Abstract
Aim
We used diffusion tensor imaging to investigate the association between white-matter integrity and reading ability in a cohort of 28 children. Nineteen preterm children (14 males, five females; mean age 11y 11mo [SD 1y 10mo], mean gestational age 30.5wks (SD 3.2), mean birthweight was 1455g [SD 625]); and nine term children (five males, four females; mean age 12y 8mo [SD 2y 5mo], mean gestational age 39.6 weeks (SD 1.2), and mean birthweight 3877g [SD 473]).
Method
We tested whether fractional anisotropy in a left hemisphere temporoparietal region and in the corpus callosum correlates with birthweight and scores on the following three subtests of the Woodcock-Johnson III Tests of Achievement: word identification, word attack, and passage comprehension.
Results
Preterm children had lower reading scores than a comparison group for all reading subtests (p<0.05). We found significant correlations between birthweight and fractional anisotropy in the whole corpus callosum (p=0.001), and between fractional anisotropy and reading skill in the genu (p=0.001) and body (p=0.001) of the corpus callosum. The correlation between reading skill and fractional anisotropy in a left temporoparietal region previously associated with reading disability was not significant (p=0.095).
Interpretation
We conclude that perinatal white-matter injury of the central corpus callosum may have long-term developmental implications for reading performance.
Brain injury is an important source of cognitive, learning, and behavioral impairment in children born preterm.1 Perinatal white-matter injury accounts for most neuropathological lesions in this population.2,3 White-matter injury results from a combination of factors, including immaturity of the vasculature in developing white matter and the vulnerability of oligodendroglial precursor cells to infection and hypoxic–ischemic injury between 24 and 32 weeks’ gestation.2 White-matter injury represents a spectrum of lesions ranging from the focal cystic necrotic lesions of periventricular leukomalacia to the non-cystic, diffuse myelination disturbances that comprise most cases.2
Diffusion tensor imaging (DTI), an application of magnetic resonance imaging, measures the microstructural properties of healthy and injured white matter.4 One commonly used measure is fractional anisotropy, a measure of the directionality of water diffusion. High fractional anisotropy values correspond to restricted diffusion in one direction; low values correspond to more isotropic diffusion. Fractional anisotropy of white matter is influenced by several factors, including myelination, packing density, and axon size.4 Reduced fractional anisotropy values in white matter indicate abnormality or damage, caused by loss or disorganization of axons or injury to myelin.5
DTI has been used to characterize differences in white matter between preterm and term infants and children.5–7 Two studies of adolescents born at low birthweight and/or preterm found reduced fractional anisotropy in the corpus callosum and other white-matter tracts.6,7 Skranes et al.5 compared very low birthweight adolescents with those of normal birthweight and found that fractional anisotropy in several specific regions correlated with visual and motor skills, IQ, and social deficits. Formal measures of receptive vocabulary and verbal IQ have also been found to correlate with fractional anisotropy values in specific white-matter tracts in preterm adolescents.6
Skilled reading is a crucial aspect of children’s academic performance. In both term and preterm children, reading difficulties at a young age often persist to adolescence and adulthood.8,9 In a retrospective study, the presence of white-matter injury, independent of gestational age, was associated with lower reading and spelling scores in school-age children.10
DTI has identified two specific white-matter regions that are associated with reading performance in children from the general population. Deutsch et al.11 first reported an association in children between reading performance scores and fractional anisotropy values in a left temporoparietal region, a finding that has since been replicated.12,13 Dougherty et al.14 have reported an association between reading skills and radial diffusivity, another measure of white-matter integrity, in callosal fibers connecting the temporal lobes. Further data are needed to understand fully the white-matter tracts that subserve reading.15
To summarize, previous studies have established connections between (1) preterm birth and damage to white matter, (2) preterm birth and reading difficulties, and (3) reading skills and damage to white matter. Our purpose in this study was to investigate the association between white-matter integrity and reading ability in a sample of children born preterm, who would be likely to have a wider range of fractional anisotropy values than participants in previous studies, and a comparison group of children born at term. We tested the hypothesis that birthweight and reading skills are associated with fractional anisotropy in regions of the brain previously found to be susceptible to white-matter injury.
Method
PARTICIPANTS
Participants (n=28; 19 preterm, nine term) were 9- to 16-year-old native English speakers (preterm: 14 males, five females; mean age 11y 11mo, SD 1y 10mo; term: five males, four females; mean age 12y 8mo, SD 2y 5mo; p=0.441). Written informed consent was obtained from all parents and assent from participants. The University of Pittsburgh Institutional Review Board and the Stanford Panel on Human Subjects in Medical and Non-Medical Research approved all procedures. Preterm participants responded to a mass mailing to families who had participated in an early intervention program for children born preterm. For preterm participants, gestational age and birthweight data were available for 18 participants. Among these participants, gestational age ranged from 24 to 36 weeks (mean 30.5wks, SD 325) and birthweight ranged from 572 to 2608g (mean 1455g, SD 625). The participant for whom gestational age and birthweight data were not available was excluded only from analyses of these factors. Participants born at term responded to advertisements in a variety of locations and periodicals. For participants born at term, gestational age ranged from 38 to 42 weeks (mean 39.6wks, SD 1.2) and birthweight ranged from 2863 to 4423g (mean 3877g, SD 473).
