Abstract
The AKT pathway is an important therapeutic target for cancer drug discovery as it functions as a main point for transducing extracellular and intracellular oncogenic signals. Moreover, alternations of the AKT pathway have been found in a wide range of cancers. In the present study, we found that an Akt1 antisense oligonucleotide (Akt1 AO) significantly downregulated the expression of AKT1 at both the mRNA and protein levels and inhibited cellular growth at nanomolar concentrations in various types of human cancer cells. Combined treatment of Akt1 AO with several cytotoxic drugs resulted in an additive growth inhibition of Caki-1 cells. The in vivo effectiveness of Akt1 AO was determined using two different xenograft nude mouse models. Akt1 AO (30 mg/kg, i.v. every 48 h) significantly inhibited the tumor growth of nude mouse subcutaneously implanted with U251 human glioblastoma cells after 27 days treatment. Akt1 AO (30 mg/kg, i.p continuously via osmotic pump) also significantly inhibited the tumor formation in nude mice implanted with luciferase-expressing MIA human pancreatic cancer cells (MIA-Luc) after 14 days of treatment. The luciferase signals from MIA-Luc cells were reduced or completely abolished after 2 weeks of treatment and the implanted tumors were barely detectable. Our findings suggest that Akt1 AO alone or in combination with other clinically approved anticancer agents should be further explored and progressed into clinical studies as a potential novel therapeutic agent.
Keywords: AKT1 AO, ANTICANCER, ANTISENSE OLIGONUCLEOTIDE, COMBINATION
AKT (also referred to as protein kinase B, PKB) is a serine/threonine kinase and was originally found as a retroviral oncogene [Staal et al., 1977; Staal, 1987; Testa and Bellacosa, 2001; Liang and Slingerland, 2003; Bellacosa et al., 2005; Cheng et al., 2005]. Currently, three AKT isoforms AKT1 (PKBα), AKT2 (PKBβ), and AKT3 (PKBγ) have been characterized. AKT1 amplification was detected in gastric carcinoma [Staal, 1987]. Enhanced AKT1 activity was reported in breast, ovarian, and prostate carcinomas [Sun et al., 2001] and some drug-resistant cancer cells such as cisplatinresistant ovarian cancer cells [Asselin et al., 2001; Yang et al., 2006; Liu et al., 2007]. Sun et al. [2001] showed that 78% of all tumors with activated AKT1 were high grade (e.g., stage III/IV carcinomas). Ectopic expression of constitutively active AKT induces oncogenic transformation of cells and tumor formation in transgenic mice and causes chemoresistance [Cheng et al., 2005].
AKT and its upstream regulators are activated or deregulated in a wide range of tumors and appear to have critical roles in cancer progression. Furthermore, AKT has downstream targets regulating tumor-associated cell progress. The activated (phosphorylated) form of AKT protein seems to promote cell survival and to inhibit apoptosis by its ability to phosphorylate and inactivate several proapoptotic targets, including Bad, mTOR, caspase-9, and forkhead box O1/forkhead transcription factor family member 1 (called as FOXO1, FOXO1A, or FKHR). Other functions of AKT in tumorigenesis include: mediation of hyper-responsiveness to ambient levels of growth factors; promotion of nuclear entry of MDM2, thus, inhibiting p53 pathway; induction of cytoplasmic localization of p21WAF1 and p27Kip1, thus, promoting cell growth; stabilization of cyclin D1/D3; enhancement of telomerase activity; activation of endothelial nitric oxide synthase (eNOS) to promote angiogenesis; and contributing to invasiveness by stimulating secretion of matrix metalloproteinase (MMP) [Testa and Bellacosa, 2001; Liang and Slingerland, 2003; Bellacosa et al., 2005; Cheng et al., 2005].
Because of important roles of AKT in tumorigenesis, targeting the AKT signaling pathway has been an active research area in pharmaceutical and academic institutions. There have been efforts to target the AKT signaling pathway by attempting to inhibit upstream kinases of AKT; however, this approach can be non-selective and affect other kinases as well as AKT. In recent years, antisense oliognucleotides have been employed to specifically target important genes in cancer progression [Jansen and Zangemeister-Wittke, 2002; Gleave and Monia, 2005]. Antisense oligonucleotides targeting Akt1, therefore, provide a promising new pharmaceutical tool for the effective modification of the expression of specific genes including Akt-1.
The objective of this study was to investigate the in vitro and in vivo effectiveness of a novel antisense oligonucleotide Akt1 AO, Akt1 inhibitor. We extensively investigated the effects of Akt1 AO on Akt1 expression and the cellular growth in various types of human cancer cells. We further determined the effects of Akt1 AO on the tumor growth in vivo using two different xenograft mouse models.
MATERIALS AND METHODS
MATERIALS
All media and reagents including Lipofectamine Plus and Lipofectamine 2000 used for transfection and M-MLV enzyme kit were obtained from Invitrogen (Carlsbad, CA). RNA-STAT kit was purchased from TEL-TEST, Inc. (Friendswood, TX). Anti-AKT1 antibody and anti-β-actin antibody were from Santa Cruz Biotechnology (Santa Cruz, CA). Antisense olionucleotides were synthesized using the 8909 Expedite DNA synthesizer from Applied Biosystems (Foster City, CA) or in some instances, purchased from TriLink BioTechnologies, Inc. (San Diego, CA). Lyophilized drug was reconstituted in distilled water to a stock concentration of 1 mM and used for both in vitro and in vivo experiments. Sense and mismatch oligonucleotides were used to demonstrate the sequence and target specificity of Akt1 AO.
