Abstract
To examine the impact of interstimulus “jitter” (i.e., randomization of the interval between successive stimulus events) on response control during continuous task performance, 41 healthy adults completed four go/no-go tasks that were identical except for interstimulus interval (ISI) jitter: a 0% jitter task with a fixed (1,000-ms) ISI, a 10% jitter task with an ISI range of 900–1,100 ms, a 30% jitter task with an ISI range of 700–1,300 ms, and a 50% jitter task with an ISI range of 500–1,500 ms. Repeated measures analysis of variance (ANOVA) revealed a quadratic effect of jitter on commissions across the group and on intrasubject reaction time variability in men; in both cases, performance was best for the 10% jitter condition. A linear effect of jitter was observed for reaction time (RT) with high levels of jitter (50%) resulting in longer RT. Findings suggest that response selection, including inhibition, is optimized by moderate increases in ISI jitter. More deliberate and controlled responding observed with increasing jitter may have important treatment implications for disorders (e.g., attention-deficit/hyperactivity disorder, ADHD), associated with impaired response control.
Keywords: Inhibitory control, Response preparation, Intrasubject variability, Supplementary motor area, Go/no-go, Attention
Response control is a basic underpinning of human behavior and reflects an individual’s ability to accurately and efficiently select a preferred response while inhibiting the selection of an unwanted response (Mostofsky & Simmonds 2008). Therefore, response control is essential in achieving a goal. Methods for improving controlled responding have been explored in relation to optimizing performance during tasks for which accurate and efficient response selection is critical (e.g., driving a car; Barkley, Murphy, O’Connell, & Connor, 2005). However, there has been a notable lack of research examining methods for improving performance on computerized tasks specifically designed to assess response selection and inhibition. These “continuous performance” tasks (CPTs) have proven useful in clinical assessment of disorders characterized by impaired behavioral control, particularly attention-deficit/hyperactivity disorder (ADHD).
In both clinical and typically developing populations the go/no-go (GNG) paradigm is a form of CPT, often used to assess response control, with weighting towards “go” stimuli creating a pre-potent tendency to respond, thereby taxing the ability to switch to withholding a response when the “no-go” stimulus appears. Response control in this design is assessed using indices of accurate response inhibition (commission errors) and consistency in response selection in reaction time (intrasubject variability: ISV; Simmonds et al., 2007). Several studies have found strong correlations between ISV and commission error rate on GNG tasks (Bellgrove, Hester, & Garavan, 2004; Klein, Wendling, Huettner, Ruder, & Peper, 2006; Simmonds et al., 2007; Verte, Geurts, Roeyers, Oosterlaan, & Sergeant, 2006), and there is substantial evidence that response selection and inhibition are closely related processes, both dependent on neural mechanisms critical to motor response preparation (Mostofsky & Simmonds, 2008). Findings from animal and human neuro-physiologic studies indicate that neural circuits centered in the rostral supplementary area (“pre-SMA”) are critical for accurate response selection and response inhibition (e.g., Isdoa & Hikosaka, 2007; Lim et al., 1994).
Given the critical role of the pre-SMA, it follows that efficient and accurate response selection and inhibition, reflected as lower GNG commission rate and lower ISV, might be associated with increased preparedness to respond (i.e., response preparation). That is, if readiness to respond can be improved, controlled responding can be facilitated. One potential method for increasing readiness to respond is to vary the interval between successive stimulus events (i.e., interstimulus interval, ISI, jitter). ISI jitter is frequently used in event-related signal processing measures (e.g., functional magnetic resonance imaging, fMRI; event-related potential, ERP) to improve measurement of event-related changes by reducing signal to noise (McCarthy, Puce, Luby, Belger, & Allison, 1996).
Multiple parameters of CPTs and GNG tasks may be manipulated, and while commonly used paradigms, such as the Conners’ CPT (Conners, 2000) and the Test of Variables of Attention (TOVA, Greenberg, 1996), incorporate ISI changes between blocks, the effect of varying ISI within blocks of trials has not been examined. Several studies have examined the effect of varying the time between a warning cue and a target (i.e., stimulus onset asynchrony or SOA)—for instance, during Posner (Correa, Lupianez, Madrid, & Tudela, 2006) or visual search tasks (Luo, Greenwood, & Parasuraman, 2001). In addition, the impact of changes in the rate of presentation of GNG stimuli on performance has been examined (Durston et al., 2007; Wiersema, van der Meere, Roeyers, Van Coster, & Baeyens, 2006). However, we are unaware of any studies examining the effect of ISI jitter—that is, varying the interval between the target stimuli themselves (as opposed to varying ISI between blocks of stimuli as employed in commercially available GNG or other CPTs).
