonvascular nervous system disease | 0.20 | |||
| none (%) | 64 | 58 | 60 | |
| mild (%) | 18 | 21 | 22 | |
| moderate (%) | 18 | 20 | 18 | |
| severe (%) | 0 | 1 | 0 | |
| Gastrointestinal and hepatobiliary disease | 0.18 | |||
| none (%) | 55 | 49 | 42 | |
| mild (%) | 20 | 22 | 24 | |
| moderate (%) | 21 | 24 | 26 | |
| severe (%) | 4 | 5 | 8 | |
| High Kt/V group (%) | 50 | 50 | 44 | 0.60 |
| High flux group (%) | 51 | 46 | 45 | 0.20 |
ANOVA was used to test differences among means for the continuous variables; Fisher’s exact test was used to compare frequency data among groups for ICED and IDS scores for cerebral vascular disease, ischemic and other heart disease, respiratory disease, and nonvascular nervous system disease; Chi-square test was used to compare all other frequency data among groups; P < 0.05 was considered statistically significant
n=1009 for group with no CVD or stroke mortality, n=175 for group with CVD mortality, n=43 for group with stroke mortality
n=1287 for group with no CVD or stroke mortality, n=277 for group with CVD mortality, n=60 for group with stroke mortality
Figure 1 presents the percent of patients consuming <1000 μg, 1000 μg and > 1000 μg of folic acid from supplements at baseline and at follow-up years 1-3. The minimum standard clinical recommendation at the time for supplementation among these hemodialysis patients was 1000 μg folic acid 40-41. Most patients consumed 1000 μg, especially during follow-up, when patients were provided with the “Nephro-Vite RX” supplement containing 1000 μg of folic acid. Baseline mean ± SD folic acid intake from supplements was 814 ± 681 μg compared to 1007 ± 652 μg at follow-up year 1, 1093 ± 1232 μg at follow-up year 2, and 1110 ± 1208 μg during follow-up year 3. Median folic acid intake from supplements was 1000 μg at baseline and all 3 years of follow-up. Among patients who consumed greater than 1000 μg folic acid, the median dose was 2000 μg at baseline and it remained so during follow-up years 1-3. For those who took < 1000 μg folic acid, the median dose was 0 μg for baseline and at follow-up year 1, at follow-up year 2 it was 71 μg and 0 μg at follow-up year 3.
Figure 1.
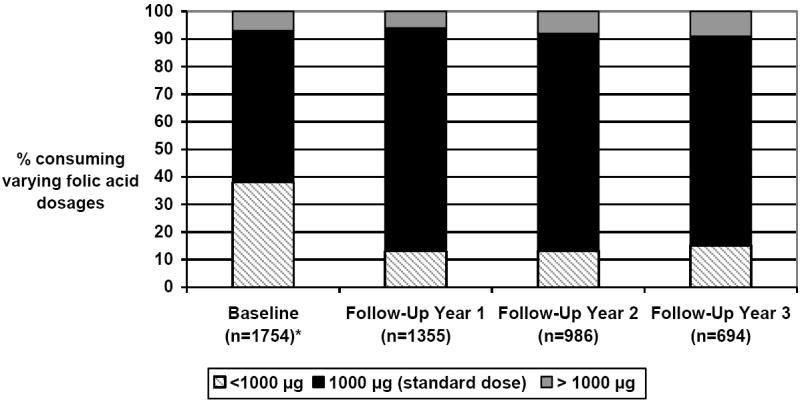
Percent of patients taking <1000 μg, 1000 μg (standard dose) and > 1000 μg of folic acid from vitamin supplements at baseline and follow-up
*n=36 with missing folic acid dosage data
Table 2 presents the results for the time-dependent Cox proportional hazards regression models for folic acid intake from vitamin supplements on cardiac mortality. Neither the continuous nor categorical measures of folic acid consumed had statistically significant effects on cardiac mortality. The findings for cardiac mortality remained non-significant after controlling for confounders. Figure 2 shows the cardiac mortality survival in time-dependent Cox proportional hazards model for consumption of the 3 folic acid supplement groups during the 3 year follow-up period. Those consuming < 1000 μg folic acid had the highest cardiac mortality followed by those consuming 1000 μg, and the > 1000 μg group with the lowest cardiac mortality, although these differences in survival rates were small and not statistically significant.