CULTURE OF HUMAN CANCER CELLS
Human cancer cell lines were obtained from the following sources: Caki-1, OVCAR-3, MCF-7, HeLa, PC3, HepG2, A549, PANC-1, and HT-29 from the American Type Culture Collection (Manassas, VA); U251 from Riken Institutes, Marunouchi, Tokyo, Japan; MKN-45 from DSMZ (German Collection of Microorganisms and Cell Cultures), Braunschweig, Germany; UMRC2 and Lox-IMVI from the United States National Cancer Institute (Bethesda, MD). All cell lines except UMRC2, Caki-1, and PANC-1 were grown in RPMI1640 medium (Invitrogen) supplemented with 10% fetal bovine serum (FBS), 1mM sodium pyruvate, 10 mM HEPES, and 100 U/ml penicillin–streptomycin (P/S). UMRC2, Caki-1, and PANC-1 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen) supplemented with 10% FBS, 10 mM HEPES, 100 U/ml P/S, and 2 mM l-glutamine. All cells were incubated at 37°C under 5% CO2 and humidified air.
CELL TRANSFECTION WITH ANTISENSE OLIGONUCLEOTIDES
Cells were seeded at 2.5 × 105 cells per well in 6-well plate or at 3,000–4,000 cells per well in 96-well plates and were attached to the well. Transfection was carried using Lipofectamine Plus for experiments of RT-PCR and Western blotting. Lipofectamine 2000 was used for the cell growth inhibition assay according to the manufacture’s manual. Lipofectamine alone was used as a control. The cells were incubated for 3–4 h with the transfection reagent containing an antisense oligonucleotide at 37°C and the medium was replaced with fresh medium containing 10% FBS and incubated for 6 h for RNA isolation, 24 h for protein isolation, and 72 h for cell growth experiment.
RT-PCR ASSAY
Total RNA was isolated with RNA-STAT kit according to the manufacture’s manual. RNA concentration was determined by spectrophotometer at 260 nm. M-MLV enzyme kit was used for RT reaction. Total RNA at 5 µg was used to synthesize cDNA in each 20 µl RT reaction. First-strand cDNA was synthesized by incubating total RNA, oligo dT (0.5 mg), and dNTP mixture (0.5 mM) at 65°C for 5 min. First-strand buffer (7.4 mM DTT and 1 µl M-MLV reverse transcriptase (200 U)) was added to the above RT reaction mixture and incubated at 37°C for 50 min and the enzyme was inactivated at 70°C for 15 min. Akt1 cDNA synthesized was determined using Sapphire PCR mix (SuperBio, Inc., Seoul, Korea) with appropriate primers. To determine Akt1 mRNA expression, primers 5′-CTGGACAAGGACGGGCACA-3′ and 5′-GGTGGGCTGAGCTTCTTCTCGTA-3′ were used. To determine β-actin as an endogenous control, primers 5′-CCCATGCCATCCTGCGTCTG-3′ and 5′-ACGGAGTACTTGCGCTCAG-3′ were used. PCR products were analyzed on 1.5% agarose gel via electrophoresis.
WESTERN BLOTTING ANALYSIS
Various cancer cell lines were transfected with Akt1 AO as described above. About 24 h after transfection, cells were washed once with PBS and resuspended with the lysis buffer containing 25 mM Tris–HCl, pH 7.5, 300 mM NaCl, 1% Triton X-100, and protease inhibitors. Total protein concentration was determined by BCA protein assay according to manufacturer’s manual (Pierce Biotechnology, Rockford, IL). Protein was separated on SDS–PAGE gel and transferred to nitrocellulose membranes. The blots were probed against anti-AKT1 antibody detecting both unphosphorylated and phosphorylated forms of AKT1. Anti-β-actin antibody was used as an internal control.
CELL GROWTH INHIBITION ASSAY
The growth inhibition by Akt1 AO against human cancer cells was assessed by the sulphorhodamine B (SRB) assay as described [Skehan et al., 1990; Vichai and Kirtikara, 2006]. Different human cancer cell lines were transfected with Akt1 AO at various concentrations. After 72 h incubation, surviving cells were fixed with trichloroacetic acid, washed and stained with SRB. For the study of combination effect of Akt1 AO with cytotoxic drugs, caki-1 cells were seeded in 96-multiwell plate and transfected with Akt1 AO for 4 h using Lipofectamine 2000. After removing Akt1 AO from the each well, cells were treated with different concentrations of the indicated cytotoxic drug. 72 h after the treatment with drugs, cells were fixed with trichloroacetic acid and processed with SRB assay. Absorbance was measured at 530 nm using Benchmark Plus Microplate reader (Bio-Rad Laboratories, Hercules, CA). The drug concentration which inhibited the cell growth by 50% (IC50) was calculated using Kaledia Graph software program (Synergy Software, Reading, PA).
IN VIVO MODEL IMPLANTED WITH U251 HUMAN GLIOBLASTOMA CELLS
Human tumor fragments (30–40 mg) from an existing in vivo passage of U251 human glioblastoma cells were implanted subcutaneously (s.c.) into athymic female nude mice near the right axillary area. The day of tumor implant was designated as day 0. After tumors were allowed to grow up to 100–200 mg in weight (100–200 mm3 in size), the treatment with Akt1 AO was initiated. Akt1 AO at 30 mg/kg per injection in normal saline was administered to mice in tail vein injections every other day for 3 weeks, following the detection of a palpable tumor mass (50–100 mm3). Control animals received normal saline alone. After Akt1 AO treatment for 3 weeks, the mice were observed for up to 30 more days to detect possible tumor regrowth. The weight of tumors was measured and the animals were weighed twice weekly starting with the first day of treatment. Tumor volume was determined by caliper and the formula for an ellipsoid sphere: L × W2/2 = mm3, where L and W refer to the larger and smaller dimensions collected at each measurement. This formula was also used to calculate tumor weight, assuming unit density.