The present study examined impact of ISI jitter on performance on a GNG task in adults. We postulated that if preparedness to respond can be increased (by introducing ISI jitter), then response selection and inhibition would be facilitated. Therefore, we hypothesized that ISI jitter would improve accuracy of GNG task performance, with a decrease in commission error rate and decreased variability. We had no a priori hypothesis specifying the level of jitter that would result in optimal performance and therefore assessed participants’ performance at four different levels of ISI jitter.
METHOD
Participants
Participants included 41 healthy adults ages 18–40 years (Mage = 27.9, SD = 5.2) who were recruited through flyers posted at Johns Hopkins University, at the Kennedy Krieger Institute, and in the community. Exclusion criteria included history of psychiatric or neurological illness (including, but not limited to depression, anxiety, traumatic brain injury requiring medical attention, seizure history); participants taking any psychotropic medication were also excluded. Demographic characteristics of the sample are presented in Table 1. The study was approved by the Johns Hopkins Medical Institutional Review Board. Participants were monetarily compensated for their participation after completing the study.
TABLE 1.
Demographic information
| Men (n = 16) |
Women (n = 25) |
|||||
|---|---|---|---|---|---|---|
| % | M | SD | % | M | SD | |
| Age | 26.8 | 5.6 | 28.4 | 4.9 | ||
| Highest level of education | ||||||
| High-school grad | 25 | 4 | ||||
| Some college | 25 | 20 | ||||
| College grad | 19 | 20 | ||||
| Postcollege | 31 | 56 | ||||
Procedure
All participants were initially screened about inclusion/exclusion criteria through a brief interview (completed either in person or over the telephone). Participants then completed four GNG conditions over two 30-minute appointments, occurring on two separate days (Mdays between appointments = 4.6, SD = 5.5, range = 1–21 days), and completed two consecutive tasks during each appointment. Participants were given a 1-minute break halfway through each GNG task and an approximate 3-minute break between tasks (i.e., three breaks per appointment). Order of condition presentation was counterbalanced to control for practice effects (i.e., participants were randomly assigned to one of four presentation orders).
Paradigms
The GNG paradigm is based on the simple GNG task described in Mostofsky and colleagues (2003) and Wodka and colleagues (2007). For all conditions, participants were seated in front of a computer that flashed red and green spaceships. Participants were instructed to push a button with their index finger as quickly as possible in response to green spaceships only. Use of familiar stimulus–response associations (green for “go;” red for “no-go”) minimized the perceptual and cognitive demands of the test (i.e., making it a “simple” GNG task). Cues were weighted towards green spaceships at a ratio of 3:1 (225 go cues; 75 no-go cues), intensifying the need to inhibit a rapid, habitual skeletomotor response. Green and red trials appeared pseudorandomly (derived using the randomization function in Microsoft Excel), with the restriction that there were never fewer than 3 green (go) trials before a red (no-go) cue and never more than 2 red (no-go) trials in a row. There were 20 practice trials followed by 300 experimental trials. Stimuli were present on-screen for 200 ms, and the time between trials was jittered around 1,000 ms (described below). The total time of each condition was identical (6 min 38 s).
There were four different GNG conditions, which varied based on percentage of ISI jitter. Condition 1 did not jitter ISI, and the ISI was constant at 1,000 ms. The remaining conditions jittered ISI by a set percentage (which differed for each of the three remaining conditions). The specific ISI presentation (based on the % jitter) was generated randomly. For Condition 2, a 10% jitter around the 1,000-ms ISI was used (i.e., ISIs of 900, 950, 1,000, 1,050, and 1,100 ms). Condition 3 employed a 30% jitter (e.g., ISIs of 700, 850, 1,000, 1,150, and 1,300 ms), and Condition 4 employed a 50% jitter (e.g., ISIs of 500, 750, 1,000, 1,250, and 1,500 ms). Percentage of commission errors, reaction time, and intrasubject variability (ISV) were used in comparisons. Of note, each participant’s ISV was calculated as the coefficient of variability (CV): (standard deviation go-reaction time)/(mean go-reaction time) × 100 (Stuss, Murphy, Binns, & Alexander, 2003).