Table 2.
Time-dependent Cox proportional hazards regression analyses for folic acid consumption and cardiac and stroke mortality*
| Consumed folic acid (continuous) | Consumption of folic acid (categorical) |
||
|---|---|---|---|
| <1000 μg vs. 1000 μg folic acid | >1000 μg vs. 1000 μg folic acid | ||
| Cardiac mortality | |||
| n** | 206 | 47 | 14 |
| Estimate | -0.011 | 0.13 | 0.17 |
| Hazard Ratio (95% CL) | 0.99 (0.97, 1.01) | 1.14 (0.81, 1.62) | 1.18 (0.69, 2.02) |
| P-value | 0.25 | 0.46 | 0.53 |
| Stroke mortality | |||
| n** | 45 | 9 | 5 |
| Estimate | 0.02 | -0.18 | 0.53 |
| Hazard Ratio (95% CL) | 1.02 (1.00, 1.03) | 0.83 (0.38, 1.80) | 1.70 (0.66, 4.39) |
| P-value | 0.06 | 0.64 | 0.27 |
Results presented are for overall models; models remained non-significant after confounders (baseline age, gender, race, dose of dialysis, membrane flux, total serum cholesterol, serum albumin, ICED and IDS) were considered (data not shown)
n=event n
n=1790 patients at baseline
n=4628 patient years
Figure 2.
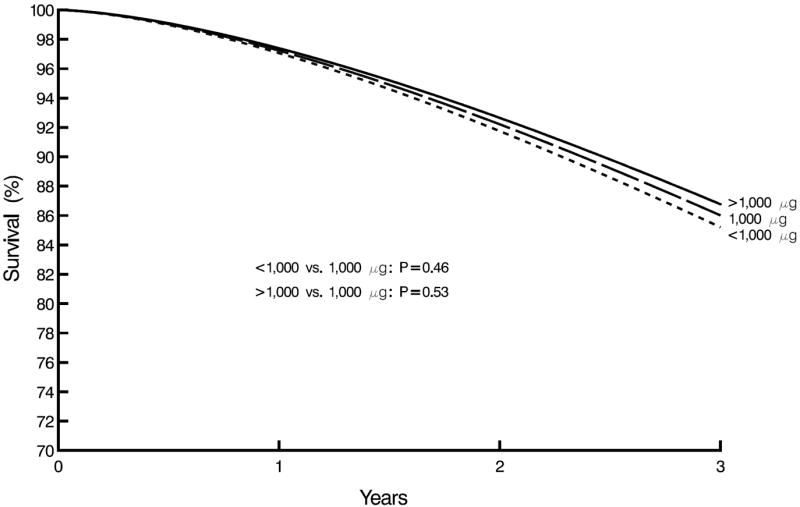
Cardiac survival by time-dependent consumed folic acid vitamins supplement group
For stroke mortality, the association between the categorical measure of folic acid intake and stroke mortality were not statistically significant (see Table 2). The results remained even after adjusting for all confounders. However, higher folic acid intakes from vitamins were associated with a slightly borderline significant increased stroke mortality risk (HR=1.02, P = 0.06) when the continuous measure of folic acid intake was used, as shown in Table 2. That is, for every 100 μg of total folic acid consumed at each annual collection date (baseline and follow-up years 1-3), patients had a 2 % increased risk of stroke mortality (P = 0.06). This association remained even after controlling for virtually all of the confounders (see Figure 3). The association became significant after controlling for ischemic and other heart disease (HR=1.02, P = 0.04). Conversely, after controlling for serum albumin, the association disappeared (HR=1.01, P = 0.28). Figure 4 shows the survival curves for stroke by time-dependent Cox proportional hazards models for consumption of the lower, standard and higher dose supplement groups during the 3 year follow-up period. Although not statistically significant, those consuming > 1000 μg folic acid had the highest stroke mortality followed by those consuming 1000 μg, and the < 1000 μg group had the lowest stroke mortality.