IN VIVO MODEL IMPLANTED WITH LUCIFERASE-EXPRESSING MIA (MIA-Luc) CELLS
The luciferase-expressing MIA (MIA-Luc) pancreatic cancer cells were established by infection with a lentivirus encoding the luciferase gene driven by an ubiquitin promoter as we described previously [Dikmen et al., 2008]. Immunodeficient nude mice (Nu/Nu; Harlan Sprague Dawley, Inc., Indianapolis, IN) were subcutaneously implanted with MIA-Luc cells at the density 2.5 × 106 in each flank area for primary tumor assessment without irradiation as we described previously [Dikmen et al., 2008]. The mice (n = 3 per group) were treated without or with Akt1 AO at 30 mg/kg using an Alzet osmotic pump inserted intraperitoneally (i.p.). This pump which has a pumping rate 0.25 µl/h (±0.05 µl/h) provides continuous infusion for 14 days. Tumor implantation and insertion of the Alzet pump were performed on the same day, designated as day 0. In vivo imaging of nude mice carrying the luciferase-expressing MIA cells were performed as we described previously [Dikmen et al., 2008]. Bioluminescence images were taken on days 7 and 14 following tumor implantation and the beginning of treatment. Relative intensities of transmitted light were shown as a pseudo-color image ranging from blue (least intense) to red (most intense).
RESULTS
AN ANTISENSE OLIGONUCLEOTIDE Akt1 AO INHIBITS Akt1 EXPRESSION IN VARIOUS TYPES OF HUMAN CANCER CELLS
Akt1 AO is a 20-mer phosphorothioate antisense oligonucleotide that is a potent inhibitor of Akt-1 and was chosen for the current study from 86 antisense oligonucleotides based on its activities on downregulation of Akt1 mRNA expression level and protein level and cellular growth of cancer cells. Akt1 AO (5′-gctgcatgatctccttggcg-3′) is specifically hybridizable to a site of the Akt1 gene (Genebank # BC000479) having the sequence of 5′-cgccaaggagatcatgcagc-3′.
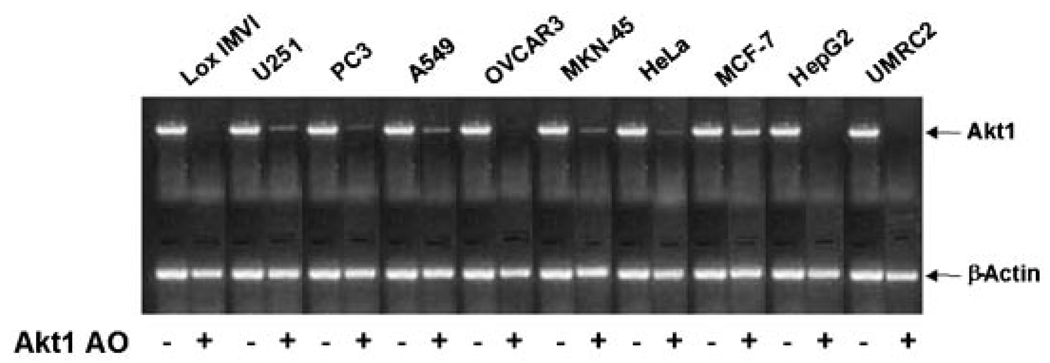
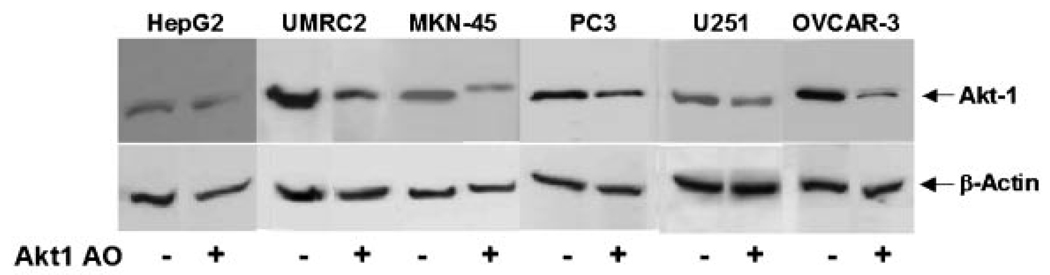
In RT-PCR analysis Akt1 AO showed strong to moderate downregulation of Akt1 mRNA expression compared to the control in the following ten different types of human cancer cell lines: PC3 (prostate), U251 (brain), HeLa (cervix), OVCAR-3 (ovary), Lox-IMVI (melanoma), HepG2 (liver), MCF-7 (breast), UMRC2 (renal), MKN-45 (stomach), and A549 (lung) (Fig. 1). Western blot analysis showed also that Akt1 AO reduced AKT1 protein expression in various types of cancer cell lines (Fig. 2).
Fig. 1.
Effect of Aktl AO on Aktl mRNA expression in various human cancer cells. The cells were transfected without (−) or with 0.3 µM of Akt1 AO (+) using Lipofectamine Plus for 3 h and incubated for 6 h with fresh media containing 10% FBS. Total RNA was isolated and Akt1 mRNA expression was determined using RT-PCR. β-Actin was determined as a loading control. Representative data are shown from two independent cell culture experiments and each RT-PCR reaction was performed in duplicate.
Fig. 2.
Effect of Akt1 AO on AKT1 protein expression in various human cancer cells. The cells were transfected without (−) or with 0.3 µM of Akt1 AO (+) using Lipofectamine Plus for 3 h and incubated for 24 h with fresh media containing 10% FBS. Cellular protein was isolated and AKT1 protein expression was determined by Western blot analysis. β-Actin was determined as a loading control. Representative data are shown from two independent cell culture experiments.
