Abstract
In an effort to discover macromolecular mimetics of heparan sulfate (HS), we previously designed sulfated lignins (Raghuraman et al. Biomacromolecules 2007, 8, 1759–1763). To probe the relevance of sulfate groups of HS in viral entry, lignins completely devoid of sulfate moieties, and yet possessing an electrostatic surface equivalent to that of HS, were designed. Two carboxylated lignins based on 4-hydroxy cinnamic acid scaffold were synthesized using enzymatic oxidative coupling in high yields, fractionated according to their sizes, and tested in cellular assays of herpes simplex virus-1 (HSV-1) infection. The two carboxylated lignins were found to not only inhibit HSV-1 entry into mammalian cells (IC50 = 8 – 56 nM), but were more potent than sulfated lignins. In addition, shorter carboxylated lignins were found to be as active as the longer chains suggesting that structural features, in addition to carboxylate groups, may be important. It can be expected that carboxylated lignins also antagonize the entry of other enveloped viruses, e.g., HIV-1, Kaposi’s sarcoma-associated herpesvirus, and hepatitis C virus, that utilize HS to gain entry into cells. The results present major opportunities for developing lignin-based anti-viral formulations for topical use.
Keywords: Enveloped viruses, Heparan Sulfate, HSV-1, Lignins, Viral Inhibition
Introduction
In an effort to discover macromolecular mimics of heparan sulfate (HS), we previously designed sulfated lignins, which are sulfated derivatives of the natural biopolymer lignin.1–4 Lignin is a complex, heterogeneous polymer made up of repeating phenylpropanoid units containing multiple hydroxyl groups.5,6 Our sulfated lignins were found to be excellent antagonists of herpes simplex virus (HSV) and human immunodeficiency virus (HIV) infection of cells.1,4
Although the biochemical chemical basis for the anti-HSV and anti-HIV activity of sulfated lignins is not known, a plausible hypothesis relies on the similarity of sulfated lignin’s electrostatic surface with that of HS.1 Naturally occurring HS is a linear biopolymer constituted of alternating sulfated glucosamine and glucuronic acid residues. It is present on virtually all cells and plays an important role in the entry of the pathogen through its interaction with several viral glycoproteins including gB, gC, gD and others.7–10
Numerous sulfated molecules have been found to inhibit HSV, HIV and other enveloped viruses to date. These include heparin, dextran sulfate, sulfated galactans, and others.11,12 Each of these belong to the general category of sulfated polysaccharides, which are difficult to synthesize and structurally manipulate in the laboratory. In contrast, the sulfated lignin scaffold that we proposed is an attractive alternative because its phenylpropanoid units afford numerous opportunities for structural variations to fine tune biological activity. In addition, lignin can be rather easily synthesized in the laboratory using enzymatic oxidative coupling.3,6,13
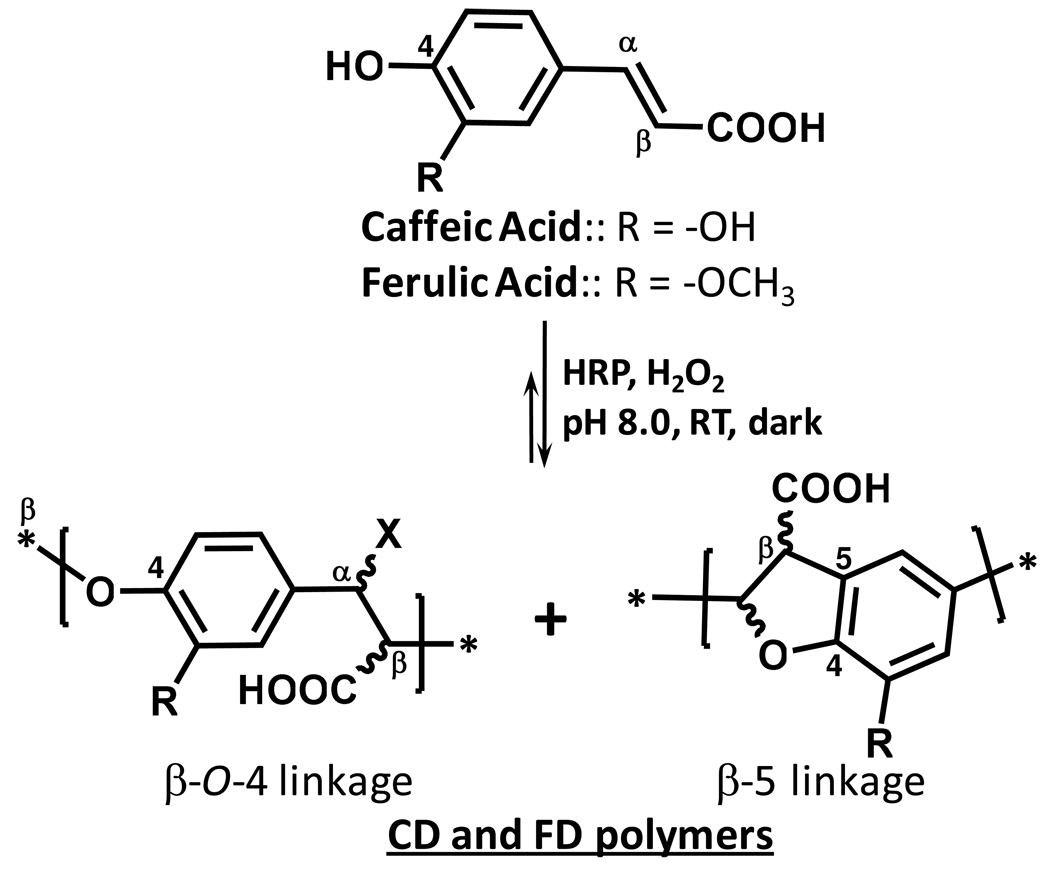
We reasoned that it should be possible to devise a lignin scaffold that is completely devoid of sulfate moieties, and yet possess an electrostatic surface equivalent to that of HS so as to retain potent antagonism of viral entry into cells. The average negative charge density of HS is nearly 1.0 – 1.4.14,15 Thus, a lignin monomer containing one negative charge, which is not a sulfate, e.g., caffeic or ferulic acid (Fig. 1), is likely to afford a non-sulfated lignin polymer with HS mimicking properties.
Figure 1.
