Abstract
Neuroimaging studies of apolipoprotein E (APOEε4) have implicated its association with brain atrophy in Alzheimer’s disease. To date, few studies have used automated morphological analysis techniques to assess APOEε4-related brain structure change in both gray and white matter in nondemented older adults. Nondemented (CDR = 0, n = 53) subjects over 60 had MRI, diffusion tensor imaging, and neurocognitive assessments. We assessed differences in cognition and brain structure associated with APOEε4 genetic variation using voxel-based morphometry techniques, and tract-based spatial statistics of fractional anisotropy change. In nondemented older adults with the ε4 allele, cognitive performance was reduced, and atrophy was present in the hippocampus and amygdala compared to APOEε4 negative participants. We also report that ε4 carriers have decreased fractional anisotropy in the left parahippocampal gyrus white matter. In conclusion, the presence of an APOEε4 allele in nondemented older adults is associated with decreases in cognition and gray and white matter changes in the medial temporal cortex. Overall we provide further evidence of the effects of genetic variance related to imaging and cognitive measures of risk for Alzheimer’s disease.
Keywords: Aging, Alzheimer’s disease, apolipoprotein (APOE), cognition, dementia, diffusion tensor imaging (DTI), fractional anisotropy (FA), genetics, hippocampus, voxel-based morphometry (VBM)
INTRODUCTION
While autosomal dominant mutations exist in Alzheimer’s disease (AD), they are exceedingly rare, with the majority of AD cases likely involving a combination of genetic and environmental risk factors [1]. New neuroimaging methods labeled “imaging genetics” [2] are being used to clarify the genetics of AD pathology, risk, and variability in an individual’s response to treatment [3–7]. Imaging genetics advantageously provides a more direct measurement of the influence of the gene at the level of neuroanatomy, with automated image analysis techniques providing an unbiased approach towards characterizing genetic effects on brain structure and function [2].
Individuals with the apolipoprotein ε4 (APOEε4) genotype have an increased risk for AD [8]. It is unclear whether individuals without clinical evidence of dementia who harbor an APOEε4 risk allele express the neuroimaging phenotype of AD. Region of interest (ROI) studies in individuals with AD have demonstrated ε4 allele-related decreases in temporal [9], hippocampal [10,11], amygdala [12,13], and whole brain [14] gray matter volume. Similar studies in healthy subjects have had mixed results. Some [7, 15–19], but not all [20–22], structural imaging studies of cognitively intact ε4-carrying adults have shown reduced brain volume, with the majority reporting trends for decreased hippocampal volume in the ε4 carriers. There has only been one voxel-based morphometry (VBM) study focused on healthy elderly, reporting decreased hippocampal gray matter density in ε4 homozygotes (but not heterozygotes) relative to noncarriers [23]. A broader VBM study of subjects ranging from 19–80 years of age found decreased density, not volume, in AΡOEε4 carriers in the medial temporal and bilateral fronto-temporal regions [24].
In addition to VBM, diffusion tensor imaging (DTI) has recently been used to examine genetically-mediated pathologic processes involved in the breakdown of brain white matter, possibly marking risk for AD. A recent ROI and voxel-based study investigating the impact of the APOEε4 allele on white matter integrity in healthy subjects suggested APOEε4-related integrity changes in the corpus callosum, occipitofronto fasciculus, and left hippocampus [25]. APOEε4-related decreases in diffusion have also been reported in the parahippocampal gyrus [26]. Finally, tract-based spatial statistics (TBSS) have been applied to analyze ε4-related white matter variation in elderly women. Smith and colleagues found reduced fractional anisotropy (FA) in ε4-carriers in the fronto-occipital, inferior temporal, and cingulum bundle fiber tracts, along with the splenium of the corpus callosum [27]. Overall, medial temporal and posterior fiber tract variability may be associated with APOEε4 genetic variation.
Studies of cognitive differences in nondemented APOEε4 carriers who are otherwise healthy are also mixed. Some studies report mild cognitive deficits in elderly individuals with the ε4 genotype [28–31], and others show no APOEε4-specific cognitive decline [15, 32,33]. Specifically, ε4-carriers may have increased age-related memory decline [31], decreases in verbal memory [28], and global cognitive deficits [34]. A review of these and other studies argued that the neurocognitive deficit associated with the APOEε4 allele in otherwise healthy elderly individuals is most likely related to risk for AD [35]. However, studies of “healthy” samples are often limited by the use of clinical techniques insensitive to the earliest stages of AD, for instance, the Mini-Mental State Examination (MMSE) or IQ testing [15–18]. Thus, those studies demonstrating hippocampal or cognitive differences [15–19] may have had individuals with mild cognitive impairment (MCI) overrepresented within their APOEε4 groups [19].
These mixed imaging and cognitive results may be the consequence of heterogeneous clinicalmethods, insensitive screening techniques, or various ROI based volumetric methods. The purpose of this study was to clarify the literature by using several new voxel-wise analysis methods to analyze both regional gray matter volume (VBM), and white matter integrity (TBSS) in a single group of subjects carefully screened to exclude early AD with intensive clinical methods. In addition we aggregated common cognitive measures to characterize gene-related cognitive variation in several domains. We also tested for a role of physical and lifestyle measures in APOEε4-related brain structure change.
METHODS
Demographics
Nondemented subjects aged 60 and over were enrolled in the University of Kansas Brain Aging Project. Participants were recruited from a referral-based memory clinic and by media appeals. A participant was considered to have a family history if either their mother or father had a diagnosis of AD. The presence or absence of dementia, and its severity if present, was determined using the Clinical Dementia Rating (CDR) scale [36]. These methods have a diagnostic accuracy for AD of 93% [37] and have been shown to be accurate in identifying the subset of individuals meeting criteria for MCI who have early stage AD [38]. The CDR assesses cognitive function in multiple domains. A global CDR score is derived from individual ratings in each domain such that CDR 0 indicates no dementia, CDR 0.5 indicates very mild, CDR 1 indicates mild, CDR 2 indicates moderate, and CDR 3 indicates severe dementia. All participants included in this analysis had a CDR rating of 0. All participants provided informed consent according to institutional guidelines. Study exclusions included neurological disease other than AD, history of ischemic heart disease, history of significant mental illness, diabetes mellitus, or other systemic illness that might impair completion of the study [39]. APOE genotyping results were obtained using restriction enzyme isotyping and were available for 53 of the healthy elderly subjects who also had brain imaging data. Subjects were divided based on if they were a carrier of the APOEε4 allele (ε4/ε4, n = 2, ε4/E3, n = 12, total ε4 carrier n = 14) or if they were a noncarrier (n = 39).
Physical and lifestyle measures
Four common assessments were selected to index physical function, daily activity, and lifestyle. The Physical Performance Test is a short battery of timed physical tasks that serves as a composite measure of physical frailty and function [40]. Peak oxygen consumption was measured during an exercise test as previously described [39] to measure cardiores-piratory fitness [41]. The participant’s level of habitual physical activity was estimated using the Physical Activity Scale in the Elderly (PASE), as described previously [42,43]. Functional independence was estimated using the Mild Cognitive Impairment Activities of Daily Living Scale (MCI-ADL).
Cognitive measures
A trained psychometrician administered a psychometric battery including standard measures of memory (Wechsler Memory Scale [WMS] – Revised Logical Memory I and II [44], Free and Cued Selective Reminding Task [45]), language (Boston Naming Test–15 item [46]), working memory (WMS III Digit Span Forwards and Backwards [44], Wechsler Adult Intelligence Scale [WAIS] letter – number sequencing [47]), executive function (Trailmaking A and B [48], Verbal Fluency [49], and Stroop Color-Word Test [50]), and visuospatial ability (WAIS Block Design [47]). The MMSE [51] was administered as a measure of global cognition. All cognitive performance scores were converted to Z scores based on the mean and SD of a larger cohort of nondemented individuals described previously [52]. The mean of each participant’s Z scores was determined to create an index of global cognitive performance.