Complete perinatal medical records were available for nine preterm participants. Newborn transcranial ultrasound noted intraventricular hemorrhage in three participants. A history of intrauterine growth restriction, chorioamnionitis, and cerebral palsy was present in four, one, and one participant(s) respectively. Other complications, each noted in a different participant, included nuchal cord, oligohydramnios, premature rupture of membranes, pneumonia, and a course of indomethacin for patent ductus arteriosis.
BEHAVIORAL TESTING
Participants completed a battery of behavioral testing, including the four subtests of the Wechsler Abbreviated Scale of Intelligence,16 generating an IQ score (standardized mean 100, SD 15), and three the following subtests of the Woodcock-Johnson III Tests of Achievement17 (all with standardized mean 100, SD 15): word identification, a letter and word recognition task; word attack, reading of pseudo-words; passage comprehension, and comprehension of single sentences and short paragraphs.
ACQUISITION OF IMAGING DATA
Structural magnetic resonance imaging and DTI data were acquired with a Siemens 3T MAGNETOM Allegra (Erlangen, Germany) system with a standard circularity-polarized head coil. Pillows and tape minimized head movement. Earplugs dampened scanner noise.
Structural images were acquired first, using a sagittal magnetization-prepared rapid gradient-echo T1-weighted sequence (repetition time 1570ms, echo time 3.04ms, flip angle 8°, inversion time 800ms, voxel size 0.78mm×0.78125mm×0.78125mm). The DTI protocol was repeated 14 times, and the images were averaged to improve signal quality. The pulse sequence was a diffusion-weighted, single-shot, spin-echo, echo-planar imaging sequence (echo time 82ms, repetition time 3.9s, field of view 200mm, matrix size 128×128, voxel size 1.5625mm×1.5625mm×4mm). We acquired 29 axial slices of 4mm thickness (no skip) for two b values, b=0 and b=850s/mm2. Diffusion was measured in six non-collinear directions.
IMAGE PREPROCESSING
T1 and DTI data were preprocessed and analysed with mrVista (http://white.stanford.edu/software) and Matlab R2006b (The Mathworks, Natick, MA, USA). For each participant, the T2-weighted (b=0) images were co-registered to the T1-weighted three-dimensional MPRAGE anatomical images. Co-registration was initiated by using the scanner coordinates stored in the image headers to achieve an approximate alignment. This alignment was refined by using a mutual-information three-dimensional rigid-body co-registration algorithm from SPM2 (http://www.fil.ion.ucl.ac.uk/spm/). Several anatomical landmarks, including the anterior commissure, the posterior commissure, and the midsagittal plane, were identified by hand in the T1 images. With these landmarks, we computed a rigid-body transform from the native image space to the conventional anterior commissure–posterior commissure aligned space. The T1 images were resampled to the anterior commissure–posterior commissure aligned space with 1mm isotropic voxels. The DTI data were resampled to this anterior commissure–posterior commissure aligned space with 2mm isotropic voxels by using a spline-based tensor interpolation algorithm.18 The six elements of the diffusion tensor were determined by multivariate regression. The eigenvalue decomposition of the diffusion tensor was computed, and the fractional anisotropy was calculated by using the resulting eigenvalues. We confirmed by visual inspection of each participant’s data set the alignment of DTI and T1 images in the brain regions of interest. To report Montreal Neurological Institute coordinates, a nonlinear transformation was computed between the B0 volume and the Montreal Neurological Institute T2 template using the spatial normalization tools from Statistical Parametric Mapping (SPM2) software (http://www.fil.ion.ucl.ac.uk/spm/). Visual inspection of each participant’s DTI data revealed that echo planar imaging distortions were not significant in our regions of interest.
REGIONS OF INTEREST DEFINITIONS AND STATISTICAL ANALYSIS
We used a region of interest (ROI) analysis in which ROIs were defined in individual brains based on Montreal Neurological Institute coordinates and anatomical markers. We examined ROIs in a temporoparietal region (Fig. 1) and in the total corpus callosum. The temporoparietal ROI was defined by using the guidelines in Deutsch et al.11 The ROI was a sphere of 5mm radius at Montreal Neurological Institute coordinates (−28, −28, 25) and at the corresponding location in the right hemisphere (28, −28, 25).
Figure 1.