Structure of carboxylated lignins CD and FD obtained from horseradish peroxidase-catalyzed oxidative coupling of caffeic and ferulic acid, respectively. Carbons designations (α, β, 4, 5 and others), unique to the lignin field, are as shown. X can be either –H or –OH. Oxidative coupling through radical intermediates give rises to oligomers with different types of inter-monomeric linkages. The most common linkages formed include β-O-4 and β-5. Other less common linkages include β-β, 5-5 and 5-O-4, for which oligomerization tends to arrest chain growth.5,6 The length of the chain greatly depends on the conditions of oligomerization.
This note reports the study of the anti-HSV-1 activity of two carboxylated, non-sulfated lignins prepared from caffeic and ferulic acid monomers. The two lignins were synthesized using enzymatic oxidative coupling in high yields, fractionated according to their sizes, and tested in cellular assays of HSV-1 infection. We find that the two carboxylated lignins are not only potent inhibitors of HSV-1 entry into mammalian cells, but are much more effective than sulfated lignins. In addition, the results show that the shorter carboxylated lignins are very potent in preventing HSV-1 infection suggesting the possibility that structural features, in addition to carboxylate groups, may be important. The results present major opportunities for developing orally available small molecular weight lignin-based anti-viral strategies.
Experimental Methods
Chemicals, Cells, and Viruses
Horseradish peroxidase (HRP) with activity of 250–330 units/mg was from Sigma (St. Louis, MO). β-Galactosidase substrate and o-nitrophenyl β-D-galactopyranoside (ONPG) were from Pierce (Rockford, IL). High purity water, obtained from NERL Diagnostics (RI, USA), was used in all experiments. Dr. Patricia Spear (Northwestern University) provided HeLa cells and the HSV reporter viruses listed here. HSV-1 virus strain carrying the lacZ gene of E. coli and capable of expressing β-galactosidase as a reporter of entry included HSV-1(KOS) gL86.16,17 Polystyrene sulfonate standards (4 – 33 kDa) were from Sigma (St. Louis, MO). Other chemicals and solvents used in the work were purchased from either Sigma-Aldrich (St. Louis, MO) or Fisher (Agawam, MA).
Synthesis of Carboxylated Lignin Polymers
The lignin polymers from caffeic acid and ferulic acid monomers, CD and FD, respectively, were prepared in a manner following earlier work.3 Briefly, caffeic acid or ferulic acid (25 mM, 200 mL) and H2O2 (75 mM, 100 mL) were added drop-wise to a 50 mL solution of 10 mg HRP in 10 mM sodium phosphate buffer, pH 8.0, over a period of 5 h at room temperature in dark. Three additional aliquots H2O2 (75 mM, 100 mL) were added over the next 72 h. At the end of polymerization the solution was freeze-dried to obtain approximately 2 g of CD and FD polymers.
Fractionation of CD and FD Using Centrifugal Filtration
The polymerization reaction mixture (~2 g) was dissolved in deionized water (8 mL) and filtered through Millipore filter (NMWC 30 kDa) at 4,000 g. Filtration was continued until the retentate volume was approximately 200 µL. This process was repeated three more times and the final retentate was labeled as F30 fraction. The filtrates from the above process were pooled, lyophilized, redissolved in deionized water and then further fractionated in the manner described above using a 10 kDa filter. The retentate so obtained was labeled as F10 fraction. This process was repeated to obtain F5 and F3 fractions using 5 kDa and 3 kDa membranes. The retentates were collected and lyophilized to obtain four fractions in yields of 15 – 20, 5 – 8, 3 – 5 and 1 – 2 % respectively.
