Abstract
Motor deficits are commonly reported in autism, with one of the most consistent findings being impaired execution of skilled movements and gestures. Given the developmental nature of autism, it is possible that deficits in motor/procedural learning contribute to impaired acquisition of motor skills. Thus, careful examination of mechanisms underlying learning and memory may be critical to understanding the neural basis of autism. A previous study reported impaired motor learning in children with high-functioning autism (HFA); however, it is unclear whether the observed deficits in motor learning are due, in part, to impaired motor execution and whether these deficits are specific to autism. In order to examine these questions, 153 children (52 with HFA, 39 with attention-deficit/hyperactivity disorder (ADHD) and 62 typically developing (TD) children) participated in two independent experiments using a Rotary Pursuit task, with change in performance across blocks as a measure of learning. For both tasks, children with HFA demonstrated significantly less change in performance than did TD children, even when differences in motor execution were minimized. Differences in learning were not seen between ADHD and TD groups on either experiment. Analyses of the pattern of findings revealed that compared with both ADHD and TD children, children with HFA showed a similar degree of improvement in performance; however, they showed significantly less decrement in performance when presented with an alternate (“interference”) pattern. The findings suggest that mechanisms underlying acquisition of novel movement patterns may differ in children with autism. These findings may help explain impaired skill development in children with autism and help to guide approaches for helping children learn novel motor, social and communicative skills.
Keywords: procedural memory, declarative memory, cerebellum, visuomotor learning
Impaired Motor Sequence Learning in Children with Autism but not in Children with ADHD
Autism is a developmental disorder characterized by delays and abnormalities in communication (both verbal and non-verbal), social interaction and a restricted range of interests. Accumulating evidence from the past decade suggests that motor impairment is also a prominent feature of the disorder [Gidley Larson & Mostofsky, 2006; Noterdaeme, Mildenberger, Minow, & Amorosa, 2002; Smith, 2004]. Evidence of anomalous motor development is a common, if not consistent, finding in children with autism spectrum disorders (ASD), equally present across high- and low-functioning individuals [Freitag, Kleser, Schneider, & von Gontard, 2007; Gidley Larson & Mostofsky, 2006; Green et al., 2002; Jansiewicz et al., 2006; Kanner, 1943; Manjiviona & Prior, 1995; Mostofsky et al., 2006; Smith & Bryson, 1994]. Early motor milestones, including sitting up and walking, are generally acquired at expected ages and while some initial retrospective studies suggested that children with autism show abnormalities in these basic motor skills [Teitelbaum et al., 2004; Teitelbaum, Teitelbaum, Nye, Fryman, & Maurer, 1998], a more recent prospective study failed to detect differences [Ozonoff et al., 2008]. In contrast, acquisition of more complex skills (e.g. riding a tricycle and pumping legs on a swing) is often delayed in children with autism [Gidley Larson & Mostofsky, 2006] and empirical studies consistently reveal impaired performance of complex motor skills and gestures in children with ASD, consistent with a developmental “dyspraxia” [Dewey, 1991; Minshew, Goldstein, & Siegel, 1997; Mostofsky et al., 2006; Rogers, Bennetto, McEvoy, & Pennington, 1996].
In the context of the developmental disorder of autism, it may be that impaired acquisition (i.e. learning) of these complex motor skills and gestures is secondary to a fundamental problem with motor skill learning. In most neuropsychological models, motor skill learning is included in a broader construct of procedural learning, which refers to the process by which skills and actions are acquired implicitly (without conscious recall) through repeated exposure to, and practice of, a task [Squire, 1986]; this is in contrast to declarative learning, which refers to acquisition of facts that can be recalled explicitly (with conscious recall). The mechanisms involved in motor skill learning are thought to also contribute to the development of social and communicative skills. More specifically, procedural learning may underlie the development of particular aspects of language—syntax and grammar [for reviews, see Ullman, 2001, 2004; Walenski, Tager-Flusberg, & Ullman, 2006], as well as the development of non-verbal social and communicative gestures (i.e. waving, blowing a kiss), which involves learning a series of complex movement sequences. Support to this hypothesis comes also from studies in typically developing (TD) children, showing a strong association between oral-motor skills and speech fluency as well as oral-motor skills and manual-motor skills [Alcock, 2006; Bishop, 2002].
Despite the fact that social, communicative and motor skills are typically impaired, parents commonly report that their children with ASD are often remarkable at memorizing facts and information (tasks dependent on declarative learning). Thus, it may be that children with ASD excessively rely on the declarative, rather than the non-declarative (i.e. procedural) learning process [Gidley Larson & Mostofsky, 2006]. Consistent with this observation, there is evidence that compared with TD children, children with autism show greater reliance on explicit/declarative approaches to learning new information and categories [Klinger & Dawson, 2001] and that they show a pattern of word retrieval consistent with enhanced declarative memory [Walenski, Mostofsky, Gidley Larson, & Ullman, 2008].
In contrast to normal and/or enhanced declarative learning abilities, research has suggested that children with high-functioning autism (HFA) may be impaired in their motor sequence learning (i.e. procedural learning) [Mostofsky, Goldberg, Landa, & Denckla, 2000]. Using a serial reaction time task (SRTT) children implicitly learned a series of repeated discrete movements; motor sequence learning was measured by a decrease in reaction time across the sequence blocks of trials. It was found that children with HFA, compared to TD children, showed significantly less improvement (decrease in reaction time) across the blocks of trials, suggestive of an impairment in motor sequence learning. Children with HFA also had significantly longer reaction times across the blocks of trials than did TD children. Given this, an impact of deficits in motor execution, reported in several studies of children with HFA [Freitag et al., 2007; Jansiewicz et al., 2006; Mari, Castiello, Marks, Marraffa, & Prior, 2003], cannot be ruled out. Further, impairments in sustained attention and associated difficulty staying on-task might also contribute to a failure to show improvement in motor skill performance across the blocks of trials. Finally, in another SRTT study, children with autism did demonstrate motor learning on an SRTT task, albeit after extensive training and fewer number of movements within the implicit sequence [Gordon & Stark, 2007].
The rotary pursuit (RP) is another visuomotor task that has often been used to examine procedural/motor skill learning in a variety of populations [Eslinger & Damasio, 1986; Gabrieli, Stebbins, Singh, Willingham, & Goetz, 1997; Heindel, Salmon, Shults, Walicke, & Butters, 1989; Jacobs et al., 1999; Roth, Baribeau, Milovan, O’Conner, & Todorov, 2004; van Gorp, Altshuler, Theberge, & Mintz, 1999], including child populations [Frith & Frith, 1974; Lord & Hulme, 1988]. Improving performance on the RP task involves learning a sequence of complex movements that anticipate the motion of a target in a novel pattern (circle or square). Unlike the SRTT, the RP provides an opportunity to control for differences in motor execution; the speed of the pursuit rotor can be adjusted so that the initial motor performance (time-on-target) is equilibrated across individual subjects. This approach has been used in prior studies investigating procedural learning in adults with bipolar disorder [van Gorp et al., 1999] and with obsessive–compulsive disorder [Roth et al., 2004].
To further investigate both motor skill learning in HFA and the contribution of motor execution to motor learning, RP was used to examine whether children with autism show impaired learning in a situation in which motor execution is similar across the groups of subjects. We conducted two independent experiments using the RP task: in Experiment 1 the pursuit rotor was set at the same speed for each subject, 20 revolutions per minute (RPMs); in Experiment 2, the speed of the pursuit rotor was calibrated for each subject at the outset of the experiment in order to minimize inter-subject differences in motor execution. We hypothesized that in both experiments, children with autism would demonstrate an impaired ability to learn the novel circular motor pattern, indicated by little change in performance across trials.
Additionally, in order to examine the effect of reliance on declarative learning, both RP experiments had an intervening block with an alternate (square) pattern. With acquisition of a motor skill, introducing a competing stimulus that forces an alternate pattern of movement sequences results in a substantial decrement in performance [Robertson, 2007; Willingham, Salidas, & Gabrieli, 2002]. In contrast to procedural memory, declarative memories are less fixed and highly flexible [Cohen, Poldrack, & Eichenbaum, 1997]. Consequently, for individuals who rely on declarative memorization to learn movement patterns, the decrement associated with a competing stimulus may be not as substantial. We therefore hypothesized that children with autism, who appear to rely more on declarative learning than do TD children, would show less interference (i.e. little change in time-on-target) with the intervening introduction of the square block than would TD controls.