Voxel-Based Morphometry (VBM)
Structural MRI data were obtained using a Siemens 3.0 Tesla Allegra MRI Scanner. High-resolution T1 weighted anatomical images were acquired (magnetization-prepared rapid gradient echo [MPRAGE]; 1 × 1 × 1 mm3 voxels, repetition time [TR] = 2,500, echo time [TE] = 4.38ms, inversion time [TI] = 1,100 ms, field of view 256×256 with 18% oversample, flip angle = 8 degrees) and processed for voxel-based analysis. Every scan was checked for image artifacts and gross anatomical abnormalities. Eleven subjects were excluded for movement artifact or inhomogeneity that distorted brain matter. Data analysis for 53 subjects was performed using the VBM5 toolbox (http://dbm.neuro.uni-jena.de), an extension of the SPM5 algorithms (Wellcome Department of Cognitive Neurology, London, UK) running under MATLAB 7.1 (The MathWorks, Natick, MA, USA) on Linux. Processing for VBM has been detailed elsewhere [53]; in short we used unified segmentation with the hidden Markov Random Field model (HMRF, 3 × 3 × 3), writing tissue probability maps without priors, nonlinear modulation, saved with affine registration only, and smoothed at a 10 mm full width at half maximum (FWHM) Gaussian kernel. Additionally, all images were visually checked after registration and segmentation for misalignment, artifact, or malfunction of segmentation. Total gray matter, white matter, and total intracranial volume were computed in cm3 using the normalized tissue maps of each study participant.
Diffusion tensor imaging
Single-shot echo-planar imaging sequence was used to obtain diffusion images with the following parameters: repetition time [TR] = 6300 ms; echo time [TE] = 84 ms. Diffusion gradients were applied in 12 non-collinear directions with 2 b-values (b = 0 and b = 1000 s/mm2). Thirty four 3 mm sections were acquired at an in-plane resolution of 128 × 128. Voxelwise statistical analysis of the FA data was carried out using TBSS [54], part of the Oxford Cemter for Functional MRI of the Brain (FMRIB) software library (FSL) [55]. First, diffusion weighted data were visually checked for motion, artifact, or poor scan quality. The data underwent an eddy current correction to minimize distortions by affine registering the baseline image with no diffusion weighting to the twelve diffusion-weighted gradient images. 53 FA images were created by fitting a tensor model to the raw diffusion data using the nonlinear registration tool (FNIRT), which uses a b-spline representation of the registration warp field [56]. A brain mask was then created with the Brain Extraction Tool for each subject on their baseline image [57]. A diffusion tensor model was fit at each voxel, aiding in the calculation of FA on a voxel-wise basis. The FA maps were registered to the high resolution FMRIB58_FA standard template. All the processing and pre-processing steps were performed on the LONI Pipeline (http://pipeline.loni.ucla.edu/).
The derived mean FA image from the actual subjects was thinned to create a mean FA skeleton representing the centre of the white matter tracts derived from the entire group. A FA threshold of 0.2 was used to keep the skeleton bound to the white matter structures and also to reduce cross-subject variability at the extremes. Each subject’s registered FA data is then projected onto the mean FA skeleton to get individual FA skeletons. Individual FA skeletons were visually inspected for orientation and artifact after automatic processing by aligning them to the group mean. These resulting individual FA skeletons were then compared on a voxel-wise basis for various statistical measures.
Statistical analyses, demographics
SPSS 16.0 was used for all statistical analysis outside of imaging space. Continuous demographic and imaging variables were compared in ε4 carrier and non-carrier groups using ANOVA. Chi-square was used to compare categorical variables between groups.
VBM statistics
To analyze brain images in SPM5, we used a full-factorial model (a 2-sample t-test) with independence between genotype groups, unequal variance, no grand mean scaling, and centered covariates on the overall mean. We used absolute threshold masking set at 0.10 to further restrict the analysis to gray matter tissue.
First, gray matter maps of APOEε4 carrier and non-carrier subjects were compared to identify areas of ε4-related brain volume difference using a 2-sample t-test with age and gender as confounding variables. We then examined the relationship of APOEε4 with medial temporal lobe volume using a small volume correction (SVC) derived from the Wake Forest University Pickatlas (http://www.fmri.wfubmc.edu) [58], as described in previous VBM studies investigating genetic variation specific to medial temporal regions [59]. The medial temporal SVC was pre-selected an early marker of disease in AD [53,60], though the literature has mixed findings of reduced medial temporal volume in cognitively intact APOEε4 carriers [16,24,61]. To correct for multiple comparisons in SVC analyses, as well as in the global analysis, all results were considered significant at p < 0.05 (family-wise error corrected, FWE). Voxels are reported with reference to the Montreal Neurological Institute standard space within SPM5 after conversion to the standard space of Talaraich and Tournoux using custom software [62].
DTI statistics
The FA skeletons were analyzed for cross-subject voxel-wise statistics using non-parametric permutation tests (Randomise [a TBSS statistical tool]; http://www.fmrib.ox.ac.uk/fsl/randomise/index.html, 5,000 iterations). The group’s mean FA skeleton was used as a mask (threshold at a mean FA value of 0.20). We report between-group differences at p < 0.05, using the threshold-free cluster-enhancement option [63]. We followed suggested methods [63] and used a nonparametric 2-sample independent t-test to compare FA between genotype groups. Age and gender were entered into this analysis as confound regressors.
RESULTS
Demographics statistical analysis
The mean age of the cohort (n = 53) was 73.4 years (SD = 6.3), with no significant difference in age between ε4 carriers (n = 14) and ε4 noncarriers (n = 39). More specifically, there were no differences in age between genotype groups (ε4/4 (n = 2); age 67 and 77: ε3/4 (n = 12); mean age = 72.8, sd = 6.3: ε3/3 (n = 39); mean age = 73.7, sd = 7.1: ANOVA, p = 0.435). Groups were similar for gender distribution and years of education. There were no significant differences in normalized whole brain volume gray and white matter volume. Subjects with an APOEε4 allele had significantly decreased global cognition (global index) compared to subjects with no ε4 allele, characterized by global cognition (p = 0.047, Table 1). Decreased global cognitive performance was primarily due to ε4-carriers performing significantly worse on two tests, Logical Memory I ε4-noncarriers, mean = 13.74, sd = 3.73; ε4-carriers, mean = 10.79, sd = 5.56), Letter Number Sequencing (ε4-noncarriers, mean = 10.26, sd = 1.618; ε4-carriers, mean = 9.14, sd = 1.834), with a difference in Delayed Logical Memory that almost reached statistical significance ε4-noncarriers, mean = 11.77, sd = 4.4; ε4-carriers, mean = 9.07, sd = 5.5, p = 0.056). There were no significant differences between ε4 carrier groups on other cognitive tests or tests of function (MCI-ADL), physical frailty (Physical Performance Test), aerobic capacity , or habitual physical activity level (PASE) as summarized in Table 1.
Table 1.
Demographics analyzed between ε4 non-Carrier and ε4 Carrier
| ε4 non Carrier (n = 39) | ε4 Carrier (n = 14) | Significance | |
|---|---|---|---|
| Age | 73.7 (6.2) | 72.7 (6.2) | 0.588 |
| Gender, % female (n) | 39% (15) | 57% (8) | 0.185 |
| Education (years) | 16.5 (2.2) | 16.2 (2.0) | 0.576 |
| Family History, % yes (n) | 33 % (13) | 57 % (8) | 0.107 |
| Whole Brain volume (mm3/L) | 0.77 (0.02) | 0.77 (0.02) | 0.815 |
| Whole Gray volume (mm3/L) | 0.43 (0.02) | 0.43 (0.02) | 0.917 |
| Whole White Matter volume (mm3/L) | 0.35 (0.02) | 0.35 (0.02) | 0.669 |
| Standardized Cognitive Score | 0.08 (0.42) | −0.20 (0.53) | *0.047 |
| PASE | 128.85 (46.37) | 148.71 (57.95) | 0.204 |
| ADL | 49.0 (3.2) | 47.6 (3.7) | 0.175 |
| VO2 lean | 37.2 (6.67) | 39.2 (7.29) | 0.336 |
| Physical Performance Test | 30.59 (3.5) | 30.07 (2.5) | 0.619 |
All data represent means (SD), unless otherwise noted.
Significant at p < 0.05.
