Abstract
Objective and methods:
Given the profound weight loss after gastric banding and bypass we compared fat compartmentalization by whole body magnetic resonance imaging in women and men after these procedures to two groups of non-surgical controls who were either matched for age, weight and height or were of lower body mass index (BMI).
Result:
In women post-surgery (n=17; BMI 31.7 kg/m2) there was lower visceral adipose tissue (VAT) (1.4 vs 2.5 kg; P<0.01) compared with matched controls (n=59; BMI 32.1 kg/m2). In contrast, VAT (5.3 vs 5.4 kg) was nearly identical in men post-surgery (n=10; BMI 34.1 kg/m2) compared with matched controls (n=10; BMI 32.1 kg/m2) even though the degree of weight reduction was not significantly different from women (27.4 vs 32.6%). Furthermore, VAT when adjusted for total adipose tissue (TAT) was 43% less in women post-surgery (1.2 vs 2.1 kg; P=0.03) than in controls with lower BMI (25.1 kg/m2). After adjustment for TAT, subcutaneous adipose tissue in women post-surgery was significantly greater than matched controls (35.1 vs 34.2 kg; P=0.03). There was a significant negative correlation of VAT and the degree of weight loss in women (r=−0.57; P=0.018) but this relationship was not significant in men (r=−0.39; P=0.27). Skeletal muscle was lower in both sexes compared with matched controls (women, 21.8 vs 23.1 kg; men, 32.5 vs 35.5 kg).
Conclusion:
Prospective studies are necessary to confirm if there is a sexual dimorphism in the effects of bariatric surgery on body composition.
Keywords: adjustable gastric banding, Roux-en-Y gastric bypass, body composition, magnetic resonance imaging, leptin, obesity
Introduction
Accumulation of visceral adipose tissue (VAT) is associated with increased insulin resistance and cardiovascular risk [1-3]. Conversely, reduction of VAT by diet is associated with improved glucose tolerance [4]. Weight loss induced by bariatric surgery is associated with even more profound metabolic improvements including remission of type 2 diabetes [5]. Given the strong associations between visceral adiposity and cardiometabolic disease risk [6] it is important to identify how bariatric procedures may affect the various fat depots and to determine if there are differences between procedures and different outcomes in men and women.
Several studies showed that after diet or drug-induced weight loss there was preferential mobilization of VAT in both men and women [7-11], yet relatively few studies have measured body composition after bariatric surgery using in-vivo measurement techniques such as magnetic resonance imaging (MRI) and computed tomography (CT), that allow for the quantifications of adipose tissue sub-depots, specifically subcutaneous adipose tissue (SAT) and VAT [12]. In one study of six women there was a preferential reduction of VAT at 8 weeks after laparoscopic adjustable gastric banding (BND) while patients were still undergoing rapid weight loss [13], however, a preferential decrease in VAT compared with abdominal SAT was not detected in another study of ten women at 12 weeks post-BND [14]. A larger study showed a greater reduction in VAT compared with SAT at one year following BND, unfortunately, results from men and women were combined so any possible sex differences could not be ascertained [15]. Using single slice measurements at the fourth lumbar vertebra level obtained by CT, Olbers et al showed that after one year there was a greater reduction in VAT in women following Roux-en-Y gastric bypass (GBP) compared with vertical banded gastroplasty, however in men, reduction in VAT was not significantly different between these procedures [16]. From these few studies, it appears that there may be sex differences in the degree of change in body composition with surgically induced weight loss and that these changes may also be dependent upon the type of surgical procedure. None of these studies examined non-surgical control groups for comparison of body composition, and to our knowledge, quantification of specific fat depots after BND or GBP with whole body MRI in a weight stable population has not been reported. Therefore, the main objective of this present study was to perform whole body MRI in weight stable women and men who had previously undergone substantial weight loss after BND or GBP and compare body composition to two non-surgical control groups from our historical database: one group was matched for age, weight and height and the other consisted of non-obese controls with significantly lower body mass index (BMI).