Further, in order to fully examine the specificity of motor learning deficits to HFA and whether motor learning deficits are due in part to impaired motor execution or problems with sustained attention/off-task behavior, we also included a clinical control group of children with attention-deficit/hyperactivity disorder (ADHD). ADHD is a developmental disorder characterized by excessive difficulty in sustaining attention and staying on-task and/or excessive hyperactivity and impulsivity [American Psychiatric Association, 1994] and, like autism, ADHD has been found to be associated with impairments in motor execution and control [Klein, Wendling, Huettner, Ruder, & Peper, 2006; Klimkeit, Mattingley, Sheppard, Lee, & Bradshaw, 2005; Mostofsky, Goldberg, Cutting, & Denckla, 2001; Mostofsky, News-chaffer, & Denckla, 2003]. We hypothesized that while children with ADHD may demonstrate deficits in motor execution (indicated by lower time-on-target during Experiment 1), the deficit in motor learning would be specific to autism.
Method
Participants
A total of 153 (52 HFA, 39 ADHD and 62 TD) children participated in this study. Participants were recruited from outpatient clinics at the Kennedy Krieger Institute, local Autism Society of America and Children and Adults With Attention-Deficit/Hyperactivity Disorder chapters, postings at schools, social skills groups, pediatrician’s offices and word of mouth. For all experiments, the participants were between the ages of 8 and 13 years. All participants had a birthweight greater than 2,000 g, had no history of seizures, neurological disorders primarily affecting motor performance, traumatic brain injury, mental retardation or known perinatal drug exposure. All participants had normal or corrected-to-normal vision.
Each experiment examined motor performance of children with HFA, with ADHD and TD children. To determine the presence or absence of ADHD, the parents of all participants were given a structured parent interview, the Diagnostic Interview for Children and Adolescents—Fourth Edition [DICA-IV; Reich, 2000] and an ADHD-specific behavior rating scale [Conners’ Parent Rating Scale—Revised (CPRS-R), long form; Conners, Sitarenios, Parker, & Epstein, 1998]. The DICA-IV was also used to examine the presence of other psychiatric diagnoses.
All children in the HFA group met Diagnostic and Statistical Manual of Mental Disorders—Fourth Edition (DSM-IV) algorithm criteria for autism, confirmed using the Autism Diagnostic Interview—Revised [Lord, Rutter, & Le Couteur, 1994] and Module 3 of the Autism Diagnostic Observation Schedule—Generic [ADOS-G; Lord et al., 2000] or an earlier edition of the ADOS [Lord et al., 1989]. Children in the HFA group were excluded from the study if they met DSM-IV criteria for conduct disorder.
For children in the ADHD group, the DICA-IV [Reich, 2000] and the CPRS [Conners et al., 1998] were used to confirm a diagnosis of ADHD. Children with DSM-IV diagnoses other than oppositional defiant disorder and simple phobia were excluded from the study. Children with ADHD were excluded from the study if they were taking longer-acting psychoactive medications (i.e. other than stimulants).
Control children were eligible for the study if they met the following criteria: no evidence of neurological disorder, no presence of ASD in any immediate family members (siblings, parents), free from diagnosis on a standardized psychiatric parent interview, the DICA-IV [Reich, 2000] (with the exception of simple phobia), no elevated scores on the CPRS [Conners et al., 1998] and no history or current use of any psychoactive medication.
Within the HFA group, 14 participants met diagnostic criteria for ADHD, 13 participants were taking stimulants, 9 were taking selective serotonin re-uptake inhibitors, 1 was taking a mood stabilizer, 4 were taking atypical neuroleptics and 1 was taking clonidine. Within the ADHD group, 33 participants were taking stimulant medication and 1 was taking clonidine. For all subjects, stimulant medications were discontinued the day prior to and the day of testing; all other medications were taken as prescribed.
Intellectual functioning was assessed using the most current version of the Wechsler Intelligence Scale for Children (WISC) at the time of testing, WISC—Third Edition [WISC-3; Wechsler, 1991] (n =52) or the WISC—Fourth Edition [WISC-4; Wechsler, 2003] (n =100), with the exception of one child whose intelligence was measured using the Differential Ability Scales [Elliott, 1990]. All children had a full-scale IQ (FSIQ) equal to or greater than 80 with the exception of three children with HFA. These children had an FSIQ within 4 points of 80 and either their perceptual or verbal score was greater than 90.
The study was approved by the Johns Hopkins Medical Institutional Review Board. Written consent was obtained from a parent/guardian and written assent was obtained from all participating children.
Apparatus and Procedures
A Lafayette instrument (photoelectric pursuit rotor, Model 30014A; Lafayette Instrument Company, Lafayette, IN) photoelectric RP was used to examine motor sequence learning in children with HFA, ADHD and TD children. The RP instrument consists of a disc, much like a turntable, with a backlit target (2 × 2 cm) on it. The disc revolves by means of an electric motor, which can be manually controlled to revolve at an RPM range of 5–99, in either a clockwise or a counterclockwise direction. Once the RPMs and direction are set by the experimenter, a plastic template, either a circle or a square in this case, is then placed over the disc so that a particular shape is visible. An “L-shaped” handheld stylus is used by the participant to follow the moving target around in the shape (circle or square) at a set RPM. Motor learning is measured by the increase of time spent on target across trials.
Using their dominant hand, which was predetermined using the Edinburgh Handedness Inventory [Oldfield, 1971], participants were instructed to keep the handheld stylus over the target as it moved in a clockwise pattern (circle or square) at a continuous speed. Two independent experiments were conducted using the above paradigm. In Experiment 1, for all subjects, the speed of the pursuit rotor was set at 20 RPM. In Experiment 2, the speed of the pursuit rotor was calibrated on an individual basis for each subject in order to achieve optimal performance while controlling for motor execution. For both Experiment 1 (“Non-Variable RPM”) and Experiment 2 (“Variable RPM”), all subjects performed four successive blocks; each block consisted of four 20-sec trials; there was a 20-min break between blocks 1 and 2. A circular pattern was presented during blocks 1, 2 and 4; during block 3 a square pattern was presented. During the 20-min break the participants performed other neuropsychological tests that are part of the testing battery. The tasks given during the break were not motor or learning based. Unless longer time was requested by the participant, the time between the other blocks was approximately 1 min. The inclusion of the intervening square block was critical to the examination of motor sequence learning; it provided a means of examining whether subjects’ improvement in time-on-target across the preceding circular blocks was due to learning the sequence of movements necessary to smoothly execute the circular pattern (reflected as a decrement in performance during the square block) or rather less specific learning involved in performing the task. Given that we are assessing motor learning, it was necessary to have separate group of children participating in each of the experiments.
Experiment 1: Non-Variable RPM
Thirty-eight children with HFA, 26 children with ADHD and 37 TD children were tested using the RP paradigm described above. Throughout all trials, participants were tested at a continuous speed of 20 RPM.
Experiment 2: Variable RPM
Fourteen children with HFA, 13 children with ADHD and 22 TD children were examined using the RP paradigm described above. In contrast to Experiment 1, Experiment 2 controlled for inter-individual subject differences in basic motor speed and function by adjusting the speed of the pursuit rotor for each child (15, 20, 25 or 30 RPM). Prior to the testing session (at least 1 day prior, time between sessions ranged from 2 days to less than 2 months, with the exception of two children (one ADHD child and one TD child) having 2.5 months between sessions and one HFA child who had 5 months between testing sessions; the mean time between sessions was less than 1 month), participants performed three 20-sec trials. The speed of the pursuit rotor was adjusted during these trials; every child started at 20 RPM, and depending on the performance the speed was either increased or decreased until the child stayed on-target in the range of 5.5–6.5 sec. This speed was then used for all trials during the learning session.
Analyses
For each experiment, multiple analyses of variance were used to examine group differences in age and intellectual ability. Recent research suggests that measures of perceptually based reasoning are more valid measures of intellectual ability in children with ASD [Mottron, 2004] than FSIQ. While all aspects of IQ are reported in Table I, the perceptual reasoning index (PRI) from the WISC-4 was considered as best suited to assess intellectual ability in children with autism and is reported within each experiment. For those subjects who received the older version of the Wechsler, the WISC-3, performance IQ (PIQ) was considered as the best possible measure of intellectual reasoning; however, the subtests used to measure PIQ were all timed (unlike those used in the WISC-4 PRI) and therefore highly dependent on the processing speed. Given the problems with speeded response noted above, PRI was therefore considered as a better measure of intellectual ability in children with autism than was PIQ.
Table I.
