Abstract
Candida albicans causes mucosal and disseminated candidiasis, which represent serious problems for the rapidly expanding immunocompromised population. Until recently, Th1-mediated immunity was thought to confer the primary protection, particularly for oral candidiasis. However, emerging data indicate that the newly-defined Th17 compartment appears to play the predominant role in mucosal candidiasis.
Keywords: Th17, IL-17, Candida albicans
1. Candida albicans causes a variety of distinct disease forms
Candida albicans is a dimorphic fungus responsible for multiple disease states in humans. Normally, C. albicans is a commensal organism found in the gastrointestinal and reproductive mucosa, and can be isolated from the oral cavity of up to 80% of healthy individuals [1]. However, in immunocompromised settings, C. albicans infections lead to oral and oropharyngeal (OPC), vulvovaginal (VVC), mucocutaneous or disseminated candidiasis. The immune responses to these different forms of disease are quite distinct, revealing the complexity of the anatomical basis for host defenses against fungi.
Mucosal and systemic C. albicans infections are a serious problem for immunocompromised populations, such as HIV+ individuals, those receiving cancer chemotherapeutics, neutropenic patients and transplant recipients [2]. OPC is one of the first clinical signs of HIV infection, and is diagnosed in up to 95% of HIV+ patients before onset of full-blown AIDS [3]. OPC is also common in neonates, the elderly, individuals with xerostomia (dry mouth) and Sjögren's Syndrome (SjS) patients (reviewed in [2]). Similarly, individuals with inherited immunodeficiency diseases such as chronic granulomatous disease (CGD), hyper-IgE syndrome (HIES) and autoimmune polyendocrinopathy candidiasis ectodermal dystrophy (APECED) present with persistent oral and mucocutaneous Candida infections [4-6]. Additionally, Candida species are the fourth most common microbe isolated from nosocomial bloodstream infections in U.S. hospitals, with a mortality rate around 40% [7]. VVC affects up to 75% of reproductive-age women at least once. In addition to the treatment costs, these episodes cause a significant decrease in quality of life [8]. Chronic mucocutaneous candidiasis (CMC) is a spectrum of chronic or recurrent diseases involving the skin, nails (onchomycosis) and mucous membranes [9]. In many cases CMC is secondary to autoimmune endocrinopathies, though this link is not fully understood [10].
2. Immune response to Candida albicans: Lessons from animal models
In order to define disease progression and understand specific cellular and molecular components involved in immunity to Candida, animal models of both mucosal and systemic candidiasis have been developed. While primates, rabbits and rats were commonly used in the past, mouse models of candidiasis now predominate due to their economic value and the availability of numerous “knockout” and transgenic strains, allowing gene-by-gene dissection of specific host components [11]. The following sections describe some of the major murine models of candidiasis and their major strengths and weaknesses (summarized in Table 1).
2A. Disseminated candidiasis models
Disseminated candidiasis induced through intravenous injection of yeast cells is lethal in wild type (WT) mice, and is the most common model used to evaluate susceptibility to C. albicans [11, 12]. Although this model has been informative for defining both Candida virulence genes and host components involved in immunity, the immune response differs from that which occurs in mucosal candidiasis, an issue that is often overlooked. This is exemplified in HIV+ individuals and HIES patients, who are highly susceptible to oral thrush but rarely develop systemic candidemia [2]. It is still debated whether systemic candidiasis in humans arises from localized mucosal colonization or from an exogenous source directly entering the bloodstream, such as an indwelling catheter. However, data from immunocompromised cohorts argue against the former [13]. Accordingly, direct administration of Candida cells into circulation may not reflect bona fide disease progression or the immune components naturally at play in the context of mucosal disease.
2B. Mucosal candidiasis models
In contrast to humans, immunocompetent rodents are not naturally colonized by C. albicans. However, mucosal candidiasis can be induced experimentally following immunosuppression or the use of estrogen [11, 14], analogous to immunocompromised settings. Vaginal candidiasis models have been developed in rats and mice under pseudoestrus conditions (reviewed in [11, 15]) These models have helped to elucidate the hormonal component of susceptibility, as estrogen is necessary for infection in this system [15]. To address this concern, in vitro studies using reconstituted human vaginal epithelia have been developed, that have also been informative in elucidating host-pathogen interactions [15]. Nonetheless, the latter model is limited since only local inflammatory events at the epithelial milieu can be evaluated.
For OPC, several oral models of candidiasis have been employed, which differ in the way colonization is initiated. These include (i) applying Candida yeast cells directly to the oral cavity and evaluating disease semi-quantitatively by oral swabbing, (ii) placing a cotton plug saturated with a Candida yeast suspension sublingually during sedation or anesthesia (Filler model) and evaluating fungal burden by CFU of tongue tissue, or (iii) an invasive model in which the tongue is scored and yeast-soaked cotton packed into the mouth for an extended period followed by CFU analysis [16-18]. Variations among the specific models may alter the course of the immune response significantly. For example, scoring the tongue before the inoculum is placed in the oral cavity may augment or fundamentally alter the inflammatory environment. Differences in the inoculum size may influence immune responses, and large bolus infections may not accurately approximate normal oral infections in humans [19]. Generally speaking, however, similar conclusions regarding Th cell biology have been found with each model (see Section 4).
While the oral models differ in some respects, each introduces C. albicans at its natural portal of entry into the body. This contrasts with a model that has been developed by Romani and colleagues to study the progression of disease in the gastrointestinal mucosa in order to approximate the inflammation seen in chronic mucocutaneous candidiasis (CMC) [11]. While C. albicans colonizes the gastrointestinal tract in low numbers, humans are not thought to develop gastric candidiasis due to the inhospitable environment of the stomach and intestine [20, 21]. The claim that the gastric model mimics the inflammation seen in CMC may not be justified, since most CMC patients suffer from oral and skin manifestations of disease, rarely esophageal candidiasis and never gastric candidiasis [9]. Moreover, inflammatory events in the gut mucosa are not necessarily reflected in other mucosal sites.
3. Innate and Adaptive Immune Mechanisms at the Mucosal-Fungal Interface
The innate and adaptive arms of the immune response play key roles in immunity to Candida. Innate immunity in mucosal infection involves many cell types: neutrophils, monocytes/macrophages, Natural Killer (NK) cells, dendritic cells (DC), certain CD4+ and CD8+ T cells, non-MHC restricted T cells such as γδ-T-cells, mucosal epithelial cells, stromal cells and keratinocytes [2]. One function of these cells is to provide a primary protective effect via direct anti-fungal activities such as phagocytosis or secretion of microbicidal compounds that neutralize fungal particles. In addition, innate cells also instruct the adaptive arm of the immune response via production of pro-inflammatory cytokines and chemokines, co-stimulatory signals and antigen uptake and presentation [2, 22].
Saliva is a highly important, often unappreciated element of innate protection against the overgrowth of C. albicans in the oral cavity. Saliva is produced from salivary glands as well as the buccal mucosa and gingival crevices, and is comprised of proteins from all these locations [23]. Salivary flow provides an important physical force to flush microbes through swallowing, thereby preventing pathogen adhesion to mucosal and dental surfaces. Saliva is also a major source of IgA antibodies (see below), many of which recognize Candida albicans and other potential pathogens. Saliva is highly enriched in antimicrobial proteins such as lysozyme, lactoferrin, histatins, cathelicidins, calprotectins and defensins, which are key factors that help to control C. albicans growth and attachment to the oral epithelium [24, 25]. Indeed a newly-recognized evasion strategy employed by Candida albicans involves protelotyic cleavage of Histatin-5 by a secreted aspartic protease [26]. The importance of saliva as a defense element in the oral cavity is starkly exemplified by the increased prevalence of OPC in individuals suffering from xerostomia due to certain medications, head-neck irradiation or SjS [27].