Methods
Study subjects
Two groups who had previously undergone either BND or GBP, and were weight stable, defined by a change in body weight < 5% over the prior 3 months were studied for analysis of body composition by MRI: women (n=17) and men (n=10). Body composition was compared to control groups from a historical database (D.G.) consisting of healthy women (n=59) and men (n=10) without a history of weight loss who had previously undergone cross-sectional or longitudinal non-interventional studies and whose mean age, weight and height were matched to the surgery groups. Surgery groups were also compared to lower BMI (≤ 27 kg/m2) female (n=7) and male (n=9) controls from the database. Adults with a body width greater than 48 cm (which is the upper limit of the MRI field of view for body composition analysis), diabetes, use of medications that may affect body weight or composition, claustrophobia, or cosmetic surgery (other than facial) were excluded from the study.
Body composition
Total-body skeletal muscle (SM) and total-body adipose tissue mass, including total (TAT), SAT, VAT, and intermuscular (IMAT), were measured from multi-slice MRI images across the whole body on a 1.5 T scanner (6X Horizon; General Electric, Milwaukee) as previously described [17]. The protocol involved the acquisition of approximately 40 axial images of 10-mm thickness at 40-mm intervals across the whole body. SliceOmatic image analysis software (version 4.2; Tomovision, Montreal, Canada) was used to analyze images. MRI volume estimates were converted to mass by using assumed density of 1.04kg/L for skeletal muscle and 0.92kg/L for AT [18]. The technical errors for four repeated readings by the same observer of MRI-derived SAT, VAT, and IMAT volumes are 1.7%, 2.3% and 5.9%, respectively. Due to the upper limit of the 48 cm field of view and/or the physical limitations of positioning, such as inability to fully extend arms overhead, data are incomplete for some parameters in certain male subjects in the surgery group. The number of men on whom data were available for the specified variables are as follows: TAT, n=8; SAT, n=8; IMAT, n=9; SM, n=9; VAT, n=10.
Hormone analysis
Plasma leptin measurements were performed in duplicate on blood samples collected in EDTA tubes with a human RIA kit (LINCO Research, Inc., St. Charles, MO) using a 125I-iodinated human leptin tracer.
Statistical methods
SAS v9.1 software (SAS Institute, Cary, NC) was used for data analysis. Separate unadjusted analysis of variance models were used to assess group differences between the surgery group and the BMI-matched non-surgical control group, and between the surgery group and the non-surgical lean control group. Separate models were constructed for women and men given the known sex differences in VAT volume [19] and leptin levels [20], and the potential sex differences in body composition after surgery. Analysis of covariance was used to estimate group differences while controlling for the confounding influence as follows: TAT and SM were adjusted for total body weight, age and height; VAT, SAT and IMAT were adjusted for TAT, age and height. Linear regression analysis was used to determine the relationships between VAT and body weight, BMI, TAT or percent weight loss. A P-value of ≤ 0.05 was considered statistically significant. Mean values ± SEM are reported.
Results
Comparison of women post-surgery to matched non-surgical controls
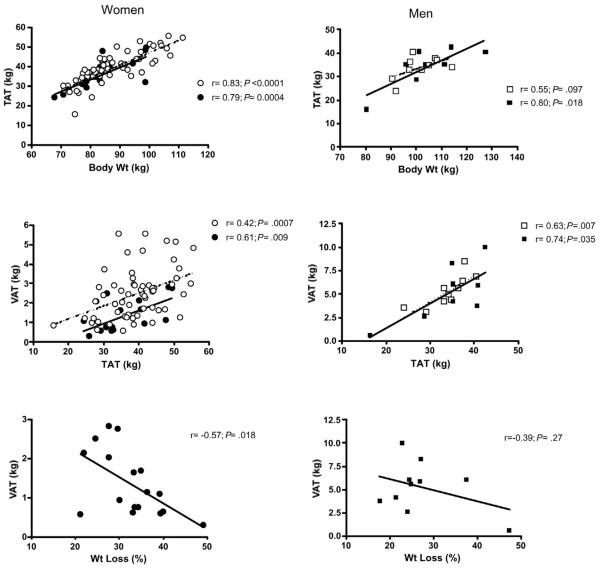
Surgery and control groups of the same sex were matched for mean age, weight and height (Table 1). In women post-surgery, VAT was 44% less than in control subjects, and the difference remained significant after adjustment for TAT and menopausal status. Surgery in women was also associated with significantly less SM compared with controls both before and after adjustment for age, height and total body weight. Although unadjusted values for SAT were not different, when adjusted for TAT, SAT was greater in the surgery group (35.1 kg) compared with matched controls (34.2 kg; P=0.03). TAT and IMAT were similar between control and surgery subjects. VAT correlated with TAT in both groups and with percent weight loss after surgery (Fig. 1). TAT, but not VAT, correlated with total body weight in both groups (Fig. 1).