Demographic Variables
| Gender |
FSIQ |
VCI/VIQ |
PRI/PIQ |
Age |
ADOS total |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | F | M | SD | M | SD | M | SD | M | SD | M | SD | |
| Experiment 1: Non-Variable RPM | ||||||||||||
| HFA (n = 38) | 34 | 4 | 98.3* | 11.3 | 100.1* | 17.7 | 101.8* | 15.4 | 10.6 | 1.7 | 14.4 | 3.6 |
| ADHD (n = 26) | 21 | 5 | 107.5* | 14.3 | 110.8* | 12.9 | 105.0 | 12.8 | 10.5 | 1.3 | – | – |
| TD (n = 37) | 34 | 3 | 115.5* | 12.2 | 111.7* | 15.2 | 111.1* | 13.4 | 10.5 | 1.5 | – | – |
| Experiment 2: Variable RPM | ||||||||||||
| HFA (n = 14) | 12 | 2 | 104.6 | 17.2 | 113.5 | 22.3 | 108.7 | 19.4 | 10.8 | 1.6 | 13.2 | 4.7 |
| ADHD (n = 13) | 9 | 4 | 102.7 | 11.1 | 110.6 | 16.6 | 103.5 | 12.5 | 10.9 | 1.3 | – | – |
| TD (n = 22) | 15 | 7 | 111.4 | 12.1 | 115.5 | 18.2 | 108.8 | 14.2 | 10.4 | 1.3 | – | – |
Note: For both experiments there was no significant difference in gender, age or total ADOS score across the three groups. In Experiment 1, post hoc analyses revealed a significant difference in the FSIQ across all three groups, in all cases P<0.018; in the VCI/VIQ between the HFA and ADHD groups (P<0.009) and the HFA and TD groups (P<0.0001); and in the PRI/PIQ between only the HFA and TD groups (P<0.005). However, given that PIQ reflects not only perceptual reasoning, but also processing speed, we conducted additional analyses including only children that were given the WISC-4 (HFA: n =18, 47%; ADHD: n =13, 50%; TD: n =17, 46%). Analysis of the PRI from the WISC-4 revealed no significant differences between the three groups [F(1, 2) =0.6; P =0.50]. In Experiment 2, all children received the WISC-4, and analyses revealed no significant differences in FSIQ, VCI or PRI across the three groups, in all cases P>0.14. FSIQ, full-scale IQ; VCI, verbal comprehension index; VIQ, verbal IQ; PRI, perceptual reasoning index; PIQ, performance IQ; ADOS, Autism Diagnostic Observation Schedule; HFA, high-functioning autism; ADHD, attention-deficit/hyperactivity disorder; TD, typically developing; WISC-4, Wechsler Intelligence Scale for Children—Fourth Edition.
In all cases, indicates that P<.05.
Motor sequence learning was measured by a change in time-on-target across the blocks. This change was statistically analyzed using a repeated measures analysis of variance (ANOVA), with mean time-on-target for each block used as dependent measures and group (diagnosis) as the independent measure. Following this, two-group repeated measures ANOVAs (TD vs. HFA, TD vs. ADHD, HFA vs. ADHD) were used to examine individual group effects.
Results
Given that each experiment had a different number of participants, age and IQ data for each sample are given in the respective results sections; see Table I for complete age and IQ data. Demographically, the entire sample was predominantly Caucasian (81% Caucasian, 13% African-American, 6% other (Asian, Hispanic, native American, bi-racial)), right-handed (87%) and male (83%). There were no significant differences between the groups on ethnicity or handedness. Similarly, there were no significant differences in age, gender distribution or ADOS scores between the groups of subjects participating in each experiment (see Table I).
Experiment 1: Non-Variable RPM
Thirty-eight children with HFA, 26 children with ADHD and 37 TD children participated in this task. There was no significant difference in the mean age across the three groups (HFA =10.6 years, ADHD =10.5 years, control =10.5 years).
There was a significant difference in PRI/PIQ across the three groups [F(2, 98) =4.22; P =0.02]; however, in light of the fact that PIQ reflects not only perceptual reasoning, but also processing speed, we conducted additional analyses including only children that were given the WISC-4 (HFA: n =18, 47% of the total sample; ADHD: n =13, 50% of the total sample; TD: n =17, 46% of the total sample). Analysis of the PRI from the WISC-4 revealed no significant differences between the three groups [F(2, 45) =0.6; P =0.5].
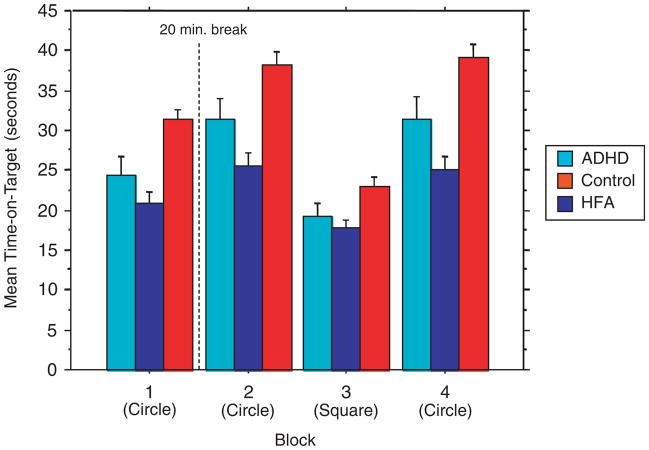
Analysis of RP performance revealed that there was a significant effect of group [F(2, 98) =13.9; P<0.0001], indicating that the groups differed in mean time-on-target across all blocks of trials. Follow-up two-group repeated measures ANOVA revealed a significant main effect of group for both TD vs. HFA [F(1, 73) =33.2; P<0.0001] and TD vs. ADHD [F(1, 61) =6.89; P =0.01], with both children with HFA and children with ADHD showing less time-on-target than TD controls. The difference between HFA and ADHD was nearly significant [F(1, 62) =3.40; P =0.07], with the HFA children showing less time-on-target than the ADHD children.
There was a significant main effect of all blocks across all subjects [F(3, 294) =131.85; P<0.0001] indicating change in performance across the blocks of trials. There was also a significant [(group) × (block)] interaction [F(3, 294) =6.17; P<0.0001], indicating that the amount of change across all blocks differed among groups (Fig. 1). Follow-up two-group repeated measures ANOVAs revealed a significant [(group) × (block)] interaction for both TD vs. HFA [F(3, 219) =12.0; P<0.0001] and ADHD vs. HFA [F(3, 186) =3.38; P =0.02], with children with HFA showing less change in time-on-target across the blocks of trials than both TD children and children with ADHD. There was no significant [(group) × (block)] interaction for TD vs. ADHD [F(3, 183) =1.94; P =0.12].
Figure 1.
Experiment 1: Non-Variable RPM. Mean time-on-target. Note: Bar graph showing mean performance (time-on-target) for blocks of trials during Experiment 1 in children with ADHD (left bars in grouping), HFA (middle bars) and TD control children (right bars in grouping). Subjects performed four blocks, each with four 20-sec trials. During the first, second and fourth blocks of trials the subjects tracked a light in a circular pattern; they tracked a square pattern in the third block. For Experiment 1, the pursuit rotor was set at the same speed (20 RPM) for each subject. Repeated measures ANOVA revealed a significant main effect of diagnosis across the blocks of trials, with TD children showing greater time-on-target than did children with HFA (P<0.0001) and children with ADHD (P<0.01). Motor learning was measured as change in time-on-target across the blocks of trials. Across all three groups there was a significant change in performance across the blocks of trials (P<0.0001). There was also a significant [(group) × (block)] interaction (P<0.0001), indicating that the amount of learning across all blocks differed among the groups. Follow-up two-group analyses revealed a significant [(group) × (block)] interaction for both TD vs. HFA (P<0.0001) and ADHD vs. HFA (P =0.019), with children with HFA showing less change in time-on-target across the blocks of trials than both TD children and children with ADHD. There was no significant [(group) × (block)] interaction for TD vs. ADHD. Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com. RPM, revolution per minute; ADHD, attention-deficit/hyperactivity disorder; HFA, high-functioning autism; TD, typically developing; ANOVA, analysis of variance. [Color figure can be viewed in the online issue which is available at www.interscience.wiley.com]
Close examination of the plots shown in Figure 1 suggests that the interaction between groups may be driven primarily by the fact that the transition to the square interference block was associated with substantially less decrement in performance for the children with HFA compared with the ADHD and TD children. To test this hypothesis, follow-up repeated measures were used to examine the effect of diagnosis on change between blocks 2 and 3. The analyses indicated that there was a significant [(group) × (block)] interaction effect [F(1, 98) =10.95; P<0.0001]; post hoc analyses revealed that the children with HFA showed significantly less decrement in performance compared with both TD children [F(1, 73) =21.45; P<0.0001] and ADHD children [F(1, 62) =6.71; P =0.01]; there was no significant difference in change between blocks 2 and 3 for the TD and ADHD children [F(1, 61) =2.634; P =0.11].
Analysis was also run in order to examine change across only the three circular blocks, blocks 1–3. Repeated measures ANOVA revealed that there was a significant effect of group [F(2, 98) =15.80; P<0.0001], a significant main effect of block across all subjects [F(2, 196) =51.01; P<0.0001], but not for the [(group) × (block)] interaction [F(2, 196) =1.34; P =.25], indicating that all groups showed a comparable increase in time-on-target across the blocks of circular trials.
Experiment 2: Variable RPM
Fourteen children with HFA, 13 children with ADHD and 22 TD children participated in this task. There were no significant differences in the mean age between the three groups (HFA =10.8 years, ADHD =10.9 years, control =10.3 years). Analysis of the PRI (all children in this experiment received WISC-4) revealed no significant difference across the three groups [F(2,46) =.56; P =0.58] (see Table I).