The adaptive arm of the immune system is also vital to prevent OPC, although the T cell compartment appears to dominate. C. albicans elicits an antibody response, yet a protective role for humoral immunity has not been strongly implicated. Secretory IgA (sIgA) is the most abundant Ig isotype found in saliva, and Candida-specific sIgA interacts with cell wall mannoproteins. Data conflict with regards to whether sIgA levels are altered during oral Candida infection. Some studies in healthy subjects suggested a protective role for IgA in inhibiting Candida colonization, yet no anti-Candida antibodies found in saliva were able to directly interfere with adherence to buccal mucosa [2]. Analysis in HIV+ individuals showed no correlation between IgA levels and OPC status. Conversely, other reports suggest that there are increased anti-Candida antibody levels in individuals with OPC, but it remains unclear why such antibodies are apparently not sufficient to prevent disease [2]. Moreover, patients with selective IgA deficiency do not normally present with oral candidiasis, arguing against a major role for this isotype [27]. Overall, most studies identify no protective role for Candida-specific antibodies at the mucosal surface during overt disease [2, 28]. Obviously, a lack of effective Ab responses has direct implications for vaccine development, suggesting that it may be hard to develop such prophylactic Abs by standard immunization strategies.
The relative contributions of innate and adaptive immunity appear to be tissue- and compartment-specific. Neutrophils are strongly implicated in defense against systemic candidiasis, since neutropenic patients are strongly prone to disseminated as well as mucosal candidiasis [29]. In contrast, a human live challenge model showed that susceptibility to VVC appears to be linked to an uncontrolled neutrophil influx, which provokes and amplifies pathogenic inflammation, rather than providing protection. Moreover, studies of women with VVC and an experimental murine animal model thereof demonstrated no protective role for cell-mediated immunity (CMI). Rather, studies indicate the importance of innate mechanisms mediated directly by vaginal mucosal epithelial cells [30]. A prevailing model states that in the vaginal mucosa, an undisturbed epithelial layer with an appropriately balanced vaginal microflora interacts with C. albicans through unknown mechanisms resulting in avoidance of this neutrophil response. The importance of maintaining the microflora may also explain the prevalence of VVC in women on antibiotic treatment [30].
Like vaginal candidiasis, OPC involves infection of mucosal tissue, but CMI is crucial for protection in the oral cavity. The importance of CD4+ T cells is strikingly illustrated in HIV+ patients, who are highly susceptible to OPC but generally not systemic candidiasis or VVC [3]. An effective neutrophil response in OPC is also necessary, but appears to be instructed by the CD4+ T cell compartment. Recent studies have demonstrated that a major mechanism of the crosstalk between neutrophils and CD4+ T cells is mediated by IL-17, the hallmark cytokine of Th17 cells (discussed in detail below). In summary, elements of the immune response interact differently against a common organism, depending on the anatomic location of the infection.
4. The Th17 paradigm: A new view of mucosal host defense
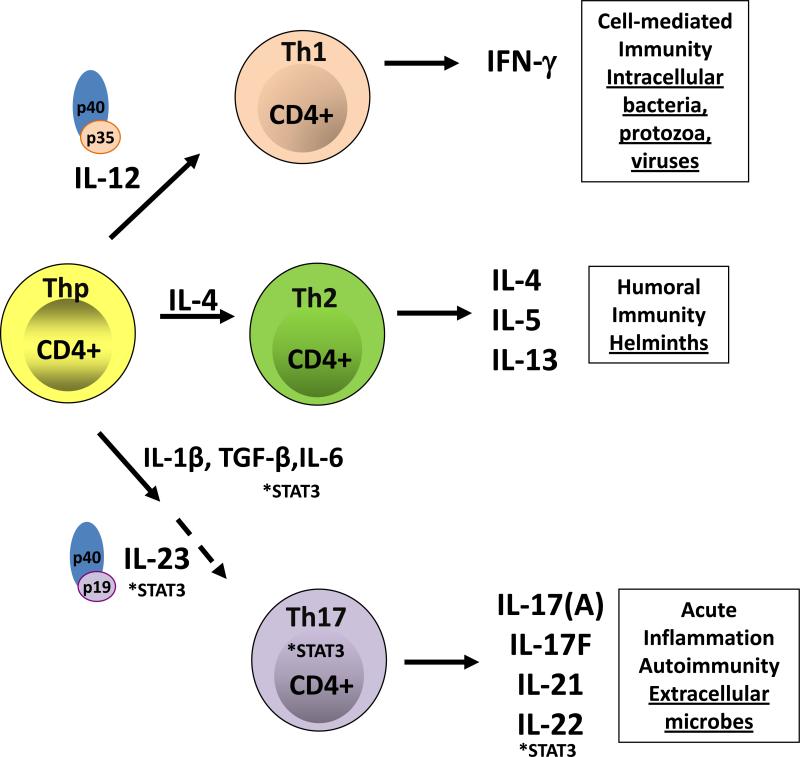
The transition from healthy to pathogenic state occurs at the mucosal-fungal interface, so understanding the host defense mechanisms employed at this junction is vital. In the last few years there has been a major shift in our understanding of the adaptive and innate immune components that confer resistance to candidiasis. Traditionally, protective adaptive responses against Candida were thought to be mediated by CD4+ Th1-effector cells [2, 31]. This view was reasonable at the time, because adaptive immunity was viewed through the lens of the Th1-Th2 paradigm put forth by Mossman and Coffman in 1986 [32]. The Th1/Th2 model posits that naïve Th-precursor cells develop into Th1 cells in the presence of IL-12, and Th2 cells in the presence of IL-4. Th1 cells are defined by their production of IFN-γ, through which they promote CMI. Th2 cells produce IL-4, IL-5 and IL-13 and promote allergy and protection against helminthes (Figure 1A).
Figure 1. T-helper cell differentiation.
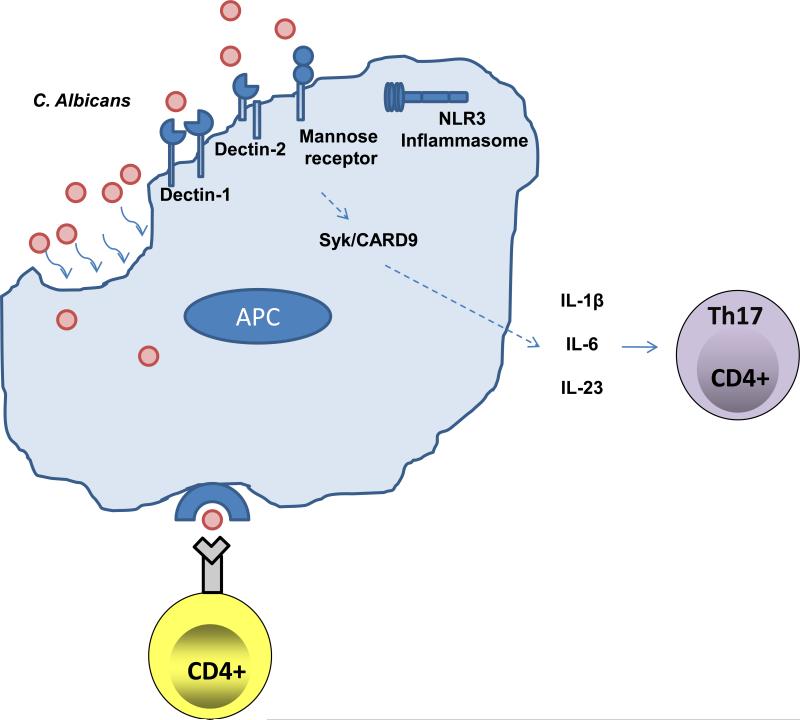
(A) Upon activation by signaling through the T-cell receptor and co-stimulatory molecules, naïve CD4+ Th precursor (Thp) cells can differentiate into three lineages of effector T helper (Th) cells, Th1, Th2 or Th17 cells depending on the local cytokine milieu. Each subset produces different cytokines that mediate distinct effector mechanisms. IL-12 is an important cytokine for the development of Th1 cells, which produce IFN-γ, through which they promote CMI and protection against intracellular pathogens. IL-4 promotes the expansion of Th2 cells, which produce IL-4, IL-5 and IL-13 and are important in allergy and protection against helminthes. On the other hand, Th17 cells develop in the presence of IL-1, IL-6 and TGF-β, while IL-23 is important for the expansion, maintenance and function of Th17 cells. Th17 cells are implicated in autoimmunity and protect against extracellular microbes, including C. albicans, a role that was erroneously ascribed to Th1 cells in many instances. The transcription factor STAT3 is very important at many steps exclusively along the Th17-development pathway. (B) C. albicans is recognized, taken up by antigen presenting cells (APCs), such as macrophages and dendritic cells (DCs), processed and presented to T cells. A balanced response to Candida includes the cooperation of many PRRs. Upon recognition of C. albicans yeast and hyphae, CLRs (dectin-1, dectin-2, and the MR) and the NLRP3 inflammasome are important in the generation of a protective Th17-response through the induction of IL-1β, IL-6, and TGF-β.