Figure 3.
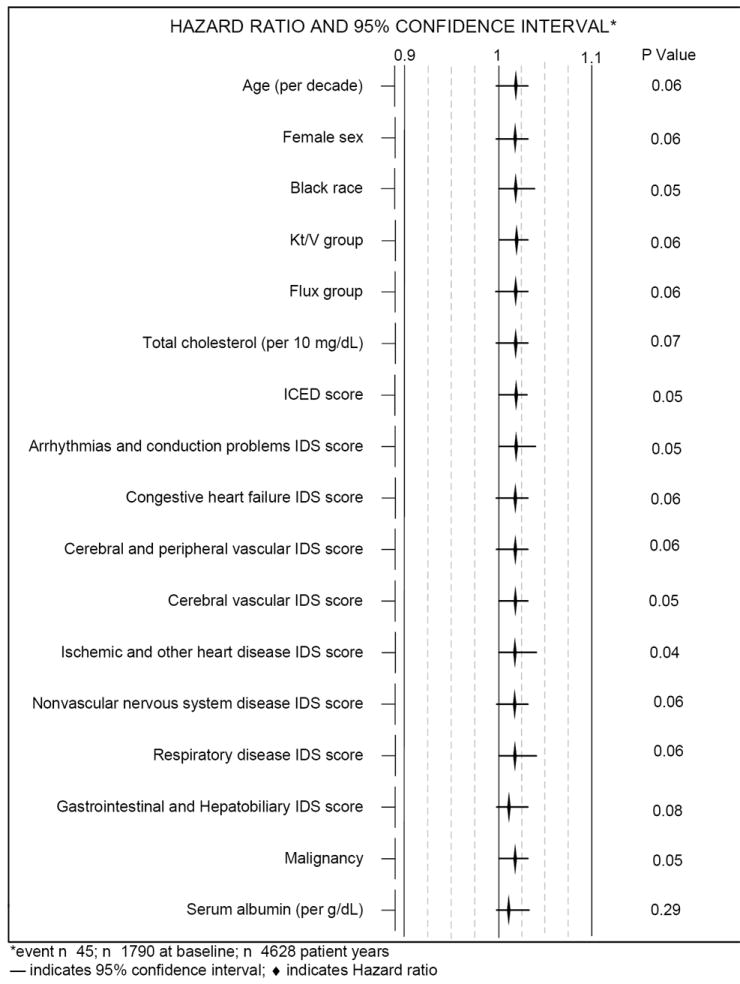
Time-dependent Cox proportional hazards regression analyses for total consumed folic acid from vitamins and stroke mortality controlling for confounders in HEMO study patients
Figure 4.
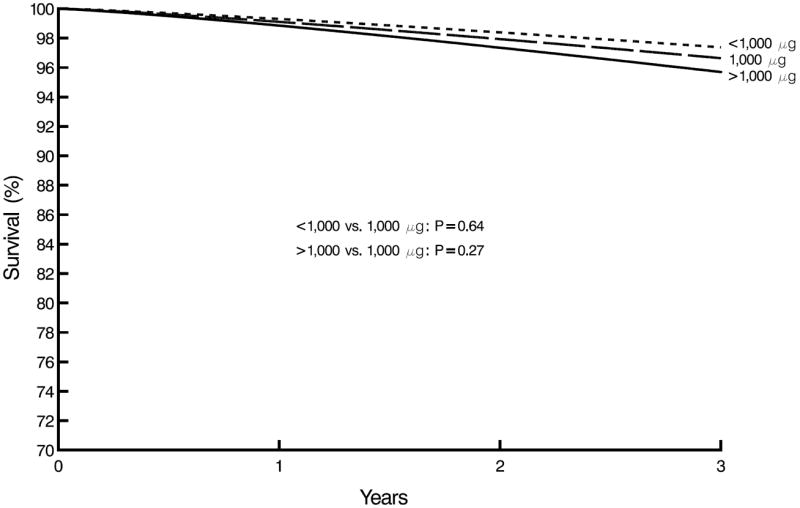
Stroke survival by time-dependent consumed folic acid vitamins supplement group
Discussion
The benefits of high potency renal formulated B-complex vitamins in protecting the nutritional status of hemodialysis patients from losses of B vitamins during dialysis are well known and justify their use 40-41. However, the utility of these vitamins in preventing cardiac or stroke mortality in hemodialysis patients is uncertain. We failed, as have others, to demonstrate that intake of folic acid containing vitamin supplements at the levels used in the HEMO Study reduced either cardiac or stroke mortality 12, 14-19, 36, 38. We were also unable to demonstrate that doses greater than standard dose of 1000 μg of folic acid compared to standard dose had beneficial effects, or that lower doses of the vitamin or no intake had adverse effects on cardiac or stroke mortality compared to a standard dose intake.