Determination of Molecular Weight of Carboxylated Lignin Fractions
The number and weight average molecular weight of CD and FD fractions was measured using gel permeation chromatography (GPC) on a Shimadzu chromatography system composed of LC10Ai pumps and a SPD-10A VP UV-Vis detector controlled by a SCL-10A VP system controller connected to a computer. Each fraction was analyzed using Phenogel 5µ (Phenomenex, Torrance, CA, 7.6 mm i.d. × 300 mm). The mobile phase consisted of 100% tetrahydrofuran at a constant flow rate of 0.7 mL/min. Polystyrene standards, caffeic acid and ferulic acid were used for calibration purposes. The relationship between logarithm of the molecular weight and the elution volume (V) of the standards was found to be linear with a correlation co-efficient of 0.99. The wavelength of detection for CD and FD fractions was set at 280 nm. Each chromatogram was sliced into 1000 time periods providing 1000 MR with the corresponding absorbance (A) values. These values were used to calculate number average molecular weight (MN), weight average molecular weight (MW) and polydispersity (P) parameters from equations 1, 2 and 3, respectively, for each CD and FD fraction.
Assay for HSV-1 Entry into Cells
Assays for infection of cells were based on the quantification of β-galactosidase expressed by the mutant HSV viral genome containing the lacZ gene, as described earlier.8,10 HeLa cells were grown in 96-well tissue culture dishes (2–4 × 104 cells/well), washed after 16 h of growth, and exposed to 10 plaque forming units (PFU) per cell of the HSV virus in 50 µL of phosphate-buffered saline (PBS) containing glucose and 1% calf serum for 6 h at 37 °C. To test for inhibitory activity, the sulfated compounds were simultaneously added to this 50 µL medium in varying amounts ranging from 0 to 2 µg. Following incubation, the cells were solubilized in 100 µL of PBS containing 0.5% NP-40 and 10 mM ONPG. The initial rate of hydrolysis of the substrate was monitored spectrophotometrically at 410 nm, which corresponds to the expressed level of β-galactosidase within HeLa K-1 cell. The initial rate of hydrolysis of the substrate in the absence of any added antagonist was used as the control and assigned a value of 100% HSV infection. Assays were performed in duplicate. Logistic equation 4 was used to fit the dose-dependence of HSV infection to obtain the IC50.
| (4) |
In this equation Y is the observed β-galactosidase activity (in %) in the presence of carboxylated lignin (L) to that in its absence, YM and YO are the maximum and minimum possible values of the β-galactosidase activity, IC50 is the concentration of L that results in 50% antagonism of HSV-1, and HC is the Hill Co-operativity Index.
Results and Discussion
Caffeic and Ferulic Acids Polymerize With Ease to Generate Long Chains
Enzymatic polymerization of phenylpropenoid monomers to produce synthetic lignins has been explored since a long time.18–22 Yet, previous reports on the oxidative coupling of 4-hydroxycinnamic acids resulted only in oligomers with an average chain length of less than 3 monomers.13,23 HRP-catalyzed polymerization of caffeic and ferulic acid monomers at pH 8.0 with gradual addition of H2O2 over a period of more than 3 days resulted in CD and FD polymers, respectively, in nearly quantitative yields (see Fig. 1). The MN and MW values of these polymeric mixtures, measured using GPC, were found to be 4264 and 13272 Da for CD and, 5322 and 11525 Da for FD (Table 1). These values indicate that CD is more polydisperse, and probably more heterogeneous, than FD. The average chain length of the two polymers was found to be 12 (CD) and 29 (FD). This is the first report of such long polymers obtained through enzymatic polymerization of 4-hydroxycinnamic acids. A probable reason for the higher level of polymerization is the higher concentration of enzyme used in the reaction as well as the prolonged addition of the oxidant.
Table 1.
