Abstract
Response control is impaired in attention-deficit hyperactivity disorder (ADHD). Given the corpus callosum's role in response control, we compared callosal morphology in 64 children with ADHD and 64 typically developing children, aged 7 to 13 years, and investigated the relationships between callosal morphology and response control. Area and circumference of 5 callosal segments (genu, rostral body, midbody, isthmus, and splenium) were normalized for cerebral volume and examined for correlation with mean reaction time, intrasubject variability, and/or commission error rate from a go/no-go task. There were no between-group differences in segment areas or circumferences. Reaction time correlated with midbody circumference for boys with ADHD and isthmus circumference for girls with ADHD. For the entire cohort, rostral body circumference correlated with intra-subject variability. Impaired response control in ADHD is associated with anomalies in frontal interhemispheric connections. Future studies examining callosal shape will illuminate the anatomic basis of correlations between callosal segment circumference and response control.
Keywords: ADHD, corpus callosum, white matter, circumference, reaction time, response control
Attention-deficit hyperactivity disorder (ADHD) is a common neuropsychiatric disorder characterized by excessive hyperactivity and impulsivity and decreased ability to maintain on-task behavior. Motor response control is crucial for selecting to withhold unwanted responses and thereby necessary for effective inhibition of the hyperactive, impulsive, and off-task behaviors associated with ADHD. Research suggests that indices of response control may be robust intermediate endophenotypes of ADHD. For many years, there was emphasis on measures of inhibitory failure, stemming in part from clinical observations suggesting that impaired inhibitory control contributes to excessive impulsivity, hyperactivity, and distractibility.1 Indeed, children with ADHD show impaired performance on a range of inhibitory control tasks.2-5 Recently attention has been brought to other aspects of response control affected in ADHD. Prominent among these is intrasubject variability, which is assessed by measuring the variability within each individual's reaction time series. Intrasubject variability is thought to represent efficiency of response preparation and selection, with lower intrasubject variability reflecting more efficient responding.6-8 Increased intrasubject variability has emerged as a highly consistent finding across several studies of ADHD.5,9-13
While response control deficits appear to be central to ADHD, their neural basis is not clear. Functional magnetic resonance imaging (MRI) has been used to better elucidate anomalies in activation patterns in children with ADHD completing tasks of response selection/response inhibition. Findings have been variable but include reduced activation of cortical regions shown to be active in adults studies of response control, in particular, the pre-supplementary motor area,14,15 right inferior frontal cortex,16,17 and right parietal cortex.15,17,18 Given that multiple cortical regions within frontal-subcortical and frontal-posterior circuits have been implicated, it may be that the white matter tracts connecting these cortical regions are themselves altered in ADHD. Anomalous white matter tracts would affect the interaction among cortical regions and could thereby result in the observed impairments in response control. As white matter connections cannot be studied with functional MRI, the use of anatomic analyses is crucial for investigating the association between white matter findings and the behavioral features of ADHD. The identification of neuroanatomic white matter markers of deficits in response control would be useful for establishing ADHD endophenotypes with utility for diagnosis and treatment.
The corpus callosum is critical for interhemispheric integration and transfer of excitatory and inhibitory information,19 making it an important white matter structure with regard to motor response control. Specifically, the corpus callosum is felt to serve several key roles in motor control: (1) transfer of lateralized information (eg, verbal input from the left hemisphere) to the opposite hemisphere to guide unilateral movement, (2) transfer of information to coordinate bilateral movements, and (3) transfer of information to inhibit contralateral movement during a unilateral motor activity.20 The importance of the corpus callosum in motor control is evidenced by impairments in synchronization of motor responses21,22 and motor planning23 demonstrated by individuals with congenital or acquired callosal anomalies.
Complementing the motor findings in individuals with primary disorders of the corpus callosum are correlations between structural features of the corpus callosum and measures of response control in both normal development/aging and other patient populations. Using diffusion tensor imaging to examine fractional anisotropy (FA), a measure of white matter integrity, 2 groups have identified associations between callosal fractional anisotropy and response times. Wilde et al reported a correlation between fractional anisotropy in the splenium and reaction time for both typically developing children and children with traumatic brain injury,24 while Aukema et al found a relationship between callosal fractional anisotropy and processing speed in a cohort of childhood survivors of cancer but not typically developing controls.25 Using morphological analysis, Anstey et al found that callosal area was negatively correlated with reaction time and intrasubject variability for adults with and without mild cognitive disorders, with the correlations being larger in magnitude for adults with mild cognitive disorders.26
The morphology of the corpus callosum in ADHD has been compared with that of typically developing children by a number of investigators; however, results have been inconsistent. These studies can be considered based on the method used for corpus callosum segmentation, dividing the structure into either 5 or 7 segments. Additionally, 2 meta-analyses have evaluated findings across studies.
Two groups previously applied slightly different methods of dividing the corpus callosum into 5 segments. Hynd et al found decreased area of the genu, posterior body, and splenium in 7 boys and girls with ADHD.27 In contrast, Baumgardner et al reported decreased rostral body area in 13 boys with ADHD.28
Five prior studies divided the corpus callosum into 7 segments using a standardized technique.29 Giedd et al found that the rostrum and rostral body were smaller in 18 boys with ADHD and also reported that the area of these regions correlated with parental report of hyperactivity and impulsivity (smaller area was associated with more hyperactivity/impulsivity).30 In 57 boys with ADHD, Castellanos et al found no difference in area of total corpus callosum or any of the 7 segments.31 However, Semrud-Clikeman et al found decreased splenium area in 15 boys with ADHD,32 Lyoo found decreased area of the isthmus and splenium in 25 children with ADHD,33 and Hill et al reported decreased area across the entire corpus callosum and the splenium in 23 boys and girls with ADHD.34
Two meta-analyses have been conducted to evaluate the differences in callosal morphology in ADHD across studies. Valera et al examined the data from those studies summarized above and reported that the only significant difference in the corpus callosum was smaller splenium size in ADHD.35 Hutchinson et al more recently completed a meta-analysis that included a total of 13 studies, including those detailed above as well as studies including children with ADHD and comorbid Tourette syndrome,36 neurofibromatosis,37 or velocardiofacial syndrome,38 and sought to identify the influence of sex and comorbidities on the findings. They reported an effect of sex, with girls with ADHD having a smaller splenium and boys with ADHD having a smaller rostral body.39 In addition, they emphasized that comorbidities may influence findings with regard to callosal size in ADHD.