The possible adverse association between folic acid and stroke mortality was only of borderline significance, however, it became non-significant after including serum albumin as a potential confounder. Malnutrition, indicated by hypoalbuminemia is highly prevalent in hemodialysis patients as are inflammation and atherosclerosis 45-46. All of these conditions are associated with increased mortality risk in CKD patients 45, 47-50.
While this study was a secondary analysis, it included the largest sample size of hemodialysis patients and the longest follow-up time to date in examining the effects of folic acid containing vitamin supplements use on cardiac and stroke mortality. Another unique strength of the analyses was its ability to define and identify the causes of mortality precisely among HEMO study patients and to use the ICED and IDS scores to quantify and explore the severity of existing comorbidities.
Our study had certain limitations. For the cardiac mortality analyses, with our sample size, the small percent (6%-9% during baseline and follow-up years 1-3) of patients consuming the higher dose of folic acid (> 1000 μg) and a cardiac mortality rate of 16%, we had only 54% power to detect a hazard ratio of 1.5 with an alpha of 0.05 and 80% power with a hazard ratio approaching 1.75. In our stroke analyses, with a relatively low stroke mortality rate of 3.4%, our sample size was even more limited in power for detecting an association between folic acid consumption and stroke mortality. Furthermore, combining the cardiac and stroke mortality outcomes would not have substantially improved the power of the analyses. The discrete nature of the folic acid vitamin supplement doses may also have been a limitation. Because the majority of the HEMO study patients took the standard dosage of 1000 μg, the dosage data had a smaller variance with dosages centered on 1000 μg and this may have impeded our ability to detect a possible beneficial effect of the higher folic acid doses on cardiac and stroke mortality. Another limitation was that folic acid intakes from other sources such as food and oral enteral nutritional supplements were not included and therefore the effect of total folic acid intakes on cardiac and stroke mortality could not be examined. If dietary folate varied markedly from person to person, this may have introduced a problem. The absence of a true control may have also hindered the observation of the effects of folic acid on cardiac and stroke mortality outcomes. Finally, plasma homocysteine and serum folate levels were not available on all HEMO study patients and so the relationship between folic acid, homocysteine, serum folate and subsequent cardiac and stroke mortality could not be ascertained.
Several large clinical trials are currently underway that address the use of folic acid as a homocysteine lowering therapy to reduce CVD related risks and mortality among patients with normal renal function and renal transplants 51-55. Although our results were negative, further examination of the association between folic acid containing vitamin supplements and CVD mortality is warranted in hemodialysis patients, because of the high risk of these outcomes in this population.