Physical Properties of Size Fractionated Carboxylated Lignins.
| MNa | MWa | MPa | Pb | LRc | |
|---|---|---|---|---|---|
| (Da) | (Da) | (Da) | |||
| FD3 | 1300 | 5300 | 700 | 4.17 | 3.6 (2–27)c |
| FD5 | 2800 | 9700 | 1300 | 3.52 | 6.5 (2–50) |
| FD10 | 5000 | 13200 | 2700 | 2.64 | 13.7 (2–68) |
| FD30 | 7600 | 16500 | 7700 | 2.18 | 39.9 (2–85) |
| FDMIX | 5300 | 11500 | 5600 | 2.16 | 29.1 (2–59) |
| CD3 | 1100 | 2100 | 860 | 1.98 | 4.7 (2–12) |
| CD5 | 1600 | 6900 | 1100 | 4.25 | 5.8 (2–38) |
| CD10 | 2500 | 8600 | 1400 | 3.45 | 5.8 (2–48) |
| CD30 | 3900 | 12800 | 1900 | 3.24 | 10.8 (2–71) |
| CDMIX | 4300 | 13200 | 2200 | 3.11 | 12.1 (2–74) |
Number, weight and peak average molecular weights were obtained by GPC analysis.
Polydispersity.
The peak average chain length was calculated from the ratio of MP and molecular weight of the two monomers. Numbers in brackets refer to the range of chain lengths. (see ‘Methods’.)
CD and FD Are Less Globular Than Natural Lignins
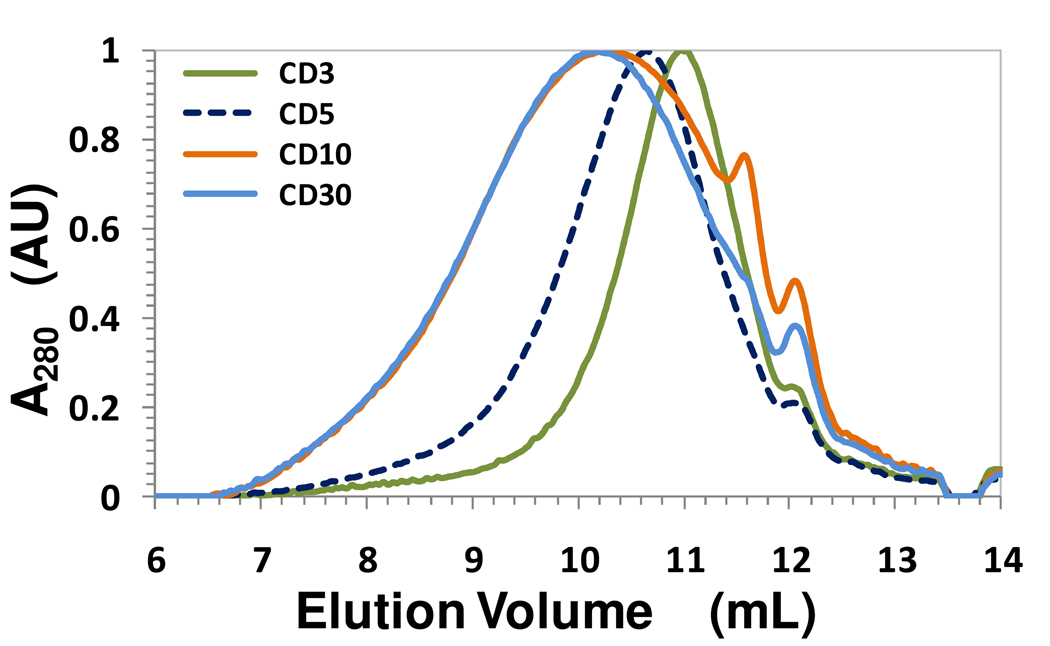
To assess the size dependence of putative anti-viral activity of CD and FD, the two polymeric mixtures were separated into four fractions of varying sizes using 3K, 5K, 10K and 30K membrane-based centrifugal filtration. The GPC profiles of fractions were found to be broad and reasonably symmetrical (Fig. 2). This implied that each fraction contained nearly all types of chains. For example, although the average chain length of FD3 and CD3 is 3.6 and 4.7, both fractions contain chains as long as 27 and 12 units, respectively (Table 1). Thus, the difference between the membrane-separated fractions arises primarily due to chains being present in different proportions. A probable explanation for why high molecular weight chains are present in CD3 and FD3 fractions is that carboxylated lignins are more linear polymers than natural lignins. The random oxidative coupling mechanism of enzymatic polymerization affords chain elongation along non-linear centers resulting in multidimensional polymerization,5,6 which can generate globular structures. Globular structures typically cannot pass through molecular membranes or sieves. In contrast, linear structures can pass through sieves through reptile-like motion. Thus, carboxylated lignins appear to be less globular than natural lignins. This is important because greater the linearity of the lignin polymer greater is its probability of mimicking HS, which has a 100% linear structure.
Figure 2.
Normalized GPC profiles of fractions CD3 through CD30 obtained from membrane-based centrifugal filtration of CD mixture. A280 refers to the absorbance at 280 nm. The profiles for FD fractions were similar (not shown). See Methods for details.