VBM results
First, we used VBM to identify areas of APOEε4-related differences in brain volume by comparing maps in nondemented elderly with and without an ε4 allele. ε4-carriers had a trend for lower gray matter regional volumes in the left lingual/parahippocampal gyrus, right inferior parietal cortex, left superior frontal cortex, left angular gyrus, left amygdala, and right pre-cuneus (p < 0.001, uncorrected, Table 2, Fig. 1). The SVC analysis of the medial temporal lobe revealed a significant cluster of reduced gray matter volume in the left anterior hippocampus and amygdala in APOEε4 carriers versus noncarriers (p < 0.05 FWE corrected, Table 2, Fig. 2).
Table 2.
Voxel-Based morphometry analysis results showing gray matter regional decreases as well as increases in Carriers compared to noncarriers
| X, Y, Z | k | Z score | P value Uncorrected (FWE Corrected) |
|
|---|---|---|---|---|
| Gray matter decreases in ε4 carriers | ||||
| L Lingual/Parahippocampal Gyrus, Precuneus | −24, −73, 1 | 4170 | 3.52 | < 0.001 |
| R Inferior Parietal Cortex | 46, −56, 42 | 1268 | 3.25 | 0.001 |
| L Superior Frontal Cortex | −22, −7, 58 | 1551 | 3.14 | 0.001 |
| L Angular/Supramarginal Gyrus | −42, −53, 22 | 2594 | 3.14 | 0.001 |
| L Amygdala | −20, −7, −12 | 6437 | 3.11 | 0.001 |
| R Precuneus | 23, −51, 49 | 766 | 3.01 | 0.001 |
| SVC-Hippocampus/Amygdala | −20, −7, −12 | 2564 | 3.11 (3.56) | 0.001 (0.049) |
| Gray matter increases in ε4 carriers | ||||
| R Medial Frontal Gyrus | 14, 51, 11 | 3196 | 3.82 | < 0.001 |
| L Middle Temporal Gyrus | −36, −67, 12 | 4147 | 3.50 | < 0.001 |
| L Middle Occipital Gyrus | −29, −83, −14 | 767 | 3.16 | 0.001 |
| R Inferior Frontal Gyrus | 49, 28, −14 | 1903 | 3.14 | 0.001 |
| R Precuneus | 15, −60, 39 | 3545 | 3.13 | 0.001 |
| R Hippocampus | 17, −24, −11 | 4128 | 3.12 | 0.001 |
| SVC-Hippocampus/Amygdala | 17, −24, −11 | 939 | 3.12 | 0.001 (0.333) |
SVC = small volume correction, ROI taken from Wakeforest PickAtlas, uncorr = uncorrected p value, L = Left, R = Right, BA = Brodmann’s Area, AD = Alzheimer’s disease, *regions in bold reached uncorrected significance, and corrected level given in parenthesis (family-wise error, FWE).
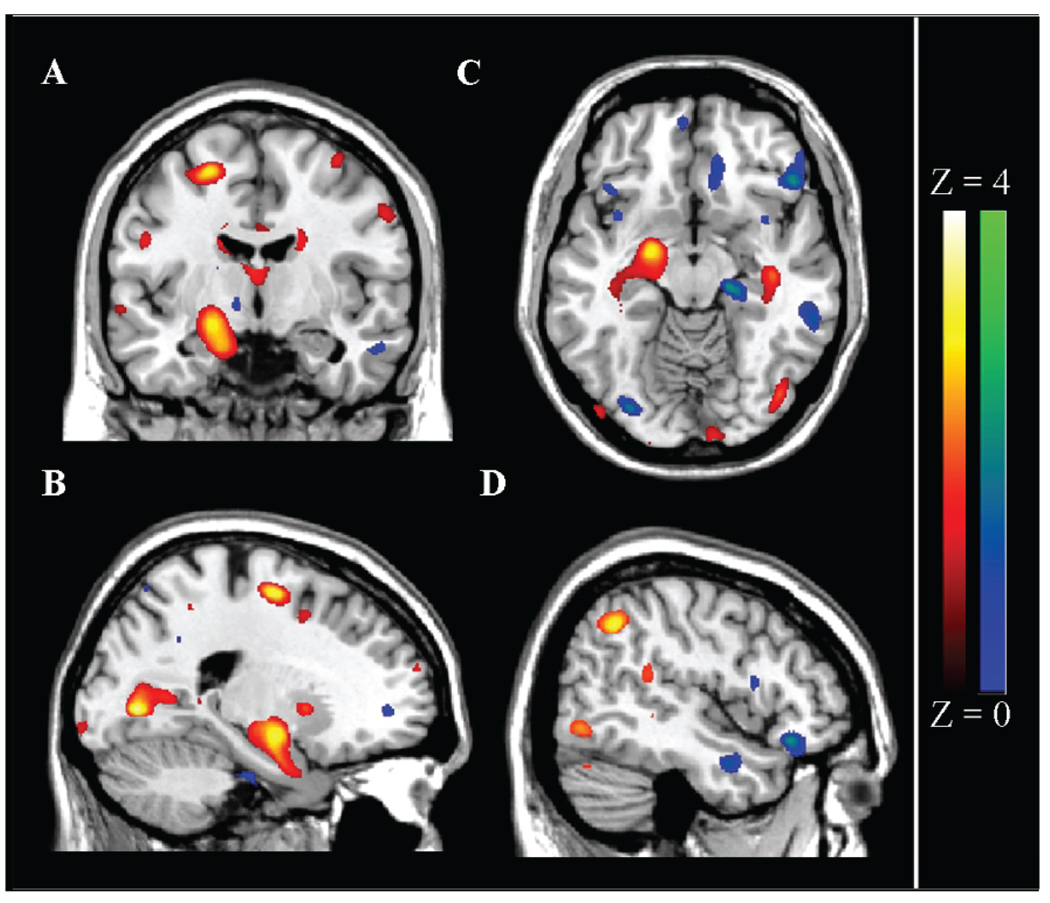
Fig. 1.
Voxel-Based Morphometry analysis of APOE carrier status affect on gray matter regional changes. Figure shows VBM results from both conditions (Noncarrier > Carrier in yellow, Carrier > Noncarrier in pink/red) overlaid on 152 MNI mean image (MRIcron). Color bar range based on Z scores, scores for individual regions listed in Table 2. Quadrant A, B, and C shows ε4 carrier decreases (red to bright yellow) in gray matter volume in left hippocampus, and decreased Precuneus volume is also visible in C. Quadrant D shows ε4 carrier increases (blue to bright green) in middle temporal and inferior frontal gyri.
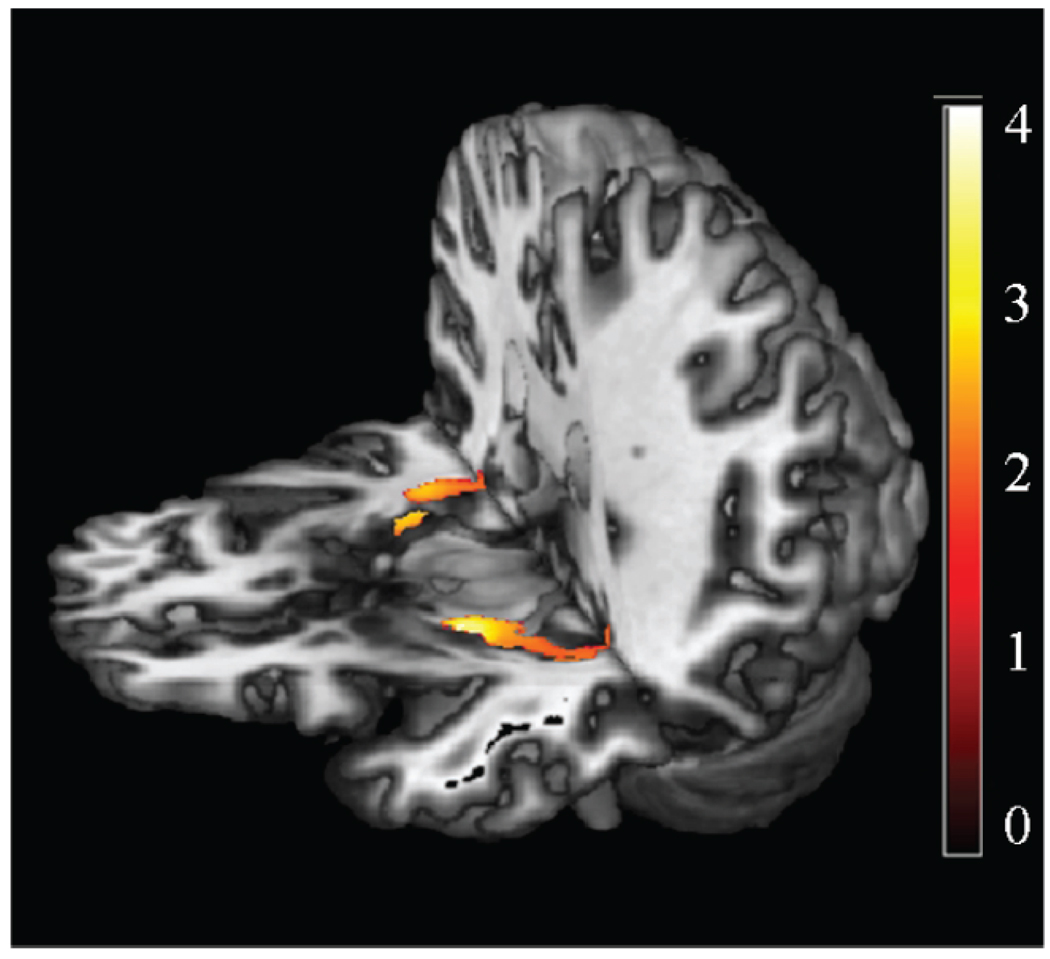
Fig. 2.