Within the constraints of the Th1/Th2 paradigm, Th1 cells were believed to confer resistance to most extracellular microbes including C. albicans, even when experimental evidence was inconsistent with this view [7]. In 2006, Farah et al. reported that mice deficient in IFN-γ, the signature Th1 cytokine, are not susceptible to oral candidiasis, using the model in which infection is evaluated semi-quantiatively by oral swabbing [17]. IL-12, which drives Th1 development, is a dimeric cytokine composed of two subunits, IL-12p35 and IL-12p40; for many years the IL-12p40-/- mouse strain was used to evaluate the role of the Th1 lineage in disease (reviewed in [33]). In surprising contrast to IFNγ-/- mice, IL-12p40-/- deficient mice were susceptible to OPC, and so Farah et al. concluded that Th1 cells act to protect the host from Candida through some sort of IFN-γ-independent mechanism. However, even at the time, it was known that IL-12p40 comprises part of another dimeric cytokine, IL-23, which was not considered in that report [34]. Hence, the stage was set for re-thinking the role of the Th1 cell in candidiasis based solely on data from the IL-12p40-knockout mouse.
In 2005, a major overhaul in understanding of T helper cell biology was made with the discovery of a new Th subset distinct from conventional Th1/Th2 populations, which centers around the shared IL-12p40 subunit (Figure 1). Termed “Th17 cells” due to production of IL-17 (a.k.a., IL-17A), these cells are generated following exposure to IL-1, IL-6 and TGF-β [35]. Notably, although IL-23 is not an inductive cytokine for the Th17 subset, it is nonetheless required for the expansion, maintenance and downstream effector functions of Th17 cells. Hence, in the absence of IL-23, no Th17 cells and very few other IL-17-producing cells are made [36]. As noted above, IL-23 is composed of IL-12p40 paired with a unique IL-23p19 component. IL-23 binds to a receptor composed of IL-12Rβ1 paired with a related but distinct IL-23R subunit, which is induced on activated Th17 cells during the process of Th17 differentiation [35]. The discovery of IL-23 explains why Th1 cells were erroneously implicated in many disease settings, including candidiasis as outlined below. [37].
In 2004 (prior to the discovery of Th17 cells per se), the IL-17 receptor (IL-17RA, the common receptor for IL-17A and IL-17F) was reported to be necessary for host protection in the disseminated candidiasis mouse model [38], which was recently confirmed by an independent group [39]. This susceptibility correlated with defects in the neutrophil compartment, and was among the first publications to link IL-17 to the PMN response to fungal infections. Combined with many emerging studies of IL-12 versus IL-23, an alternative and more plausible explanation of the results obtained bin the oral candidiasis model using IL-12p40-/- mice [17] was that IL-23/Th17 cells, rather than IL-12/Th1 cells, are required for host defense against OPC. In a more direct test of this hypothesis, Conti et al. evaluated OPC in mice deficient in IL-12p35 (i.e., Th1-deficient) or IL-23p19 (Th17-deficient) in the Filler model of OPC [16, 40]. Confirming a dominant role of IL-23 rather than IL-12, these studies revealed that IL-23p19-/- mice experienced high fungal burdens of Candida in the oral cavity and showed signs of overt disease such as hyphal lesions and weight loss. In contrast, IL-12p35-/- mice showed only mild increases in oral fungal burden with no evidence of fungal thrush lesions or weight loss. Consistently, mice deficient in IL-17RA were also highly susceptible to OPC. Therefore, in mouse models at least, the Th1 subset does not appear to play an essential role in preventing the transition of C. albicans to a pathogenic form, whereas IL-17 and Th17 cells are vital.
The timing of Th-mediated events in mouse models of OPC is curious, since protection in this setting is conferred within 5 days, rather than the 2-3 week time frame generally required for typical adaptive responses. Although Th17 cells are part of the adaptive immune response, they serve mainly to regulate innate immune responses [41]. The dual nature of IL-23 is also indicated by the role it plays in the expansion of other more innate type cells, including those that express γδ T cell receptors (TCR). Under the influence of IL-23, γδ-T cells produce a “Th17-like” cytokine profile and function in a capacity similar to Th17 cells, generally at early time frames consistent with innate responses [42, 43]. However, in murine OPC, the αβ+ TCR subset of IL-17-producing cells appears to be more important than the γδ+ subset, and γδ-T cells are only found in low numbers in the oral mucosa [44]. Recently, “natural” Th17 (nTh17) cells have been identified that are also an important source of IL-17A and IFN-γ, acting prior to the establishment of adaptive immunity. This memory-like T cell subset has been implicated in early phases of pulmonary host defense, and is likely to play a role in protection at other mucosal surfaces [45]. Intriguingly, Th17 cells are lost preferentially during HIV and SIV infection [46], although studies have focused on the gut mucosa rather than the oral cavity.
5. Th17 cells coordinate mucosal immune responses
Mucosal immune mechanisms are vital to prevent the systemic spread of pathogens from a localized infection. The discovery of the Th17 pathway has resolved many of the discrepancies existing in the CD4+ T cell field in mucosal infections. As illustration, IFN-γ-producing CD4+ T cells found at a site of infection were broadly deemed Th1 cells, when in fact they probably included IFN-γ and IL-17 double-producing Th17 cells [37]. These findings also align with the recently described and expanding role of Th17 cells in barrier immunity against microorganisms at epithelial and mucosal surfaces [47, 48]. Th17 cells express the chemokine receptors CCR6 and CCR4, which targets them to mucosal areas [7]. Subsequently, cytokines produced by these Th17 or Th17-like cells act upon mucosal epithelial cells to mediate various host defense mechanisms. IL-17 and IL-22, in particular, drive production of pro-inflammatory cytokines, chemokines and antimicrobial proteins by epithelial and stromal cells (reviewed in [49]). Initially the protective mechanism of IL-17 at mucosal surfaces was shown to be mediated through the production of chemoattractants, such as CXCL1, CXCL5 and IL-8, which contribute to a targeted neutrophil response [41]. While the essential activity of the neutrophil response to candidiasis is indicated by the susceptibility of neutropenic patients or mice lacking a proper neutrophil response, it is apparent that an appropriate mucosa-specific response also requires the action of various antimicrobial proteins such as β-defensins, cathelicidins and S100 proteins (calprotectins), which can directly kill fungi and other pathogens [16]. Thus, Th17 cells and the cytokines they produce serve as a link between the adaptive and epithelial host defense responses.