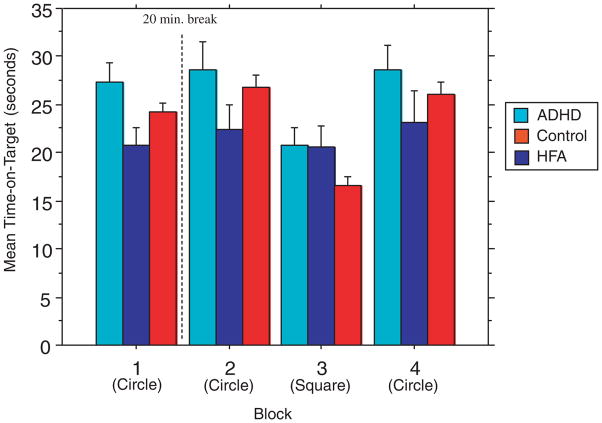
In contrast to Experiment 1, the analysis of RP performance revealed that there was no significant effect of group [F(2, 46) =1.77; P =0.18], indicating that the groups did not differ in mean time-on-target across the blocks of trials.
There was a significant main effect of block across all subjects [F(3, 138) =19.89; P<0.0001] indicating change in performance across the blocks of trials. There was also a significant [(group) × (block)] interaction [F(3, 138) =2.88; P =0.01], indicating that the amount of change across all blocks differed among groups (Fig. 1). Follow-up two-group repeated measures ANOVA revealed a significant [(group) × (block)] interaction only for TD vs. HFA [F(1, 34) =5.77; P =0.001] and not for TD vs. ADHD [F(1, 33) =.436; P =0.73], indicating that, as with the Non-Variable RP task, only children with HFA showed no change in performance compared to TD children (Fig. 2). There was no significant [(group) × (block)] interaction for ADHD vs. HFA [F(1, 25) =2.03; P =0.12].
Figure 2.
Experiment 2: Variable RPM. Mean time-on-target. Note: Bar graph showing mean performance (time-on-target) for blocks of trials during Experiment 1 in children with ADHD (left bars in groupings), HFA (middle bars) and TD control children (right bars in grouping). Subjects performed four blocks, each with four 20-sec trials. During the first, second and fourth blocks of trials the subjects tracked a light in a circular pattern; they tracked a square pattern in the third block. For Experiment 2, the speed of the pursuit rotor was calibrated on an individual basis to minimize differences in motor execution. Repeated measures ANOVA revealed no significant effect of diagnosis across the blocks of trials (P>0.05). Motor learning was measured as a change in time-on-target across the blocks of trials. Across all three groups there was a significant change in performance across the blocks of trials (P<0.0001). There was also a significant [(group) × (block)] interaction (P =0.011), indicating that the amount of learning across all blocks differed among the groups. Consistent with Experiment 1, follow-up two-group analyses revealed a significant [(group) × (block)] interaction for the TD vs. HFA (P =0.001), with children with HFA showing less change in time-on-target across the blocks of trials than the TD children. However, there was no significant [(group) × (block)] interaction for TD vs. ADHD or the ADHD vs. HFA. Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com. RPM, revolution per minute; ADHD, attention-deficit/hyperactiv-ity disorder; HFA, high-functioning autism; TD, typically developing; ANOVA, analysis of variance. [Color figure can be viewed in the online issue which is available at www.interscience.wiley.com]
Similar to the results of Experiment 1, close examination of the plots shown in Figure 2 suggests that the interaction between groups may be driven primarily by the fact that the transition to the square interference block was associated with substantially less decrement in performance for the children with HFA compared with the ADHD and TD children. To test this hypothesis, follow-up repeated measures were used to examine the effect of diagnosis on change between blocks 2 and 3. The analyses indicated that there was a significant [(group) × (block)] interaction effect [F(1, 46) =6.55; P =0.003]; post hoc analyses revealed that the children with HFA showed significantly less decrement in performance compared with both TD children [F(1, 34) =14.74; P =0.0005] and ADHD children [F(1, 25) =4.51; P =0.44]; there was no significant difference in change between blocks 2 and 3 for the TD and ADHD children [F(1, 33) =0.932; P =0.34].
Again, an analysis was run in order to examine the change across only the three circular blocks (blocks 1–3). Repeated measures ANOVA revealed a trend toward significant effects of group [F(2, 46) =2.60; P =0.085] and of block [F(2, 92) =2.69; P =0.07], and no significant [(group) × (block)] interaction [F(2, 92) =0.173; P =0.95], indicating that the groups did not show much change in performance across blocks of circular trials.
Lastly, given that we controlled for inter-individual subject differences in basic motor speed and function by adjusting the speed of the pursuit rotor for each child, an analysis was run to examine group differences in the rotary speed. A one-way ANOVA revealed a significant difference between groups [F(2, 46) =8.474; P =0.0007], with the controls having a significantly faster speed than both the HFA (P =0.0005) and the ADHD (P =0.0047) groups. There were no significant differences between the HFA and ADHD groups (P =0.52). Simple regression examining speed and total performance (total time-on-target summed across all blocks) indicated that speed of the rotary was not related to task performance (P =0.72).
Discussion
To our knowledge, this is the first study to explore motor learning in children with autism compared to both a TD control group and a clinical control group (ADHD group) known to be also associated with motor impairments [Denckla & Rudel, 1978; Klein et al., 2006; Klimkeit et al., 2005; Mostofsky et al., 2001, 2003]. Inconsistent with our first hypothesis, we found that children with HFA showed similar degrees of learning (i.e., similar rates of improvement in time on target) across the circular blocks compared to TD children. This lack of difference suggests that children with autism were able to learn the motor sequence. However, despite this similarity in performance, our findings of significant between-group differences in the effect of the interference (square) block suggest that, compared to TD children, children with HFA demonstrate differences in the pattern (i.e., approach) of learning a novel motor sequence necessary to optimize accurate performance of a task. This was seen when all subjects performed the RP task under the same conditions (Experiment 1) as well as when the task was adjusted to minimize differences in motor execution (Experiment 2). In contrast, children with ADHD did not show impaired learning (demonstrated by a change in performance) on either task compared to TD children.
Our findings using the traditional RP design, with speed held constant across subjects (Experiment 1), revealed that children with autism stayed on-target for significantly less time and showed less change across trials than both the ADHD and the TD groups independently. Therefore, while the findings from Experiment 1 reveal that children with HFA demonstrate less change across the blocks of trials, they left open the question of whether differences in visuomotor learning could be accounted for by problems with motor execution, manifested as lower time-on-target. The fact that children with ADHD show a similar pattern of performance as do the TD children despite having significantly poorer motor execution suggested otherwise. More critically, we were able to directly address the question of the impact of motor execution in Experiment 2. Using an approach adapted from van Gorp et al. [1999], we were successful in minimizing group differences in motor execution, while keeping all other components of the testing the same. In contrast to Experiment 1, analyses of Experiment 2 revealed no significant differences in motor execution (measured as time-on-target) across the three groups. Consistent with Experiment 1, we found that even after minimizing group differences in motor execution, children with HFA showed significantly less change in performance across trials than did the TD group. Once again, change across the blocks of trials in the ADHD group did not significantly differ from the TD group, suggesting that differences in motor learning may be specific to autism.
More detailed analysis of the pattern of findings revealed that the HFA-associated differences in change across trials were primarily driven by an effect of the intervening square block (block 3). For both experiments, the children with ADHD and the TD children demonstrated a marked interference effect with the square block, while the children with HFA showed very little interference. This is despite the fact that children with HFA often show cognitive inflexibility and have associated difficulty with shifting (“transitioning”) from one task to another. The children with HFA did, however, show improvement in performance across the circular blocks of trials comparable to that seen in TD children and children with ADHD. The pattern of findings suggests that while children with HFA were able to improve their RP performance, the manner in which they did so differed from TD children (and children with ADHD). Likewise, other studies have revealed that despite seemingly intact performance by children with autism, their approach to the task either differed or they required greater resources (i.e., worked harder) than their comparison groups [Bowler, 1992; Eisenmajer & Prior, 1991; Happe, 1995].
Evidence from prior studies suggests that children with autism show an over-reliance on declarative, rather than procedural, processes typically used to acquire motor skills [Klinger & Dawson, 2001; Walenski et al., 2008]. Increased reliance on declarative learning during RP would result in less “proceduralization” of the visuomotor (circular) sequence such that there would be less interference when a competing (square) pattern was introduced.
Declarative and procedural memory systems are not entirely independent systems. Instead, they appear to dynamically interact in both a cooperative and competitive manner during motor sequence learning. Declarative mechanisms are favored during the initial learning stages; however, once the sequence is acquired, improvement in terms of speed of performance appears to be related to procedural mechanisms [Brown & Robertson, 2007a, b]. Given our findings, it may be that the pattern of circular motion was less well ingrained in the children with HFA than in TD children, and that the children with HFA had not yet recruited procedural mechanisms.