Table 1.
Characteristics of women post-surgery with matched and non-obese controls.
| Characteristic | Non-obese controls (n=7) |
Matched controls (n=59) |
Surgery 7 BND/ 10 GBP (n=17) |
|---|---|---|---|
| Age (yr) | 48.9 ± 4.4 | 47.4 ± 1.6 | 48.5 ± 2.2 |
| Wt (kg) | 74.0 ± 1.3** | 86.9 ± 1.4 | 83.3 ± 2.3 |
| BMI (kg/m2) | 25.1 ± 0.5*** | 32.1 ± 0.5 | 31.7 ± 1.1 |
| Wt loss (%) | na | na | 32.6 ± 1.7 [21 – 49] |
| Post-op period (mo) |
na | na | 25.1 ± 2.0 |
| TAT (kg) | 26.8 ± 2.1** | 39.0 ± 1.1 | 35.8 ± 1.8 |
| VAT (kg) | 1.6 ± 0.4† | 2.5 ± 0.2**†† | 1.4 ± 0.2 |
| SAT (kg) | 24.2 ± 1.9** | 34.9 ± 1.0† | 32.9 ± 1.7 |
| IMAT (kg) | 0.9 ± 0.2 | 1.6 ± 0.1 | 1.5 ± 0.3 |
| SM (kg) | 23.1 ± 2.0 | 23.3 ± 0.5*† | 21.1 ± 0.7 |
Numbers represent unadjusted mean values±SEM. Numbers in brackets represent range.
P≤0.05
P<0.01
P<0.001 compared with surgery group.
P≤0.05
P<0.01 compared with surgery group after values were adjusted as follows: TAT and SM were adjusted for total body weight, age and height; VAT, SAT and IMAT were adjusted for TAT, age and height. na = not applicable.
Figure 1.
Relationships between TAT and body weight, and between VAT and TAT and percent weight loss in women (left panel) and men (right panel). Open circles and squares represent non-surgical controls matched for age, weight, and height to the post-surgery subjects represented by closed circles and squares. Solid lines represent linear regression from post-surgery subjects; dotted lines represent linear regression from non-surgical matched controls. r indicates Pearson correlation coefficients.
Comparison of women post-surgery to non-obese non-surgical controls
Body composition after surgery was also compared with a database of non-obese (BMI ≤ 27 kg/m2) controls (Table 1). When adjusted for TAT, the amount of VAT in the post-surgery group was 43% less (1.2 kg) compared with VAT in women with lower BMI (2.1 kg; P=0.03). The difference remained significant when adjusted for menopausal status. TAT and SAT were non-significantly different between groups after adjustments for body weight and TAT, respectively.
Comparison of men post-surgery to BMI-matched non-surgical controls
In contrast to women, VAT in men post-surgery was similar to non-surgical controls (Table 2). SM was significantly lower post-surgery (32.5 kg) compared to matched controls (35.5 kg; P=0.0099) after adjustments for age, height and total body weight. IMAT was significantly greater in the surgery group, however, after adjustment for TAT this difference was no longer significant. VAT correlated with TAT, but not with total body weight, in both groups (Fig. 1). VAT did not correlate with percent weight loss.
Table 2.
Characteristics of men post-surgery with matched and non-obese controls.
| Characteristic | Non-obese controls (n=9) |
Matched controls (n=10) |
Surgery 4 BND/ 6 GBP (n=10) |
|---|---|---|---|
| Age (yr) | 52.9 ± 3.4 | 54.3 ± 3.5 | 55.0 ± 3.9 |
| Wt (kg) | 86.8 ± 1.6** | 100.3 ± 2.8 | 103.4 ± 3.9 |
| BMI (kg/m2) | 26.0 ± 0.3*** | 32.1 ± 1.0 | 34.1 ± 1.3 |
| Wt loss (%) | na | na | 27.4 ± 2.7 [18 – 47] |
| Post-op period (mo) |
na | na | 19.2 ± 1.1 |
| TAT (kg) | 21.5 ± 1.3*** | 33.9 ± 1.6 | 34.3 ± 3.0 |
| VAT (kg) | 2.6 ± 0.3** | 5.4 ± 0.5 | 5.3 ± 0.9 |
| SAT (kg) | 18.2 ± 1.0*** | 27.0 ± 1.3 | 28.9 ± 2.2 |
| IMAT (kg) | 0.7 ± 0.1*** | 1.5 ± 0.1* | 2.5 ± 0.5 |
| SM (kg) | 34.6 ± 1.2† | 35.2 ± 1.4†† | 32.8 ± 2.0 |
Numbers represent unadjusted mean values±SEM. Numbers in brackets represent range.