Voxel-Based Morphometry analysis of APOE carrier status effect on medial temporal cortex. VBM results overlaid on 3D mean. The color bar range is based on Z scores. Yellow regions represent significant medial temporal decreased gray matter volume in nondemented APOEε4 carriers compared to APOEε4 noncarriers, from small volume-corrected analysis (p < 0.05, corrected, significant for left hippocampus/amygdala).
We also found a trend for increased gray matter volume APOEε4 carriers compared to those noncarriers in the right medial frontal gyrus, the left middle temporal gyrus, the left middle occipital gyrus, right inferior frontal cortex, right precuneus, and right hippocampus (p < 0.001 uncorrected, Table 2, Fig. 1). SVC analyses confined to the medial temporal lobes demonstrated no significant gray matter volume increase or decrease.
DTI results
There were no regions where FA was significantly higher or lower in healthy elderly ε4 carriers versus noncarriers at p < 0.05 corrected level of significance. However a large cluster of decreased FA in ε4 carriers in the left parahippocampal gyrus white matter (−27, −24, −23, x,y,z) trended toward significance (p < 0.001 uncorrected; Fig. 3).
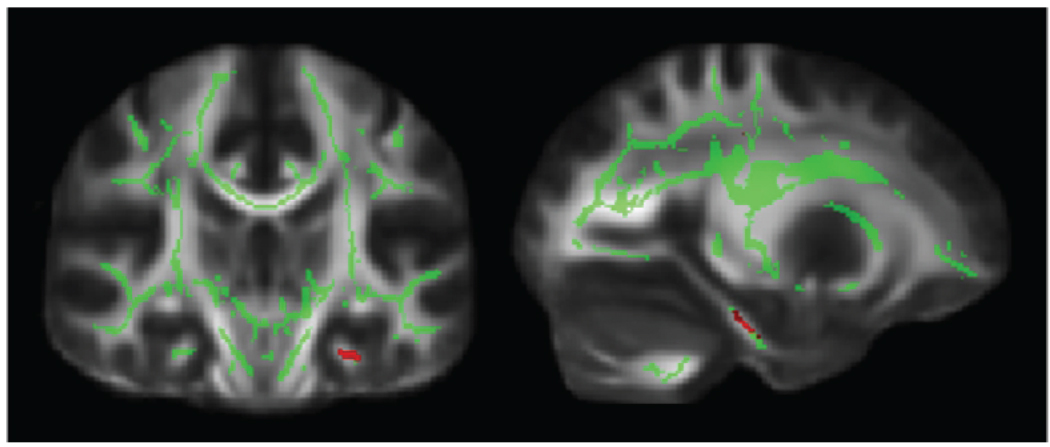
Fig. 3.
DTI analysis of APOE carrier status effect on FA. Spatial maps of the results of between group voxel-wise statistics. Differences in WM tract integrity are displayed in red for ε4-Carriers, who had less FA than ε4-noncarriers in the left parahippocampal gyrus. Images (coronal and sagittal view) are t-statistics displayed at the threshold p < 0.001, uncorrected. In green, the “mean FA skeleton” isn’t in document of all participants is shown, only FA values projected on the skeleton were compared. These images are overlaid on the MNI152 standard diffusion brain. The left hemisphere of the brain corresponds to the right side of the image.
APOE interaction with lifestyle measures: Effect on gray matter volume
To examine the relationship of several environmental factors on moderating the relationship of APOEε4-related brain structure variation, we next extracted the most significant result for each contrast; the hippocampal/amygdalar volume decrease in ε4 carriers and the trend for right medial frontal volume increase in ε4 carriers using the volume of interest (eigenvector) tool in SPM5. Data were then analyzed outside of imaging space using SPSS 16.0 to test for an influence of physical activity or lifestyle on APOEε4 related decreased or increased gray matter volume. Partial correlation analysis, controlling for age and gender, did not reveal a significant contribution of physical function or lifestyle (MCI-ADL, PASE, Physical Performance Test, ) to extracted gray matter volume measures in either the ε4 noncarriers or the carriers.
DISCUSSION
We applied novel voxel-wise VBM and TBSS analyses to assess the relationship of APOEε4 genetic variation on brain structure in healthy elderly subjects. We found decreased medial temporal gray matter volume and global cognitive performance in carriers of the ε4 allele. APOE is the only established genetic risk factor for late-onset AD [8], and carriers of the ε4 allele, despite being nondemented, express small measurable endophenotypes of brain structure and function that are similar to individuals with AD.
We found that those cognitively intact, nondemented subjects with an ε4 allele had significantly decreased global cognitive performance compared to APOEε4 negative participants. Interestingly, this decrease was primarily due to ε4-carriers performing significantly worse on Logical Memory I and Letter Number Sequencing and a trend for decreased performance in Delayed Logical Memory. Poorer performance on Logical Memory I has been reported to be an indicator of risk for AD in healthy subjects [64] and both Logical Memory and Letter Number Sequencing have been noted as markers of early dysfunction in AD [65,66]. While presence of an ε4 allele along with these deficits may mark risk for AD, decline in memory performance and hippocampal volume are also commonly observed in normal aging [67].
Some have argued that morphological changes in ε4 carriers, such as decreased hippocampal volume, represent brain changes associated with preclinical AD [7]. Modest APOEε4-related brain volume difference was observed in regions known to be affected in AD, namely the medial temporal cortex, including the hippocampus and amygdala. Decreased hippocampal volume is an early feature of AD and most likely occurs several years before the onset of symptoms [68], perhaps even in childhood for those carrying an APOEε4 allele [69]. AD subjects with an APOEε4 allele have lower hippocampal [10,11] and amygdala [12,13] gray matter volumes. Past VBM studies reported reduced entorinal and subiculum cortical thickness in nondemented ε4 carriers [70] and decreased hippocampal gray matter density in ε4 homozygotes (but not heterozygotes) relative to noncarriers [23].
This study demonstrates ε4-related decreased hippocampal and amygdala volume in nondemented elderly men and women who have been carefully screened for evidence of clinical change using sensitive diagnostic methods (CDR). ε4-carriers also had a trend for lower GM regional volumes in the left parahippocampal gyrus, right inferior parietal cortex, left superior frontal cortex, left angular gyrus, and right precuneus. Decreased gray matter volume in the parahippocampal gyrus, posterior temporal (supramarginal gyrus and angular gyrus), inferior parietal cortex have been reported in early AD subjects compared to healthy controls [53, 71–74]. However, our current, cross-sectional findings cannot be interpreted to be either a direct manifestation of the disease process or predisposition to later development. Longitudinal follow-up will determine how these morphological differences represent gray matter changes that may lead to a disease state.