Alternatively, the findings of decreased interference effect might be explained by alterations in the mechanisms underlying procedural learning. Findings from previous studies suggest that autism may be associated with a preference for reliance on proprioceptive, rather than visual, information to guide acquisition of novel movement patterns [Masterton & Biederman, 1983]. This might explain why the switch to a different visual presentation (from circle to square) disrupted performance to a significantly lesser degree in the group of autism subjects.
The altered pattern of motor sequence learning might help to explain impaired motor skill development in children with autism. In particular, parents often report that their children with ASD are delayed in the acquisition of motor skills that involve learning novel patterns of sequenced movements, such as peddling a tricycle, pumping legs on a swing and a range of fine motor skills (e.g. buttoning, zippering, tying shoelaces). These reports are consistent with a description by Wing [1969, in Smith and Bryson] that, “clumsy children with autism reportedly have particular difficulty with learning organized patterns of movements (e.g. skipping and dancing)” (p 267). Future studies should begin to focus on teasing apart whether these impairments are due to decreased reliance on, or abnormality in, procedural learning. A better understanding could help to shape intervention and treatment for children with ASD.
The findings that children with autism use a different approach to motor sequence learning may also provide insight into neurologic abnormalities associated with autism. Motor sequence learning relies on a broad neural network principally involving connections between frontal and parietal cortices and subcortical regions [Doyon, Penhune, & Ungerleider, 2003; Eslinger & Damasio, 1986]. The frontal regions, including motor and premotor areas, are particularly important for the storage of motor representations of movement sequences [Doyon et al., 2003] and the retrieval of sequenced motor responses necessary to guide proper execution [Doyon et al., 1997], while parietal regions are critical for the storage and retrieval of spatial and temporal representations of this movement [Heilman & Gonzalez Rothi, 2003]. Findings from imaging studies point to additional distinctions in contributions from subcortical regions, including the cerebellum and basal ganglia. Using positron emission tomography, Grafton, Woods, and Mike [1994] found robust learning-dependent cerebellar activation in early RP learning. However, once participants learned the motor sequence and demonstrated optimal task performance, activity within the cerebellum became undetectable, suggesting that the cerebellum is particularly important for the early learning stages and acquisition of a motor sequence program [Doyon, Owen, Petrides, Sziklas, & Evans, 1996; Jueptner, Frith, Brooks, Frackowiak, & Passingham, 1997; Jueptner, Stephan et al., 1997]. This appears to involve neuronal mechanisms of long-term depression (LTD) which contribute to refinement of the motor sequence based on internal models involving error detection through comparison of intended and actual response [Ito, 2005]. In contrast, the basal ganglia, which is critical for movement selection, has been implicated in the acquisition of motor sequences as well as in the encoding and long-term storage of well-learned sequenced movements [Doyon et al., 2003].
There is a good deal of evidence for autism-related abnormalities in regions within these cortical–subcortical circuits, providing a neuroanatomic basis for deficits in procedural/motor skill learning. Neurologic, neuropsychologic, postmortem, neurophysiologic and neuroimaging studies have long since suggested that motor impairments observed in autism may stem from dysfunction in frontal and subcortical structures and circuits [Bailey et al., 1998; Carper & Courchesne, 2005; Casanova, Buxhoeveden, & Brown, 2002; Chugani et al., 1999; Hardan, Kilpatrick, Keshavan, & Minshew, 2003; Hughes, 1996; Mostofsky et al., 2000; Muller, Kleinhans, Kemmotsu, Pierce, & Courchesne, 2003; Rinehart et al., 2006]. Abnormalities in many of these structures are common findings in studies of autism. Abnormalities in frontal and parietal cortices as well as the basal ganglia and cerebellum have been reported in several imaging studies [Abell et al., 1999; Carper & Courchesne, 2000, 2005; Carper, Moses, Tigue, & Courchesne, 2002; Hardan et al., 2003; Kates et al., 1998; McAlonan et al., 2002; Muller et al., 2003; Piven, Arndt, Bailey, & Andreasen, 1996; Sears et al., 1999] and histological studies reveal abnormalities in the minicolumn structure within the frontal cortex [Buxhoeveden et al., 2006; Casanova et al., 2002]. Particularly compelling is the fact that the decreased Purkinje cell count in the cerebellum is the most consistent finding in postmortem studies of autism [Bailey et al., 1998; Bauman & Kemper, 1994; Fatemi et al., 2002; Ritvo et al., 1986; Williams, Hauser, Purpura, DeLong, & Swisher, 1980], which would prompt speculation that cerebellar dysfunction, perhaps related to impairments in LTD within the Purkinje cell, contributes to impaired motor sequence learning. However, findings revealing that children with HFA have intact motor adaptation [Mostofsky et al., 2004] might suggest otherwise.
It is also worth considering whether the observed difficulties with motor sequence learning might not be related to dysfunction within a particular brain region, but rather whether it is a consequence of abnormalities in connections between these regions. There is increasing evidence in the current literature that suggests reduced connectivity between these distant brain regions in individuals with autism [Herbert et al., 2004, 2005]. Anatomic imaging studies reveal an overgrowth of radiate white matter regions immediately underlying the cortex [Herbert et al., 2004] with increased radiate white matter volume in primary motor cortex being a robust predictor of motor impairment in children with autism [Mostofsky, Burgess, & Gidley Larson, 2007]. Investigators have further suggested that a relative undergrowth of more distant connections between cerebral cortical regions and subcortical structures [Happe & Frith, 2006; Herbert et al., 2004, 2005] results in impaired complex information processing [Minshew et al., 1997] and “weak central coherence” [Shah & Frith, 1993]. Given that motor learning relies on a distributed neural network, including cortico-cortical, cortico-striatal and cortico-cerebellar connections, it follows that individuals with autism may demonstrate a deficit in motor learning due to reduced connectivity between distant brain regions. Future research employing imaging techniques, such as diffuse tensor imaging and functional magnetic resonance imaging examinations of functional connectivity, may provide insight into abnormalities in the interconnections between brain regions critical for motor sequence learning.
The current findings that children with HFA utilize a different approach to motor skill learning may also have implications for development of the broader phenotype of social and communicative deficits in autism. After an extensive review of the literature describing motor deficits in autism, Leary and Hill [1996] concluded that the extent of the motor disturbances present in autism “can clearly have a profound effect on a person’s ability to regulate movement in order to effectively communicate, relate, and participate with others” (p 44).
Alternatively (or perhaps in parallel), abnormalities in procedural learning have been shown to be important not only for the acquisition of motor skills, but also for language and other aspects of social communication. There is robust evidence from behavioral, electrophysiology and imaging studies that support a model in which procedural learning is critical in the development of syntax and grammar, in contrast to lexical/semantic domains that appear to be dependent on declarative learning [for reviews, see Ullman, 2001, 2004]. Differences in procedural learning may thereby contribute to problems with syntactic formulation in autism [Walenski et al., 2006] and help to explain why children with autism frequently display overly “scripted” speech in which memorized, rote phrases are sometimes used in conversation instead of self-generated syntax.
Abnormalities in procedural learning might also impact the development of non-verbal communication and socialization. Recent findings from multiple groups of investigators reveal that for children with autism there exist robust correlations between performance of skilled gestures and measures of social/communicative impairment [Dziuk et al., 2007; Freitag et al., 2007]. It may be that procedural learning mechanisms critical for the development of motor skills may also contribute to impaired development of social and communicative gestures in autism. For TD populations, it would appear to be unlikely that social and communicative gestures (i.e. waving, blowing a kiss) are acquired through declarative learning mechanisms involving explicit memorization; rather it is more plausible that the automaticity of these social and communicative gestures is achieved through procedural means involving motor sequence learning.
While the experimental design and use of a clinical control group are strengths of the current study, there are limitations. Given the nature of learning tasks, it was necessary to use two independent groups of children for the two experiments. While this does introduce inter-individual variability as a confound, the groups were phenotypically similar; both groups met the same diagnostic and eligibility criteria and there were no statistically significant differences in gender distribution, age, IQ (as measured by PRI, with the exception of the HFA groups) or ADOS scores across the two groups. Furthermore, this study comprised a single session of learning. Research has suggested that practice and delay are integral to learning a novel motor sequence [Savion-Lemieux & Penhune, 2005]. Thus, the examination of motor learning in children with ASD over days and months is an important area of future study.
It is also important to note that motor learning during RP and other tasks used in studies of autism (e.g. SRTT) are visually guided. Previous research has suggested that children with autism tend to rely on proprioceptive rather than visual feedback to adapt arm movements [Masterton & Biederman, 1983]; and more recently abnormalities in motion perception, processing and coherence [Bertone, Mottron, Jelenic, & Faubert, 2005; Dakin & Frith, 2005; Milne et al., 2002] have been reported in children with ASD. Examining motor learning across other modalities where visual guidance/feedback is not, or is less, necessary (i.e. tasks in which learning depends on somatosensory/proprioceptive feedback) is an important area of future study.