THE SEQUENCE OF Akt1 AO IS SPECIFIC FOR INHIBITING Akt1 mRNA EXPRESSION
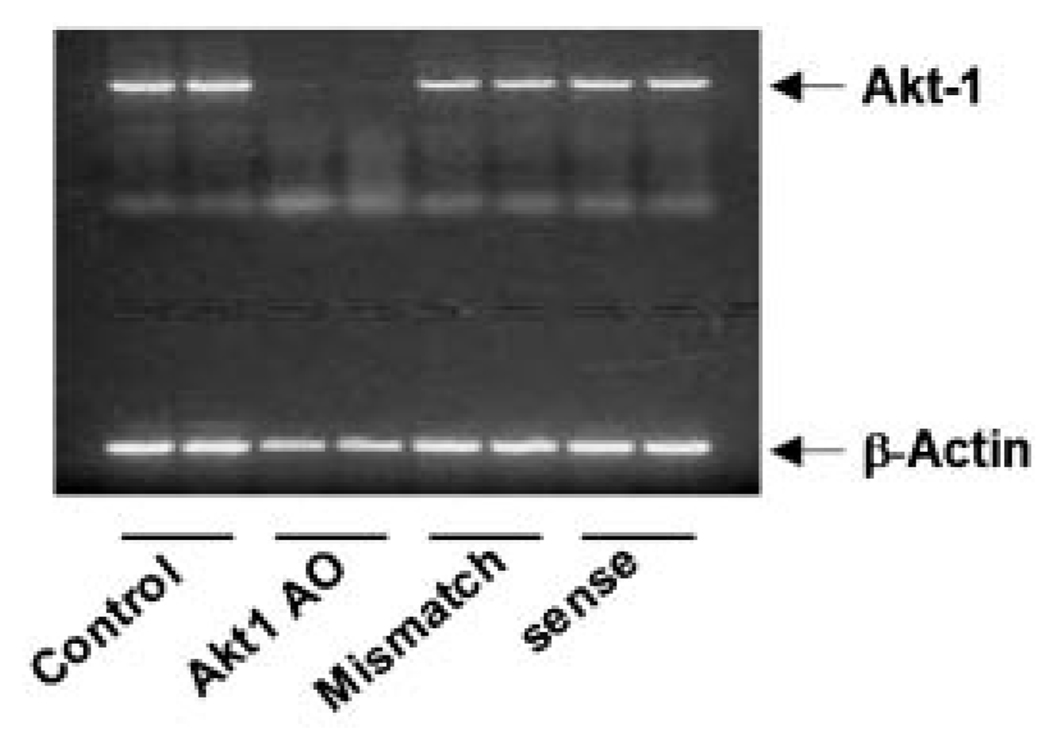
In order to confirm whether Akt1 AO antisense oligonucleotide possesses the sequence specificity to inhibit Akt1 mRNA expression, the effects of two oligonucleotides (sense sequences of Akt1 AO and mismatch sequences at 20 nucleotides in lengths) on Akt1 mRNA expression were determined. UMRC2 cells were transfected with Akt1 AO, sense sequences of Akt1 AO, and mismatch sequences at 0.3 µM. Results indicated that the sense sequences of Akt1 AO and the mismatch sequences had no effects on the inhibition of Akt1 mRNA expression demonstrating the sequence-specific effects of Akt1 AO on Akt1 gene expression (Fig. 3).
Fig. 3.
The sequence of Aktl AO is specific to inhibit Aktl mRNA expression in UMRC2 cells. The cells were transfected with 0.3 µM of Akt1 AO, sense sequence oligonucleotide of Akt1 AO, and mismatch sequence oligonucleotide using Lipofectamine Plus for 3 h and incubated for 6 h with fresh media containing 10% FBS. Total mRNA were isolated and Akt1 mRNA expression was determined using RT-PCR. β-Actin was determined as a loading control. Each RT-PCR reaction was performed in duplicate. Representative data are shown from two independent cell culture experiments.
AN ANTISENSE OLIGONUCLEOTIDE Akt1 AO INHIBITS THE GROWTH OF VARIOUS TYPES OF HUMAN CANCER CELLS
The growth inhibition of cancer cells by Akt1 AO was examined in various human cancer cells. Akt1 AO strongly inhibited proliferation of human cancer cells of the brain, breast, cervix, colon, kidney, liver, lung, ovary, pancreas, prostate, skin, stomach, and skin (melanoma). The growth inhibition by Akt1 AO appeared to be dose-dependent and the IC50 for cell growth ranged from 3.3 to 42 nM in the human cancer cells tested (see Table I).
TABLE I.
In Vitro Growth Inhibition of Human Cancer Cells by Akt1 AO
| Cell line | Types of cancer cells | IC50 (nM) ± SD |
|---|---|---|
| U251 | Brain | 5.0 ± 1.2 |
| MCF-7 | Breast | 20.0 ± 0.45 |
| HeLa | Cervix | 12.0 ± 0.93 |
| HT-29 | Colon | 42.0 ± 13.0 |
| Caki-1 | Kidney | 25.7 ± 0.61 |
| UMRC-2 | Kidney | 15.0 ± 1.6 |
| HepG2 | Liver | 19.0 ± 4.8 |
| A549 | Lung | 6.7 ± 1.3 |
| OVCAR-3 | Ovary | 3.3 ± 0.72 |
| PANC-1 | Pancreas | 28.0 ± 0.98 |
| PC-3 | Prostate | 18.0 ± 4.25 |
| Lox-IMVI | Skin | 18.0 ± 3.3 |
| MKN-45 | Stomach | 6.4 ± 1.4 |
The growth inhibition was determined as described in Materials and Methods Section using the sulforhodamine B (SRB) assay. Akt1 AO treatment at various concentrations was performed in triplicates. Representative data are shown from three independent cell culture experiments (IC50 ± SD).