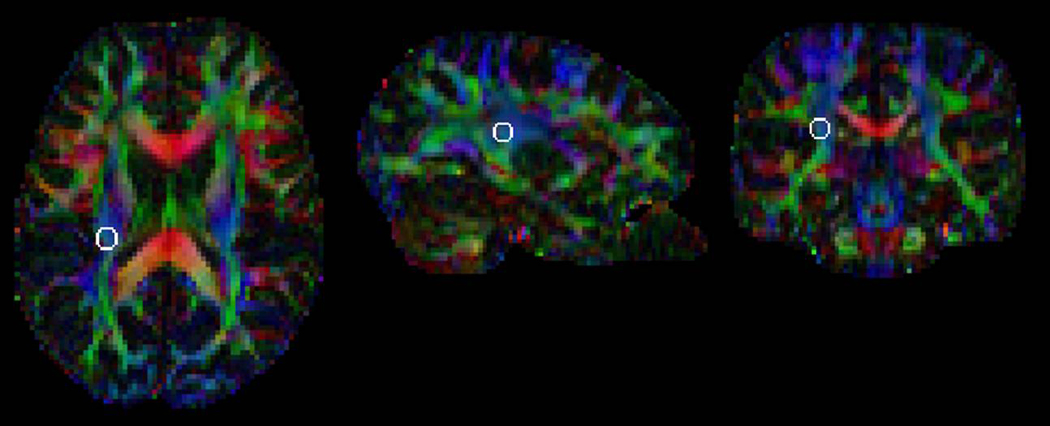
Temporoparietal Region of Interest (white circle) in a typical participant. The region of interest is overlaid on a background image that color-codes the principal diffusion direction (red, left-right; green, anterior-posterior; blue, superior-inferior). The region of interest is shown in the left hemisphere. A similar right hemisphere region of interest was also studied (not shown).
We next segmented the callosum by generating callosal ROIs following a standard anatomical scheme that has been previously applied to healthy and patient populations.19 In the midsagittal slice in each individual’s fractional anisotropy image, we created four callosal ROIs: (1) genu, (2) body, (3) isthmus, and (4) splenium. Following the standard method, the length of the callosum on the y-axis (L) was divided into thirds. The anterior third (L/3) was the genu, and the middle third (L/3) was the body. The posterior third was further divided such that the posterior segment (L/5) was the splenium and the remaining segment (L/3–L/5) was the isthmus. Each callosal ROI was defined manually on the fractional anisotropy map by a single researcher (JA) using a conservative fractional anisotropy threshold of 0.2 to confine the data to white matter only. A fractional anisotropy value of 0.15 was shown previously to provide a reliable threshold between gray matter and white matter.20 We simplified the standard scheme to increase the number of voxels in each ROI in order to increase the ROIs’ reliability (Fig. 2). In each ROI we calculated the mean fractional anisotropy.
Figure 2.
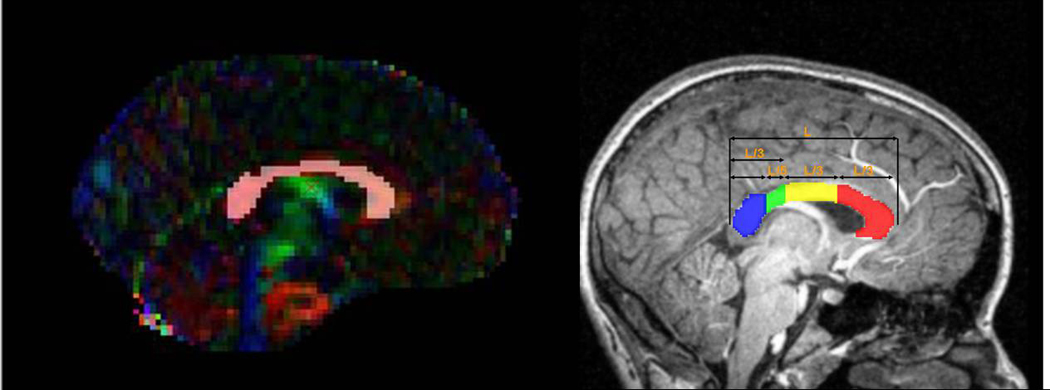
Left Panel: Total corpus callosum region of interest projected on a typical participant’s RBG map (color coded as in Fig. 1). Right panel, the corpus callosum segmentation (Adapted from Rotarska-Jagiela et al19, 2008).
Last, we generated the callosal ROIs in each individual’s T1 image. These T1 ROIs were used to test whether the ROI volume correlated with birthweight and/or reading scores. The T1 data, rather than the DTI data, were used to test for these correlations because of the greater spatial resolution of the T1 images compared with the DTI images. Each ROI’s volume (in cubic millimeters) was calculated. To control for differences in whole-brain white-matter volume, we created a white-matter mask, using a threshold of fractional anisotropy greater than 0.2, from each participant’s DTI image. The volume of this white-matter mask gave us a measure of whole-brain white-matter volume.