The MN, MW and polydispersity (P) values calculated from the GPC profiles confirm the above conclusions. The MN values ranged from 1052 to 7558, while MW was found to be in the range of 2084 and 16464 (Table 1). The molecular weight at the peak maximum (MP) was found to more closely resemble MN than MW suggesting a larger proportion of smaller chains. The average chain length varied from 3.6 to 39.9 for FD fractions, while it was between 4.7 and 12.1 oligomeric units for CD fractions (Table 1). This implies that the chain length range was found to be much greater for FD (~11-fold) than for CD (2.5-fold).
CD and FD are Potent Antagonists of HSV-1 Entry into Cells
The ability of CD and FD to prevent the entry of HSV-1 particles into mammalian cells was studied using a standard cellular assay.7,8 In this assay, HeLa cells were exposed to a reporter HSV-1 containing the lacZ gene in the presence and absence of CD, FD and their size fractionated preparations. The large size and significant anionic character of the lignins prevents them from entering the mammalian cell, while the viral particle is internalized through the concerted action of multiple cellular receptors including HS.7–10 Assuming CD and FD mimic cell membrane-bound HS, the lignins would competitively reduce the levels of the virus internalized by HeLa cells. Thus, the β-galactosidase activity of the internalized HSV-1 serves as a measure of the activity of lignins in preventing viral entry.
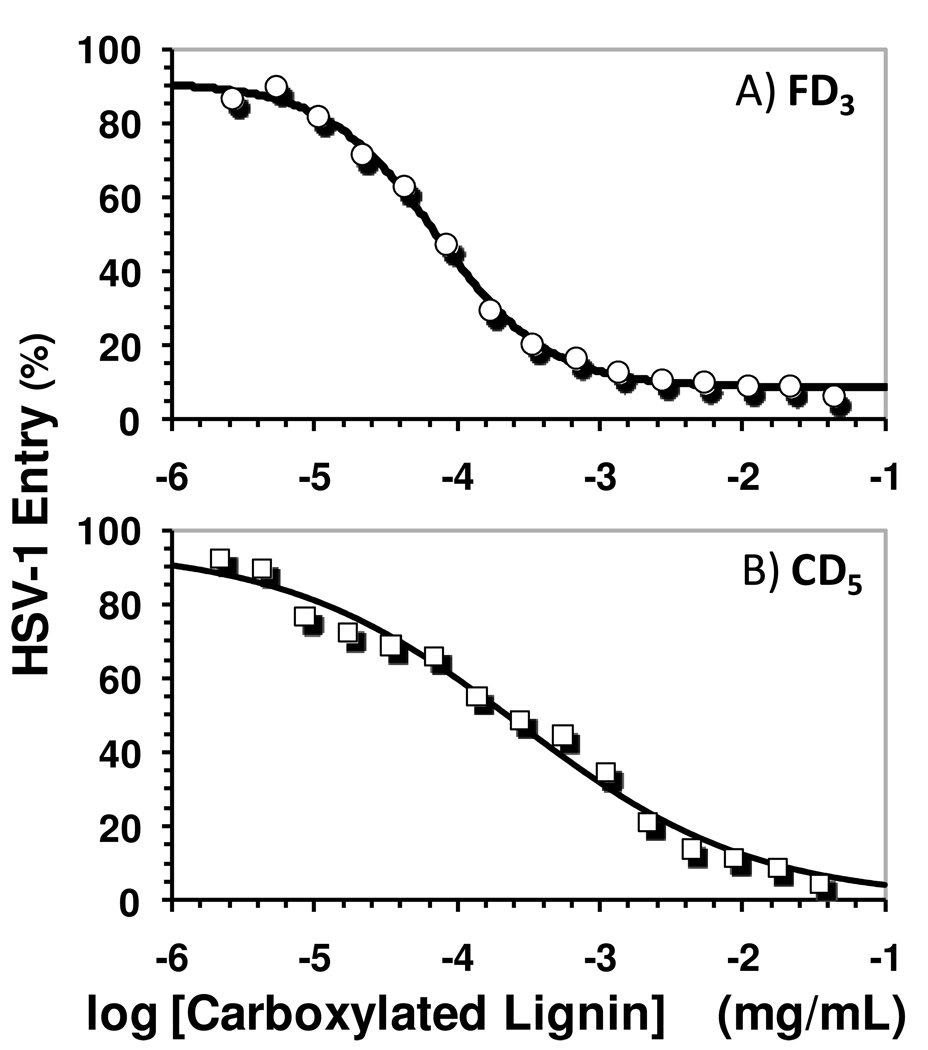
Figure 3 shows the viral entry profile for two representative lignin fractions – FD3 and CD5. The profiles for other fractions were similar (not shown). A concentration-dependent antagonism of entry of HSV-1 into HeLa was noted for all preparations, which was fitted using the standard dose–response equation to calculate the concentration of carboxylated lignin required for 50% antagonism (IC50) (Table 2). For size-fractioned carboxylated lignins FD and CD, the IC50 values were found to be in the range of 72 and 284 ng/mL, which correspond to nanomolar potencies (13 to 56 nM). This indicates that our new carboxylated lignin scaffold is highly effective in preventing the entry of HSV-1.