P≤0.05
P<0.01
P<0.001 compared with surgery group.
P≤0.05
P<0.01 compared with surgery group after SM was adjusted for total body weight, age and height. na = not applicable.
Comparison of men post-surgery to non-obese non-surgical controls
TAT, VAT, SAT and IMAT were all significantly lower in the non-obese controls compared with the post-surgery group (Table 2), however, when adjustments were made as described in Table 1, none of these differences remained statistically significant. SM was significantly lower post-surgery (31.8 kg) compared to non-obese controls (35.7 kg; P=0.022) after adjustments for age, height and total body weight.
Comparison of BND vs GBP in women
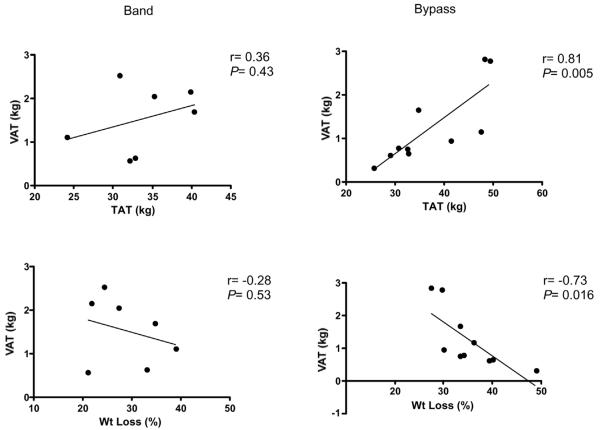
Age, menopause status, weight, BMI and post-operative period were not significantly different in BND and GBP groups (Table 3). Weight loss in women tended to be greater in GBP compared with BND (P=0.065). Comparison of body composition in women post-BND and GBP did not reveal significant differences, however, after adjustment for percent weight loss, TAT (31.1 vs 39.1 kg; P=0.042) and SAT (28.8 vs 35.8 kg; P=0.045) were significantly lower in the BND patients (Table 3). Following GBP there were strong correlations between VAT and TAT and percent weight loss, whereas these relationships were not significant in the BND group (Fig. 2).
Table 3.
Comparison of band and bypass surgery in women.
| Patient characteristic and measurement |
BND (n=7) | GBP (n=10) |
|---|---|---|
| Age (yr) | 44.6 ± 3.0 | 51.2 ± 2.8 |
| Weight (kg) | 82.5 ± 3.5 | 83.9 ± 3.2 |
| BMI | 31.9 ± 2.2 | 31.5 ± 1.2 |
| Post-op period (mo) | 24.7 ± 3.1 | 25.3 ± 2.8 |
| Weight loss (%) | 28.8 ± 2.6 | 35.2 ± 2.0 |
| TAT (kg) | 33.7 ± 2.1† | 37.2 ± 2.6 |
| VAT (kg) | 1.53 ± 0.29 | 1.24 ± 0.28 |
| SAT (kg) | 31.0 ± 2.1† | 34.2 ± 2.4 |
| IMAT (kg) | 1.09 ± 0.21 | 1.77 ± 0.40 |
| SM (kg) | 20.8 ± 1.0 | 21.3 ± 0.9 |
| Leptin (ng/ml) | 18.6 ± 3.6 | 14.1 ± 2.4 |
| Leptin/ kg TAT (ng/ml/kg) | 0.54 ± 0.10 | 0.37 ± 0.05 |
Numbers represent unadjusted mean values±SEM. There were no significant differences between groups in unadjusted values.
P≤0.05 compared with GBP group after values were adjusted for percent weight loss.
Figure 2.
Relationships between VAT with TAT and percent weight loss in women post-BND (left panel) and post-GBP (right panel). Solid lines represent linear regression. r indicates Pearson correlation coefficients.