DATA ANALYSIS
We conducted group comparisons using Student’s t-test, and we tested for associations using Spearman’s rank correlation coefficient as defined by SPSS for Windows (version 16.0; SPSS Inc., Chicago, IL USA). Significance tests were two-tailed. We used the non-parametric correlation test because of the relatively small sample size in which data for fractional anisotropy, gestational age, birthweight, and reading score were not normally distributed. We also tested for a correlation between ROI volume and reading scores while partialling out the effect of whole-brain white-matter volume. We used linear regression to test which of the above factors predicted reading scores. All statistical calculations were performed using with SPSS. We set statistical significance at p<0.05. Given the small number of hypothesis-driven comparisons, we did not correct the significance threshold for multiple comparisons.21
Results
BEHAVIORAL RESULTS
Table I summarizes the participants’ IQ and reading scores. Participants, as a group, performed in the normal range. However, group differences in mean scores on the Wechsler Abbreviated Scale of Intelligence (WASI: p=0.02), word identification (p=0.001), word attack subtest (p=0.001), and passage comprehension (p=0.021) were significant. Scores on all subtests correlated at p<0.05, except word attack and passage comprehension (rho=0.218 and p=0.319 respectively). The correlations of birthweight with word identification and word attack scores were significant (identification: rho=0.472, p=0.011; attack: rho=0.533, p=0.004).
Table I.
Comparison of reading scores and fractional anisotropy of regions of interest (ROI) between preterm and term participants, mean (SD)
| Preterm (n=19) |
Term (n=9) | p value | |
|---|---|---|---|
| Standardized test | |||
| Wechsler Abbreviated Scale of Intelligence (n=23) |
100.3 (13.25) |
112.9 (5.69) | 0.002 |
| WJ-III word identification (n=24) |
98.5 (11.33) |
114.5 (9.12) | 0.001 |
| WJ-III passage comprehension (n=24) |
97.5 (15.19) |
107.8 (7.25) | 0.021 |
| WJ-III word attack (n=23) | 99.1 (9.75) | 114.5 (8.60) | 0.001 |
| Fractional anisotropy | |||
| Left temporoparietal ROI | 0.482 (0.069) |
0.515 (0.066) |
0.224 |
| Right temporoparietal ROI | 0.476 (0.088) |
0.513 (0.051) |
0.174 |
| Total corpus callosum | 0.599 (0.055) |
0.668 (0.024) |
0.001 |
| Genu of the corpus callosum | 0.614 (0.029) |
0.678 (0.025) |
0.001 |
| Body of the corpus callosum | 0.498 (0.060) |
0.618 (0.050) |
0.001 |
| Isthmus of the corpus callosum | 0.514 (0.100) |
0.568 (0.042) |
0.057 |
| Splenium of the corpus callosum |
0.665 (0.066) |
0.713 (0.046) |
0.036 |
Significant values in bold type. WJ-III, Woodcock-Johnson III Tests of Cognitive Abilities.
CONVENTIONAL MAGNETIC RESONANCE IMAGING FINDINGS
Radiological findings on T1 images were within the range of normal for all participants who were born at term except one who was noted to have a cerebellar Chiari I malformation. Among the preterm participants, 13 had normal imaging appearances, three had ventricular dilatation (one mild, two moderate), three had moderate thinning of the posterior corpus callosum, and one had persistent cavum septum pellucidum and vergae.
FRACTIONAL ANISOTROPY VALUES
The mean fractional anisotropy values for callosal ROIs in preterm and term participants are summarized in Table I. Fractional anisotropy differed significantly between these two groups in the total corpus callosum (p=0.001); differences within the genu (p=0.01), body (p=0.001), and splenium (p=0.036), but not isthmus, were significant. The association of fractional anisotropy in the whole callosum and birthweight was significant (ρ=0.653, p=0.001; Table II). Birthweight correlated with fractional anisotropy in the genu (rho=0.656, p=0.001) and body (rho=0.601, p=0.001) of the callosum. Interestingly, fractional anisotropy did not differ significantly between preterm and term participants in the left or right temporoparietal ROIs, though the correlation between fractional anisotropy values in the left and right temporoparietal ROIs was significant (rho=0.659, p=0.001).
Table II.