Susceptibility to mucosal infections is also dependent on the availability of niches to allow colonization. Commensal flora in the oral, GI and vaginal tracts play a vital role in limiting infections. It is well known, for example, that antibiotic use predisposes to VVC and OPC. In this regard, Th17 cells play a central role in controlling the composition of commensal flora in the gut [50], and hence may indirectly influence Candida colonization by virtue of altering local flora at mucosal surfaces.
6. Th17-derived cytokines: IL-17 and IL-22
The IL-17 family comprises six cytokines (IL-17A through IL-17F) and five receptors (IL-17RA through IL-17RD) [51]. IL-17A and IL-17F are 50% homologous, and both signal through a heteromeric complex of IL-17RA and IL-17RC. IL-17A and IL-17F homodimers and heterodimers have been identified, though the in vivo importance of the heterodimer has not been completely delineated. Although IL-17A and IL-17F signal through the same receptor subunits, they display unique biological attributes. IL-17A appears more pathogenic than IL-17F in autoimmune settings, likely due to stronger signaling strength through IL-17RA [51]. IL-17F displays a stronger affinity for IL-17RC [52], and IL-17F-/- mice are more susceptible to Staphylococcal infections [53, 54]. IL-17RA has also been shown to associate with IL-17RB and IL-17RD, although the consequences of this interaction are not well understood [55]. IL-17E (IL-25) is a Th2 cytokine that signals through IL-17RB, and expression of IL-9 is controlled by IL-25, leading to enhanced allergic responses in lung [56].
Th17 cells are normally associated with production of IL-17 (IL-17A), but are also an important source of IL-22, an IL-10-family cytokine that is enriched at mucosal areas [57]. IL-22 binds to a hematopoietic receptor complex composed of IL-22R1 and IL-10R2 [58]. Notably, whereas the IL-17R activates typical innate inflammatory signals such as NF-κB and C/EBP transcription factors, IL-22 activates the JAK/STAT3 and MAPK pathways [51]. Nonetheless, subsequent signaling through both the IL-17 and IL-22 receptors results in the cooperative upregulation of neutrophil chemokines, antimicrobial peptides, and other pro-inflammatory cytokines. Collectively, these downstream effector molecules provide a means by which the Th17 pathway mediates clearance of extracellular pathogens. Interestingly, IL-22 has been shown to play a more vital role than IL-17 in host defense against certain gut and lung mucosal pathogens [57]. Surprisingly, however, studies in the IL-22-/- mouse suggest that this cytokine plays a relatively minor role in protection in the oral cavity in OPC, at least in the mouse model [16]. However, an important role for IL-22 in antifungal mechanisms may exist, because CD4+ memory T cells specific for C. albicans have been detected in humans [59], and neutralizing antibodies to IL-22 are found at high levels in humans with APECED-induced candidiasis [5, 6].
7. IL-23/Th17 pathway is protective in candidiasis: From pattern recognition receptors to antimicrobial proteins
A precipitous increase in publications related to antifungal responses has begun to delineate more clearly the early events in anti-Candida immunity. Balanced recognition of the myriad of microorganisms found at mucosal surfaces is managed by various pattern recognition receptors (PRRs) expressed on host cells. PRRs recognize pathogen associated molecular patterns (PAMPs) found on or within microbes, and help to shape the magnitude and nature of the host inflammatory response.
Recognition of C. albicans by the host involves the cooperation of many different PRRs, and how their various downstream signaling pathways augment a protective response continues to be investigated. The outer layer of the cell wall of C. albicans contains protein and carbohydrate PAMPs, such as β1,3- and β1,6-glucans, chitin, and mannose derivatives. Two families of PRRs that have been implicated in fungal immunity are the toll-like receptors (TLRs) and the C-type lectin receptors (CLRs). PRRs can be distinguished between those that are involved in immediate immune effector functions and those that are capable of regulating gene expression. The TLRs have previously been shown to couple innate and adaptive immunity by regulating the expression of innate response genes, such as those encoding co-stimulatory molecules, cytokines and chemokines. Various TLRs (namely, TLR2, TLR4 TLR6 and TLR9) have been linked to recognition of C. albicans [60, 61]. Most TLRs (except TLR3) transduce a signal using the downstream adaptor molecule, MyD88 (myeloid differentiation primary response gene 88). TLR3 and TLR4 also signal independently of Myd88, using the adaptor TRIF (TIR-domain-containing adapter-inducing interferon-β) instead. It appears that Myd88-dependent and Myd88–independent TLR signaling alone cannot fully account for the response to fungal microorganisms. [62].
More recently it has been demonstrated that Candida activates the C-type lectin receptors (CLRs) dectin-1, dectin-2 and Mincle, via the carbohydrate components of the yeast cell wall, although there remains some controversy about whether Dectin-1 is required for disseminated candidiasis protection [63-66]. These receptors couple downstream directly or indirectly to the spleen tyrosine kinase (Syk), which engages the signaling complex Card9, ultimately promoting a Th17-skewed response [67, 68]. The importance of a coordinated response was also substantiated by a report showing the macrophage mannose receptor, in conjunction with TLR2/dectin-1 signaling, is important for inducing IL-17 in response to Candida cell wall mannan moieties [69]. In addition, human studies verify that DECTIN-1 and CARD9 polymorphisms are linked to susceptibility to Candida infections [70, 71].
The integrated recognition of C. albicans involves other PRRs. Several groups have recently demonstrated a role for the NOD-like receptor (NLR) NLRP3 in defense against C. albicans infection in mice [18, 72, 73]. Engagement of the NLRP3 mobilizes the inflammasome, a multi-protein complex composed of multiple adaptors and signaling intermediates (reviewed in [74]). Engagement of the inflammasome leads to activation of caspase-1, which cleaves pro-IL-1β leading to its secretion. IL-1, of course, is an important proinflammatory cytokine that is upstream of Th17 activation as well as a myriad of inflammatory events [75]. The NLRP3/inflammasome was previously understood to be important in the recognition of various bacterial and viral species, but this is the first time fungi have been shown to activate this pathway. Interestingly, the hyphal form of C. albicans preferentially activated the NLRP3 inflammasome, perhaps serving as a pathogenic signal for induction of inflammation [18, 72, 73]. Similar to dectin-1 and dectin-2, NLRP3 inflammasome-mediated protection is dependent on the Syk/CARD9-signaling axis, illustrating the centrality of these signaling mediators in antifungal defense [72]. It should be noted, however, that one group has found no role for the NLRP3 inflammasome pathway in candidiasis, and therefore its significance is still controversial (T. Kanneganti, personal communication). In sum, the response to C. albicans appears to be initiated by various recognition systems that work collaboratively to direct a protective response dominated by various innate and Th17-dominated adaptive mechanisms (Figure 1B).
8. IL-23/Th17 pathway is protective in candidiasis: Evidence from human studies
HIV+ patients provided the first indication that CD4+ T cells, which of course include Th17 cells, play a particularly important role in defense against C. albicans infection in the oral cavity. More direct evidence regarding the importance of Th17 cells comes from two rare genetic immunodeficiency syndromes: hyperimmunoglobulin-E syndrome (HIES) and APECED (autoimmune polyendocrinopathy candidiasis ectodermal dystrophy, also known as autoimmune polyendocrine syndrome 1, APS-1). Autosomal dominant (AD)-HIES (also known as Job's syndrome) is a primary immunodeficiency disorder characterized by both immunological and non-immunological defects [4]. HIES patients suffer from elevated serum IgE levels, eczema, and recurrent skin and lung infections, and are particularly susceptible to staphylococcal infections and oral and mucocutaneous candidiasis. The major genetic lesion leading to HIES was initially defined as dominant-negative mutations in the transcription factor, STAT3 [76-78]. STAT3 functions promiscuously but has been shown to be necessary for signaling downstream of IL-6, IL-21 and IL-23 as well as expression of IL-17 [79, 80]. A subsequent series of publications indicated that an assortment of STAT3 genetic lesions results in impairment of Th17 cell differentiation in these patient cohorts. This finding verifies the role of Th17 cells in human mucosal candidiasis, and dovetails well with the contemporaneous data in mouse systems (Table 1) [77, 81, 82].