Paradigm programming was done using Presentation software (Neurobehavioral Systems, Albany, CA, USA) using Windows XP.
Data analyses
A series of three repeated measures analyses of variance (ANOVAs) were used to examine performance by jitter condition for each of the three dependent variables (percentage of commission errors; ISV; reaction time, RT) as well as the interaction between jitter condition and sex. As we had no prior hypothesis specifying level of jitter that would optimize performance, linear and quadratic relationships between jitter condition and dependent variables were explored to protect against Type II error. For those dependent measures yielding a significant effect for jitter, six separate paired t tests were used to compare individual conditions (e.g., 0% vs. 10%, 0% vs. 30%, etc.). For those dependent measures yielding a significant interaction effect, post hoc repeated measures ANOVA was used for each sex individually to examine performance by jitter condition, and paired samples t tests were used to examine performance on each jitter condition within men and women separately. Effect size values were computed using the d statistic. Effect size is a standardized quantitative index that can represent the magnitude of change that one variable produces in another variable as reflected in the difference between two means, independent of sample size (Cohen, 1988). Interpretation of the effect size d is based on a convention suggested by Cohen, such that 0.20 is considered a “small” effect size, 0.50 considered “medium,” and 0.80 or greater a “large” effect size.
RESULTS
Preliminary analyses
Pearson correlations were used to examine the association of demographic variables (i.e., age, education, and race) and outcome measures (commission errors, ISV, and RT) by jitter condition. Education was significantly correlated with commission errors on the 10% jitter condition (r = −.35, p = .025), with individuals with lower levels of education committing more commission errors. As only 1 of 24 correlations was significant at p < .05, there were no demographic variables chosen to use as covariates.
Percentage of commission error performance by jitter condition
Results of repeated measures ANOVA did not reveal a significant main effect (linear) or interaction effect (jitter by sex) on percentage of commission errors; however, within-subjects contrasts revealed a significant quadratic effect, F(1, 39) = 21.4, p < .001, for the whole sample, with the fewest commissions observed at the 10% jitter condition (Figure 1).
Figure 1.
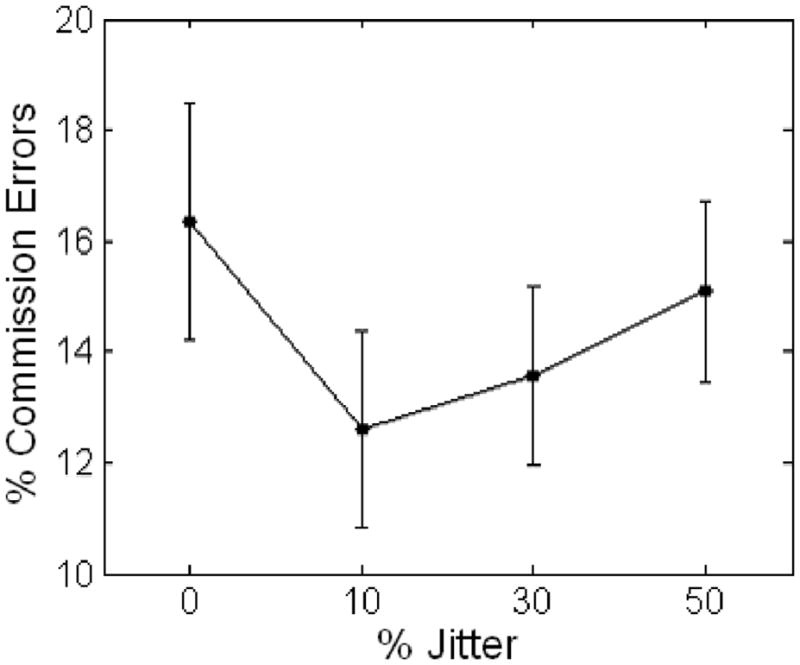
Within-subjects quadratic effect of jitter on commission errors: analysis of variance (ANOVA). Significant within-subjects quadratic effect (p < .001). Error bars represent standard error.