Acknowledgments
Supported by the National Institute of Diabetes, Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health and the National Kidney Foundation Council on Renal Nutrition Research Grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.United States Renal Data System. USRDS 2002 Annual Data Report. Bethesda, MD: US Department of Health and Human Services, Public Health Service, National Institutes of Health; 2002. [Google Scholar]
- 2.Levey AS, Beto JA, Coronado BE, et al. Controlling the Epidemic of Cardiovascular Disease in Chronic Renal Disease: What Do We Know? What Do We Need to Learn? Where do We Go From Here? Am J Kidney Dis. 1998;32:853–906. doi: 10.1016/s0272-6386(98)70145-3. [DOI] [PubMed] [Google Scholar]
- 3.National Kidney Foundation (NKF) Kidney Disease Outcome Quality Initiative (K/DOQI) Advisory Board K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Kidney Disease Outcome Quality Initiative. Am J Kidney Dis. 2002;39(suppl 2):S1–246. [PubMed] [Google Scholar]
- 4.De Vecchi AF, Bamonti-Catena F, Finazzi S, et al. Homocysteine, vitamin B12, and serum and erythrocyte folate in peritoneal dialysis and hemodialysis patients. Perit Dial Int. 2000;20:169–173. [PubMed] [Google Scholar]
- 5.Suliman ME, Qureshi AR, Barany P, et al. Hyperhomocysteinemia, nutritional status, and cardiovascular disease in hemodialysis patients. Kidney Int. 2000;57:1727–1735. doi: 10.1046/j.1523-1755.2000.00018.x. [DOI] [PubMed] [Google Scholar]
- 6.Bostom AG, Shemin D, Verhoef P, et al. Elevated fasting total plasma homocysteine levels and cardiovascular disease outcomes in maintenance dialysis patients. A prospective study. Arterioscler Thromb Vasc Biol. 1997;17:2554–2558. doi: 10.1161/01.atv.17.11.2554. [DOI] [PubMed] [Google Scholar]
- 7.Friedman AN, Bostom AG, Selhub J, et al. The kidney and homocysteine metabolism. J Am Soc Nephrol. 2001;12:2181–2189. doi: 10.1681/ASN.V12102181. [DOI] [PubMed] [Google Scholar]
- 8.Manns BJ, Burgess ED, Hyndman ME, et al. Hyperhomocysteinemia and the prevalence of atherosclerotic vascular disease in patients with end stage renal disease. Am J Kidney Dis. 1998;32:669–677. doi: 10.1016/S0272-6386(99)70392-6. [DOI] [PubMed] [Google Scholar]
- 9.Moustapha A, Gupta A, Robinson K, et al. Prevalence and determinants of hyperhomocysteinemia in hemodialysis and peritoneal dialysis. Kidney Int. 1999;55:1470–1475. doi: 10.1046/j.1523-1755.1999.00378.x. [DOI] [PubMed] [Google Scholar]
- 10.Bostom AG, Shemin D, Lapane KL, et al. Hyperhomocysteinemia and traditional cardiovascular disease risk factors in end-stage renal disease patients on dialysis: a case-control study. Atherosclerosis. 1995;114:93–103. doi: 10.1016/0021-9150(94)05470-4. [DOI] [PubMed] [Google Scholar]
- 11.Rimm EB, Willett WC, Hu FB, et al. Folate and vitamin B6 from diet and supplements in relation to risk of coronary heart disease among women. J Am Med Assoc. 1998;279:359–364. doi: 10.1001/jama.279.5.359. [DOI] [PubMed] [Google Scholar]
- 12.Toole JF, Malinow MR, Chambless LE, et al. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: the Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial. J Am Med Assoc. 2004;291:565–575. doi: 10.1001/jama.291.5.565. [DOI] [PubMed] [Google Scholar]
- 13.Schnyder G, Roffi M, Flammer Y, et al. Effect of homocysteine-lowering therapy with folic acid, vitamin B12, and vitamin B6 on clinical outcome after percutaneous coronary intervention: the Swiss Heart study: a randomized controlled trial. J Am Med Assoc. 2002;288:973–979. doi: 10.1001/jama.288.8.973. [DOI] [PubMed] [Google Scholar]