EFFECTS OF Akt1 AO IN COMBINATION WITH CYTOTOXIC DRUGS
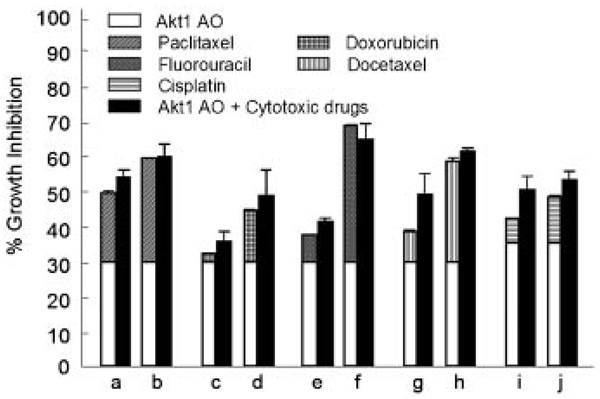
To assess the combination effects of Akt1 AO with known anti-cancer drugs, Caki-1 cells, renal cancer cell line, was used. Akt1 AO alone at a dose of 0.015 µM showed an inhibition of Caki-1 cell growth of ∼30% (Fig. 4). Therefore, we selected this low concentration to study whether any cooperative antiproliferative effect may occur between Akt1 AO and a series of cytotoxic drugs acting by different mechanisms of action. When Caki-1 cells was treated with Akt1 AO and paclitaxel, doxorubicin or fluorouracil, an additive growth inhibitory effect was observed (Fig. 4). Treatment with 0.015 µM Akt1 AO, which inhibits 29.5% of the growth of Caki-1 cells, in combination with 2 or 3 nM paclitaxel, which used alone showed 19.5% and 29.5% growth inhibition, respectively, caused a 53.5% and 59.4% inhibition, respectively. In cells treated with 5 or 10 nM doxorubicin, which alone cause 2.5% and 14.7% growth inhibition, respectively, addition of 0.015 µM Akt1 AO showed a growth inhibition of 35.4% and 48.1%, respectively. The growth inhibitory effect by 5-FU at 2 µM (7.5%) or 5 µM (38.6%) was increased to 40.9% and 64.2%, respectively, with the addition of 0.015 µM Akt1 AO to Caki-1 cells. Further study with docetaxel, drug of the same class with paclitaxel, or cisplatin occurred also the additive inhibition of growth of Caki-1 cells. In fact, in cells treated with 0.5 or 1 nM docetaxel, which alone cause 8.7% and 28.6% growth inhibition, respectively, addition of 0.015 µM Akt1 AO caused a growth inhibition of 48.7% and 60.8%, respectively. Cisplatin at 0.5 µM (7.0%) or 1.0 µM (13.1%) showed also 49.9% and 52.9% of inhibition with the addition of 0.015 µM Akt1 AO, respectively.
Fig. 4.
Combination effect of Akt1 AO with different cytotoxic drugs on the growth of Caki-1 cancer cells. The drugs were used at the following doses: a,b, 2 and 3 nM paclitaxel; c,d, 5 and 10 nM doxorubicin; e,f, 2 and 5 µM fluorouracil; g,h, 0.5 and 1 nM docetaxel, i,j 0.5 and 1.0 µM cisplatin. Data are expressed as percentage growth inhibition in reference to the growth of untreated control cells. Lipofectamine alone (no Akt1 AO) and 0.25% DMSO in media were used as a control for drugs except cisplatin. Lipofectamine alone and water were used as a control for cisplatin. The open portion of the bars represents the percentage growth inhibition values for Akt1 AO. The striped or squared portion of the bars represents the percentage growth inhibition values for the cytotoxic drugs as indicated in the respective legends. The height of the bars on the left represents the sum of the individual agents’ effects and the expected percentage growth inhibition if drugs are additive when used in combination. The total height of the solid bar indicates the actual observed growth inhibition when drugs were used in combination. The data represent means and standard errors of triplicate determination of at least two experiments.
Akt1 AO INHIBITS TUMOR FORMATION IN VIVO
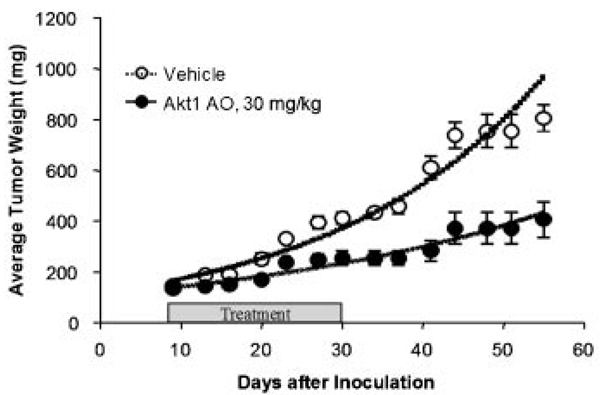
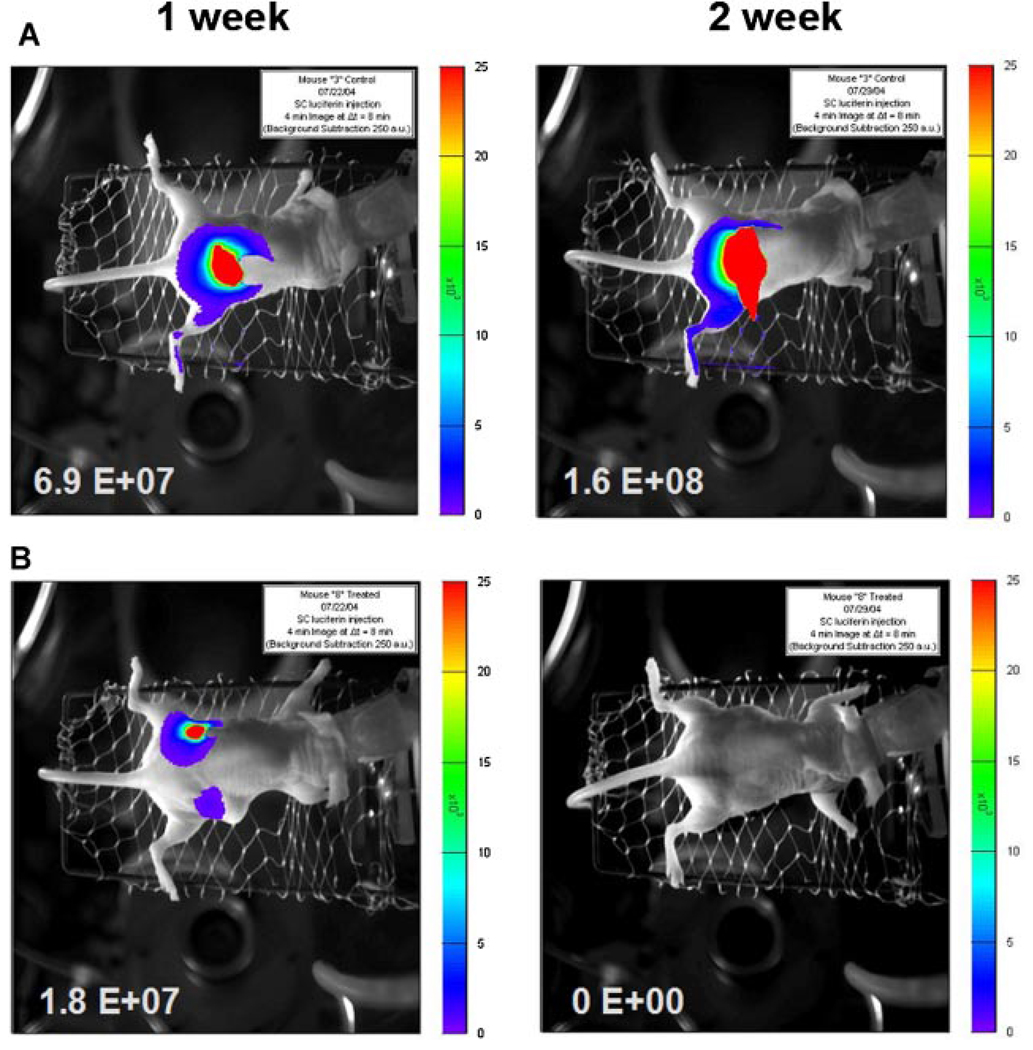