We have previously reported that absolute leptin levels tended to be greater in women post-BND vs. GBP (P=0.079) who were matched for BMI, but for whom body composition analysis was not performed [21]. We now show that leptin levels per kg TAT were not significantly different between BND and GBP groups (P=0.109; Table 3), and that values were nearly identical after adjustment for weight loss (0.47 vs 0.42 ng/ml/kg, respectively; P=0.6). Comparison of the different surgical procedures in men was not performed due to the fewer number of subjects.
Discussion
To date, most investigations using CT or MRI to evaluate body composition after bariatric surgery have involved acquisition of single slice cross-sectional abdominal images, however, the accuracy of the estimation of total VAT from single slice measurements is largely dependent upon the level studied [12, 22, 23], and the association between VAT and the presence of cardiometablic risk also depends on the site of measurement [24]. In this report we used whole body MRI to demonstrate that in women, surgery was associated with significantly lower VAT compared with matched controls. In contrast, men post-surgery had nearly an identical amount of VAT compared with controls. The shorter duration after surgery at the time of study in men (19 vs 25 months, P=0.046) is unlikely to contribute to the difference in VAT, since both sexes were studied at a minimum of three months of weight stability. In addition, multiple regression analysis confirmed that the difference in VAT between sexes was not dependent upon percent weight loss (P=0.54). When adjusted for body weight, age and height, SM was lower in both sexes after surgery compared with their respective matched controls. Less SM may be one of the causes of lower resting energy expenditure shown in women after BND and GBP [25, 26].
While generalized adiposity is clearly associated with insulin resistance and the accompanying metabolic complications, data on the association with adipose tissue accumulation in particular anatomical compartments are somewhat discrepant [27, 28]. As reviewed by Garg, most studies have demonstrated a strong association between VAT and insulin resistance [27]. However, it is unclear whether greater VAT is a marker of insulin resistance or plays a causal role [29, 30]. In one study, reduction in VAT was associated with improvement in oral glucose tolerance even after adjusting for overall weight loss [31]. In another study, insulin sensitivity correlated with generalized and regional adiposity, however, the magnitude of improvement in insulin sensitivity was predicted by the percent decrease in VAT, but not by other changes in body composition [32]. Decreasing SAT by large volume liposuction does not appear to significantly alter insulin sensitivity in obese women [33], however, some studies have shown an association between SAT or particular subdivisions of SAT and insulin resistance [27, 34, 35].
Weight loss after bariatric surgery is usually associated with an improvement in insulin resistance [5, 36]. Busetto et al reported that in six pre-menopausal non-diabetic women at baseline there was no significant correlation between insulin levels and fat distribution measured by MRI, whereas after a 16% reduction in total body weight and 36% reduction in VAT at 24 wks post-BND there was a significant correlation between the percentage change of insulin levels from baseline with changes in both VAT and abdominal SAT [13]. We previously demonstrated that women who had lost weight after BND or GBP had significantly lower fasting insulin levels and HOMAIR compared with BMI-matched controls who did not undergo weight reduction [21, 37]. Collectively, these data suggest that the lower amount of VAT in women post-bariatric surgery may be a major contributor to reduced insulin resistance. In non-diabetic men who have not undergone weight loss (n=9; BMI 36 kg/m2) we observed that HOMA-IR was markedly greater compared to the post-surgery group in this study (5.1 vs 2.1 μU·mol−1·l−3; JK unpublished observations) despite similar BMI values. Although indirect, these results suggest that in men the benefits of weight loss are still manifested with regard to insulin resistance even though the amount of VAT and SAT are equivalent to matched controls. In fact, Smith et al have shown that in men, but not in women, deep SAT explained a greater proportion of the variance in fasting insulin than superficial SAT or VAT [35]. Other factors such as fat cell size, cortisol metabolism, levels of intraheptatic fat, adiponectin and/or inflammatory markers [38] likely play important roles in metabolic improvement.
Comparisons of gastric banding with Roux-en-Y gastric bypass have shown that the latter procedure is more effective in producing improvements in insulin resistance and even remission of type 2 diabetes [36]. Whether this difference is due to differences in the degree of weight loss, body fat distribution and/or certain hormones is unclear [39]. In this small study we were unable to detect a significant difference in VAT mass between these procedures in women, however, SAT was significantly greater in the GBP group when values were adjusted for weight loss. It is unlikely that sex hormones contributed to this difference, since both groups had a similar distribution of menopausal women. Kelley et. al. have shown that SAT in the abdomen may be divided into superficial and deep compartments and it is only the latter compartment that has a significant negative relation to insulin resistance [34]. Unfortunately, we did not delineate between these compartments, neither did we subdivide VAT into intra- and retroperitoneal compartments which also may be functionally distinct [27]. Our data also suggest that determinants of VAT mass may be different in BND compared with GBP since significant correlations between VAT and TAT and percent weight loss were detected only in the latter. However, given the small sample size we cannot rule out entirely that more subtle interactions exist between VAT and TAT and percent weight loss after BND.