- 14.Bonaa KH, Njolstad I, Ueland PM, et al. Homocysteine lowering and cardiovascular events after acute myocardial infarction. N Engl J Med. 2006;354:1578–1588. doi: 10.1056/NEJMoa055227. [DOI] [PubMed] [Google Scholar]
- 15.Lonn E, Yusuf S, Arnold MJ, et al. Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med. 2006;354:1567–1577. doi: 10.1056/NEJMoa060900. [DOI] [PubMed] [Google Scholar]
- 16.Albert CM, Cook NR, Gaziano JM, et al. Effect of folic acid and B vitamins on risk of cardiovascular events and total mortality among women at high risk for cardiovascular disease. J Am Med Assoc. 2008;299:2027–2036. doi: 10.1001/jama.299.17.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ebbing M, Bleie O, Ueland PM, et al. Mortality and cardiovascular events in patients treated with homocysteine-lowering B vitamins after coronary angiography. J Am Med Assoc. 2008;300:795–804. doi: 10.1001/jama.300.7.795. [DOI] [PubMed] [Google Scholar]
- 18.Baker F, Picton D, Blackwood S. Blinded comparison of folic acid and placebo in patients with ischemic heart disease: an outcome trial. Circulation. 2002;19:A3642. [Google Scholar]
- 19.Bazzano LA, Reynolds K, Holder KN, et al. Effect of folic acid supplementation on risk of cardiovascular diseases: a meta-analysis of randomized controlled trials. J Am Med Assoc. 2006;296:2720–2726. doi: 10.1001/jama.296.22.2720. [DOI] [PubMed] [Google Scholar]
- 20.Wang X, Qin X, Demirtas H, et al. Efficacy of folic acid supplementation in stroke prevention: a meta-analysis. Lancet. 2007;369:1876–1882. doi: 10.1016/S0140-6736(07)60854-X. [DOI] [PubMed] [Google Scholar]
- 21.Peterson JC, Spence JD. Vitamins and progression of atherosclerosis in hyper-homocyst(e)inaemia. Lancet. 1998;351:263. doi: 10.1016/S0140-6736(05)78275-1. [DOI] [PubMed] [Google Scholar]
- 22.Anonymous. Lowering blood homocysteine with folic acid based supplements: meta-analysis of randomised trials. Homocysteine Lowering Trialists’ Collaboration. Br Med J. 1998;316:894–898. [PMC free article] [PubMed] [Google Scholar]
- 23.Brattstrom L, Israelsson B, Norrving B, et al. Impaired homocysteine metabolism in early-onset cerebral and peripheral occlusive arterial disease. Effects of pyridoxine and folic acid treatment. Atherosclerosis. 1990;81:51–60. doi: 10.1016/0021-9150(90)90058-q. [DOI] [PubMed] [Google Scholar]
- 24.Ubbink JB, Vermaak WJ, van der Merwe A, et al. Vitamin requirements for the treatment of hyperhomocysteinemia in humans. J Nutr. 1994;124:1927–1933. doi: 10.1093/jn/124.10.1927. [DOI] [PubMed] [Google Scholar]
- 25.Arnadottir M, Brattstrom L, Simonsen O, et al. The effect of high-dose pyridoxine and folic acid supplementation on serum lipid and plasma homocysteine concentrations in dialysis patients. Clin Nephrol. 1993;40:236–240. [PubMed] [Google Scholar]
- 26.Hyndman ME, Manns BJ, Snyder FF, et al. Vitamin B12 decreases, but does not normalize, homocysteine and methylmalonic acid in end-stage renal disease: a link with glycine metabolism and possible explanation of hyperhomocysteinemia in end-stage renal disease. Metabolism. 2003;52:168–172. doi: 10.1053/meta.2003.50022. [DOI] [PubMed] [Google Scholar]
- 27.Ziakka S, Rammos G, Kountouris S, et al. The effect of vitamin B6 and folate supplements on plasma homocysteine and serum lipids levels in patients on regular hemodialysis. Int Urol Nephrol. 2001;33:559–562. doi: 10.1023/a:1019559328424. [DOI] [PubMed] [Google Scholar]
- 28.Trimarchi H, Schiel A, Freixas E, et al. Randomized trial of methylcobalamin and folate effects on homocysteine in hemodialysis patients. Nephron. 2002;91:58–63. doi: 10.1159/000057605. [DOI] [PubMed] [Google Scholar]