The inhibitory ability of Akt1 AO on tumor formation in vivo was determined using two different xenograft nude mouse models. In athymic nude mice subcutaneously implanted with U251 human glioblastoma fragments, Akt1 AO was well tolerated without deaths and no more than 1 g body weight fluctuations was observed. After day 27, the tumor weights were significantly reduced in the mice treated with Akt1 AO at 30 mg/kg compared to the control vehicle-treated mice (Fig. 5). Progressive tumor development was quantified via live animal bioluminescence imaging in nude mice subcutaneously implanted in the flank area with luciferase-expressing MIA human pancreatic cancer cells (MIA-Luc). The intraperitoneal treatment of Akt1 AO (30 mg/kg) for 2 weeks reduced the size of tumors, with a complete loss of luciferase signals from MIA-Luc tumors in two of the three treated mice, compared to the control group (Fig. 6).
Fig. 5.
Effect of Akt1 AO on the human glioblastoma U251 tumor xenograft. Nude micebearing U251 tumorsweretreated bytail veil injections every other day for 3 weeks with either saline as vehicle control (○) or Akt1 AO at dose of 30 mg/kg (●) per injection. After Akt1 AO treatment for 3 weeks, the mice were observed for up to 30 more days to detect possible tumor regrowth. Data shown are average tumor weight ±SEM (n = 6–8 per group).
Fig. 6.
Representative in vivo bioluminescence images of Aktl AO treatment on luciferase-expressing MIA (MIA-Luc) human pancreatic cancer cells. Nude mice were subcutaneously implanted with MIA-Luc cells at 2.5 × 106 in each flank area. Akt1 AO (30 mg/kg) was continuously and intraperitoneally infused using osmotic pump to the mice. The images on the left hand are the control and the images on the right hand are Akt1 AO treated. A: Control group: The images on the left is 1 week after the injection of MIA-Luc cells and the image on the right is 2 weeks after the injection of cells. B: Akt1 AO treatment group (30 mg/kg): The images on the left is 1 week after treatment with Akt1 AO and the image on the right is 2 weeks after treatment with Akt1 AO. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
DISCUSSION
Antisense oliognucleotide approaches can specifically target genes involved in cancer progression, mainly those that are not easily inhibited by small molecules or antibody inhibition. An antisense oligonucleotide is a single-stranded, chemically modified DNA-like molecule (17–22 nucleotides in length) that is designed to be complementary to a specific gene’s mRNA and thereby specifically inhibit expression of that gene and finally inhibit the target protein and alter the subsequent cascades regulating cellular proliferation, differentiation, apoptosis, and tumor homeostasis [Jansen and Zangemeister-Wittke, 2002; Gleave and Monia, 2005]. Some antisense oligonucleotides show increased specificity for malignant cells and less side effects due to their well-defined mechanisms of action [Jansen and Zangemeister-Wittke, 2002; Gleave and Monia, 2005]. Recently, prostate cancer pre-surgery phase I trials showed that the second generation antisense oligonucleotide 2′-O-methoxy or 2′-methoxy-ethyl (MOE) OGX011 potently inhibited the target protein clusterin and was well tolerated in prostate cancer patients [Gleave and Monia, 2005]. This suggests the potential use of antisense oligonucleotides targeting specific genes as anticancer agents.