In summary, the present findings suggest that children with HFA show differences in the pattern of visuomotor sequence learning and that these differences persist even after minimizing individual differences in motor execution. Evidence for specificity of this impairment is demonstrated in that children with ADHD did not show differences in the pattern of motor learning compared with the same group of TD children. Detailed analysis revealed that while children with HFA showed similar gains across blocks of trials, they failed to show an expected decline in performance when an interfering pattern was introduced. The pattern suggests that autism may be associated with increased reliance on declarative learning, which could interfere with procedural learning, or, alternatively, might represent a mechanism by which children with autism compensate for underlying deficits in procedural learning. Future studies examining correlations between RP learning and measures of declarative memory would help to resolve this question. Furthermore, given that declarative memories are prone to decay, are less fixed and are highly flexible [Cohen et al., 1997], studies of motor learning and retention over days to weeks would also help to understand the pattern of learning deficits in autism. Understanding autism-associated differences in learning could help to guide treatment intervention and might also provide insight into the biological basis of the motor deficits associated with autism, as well as impairments in socialization and communication that are the hallmarks of autism.
Acknowledgments
This work was supported by grants from the National Alliance for Autism Research/Autism Speaks and from NIH: K02 NS 044850 (S. H. M), RO1NS048527, M01 RR00052 (Johns Hopkins General Clinical Research Center). We also thank Megan Roeder for the help with subject recruitment and testing.
References
- Abell F, Krams M, Ashburner J, Passingham R, Friston K, et al. The neuroanatomy of autism: A voxel-based whole brain analysis of structural scans. Neuroreport. 1999;10:1647–1651. doi: 10.1097/00001756-199906030-00005. [DOI] [PubMed] [Google Scholar]
- Alcock K. The development of oral motor control and language. Down’s Syndrome, Research and Practice. 2006;11:1–8. doi: 10.3104/reports.310. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 4(DSM-IV)e. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Bailey A, Luthert P, Dean A, Harding B, Janota I, et al. A clinicopathological study of autism. Brain. 1998;121:889–905. doi: 10.1093/brain/121.5.889. [DOI] [PubMed] [Google Scholar]
- Bauman ML, Kemper TL. Neuroanatomic observations of the brain in autism. Baltimore, MD: The Johns Hopkins University Press; 1994. [Google Scholar]
- Bertone A, Mottron L, Jelenic P, Faubert J. Enhanced and diminished visuo-spatial information processing in autism depends on stimulus complexity. Brain. 2005;128:2430–2441. doi: 10.1093/brain/awh561. [DOI] [PubMed] [Google Scholar]
- Bishop DV. Motor immaturity and specific speech and language impairment: Evidence for a common genetic basis. American Journal of Medical Genetics. 2002;114:56–63. doi: 10.1002/ajmg.1630. [DOI] [PubMed] [Google Scholar]
- Bowler DM. Theory of mind in Asperger’s syndrome. J Child Psychol Psychiatry. 1992;33(2):877–893. doi: 10.1111/j.1469-7610.1992.tb01962.x. [DOI] [PubMed] [Google Scholar]
- Brown RM, Robertson EM. Inducing motor skill improvements with a declarative task. Nature Neuroscience. 2007a;10:148–149. doi: 10.1038/nn1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RM, Robertson EM. Off-line processing: Reciprocal interactions between declarative and procedural memories. The Journal of Neuroscience. 2007b;27:10468–10475. doi: 10.1523/JNEUROSCI.2799-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxhoeveden DP, Semendeferi K, Buckwalter J, Schenker N, Switzer R, Courchesne E. Reduced minicolumns in the frontal cortex of patients with autism. Neuropathology and Applied Neurobiology. 2006;32:483–491. doi: 10.1111/j.1365-2990.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- Carper RA, Courchesne E. Inverse correlation between frontal lobe and cerebellum sizes in children with autism. Brain. 2000;123:836–844. doi: 10.1093/brain/123.4.836. [DOI] [PubMed] [Google Scholar]
- Carper RA, Courchesne E. Localized enlargement of the frontal cortex in early autism. Biological Psychiatry. 2005;57:126–133. doi: 10.1016/j.biopsych.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Carper RA, Moses P, Tigue ZD, Courchesne E. Cerebral lobes in autism: Early hyperplasia and abnormal age effects. Neuroimage. 2002;16:1038–1051. doi: 10.1006/nimg.2002.1099. [DOI] [PubMed] [Google Scholar]
- Casanova MF, Buxhoeveden DP, Brown C. Clinical and macroscopic correlates of minicolumnar pathology in autism. Journal of Child Neurology. 2002;17:692–695. doi: 10.1177/088307380201700908. [DOI] [PubMed] [Google Scholar]
- Chugani DC, Muzik O, Behen M, Rothermel R, Janisse JJ, et al. Developmental changes in brain serotonin synthesis capacity in autistic and nonautistic children. Annals of Neurology. 1999;45:287–295. doi: 10.1002/1531-8249(199903)45:3<287::aid-ana3>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Cohen N, Poldrack R, Eichenbaum H. Memory of items and memory for relations in the procedural/declarative memory framework. Memory. 1997;5:131–178. doi: 10.1080/741941149. [DOI] [PubMed] [Google Scholar]
- Conners C, Sitarenios G, Parker J, Epstein J. The Revised Conners’ Parent Rating Scale (CPRS-R): Factor structure, reliability and criterion validity. Journal of Abnormal Child Psychology. 1998;26:257–268. doi: 10.1023/a:1022602400621. [DOI] [PubMed] [Google Scholar]
- Dakin S, Frith U. Vagaries of visual perception in autism. Neuron. 2005;48:497–507. doi: 10.1016/j.neuron.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Denckla MB, Rudel R. Anomalies of motor development in hyperactive boys. Annals of Neurology. 1978;3:231–233. doi: 10.1002/ana.410030308. [DOI] [PubMed] [Google Scholar]
- Dewey D. Praxis and sequencing skills in children with sensorimotor dysfunction. Developmental Neuropsychology. 1991;7:197–206. [Google Scholar]
- Doyon J, Gaudreau D, Laforce R, Jr, Castonguay M, Bedard PJ, et al. Role of the striatum, cerebellum, and frontal lobes in the learning of a visuomotor sequence. Brain and Cognition. 1997;34:218–245. doi: 10.1006/brcg.1997.0899. [DOI] [PubMed] [Google Scholar]
- Doyon J, Owen AM, Petrides M, Sziklas V, Evans A. Functional anatomy of visuomotor skill learning in human subjects examined with positron emission tomography. European Journal of Neuroscience. 1996;8:637–648. doi: 10.1111/j.1460-9568.1996.tb01249.x. [DOI] [PubMed] [Google Scholar]
- Doyon J, Penhune V, Ungerleider LG. Distinct contribution of the cortico-striatal and cortico-cerebellar systems to motor skill learning. Neuropsychologia. 2003;41:252–262. doi: 10.1016/s0028-3932(02)00158-6. [DOI] [PubMed] [Google Scholar]
- Dziuk M, Gidley Larson J, Apostu A, Mahone E, Denckla M, Mostofsky S. Dyspraxia in autism: Association with motor, social, and communicative deficits. Developmental Medicine and Child Neurology. 2007;49:734–739. doi: 10.1111/j.1469-8749.2007.00734.x. [DOI] [PubMed] [Google Scholar]
- Eisenmajer R, Prior M. Congnitive linguistic correlates of theory of mind ability in autistic children. British Journal of Developmental Psychology. 1991;9:351–364. [Google Scholar]
- Elliott CD. Differential Ability Scales. San Antonio, TX: Harcourt Assessment; 1990. [Google Scholar]
- Eslinger P, Damasio AR. Preserved motor learning in Alzheimer’s disease: Implications for anatomy and behavior. The Journal of Neuroscience. 1986;6:3006–3009. doi: 10.1523/JNEUROSCI.06-10-03006.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Halt AR, Realmuto G, Earle J, Kist DA, et al. Purkinje cell size is reduced in cerebellum of patients with autism. Cellular and Molecular Neurobiology. 2002;22:171–175. doi: 10.1023/A:1019861721160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitag C, Kleser C, Schneider M, von Gontard A. Quantitative assessment of neuromotor function in adolescents with high functioning autism and Asperger syndrome. Journal of Autism and Developmental Disorders. 2007;37:948–959. doi: 10.1007/s10803-006-0235-6. [DOI] [PubMed] [Google Scholar]