- 29.Dierkes J, Domrose U, Bosselmann KP, et al. Homocysteine lowering effect of different multi-vitamin preparations in patients with end-stage renal disease. J Ren Nutr. 2001;11:67–72. doi: 10.1016/s1051-2276(01)31274-8. [DOI] [PubMed] [Google Scholar]
- 30.Stanford JL, Molina H, Phillips J, et al. Oral folate reduces plasma homocyst(e)ine levels in hemodialysis patients with cardiovascular disease. J Thorac Cardiovasc Surg. 2000;8:567–571. doi: 10.1016/s0967-2109(00)00062-4. [DOI] [PubMed] [Google Scholar]
- 31.Bostom AG, Shemin D, Bagley P, et al. Controlled comparison of L-5-methyltetrahydrofolate versus folic acid for the treatment of hyperhomocysteinemia in hemodialysis patients. Circulation. 2000;101(24):2829–2832. doi: 10.1161/01.cir.101.24.2829. [DOI] [PubMed] [Google Scholar]
- 32.Yango A, Shemin D, Hsu N, et al. Rapid communication: L-folinic acid versus folic acid for the treatment of hyperhomocysteinemia in hemodialysis patients. Kidney Int. 2001;59:324–327. doi: 10.1046/j.1523-1755.2001.00507.x. [DOI] [PubMed] [Google Scholar]
- 33.Arnadottir M, Gudnason V, Hultberg B. Treatment with different doses of folic acid in haemodialysis patients: effects on folate distribution and aminothiol concentrations. Nephrol Dial Transplant. 2000;15:524–528. doi: 10.1093/ndt/15.4.524. [DOI] [PubMed] [Google Scholar]
- 34.Sunder-Plassmann G, Fodinger M, Buchmayer H, et al. Effect of high dose folic acid therapy on hyperhomocysteinemia in hemodialysis patients: results of the Vienna multicenter study. J Am Soc Nephrol. 2000;11:1106–1116. doi: 10.1681/ASN.V1161106. [DOI] [PubMed] [Google Scholar]
- 35.Gonin JM, Nguyen H, Gonin R, et al. Controlled trials of very high dose folic acid, vitamins B12 and B6, intravenous folinic acid and serine for treatment of hyperhomocysteinemia in ESRD. J Nephrol. 2003;16:522–534. [PubMed] [Google Scholar]
- 36.Wrone EM, Hornberger JM, Zehnder JL, et al. Randomized trial of folic acid for prevention of cardiovascular events in end-stage renal disease. J Am Soc Nephrol. 2004;15:420–426. doi: 10.1097/01.asn.0000110181.64655.6c. [DOI] [PubMed] [Google Scholar]
- 37.Righetti M, Serbelloni P, Milani S, et al. Homocysteine-lowering vitamin B treatment decreases cardiovascular events in hemodialysis patients. Blood Purif. 2006;24:379–386. doi: 10.1159/000093680. [DOI] [PubMed] [Google Scholar]
- 38.Zoungas S, McGrath BP, Branley P, et al. Cardiovascular morbidity and mortality in the Atherosclerosis and Folic Acid Supplementation Trial (ASFAST) in chronic renal failure: a multicenter, randomized, controlled trial. J Am Coll Cardiol. 2006;47:1108–1116. doi: 10.1016/j.jacc.2005.10.064. [DOI] [PubMed] [Google Scholar]
- 39.Jamison RL, Hartigan P, Kaufman JS, et al. Effect of homocysteine lowering on mortality and vascular disease in advanced chronic kidney disease and end stage renal disease. J Am Med Assoc. 2007;298:1163–1170. doi: 10.1001/jama.298.10.1163. [DOI] [PubMed] [Google Scholar]
- 40.Eknoyan G, Beck GJ, Cheung AK, et al. Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med. 2002;347:2010–2019. doi: 10.1056/NEJMoa021583. [DOI] [PubMed] [Google Scholar]
- 41.Makoff R. Water-soluble vitamin status in patients with renal disease treated with hemodialysis or peritoneal dialysis. J Ren Nutr. 1991;1:56–73. [Google Scholar]
- 42.Makoff R. Vitamin replacement therapy in renal failure patients. Miner Electrolyte Metab. 1999;25:349–351. doi: 10.1159/000057473. [DOI] [PubMed] [Google Scholar]