ISV for reaction time performance by jitter condition
Results of repeated measures ANOVA did not reveal a significant main effect of jitter on ISV; however, there was a significant interaction effect of jitter by sex on ISV, F(3, 37) = 2.8, p < .05, d = 0.20. Post hoc repeated measures ANOVA for women was not significant; however, a significant quadratic effect was observed for men, F(3, 13) = 5.6, p = .031, d = 0.30, with the lowest level of variability observed at the 10% jitter condition (Figure 2). Further, paired samples t tests indicated that women were significantly less variable than men on the 0%, t(40) = −3.2, p = .002; 30%, t(40) = −2.2, p = .032; and 50%, t(40) = −2.8, p = .006, conditions, but not the 10% condition, t(40) = −0.7, p = .503.
Figure 2.
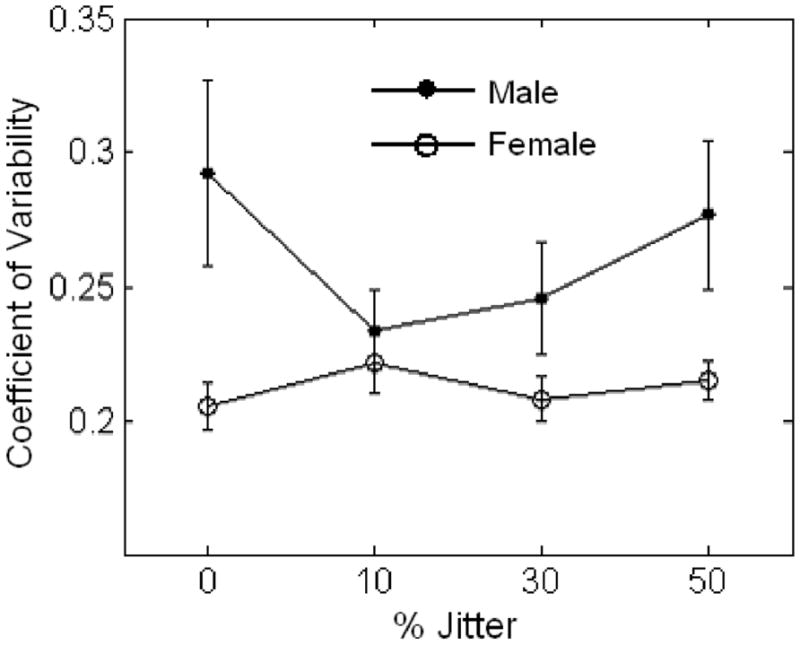
Jitter by sex interaction effect on intrasubject variability (ISV): analysis of variance (ANOVA). Significant jitter by sex interaction on reaction time (RT) variability (p = .05). Significant quadratic effect for men only (p = .032). Error bars represent standard error.
Reaction time performance by jitter condition
Repeated measures ANOVA revealed a significant main effect of jitter condition on reaction time, F(3, 37) = 8.0, p < .001, d = 0.40, and within-subjects contrasts revealed a significant linear effect, F(1, 39) = 21.4, p < .001 (Figure 3) for the whole sample—such that greater jitter was associated with increased reaction time. There was no significant sex by jitter interaction. Post hoc paired samples t tests revealed that RT was significantly slower on the 50% jitter condition than on all other conditions: 0%, t(1, 40) = −5.1, p < .001; 10%, t(1, 40) = −2.6, p = .014; 30%, t(1, 40) = −3.2, p = .002.
Figure 3.