Evidence to date suggests that there may be ADHD-associated differences in the structure of the corpus callosum and that these differences may be important for understanding anomalies of motor response control in ADHD. We examined, in the largest cohort to date, the relationship of callosal segment area and circumference in children with ADHD in comparison to that of typically developing children as well as the relationship between these morphological features and behavioral measures of response control derived from a go/no-go task: mean reaction time, intrasubject variability, and commission error rate. Based on evidence of structural and functional anomalies of the pre-supplementary motor area in ADHD15,40,41 and the importance of the pre-supplementary motor area with regard to response control,42-44 we hypothesized that for children with ADHD smaller area and circumference of the segment of the corpus callosum containing white matter tracts from this cortical region would correlate with impaired motor response control. In addition, given the sex-based differences in callosal morphology reported by Hutchinson et al39 as well reported effects of sex on response preparation/selection in ADHD,45 we were interested in examining the sex-based effects on corpus callosum morphology and brain-behavior relationships.
Methods and Materials
Participants
A total of 64 children with ADHD and 64 age- and sex-matched typically developing children (controls) were included in this study. Participants were recruited from outpatient clinics at the Kennedy Krieger Institute, from local area pediatricians, schools, social/service organizations (eg, Boy/Girl Scouts) and chapters of children and adults with ADHD, and from advertisements in the community. Children were between the ages of 7 and 13 years and had Full-Scale IQ scores greater than or equal to 85 on Wechsler Intelligence Scale for Children (WISC) Third46 or Fourth47 Edition. No child had a history of speech/language disorder or reading disability; all had standard scores of 85 or higher on the basic or word reading subtests of the Wechsler Individual Achievement Test48 or Wechsler Individual Achievement Test II.49
The structured parent interview, Diagnostic Interview for Children and Adolescents Fourth edition50 and an ADHD-specific broad behavior rating scale (Conners Parent and Teacher Rating Scales Revised, long form51) were used to confirm ADHD diagnosis and to evaluate ADHD subtype. Children were assigned to the predominantly inattentive subtype if they met the criteria for inattentiveness but not hyperactivity/impulsivity on the Diagnostic Interview for Children and Adolescents Fourth edition, and a T-score of 65 or greater on the Conners Parent and Teacher Rating Scales–Revised Scale L (Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition criteria for Predominantly Inattentive Type), and a T-score of 60 or less on the Conners Parent and Teacher Rating Scales–Revised Scale M (Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition criteria for predominantly hyperactive-impulsive type). Children were assigned to the predominantly hyperactive/impulsive subtype if they met the criteria for hyperactivity/impulsivity but not inattention on the Diagnostic Interview for Children and Adolescents–Fourth edition, and a T-score of 65 or greater on the Conners Parent and Teacher Rating Scales–Revised Scale M and a T-score of 60 or less on the Conners Parent and Teacher Rating Scales–Revised Scale L (Diagnostic and Statistical Manual of Mental Disorders criteria for predominantly inattentive type). All other children who met the criteria for ADHD were assigned to the combined subtype. A total of 37 children with ADHD met the criteria for the combined subtype, 26 met the criteria for the predominantly inattentive subtype, and 1 met the criteria for the predominantly hyperactive/impulsive subtype. The Conners Parent and Teacher Rating Scales–Revised Scale N (total Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition symptoms) was used as a global measure of ADHD symptom severity.
The Diagnostic Interview for Children and Adolescents–Fourth edition was additionally used to examine for other psychiatric disorders in all children. Children with comorbid conduct disorder, mood disorder, generalized anxiety disorder, separation anxiety disorder, or obsessive–compulsive disorder were excluded from this study. Children with ADHD and comorbid oppositional defiant disorder or Specific Phobia were included; 24 participants with ADHD met the criteria for oppositional defiant disorder, and 12 children with ADHD met the criteria for specific phobia (including 7 children with oppositional defiant disorder). No participants had a history of other neurological disorders, including Tourette syndrome. Of the children with ADHD, 35 were being treated with stimulant medication, and their parents were requested to withhold the medication the day of and the day prior to testing. Withholding of medication was confirmed by parent report on the day of testing. Children with ADHD who were taking other types of psychoactive medications were excluded.
Children were included in the typically developing group only if they did not meet ADHD diagnostic criteria on any of the administered rating scales and questionnaires. Four of the typically developing children met the criteria for specific phobia on the Diagnostic Interview for Children and Adolescents–Fourth edition. No other children met the criteria for psychiatric disorder on the Diagnostic Interview for Children and Adolescents–Fourth edition, and none of the control children were taking psychoactive medications.
This study was approved by the Johns Hopkins Medicine Institutional Review Board. After complete description of the study to the participants, written informed consent was obtained from a parent/guardian, and assent was obtained from the participating child.
Imaging Acquisitions
High-resolution magnetization prepared rapid gradient recalled echo images were gathered on a 1.5 T Philips Gyroscan NT with the following parameters: repetition time = 7 ms, echo time = 3 ms, matrix = 256 × 256, field of view = 260 mm, slice thickness = 1.2 mm. Diffusion tensor images were acquired on a 3.0 T Philips Gyroscan NT: single-shot EPI with SENSE acquisition (factor = 2.5) with repetition time = 6.346 s, echo time = 75 ms, matrix = 96 × 96, field of view = 212 mm, slice thickness = 2.2 mm. Diffusion weighting was applied along 30 independent axes (Jones-30) with b = 800 s/mm2 in addition to a minimally weighted b0 image.
Corpus Callosum Measurements
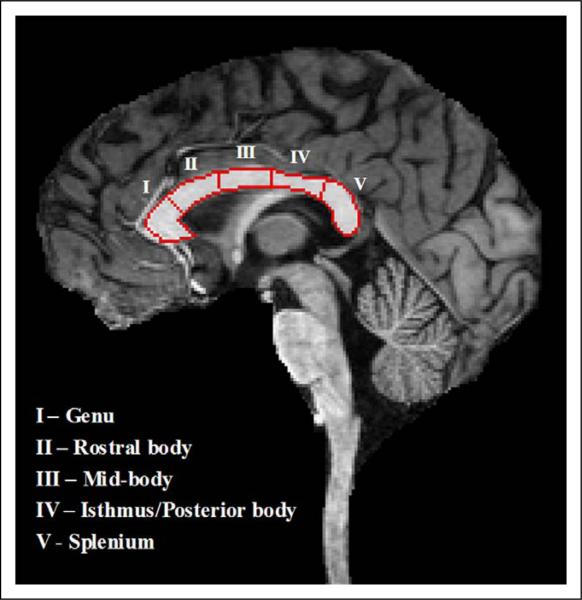
The corpus callosum was manually delineated on images aligned along the midsagittal plane using the Medical Image Processing, Analysis, and Visualization program.52 Binary masks were imported into the program BrainImage (http://spnl.stanford.edu/tools/brainimage.htm) for further segmentation based on the procedure used by Peterson et al.53 In brief, a medial axis transform of the corpus callosum area was computed with endpoints extending to the region boundary, creating a centerline. The centerline length was divided into five subdivisions to determine the segment length along the centerline for a single subregion. Each subregion was subsequently formed by tracking the normal (in both directions) at the endpoints of each corresponding medial axis segment to the region boundary. See Figure 1 for an example of the segmentation. Segment areas and circumferences were computed by the automated BrainImage program. All measurements were normalized by total cerebral volume generated using FreeSurfer (http://www.nmr.mgh.harvard.edu). Specifically, each raw segment area and circumference was multiplied by the ratio of the mean total cerebral volume of the entire cohort to the total cerebral volume of the individual to yield segment areas and circumferences normalized for total cerebral volume. The normalized areas and circumferences were used in the analyses described below.