- 43.Cheung AK, Sarnak MJ, Yan G, et al. Cardiac diseases in maintenance hemodialysis patients: results of the HEMO Study. Kidney Int. 2004;65:2380–2389. doi: 10.1111/j.1523-1755.2004.00657.x. [DOI] [PubMed] [Google Scholar]
- 44.Miskulin DC, Athienites NV, Yan G, et al. Comorbidity assessment using the Index of Coexistent Diseases in a multicenter clinical trial. Kidney Int. 2001;60:1498–1510. doi: 10.1046/j.1523-1755.2001.00954.x. [DOI] [PubMed] [Google Scholar]
- 45.Stenvinkel P, Heimburger O, Lindholm B, et al. Are there two types of malnutrition in chronic renal failure? Evidence for relationships between malnutrition, inflammation and atherosclerosis (MIA syndrome) Nephrol Dial Transplant. 2000;15:953–60. doi: 10.1093/ndt/15.7.953. [DOI] [PubMed] [Google Scholar]
- 46.Stenvinkel P, Heimburger O, Paultre F, et al. Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int. 1999;55:1899–911. doi: 10.1046/j.1523-1755.1999.00422.x. [DOI] [PubMed] [Google Scholar]
- 47.Lowrie EG, Lew NL. Death risk in hemodialysis patients: the predictive value of commonly measured variables and an evaluation of death rate differences between facilities. Am J Kidney Dis. 1990;15:458–82. doi: 10.1016/s0272-6386(12)70364-5. [DOI] [PubMed] [Google Scholar]
- 48.Bergstrom J, Heimburger O, Lindholm B, et al. Elevated serum C-reactive protein is a strong predictor of increased mortality and low serum albumin in hemodialysis (HD) patients. J Am Soc Nephrol. 1995;6:573. abstr. [Google Scholar]
- 49.Zimmermann J, Herrlinger S, Pruy A, et al. Inflammation enhances cardiovascular risk and mortality in hemodialysis patients. Kidney Int. 1999;55:648–658. doi: 10.1046/j.1523-1755.1999.00273.x. [DOI] [PubMed] [Google Scholar]
- 50.Ikizler TA, Wingard RL, Harvell J, et al. Association of morbidity with markers of nutrition and inflammation in chronic hemodialysis patients: a prospective study. Kidney Int. 1999;55:1945–1951. doi: 10.1046/j.1523-1755.1999.00410.x. [DOI] [PubMed] [Google Scholar]
- 51.MacMahon M, Kirkpatrick C, Cummings CE, et al. A pilot study with simvastatin and folic acid/vitamin B12 in preparation for the Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine (SEARCH) Nutr Metab Cardiovasc Dis. 2000;10:195–203. [PubMed] [Google Scholar]
- 52.VITATOPS Trial Study Group. The VITATOPS (Vitamins to Prevent Stroke) Trial: rationale and design of an international, large, simple, randomised trial of homocysteine-lowering multivitamin therapy in patients with recent transient ischaemic attack or stroke. Cerebrovasc Dis. 2002;13:120–126. doi: 10.1159/000047761. [DOI] [PubMed] [Google Scholar]
- 53.Galan P, de Bree A, Mennen L, et al. Background and rationale of the SU.FOL.OM3 study: double-blind randomized placebo-controlled secondary prevention trial to test the impact of supplementation with folate, vitamin B6 and B12 and/or omega-3 fatty acids on the prevention of recurrent ischemic events in subjects with atherosclerosis in the coronary or cerebral arteries. J Nutr Health Aging. 2003;7:428–35. [PubMed] [Google Scholar]
- 54.Bostom AG, Carpenter MA, Kusek JW, et al. Rationale and design of the Folic Acid for Vascular Outcome Reduction in Transplantation (FAVORIT) trial. Am Heart J. 2006;152:448.e1–448.e7. doi: 10.1016/j.ahj.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 55.B Vitamin Treatment Trialists’ Collaboration. Homocysteine-lowering trials for prevention of cardiovascular events: a review of the design and power of the large randomized trials. Am Heart J. 2006;151:282–287. doi: 10.1016/j.ahj.2005.04.025. [DOI] [PubMed] [Google Scholar]