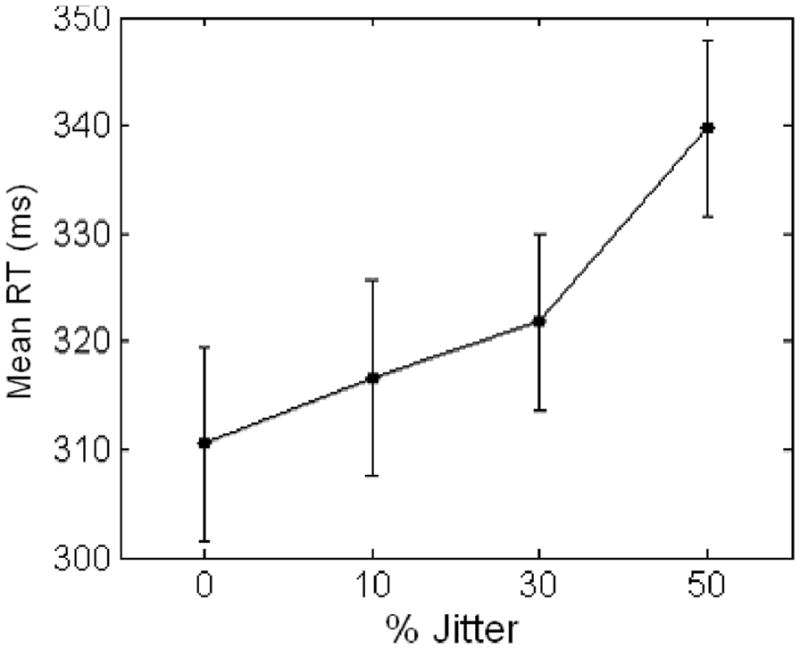
Main effect of jitter on reaction time: analysis of variance (ANOVA). Significant linear main effect of jitter on reaction time (p < .001). Paired samples t test: 50% > 0%, p < .001; 50% > 10%, p = .014; and 50% > 30%, p = .002. Error bars represent standard error.
DISCUSSION
The purpose of this study was to examine the impact of ISI jitter on GNG task performance (i.e., response preparation, selection, and inhibition). While there is evidence that manipulating aspects of GNG tasks (e.g., cognitive demands) impacts performance and neurological correlates (Mostofsky et al., 2003; Wodka et al., 2007), there has been no prior research examining how introducing uneven intervals between stimulus presentation (i.e., ISI jitter) impacts performance on GNG tasks. Consistent with our hypothesis, results showed that a moderate level of ISI jitter (i.e., 10%) facilitates inhibitory control; however, a high level of ISI jitter (i.e., 50%) does not support inhibitory control. Similarly, improvement in variability of responding was also observed at the 10% jitter condition in men; however, this effect was not observed for women. The findings suggest that improvements in accuracy in responding, with lower inhibitory error rate (men and women) and decreased variability (in men), can be optimized with moderate amounts of jitter.
Effects of ISI jitter on RT showed a different pattern than that seen with commission rate and ISV. There was an observed linear relationship between ISI jitter and RT (as ISI jitter increased, so did RT) with significant differences for the largest ISI jitter condition (50%) in comparison to each other smaller ISI jitter conditions (0%, 10%, & 30%), thus implying that high levels of ISI jitter impede speed of responding.
Overall, for the total sample, moderate amounts of ISI jitter (approximately 10%) appears to optimize response control, balancing speed (RT) with accuracy (lower commission rate and ISV). It may be that the introduction of uneven intervals between successive stimuli (“jitter”) increases one’s response preparatory state, effectively keeping people “on their toes” and in doing so improving one’s ability to efficiently (lower ISV) and effectively (lower commissions) control responding. In contrast, high rates of jitter could begin to interfere with ability to maintain response set, resulting in poorer control with decrements in both speed and accuracy.
Considering the neuroanatomical correlates of response preparation and inhibition, the increased deliberate and controlled responding observed with a moderate level of ISI jitter (e.g., 10%) may reflect increased recruitment of premotor circuits, particularly those in the pre-SMA, critical to motor response preparation and selection (Isoda & Hikosaka, 2007; Matsuzaka, Aizawa, & Tanji, 1992; Mostofsky et al., 2003).
The parallel quadratic trajectories for commission rate and ISV, with both optimized at 10% jitter, provide additional support for a construct in which response inhibition is viewed as a facet of response selection, with response preparation being critical to both processes (Mostofsky & Simmonds, 2008). This, however, may be particularly true for a simplified GNG task (with green = go, and red = no-go), in which response inhibition is particularly dependent on efficient switching of motor set (from repeated responding to withholding the motor response; Isoda & Hikosaka, 2007). Different effects might be seen for other tasks—for instance, those in which response inhibition is dependent upon resolution of a perceptual conflict (e.g., Stroop or flanker) or use of higher order reasoning (e.g., working memory). Findings of task-dependent differences would support a multidomain hypothesis in which the regions/circuits recruited for response inhibition depend on the nature of the task (Mostofsky et al., 2003).