We identified a trend for regional volume increases in ε4 carriers compared to non-carriers, primarily in the frontal cortex. A number of studies have noted increased frontal lobe volume [10,14,75] as well as increased executive function in APOEε4 carriers with AD [76], although the same has not been reported in nondemented subjects. Regionally increased brain volume in nondemented ε4 carriers may represent preservation of brain health, or alternate protective genetic or environmental mechanisms at work. Indeed, our sample of elderly ε4-carriers are nondemented despite the high risk for AD imparted by the ε4-allele. ε4 carriers typically manifest AD at an earlier age than our present cohort. Alternatively, it is possible these results represent a false positive. Given that our hippocampal cluster extended into subgyral space, the SVC did not reveal a significant increase in this region, and lacking a mechanistic model for increased volume, this possibility cannot be ruled out in the present study. It is possible that our individuals selected out for being nondemented are a residual group of ε4-carriers not fully representative of the population of ε4-carriers. Nevertheless, the present data suggest that despite the impact of the ε4 allele on cognitive and imaging measures in healthy elderly, there are mediating protective mechanisms preventing disease onset in this particular group.
As a post-hoc analysis we extracted the cluster of significantly decreased volume in carriers of the ε4 allele and tested if various factors related to lifestyle, physical activity or cardiorespiratory fitness were related to APOEε4 related brain volume decreases. None of these factors significantly covaried with change in brain morphology associated with the APOE gene. There may be other factors influencing brain volume changes that we have not controlled for such as other genes or medication, and future studies looking at epistasis between interacting gene variants of interest may shed light on more complex genetic mechanisms at work. Several studies have linked APOE risk effects to lifestyle [77], and while our study consisted of a variety of lifestyle measures, these measures and our sample size may have limited sensitivity for identifying this type of geneenvironment interaction.
We found that APOEε4 genetic variation may be associated with disrupted white matter integrity in the parahippocampal gyrus of healthy elderly subjects. A large number of studies have found decreased white matter strength in posterior temporal regions, including parahippocampal tracts, in subjects with AD [78–83]. AD-related hippocampal atrophy has been correlated with abnormalities in the cingulum bundle fiber tract [84,85], which mainly includes fibers connecting the parahippocampal gyrus and hippocampus proper to the posterior cingulate cortex [86,87]. Moreover several DTI studies in nondemented subjects have reported a significant relationship between the APOEε4 allele and decreased white matter integrity in temporo-parietal regions [25–27]. A recent DTI study focused on the parahippocampal gyrus and found decreased FA in healthy carriers of the ε4 allele, similar to our own results [26]. Another region of interest study found decreased FA in the posterior corpus callosum of individuals carrying a copy of the APOEε4 allele [25]. Taken together, these results and ours suggest that genetic variation in APOEε4 may influence disease-related white matter integrity in posterior white matter tracts, especially those branching from the medial temporal cortex.
TBSS was created to alleviate alignment-related problems in DTI data, and thus restricts the analysis to a group-estimated skeleton strictly of white matter. Ideally this would increase the power of the analysis to detect the small fractional anisotropy changes that might occur in a cognitively healthy group. Although it is possible that restriction in white matter to the FA skeleton could increase the chance of a Type II error, a recent study using TBSS in AD subjects reported disease-related FA decreases at a statistical threshold similar to our own in the parahippocampal gyrus [88]. Usage of the FMRIB58_FA standard template might also introduce bias through the registration process, due to the age discrepancy between the template subjects and the age of our subjects. It is also possible that TB-SS could be vulnerable to detecting the vascular burden of the white matter, and that this, in turn, is affecting the APOEε4 results in the white matter. While TBSS confines analyses to a narrow skeleton within the white matter tracts of greatest integrity, the presence of white matter lesions may have influenced the results. We excluded individuals with a history of clinical stroke, diabetes, and cardiovascular disease, minimizing the potential influence of vascular-related changes.
Overall we sought to use automated morphological analysis techniques to characterize the genetic variation in APOE associated with both gray matter volume and white matter viability in carefully screened, non-demented elderly subjects. Using voxel-based analyses in several imaging modalities, we validate previous studies identifying decreased hippocampal and amygdala volume in APOEε4 carriers and decreased FA in the parahippocampal gyrus in one group of subjects. We also identify selective decreases in memory-related cognitive performance in otherwise healthy elderly individuals carrying the ε4 allele. Our finding of a trend for increased frontal gray matter associated with the risk allele sets the groundwork for future studies to identify potential protective mechanisms at work in individuals at risk for AD. More complex approaches to characterizing genetic epistasis using these comprehensive, unbiased image analyses in a larger group, along with relevant environmental, lifestyle, or other genetic measures may inform risk for the disease as well as the mechanisms involved in the disease pathology.
ACKNOWLEDGMENTS
This study was supported by grants R03 AG026374 and R21 AG029615 from the National Institutes of Aging and K23NS058252 from the National Institute on Neurological Disorders and Stroke. The University of Kansas General Clinical Research Center (M01RR023940) provided essential space, expertise, and nursing support. The Hoglund Brain Imaging Center is supported by grant C76 HF00201.
The authors thank members of the KU Brain Aging Project team, especially Pat Laubinger, Phyllis Switzer, Diane Cunningham, and George Thomas for their assistance with data collection and study as well as helpful suggestions. Finally, they thank the research participants of the KU Brain Aging Project for their generosity of time and spirit, which makes this research possible.
Footnotes
Authors’ disclosures available online (http://www.j-alz.com/disclosures/view.php?id=49).
REFERENCES
- 1.Reiman EM. Linking brain imaging and genomics in the study of Alzheimer’s disease and aging. Ann N Y Acad Sci. 2007;1097:94–113. doi: 10.1196/annals.1379.011. [DOI] [PubMed] [Google Scholar]
- 2.Mattay VS, Goldberg TE, Sambataro F, Weinberger DR. Neurobiology of cognitive aging: Insights from imaging genetics. Biol Psychol. 2008;79:9–22. doi: 10.1016/j.biopsycho.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rovio S, Kareholt I, Helkala EL, Viitanen M, Winblad B, Tuomilehto J, Soininen H, Nissinen A, Kivipelto M. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer’s disease. Lancet Neurol. 2005;4:705–711. doi: 10.1016/S1474-4422(05)70198-8. [DOI] [PubMed] [Google Scholar]