APECED is a rare, severe autoimmune syndrome arising from mutations in the autoimmune regulator (AIRE), mutations in which result in aberrant thymic self-tolerance mechanisms and multi-organ autoimmune disease. Although the basis for multi-organ autoimmunity was clarified with the recognition that AIRE governs expression of self-antigens within the thymus, it was unclear why CMC is the first sign of disease in the majority of these patients. New studies reveal that the susceptibility to Candida infections appears to be due to high levels of neutralizing autoantibodies to Th17-related cytokines, including IL-17A, IL-17F and IL-22 [5, 6]. These studies provide compelling new evidence for the protective role of the Th17-pathway in CMC, and are also the first to demonstrate an autoimmune component to fungal infection susceptibility. Interestingly, anti-IL-17 and anti-IL-22 Abs are also found in certain thymoma patients, correlating tightly with susceptibility to candidiasis. Finally, genetic studies corroborating the animal studies outlined above show that dectin-1 polymorphisms are linked to susceptibility to mucocutaneous and invasive Candida infections [71, 83], and CARD9 mutations were found in a consanguineous family with persistent fungal infections [70].
Th17 cells and IL-17 can be protective or pathogenic depending on the context. Just as the Th17 pathway is pathogenic in autoimmune settings, it may play a negative role in antifungal mechanisms in the stomach and gut. IL-23 and IL-17 appear to be counterproductive in a murine gastric model of candidiasis. Evidence in this setting suggests that the Th17 pathway limits protective IFN-γ/IL-12 (Th1) responses, leading to an exacerbated state of inflammation which promotes disease progression [84]. Although the relevance to other forms of mucosal candidiasis is unclear, the IL-23/Th17 pathway does promote deleterious inflammation in gut, and humans with polymorphisms in the IL-23R are protected from inflammatory bowel disease [85]. Since the development of candidiasis is compartment-dependent, the immune response in gastric versus the other forms of infection may not proceed identically. Thus, if the disparate results surrounding the Th17 pathway cannot be explained by model differences, it must be considered that the protective role carried out by the Th17 cell in host defense to C. albicans in the oral cavity is not applicable to all mucosal surfaces.
9. Summary and Perspectives
Investigations in both mice and humans have provided strong evidence for a protective role of the Th17 pathway in antifungal immunity at most mucosal and epithelial surfaces, particularly the oral cavity and skin. Overall, a well-balanced response to C. albicans appears to be initiated by recognition systems that work collaboratively to direct a protective response involving Th17-dominated innate and adaptive mechanisms. An understanding of how C. albicans is recognized by the host has direct implications for vaccine development. Differential expression of PRRs may lead to compartment- or tissue-specific responses that optimally keep fungal overgrowth at bay. Effective vaccines would need to selectively initiate these protective pathways, though this remains a considerable challenge.
OPC is already a major problem for immunocompromised humans, and the studies outlined here predict other segments of the population may also be affected due to impending treatments strategies aimed at limiting Th17-mediated pathology. Since IL-17 is pathogenic in autoimmune settings, it is considered an attractive target for therapeutic blocking agents, which have proven clinically effective thus far [86]. However, the studies outlined here suggest that when these drugs are used to dampen the Th17 response, protective antifungal qualities will also be compromised.
Acknowledgments
SLG was supported by the NIH (DE018822, AR054389) and the University of Pittsburgh School of Medicine, and HRC was supported by a Training Grant to the Department of Oral Biology at SUNY Buffalo (DE007034). We thank Dr. M. Edgerton (SUNY Buffalo) for critical reading, and Dr. T. Kanneganti (St. Jude's Children's Research Hospital) for sharing unpublished information.
Abbreviations
- AIRE
autoimmune regulator
- APECED
autoimmune polyendocrinopathy candidiasis ectodermal dystrophy
- APS-1
also known as autoimmune polyendocrine syndrome 1
- BD
β defensin
- CMC
chronic mucocutaneous candidiasis
- CLR
C-type lectin receptor
- CMI
cell mediated immunity
- HIES
hyper-IgE syndrome
- JAK
Janus kinase
- NLR
NOD-like receptor
- OPC
oropharyngeal candidiasis
- PAMP
pathogen associated molecular pattern
- PRR
pattern recognition receptor
- SjS
Sjögren's Syndrome
- STAT
signal transducer and activator of transcription
- TLR
toll-like receptor
- VVC
vulvovaginal candidiasis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Villar CC, Dongari-Bagtzoglou A. Immune defence mechanisms and immunoenhancement strategies in oropharyngeal candidiasis. Expert Rev Mol Med. 2008;10:e29. doi: 10.1017/S1462399408000835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dongari-Bagtzoglou A, Fidel PL., Jr. The host cytokine responses and protective immunity in oropharyngeal candidiasis. J Dent Res. 2005;84:966–977. doi: 10.1177/154405910508401101. [DOI] [PubMed] [Google Scholar]
- 3.Fidel PL., Jr. Candida-host interactions in HIV disease: relationships in oropharyngeal candidiasis. Adv Dent Res. 2006;19:80–84. doi: 10.1177/154407370601900116. [DOI] [PubMed] [Google Scholar]
- 4.Domingo DL, Freeman AF, Davis J, Puck JM, Tianxia W, Holland SM, Hart TC. Novel intraoral phenotypes in hyperimmunoglobulin-E syndrome. Oral Dis. 2008;14:73–81. doi: 10.1111/j.1601-0825.2007.01363.x. [DOI] [PubMed] [Google Scholar]
- 5.Kisand K, Boe Wolff AS, Podkrajsek KT, Tserel L, Link M, Kisand KV, Ersvaer E, Perheentupa J, Erichsen MM, Bratanic N, Meloni A, Cetani F, Perniola R, Ergun-Longmire B, Maclaren N, Krohn KJ, Pura M, Schalke B, Strobel P, Leite MI, Battelino T, Husebye ES, Peterson P, Willcox N, Meager A. Chronic mucocutaneous candidiasis in APECED or thymoma patients correlates with autoimmunity to Th17-associated cytokines. J Exp Med. 2010;207:299–308. doi: 10.1084/jem.20091669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Puel A, Doffinger R, Natividad A, Chrabieh M, Barcenas-Morales G, Picard C, Cobat A, Ouachee-Chardin M, Toulon A, Bustamante J, Al-Muhsen S, Al-Owain M, Arkwright PD, Costigan C, McConnell V, Cant AJ, Abinun M, Polak M, Bougneres PF, Kumararatne D, Marodi L, Nahum A, Roifman C, Blanche S, Fischer A, Bodemer C, Abel L, Lilic D, Casanova JL. Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. J Exp Med. 2010;207:291–297. doi: 10.1084/jem.20091983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khader SA, Gaffen SL, Kolls JK. Th17 cells at the crossroads of innate and adaptive immunity against infectious diseases at the mucosa. Mucosal Immunol. 2009;2:403–411. doi: 10.1038/mi.2009.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McClelland RS, Richardson BA, Hassan WM, Graham SM, Kiarie J, Baeten JM, Mandaliya K, Jaoko W, Ndinya-Achola JO, Holmes KK. Prospective study of vaginal bacterial flora and other risk factors for vulvovaginal candidiasis. J Infect Dis. 2009;199:1883–1890. doi: 10.1086/599213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirkpatrick CH. Chronic mucocutaneous candidiasis. Pediatr Infect Dis J. 2001;20:197–206. doi: 10.1097/00006454-200102000-00017. [DOI] [PubMed] [Google Scholar]