Figure 1.
Magnetization prepared rapid gradient recalled echo image demonstrating delineation of boundaries of the 5 segments of the corpus callosum.
Diffusion Tensor Imaging
Diffusion tensor imaging tractography was performed in MRIStudio (www.mristudio.org) to localize the cortical projections of white matter tracts contained in each callosal segment. Diffusion tensor imaging and magnetization prepared rapid gradient recalled echo images from the same participant were coregistered, and the corpus callosum segmental masks, created as described above, were imported as regions of interest into MRIStudio. Tractography was performed through each region of interest using parameters to stop tracking for fractional anisotropy below .20 or turning angle greater than 41 degrees.
Go/No-Go Task
Forty-five participants from the typically developing group (M:F, 24:21) and 56 participants from the ADHD group (M:F, 34:22) completed a go/no-go task previously described by Wodka et al.5 Briefly, participants were instructed to push a button when they viewed green spaceships (Go stimuli) on a computer screen but not when they viewed red spaceships (No-go stimuli). Stimuli were displayed for 300 ms once every 1800 ms (1500 ms interstimulus interval). Green spaceships were presented more frequently (3:1) than red spaceships (173 go cues; 44 no-go cues) to reinforce the prepotent “Go” response. Total task time was 8 minutes 19 seconds. Mean reaction time for correct Go responses, intrasubject variability (calculated as [standard deviation of reaction time]/[mean reaction time]), and commission error rate were investigated as potential correlates with callosal segment areas and circumferences to evaluate the relationship between corpus callosum morphology and response control.
Statistical Analyses
Between-group comparisons for demographic data were completed using 2-tailed, unpaired t tests or chi-square analyses. Between-group comparisons in segment areas and circumferences were evaluated using analyses of covariance, covarying for full-scale IQ. Due to skewed data, reaction time and intrasubject variability variables were log-transformed prior to further analyses. Unpaired t tests were subsequently used to evaluate for between-group differences in behavioral data. Pearson correlations were completed to evaluate relationships between go/no-go variables and corpus callosum measurements. For the correlational analyses, Bonferroni correction was made for comparisons across 5 segments. Fisher r-to-z transformations were used to examine for between-group differences in brain-behavior correlations. For all analyses, P values less than or equal to .05 were considered significant.
Results
Group Characteristics
Demographic data are presented in Table 1. Groups were age- and sex-matched. Full-Scale IQ was lower in the ADHD group (P < .001). For the ADHD group, mean T-score for ADHD symptom severity was 73.9, with a standard deviation of 12.
Table 1.
Demographic and Imaging Variablesa
| Variable | TD n = 64 | ADHD n = 64 | P value |
|---|---|---|---|
| Demographic variables | |||
| Gender (number of male/female) | 38/26 | 38/26 | l |
| Age at scan (years) | 10.3 (1.2) | l0.5 (l.3) | .50 |
| Full-scale IQ | 116 (11) | l08 (l4) | <.00l |
| Imaging variables | |||
| Total cerebral volume (cm3) | 1160 (103) | 1110 (95) | .003 |
| Corpus callosum segment area (mm2) | |||
| Genu | 114.4 (17) | ll4.2 (l6) | .45l |
| Rostral body | 91.9 (l4) | 95.7 (l6) | .202 |
| Midbody | 83.8 (l6) | 84.0 (l2) | .896 |
| Isthmus | 73.0 (l6) | 73.4 (l4) | .847 |
| Splenium | l29.0 (l7) | l34.l (22) | .226 |
| Corpus callosum segment circumference (mm) | |||
| Genu | 59.2 (8) | 59.7 (7) | .825 |
| Rostral body | 60.9 (8) | 62.l (9) | .904 |
| Midbody | 48.8 (8) | 49.6 (8) | .922 |
| Isthmus | 60.3 (l4) | 59.0 (9) | .l5l |
| Splenium | 5l.l (7) | 52.5 (7) | .703 |
NOTE: TD, typically developing; ADHD, attention-deficit hyperactivity disorder.
All values presented as mean (SD) unless otherwise specified. P values for demographic variables obtained from independent group's t tests or chi-square analyses. P values for between-group comparisons of imaging variables are derived from the analyses of covariance including Full-Scale IQ as a covariate.
Morphological Data
Morphological data are summarized in Table 1. Total cerebral volume was reduced in the ADHD group compared with the typically developing group (P = .003). For each of the five segments, no significant between-group differences were detected in segment area or circumference; there were also no significant sex by diagnosis interactions for any of the callosal segments.
Corpus Callosum Segment Projections (Diffusion Tensor Imaging Analyses)
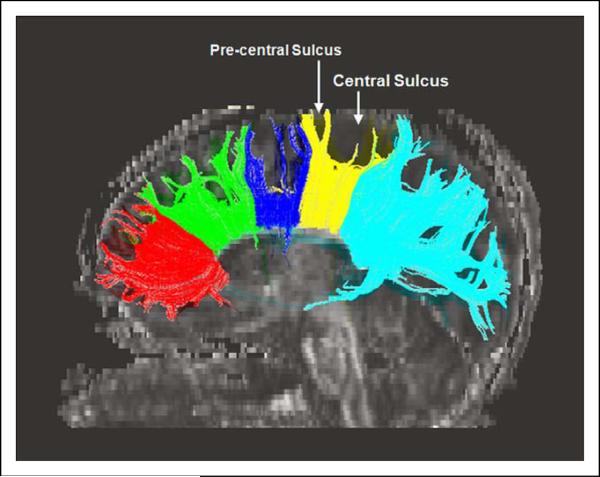
Figure 2 demonstrates the cortical projections from each of the 5 segments of the corpus callosum. With the exception of the splenium, all callosal segments demonstrated white matter projections to medial frontal lobe cortical regions. Projections from the genu localized to the medial orbitofrontal cortex (Brodmann area [BA]9/10). Projections from the rostral body localized to medial prefrontal (Brodmann area 8) and premotor (Brodmann area 6) cortex. Projections from the midbody localized to medial premotor cortex (Brodmann area 6). Projections from the isthmus localized primarily to medial primary motor (Brodmann area 4) and primary somatosensory (Brodmann areas 3,2,1) cortices with a few isolated projections anteriorly to premotor cortex (Brodmann area 6). Projections from the splenium localized to primary sensory (Brodmann area 2), posterior parietal (Brodmann area 7), and occipital (Brodmann area 18/19) cortices.