- 4.Etnier JL, Caselli RJ, Reiman EM, Alexander GE, Sibley BA, Tessier D, McLemore EC. Cognitive performance in older women relative to ApoE-epsilon4 genotype and aerobic fitness. Med Sci Sports Exerc. 2007;39:199–207. doi: 10.1249/01.mss.0000239399.85955.5e. [DOI] [PubMed] [Google Scholar]
- 5.Podewils LJ, Guallar E, Kuller LH, Fried LP, Lopez OL, Carlson M, Lyketsos CG. Physical activity, APOE genotype, and dementia risk: findings from the Cardiovascular Health Cognition Study. Am J Epidemiol. 2005;161:639–651. doi: 10.1093/aje/kwi092. [DOI] [PubMed] [Google Scholar]
- 6.Schuit AJ, Feskens EJ, Launer LJ, Kromhout D. Physical activity and cognitive decline, the role of the apolipoprotein e4 allele. Med Sci Sports Exerc. 2001;33:772–777. doi: 10.1097/00005768-200105000-00015. [DOI] [PubMed] [Google Scholar]
- 7.Reiman EM, Caselli RJ, Yun LS, Chen K, Bandy D, Minoshima S, Thibodeau SN, Osborne D. Preclinical evidence of Alzheimer’s disease in persons homozygous for the ε4 allele for apolipoprotein E. New Engl J Med. 1996;334:752–758. doi: 10.1056/NEJM199603213341202. [DOI] [PubMed] [Google Scholar]
- 8.Saunders AM, Strittmatter WJ, Schmechel D, George-Hyslop PH, Pericak-Vance MA, Joo SH, Rosi BL, Gusella JF, Crapper-MacLachlan DR, Alberts MJ, et al. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer’s disease. Neurology. 1993;43:1467–1472. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- 9.Geroldi C, Pihlajamaki M, Laakso MP, DeCarli C, Beltramello A, Bianchetti A, Soininen H, Trabucchi M, Frisoni GB. APOE-epsilon4 is associated with less frontal and more medial temporal lobe atrophy in AD. Neurology. 1999;53:1825–1832. doi: 10.1212/wnl.53.8.1825. [DOI] [PubMed] [Google Scholar]
- 10.Lehtovirta M, Soininen H, Laakso MP, Partanen K, Helisalmi S, Mannermaa A, Ryynanen M, Kuikka J, Hartikainen P, Riekkinen PJ., Sr SPECT and MRI analysis in Alzheimer’s disease: relation to apolipoprotein E epsilon 4 allele. J Neurol Neurosurg Psychiatry. 1996;60:644–649. doi: 10.1136/jnnp.60.6.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bigler ED, Lowry CM, Anderson CV, Johnson SC, Terry J, Steed M. Dementia, quantitative neuroimaging, and apolipoprotein E genotype. AJNR Am J Neuroradiol. 2000;21:1857–1868. [PMC free article] [PubMed] [Google Scholar]
- 12.Hashimoto M, Yasuda M, Tanimukai S, Matsui M, Hirono N, Kazui H, Mori E. Apolipoprotein E epsilon 4 and the pattern of regional brain atrophy in Alzheimer’s disease. Neurology. 2001;57:1461–1466. doi: 10.1212/wnl.57.8.1461. [DOI] [PubMed] [Google Scholar]
- 13.Basso M, Gelernter J, Yang J, MacAvoy MG, Varma P, Bronen RA, van Dyck CH. Apolipoprotein E epsilon4 is associated with atrophy of the amygdala in Alzheimer’s disease. Neurobiol Aging. 2006;27:1416–1424. doi: 10.1016/j.neurobiolaging.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 14.Yasuda M, Mori E, Kitagaki H, Yamashita H, Hirono N, Shimada K, Maeda K, Tanaka C. Apolipoprotein E epsilon 4 allele and whole brain atrophy in late-onset Alzheimer’s disease. Am J Psychiatry. 1998;155:779–784. doi: 10.1176/ajp.155.6.779. [DOI] [PubMed] [Google Scholar]
- 15.Cohen RM, Small C, Lalonde F, Friz J, Sunderland T. Effect of apolipoprotein E genotype on hippocampal volume loss in aging healthy women. Neurology. 2001;57:2223–2228. doi: 10.1212/wnl.57.12.2223. [DOI] [PubMed] [Google Scholar]
- 16.den Heijer T, Oudkerk M, Launer LJ, van Duijn CM, Hofman A, Breteler MM. Hippocampal, amygdalar, and global brain atrophy in different apolipoprotein E genotypes. Neurology. 2002;59:746–748. doi: 10.1212/wnl.59.5.746. [DOI] [PubMed] [Google Scholar]
- 17.Tohgi H, Takahashi S, Kato E, Homma A, Niina R, Sasaki K, Yonezawa H, Sasaki M. Reduced size of right hippocampus in 39- to 80-year-old normal subjects carrying the apolipoprotein E epsilon4 allele. Neurosci Lett. 1997;236:21–24. doi: 10.1016/s0304-3940(97)00743-x. [DOI] [PubMed] [Google Scholar]
- 18.Lind J, Larsson A, Persson J, Ingvar M, Nilsson LG, Back-man L, Adolfsson R, Cruts M, Sleegers K, Van Broeckhoven C, Nyberg L. Reduced hippocampal volume in non-demented carriers of the apolipoprotein E epsilon4: relation to chronological age and recognition memory. Neurosci Lett. 2006;396:23–27. doi: 10.1016/j.neulet.2005.11.070. [DOI] [PubMed] [Google Scholar]
- 19.Jak AJ, Houston WS, Nagel BJ, Corey-Bloom J, Bondi MW. Differential cross-sectional and longitudinal impact of APOE genotype on hippocampal volumes in nondemented older adults. Dement Geriatr Cogn Disord. 2007;23:382–389. doi: 10.1159/000101340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reiman EM, Uecker A, Caselli RJ, Lewis S, Bandy D, de Leon MJ, De Santi S, Convit A, Osborne D, Weaver A, Thibodeau SN. Hippocampal volumes in cognitively normal persons at genetic risk for Alzheimer’s disease. Ann Neurol. 1998;44:288–291. doi: 10.1002/ana.410440226. [DOI] [PubMed] [Google Scholar]
- 21.Cherbuin N, Anstey KJ, Sachdev PS, Maller JJ, Meslin C, Mack HA, Wen W, Easteal S. Total and regional gray matter volume is not related to APOE*ε4 status in a community sample of middle-aged individuals. J Gerontol A Biol Sci Med Sci. 2008;63:501–504. doi: 10.1093/gerona/63.5.501. [DOI] [PubMed] [Google Scholar]
- 22.Bigler ED, Lowry CM, Kerr B, Tate DF, Hessel CD, Earl HD, Miller MJ, Rice SA, Smith KH, Tschanz JT, Welsh-Bohmer K, Plassman B, Victoroff J. Role of white matter lesions, cerebral atrophy, and APOE on cognition in older persons with and without dementia: the Cache County, Utah, study of memory and aging. Neuropsychology. 2003;17:339–352. doi: 10.1037/0894-4105.17.3.339. [DOI] [PubMed] [Google Scholar]
- 23.Lemaitre H, Crivello F, Dufouil C, Grassiot B, Tzourio C, Alperovitch A, Mazoyer B. No epsilon4 gene dose effect on hippocampal atrophy in a large MRI database of healthy elderly subjects. Neuroimage. 2005;24:1205–1213. doi: 10.1016/j.neuroimage.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 24.Wishart HA, Saykin AJ, McAllister TW, Rabin LA, McDonald BC, Flashman LA, Roth RM, Mamourian AC, Tsongalis GJ, Rhodes CH. Regional brain atrophy in cognitively intact adults with a single APOE epsilon4 allele. Neurology. 2006;67:1221–1224. doi: 10.1212/01.wnl.0000238079.00472.3a. [DOI] [PubMed] [Google Scholar]
- 25.Persson J, Lind J, Larsson A, Ingvar M, Cruts M, Van Broeckhoven C, Adolfsson R, Nilsson LG, Nyberg L. Altered brain white matter integrity in healthy carriers of the APOE epsilon4 allele: a risk for AD? Neurology. 2006;66:1029–1033. doi: 10.1212/01.wnl.0000204180.25361.48. [DOI] [PubMed] [Google Scholar]
- 26.Nierenberg J, Pomara N, Hoptman MJ, Sidtis JJ, Ardekani BA, Lim KO. Abnormal white matter integrity in healthy apolipoprotein E epsilon4 carriers. Neuroreport. 2005;16:1369–1372. doi: 10.1097/01.wnr.0000174058.49521.16. [DOI] [PubMed] [Google Scholar]
- 27.Smith CD, Chebrolu H, Andersen AH, Powell DA, Lovell MA, Xiong S, Gold BT. White matter diffusion alterations in normal women at risk of Alzheimer’s disease. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.08.006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deary IJ, Whiteman MC, Pattie A, Starr JM, Hayward C, Wright AF, Visscher PM, Tynan MC, Whalley LJ. Apolipoprotein e gene variability and cognitive functions at age 79: a follow-up of the Scottish mental survey of 1932. Psychol Aging. 2004;19:367–371. doi: 10.1037/0882-7974.19.2.367. [DOI] [PubMed] [Google Scholar]