- 10.Eyerich K, Foerster S, Rombold S, Seidl HP, Behrendt H, Hofmann H, Ring J, Traidl-Hoffmann C. Patients with chronic mucocutaneous candidiasis exhibit reduced production of Th17-associated cytokines IL-17 and IL-22. J Invest Dermatol. 2008;128:2640–2645. doi: 10.1038/jid.2008.139. [DOI] [PubMed] [Google Scholar]
- 11.de Repentigny L. Animal models in the analysis of Candida host-pathogen interactions. Curr Opin Microbiol. 2004;7:324–329. doi: 10.1016/j.mib.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 12.Spellberg B, Ibrahim AS, Edwards JE, Jr., Filler SG. Mice with disseminated candidiasis die of progressive sepsis. J Infect Dis. 2005;192:336–343. doi: 10.1086/430952. [DOI] [PubMed] [Google Scholar]
- 13.Miranda LN, van der Heijden IM, Costa SF, Sousa AP, Sienra RA, Gobara S, Santos CR, Lobo RD, Pessoa VP, Jr., Levin AS. Candida colonisation as a source for candidaemia. J Hosp Infect. 2009;72:9–16. doi: 10.1016/j.jhin.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 14.Naglik JR, Fidel PL, Jr., Odds FC. Animal models of mucosal Candida infection. FEMS Microbiol Lett. 2008;283:129–139. doi: 10.1111/j.1574-6968.2008.01160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cassone A, De Bernardis F, Santoni G. Anticandidal immunity and vaginitis: novel opportunities for immune intervention. Infect Immun. 2007;75:4675–4686. doi: 10.1128/IAI.00083-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conti HR, Shen F, Nayyar N, Stocum E, Sun JN, Lindemann MJ, Ho AW, Hai JH, Yu JJ, Jung JW, Filler SG, Masso-Welch P, Edgerton M, Gaffen SL. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med. 2009;206:299–311. doi: 10.1084/jem.20081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farah CS, Hu Y, Riminton S, Ashman RB. Distinct roles for interleukin-12p40 and tumour necrosis factor in resistance to oral candidiasis defined by gene-targeting. Oral Microbiol Immunol. 2006;21:252–255. doi: 10.1111/j.1399-302X.2006.00288.x. [DOI] [PubMed] [Google Scholar]
- 18.Hise AG, Tomalka J, Ganesan S, Patel K, Hall BA, Brown GD, Fitzgerald KA. An essential role for the NLRP3 inflammasome in host defense against the human fungal pathogen Candida albicans. Cell Host Microbe. 2009;5:487–497. doi: 10.1016/j.chom.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pirofski LA, Casadevall A. Rethinking T cell immunity in oropharyngeal candidiasis. J Exp Med. 2009;206:269–273. doi: 10.1084/jem.20090093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ashman RB. Protective and pathologic immune responses against Candida albicans infection. Front Biosci. 2008;13:3334–3351. doi: 10.2741/2929. [DOI] [PubMed] [Google Scholar]
- 21.Noverr MC, Huffnagle GB. Does the microbiota regulate immune responses outside the gut? Trends in microbiology. 2004;12:562–568. doi: 10.1016/j.tim.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 22.Romani L, Montagnoli C, Bozza S, Perruccio K, Spreca A, Allavena P, Verbeek S, Calderone RA, Bistoni F, Puccetti P. The exploitation of distinct recognition receptors in dendritic cells determines the full range of host immune relationships with Candida albicans. Int Immunol. 2004;16:149–161. doi: 10.1093/intimm/dxh012. [DOI] [PubMed] [Google Scholar]
- 23.Gorr SU. Antimicrobial peptides of the oral cavity. Periodontol 2000. 2009;51:152–180. doi: 10.1111/j.1600-0757.2009.00310.x. [DOI] [PubMed] [Google Scholar]
- 24.Hibino K, Samaranayake LP, Hagg U, Wong RW, Lee W. The role of salivary factors in persistent oral carriage of Candida in humans. Arch Oral Biol. 2009;54:678–683. doi: 10.1016/j.archoralbio.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 25.Edgerton M, Koshlukova SE, Lo TE, Chrzan BG, Straubinger RM, Raj PA. Candidacidal activity of salivary histatins. J. Biol. Chem. 1998;273:20438–20447. doi: 10.1074/jbc.273.32.20438. [DOI] [PubMed] [Google Scholar]
- 26.Meiller TF, Hube B, Schild L, Shirtliff ME, Scheper MA, Winkler R, Ton A, Jabra-Rizk MA. A novel immune evasion strategy of candida albicans: proteolytic cleavage of a salivary antimicrobial peptide. PLoS One. 2009;4:e5039. doi: 10.1371/journal.pone.0005039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Repentigny L, Lewandowski D, Jolicoeur P. Immunopathogenesis of oropharyngeal candidiasis in human immunodeficiency virus infection. Clin Microbiol Rev. 2004;17:729–759. doi: 10.1128/CMR.17.4.729-759.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cassone A, De Bernardis F, Torososantucci A. An outline of the role of anti-Candida antibodies within the context of passive immunization and protection from candidiasis. Curr Mol Med. 2005;5:377–382. doi: 10.2174/1566524054022549. [DOI] [PubMed] [Google Scholar]
- 29.Gardner A. Diagnosing fungal infections in neutropenic patients. Clin J Oncol Nurs. 2007;11:29–32. doi: 10.1188/07.CJON.29-32. [DOI] [PubMed] [Google Scholar]
- 30.Fidel PL., Jr. History and update on host defense against vaginal candidiasis. Am J Reprod Immunol. 2007;57:2–12. doi: 10.1111/j.1600-0897.2006.00450.x. [DOI] [PubMed] [Google Scholar]
- 31.Cenci E, Mencacci A, Spaccapelo R, Tonnetti L, Mosci P, Enssle KH, Puccetti P, Romani L, Bistoni F. T helper cell type 1 (Th1)- and Th2-like responses are present in mice with gastric candidiasis but protective immunity is associated with Th1 development. J Infect Dis. 1995;171:1279–1288. doi: 10.1093/infdis/171.5.1279. [DOI] [PubMed] [Google Scholar]
- 32.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 33.Steinman L. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nature Med. 2007;13:139–145. doi: 10.1038/nm1551. [DOI] [PubMed] [Google Scholar]
- 34.Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, Vega F, Yu N, Wang J, Singh K, Zonin F, Vaisberg E, Churakova T, Liu M, Gorman D, Wagner J, Zurawski S, Liu Y, Abrams JS, Moore KW, Rennick D, de Waal-Malefyt R, Hannum C, Bazan JF, Kastelein RA. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–725. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 35.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 Family Cytokines and the Expanding Diversity of Effector T Cell Lineages. Annu Rev Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 36.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 37.O'Quinn DB, Palmer MT, Lee YK, Weaver CT. Emergence of the Th17 pathway and its role in host defense. Adv Immunol. 2008;99:115–163. doi: 10.1016/S0065-2776(08)00605-6. [DOI] [PubMed] [Google Scholar]