Correlations between birthweight, Woodcock-Johnson III (WJ-III) reading scores, and fractional anisotropy for the temporoparietal regions of interest and corpus callosum
| Fractional anisotropy of temporoparietal regions of interest |
Fractional anisotropy of the corpus callosum | |||||||
|---|---|---|---|---|---|---|---|---|
| Birthweight | Left | Right | Total | Genu | Body | Isthmus | Splenium | |
| Birthweight | 0.057 (−0.323 to 0.421) |
0.028 (−0.349 to 0.397) |
0.653a (0.370 to 0.825) |
0.656a (0.375 to 0.827) |
0.601a (0.294 to 0.796) |
0.335 (−0.044 to 0.629) |
0.379 (−0.007 to 0.659) |
|
| WJ-III ID |
0.472b (0.120 to 0.719) |
0.322 (−0.058 to 0.621) |
0.371 (−0.002 to 0.654) |
0.553b (0.227 to 0.768) |
0.602a (0.295 to 0.796) |
0.755a (0.532 to 0.880) |
0.256 (−0.129 to 0.574) |
0.154 (−0.232 to 0.498) |
| WJ-III WA |
0.533b (0.200 to 0.756) |
0.254 (−0.132 to 0.573) |
0.093 (−0.290 to 0.450) |
0.537b (0.205 to 0.758) |
0.543b (0.213 to 0.762) |
0.537b (0.205 to 0.758) |
0.215 (−0.172 to 0.544) |
0.400b (0.032 to 0.673) |
| WJ-III PC |
0.279 (−0.105 to 0.591) |
0.417b (0.052 to 0.684) |
0.459b (0.104 to 0.710) |
0.310 (−0.071 to 0.612) |
0.348 (−0.029 to 0.638) |
0.527b (0.192 to 0.752) |
0.062 (−0.318 to 0.425) |
0.075 (−0.307 to 0.436) |
p<0.001;
p<0.05;
ID, word identification; WA, word attack, PC, passage comprehension; values in parentheses are 95% confidence intervals.
CORRELATIONS WITH READING
Table II summarizes the correlations of birthweight and fractional anisotropy with reading scores. The correlation of fractional anisotropy of the left and right temporoparietal ROIs with word identification scores approached but did not reach statistical significance (left: rho=0.322, p=0.095; right: rho=0.371, p=0.052), though the correlation of fractional anisotropy of these regions with passage comprehension was significant (left: rho=0.416, p=0.027; right: rho=0.451, p=0.014). The correlation of fractional anisotropy of the whole callosum and word identification scores was significant (rho=0.553, p=0.002). Word identification scores and fractional anisotropy of the genu (rho=0.602, p=0.001) and body (rho=0.755, p=0.001) of the callosum showed a strong positive correlation (Fig. 3). The correlation between fractional anisotropy and word identification in the body, not in the genu, remained highly significant even after controlling for birthweight (rho=0.531, p=0.009) and then for WASI IQ score (rho=0.519, p=0.008). Stepwise linear regression revealed that fractional anisotropy of the body of the callosum was significantly associated with word identification scores (β=0.679, p=0.001), whereas birthweight, WASI, and fractional anisotropy of temporoparietal and all other callosal ROIs were not. Stepwise linear regression also revealed that WASI scores were significantly associated with passage comprehension (β=0.698, p=0.001), whereas birthweight and fractional anisotropy in temporoparietal and all callosal ROIs were not.
Figure 3.
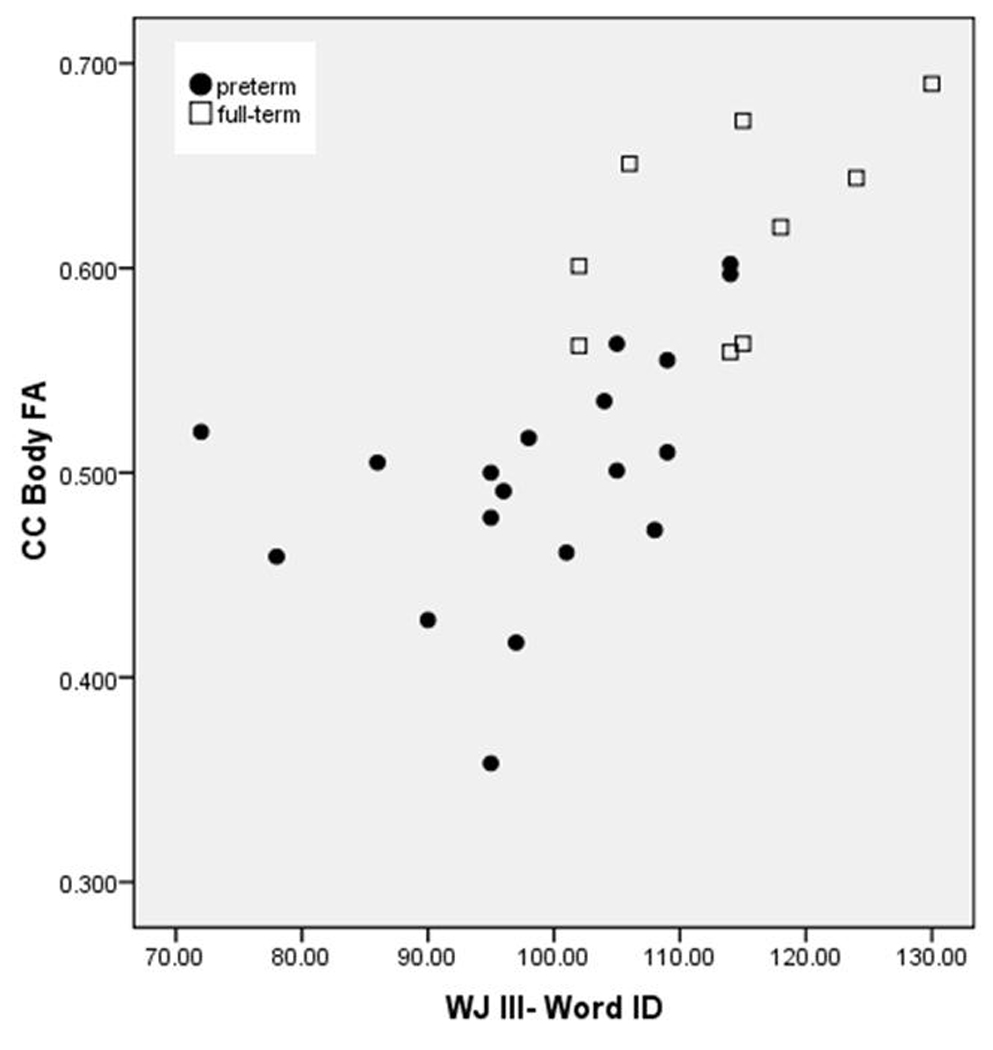
Woodcock-Johnson III Tests of Achievement word identification (WJ III-Word ID) scores and fractional anisotropy (FA) in the body of the corpus callosum (CC) in preterm and term participants‥
[Typesetter: preterm should be Preterm; Full-term should be Term. Please remove background shading and borders]
We did not detect a significant correlation between volume and word identification scores or between volume and birthweight in any callosal segment. Stepwise linear regression revealed that fractional anisotropy of the body of the callosum, but not white-matter volume, was significantly associated with word identification scores.