- 29.Linn RT, Wolf PA, Bachman DL, Knoefel JE, Cobb JL, Belanger AJ, Kaplan EF, D’Agostino RB. The ‘preclinical phase’ of probable Alzheimer’s disease. A 13-year prospective study of the Framingham cohort. Arch Neurol. 1995;52:485–490. doi: 10.1001/archneur.1995.00540290075020. [DOI] [PubMed] [Google Scholar]
- 30.Bondi MW, Salmon DP, Monsch AU, Galasko D, Butters N, Klauber MR, Thal LJ, Saitoh T. Episodic memory changes are associated with the APOE-epsilon 4 allele in nondemented older adults. Neurology. 1995;45:2203–2206. doi: 10.1212/wnl.45.12.2203. [DOI] [PubMed] [Google Scholar]
- 31.Deary IJ, Whiteman MC, Pattie A, Starr JM, Hayward C, Wright AF, Carothers A, Whalley LJ. Cognitive change and the APOE epsilon 4 allele. Nature. 2002;418:932. doi: 10.1038/418932a. [DOI] [PubMed] [Google Scholar]
- 32.Plassman BL, Welsh-Bohmer KA, Bigler ED, Johnson SC, Anderson CV, Helms MJ, Saunders AM, Breitner JC. Apolipoprotein E epsilon 4 allele and hippocampal volume in twins with normal cognition. Neurology. 1997;48:985–989. doi: 10.1212/wnl.48.4.985. [DOI] [PubMed] [Google Scholar]
- 33.Jorm AF, Mather KA, Butterworth P, Anstey KJ, Christensen H, Easteal S. APOE genotype and cognitive functioning in a large age-stratified population sample. Neuropsychology. 2007;21:1–8. doi: 10.1037/0894-4105.21.1.1. [DOI] [PubMed] [Google Scholar]
- 34.Caselli RJ, Graff-Radford NR, Reiman EM, Weaver A, Osborne D, Lucas J, Uecker A, Thibodeau SN. Preclinical memory decline in cognitively normal apolipoprotein E-î homozygotes. Neurology. 1999;53:201–207. doi: 10.1212/wnl.53.1.201. [DOI] [PubMed] [Google Scholar]
- 35.Savitz J, Solms M, Ramesar R. Apolipoprotein E variants and cognition in healthy individuals: a critical opinion. Brain Res Rev. 2006;51:125–135. doi: 10.1016/j.brainresrev.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 36.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412b–2414b. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 37.Berg L, McKeel DW, Jr, Miller JP, Storandt M, Rubin EH, Morris JC, Baty J, Coats M, Norton J, Goate AM, Price JL, Gearing M, Mirra SS, Saunders AM. Clinicopathologic studies in cognitively healthy aging and Alzheimer disease: relation of histologic markers to dementia severity, age, sex, and apolipoprotein E genotype. Arch Neurol. 1998;55:326–335. doi: 10.1001/archneur.55.3.326. [DOI] [PubMed] [Google Scholar]
- 38.Morris JC, Storandt M, Miller JP, McKeel DW, Price JL, Rubin EH, Berg L. Mild cognitive impairment represents early-stage Alzheimer disease. Arch Neurol. 2001;58:397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- 39.Burns JM, Cronk BB, Anderson HS, Donnelly JE, Thomas GP, Harsha A, Brooks WM, Swerdlow RH. Cardiorespiratory fitness and brain atrophy in early Alzheimer disease. Neurology. 2008;71:210–216. doi: 10.1212/01.wnl.0000317094.86209.cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shah KR, Carr D, Roe CM, Miller JP, Coats M, Morris JC. Impaired physical performance and the assessment of dementia of the Alzheimer type. Alzheimer Dis Assoc Disord. 2004;18:112–118. doi: 10.1097/01.wad.0000127441.77570.f3. [DOI] [PubMed] [Google Scholar]
- 41.Balady GJ, Berra KA, Golding LA, Gordon NF, A MD, N MJ, Sheldahl LM. American College of Sport Medicine’s Guidelines for Exercise Testing and Prescription. Baltimore: Williams & Wilkins; 2007. [Google Scholar]
- 42.Burns JM, Mayo MS, Anderson HS, Smith HJ, Donnelly JE. Cardiorespiratory fitness in early-stage Alzheimer disease. Alzheimer Dis Assoc Disord. 2008;22:39–46. doi: 10.1097/WAD.0b013e31815a9ddc. [DOI] [PubMed] [Google Scholar]
- 43.Washburn RA, McAuley E, Katula J, Mihalko SL, Boileau RA. The physical activity scale for the elderly (PASE): evidence for validity. J Clin Epidemiol. 1999;52:643–651. doi: 10.1016/s0895-4356(99)00049-9. [DOI] [PubMed] [Google Scholar]
- 44.Wechsler D, Stone CP. Manual: Wechsler Memory Scale. New York: Psychological Corporation; 1973. [Google Scholar]
- 45.Grober E, Buschke H, Crystal H, Bang S, Dresner R. Screening for dementia by memory testing. Neurology. 1988;38:900–903. doi: 10.1212/wnl.38.6.900. [DOI] [PubMed] [Google Scholar]
- 46.Goodglass H, Kaplan E. Boston Naming Test Scoring Booklet. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- 47.Wechsler D. Manual: Wechsler Adult Intelligence Scale. New York: Psychological Corporation; 1955. [Google Scholar]
- 48.Armitage SG. An analysis of certain psychological tests used in the evaluation of brain injury. Psychological Monographs. 1946;60:1–48. [Google Scholar]
- 49.Hänninen T, Reinikainen KJ, Helkala EL, Koivisto K, MykkÎnen L, Laakso M, PyrÎlÎ K, Riekkinen P. Subjective memory complaints and personality traits in normal elderly subjects. J Am Geriatrics Soc. 1994;42:1–4. doi: 10.1111/j.1532-5415.1994.tb06064.x. [DOI] [PubMed] [Google Scholar]
- 50.Stroop J. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18:643–662. [Google Scholar]
- 51.Folstein MF, Folstein SE, McHugh PR. Mini-mental State: A practical method for grading the cognitive state of patients for the clinicians. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 52.Burns JM, Donnelly JE, Anderson HS, Mayo MS, Spencer-Gardner L, Thomas G, Cronk BB, Haddad Z, Klima D, Hansen D, Brooks WM. Peripheral insulin and brain structure in early Alzheimer disease. Neurology. 2007;69:1094–1104. doi: 10.1212/01.wnl.0000276952.91704.af. [DOI] [PubMed] [Google Scholar]
- 53.Honea RA, Thomas GP, Harsha A, Anderson HS, Donnelly JE, Brooks WM, Burns JM. Cardiorespiratory fitness and preserved medial temporal lobe volume in Alzheimer’s Disease. Alzheimer Dis Assoc Disord. 2009 doi: 10.1097/WAD.0b013e31819cb8a2. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 55.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23 Suppl 1:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 56.Rueckert D, Sonoda LI, Hayes C, Hill DL, Leach MO, Hawkes DJ. Nonrigid registration using free-form deformations: application to breast MR images. IEEE Trans Med Imaging. 1999;18:712–721. doi: 10.1109/42.796284. [DOI] [PubMed] [Google Scholar]
- 57.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 59.Honea R, Verchinski BA, Pezawas L, Kolachana BS, Callicott JH, Mattay VS, Weinberger DR, Meyer-Lindenberg A. Impact of interacting functional variants in COMT on regional gray matter volume in human brain. Neuroimage. 2009;45:44–51. doi: 10.1016/j.neuroimage.2008.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol (Berl) 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 61.Jack CR, Petersen RC, Xu YC, O’Brien PC, Waring SC, Tangalos EG, Smith GE, Ivnik RJ, Thibodeau SN, Kokmen E. Hippocampal atrophy and apolipoprotein E genotype are independently associated with Alzheimer’s disease. Ann Neurol. 1998;43:303–310. doi: 10.1002/ana.410430307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Honea RA, Meyer-Lindenberg A, Hobbs KB, Pezawas L, Mattay VS, Egan MF, Verchinski B, Passingham RE, Weinberger DR, Callicott JH. Is gray matter volume an intermediate phenotype for schizophrenia? A voxel-based morphometry study of patients with schizophrenia and their healthy siblings. Biol Psychiatry. 2008;63:465–474. doi: 10.1016/j.biopsych.2007.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smith SM, Johansen-Berg H, Jenkinson M, Rueckert D, Nichols TE, Miller KL, Robson MD, Jones DK, Klein JC, Bartsch AJ, Behrens TE. Acquisition and voxelwise analysis of multi-subject diffusion data with tract-based spatial statistics. Nat Protoc. 2007;2:499–503. doi: 10.1038/nprot.2007.45. [DOI] [PubMed] [Google Scholar]