- 38.Huang W, Na L, Fidel PL, Schwarzenberger P. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J Infect Dis. 2004;190:624–631. doi: 10.1086/422329. [DOI] [PubMed] [Google Scholar]
- 39.van de Veerdonk FL, Kullberg BJ, Verschueren IC, Hendriks T, van der Meer JW, Joosten LA, Netea MG. Differential effects of IL-17 pathway in disseminated candidiasis and zymosan-induced multiple organ failure. Shock. 2010 doi: 10.1097/SHK.0b013e3181d67041. in press. [DOI] [PubMed] [Google Scholar]
- 40.Kamai Y, Kubota M, Hosokawa T, Fukuoka T, Filler SG. New model of oropharyngeal candidiasis in mice. Antimicrob Agents Chemother. 2001;45:3195–3197. doi: 10.1128/AAC.45.11.3195-3197.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu JJ, Gaffen SL. Interleukin-17: a novel inflammatory cytokine that bridges innate and adaptive immunity. Front Biosci. 2008;13:170–177. doi: 10.2741/2667. [DOI] [PubMed] [Google Scholar]
- 42.Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M. Interleukin-17-producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity. 2009;31:321–330. doi: 10.1016/j.immuni.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 43.Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 44.Pepin LF, Roger T, Morisset J, Seman M. Preferential V delta 1 expression among TcR gamma/delta-bearing T cells in human oral epithelium. Scan J Immunol. 1993;37:289–294. doi: 10.1111/j.1365-3083.1993.tb02556.x. [DOI] [PubMed] [Google Scholar]
- 45.Tanaka S, Yoshimoto T, Naka T, Nakae S, Iwakura Y, Cua D, Kubo M. Natural occurring IL-17 producing T cells regulate the initial phase of neutrophil mediated airway responses. J Immunol. 2009;183:7523–7530. doi: 10.4049/jimmunol.0803828. [DOI] [PubMed] [Google Scholar]
- 46.Raffatellu M, Santos RL, Verhoeven DE, George MD, Wilson RP, Winter SE, Godinez I, Sankaran S, Paixao TA, Gordon MA, Kolls JK, Dandekar S, Baumler AJ. Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat Med. 2008;14:421–428. doi: 10.1038/nm1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu JZ, Pezeshki M, Raffatellu M. Th17 cytokines and host-pathogen interactions at the mucosa: dichotomies of help and harm. Cytokine. 2009;48:156–160. doi: 10.1016/j.cyto.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marks BR, Craft J. Barrier immunity and IL-17. Semin Immunol. 2009;21:164–171. doi: 10.1016/j.smim.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Onishi R, Gaffen SL. IL-17 and its Target Genes: Mechanisms of IL-17 Function in Disease. Immunology. 2010 doi: 10.1111/j.1365-2567.2009.03240.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, Tanoue T, Imaoka A, Itoh K, Takeda K, Umesaki Y, Honda K, Littman DR. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gaffen SL. Structure and signalling in the IL-17 receptor family. Nat Rev Immunol. 2009;9:556–567. doi: 10.1038/nri2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kuestner RE, Taft DW, Haran A, Brandt CS, Brender T, Lum K, Harder B, Okada S, Ostrander CD, Kreindler JL, Aujla SJ, Reardon B, Moore M, Shea P, Schreckhise R, Bukowski TR, Presnell S, Guerra-Lewis P, Parrish-Novak J, Ellsworth JL, Jaspers S, Lewis KE, Appleby M, Kolls JK, Rixon M, West JW, Gao Z, Levin SD. Identification of the IL-17 receptor related molecule IL-17RC as the receptor for IL-17F. J Immunol. 2007;179:5462–5473. doi: 10.4049/jimmunol.179.8.5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ishigame H, Kakuta S, Nagai T, Kadoki M, Nambu A, Komiyama Y, Fujikado N, Tanahashi Y, Akitsu A, Kotaki H, Sudo K, Nakae S, Sasakawa C, Iwakura Y. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity. 2009;30:108–119. doi: 10.1016/j.immuni.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 54.Yang XO, Chang SH, Park H, Nurieva R, Shah B, Acero L, Wang YH, Schluns KS, Broaddus RR, Zhu Z, Dong C. Regulation of inflammatory responses by IL-17F. J Exp Med. 2008;205:1063–1075. doi: 10.1084/jem.20071978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rickel EA, Siegel LA, Yoon BR, Rottman JB, Kugler DG, Swart DA, Anders PM, Tocker JE, Comeau MR, Budelsky AL. Identification of functional roles for both IL-17RB and IL-17RA in mediating IL-25-induced activities. J Immunol. 2008;181:4299–4310. doi: 10.4049/jimmunol.181.6.4299. [DOI] [PubMed] [Google Scholar]
- 56.Angkasekwinai P, Chang SH, Thapa M, Watarai H, Dong C. Regulation of IL-9 expression by IL-25 signaling. Nat Immunol. 2010;11:250–256. doi: 10.1038/ni.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kolls JK, McCray PB, Jr., Chan YR. Cytokine-mediated regulation of antimicrobial proteins. Nat Rev Immunol. 2008;8:829–835. doi: 10.1038/nri2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shen F, Gaffen SL. Structure-function relationships in the IL-17 receptor: implications for signal transduction and therapy. Cytokine. 2008;41:92–104. doi: 10.1016/j.cyto.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu Y, Yang B, Zhou M, Li L, Zhou H, Zhang J, Chen H, Wu C. Memory IL-22-producing CD4+ T cells specific for Candida albicans are present in humans. Eur J Immunol. 2009;39:1472–1479. doi: 10.1002/eji.200838811. [DOI] [PubMed] [Google Scholar]
- 60.Reid DM, Gow NA, Brown GD. Pattern recognition: recent insights from Dectin-1. Curr Opin Immunol. 2009;21:30–37. doi: 10.1016/j.coi.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van de Veerdonk FL, Kullberg BJ, van der Meer JW, Gow NA, Netea MG. Host-microbe interactions: innate pattern recognition of fungal pathogens. Curr Opin Microbiol. 2008;11:305–312. doi: 10.1016/j.mib.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 62.Netea MG, Brown GD, Kullberg BJ, Gow NA. An integrated model of the recognition of Candida albicans by the innate immune system. Nat Rev Microbiol. 2008;6:67–78. doi: 10.1038/nrmicro1815. [DOI] [PubMed] [Google Scholar]
- 63.Saijo S, Fujikado N, Furuta T, Chung SH, Kotaki H, Seki K, Sudo K, Akira S, Adachi Y, Ohno N, Kinjo T, Nakamura K, Kawakami K, Iwakura Y. Dectin-1 is required for host defense against Pneumocystis carinii but not against Candida albicans. Nat Immunol. 2007;8:39–46. doi: 10.1038/ni1425. [DOI] [PubMed] [Google Scholar]
- 64.Taylor PR, Tsoni SV, Willment JA, Dennehy KM, Rosas M, Findon H, Haynes K, Steele C, Botto M, Gordon S, Brown GD. Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat Immunol. 2007;8:31–38. doi: 10.1038/ni1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bugarcic A, Hitchens K, Beckhouse AG, Wells CA, Ashman RB, Blanchard H. Human and Mouse Macrophage-Inducible C-type Lectin (Mincle) bind Candida albicans. Glycobiology. 2008;18:679–685. doi: 10.1093/glycob/cwn046. [DOI] [PubMed] [Google Scholar]