Another important difference in outcome after gastric banding compared with gastric bypass is that the latter procedure usually results in greater weight loss [36]. Since leptin is an adipocyte-derived hormone that mediates several aspects of energy homeostasis [40], we compared leptin levels in women after BND and GBP. Similar, to our previous data [21], absolute values of leptin were greater in BND compared with GBP, but the difference was not statistically difference. We now have been able to demonstrate that when adjusted for TAT and percent weight loss, leptin values are nearly identical between both procedures. It, therefore, seems unlikely that the differences in weight loss are due to the effect of these procedures on leptin concentrations.
A limitation of this study is the lack of information on pre-operative body composition and metabolic data such as glucose and insulin values. Therefore, we cannot comment on the relative change in different anatomic compartments, changes in insulin resistance and comparisons of metabolic parameters with our control populations. Nor are we able to determine if there were differences in physical activity between groups since this variable was not measured. Prospective studies are warranted to determine the contribution of the change in different anatomical compartments to the change in insulin sensitivity, however, most current MRI scanners have limited field of view and maximum body weight restrictions that prohibit complete visualization of all tissues in morbidly obese patients. Clearly there are sex differences in body composition that deserve further investigation and additional subjects should be studied to address whether the different bariatric procedures result in subtle differences in body composition and insulin sensitivity that could not be detected in this small study. Our data are consistent with current clinical recommendations advising patients after surgery to engage in regular physical activity in order to maximize muscle mass and metabolic rate, thus aiding weight reduction and weight loss maintenance.
Acknowledgments
This work was supported by National Institutes of Health grant R01-DK072011 (J.K.) and P30 DK26687. Special thanks to Stephen Dashnaw for performance of MRI in post-surgical subjects and Irene M. Conwell for measurement of leptin levels.
References
- 1.Klein S, Allison DB, Heymsfield SB, et al. Waist circumference and cardiometabolic risk: a consensus statement from shaping America's health: Association for Weight Management and Obesity Prevention; NAASO, the Obesity Society; the American Society for Nutrition; and the American Diabetes Association. Diabetes Care. 2007;30:1647–52. doi: 10.2337/dc07-9921. [DOI] [PubMed] [Google Scholar]
- 2.Bergman RN, Kim SP, Hsu IR, et al. Abdominal obesity: role in the pathophysiology of metabolic disease and cardiovascular risk. Am J Med. 2007;120:S3–8. doi: 10.1016/j.amjmed.2006.11.012. discussion S29-32. [DOI] [PubMed] [Google Scholar]
- 3.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–28. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 4.Fujioka S, Matsuzawa Y, Tokunaga K, et al. Improvement of glucose and lipid metabolism associated with selective reduction of intra-abdominal visceral fat in premenopausal women with visceral fat obesity. Int J Obes. 1991;15:853–9. [PubMed] [Google Scholar]
- 5.Sjostrom L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683–93. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 6.Pouliot MC, Despres JP, Lemieux S, et al. Waist circumference and abdominal sagittal diameter: best simple anthropometric indexes of abdominal visceral adipose tissue accumulation and related cardiovascular risk in men and women. Am J Cardiol. 1994;73:460–8. doi: 10.1016/0002-9149(94)90676-9. [DOI] [PubMed] [Google Scholar]