- 64.Schiffman SS, Graham BG, Sattely-Miller EA, Zervakis J, Welsh-Bohmer K. Taste, smell and neuropsychological performance of individuals at familial risk for Alzheimer’s disease. Neurobiol Aging. 2002;23:397–404. doi: 10.1016/s0197-4580(01)00337-2. [DOI] [PubMed] [Google Scholar]
- 65.Martin RC, Annis SM, Darling LZ, Wadley V, Harrell L, Marson DC. Loss of calculation abilities in patients with mild and moderate Alzheimer disease. Arch Neurol. 2003;60:1585–1589. doi: 10.1001/archneur.60.11.1585. [DOI] [PubMed] [Google Scholar]
- 66.Li M, Ng TP, Kua EH, Ko SM. Brief informant screening test for mild cognitive impairment and early Alzheimer’s disease. Dement Geriatr Cogn Disord. 2006;21:392–402. doi: 10.1159/000092808. [DOI] [PubMed] [Google Scholar]
- 67.Tupler LA, Krishnan KR, Greenberg DL, Marcovina SM, Payne ME, MacFall JR, Charles HC, Doraiswamy PM. Predicting memory decline in normal elderly: genetics, MRI, and cognitive reserve. Neurobiol Aging. 2007;28:1644–1656. doi: 10.1016/j.neurobiolaging.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 68.Schott JM, Fox NC, Frost C, Scahill RI, Janssen JC, Chan D, Jenkins R, Rossor MN. Assessing the onset of structural change in familial Alzheimer’s disease. Ann Neurol. 2003;53:181–188. doi: 10.1002/ana.10424. [DOI] [PubMed] [Google Scholar]
- 69.Shaw P, Lerch JP, Pruessner JC, Taylor KN, Rose AB, Greenstein D, Clasen L, Evans A, Rapoport JL, Giedd JN. Cortical morphology in children and adolescents with different apolipoprotein E gene polymorphisms: an observational study. Lancet Neurol. 2007;6:494–500. doi: 10.1016/S1474-4422(07)70106-0. [DOI] [PubMed] [Google Scholar]
- 70.Burggren AC, Zeineh MM, Ekstrom AD, Braskie MN, Thompson PM, Small GW, Bookheimer SY. Reduced cortical thickness in hippocampal subregions among cognitively normal apolipoprotein E e4 carriers. Neuroimage. 2008;41:1177–1183. doi: 10.1016/j.neuroimage.2008.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Duarte A, Hayasaka S, Du A, Schuff N, Jahng GH, Kramer J, Miller B, Weiner M. Volumetric correlates of memory and executive function in normal elderly, mild cognitive impairment and Alzheimer’s disease. Neurosci Lett. 2006;406:60–65. doi: 10.1016/j.neulet.2006.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Busatto GF, Garrido GE, Almeida OP, Castro CC, Camargo CH, Cid CG, Buchpiguel CA, Furuie S, Bottino CM. A voxel-based morphometry study of temporal lobe gray matter reductions in Alzheimer’s disease. Neurobiol Aging. 2003;24:221–231. doi: 10.1016/s0197-4580(02)00084-2. [DOI] [PubMed] [Google Scholar]
- 73.Matsuda H, Kitayama N, Ohnishi T, Asada T, Nakano S, Sakamoto S, Imabayashi E, Katoh A. Longitudinal evaluation of both morphologic and functional changes in the same individuals with Alzheimer’s disease. J Nucl Med. 2002;43:304–311. [PubMed] [Google Scholar]
- 74.Baron JC, Chetelat G, Desgranges B, Perchey G, Landeau B, de la Sayette V, Eustache F. In vivo mapping of gray matter loss with voxel-based morphometry in mild Alzheimer’s disease. Neuroimage. 2001;14:298–309. doi: 10.1006/nimg.2001.0848. [DOI] [PubMed] [Google Scholar]
- 75.Geroldi C, Laakso MP, DeCarli C, Beltramello A, Bianchetti A, Soininen H, Trabucchi M, Frisoni GB. Apolipoprotein E genotype and hippocampal asymmetry in Alzheimer’s disease: a volumetric MRI study. J Neurol Neurosurg Psychiatry. 2000;68:93–96. doi: 10.1136/jnnp.68.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.van der Vlies AE, Pijnenburg YA, Koene T, Klein M, Kok A, Scheltens P, van der Flier WM. Cognitive impairment in Alzheimer’s disease is modified by APOE genotype. Dement Geriatr Cogn Disord. 2007;24:98–103. doi: 10.1159/000104467. [DOI] [PubMed] [Google Scholar]
- 77.Kivipelto M, Rovio S, Ngandu T, Kareholt I, Eskelinen M, Winblad B, Hachinski V, Cedazo-Minguez A, Soininen H, Tuomilehto J, Nissinen A. Apolipoprotein E epsilon4 magnifies lifestyle risks for dementia: a population-based study. J Cell Mol Med. 2008;12:2762–2771. doi: 10.1111/j.1582-4934.2008.00296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang Y, Schuff N, Jahng GH, Bayne W, Mori S, Schad L, Mueller S, Du AT, Kramer JH, Yaffe K, Chui H, Jagust WJ, Miller BL, Weiner MW. Diffusion tensor imaging of cingulum fibers in mild cognitive impairment and Alzheimer disease. Neurology. 2007;68:13–19. doi: 10.1212/01.wnl.0000250326.77323.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fellgiebel A, Muller MJ, Wille P, Dellani PR, Scheurich A, Schmidt LG, Stoeter P. Color-coded diffusion-tensor-imaging of posterior cingulate fiber tracts in mild cognitive impairment. Neurobiol Aging. 2005;26:1193–1198. doi: 10.1016/j.neurobiolaging.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 80.Rose SE, McMahon KL, Janke AL, O’Dowd B, de Zubicaray G, Strudwick MW, Chalk JB. Diffusion indices on magnetic resonance imaging and neuropsychological performance in amnestic mild cognitive impairment. J Neurol Neurosurg Psychiatry. 2006;77:1122–1128. doi: 10.1136/jnnp.2005.074336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Teipel SJ, Stahl R, Dietrich O, Schoenberg SO, Perneczky R, Bokde AL, Reiser MF, Moller HJ, Hampel H. Multivariate network analysis of fiber tract integrity in Alzheimer’s disease. Neuroimage. 2007;34:985–995. doi: 10.1016/j.neuroimage.2006.07.047. [DOI] [PubMed] [Google Scholar]
- 82.Chen SQ, Kang Z, Hu XQ, Hu B, Zou Y. Diffusion tensor imaging of the brain in patients with Alzheimer’s disease and cerebrovascular lesions. J Zhejiang Univ Sci B. 2007;8:242–247. doi: 10.1631/jzus.2007.B0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Teipel SJ, Pogarell O, Meindl T, Dietrich O, Sydykova D, Hunklinger U, Georgii B, Mulert C, Reiser MF, Moller HJ, Hampel H. Regional networks underlying interhemispheric connectivity: An EEG and DTI study in healthy ageing and amnestic mild cognitive impairment. Hum Brain Mapp. 2009;30:2098–2119. doi: 10.1002/hbm.20652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Villain N, Desgranges B, Viader F, de la Sayette V, Mezenge F, Landeau B, Baron JC, Eustache F, Chetelat G. Relationships between hippocampal atrophy, white matter disruption, and gray matter hypometabolism in Alzheimer’s disease. J Neurosci. 2008;28:6174–6181. doi: 10.1523/JNEUROSCI.1392-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Firbank MJ, Blamire AM, Krishnan MS, Teodorczuk A, English P, Gholkar A, Harrison R, O’Brien JT. Atrophy is associated with posterior cingulate white matter disruption in dementia with Lewy bodies and Alzheimer’s disease. Neuroimage. 2007;36:1–7. doi: 10.1016/j.neuroimage.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 86.Mori S, Wakana S, Nagae-Poetscher LM, van Zijl PCM. MRI atlas of human white matter. Amsterdam, The Netherlands: Elsevier B. V.; 2005. [Google Scholar]
- 87.Mufson EJ, Pandya DN. Some observations on the course and composition of the cingulum bundle in the rhesus monkey. J Comp Neurol. 1984;225:31–43. doi: 10.1002/cne.902250105. [DOI] [PubMed] [Google Scholar]
- 88.Damoiseaux JS, Smith SM, Witter MP, Sanz-Arigita EJ, Barkhof F, Scheltens P, Stam CJ, Zarei M, Rombouts SA. White matter tract integrity in aging and Alzheimer’s disease. Hum Brain Mapp. 2009;30:1051–1059. doi: 10.1002/hbm.20563. [DOI] [PMC free article] [PubMed] [Google Scholar]