- 66.Willment JA, Brown GD. C-type lectin receptors in antifungal immunity. Trends Microbiol. 2008;16:27–32. doi: 10.1016/j.tim.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 67.LeibundGut-Landmann S, Gross O, Robinson MJ, Osorio F, Slack EC, Tsoni SV, Schweighoffer E, Tybulewicz V, Brown GD, Ruland J, Reis e Sousa C. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol. 2007;8:630–638. doi: 10.1038/ni1460. [DOI] [PubMed] [Google Scholar]
- 68.Robinson MJ, Osorio F, Rosas M, Freitas RP, Schweighoffer E, Gross O, Verbeek JS, Ruland J, Tybulewicz V, Brown GD, Moita LF, Taylor PR, Reis e Sousa C. Dectin-2 is a Syk-coupled pattern recognition receptor crucial for Th17 responses to fungal infection. J Exp Med. 2009;206:2037–2051. doi: 10.1084/jem.20082818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van de Veerdonk FL, Marijnissen RJ, Kullberg BJ, Koenen HJ, Cheng SC, Joosten I, van den Berg WB, Williams DL, van der Meer JW, Joosten LA, Netea MG. The macrophage mannose receptor induces IL-17 in response to Candida albicans. Cell Host Microbe. 2009;5:329–340. doi: 10.1016/j.chom.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 70.Glocker EO, Hennigs A, Nabavi M, Schaffer AA, Woellner C, Salzer U, Pfeifer D, Veelken H, Warnatz K, Tahami F, Jamal S, Manguiat A, Rezaei N, Amirzargar AA, Plebani A, Hannesschlager N, Gross O, Ruland J, Grimbacher B. A homozygous CARD9 mutation in a family with susceptibility to fungal infections. N Engl J Med. 2009;361:1727–1735. doi: 10.1056/NEJMoa0810719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Plantinga TS, van der Velden WJ, Ferwerda B, van Spriel AB, Adema G, Feuth T, Donnelly JP, Brown GD, Kullberg BJ, Blijlevens NM, Netea MG. Early stop polymorphism in human DECTIN-1 is associated with increased candida colonization in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2009;49:724–732. doi: 10.1086/604714. [DOI] [PubMed] [Google Scholar]
- 72.Gross O, Poeck H, Bscheider M, Dostert C, Hannesschlager N, Endres S, Hartmann G, Tardivel A, Schweighoffer E, Tybulewicz V, Mocsai A, Tschopp J, Ruland J. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature. 2009;459:433–436. doi: 10.1038/nature07965. [DOI] [PubMed] [Google Scholar]
- 73.Joly S, Ma N, Sadler JJ, Soll DR, Cassel SL, Sutterwala FS. Cutting edge: Candida albicans hyphae formation triggers activation of the Nlrp3 inflammasome. J Immunol. 2009;183:3578–3581. doi: 10.4049/jimmunol.0901323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tschopp J, Schroder K. NLRP3 inflammasome activation: the convergence of multiple signalling pathways on ROS production? Nat Rev Immunol. 2010;10:210–215. doi: 10.1038/nri2725. [DOI] [PubMed] [Google Scholar]
- 75.Vonk AG, Netea MG, van Krieken JH, Iwakura Y, van der Meer JW, Kullberg BJ. Endogenous interleukin (IL)-1 alpha and IL-1 beta are crucial for host defense against disseminated candidiasis. J Infect Dis. 2006;193:1419–1426. doi: 10.1086/503363. [DOI] [PubMed] [Google Scholar]
- 76.Minegishi Y, Saito M, Tsuchiya S, Tsuge I, Takada H, Hara T, Kawamura N, Ariga T, Pasic S, Stojkovic O, Metin A, Karasuyama H. Dominant-negative mutations in the DNA-binding domain of STAT3 cause hyper-IgE syndrome. Nature. 2007;448:1058–1062. doi: 10.1038/nature06096. [DOI] [PubMed] [Google Scholar]
- 77.de Beaucoudrey L, Puel A, Filipe-Santos O, Cobat A, Ghandil P, Chrabieh M, Feinberg J, von Bernuth H, Samarina A, Janniere L, Fieschi C, Stephan JL, Boileau C, Lyonnet S, Jondeau G, Cormier-Daire V, Le Merrer M, Hoarau C, Lebranchu Y, Lortholary O, Chandesris MO, Tron F, Gambineri E, Bianchi L, Rodriguez-Gallego C, Zitnik SE, Vasconcelos J, Guedes M, Vitor AB, Marodi L, Chapel H, Reid B, Roifman C, Nadal D, Reichenbach J, Caragol I, Garty BZ, Dogu F, Camcioglu Y, Gulle S, Sanal O, Fischer A, Abel L, Stockinger B, Picard C, Casanova JL. Mutations in STAT3 and IL12RB1 impair the development of human IL-17-producing T cells. J Exp Med. 2008;205:1543–1550. doi: 10.1084/jem.20080321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Renner ED, Rylaarsdam S, Anover-Sombke S, Rack AL, Reichenbach J, Carey JC, Zhu Q, Jansson AF, Barboza J, Schimke LF, Leppert MF, Getz MM, Seger RA, Hill HR, Belohradsky BH, Torgerson TR, Ochs HD. Novel signal transducer and activator of transcription 3 (STAT3) mutations, reduced T(H)17 cell numbers, and variably defective STAT3 phosphorylation in hyper-IgE syndrome. J Allergy Clin Immunol. 2008;122:181–187. doi: 10.1016/j.jaci.2008.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ivanov II, Zhou L, Littman DR. Transcriptional regulation of Th17 cell differentiation. Semin Immunol. 2007;19:409–417. doi: 10.1016/j.smim.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen Z, Laurence A, Kanno Y, Pacher-Zavisin M, Zhu BM, Tato C, Yoshimura A, Hennighausen L, O'Shea J J. Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells. Proc Natl Acad Sci U S A. 2006;103:8137–8142. doi: 10.1073/pnas.0600666103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ma CS, Chew GY, Simpson N, Priyadarshi A, Wong M, Grimbacher B, Fulcher DA, Tangye SG, Cook MC. Deficiency of Th17 cells in hyper IgE syndrome due to mutations in STAT3. J Exp Med. 2008;205:1551–1557. doi: 10.1084/jem.20080218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Milner JD, Brenchley JM, Laurence A, Freeman AF, Hill BJ, Elias KM, Kanno Y, Spalding C, Elloumi HZ, Paulson ML, Davis J, Hsu A, Asher AI, O'Shea J, Holland SM, Paul WE, Douek DC. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature. 2008;452:773–776. doi: 10.1038/nature06764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ferwerda B, Ferwerda G, Plantinga TS, Willment JA, van Spriel AB, Venselaar H, Elbers CC, Johnson MD, Cambi A, Huysamen C, Jacobs L, Jansen T, Verheijen K, Masthoff L, Morre SA, Vriend G, Williams DL, Perfect JR, Joosten LA, Wijmenga C, van der Meer JW, Adema GJ, Kullberg BJ, Brown GD, Netea MG. Human dectin-1 deficiency and mucocutaneous fungal infections. N Engl J Med. 2009;361:1760–1767. doi: 10.1056/NEJMoa0901053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zelante T, De Luca A, Bonifazi P, Montagnoli C, Bozza S, Moretti S, Belladonna ML, Vacca C, Conte C, Mosci P, Bistoni F, Puccetti P, Kastelein RA, Kopf M, Romani L. IL-23 and the Th17 pathway promote inflammation and impair antifungal immune resistance. Eur J Immunol. 2007;37:2695–2706. doi: 10.1002/eji.200737409. [DOI] [PubMed] [Google Scholar]
- 85.Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, Daly MJ, Steinhart AH, Abraham C, Regueiro M, Griffiths A, Dassopoulos T, Bitton A, Yang H, Targan S, Datta LW, Kistner EO, Schumm LP, Lee A, Gregersen PK, Barmada MM, Rotter JI, Nicolae DL, Cho JH. A Genome-Wide Association Study Identifies IL23R as an Inflammatory Bowel Disease Gene. Science. 2006;314:1461–1463. doi: 10.1126/science.1135245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gaffen SL. The role of interleukin-17 in the pathogenesis of rheumatoid arthritis. Curr Rheumatol Rep. 2009;11:365–370. doi: 10.1007/s11926-009-0052-y. [DOI] [PMC free article] [PubMed] [Google Scholar]