In the current study, we demonstrated that our novel antisense oligonucleotide Akt1 AO downregulated Akt1 mRNA and protein expression in several different types of human cancer cell lines. Among the cell lines examined, in OVCAR3 (ovary), MKN45 (stomach), and UMRC2 (kidney) cells, Akt1 AO showed the strongest inhibition of AKT1 expressions at both the mRNA and protein levels. Akt1 AO showed growth inhibitory activity at nanomolar concentrations in 13 different human cancer cell lines. In OVCAR-3 cells, Akt1 AO exhibited the lowest IC50 as 3.3 nM, followed by in U251 (brain) cells as 5.0 nM, in MKN45 cells at 6.4 nM, and in A549 (lung) cells at 6.7 nM, while in HT-29 (colon) and PANC1 (pancreas) cells, Akt1 AO showed higher IC50 as 42 nM and 28 nM, respectively. This different sensitivity of different types of human cancer cells to the growth inhibitory activity of Akt1 AO is maybe from the result of different levels of AKT1 protein expression and/or AKT1 activation/phosphorylation in different cancer cell lines and/or different uptake levels of oligonucleotides in different cancer cells.
To evaluate whether any cooperative effect may exist between cytotoxic drugs and a novel Akt1 inhibitor, Akt1 AO, we tested the effect of Akt1 AO in combination with cytotoxic drugs of different classes in the Caki-1 renal cancer cell line. An additive effect was observed when Akt1 AO was used in combination with the taxanes paclitaxel and docetaxel, the platinum derivative cisplatin, the topoisomerase II-selective agent doxorubicin, and the anti-metabolite fluorouracil. This result suggests that the inhibition of Akt pathway by Akt1 AO plays a key role in the observed cooperative effect with these cytotoxic drugs. Moreover, in vivo studies demonstrated that, Akt1 AO inhibited the tumor formation in two different xenograft mice models. Thus, this tumor growth inhibitory activity of Akt1 AO in both in vitro and in vivo suggests a potential use of Akt1 AO in combination with other anticancer agents to increase the therapeutic index. As the importance of AKT signaling as a therapeutic target is emerging, Akt inhibitors have been extensively investigated in clinical trials for cancer treatment. Akt inhibitors currently in clinical trails include GSK690693, XL418, Perifosine, Triciribine phosphate monohydrate (VQD-002, TCN-P, TCN-PM), and Akt1 AO. Among these drugs, Akt1 AO is the only antisense oligonucleotide compound that selectively inhibits Akt1 [Li, 2007]. Furthermore, although several antisense oligonucleotides targeting Akt1 were synthesized by other researchers [Monia and Cowsert, 2000; Liu et al., 2001; Sale et al., 2007], Akt1 AO is the only known antisense compound currently in clinical trials.
Many potential chemotherapeutic agents have been shown to kill tumor cells by inducing apoptosis (programmed cell death), without eliciting an inflammatory response in the surrounding normal tissue. For example, gemcitabine used as an anti-cancer agent in various carcinomas including pancreatic, non-small cell lung, bladder, and breast cancers induces apoptosis [Plunkett et al., 1995; Li et al., 1999; Rundall et al., 2005]. Nonsteroidal anti-inflammatory drugs (NSAID) such as aspirin [Qiao et al., 1998], sulindac [Piazza et al., 1997], piroxicam [Waddell, 1998], and indomethacin [Erickson et al., 1999] inhibit colon carcinogenesis via induction of apoptosis. We confirmed the occurrence of apoptosis by Akt1 AO treatment in cancer cells using APOPercentage™ Apoptosis Assay that detects the membrane alteration in apoptotic cells (unpublished result). This implies that Akt1 AO may increase apoptosis by inhibiting AKT1 expression and activation.
In conclusion, a novel antisense oligonucleotide Akt1 AO specifically downregulates AKT1 expression at both the mRNA and protein levels and inhibits the growth of various types of human cancer cell lines as well as tumor formation in vivo. Our in vitro and in vivo findings provide a rational for initiating clinical trials with Akt1 AO as a potential anti-cancer agent.
ACKNOWLEDGMENTS
We wish to thank Ms. Lingjun Mao for her technical assistance on RT-PCR and Western blotting analysis. We are grateful to Cancer imaging group (Ralph Mason, Peter Antich, Edmond Richer, Bob Bollinger, Allen Harper) of the University of Texas Southwestern Medical Center for assistance with technical development. This study was supported by the University of Texas Southwestern/MD Anderson Lung SPORE CA70907, the University of Nebraska Medical Center Gastrointestinal Cancer SPORE CA127297, the Cancer Imaging Program P20 (pre-ICMIC) CA 86354, and Rexahn Pharmaceuticals, Inc.
Grant sponsor: University of Texas Southwestern/MD Anderson Lung SPORE; Grant number: CA70907; Grant sponsor: University of Nebraska Medical Center Gastrointestinal Cancer SPORE; Grant number: CA127297; Grant sponsor: Cancer Imaging Program P20 (pre-ICMIC); Grant number: CA 86354; Grant sponsor: Rexahn Pharmaceuticals, Inc.