- Frith U, Frith CD. Specific motor disabilities in Down’s syndrome. Journal of Child Psychology and Psychiatry. 1974;15:293–301. doi: 10.1111/j.1469-7610.1974.tb01253.x. [DOI] [PubMed] [Google Scholar]
- Gabrieli JD, Stebbins GT, Singh J, Willingham DB, Goetz CG. Intact mirror-tracing and impaired rotary-pursuit skill learning in patients with Huntington’s disease: Evidence for dissociable memory systems in skill learning. Neuropsychology. 1997;11:272–281. doi: 10.1037//0894-4105.11.2.272. [DOI] [PubMed] [Google Scholar]
- Gidley Larson J, Mostofsky S. Motor deficits in autism. In: Tuchman R, Rapin I, editors. Autism: A neurological disorder of early brain development. London: MacKeith Press; 2006. [Google Scholar]
- Gordon B, Stark S. Procedural learning of a visual sequence in individuals with autism. Focus on Autism and Other Developmental Disabilities. 2007;22:14–22. [Google Scholar]
- Grafton ST, Woods RP, Mike T. Functional imaging of procedural motor learning: Relating cerebral blood flow with individual subject performance. Human Brain Mapping. 1994;1:221–234. doi: 10.1002/hbm.460010307. [DOI] [PubMed] [Google Scholar]
- Green D, Baird G, Barnett AL, Henderson L, Huber J, Henderson SE. The severity and nature of motor impairment in Asperger’s syndrome: A comparison with specific developmental disorder of motor function. Journal of Child Psychology and Psychiatry. 2002;43:655–668. doi: 10.1111/1469-7610.00054. [DOI] [PubMed] [Google Scholar]
- Happe FG. The role of age and verbal ability in the theory of mind task performance of subjects with autism. Child Dev. 1995;66(3):843–855. [PubMed] [Google Scholar]
- Happe F, Frith U. The weak coherence account: Detail-focused cognitive style in autism spectrum disorders. Journal of Autism and Developmental Disorders. 2006;36:5–25. doi: 10.1007/s10803-005-0039-0. [DOI] [PubMed] [Google Scholar]
- Hardan AY, Kilpatrick M, Keshavan MS, Minshew NJ. Motor performance and anatomic magnetic resonance imaging (MRI) of the basal ganglia in autism. Journal of Child Neurology. 2003;18:317–324. doi: 10.1177/08830738030180050801. [DOI] [PubMed] [Google Scholar]
- Heilman KM, Gonzalez Rothi LJ. Apraxia. 4. New York: Oxford University Press; 2003. [Google Scholar]
- Heindel W, Salmon D, Shults C, Walicke P, Butters N. Neuropsychological evidence for multiple implicit memory systems: A comparison of Alzheimer’s, Huntington’s, and Parkinson’s disease patients. The Journal of Neuroscience. 1989;9:582–587. doi: 10.1523/JNEUROSCI.09-02-00582.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert MR, Ziegler DA, Deutsch CK, O’Brien LM, Kennedy DN, et al. Brain asymmetries in autism and developmental language disorder: A nested whole-brain analysis. Brain. 2005;128:213–226. doi: 10.1093/brain/awh330. [DOI] [PubMed] [Google Scholar]
- Herbert MR, Ziegler DA, Makris N, Filipek PA, Kemper TL, et al. Localization of white matter volume increase in autism and developmental language disorder. Annals of Neurology. 2004;55:530–540. doi: 10.1002/ana.20032. [DOI] [PubMed] [Google Scholar]
- Hughes C. Brief report: Planning problems in autism at the level of motor control. Journal of Autism and Developmental Disorders. 1996;26:99–107. doi: 10.1007/BF02276237. [DOI] [PubMed] [Google Scholar]
- Ito M. Bases and implications of learning in the cerebellum—adaptive control and internal model mechanism. Progress in Brain Research. 2005;148:95–109. doi: 10.1016/S0079-6123(04)48009-1. [DOI] [PubMed] [Google Scholar]
- Jacobs DH, Adair JC, Williamson DJ, Na DL, Gold M, et al. Apraxia and motor-skill acquisition in Alzheimer’s disease are dissociable. Neuropsychologia. 1999;37:875–880. doi: 10.1016/s0028-3932(98)00139-0. [DOI] [PubMed] [Google Scholar]
- Jansiewicz EM, Goldberg MC, Newschaffer CJ, Denckla M, Landa RJ, Mostofsky S. Motor signs distinguish children with high functioning autism and Asperger’s syndrome from controls. Journal of Autism and Developmental Disorders. 2006;36:613–621. doi: 10.1007/s10803-006-0109-y. [DOI] [PubMed] [Google Scholar]
- Jueptner M, Frith CD, Brooks DJ, Frackowiak RS, Passingham RE. Anatomy of motor learning. II. Subcortical structures and learning by trial and error. Journal of Neurophysiology. 1997;77:1325–1337. doi: 10.1152/jn.1997.77.3.1325. [DOI] [PubMed] [Google Scholar]
- Jueptner M, Stephan KM, Frith CD, Brooks DJ, Frackowiak RS, Passingham RE. Anatomy of motor learning. I. Frontal cortex and attention to action. Journal of Neurophysiology. 1997;77:1313–1324. doi: 10.1152/jn.1997.77.3.1313. [DOI] [PubMed] [Google Scholar]
- Kanner L. Autistic disturbances of affective contact. Nervous Child. 1943;2:217–250. [PubMed] [Google Scholar]
- Kates WR, Mostofsky S, Zimmerman AW, Mazzocco MM, Landa R, et al. Neuroanatomical and neurocognitive differences in a pair of monozygous twins discordant for strictly defined autism. Annals of Neurology. 1998;43:782–791. doi: 10.1002/ana.410430613. [DOI] [PubMed] [Google Scholar]
- Klein C, Wendling K, Huettner P, Ruder H, Peper M. Intra-subject variability in attention-deficit hyperactivity disorder. Biological Psychiatry. 2006;60:1088–1097. doi: 10.1016/j.biopsych.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Klimkeit E, Mattingley J, Sheppard D, Lee P, Bradshaw JL. Motor preparation, motor execution, attention, and executive functions in attention deficit/hyperactivity disorder (ADHD) Child Neuropsychology. 2005;11:153–173. doi: 10.1080/092970490911298. [DOI] [PubMed] [Google Scholar]
- Klinger L, Dawson G. Prototype formation in autism. Development and Psychopathology. 2001;13:111–124. doi: 10.1017/s0954579401001080. [DOI] [PubMed] [Google Scholar]
- Leary MR, Hill DA. Moving on: Autism and movement disturbance. Mental Retardation. 1996;34:39–53. [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, et al. The autism diagnostic observation schedule—generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30:205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, Goode S, Heemsbergen J, Jordan H, et al. Autism diagnostic observation schedule: A standardized observation of communicative and social behavior. Journal of Autism and Developmental Disorders. 1989;19:185–212. doi: 10.1007/BF02211841. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview—Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Lord R, Hulme C. Patterns of rotary pursuit performance in clumsy and normal children. Journal of Child Psychology and Psychiatry. 1988;29:691–701. doi: 10.1111/j.1469-7610.1988.tb01889.x. [DOI] [PubMed] [Google Scholar]
- Manjiviona J, Prior M. Comparison of Asperger syndrome and high-functioning autistic children on a test of motor impairment. Journal of Autism and Developmental Disorders. 1995;25:23–39. doi: 10.1007/BF02178165. [DOI] [PubMed] [Google Scholar]
- Mari M, Castiello U, Marks D, Marraffa C, Prior M. The reach-to-grasp movement in children with autism spectrum disorder. Philosophical Transactions of the Royal Society B. 2003;358:393–403. doi: 10.1098/rstb.2002.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masterton B, Biederman G. Proprioceptive versus visual control in autistic children. Journal of Autism and Developmental Disorders. 1983;13:141–152. doi: 10.1007/BF01531815. [DOI] [PubMed] [Google Scholar]
- McAlonan GM, Daly E, Kumari V, Critchley HD, van Amelsvoort T, et al. Brain anatomy and sensorimotor gating in Asperger’s syndrome. Brain. 2002;125:1594–1606. doi: 10.1093/brain/awf150. [DOI] [PubMed] [Google Scholar]
- Milne E, Swettenham J, Hansen P, Campbell R, Jeffries H, Plaisted K. High motion coherence thresholds in children with autism. Journal of Child Psychology and Psychiatry. 2002;43:255–263. doi: 10.1111/1469-7610.00018. [DOI] [PubMed] [Google Scholar]
- Minshew NJ, Goldstein G, Siegel DJ. Neuropsychologic functioning in autism: Profile of a complex information processing disorder. Journal of the International Neuropsychological Society. 1997;3:303–316. [PubMed] [Google Scholar]
- Mostofsky S, Burgess M, Gidley Larson J. Increased motor cortex white matter volume predicts motor impairment in autism. Brain. 2007;32:543–562. doi: 10.1093/brain/awm129. [DOI] [PubMed] [Google Scholar]