Discussion
We report a novel finding that reading performance correlates with fractional anisotropy in the body of the callosum in a group of children in a ranging of gestational ages. In addition, we identified a difference in mean reading scores between preterm and term participants, and we corroborated a significant difference in mean fractional anisotropy of the corpus callosum in preterm versus term children.6,7 The correlation between decoding skill and fractional anisotropy of a previously studied left temporoparietal ROI was not significant; however, the correlation of passage comprehension and fractional anisotropy in this region was significant.15
The correlation between fractional anisotropy and reading in the body of the corpus callosum persisted when we controlled for birthweight and IQ, indicating that this correlation is not explained by differences in the degree of preterm birth or overall IQ. Rather, this trend probably reflects how underlying differences in properties of white-matter microstructure influence reading skill. The presence of this correlation between fractional anisotropy and reading among children of varying degrees of preterm birth suggests that these regions of the callosum may be particularly important to reading performance.
As in previous studies,7,22 we found that white-matter microstructural abnormalities, the sequelae of preterm birth and white-matter injury, persist into adolescence and are detectable by DTI. Moreover, DTI detected these abnormalities despite a normal appearance on conventional T1 imaging for nearly all participants. We also observed a correlation with specific cognitive function: skilled reading performance. Perinatal white-matter injury of the central callosum, may, therefore, have long-term developmental implications for reading performance throughout childhood and into young adulthood. DTI measurements, such as fractional anisotropy, may be useful in identifying which school-age children will experience reading difficulties as young adults.
Others have reported reduced fractional anisotropy throughout the callosum, including in the body, in preterm children,7 and a correlation between cognitive language skills and fractional anisotropy in the posterior callosum in term children.14,23 Recent tractography studies have depicted the projections from the body of the callosum as terminating in the posterior frontal and anterior parietal cortices.14,24 These projections to the posterior frontal cortex may include projections to the posterior region of the inferior frontal gyrus, which is extensively implicated in phonological processing.25 We recognize that in children with white-matter injury, organization of white-matter tracts may be altered.26 Thus we are cautious to interpret which tracts are implicated in our cohort.
Volume did not correlate with reading in any of the callosal segments, which suggests that the relation between fractional anisotropy and reading is the result of white-matter microstructural differences detected by DTI rather than regional differences in white-matter volume. Fractional anisotropy reductions reflect disruptions of axonal membrane organization and myelination.27 For a cognitive process, such as reading, which relies on widely distributed neural pathways throughout the brain, disruptions of the relevant white-matter tracts may, therefore, be associated with reduced cognitive functioning.