Figure 3.
Profile of HSV-1 entry into cells in the presence of FD3 (A) and CD5 (B) using a β-galactosidase activity-based viral entry assay. Profiles of other fractions were similar and not shown. IC50 values calculated from these profiles are listed in Table 2. See Methods for details.
Table 2.
Inhibition of HSV-1 Entry into Mammalian Cells by Size-fractionated Carboxylated Lignins CD and FD.a
| Fractions | IC50 | IC50b | HC |
|---|---|---|---|
| (ng/mL) | (nM) | ||
| FD3 | 72±1c | 13.6±0.2 | 1.1±0.1 |
| FD5 | 127±4 | 13.1±0.4 | 1.0±0.1 |
| FD10 | 111±2 | 8.4±0.2 | 1.0±0.1 |
| FD30 | 284±12 | 17.3±0.4 | 1.2±0.1 |
| CD3 | 153±7 | 28.9±1.4 | 0.5±0.1 |
| CD5 | 269±21 | 50.8±4.0 | 0.5±0.1 |
| CD10 | 295±14 | 55.7±2.7 | 0.6±0.1 |
| CD30 | 220±8 | 17.2±0.6 | 0.8±0.2 |
The IC50 values are concentrations of carboxylated lignins that result in 50% reduction in level of HSV-1 entry. IC50 and HC values were obtained through non-linear regression analysis of viral entry data in the presence and absence of carboxylated lignins (see ‘Experimental Methods’).
Calculated using MW values from Table 1.
Errors represent ± 2 S. E.
A closer look reveals that FD fractions are typically more potent than corresponding CD fractions (Table 2). For example, FD3 is approximately 2-fold more potent than CD3. Another difference between the two polymers appears in the Hill Co-operativity indices. Whereas FD fractions display a HC of ~1, CD fractions show a value of ~0.5. In a traditional multi-site interaction of a macromolecule with its receptor, HC corresponds to co-operativity of interaction. A value of 1.0 implies no co-operativity, while values less than 1.0 imply negative co-operativity. It is difficult to ascribe the phenomenon of co-operativity in the antagonism of HSV-1 by CD and FD because both are heterogeneous and polydisperse. Rather, HC values may reflect the degree of structural complexity of the mixture, which can generate many different types of ligand – receptor complexes. This possibility is greater for CD due to the presence of an additional –OH group that is known to be more reactive than the corresponding –OMe in FD.
The potency of inhibition is minimally affected for FD fractions despite the change in their molecular weights. For example, FD3 exhibits an IC50 of 13 nM, which is not different from the value of 17 nM for FD30, although the average chain length increases nearly 11-fold (Tables 1 & 2). The results for CD fractions are similar. Although the IC50 increases from 29 nM for the CD3 to 56 nM for CD10 and then decreases to 17 nM for CD30 (Table 2), the apparently nonlinear change is most likely due to its greater heterogeneity.
The viral inhibition profile of non-sulfated carboxylated lignins is dramatically different from that of the sulfated lignins. For sulfated lignins, as the average chain length increased so did their ability to antagonize viral entry.1,24 The reason for this size dependence is the well known statistical advantage offered by multiple binding sites likely to be present in longer polymers. Yet, size-dependence is nearly completely eliminated for carboxylated lignins. This implies that even the smallest FD and CD chains are able to effectively prevent HSV-1 entry into cells, an observation which carries major pharmaceutical value.
Conclusions
Although a large number of polymers have been designed to inhibit viral entry into mammalian cells, nearly each is a sulfated polysaccharide. Our success with sulfated lignins led to the current work on carboxylated lignins, which do not contain any sulfate group. The two enzymatically synthesized carboxylated lignins are polymers of phenylpropanoid residues connected primarily through β-O-4 and β-5 linkages (Fig. 1).1–4 Other linkages including β-β and 5-5 are also likely. These varying inter-residue linkages and chain lengths introduce considerable structural heterogeneity in the polymeric mixture. Structural heterogeneity is a major impediment in the drug discovery process. Yet, it affords a high probability of discovering an initial lead, which may be fine tuned to result in a new pharmaceutical agent. The potent antagonism of HSV-1 entry into mammalian cells by all FD and CD fractions highlights the importance of investigating scaffolds outside of the traditional saccharide scaffolds to discover novel pharmaceuticals.