The impact of jitter on ISV was different for men and women. Men responded favorably to the addition of jitter (10%), while the provision of jitter did not affect women’s performance. Further, at all levels of jitter except the 10% condition, men were significantly more variable than women. As men had an overall higher ISV it may be that the comparative lack of improvement in women reflects a ceiling effect. The better response seen in the sex with poorer baseline level of control would suggest that clinical populations associated with higher baseline ISV (poorer control) would respond best to moderate levels of jitter.
It follows that use of ISI jitter may have implications for treatment in disorders associated with impairments in response control, including ADHD and mild traumatic brain injury (TBI). The effect of infrequent, unexpected timing of go and no-go stimuli (in which nearly all stimuli appeared at 4-s intervals, and 12% appeared at an unexpected interval of 2 s) was recently examined in children with ADHD (Durston et al., 2007), with findings suggesting that children with ADHD do not prepare their responses as well as control children. It would be particularly relevant to determine how children with ADHD perform under conditions in which the timing of stimuli varies (i.e., is jittered) throughout the task and to determine how different degrees of jittering affect their performance. Uncovering a pattern of performance similar to that seen in the present study (i.e., an optimized ISI jitter) in a sample of individuals with disorders associated with impaired inhibitory control (i.e., ADHD or mild TBI) could help to guide intervention. For instance, it may be expected that some level of jitter would support response preparation, selection, and inhibition in those with deficits in these areas. If so, some level of unpredictability of lecture presentation may support one’s ability to respond consistently and accurately. For instance, class settings in which children do not know when they will be called upon may increase their state of readiness, while those in which is it more predictable may decrease preparedness to respond. Based on findings from the present evaluation, an increase in task difficulty/change in timing of presentation must be balanced to ensure that it is not too difficult or fast, given the observed quadratic relationship.
The results of the present study are limited by several methodological issues that should be addressed in future research endeavors. Specifically, although men and women demonstrated a significant difference in pattern of performance in reaction time variability, our sample was biased towards women participants, and other sex differences may be evident if our sample was more balanced between sexes. Follow-up studies with a greater proportion of men may provide further insight into the effects of jitter. This may have important implications for optimizing performance during tasks requiring controlled responding (e.g., driving a car). Further, future research should examine the impact of ISI jitter in individuals with disorders that impact performance on GNG tests, in particular children with ADHD.
Acknowledgments
The research was supported by the following grants from the National Institutes of Health (NIH): K02 NS044850 and R01 NS047781; P30 HD24061 (Mental Retardation and Developmental Disabilities Research Center). We would like to thank Howard Egeth for his helpful guidance in literature review.
Footnotes
Publisher's Disclaimer: Full terms and conditions of use: http://www.informaworld.com/terms-and-conditions-of-access.pdf
This article may be used for research, teaching and private study purposes. Any substantial or systematic reproduction, re-distribution, re-selling, loan or sub-licensing, systematic supply or distribution in any form to anyone is expressly forbidden.
The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.