Abbreviations used
- Akt1 AO
Akt1 antisense oligonucleotide
- DTT
dithiothreitol
- SRB
sulphorhodamine B
REFERENCES
- Asselin E, Mills GB, Tsang BK. XIAP regulates Akt activity and caspase-3-dependent cleavage during cisplatin-induced apoptosis in human ovarian epithelial cancer cells. Cancer Res. 2001;61:1862–1868. [PubMed] [Google Scholar]
- Bellacosa A, Kumar CC, Cristofano AD, Testa JR. Activation of AKT kinases in cancer: Implications for therapeutic targeting. Adv Cancer Res. 2005;94:29–86. doi: 10.1016/S0065-230X(05)94002-5. [DOI] [PubMed] [Google Scholar]
- Cheng JQ, Lindsley CW, Cheng GZ, Yang H, Nicosia SV. The Akt/PKB pathway: Molecular target for cancer drug discovery. Oncogene. 2005;24:7482–7492. doi: 10.1038/sj.onc.1209088. [DOI] [PubMed] [Google Scholar]
- Dikmen ZG, Gellert GC, Dogan P, Yoon H, Lee YB, Ahn CH, Shay JW. In vivo and in vitro effects of a HIF-1A inhibitor, RX-0047. J Cell Biochem. 2008;104:985–994. doi: 10.1002/jcb.21681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson BA, Longo WE, Panesar N, Mazuski JE, Kaminski DL. The effect of selective cyclooxygenase inhibitors on intestinal epithelial cell mitogenesis. J Surg Res. 1999;81:101–107. doi: 10.1006/jsre.1998.5511. [DOI] [PubMed] [Google Scholar]
- Gleave ME, Monia BP. Antisense therapy for cancer. Nat Rev Cancer. 2005;5:468–479. doi: 10.1038/nrc1631. [DOI] [PubMed] [Google Scholar]
- Jansen B, Zangemeister-Wittke U. Antisense therapy for cancer – The time of truth. Lancet Oncol. 2002;3:672–683. doi: 10.1016/s1470-2045(02)00903-8. [DOI] [PubMed] [Google Scholar]
- Li Q. Recent progress in the discovery of Akt inhibitors as anticancer agents. Expert Opin Ther Patents. 2007;17:1077–1130. [Google Scholar]
- Li YW, Singh B, Ali N, Sarkar FH. Induction of growth inhibition and apoptosis in pancreatic cancer cells by auristatin-PE and gemcitabine. Int J Mol Med. 1999;3:647–653. doi: 10.3892/ijmm.3.6.647. [DOI] [PubMed] [Google Scholar]
- Liang J, Slingerland JM. Multiple roles of the PI3K/PKB (Akt) pathway in cell cycle progression. Cell Cycle. 2003;2:339–345. [PubMed] [Google Scholar]
- Liu X, Shi Y, Han EKH, Chen Z, Rosenberg SH, Giranda VL, Luo Y, Ng SC. Downregulation of Akt1 inhibits anchorage-independent cell growth and induces apoptosis in cancer cells. Neoplasia. 2001;3:278–286. doi: 10.1038/sj.neo.7900163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu LZ, Zhou XD, Qian G, Shi X, Fang J, Jiang BH. AKT1 amplification regulates cisplatin resistance in human lung cancer cells through the mammalian target of rapamycin/p70S6K1 pathway. Cancer Res. 2007;67:6325–6332. doi: 10.1158/0008-5472.CAN-06-4261. [DOI] [PubMed] [Google Scholar]
- Monia BP, Cowsert LM. Antisense modulation of Akt-1 expression. World Intellectual Property Organization, International Publication No. WO2000/036149. 2000 [Google Scholar]
- Piazza GA, Rahm AK, Finn TS, Fryer BH, Li H, Stoumen AL, Pamukcu R, Ahnen DJ. Apoptosis primarily accounts for the growth-inhibitory properties of sulindac metabolites and involves a mechanism that is independent of cyclooxygenase inhibition, cell cycle arrest, and p53 induction. Cancer Res. 1997;57:2452–2459. [PubMed] [Google Scholar]
- Plunkett W, Huang P, Xu YZ, Heinemann V, Grunewald R, Gandhi V. Gemcitabine: Metabolism, mechanisms of action, and self-potentiation. Semin Oncol. 1995;22(4 Suppl 11):3–10. [PubMed] [Google Scholar]
- Qiao L, Hanif R, Sphicas E, Shiff SJ, Rigas B. Effect of aspirin on induction of apoptosis in HT-29 human colon adenocarcinoma cells. Biochem Pharmacol. 1998;55:53–64. doi: 10.1016/s0006-2952(97)00400-0. [DOI] [PubMed] [Google Scholar]
- Rundall BK, Denlinger CE, Jones DR. Suberoylanilide hydroxamic acid combined with gemcitabine enhances apoptosis in non-small cell lung cancer. Surgery. 2005;138:360–367. doi: 10.1016/j.surg.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Sale G, Sale E, Hodgkinson C, Jones N. Antisense oligonucleotides against protein kinase isoforms alpha, beta, and gamma. World Intellectual Property Organization, International Publication No. WO 2007/020459. 2007 [Google Scholar]
- Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990;82:1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- Staal SP. Molecular cloning of the akt oncogene and its human homologues AKT1 and AK T2: Amplification of AKT1 in a primary human gastric adenocarcinoma. Proc Natl Acad Sci USA. 1987;84:5034–5037. doi: 10.1073/pnas.84.14.5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staal SP, Hartley JW, Rowe WP. Isolation of transforming murine leukemia viruses from mice with a high incidence of spontaneous lymphoma. Proc Natl Acad Sci USA. 1977;74:3065–3067. doi: 10.1073/pnas.74.7.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M, Wang G, Paciga JE, Feldman RI, Yuan Z-Q, Ma X-L, Shelley SA, Jove R, Tsichlis PN, Nicosia SV, Cheng JQ. AKT1/PKB kinase is frequently elevated in human cancers and its constitutive activation is required for oncogenic transformation in NIH3T3 cells. Am J Pathol. 2001;159:431–437. doi: 10.1016/s0002-9440(10)61714-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa JR, Bellacosa A. AKT plays a central role in tumorigenesis. Proc Natl Acad Sci USA. 2001;98:10983–10985. doi: 10.1073/pnas.211430998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vichai V, Kirtikara K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat Protoc. 2006;1:1112–1116. doi: 10.1038/nprot.2006.179. [DOI] [PubMed] [Google Scholar]
- Waddell WR. Stimulation of apoptosis by sulindac and piroxicam. Clin Sci (Lond) 1998;95:385–388. [PubMed] [Google Scholar]
- Yang X, Fraser M, Moll UM, Basak A, Tsang BK. Akt-mediated cisplatin resistance in ovarian cancer: Modulation of p53 action on caspase-dependent mitochondrialdeath pathway. Cancer Res. 2006;66:3126–3136. doi: 10.1158/0008-5472.CAN-05-0425. [DOI] [PubMed] [Google Scholar]