The IC50 value of HSV-1 inhibition by FD and CD fractions range from 8 to 56 nM (Table 2). In comparison, our previous study with sulfated lignins demonstrated values in the range of 1.7 to 1450 nM.1 This indicates that most carboxylated lignins are better antagonists of HSV-1 infection of cells. More specifically, FD3 (MP= 698 Da) and CD3 (MP =854 Da) displayed IC50 of 13 and 29 nM, respectively, which are 50 – 106-fold lower than that found for the smallest sulfated lignin (MP = 1900 Da) earlier.1 This implies that either carboxylated lignins are better at mimicking HS in comparison to sulfated lignins or that these new molecules possess a completely different mechanism of antagonism of HSV-1.
A key discovery of this work is the high potency of FD (and CD) irrespective of chain length. For example, FD3 is almost as anti-viral as FD30 (Table 2). A plausible explanation for this observation is that FD possesses discrete structure(s) that induce anti-HSV-1 activity. The average chain length of FD3 is 3.6. This implies that small, carboxylate group containing, FD-based molecules, e.g., trimers with molecular weight of 582 (194×3) are likely exhibit significant anti-HSV-1 activity. In addition to the small size, the presence of carboxylic acid groups in these polymers affords an excellent route to design orally bioavailable anti-HSV-1 agents. Orally bioavailable designs would contain ester protecting groups, which will undergo non-specific hydrolysis in vivo to release the small, carboxylated lignin-based inhibitor.
The carboxylated lignins studied here have additional advantages over sulfated lignins as well as other polysaccharide-based agents. These polymers can be easily synthesized in high yields in a fairly consistent manner. Chemical sulfation, on the other hand, is a difficult reaction and is typically considerably variable.25,26 A large number of structural variants of ferulic and caffeic acid monomers are available, which implies that new agents can be studied for fine tuning anti-HSV activity.
Overall, this study puts forward a carboxylated lignin polymer as a potent antagonist of HSV-1 entry into mammalian cells. It can be expected that carboxylated lignins also antagonize the entry of other enveloped viruses, e.g., HIV-1, dengue, Kaposi’s sarcoma-associated herpesvirus and hepatitis C virus. In fact, a review of literature suggests that effort to develop carboxylated lignins as anti-HIV agents has been made in the past.27–29 Interestingly, the carboxylated lignins discussed here are about 1000-fold more potent at inhibiting HSV-1 than those studied earlier as anti-HIV agents.29 The reason for this massive selectivity in targeting HSV is not clear at the present time, but in combination the results present the possibility of preparing topical formulations of these polymers for preventing infections arising from multiple pathogens.
Acknowledgments
This work was supported by grants from the National Institutes of Health (HL090586 and HL099420), the American Heart Association (EIA 0640053N), and from the Mizutani Foundation for Glycoscience, Japan.
References and Notes
- 1.Raghuraman A, Tiwari V, Zhao Q, Shukla D, Debnath AK, Desai UR. Biomacromolecules. 2007;8:1759–1763. doi: 10.1021/bm0701651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henry BL, Monien BH, Bock PE, Desai UR. J. Biol. Chem. 2007;282:31891–31899. doi: 10.1074/jbc.M704257200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monien BH, Henry BL, Raghuraman A, Hindle M, Desai UR. Bioorg. Med. Chem. 2006;14:7988–7998. doi: 10.1016/j.bmc.2006.07.066. [DOI] [PubMed] [Google Scholar]
- 4.Raghuraman A, Tiwari V, Thakkar JN, Gunnarsson GT, Shukla D, Hindle M, Desai UR. Biomacromolecules. 2005;6:2822–2832. doi: 10.1021/bm0503064. [DOI] [PubMed] [Google Scholar]
- 5.Reale S, Di Tullio A, Spreti N, De Angelis F. Mass Spectrom. Rev. 2004;24:87–126. doi: 10.1002/mas.10072. [DOI] [PubMed] [Google Scholar]
- 6.Boerjan W, Ralph J, Baucher M. Annu. Rev. Plant Biol. 2003;54:519–546. doi: 10.1146/annurev.arplant.54.031902.134938. [DOI] [PubMed] [Google Scholar]