- 7.Zamboni M, Armellini F, Turcato E, et al. Effect of weight loss on regional body fat distribution in premenopausal women. Am J Clin Nutr. 1993;58:29–34. doi: 10.1093/ajcn/58.1.29. [DOI] [PubMed] [Google Scholar]
- 8.Ross R, Rissanen J. Mobilization of visceral and subcutaneous adipose tissue in response to energy restriction and exercise. Am J Clin Nutr. 1994;60:695–703. doi: 10.1093/ajcn/60.5.695. [DOI] [PubMed] [Google Scholar]
- 9.Kamel EG, McNeill G, Van Wijk MC. Change in intra-abdominal adipose tissue volume during weight loss in obese men and women: correlation between magnetic resonance imaging and anthropometric measurements. Int J Obes Relat Metab Disord. 2000;24:607–13. doi: 10.1038/sj.ijo.0801204. [DOI] [PubMed] [Google Scholar]
- 10.Pare A, Dumont M, Lemieux I, et al. Is the relationship between adipose tissue and waist girth altered by weight loss in obese men? Obes Res. 2001;9:526–34. doi: 10.1038/oby.2001.69. [DOI] [PubMed] [Google Scholar]
- 11.Doucet E, St-Pierre S, Almeras N, et al. Reduction of visceral adipose tissue during weight loss. European journal of clinical nutrition. 2002;56:297–304. doi: 10.1038/sj.ejcn.1601334. [DOI] [PubMed] [Google Scholar]
- 12.Shen W, Wang Z, Punyanita M, et al. Adipose tissue quantification by imaging methods: a proposed classification. Obes Res. 2003;11:5–16. doi: 10.1038/oby.2003.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Busetto L, Tregnaghi A, Bussolotto M, et al. Visceral fat loss evaluated by total body magnetic resonance imaging in obese women operated with laparascopic adjustable silicone gastric banding. Int J Obes Relat Metab Disord. 2000;24:60–9. doi: 10.1038/sj.ijo.0801086. [DOI] [PubMed] [Google Scholar]
- 14.Phillips ML, Lewis MC, Chew V, et al. The early effects of weight loss surgery on regional adiposity. Obes Surg. 2005;15:1449–55. doi: 10.1381/096089205774859353. [DOI] [PubMed] [Google Scholar]
- 15.Pontiroli AE, Pizzocri P, Librenti MC, et al. Laparoscopic adjustable gastric banding for the treatment of morbid (grade 3) obesity and its metabolic complications: a three-year study. J Clin Endocrinol Metab. 2002;87:3555–61. doi: 10.1210/jcem.87.8.8708. [DOI] [PubMed] [Google Scholar]
- 16.Olbers T, Bjorkman S, Lindroos A, et al. Body composition, dietary intake, and energy expenditure after laparoscopic Roux-en-Y gastric bypass and laparoscopic vertical banded gastroplasty: a randomized clinical trial. Ann Surg. 2006;244:715–22. doi: 10.1097/01.sla.0000218085.25902.f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gallagher D, Kuznia P, Heshka S, et al. Adipose tissue in muscle: a novel depot similar in size to visceral adipose tissue. Am J Clin Nutr. 2005;81:903–10. doi: 10.1093/ajcn/81.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Snyder WS, Cook MJ, Nasset ES, et al. Report of the task group on reference men. Pergamon Press; Oxford, United Kingdom: 1975. International Commission on Radiological Protection No. 23. [Google Scholar]
- 19.Lemieux S, Prud'homme D, Bouchard C, et al. Sex differences in the relation of visceral adipose tissue accumulation to total body fatness. Am J Clin Nutr. 1993;58:463–7. doi: 10.1093/ajcn/58.4.463. [DOI] [PubMed] [Google Scholar]
- 20.Rosenbaum M, Nicolson M, Hirsch J, et al. Effects of gender, body composition, and menopause on plasma concentrations of leptin. J Clin Endocrinol Metab. 1996;81:3424–7. doi: 10.1210/jcem.81.9.8784109. [DOI] [PubMed] [Google Scholar]
- 21.Korner J, Inabnet W, Conwell IM, et al. Differential effects of gastric bypass and banding on circulating gut hormone and leptin levels. Obesity. 2006;14:1553–61. doi: 10.1038/oby.2006.179. [DOI] [PubMed] [Google Scholar]
- 22.Shen W, Punyanitya M, Wang Z, et al. Visceral adipose tissue: relations between single-slice areas and total volume. Am J Clin Nutr. 2004;80:271–8. doi: 10.1093/ajcn/80.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen W, Punyanitya M, Wang Z, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol. 2004;97:2333–8. doi: 10.1152/japplphysiol.00744.2004. [DOI] [PubMed] [Google Scholar]