Previous studies11,28 have reported a correlation between fractional anisotropy and reading performance in a left temporoparietal ROI. In our cohort, the correlation between fractional anisotropy of this ROI and word identification, a measure of decoding, approached but did not reach statistical significance. However, the correlation with passage comprehension, a measure associated with overall IQ, was significant. We may not have identified this association because the participants in our study had higher reading scores than the poor readers in previous studies. It is also possible that this negative finding reflects difficulties of the normalization process in aligning neuroanatomical structures for preterm children with diffuse white-matter injury. We favor the latter explanation, and we hypothesize that positional shifting of one or more of the major white-matter tracts traversing this region may explain why the correlation with word identification failed to reach statistical significance in our cohort. Fractional anisotropy values in the left and right temporoparietal ROIs were highly correlated (rho=0.659), which argues against explaining our negative finding in the left hemisphere by increased lateralization of the relevant white-matter tracts to the right hemisphere. Moreover, our ability to detect the correlations between fractional anisotropy and passage comprehension in the temporoparietal ROI and between fractional anisotropy and word identification in the callosum suggests that our study was sufficiently powered to detect the trends of interest.
Our study is limited by a relatively small sample size (n=28) and by our inability to obtain perinatal medical records on some preterm participants. Increased spatial resolution and isotropic voxels would have enabled us to dissect the callosum using fiber-tracking techniques that detect individual variability in callosal shape and organization.
Conclusion
White-matter microstructural measures in the body of the callosum significantly correlated with reading skills in a sample of preterm and term children. These findings suggest that this region is important to reading performance. Future research will examine the fiber-tract projections of this region of the callosum and elucidate the cortical regions subserved to understand the cognitive sequelae of diffuse white-matter injury. Characterizing white-matter integrity, tract organization, and cognitive abilities may lead to understanding of neural plasticity and re-organization after the white-matter injury of preterm birth.
ACKNOWLEDGMENTS
We thank the participants and their families for their contributions. We also thank Barbara Fritz, Laura Mazurkewicz, and Gina Acquavita for data collection; Bob Dougherty, Brian Wandell, Miya Asato, and Robert Terwilliger for assistance with methodology; and Susan R Hintz for comments on an earlier version of the manuscript. This work was supported by grants from the Stanford University Medical School Medical Scholars Program and the National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development, RO1 HD046500.
References
- 1.Vohr BR, Allan WC, Westerveld M, et al. School-age outcomes of very low birth weight infants in the indomethacin intraventricular hemorrhage prevention trial. Pediatrics. 2003;111(4 Pt 1):e340–e346. doi: 10.1542/peds.111.4.e340. [DOI] [PubMed] [Google Scholar]
- 2.Back SA. Perinatal white matter injury: the changing spectrum of pathology and emerging insights into pathogenetic mechanisms. Ment Retard Dev Disabil Res Rev. 2006;12:129–140. doi: 10.1002/mrdd.20107. [DOI] [PubMed] [Google Scholar]
- 3.Volpe JJ. Neurobiology of periventricular leukomalacia in the premature infant. Pediatr Res. 2001;50:553–562. doi: 10.1203/00006450-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Le Bihan D, Mangin JF, Poupon C, et al. Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging. 2001;13:534–546. doi: 10.1002/jmri.1076. [DOI] [PubMed] [Google Scholar]
- 5.Skranes J, Vangberg TR, Kulseng S, et al. Clinical findings and white matter abnormalities seen on diffusion tensor imaging in adolescents with very low birth weight. Brain. 2007;130:654–666. doi: 10.1093/brain/awm001. [DOI] [PubMed] [Google Scholar]
- 6.Constable RT, Ment LR, Vohr BR, et al. Prematurely born children demonstrate white matter microstructural differences at 12 years of age, relative to term control subjects: an investigation of group and gender effects. Pediatrics. 2008;121:306–316. doi: 10.1542/peds.2007-0414. [DOI] [PubMed] [Google Scholar]
- 7.Vangberg TR, Skranes J, Dale AM, Martinussen M, Brubakk AM, Haraldseth O. Changes in white matter diffusion anisotropy in adolescents born prematurely. Neuroimage. 2006;32:1538–1548. doi: 10.1016/j.neuroimage.2006.04.230. [DOI] [PubMed] [Google Scholar]