- 7.Shukla D, Liu J, Blaiklock P, Shworak NW, Bai X, Esko JD, Cohen GH, Eisenberg RJ, Rosenberg RD, Spear PG. Cell. 1999;99:13–22. doi: 10.1016/s0092-8674(00)80058-6. [DOI] [PubMed] [Google Scholar]
- 8.Tiwari V, Clement C, Duncan MB, Chen J, Liu J, Shukla D. J. Gen. Virol. 2004;85:805–809. doi: 10.1099/vir.0.19641-0. [DOI] [PubMed] [Google Scholar]
- 9.O'Donnell CD, Shukla D. Virol. Sin. 2008;23:383–393. doi: 10.1007/s12250-008-2992-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Donnell CD, Kovacs M, Akhtar J, Valyi-Nagy T, Shukla D. Virology. 2010;397:389–398. doi: 10.1016/j.virol.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Damonte EB, Matulewicz MC, Cerezo AS. Curr. Med. Chem. 2004;11:2399–2419. doi: 10.2174/0929867043364504. [DOI] [PubMed] [Google Scholar]
- 12.Rusnati M, Urbinati C. Curr. Pharm. Des. 2009;15:2946–2957. doi: 10.2174/138161209789058156. [DOI] [PubMed] [Google Scholar]
- 13.Ward G, Hadar Y, Bilkis I, Konstantinovsky L, Dosoretz CG. J. Biol. Chem. 2001;276:18734–18741. doi: 10.1074/jbc.M009785200. [DOI] [PubMed] [Google Scholar]
- 14.Warda M, Gouda EM, Toida T, Chi L, Linhardt RJ. Comp. Biochem. Physiol. B. 2003;136:357–365. doi: 10.1016/j.cca.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 15.Warda M, Linhardt RJ. Comp Biochem. Physiol. B Biochem. Mol. Biol. 2006;143:37–43. doi: 10.1016/j.cbpb.2005.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Montgomery RI, Warner MS, Lum BJ, Spear PG. Cell. 1996;87:427–436. doi: 10.1016/s0092-8674(00)81363-x. [DOI] [PubMed] [Google Scholar]
- 17.Warner MS, Geraghty RJ, Martinez WM, Montgomery RI, Whitbeck JC, Xu R, Eisenberg RJ, Cohen GH, Spear PG. Virology. 1998;246:179–189. doi: 10.1006/viro.1998.9218. [DOI] [PubMed] [Google Scholar]
- 18.Holmgren A, Henriksson G, Zhang LM. Biomacromolecules. 2008;9:3378–3382. doi: 10.1021/bm800704k. [DOI] [PubMed] [Google Scholar]
- 19.Bunzel M, Heuermann B, Kim H, Ralph J. J. Agric. Food Chem. 2008;56:10368–10375. doi: 10.1021/jf801825z. [DOI] [PubMed] [Google Scholar]
- 20.Touzel JP, Chabbert B, Monties B, Debeire P, Cathala B. J. Agric. Food Chem. 2003;51:981–986. doi: 10.1021/jf020200p. [DOI] [PubMed] [Google Scholar]
- 21.Kim H, Ralph J, Lu FC, Ralph SA, Boudet AM, MacKay JJ, Sederoff RR, Ito T, Kawai S, Ohashi H, Higuchi T. Org. Biomol. Chem. 2003;1:268–281. doi: 10.1039/b209686b. [DOI] [PubMed] [Google Scholar]
- 22.Holmgren A, Norgren M, Zhang L, Henriksson G. Phytochemistry. 2009;70:147–155. doi: 10.1016/j.phytochem.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 23.Pan GX, Spencer L, Leary GJ. J. Agric. Food Chem. 1999;47:3325–3331. doi: 10.1021/jf9902494. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki H, Tochikura TS, Iiyama K, Yamazaki S, Yamamoto N, Toda S. Lignosulfonate, a water-solubilized lignin from the waste liquor of the pulping process, inhibits the infectivity and cytopathic effects of human immunodeficiency virus in vitro. Agric. Biol. Chem. 1989;53:3369–3372. [Google Scholar]
- 25.Raghuraman A, Riaz M, Hindle M, Desai UR. Tetrahedron Lett. 2007;48:6754–6758. doi: 10.1016/j.tetlet.2007.07.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Horani RA, Desai UR. Tetrahedron. 2010;66:2907–2918. doi: 10.1016/j.tet.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakashima H, Murakami T, Yamamoto N, Naoe T, Kawazoe Y, Konno K, Sakagami H. Lignified materials as medicinal resources. V. Anti-HIV (human immunodeficiency virus) activity of some synthetic lignins. Chem. Pharm. Bull. 1992;40:2102–2105. doi: 10.1248/cpb.40.2102. [DOI] [PubMed] [Google Scholar]
- 28.Shimizu N, Naoe T, Kawazoe Y, Sakagami H, Nakashima H, Murakamim T, Yamamoto N. Lignified materials as medicinal resources. VI. Anti-HIV activity of dehydrogenation polymer of p-coumaric acid, a synthetic lignin, in a quasi-in-vivo assay system as an intermediary step to clinical trials. Biol. Pharm. Bull. 1993;16:434–436. doi: 10.1248/bpb.16.434. [DOI] [PubMed] [Google Scholar]
- 29.Ichimura T, Otake T, Mori H, Maruyama S. HIV-1 protease inhibition and anti-HIV effect of natural and synthetic water-soluble lignin-like substances. Biosci. Biotech. Biochem. 1999;63:2202–2204. doi: 10.1271/bbb.63.2202. [DOI] [PubMed] [Google Scholar]