- Mostofsky S, Dubey P, Jerath VK, Jansiewicz EM, Goldberg MC, Denckla MB. Developmental dyspraxia is not limited to imitation in children with autism spectrum disorders. Journal of the International Neuropsychological Society. 2006;12:314–326. doi: 10.1017/s1355617706060437. [DOI] [PubMed] [Google Scholar]
- Mostofsky S, Goldberg MC, Cutting L, Denckla M. Impaired procedural learning of rotary pursuit in children with autism. Paper presented at the international meeting for autism research; San Diego, CA. 2001. [Google Scholar]
- Mostofsky S, Goldberg MC, Landa RJ, Denckla MB. Evidence for a deficit in procedural learning in children and adolescents with autism: Implications for cerebellar contribution. Journal of International Neuropsychological Society. 2000;6:752–759. doi: 10.1017/s1355617700677020. [DOI] [PubMed] [Google Scholar]
- Mostofsky S, Newschaffer CJ, Denckla M. Overflow movements predict impaired response inhibition in children with ADHD. Perceptual and Motor Skills. 2003;97:1315–1331. doi: 10.2466/pms.2003.97.3f.1315. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Bunoski R, Morton SM, Goldberg MC, Bastian AJ. Children with autism adapt normally during a catching task requiring the cerebellum. Neurocase. 2004;10:60–64. doi: 10.1080/13554790490960503. [DOI] [PubMed] [Google Scholar]
- Mottron L. Matching strategies in cognitive research with individuals with high-functioning autism: Current practices, instrument biases, and recommendations. Journal of Autism and Developmental Disorders. 2004;34:19–27. doi: 10.1023/b:jadd.0000018070.88380.83. [DOI] [PubMed] [Google Scholar]
- Muller RA, Kleinhans N, Kemmotsu N, Pierce K, Courchesne E. Abnormal variability and distribution of functional maps in autism: An FMRI study of visuomotor learning. The American Journal of Psychiatry. 2003;160:1847–1862. doi: 10.1176/appi.ajp.160.10.1847. [DOI] [PubMed] [Google Scholar]
- Noterdaeme M, Mildenberger K, Minow F, Amorosa H. Evaluation of neuromotor deficits in children with autism and children with specific speech and language disorder. European Child & Adolescent Psychiatry. 2002;11:219–225. doi: 10.1007/s00787-002-0285-z. [DOI] [PubMed] [Google Scholar]
- Oldfield R. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Goldring S, Greiss-Hess L, Herrera AM, et al. Gross motor development, movement abnormalities, and early identification of autism. Journal of Autism and Developmental Disorders. 2008;38:644–656. doi: 10.1007/s10803-007-0430-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piven J, Arndt S, Bailey J, Andreasen N. Regional brain enlargement in autism: A magnetic resonance imaging study. Journal of the American Academy of Child and Adolescent Psychiatry. 1996;35:530–536. doi: 10.1097/00004583-199604000-00020. [DOI] [PubMed] [Google Scholar]
- Reich W. Diagnostic interview for children and adolescents (DICA) Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:59–66. doi: 10.1097/00004583-200001000-00017. [DOI] [PubMed] [Google Scholar]
- Rinehart NJ, Tonge BJ, Iansek R, McGinley J, Brereton AV, et al. Gait function in newly diagnosed children with autism: Cerebellar and basal ganglia related motor disorder. Developmental Medicine and Child Neurology. 2006;48:819–824. doi: 10.1017/S0012162206001769. [DOI] [PubMed] [Google Scholar]
- Ritvo ER, Freeman BJ, Scheibel AB, Duong T, Robinson H, et al. Lower Purkinje cell counts in the cerebella of four autistic subjects: Initial findings of the UCLA-NSAC Autopsy Research Report. The American Journal of Psychiatry. 1986;143:862–866. doi: 10.1176/ajp.143.7.862. [DOI] [PubMed] [Google Scholar]
- Robertson E. The serial reaction time task: Implicit motor skill learning? Journal of Neuroscience. 2007;27:10073–10075. doi: 10.1523/JNEUROSCI.2747-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers S, Bennetto L, McEvoy R, Pennington B. Imitation and pantomime in high-functioning adolescents with autism spectrum disorders. Child Development. 1996;67:2060–2073. [PubMed] [Google Scholar]
- Roth R, Baribeau J, Milovan D, O’Conner K, Todorov C. Procedural and declarative memory in obsessive–compulsive disorder. Journal of the International Neuropsy-chological Society. 2004;10:647–654. doi: 10.1017/S1355617704105018. [DOI] [PubMed] [Google Scholar]
- Savion-Lemieux T, Penhune VB. The effects of practice and delay on motor skill learning and retention. Experimental Brain Research. 2005;161:423–431. doi: 10.1007/s00221-004-2085-9. [DOI] [PubMed] [Google Scholar]
- Sears LL, Vest C, Mohamed S, Bailey J, Ranson BJ, Piven J. An MRI study of the basal ganglia in autism. Progress in Neuropsychopharmacology & Biological Psychiatry. 1999;23:613–624. doi: 10.1016/s0278-5846(99)00020-2. [DOI] [PubMed] [Google Scholar]
- Shah A, Frith U. Why do autistic individuals show superior performance on the block design task? Journal of Child Psychology and Psychiatry. 1993;34:1351–1364. doi: 10.1111/j.1469-7610.1993.tb02095.x. [DOI] [PubMed] [Google Scholar]
- Smith IM. Motor problems in children with autistic spectrum disorders. In: Dewey D, Tupper D, editors. Developmental motor disorders: A neuropsychological perspective. New York: Guilford Press; 2004. [Google Scholar]
- Smith IM, Bryson SE. Imitation and action in autism: A critical review. Psychological Bulletin. 1994;116:259–273. doi: 10.1037/0033-2909.116.2.259. [DOI] [PubMed] [Google Scholar]
- Squire LR. Mechanisms of memory. Science. 1986;232:1612–1619. doi: 10.1126/science.3086978. [DOI] [PubMed] [Google Scholar]
- Teitelbaum O, Benton T, Shah PK, Prince A, Kelly JL, Teitelbaum P. Eshkol–Wachman movement notation in diagnosis: The early detection of Asperger’s syndrome. Proceedings of the National Academy of Sciences. 2004;101:11909–11914. doi: 10.1073/pnas.0403919101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelbaum P, Teitelbaum O, Nye J, Fryman J, Maurer RG. Movement analysis in infancy may be useful for early diagnosis of autism. Proceedings of the National Academy of Sciences. 1998;95:13982–13987. doi: 10.1073/pnas.95.23.13982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullman MT. The declarative/procedural model of lexicon and grammar. Journal of Psycholinguistic Research. 2001;30:37–69. doi: 10.1023/a:1005204207369. [DOI] [PubMed] [Google Scholar]
- Ullman MT. Contributions of memory circuits to language: The declarative/procedural model. Cognition. 2004;92:231–270. doi: 10.1016/j.cognition.2003.10.008. [DOI] [PubMed] [Google Scholar]
- van Gorp WG, Altshuler L, Theberge DC, Mintz J. Declarative and procedural memory in bipolar disorder. Biological Psychiatry. 1999;46:525–531. doi: 10.1016/s0006-3223(98)00336-9. [DOI] [PubMed] [Google Scholar]
- Walenski M, Mostofsky S, Gidley Larson J, Ullman MT. Brief report: enhanced picture naming in autism. J Autism Dev Disord. 2008;38:1395–1399. doi: 10.1007/s10803-007-0513-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walenski M, Tager-Flusberg H, Ullman MT. Language in autism. In: Moldin S, Rubenstein J, editors. Understanding autism: From basic neuroscience to treatment. Boca Raton, FL: Taylor & Francis Books; 2006. pp. 175–203. [Google Scholar]
- Wechsler D. Manual for the Wechsler Intelligence Scale for Children—3rd Edition. 3. San Antonio, TX: The Psychological Corporation; 1991. [Google Scholar]
- Wechsler D. Manual for the Wechsler Intelligence Scale for Children—4th Edition. San Antonio, TX: The Psychological Corporation; 2003. [Google Scholar]
- Williams RS, Hauser SL, Purpura DP, DeLong GR, Swisher CN. Autism and mental retardation: Neuropathologic studies performed in four retarded persons with autistic behavior. Archives of Neurology. 1980;37:749–753. doi: 10.1001/archneur.1980.00500610029003. [DOI] [PubMed] [Google Scholar]
- Willingham DB, Salidas J, Gabrieli JD. Direct comparison of neural systems mediating conscious and unconscious skill learning. Journal of Neurophysiology. 2002;88:1451–1460. doi: 10.1152/jn.2002.88.3.1451. [DOI] [PubMed] [Google Scholar]
- Wing L. The handicaps of autistic children—a comparative study. J Child Psychol Psychiatry. 1969;10:1–40. doi: 10.1111/j.1469-7610.1969.tb02066.x. [DOI] [PubMed] [Google Scholar]