Figure 2.
Diffusion tensor imaging tractography demonstrating cortical projections from each of the 5 segments of the corpus callosum. Projections were localized as follows: genu –> orbitofrontal cortex (Brodmann area 9/10); rostral body –> prefrontal (Brodmann area 8) and premotor (Brodmann area 6) cortex; midbody –> premotor cortex (Brodmann area 6); isthus –> primary motor (Brodmann area 4) cortex; splenium –> primary sensory (Brodmann area 2), posterior parietal (Brodmann area 7), and occipital (Brodmann area 18/19) cortex.
Go/No-Go Task Performance
There were no significant between-group differences in mean reaction time in whole group and sex by diagnosis comparisons. Intrasubject variability was increased in the children with ADHD compared with the typically developing group (log-transformed means –.434 vs –.539, P = .002; raw means .394 vs. .317). Examining by sex, intrasubject variability was increased significantly for the girls with ADHD compared with the typically developing girls (log-transformed means –.414 vs –.548, P = .005; raw means .406 vs .305), and there was a trend toward increased intrasubject variability for the ADHD boys compared with the typically developing boys (log-transformed means –.447 vs –.531, P = .08; raw means .387 vs .328). There was a trend toward a higher percentage of commission errors in the entire ADHD group compared with typically developing children (raw means 39.4% vs 33.0%, P = .08); there was no significant difference in commission errors in sex by diagnosis comparisons.
Brain-Behavior Correlations
As in the full cohort, within the subset of participants for whom behavioral data was available, no significant between-group differences were detected in callosal segment areas or circumferences. There were no significant correlations between callosal segment areas and reaction time, intrasubject variability, or commission errors. Significant correlations between segment circumference and behavioral variables are displayed in Table 2 and detailed below.
Table 2.
Correlation Coefficients for Brain-Behavior Relationships Between Corpus Callosum Segment Circumference and Behavioral Performance on Go/No-Go Task
| Genu | Rostral body | Midbody | Isthmus | Splenium | |
|---|---|---|---|---|---|
| Mean reaction timea | |||||
| ADHD + TD (n = 101) | 0.09 | 0.18 | 0.16 | 0.11 | 0.04 |
| Male | 0.25 | 0.25 | 0.21 | 0.06 | 0.08 |
| Female | 0.14 | 0.01 | 0.04 | 0.16 | –0.04 |
| TD only (n = 45) | 0.05 | 0.18 | –0.06 | –0.10 | –0.16 |
| Male | 0.02 | 0.14 | –0.06 | –0.24 | –0.26 |
| Female | 0.03 | 0.21 | –0.10 | –0.01 | –0.09 |
| ADHD only (n = 56) | 0.13 | 0.17 | 0.40b | 0.40b | 0.21 |
| Male | 0.04 | 0.33 | 0.47b | 0.34 | 0.29 |
| Female | 0.35 | –0.40 | 0.24 | 0.54b | 0.01 |
| Intrasubject variabilitya | |||||
| ADHD + TD | 0.05 | 0.33c | 0.04 | 0.03 | 0.11 |
| Male | 0.05 | 0.40b | –0.02 | –0.10 | 0.05 |
| Female | 0.05 | 0.22 | 0.15 | 0.18 | 0.20 |
| TD only | 0.06 | 0.30 | 0.15 | 0.00 | 0.08 |
| Male | 0.00 | 0.51 | 0.07 | –0.39 | –0.11 |
| Female | 0.22 | 0.10 | 0.32 | 0.39 | 0.34 |
| ADHD only | 0.05 | 0.30 | –0.07 | 0.10 | 0.12 |
| Male | 0.04 | 0.27 | –0.14 | 0.16 | 0.13 |
| Female | 0.03 | 0.35 | 0.07 | 0.02 | 0.05 |
| Commission errors | |||||
| ADHD + TD | 0.07 | 0.14 | 0.05 | 0.11 | 0.07 |
| Male | 0.14 | 0.28 | 0.04 | 0.09 | 0.06 |
| Female | 0.08 | 0.00 | 0.10 | 0.18 | 0.16 |
| TD only | 0.09 | 0.17 | 0.31 | 0.18 | 0.12 |
| Male | –0.02 | 0.54b | 0.44 | –0.01 | 0.14 |
| Female | 0.30 | –0.14 | 0.17 | 0.35 | 0.15 |
| ADHD only | 0.06 | 0.08 | –0.19 | 0.06 | 0.02 |
| Male | 0.20 | 0.10 | –0.27 | 0.14 | 0.00 |
| Female | –0.18 | 0.18 | 0.03 | –0.10 | 0.17 |
NOTE: TD, typically developing; ADHD, attention-deficit hyperactivity disorder.
Behavioral data were log-transformed.
P <.05
P < .01, after Bonferroni correction.
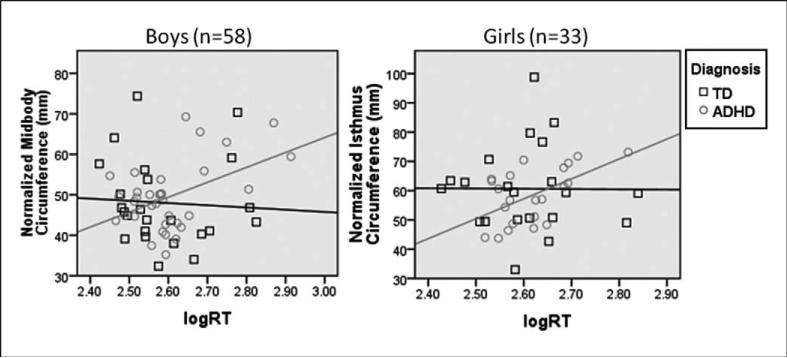
In the children with ADHD, reaction time correlated with midbody and isthmus circumferences (r = .4, P = .01 for both), reflecting a relationship between reaction time and white matter projections to the premotor and motor cortices. Segment circumferences and reaction time were not correlated in the typically children. Reaction time and midbody circumference (reflecting white matter tracts projecting to the premotor cortex) were significantly correlated for boys with ADHD (r = .47, P = .03) but not girls with ADHD (r = .24, P = .28). There was a statistically significant difference in the reaction time-midbody circumference correlation between the boys with ADHD and typically developing boys (z = 2.014; Figure 3). Within the ADHD group, reaction time and isthmus circumference (reflecting white matter tracts projecting to primary motor cortex) were significantly correlated for girls (r = .54, P < .05) but not boys (r = .34, P = .23). Comparing the reaction time–isthmus circumference correlations between girls with ADHD and typically developing girls, the difference approaches significance (z = 1.854; Figure 3).