- 24.Kuk JL, Church TS, Blair SN, et al. Does measurement site for visceral and abdominal subcutaneous adipose tissue alter associations with the metabolic syndrome? Diabetes Care. 2006;29:679–84. doi: 10.2337/diacare.29.03.06.dc05-1500. [DOI] [PubMed] [Google Scholar]
- 25.Galtier F, Farret A, Verdier R, et al. Resting energy expenditure and fuel metabolism following laparoscopic adjustable gastric banding in severely obese women: relationships with excess weight lost. Int J Obesity. 2006;30:1104–10. doi: 10.1038/sj.ijo.0803247. [DOI] [PubMed] [Google Scholar]
- 26.Bobbioni-Harsch E, Morel P, Huber O, et al. Energy economy hampers body weight loss after gastric bypass. J Clin Endocrinol Metab. 2000;85:4695–700. doi: 10.1210/jcem.85.12.7083. [DOI] [PubMed] [Google Scholar]
- 27.Garg A. Regional adiposity and insulin resistance. J Clin Endocrinol Metab. 2004;89:4206–10. doi: 10.1210/jc.2004-0631. [DOI] [PubMed] [Google Scholar]
- 28.Reaven G. All obese individuals are not created equal: insulin resistance is the major determinant of cardiovascular disease in overweight/obese individuals. Diab Vasc Dis Res. 2005;2:105–12. doi: 10.3132/dvdr.2005.017. [DOI] [PubMed] [Google Scholar]
- 29.Lebovitz HE, Banerji MA. Point: visceral adiposity is causally related to insulin resistance. Diabetes Care. 2005;28:2322–5. doi: 10.2337/diacare.28.9.2322. [DOI] [PubMed] [Google Scholar]
- 30.Miles JM, Jensen MD. Counterpoint: visceral adiposity is not causally related to insulin resistance. Diabetes Care. 2005;28:2326–8. doi: 10.2337/diacare.28.9.2326. [DOI] [PubMed] [Google Scholar]
- 31.Fujioka S, Matsuzawa Y, Tokunaga K, et al. Contribution of intra-abdominal fat accumulation to the impairment of glucose and lipid metabolism in human obesity. Metabolism. 1987;36:54–9. doi: 10.1016/0026-0495(87)90063-1. [DOI] [PubMed] [Google Scholar]
- 32.Goodpaster BH, Kelley DE, Wing RR, et al. Effects of weight loss on regional fat distribution and insulin sensitivity in obesity. Diabetes. 1999;48:839–47. doi: 10.2337/diabetes.48.4.839. [DOI] [PubMed] [Google Scholar]
- 33.Klein S, Fontana L, Young VL, et al. Absence of an effect of liposuction on insulin action and risk factors for coronary heart disease. N Engl J Med. 2004;350:2549–57. doi: 10.1056/NEJMoa033179. [DOI] [PubMed] [Google Scholar]
- 34.Kelley DE, Thaete FL, Troost F, et al. Subdivisions of subcutaneous abdominal adipose tissue and insulin resistance. Am J Physiol Endocrinol Metab. 2000;278:E941–8. doi: 10.1152/ajpendo.2000.278.5.E941. [DOI] [PubMed] [Google Scholar]
- 35.Smith SR, Lovejoy JC, Greenway F, et al. Contributions of total body fat, abdominal subcutaneous adipose tissue compartments, and visceral adipose tissue to the metabolic complications of obesity. Metabolism. 2001;50:425–35. doi: 10.1053/meta.2001.21693. [DOI] [PubMed] [Google Scholar]
- 36.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–37. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 37.Korner J, Bessler M, Cirilo LJ, et al. Effects of Roux-en-Y gastric bypass surgery on fasting and postprandial concentrations of plasma ghrelin, peptide YY, and insulin. J Clin Endocrinol Metab. 2005;90:359–65. doi: 10.1210/jc.2004-1076. [DOI] [PubMed] [Google Scholar]
- 38.Bloomgarden ZT. Fat metabolism and diabetes: 2003 American Diabetes Association postgraduate course. Diabetes Care. 2003;26:2198–203. doi: 10.2337/diacare.26.7.2198. [DOI] [PubMed] [Google Scholar]
- 39.Cummings DE, Overduin J, Foster-Schubert KE, et al. Role of the bypassed proximal intestine in the anti-diabetic effects of bariatric surgery. Surg Obes Relat Dis. 2007;3:109–15. doi: 10.1016/j.soard.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leibel RL. The role of leptin in the control of body weight. Nutr Rev. 2002;60:S15–9. doi: 10.1301/002966402320634788. [DOI] [PubMed] [Google Scholar]