- 8.Hack M, Flannery DJ, Schluchter M, Cartar L, Borawski E, Klein N. Outcomes in young adulthood for very-low-birth-weight infants. N Engl J Med. 2002;346:149–157. doi: 10.1056/NEJMoa010856. [DOI] [PubMed] [Google Scholar]
- 9.Shaywitz SE, Morris R, Shaywitz BA. The education of dyslexic children from childhood to young adulthood. Annu Rev Psychol. 2008;59:451–475. doi: 10.1146/annurev.psych.59.103006.093633. [DOI] [PubMed] [Google Scholar]
- 10.Downie AL, Frisk V, Jakobson LS. The impact of periventricular brain injury on reading and spelling abilities in the late elementary and adolescent years. Child Neuropsychol. 2005;11:479–495. doi: 10.1080/09297040591001085. [DOI] [PubMed] [Google Scholar]
- 11.Deutsch GK, Dougherty RF, Bammer R, Siok WT, Gabrieli JD, Wandell B. Children’s reading performance is correlated with white matter structure measured by diffusion tensor imaging. Cortex. 2005;41:354–363. doi: 10.1016/s0010-9452(08)70272-7. [DOI] [PubMed] [Google Scholar]
- 12.Beaulieu C, Plewes C, Paulson LA, et al. Imaging brain connectivity in children with diverse reading ability. Neuroimage. 2005;25:1266–1271. doi: 10.1016/j.neuroimage.2004.12.053. [DOI] [PubMed] [Google Scholar]
- 13.Niogi SN, McCandliss BD. Left lateralized white matter microstructure accounts for individual differences in reading ability and disability. Neuropsychologia. 2006;44:2178–2188. doi: 10.1016/j.neuropsychologia.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 14.Dougherty RF, Ben-Shachar M, Deutsch GK, Hernandez A, Fox GR, Wandell BA. Temporal-callosal pathway diffusivity predicts phonological skills in children. Proc Natl Acad Sci U S A. 2007;104:8556–8561. doi: 10.1073/pnas.0608961104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ben-Shachar M, Dougherty RF, Wandell BA. White matter pathways in reading. Curr Opin Neurobiol. 2007;17:258–270. doi: 10.1016/j.conb.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 16.Wechsler D. Wechsler Abbreviated Scale of Intelligence Manual. San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- 17.Woodcock R, McGrew K, Mather N. Woodcock-Johnson III Tests of Achievement. Itasca, IL: Riverside Publishing; 2001. [Google Scholar]
- 18.Pajevic S, Aldroubi A, Basser PJ. A continuous tensor field approximation of discrete DT-MRI data for extracting microstructural and architectural features of tissue. J Magn Reson. 2002;154:85–100. doi: 10.1006/jmre.2001.2452. [DOI] [PubMed] [Google Scholar]
- 19.Rotarska-Jagiela A, Schonmeyer R, Oertel V, Haenschel C, Vogeley K, Linden DE. The corpus callosum in schizophrenia – volume and connectivity changes affect specific regions. Neuroimage. 2008;39:1522–1532. doi: 10.1016/j.neuroimage.2007.10.063. [DOI] [PubMed] [Google Scholar]
- 20.Jones DK, Simmons A, Williams SC, Horsfield MA. Non-invasive assessment of axonal fiber connectivity in the human brain via diffusion tensor MRI. Magn Reson Med. 1999;42:37–41. doi: 10.1002/(sici)1522-2594(199907)42:1<37::aid-mrm7>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 21.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–46. [PubMed] [Google Scholar]
- 22.Nagy Z, Westerberg H, Skare S, et al. Preterm children have disturbances of white matter at 11 years of age as shown by diffusion tensor imaging. Pediatr Res. 2003;54:672–679. doi: 10.1203/01.PDR.0000084083.71422.16. [DOI] [PubMed] [Google Scholar]
- 23.Frye RE, Hasan K, Xue L, et al. Splenium microstructure is related to two dimensions of reading skill. Neuroreport. 2008;19:1627–1631. doi: 10.1097/WNR.0b013e328314b8ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hofer S, Frahm J. Topography of the human corpus callosum revisited – comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. Neuroimage. 2006;32:989–994. doi: 10.1016/j.neuroimage.2006.05.044. [DOI] [PubMed] [Google Scholar]
- 25.Vigneau M, Beaucousin V, Herve PY, et al. Meta-analyzing left hemisphere language areas: phonology, semantics, and sentence processing. Neuroimage. 2006;30:1414–1432. doi: 10.1016/j.neuroimage.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 26.Yeatman JD, Ben Shachar M, Bammer R, Feldman HM. Using diffusion tensor imaging and fiber tracking to characterize diffuse perinatal white matter injury: a case report. J Child Neurol. 2009 doi: 10.1177/0883073808331080. doi:10.1177/0883073808331080: published online 11 May 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beaulieu C. The basis of anisotropic water diffusion in the nervous system – a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- 28.Klingberg T, Hedehus M, Temple E, et al. Microstructure of temporoparietal white matter as a basis for reading ability: evidence from diffusion tensor magnetic resonance imaging. Neuron. 2000;25:493–500. doi: 10.1016/s0896-6273(00)80911-3. [DOI] [PubMed] [Google Scholar]