Figure 3.
Correlations between corpus callosum segment circumference and reaction time, by sex and diagnosis. A, For boys with ADHD, midbody circumference is correlated with reaction time (r = .469, P = .025, corrected for multiple comparisons). For typically developing boys, there is not a significant correlation between midbody circumference and reaction time. There is a statistically significant difference in the relationship between midbody circumference and reaction time for boys with ADHD vs typically developing boys (z = 2.014). B, For girls with ADHD, isthmus circumference is correlated with reaction time (r = .54, P = .045, corrected for multiple comparisons). For typically developing girls, there is not a statistically significant correlation between isthmus circumference and reaction time. The difference in the relationship between isthmus circumference and reaction time for girls with ADHD vs typically developing girls approaches but does not reach significance (z = 1.854). ADHD, attention-deficit hyperactivity disorder; RT, reaction time; TD, typically developing; logRT, log-transformed mean reaction time.
In the entire cohort of children (ADHD+typically developing), intrasubject variability correlated with rostral þ body circumference (r = .3, P = .01), reflecting an association between intrasubject variability and white matter tracts projecting to prefrontal and premotor cortices. Within the entire group, this correlation was significant for the boys (r = 0.4, P = 0.01) but not for the girls (r = .2, P = .16). There was a trend toward correlation between intrasubject variability and rostral body circumference in the typically developing boys (r = .51, P = .06 [P = .012 prior to correction for multiple comparisons]). Intrasubject variability did not correlate with segment circumferences in the ADHD cohort.
Typically developing boys exhibited a significant correlation between commission errors and rostral body circumference (r = .54, P = .04), reflecting a relationship between commission errors and white matter tracts projecting to prefrontal and premotor cortices. Segment circumferences did not correlate with commission errors in the typically developing girls or within the ADHD cohort.
Discussion
To better understand the neural basis for anomalies of motor response control in children with ADHD, we examined the relationship of corpus callosum segment area and circumference in children with ADHD to that of typically developing children and examined the relationship between these callosal morphological features and behavioral measures of response control derived from a go/no-go task: reaction time, intrasubject variability, and commission error rate. Area and circumference were obtained for the midsagittal section of 5 callosal segments, and diffusion tensor imaging tractography was used to identify the cortical projections from each segment.
The children with ADHD in this cohort demonstrated significantly smaller total cerebral volume, consistent with previous reports.54-56 After controlling for total cerebral volume, no significant differences in corpus callosum segment area were identified between children with ADHD and typically developing children. This finding is consistent with the work by Castellanos et al31 in a similarly sized cohort, in which no differences in callosal morphology were identified between boys with ADHD and typically developing boys. However, this finding contrasts findings from multiple previous studies27,28,32,33 and 2 meta-analyses,35,39 in which smaller segment areas were identified in children with ADHD. As has previously been identified, the presence of comorbidities may have influenced the prior findings,39 and our careful screening to eliminate many comorbidities commonly associated with ADHD may in part explain the lack of differences between corpus callosum segment areas in the ADHD and typically developing groups. Furthermore, as the largest single-study ADHD cohort in which corpus callosum morphology has been examined, our data are less sensitive to outlier measurements, potentially related to comorbidities, which may have influenced findings in prior studies with smaller cohorts.
We also found no significant between-group differences in callosal segment circumferences. In the past, corpus callosum segment circumference has not been routinely reported along with segment area. In one prior study, total corpus callosum perimeter length was reported for 13 children with ADHD and 27 typically developing children; while mean perimeter length was shorter for the ADHD cohort, no statistically significant difference was reported.28 Although the segment circumference has not been as commonly studied as segment area, we believe that this may be an important measurement, as circumference could be more sensitive than area to subtle changes in the shape of callosal segments, as described below.
The children in this study completed a simplified go/no-go task in which extraneous cognitive demands were minimized. The children with ADHD demonstrated increased intrasubject variability compared with the typically developing children. This is consistent with recent studies identifying increased intrasubject variability as an important behavioral endopheno-type for ADHD.2,5,10-12,57-59 Interestingly, the between-group difference in commission error rate did not meet statistical significance, suggesting that intrasubject variability may be a more robust marker of ADHD than is impaired inhibitory control.
While no between-group behavioral difference was found in mean reaction time, we identified correlations between reaction time and midbody and isthmus circumferences in children with ADHD but not in typically developing children. The correlation between reaction time and midbody was driven by boys with ADHD, while the correlation between reaction time and isthmus was driven by girls with ADHD. Given the localization of midbody projections to premotor (Brodmann area 6) cortex and projections from the isthmus primarily to primary motor cortex (Brodmann area 4), this suggests that anatomic variability in premotor and motor circuits may influence motor control in ADHD in a manner that does not occur in typically developing children and that there may be sex-based differences in these associations. Specifically, among boys with ADHD, slower reaction time may reflect structural anomalies in the premotor system, whereas among girls with ADHD, slower reaction time may be due to structural differences in primary sensorimotor circuits. Furthermore, anatomic variability in ADHD may underlie the anomalous functional activation of premotor and primary motor cortices during performance of motor response tasks, which we have previously identified in children with ADHD.15,40,60
The identified correlations between rostral body circumference and intrasubject variability (in the entire cohort of children driven by a trend for typically developing boys) and commission error rate (in the typically developing boys only) emphasize the importance of midline premotor (Brodmann area 6) and prefrontal (Brodmann area 8) regions in response preparation and selection. This lends further support to the role of these regions, including the pre-supplementary motor area, in response control, as has previously been demonstrated through lesion, electrophysiological, and imaging studies.42,61,62 While the relationship between intrasubject variability and rostral body circumference did not remain statistically significant after correction for multiple comparisions, the high r value (.51) and the relatively small sample size (n = 24) suggest that a meaningful relationship exists, which should be confirmed with a larger sample size. It is interesting that the correlations between rostral body circumference and intrasubject variability and commission error rate were only observed in the typically developing boys, perhaps suggesting that differences in the anatomy and/or functional activation of these premotor and prefrontal circuits, as related to response inhibition, exist for sex and diagnosis groups.