References
- Barkley RA, Murphy KR, O’Connell T, Connor DF. Effects of two doses of methylphenidate on simulator driving performance in adults with attention deficit hyperactivity disorder. Journal of Safety Research. 2005;36:121–131. doi: 10.1016/j.jsr.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Bellgrove MA, Hester R, Garavan H. The functional neuroanatomical correlates of response inhibition variability: Evidence from a response inhibition task. Neuropsychologia. 2004;42:1910–1916. doi: 10.1016/j.neuropsychologia.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- Conners CK. Conners’ Continuous Performance Test-II. Toronto, Canada: Multi-Health Systems; 2000. [Google Scholar]
- Correa A, Lupianez J, Madrid E, Tudela P. Temporal attention enhances early visual processing: A review and new evidence from event-related potentials. Brain Research. 2006;1076:116–128. doi: 10.1016/j.brainres.2005.11.074. [DOI] [PubMed] [Google Scholar]
- Durston S, Davidson MC, Mulder MJ, Spicer JA, Galvan A, Tottenham N, et al. Neural and behavioral correlates of expectancy violations in attention-deficit hyperactivity disorder. Journal of Child Psychology and Psychiatry. 2007;48:881–889. doi: 10.1111/j.1469-7610.2007.01754.x. [DOI] [PubMed] [Google Scholar]
- Greenberg LM. Test of Variables of Attention. Los Alamitos, CA: Universal Attention Disorders; 1996. [Google Scholar]
- Isoda M, Hikosaka O. Switching from automatic to controlled action by monkey medical frontal cortex. Nature Neuroscience. 2007;10:240–248. doi: 10.1038/nn1830. [DOI] [PubMed] [Google Scholar]
- Klein C, Wendling K, Huettner P, Ruder H, Peper M. Intra-subject variability in attention-deficit hyperactivity disorder. Biological Psychiatry. 2006;60:1088–1097. doi: 10.1016/j.biopsych.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Lim SH, Dinner DS, Pillay PK, Luders H, Morrise HH, Klem G, et al. Functional anatomy of the human supplementary sensorimotor area: Results of extraoperative electrical stimulation. Electroencephalography and Clinical Neurophysiology. 1994;91:179–193. doi: 10.1016/0013-4694(94)90068-x. [DOI] [PubMed] [Google Scholar]
- Luo Y, Greenwood PM, Parasuraman R. Dynamics of the spatial scale of visual attention revealed by brain event-related potentials. Cognitive Brain Research. 2001;12:371–381. doi: 10.1016/s0926-6410(01)00065-9. [DOI] [PubMed] [Google Scholar]
- Matsuzaka Y, Aizawa H, Tanji J. A motor area rostral to the supplementary motor area (presupplementary motor area) in the monkey: Neuronal activity during a learned motor task. Journal of Neurophysiology. 1992;68:653–662. doi: 10.1152/jn.1992.68.3.653. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Puce A, Luby M, Belger A, Allison T. Magnetic resonance imaging studies of functional brain activation: Analysis and interpretation. Electroencephalography and Clinical Neurophysiology Supplement. 1996;47:15–31. [PubMed] [Google Scholar]
- Mostofsky SH, Schafer JGB, Abrams MT, Goldberg MC, Flower AA, Boyce A, et al. fMRI evidence that the neural basis of response inhibition is task-dependent. Cognitive Brain Research. 2003;17:419–430. doi: 10.1016/s0926-6410(03)00144-7. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Simmonds DJ. Response inhibition and response selection: Two sides of the same coin. Journal of Cognitive Neuroscience. 2008;20:1–11. doi: 10.1162/jocn.2008.20500. [DOI] [PubMed] [Google Scholar]
- Simmonds DJ, Fotedar SG, Suskauer SJ, Pekar JJ, Denckla MB, Mostofsky SH. Functional brain correlates of response time variability in children. Neuropsychologia. 2007;45:2147–2157. doi: 10.1016/j.neuropsychologia.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Murphy KJ, Binns MA, Alexander MP. Staying on the job: The frontal lobes control individual performance variability. Brain. 2003;126:2363–2380. doi: 10.1093/brain/awg237. [DOI] [PubMed] [Google Scholar]
- Verte S, Geurts HM, Roeyers H, Oosterlaan J, Sergeant JA. The relationship of working memory, inhibition, and response variability in child psychopathology. Journal of Neuroscience Methods. 2006;151:5–14. doi: 10.1016/j.jneumeth.2005.08.023. [DOI] [PubMed] [Google Scholar]
- Wiersema R, van der Meere J, Roeyers H, Van Coster R, Baeyens D. Event rate and event-related potentials in ADHD. Journal of Clinical Psychology and Psychiatry. 2006;47:560–567. doi: 10.1111/j.1469-7610.2005.01592.x. [DOI] [PubMed] [Google Scholar]
- Wodka EL, Mahone EM, Blackner JG, Gidley Larson JC, Fotedar S, et al. Evidence that response inhibition is a primary deficit in ADHD. Journal of Clinical and Experimental Neuropsychology. 2007;29:345–356. doi: 10.1080/13803390600678046. [DOI] [PubMed] [Google Scholar]