For the significant brain-behavior correlations identified here, increased circumference was associated with worse behavioral performance (increased mean reaction time, intrasubject variability, or commission errors). Given that similar correlations were not identified with increased segment area, it may be that these findings are indicative of increased circumference due to invagination in the segment perimeter, due to decreased size of specific white matter tracts that course through the corpus callosum and are important for response preparation, rather than enlargement of the segment area resulting in the larger circumference. If the volume of a white matter tract is decreased, this could result in changes in the cross-sectional shape of the tract as it passes through midsagittal section of the corpus callosum segment which, on 2-dimensional inspection, would be appreciated as invagination along the perimeter. Highly localized variations in corpus callosum segment morphology due to variability in the cross-sectional appearance of individual white matter tracts may be better reflected in variability in circumference rather than changes in area, explaining why we identified relationships between segment circumference, but not segment area, and behavioral performance. Interestingly, the rostral body was the only segment for which area and circumference were not correlated and was also the segment in which circumference correlated with intrasubject variability and commission errors in typically developing boys. This may suggest that the rostral body shape is particularly complex with regard to structure and, again, that sex differences will be important to explore in future analyses.
These data demonstrate that careful examination of the corpus callosum in ADHD may yield important findings related to a number of cerebral circuits. Further inferences regarding the neuroanatomical basis of the current findings are limited due to the incomplete information that circumference and area provide about variability in shape of callosal segments. Future studies using more sophisticated approaches to examining the shape of these structures may be useful for confirming our hypothesis and for localizing, in ADHD, the neuroanatomical differences associated with behavioral variability, which are suggested by the current data.
Funding
The authors disclosed receipt of the following financial support for the research and/or authorship of this article: This work was supported by the National Institute of Neurological Disorders and Stroke (grant numbers K02NS044850 [SHM], R01NS047781 [SHM], R01NS043480 [MBD]); the National Institute of Child Health and Human Development (grant numbers K12HD001097 [SJS], P30HD024061 [Intellectual and Developmental Disabilities Research Center]); and the Johns Hopkins University School of Medicine Institute for Clinical and Translational Research, an NIH/NCRR CTSA Program (grant number UL1-RR025005).
Footnotes
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- 1.Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol Bull. 1997;121(1):65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- 2.Lijffijt M, Kenemans JL, Verbaten MN, van Engeland H. A meta-analytic review of stopping performance in attention-deficit/hyper-activity disorder: deficient inhibitory motor control? J Abnorm Psychol. 2005;114(2):216–222. doi: 10.1037/0021-843X.114.2.216. [DOI] [PubMed] [Google Scholar]
- 3.Berlin L, Bohlin G, Nyberg L, Janols LO. How well do measures of inhibition and other executive functions discriminate between children with ADHD and controls? Child Neuropsychol. 2004;10(1):1–13. doi: 10.1076/chin.10.1.1.26243. [DOI] [PubMed] [Google Scholar]
- 4.Hanisch C, Radach R, Holtkamp K, Herpertz-Dahlmann B, Konrad K. Oculomotor inhibition in children with and without attention-deficit hyperactivity disorder (ADHD). J Neural Transm. 2006;113(5):671–684. doi: 10.1007/s00702-005-0344-y. [DOI] [PubMed] [Google Scholar]
- 5.Wodka EL, Mahone EM, Blankner JG, et al. Evidence that response inhibition is a primary deficit in ADHD. J Clin Exp Neuropsychol. 2007;29(4):345–356. doi: 10.1080/13803390600678046. [DOI] [PubMed] [Google Scholar]
- 6.Simmonds DJ, Fotedar SG, Suskauer SJ, Pekar JJ, Denckla MB, Mostofsky SH. Functional brain correlates of response time variability in children. Neuropsychologia. 2007;45(9):2147–2157. doi: 10.1016/j.neuropsychologia.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 7.Mostofsky SH, Simmonds DJ. Response inhibition and response selection: two sides of the same coin. J Cogn Neurosci. 2008;20(5):751–761. doi: 10.1162/jocn.2008.20500. [DOI] [PubMed] [Google Scholar]
- 8.Bellgrove MA, Hester R, Garavan H. The functional neuroanatomical correlates of response variability: evidence from a response inhibition task. Neuropsychologia. 2004;42(14):1910–1916. doi: 10.1016/j.neuropsychologia.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 9.Castellanos FX, Sonuga-Barke EJ, Scheres A, Di Martino A, Hyde C, Walters JR. Varieties of attention-deficit/hyperactivity disorder-related intra-individual variability. Biol Psychiatry. 2005;57(11):1416–1423. doi: 10.1016/j.biopsych.2004.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson KA, Kelly SP, Bellgrove MA, et al. Response variability in attention deficit hyperactivity disorder: evidence for neuropsychological heterogeneity. Neuropsychologia. 2007;45(4):630–638. doi: 10.1016/j.neuropsychologia.2006.03.034. [DOI] [PubMed] [Google Scholar]
- 11.Klein C, Wendling K, Huettner P, Ruder H, Peper M. Intra-subject variability in attention-deficit hyperactivity disorder. Biol Psychiatry. 2006;60(10):1088–1097. doi: 10.1016/j.biopsych.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 12.Vaurio RG, Simmonds DJ, Mostofsky SH. Increased intra-individual reaction time variability in attention-deficit/hyperactivity disorder across response inhibition tasks with different cognitive demands. Neuropsychologia. 2009;47(12):2389–2396. doi: 10.1016/j.neuropsychologia.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams BR, Strauss EH, Hultsch DF, Hunter MA, Tannock R. Reaction time performance in adolescents with attention deficit/ hyperactivity disorder: evidence of inconsistency in the fast and slow portions of the RT distribution. J Clin Exp Neuropsychol. 2007;29(3):277–289. doi: 10.1080/13803390600678020. [DOI] [PubMed] [Google Scholar]
- 14.Tamm L, Menon V, Ringel J, Reiss AL. Event-related FMRI evidence of frontotemporal involvement in aberrant response inhibition and task switching in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2004;43(11):1430–1440. doi: 10.1097/01.chi.0000140452.51205.8d. [DOI] [PubMed] [Google Scholar]
- 15.Suskauer SJ, Simmonds DJ, Fotedar S, et al. Functional magnetic resonance imaging evidence for abnormalities in response selection in attention deficit hyperactivity disorder: differences in activation associated with response inhibition but not habitual motor response. J Cogn Neurosci. 2008;20(3):478–493. doi: 10.1162/jocn.2008.20032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aron AR, Poldrack RA. The cognitive neuroscience of response inhibition: relevance for genetic research in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57(11):1285–1292. doi: 10.1016/j.biopsych.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 17.Rubia K, Overmeyer S, Taylor E, et al. Hypofrontality in attention deficit hyperactivity disorder during higher-order motor control: a study with functional MRI. Am J Psychiatry. 1999;156(6):891–896. doi: 10.1176/ajp.156.6.891. [DOI] [PubMed] [Google Scholar]
- 18.Smith AB, Taylor E, Brammer M, Toone B, Rubia K. Task-specific hypoactivation in prefrontal and temporoparietal brain regions during motor inhibition and task switching in medication-naive children and adolescents with attention deficit hyperactivity disorder. Am J Psychiatry. 2006;163(6):1044–1051. doi: 10.1176/ajp.2006.163.6.1044. [DOI] [PubMed] [Google Scholar]
- 19.Bloom JS, Hynd GW. The role of the corpus callosum in interhemispheric transfer of information: excitation or inhibition? Neuropsychol Rev. 2005;15(2):59–71. doi: 10.1007/s11065-005-6252-y. [DOI] [PubMed] [Google Scholar]
- 20.Geffen GM, Jones DL, Geffen LB. Interhemispheric control of manual motor activity. Behav Brain Res. 1994;64(1-2):131–140. doi: 10.1016/0166-4328(94)90125-2. [DOI] [PubMed] [Google Scholar]
- 21.Midorikawa A, Kawamura M, Takaya R. A disconnection syndrome due to agenesis of the corpus callosum: disturbance of unilateral synchronization. Cortex. 2006;42(3):356–365. doi: 10.1016/s0010-9452(08)70362-9. [DOI] [PubMed] [Google Scholar]
- 22.Serrien DJ, Nirkko AC, Wiesendanger M. Role of the corpus callosum in bimanual coordination: a comparison of patients with congenital and acquired callosal damage. Eur J Neurosci. 2001;14(11):1897–1905. doi: 10.1046/j.0953-816x.2001.01798.x. [DOI] [PubMed] [Google Scholar]
- 23.Caille S, Sauerwein HC, Schiavetto A, Villemure JG, Lassonde M. Sensory and motor interhemispheric integration after section of different portions of the anterior corpus callosum in nonepileptic patients. Neurosurgery. 2005;57(1):50–59. doi: 10.1227/01.neu.0000163089.31657.08. discussion 50-59. [DOI] [PubMed] [Google Scholar]
- 24.Wilde EA, Chu Z, Bigler ED, et al. Diffusion tensor imaging in the corpus callosum in children after moderate to severe traumatic brain injury. J Neurotrauma. 2006;23(10):1412–1426. doi: 10.1089/neu.2006.23.1412. [DOI] [PubMed] [Google Scholar]
- 25.Aukema EJ, Caan MW, Oudhuis N, et al. White matter fractional anisotropy correlates with speed of processing and motor speed in young childhood cancer survivors. Int J Radiat Oncol Biol Phys. 2009;74(3):837–843. doi: 10.1016/j.ijrobp.2008.08.060. [DOI] [PubMed] [Google Scholar]
- 26.Anstey KJ, Mack HA, Christensen H, et al. Corpus callosum size, reaction time speed and variability in mild cognitive disorders and in a normative sample. Neuropsychologia. 2007;45(8):1911–1920. doi: 10.1016/j.neuropsychologia.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 27.Hynd GW, Semrud-Clikeman M, Lorys AR, Novey ES, Eliopulos D, Lyytinen H. Corpus callosum morphology in attention deficit-hyperactivity disorder: morphometric analysis of MRI. J Learn Disabil. 1991;24(3):141–146. doi: 10.1177/002221949102400302. [DOI] [PubMed] [Google Scholar]
- 28.Baumgardner TL, Singer HS, Denckla MB, et al. Corpus callosum morphology in children with Tourette syndrome and attention deficit hyperactivity disorder. Neurology. 1996;47(2):477–482. doi: 10.1212/wnl.47.2.477. [DOI] [PubMed] [Google Scholar]
- 29.Witelson SF. Hand and sex differences in the isthmus and genu of the human corpus callosum. A postmortem morphological study. Brain. 1989;112(pt 3):799–835. doi: 10.1093/brain/112.3.799. [DOI] [PubMed] [Google Scholar]
- 30.Giedd JN, Castellanos FX, Casey BJ, et al. Quantitative morphology of the corpus callosum in attention deficit hyperactivity disorder. Am J Psychiatry. 1994;151(5):665–669. doi: 10.1176/ajp.151.5.665. [DOI] [PubMed] [Google Scholar]
- 31.Castellanos FX, Giedd JN, Marsh WL, et al. Quantitative brain magnetic resonance imaging in attention-deficit hyperactivity disorder. Arch Gen Psychiatry. 1996;53(7):607–616. doi: 10.1001/archpsyc.1996.01830070053009. [DOI] [PubMed] [Google Scholar]
- 32.Semrud-Clikeman M, Filipek PA, Biederman J, et al. Attention-deficit hyperactivity disorder: magnetic resonance imaging morphometric analysis of the corpus callosum. J Am Acad Child Adolesc Psychiatry. 1994;33(6):875–881. doi: 10.1097/00004583-199407000-00014. [DOI] [PubMed] [Google Scholar]
- 33.Lyoo IK, Noam GG, Lee CK, Lee HK, Kennedy BP, Renshaw PF. The corpus callosum and lateral ventricles in children with attention-deficit hyperactivity disorder: a brain magnetic resonance imaging study. Biol Psychiatry. 1996;40(10):1060–1063. doi: 10.1016/s0006-3223(96)00349-6. [DOI] [PubMed] [Google Scholar]
- 34.Hill DE, Yeo RA, Campbell RA, Hart B, Vigil J, Brooks W. Magnetic resonance imaging correlates of attention-deficit/hyperactivity disorder in children. Neuropsychology. 2003;17(3):496–506. doi: 10.1037/0894-4105.17.3.496. [DOI] [PubMed] [Google Scholar]
- 35.Valera EM, Faraone SV, Murray KE, Seidman LJ. Meta-analysis of structural imaging findings in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2007;61(12):1361–1369. doi: 10.1016/j.biopsych.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 36.Mostofsky SH, Wendlandt J, Cutting L, Denckla MB, Singer HS. Corpus callosum measurements in girls with Tourette syndrome. Neurology. 1999;53(6):1345–1347. doi: 10.1212/wnl.53.6.1345. [DOI] [PubMed] [Google Scholar]
- 37.Kayl AE, Moore BD, 3rd, Slopis JM, Jackson EF, Leeds NE. Quantitative morphology of the corpus callosum in children with neurofibromatosis and attention-deficit hyperactivity disorder. J Child Neurol. 2000;15(2):90–96. doi: 10.1177/088307380001500206. [DOI] [PubMed] [Google Scholar]
- 38.Antshel KM, Conchelos J, Lanzetta G, Fremont W, Kates WR. Behavior and corpus callosum morphology relationships in velocardiofacial syndrome (22q11.2 deletion syndrome). Psychiatry Res. 2005;138(3):235–245. doi: 10.1016/j.pscychresns.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 39.Hutchinson AD, Mathias JL, Banich MT. Corpus callosum morphology in children and adolescents with attention deficit hyper-activity disorder: a meta-analytic review. Neuropsychology. 2008;22(3):341–349. doi: 10.1037/0894-4105.22.3.341. [DOI] [PubMed] [Google Scholar]
- 40.Suskauer SJ, Simmonds DJ, Caffo BS, Denckla MB, Pekar JJ, Mostofsky SH. fMRI of intrasubject variability in ADHD: anomalous premotor activity with prefrontal compensation. J Am Acad Child Adolesc Psychiatry. 2008 doi: 10.1097/CHI.0b013e3181825b1f. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shaw P, Lerch J, Greenstein D, et al. Longitudinal mapping of cortical thickness and clinical outcome in children and adolescents with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2006;63(5):540–549. doi: 10.1001/archpsyc.63.5.540. [DOI] [PubMed] [Google Scholar]
- 42.Picton TW, Stuss DT, Alexander MP, Shallice T, Binns MA, Gillingham S. Effects of focal frontal lesions on response inhibition. Cereb Cortex. 2007;17(4):826–838. doi: 10.1093/cercor/bhk031. [DOI] [PubMed] [Google Scholar]
- 43.Shibasaki H, Hallett M. What is the Bereitschaftspotential? Clin Neurophysiol. 2006;117(11):2341–2356. doi: 10.1016/j.clinph.2006.04.025. [DOI] [PubMed] [Google Scholar]
- 44.Toma K, Honda M, Hanakawa T, et al. Activities of the primary and supplementary motor areas increase in preparation and execution of voluntary muscle relaxation: an event-related fMRI study. J Neurosci. 1999;19(9):3527–3534. doi: 10.1523/JNEUROSCI.19-09-03527.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mahone EM, Mostofsky SH, Lasker AG, Zee D, Denckla MB. Oculomotor anomalies in attention-deficit/hyperactivity disorder: evidence for deficits in response preparation and inhibition. J Am Acad Child Adolesc Psychiatry. 2009;48(7):749–756. doi: 10.1097/CHI.0b013e3181a565f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wechsler D. Wechsler Intelligence Scale for Children—III. The Psychological Corporation; San Antonio, TX: 1991. [Google Scholar]
- 47.Wechsler D. Wechsler Intelligence Scale for Children—IV. The Psychological Corporation; San Antonio, TX: 2003. [Google Scholar]
- 48.Wechsler D. Wechsler Individual Achievement Test. The Psychological Corporation; San Antonio, TX: 1992. [Google Scholar]
- 49.Wechsler D. Wechsler Individual Achievement Test—II. The Psychological Corporation; San Antonio, TX: 2002. [Google Scholar]
- 50.Reich W. Diagnostic interview for children and adolescents (DICA). J Am Acad Child Adolesc Psychiatry. 2000;39(1):59–66. doi: 10.1097/00004583-200001000-00017. [DOI] [PubMed] [Google Scholar]
- 51.Conners CK. Conners Rating Scales—Revised. Multihealth Systems; North Tonawanda, NY: 1997. [Google Scholar]
- 52.McAuliffe M, Lalonde E, McGarry D, Gandler W, Csaky K, Trus B. Medical image processing, analysis and visualization in clinical research. IEEE Symposium on Computer-Based Medical Systems. 2001:381–386. [Google Scholar]
- 53.Peterson BS, Leckman JF, Duncan JS, et al. Corpus callosum morphology from magnetic resonance images in Tourette's syndrome. Psychiatry Res. 1994;55(2):85–99. doi: 10.1016/0925-4927(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 54.Mostofsky SH, Cooper KL, Kates WR, Denckla MB, Kaufmann WE. Smaller prefrontal and premotor volumes in boys with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2002;52(8):785–794. doi: 10.1016/s0006-3223(02)01412-9. [DOI] [PubMed] [Google Scholar]
- 55.Castellanos FX, Acosta MT. The neuroanatomy of attention deficit/hyperactivity disorder [in Spanish]. Rev Neurol. 2004;38(suppl 1):S131–S136. [PubMed] [Google Scholar]
- 56.Wolosin SM, Richardson ME, Hennessey JG, Denckla MB, Mostofsky SH. Abnormal cerebral cortex structure in children with ADHD. Hum Brain Mapp. 2009;30(1):175–184. doi: 10.1002/hbm.20496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nigg JT. The ADHD response-inhibition deficit as measured by the stop task: replication with DSM-IV combined type, extension, and qualification. J Abnorm Child Psychol. 1999;27(5):393–402. doi: 10.1023/a:1021980002473. [DOI] [PubMed] [Google Scholar]
- 58.Mostofsky SH, Lasker AG, Singer HS, Denckla MB, Zee DS. Oculomotor abnormalities in boys with tourette syndrome with and without ADHD. J Am Acad Child Adolesc Psychiatry. 2001;40(12):1464–1472. doi: 10.1097/00004583-200112000-00018. [DOI] [PubMed] [Google Scholar]
- 59.de Zeeuw P, Aarnoudse-Moens C, Bijlhout J, et al. Inhibitory performance, response speed, intraindividual variability, and response accuracy in ADHD. J Am Acad Child Adolesc Psychiatry. 2008;47(7):808–816. doi: 10.1097/CHI.0b013e318172eee9. [DOI] [PubMed] [Google Scholar]
- 60.Mostofsky SH, Rimrodt SL, Schafer JG, et al. Atypical motor and sensory cortex activation in attention-deficit/hyperactivity disorder: a functional magnetic resonance imaging study of simple sequential finger tapping. Biol Psychiatry. 2006;59(1):48–56. doi: 10.1016/j.biopsych.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 61.Matsuzaka Y, Aizawa H, Tanji J. A motor area rostral to the supplementary motor area (presupplementary motor area) in the monkey: neuronal activity during a learned motor task. J Neurophysiol. 1992;68(3):653–662. doi: 10.1152/jn.1992.68.3.653. [DOI] [PubMed] [Google Scholar]
- 62.Connolly JD, Goodale MA, Desouza JF, Menon RS, Vilis T. A comparison of frontoparietal fMRI activation during anti-saccades and anti-pointing. J Neurophysiol. 2000;84(3):1645–1655. doi: 10.1152/jn.2000.84.3.1645. [DOI] [PubMed] [Google Scholar]