Abstract
The arctic biome is a relatively young ecosystem with ~2300 species of vascular plants. We studied the genus Ranunculus as an example of the origin and evolution of the arctic flora. For this purpose we used molecular phylogenetic and clock analyses based on evaluation of nuclear ITS and chloroplast matK-trnK DNA sequences in 194 taxa of Ranunculus and closely related genera. Taxa occurring in the Arctic arose form seven phylogenetic lineages of Ranunculus and also in the genera Coptidium and Halerpestes. Two clades of Ranunculus are species-rich in the Arctic, i.e., Ranunculus sect. Ranunculus and R. sect. Auricomus (both from R. subg. Ranunculus), but this is due to a number of arctic “microtaxa” morphologically barely separate from R. acris in the former clade and the widely agamospermic species complex of R. auricomus in the latter. Lineages with species adapted to wetlands or aquatic habitats are significant groups represented in the arctic flora (R. subg. Ranunculus sectt. Flammula and Hecatonia/Xanthobatrachium, R. subg. Batrachium, genus Coptidium) but show no clear signs of radiation in the Arctic or the northern boreal zone, except for sectt. Hecatonia/Xanthobatrachium, with R. hyperboreus and R. sceleratus subsp. reptabundus. Astonishingly few of the otherwise numerous lineages of Ranunculus with distributions in the higher mountain systems of Eurasia and North America have acted as “founding sources” for the arctic flora. The only clear example is that of the arctic-alpine R. glacialis and the Beringian R. chamissonis from the lineage of subg. R. sectt. Aconitifolii/Crymodes, although there might be others in sect. Auricomus not recovered in the current molecular data. Lineages that gave rise to arctic taxa diverged from each other from the early Miocene (R. glacialis/R. chamissonis, Coptidium, lineages in Halerpestes) and continued at an even rate throughout the Tertiary. There are no signs that the intense climate changes of the late Pliocene and the Quaternary substantially accelerated or impeded diversification in Ranunculus. Only the crown group split of R. acris and its relatives is clearly of Quaternary age. A detailed comparison concerning morphology, karyology, and life form excludes fundamental differences between taxa of Ranunculus in the Arctic and their respective closest relatives in regions south of it. Ecological traits, e.g., preferences for dry or moist soils or growth in open and sheltered conditions, also do not differ between arctic and nonarctic̣ taxa. Migration into the Arctic thus started from different phylogenetic lineages and at different times, without development of obvious special traits in the adaptation to arctic environments. This recurrent pattern in Ranunculus differs from that seen in other arctic genera, e.g., Artemisia, in which special traits of adaptation to arctic environments are found. In Ranunculus, the origin of the open arctic biome primarily favored range expansions of taxa/species already adapted to wet habitats in cold areas and depending on rapid dispersal.
Keywords: Ranunculus, Halerpestes, Arctic, molecular clock, phylogeny, biogeography
Introduction
The Arctic is a large area with a relatively low number of vascular plant species. The Eurasian part comprises ~1690 species (Tkach et al. 2008c), and some 500 further species may be added if arctic North America is included. The relative paucity of the arctic flora is considered to result from a rather recent origin of this ecosystem (Matthews and Ovenden 1990) and the absence of large radiations (Hoffmann and Röser 2009). Kinetic effects of temperature are discussed as a further factor because low temperatures and the short vegetation period in the Arctic negatively impact genetic divergence and speciation (Allen et al. 2006). During much of the Tertiary, the landmasses presently situated in the Arctic supported the Arcto-Tertiary or boreal flora (Mai 1995), consisting of extensive forests. Temperatures decreased in the Middle Eocene ~45 Myr ago, and the first temporary ice sheets were formed (Moran et al. 2006). The ice sheet expansion in Greenland, about 3.2 Myr ago, corresponds with the emergence of the arctic tundra ecosystem (Matthews 1979; Matthews and Ovenden 1990), whose extensions varied widely during the times (Svendsen et al. 2004). Tertiary fossils from mostly riparian habitats or bogs, but also from well-drained uplands with herb and dwarf-shrub communities, indicate that many genera or species currently found in the arctic tundra were already present in the North before the arctic biome established, e.g., species of Empetrum, Vaccinium, or Papaver (Bennike and Bøcher 1990; Matthews and Ovenden 1990; Murray 1995). Ranunculus and Coptidium macrofossils have been frequently reported from fossil deposits in arctic areas from the mid-Miocene onward (Bennike and Bøcher 1990; Matthews and Ovenden 1990) and seem to belong to Ranunculus hyperboreus and Coptidium lapponicum or their close extant relatives. Both species are still important and abundant in the arctic flora but differ in their distribution ranges; R. hyperboreus is almost confined to the Arctic, whereas C. lapponicum occurs only in the southern Arctic and is more widespread in the boreal zone.
The relatively young ecosystem of the Arctic raises questions on the origin of its flora. It may have evolved from autochthonous taxa of the Arcto-Tertiary forest vegetation by adaptation to colder climates. Alternatively, the flora could have recruited from more recent Quaternary migrants that were somewhat preadapted to arctic environmental conditions. It is thought that the latter were derived from taxa of ancient high-mountain ranges in Asia and North America (Hultén 1937, 1958; Tolmachev 1960; Weber 1965; Hedberg 1992), though this has been hard to prove precisely (Mai 1995). Other arctic plants are widespread taxa with wide ecological amplitudes enabling them to cope easily with arctic conditions (Tkach et al. 2008c). As emphasized by Abbott and Brochmann (2003), the origins of the founding stocks of the present-day arctic flora are largely unknown, although some progress has been made on the basis of fossil and molecular phylogeographic evidence. In studies on Artemisia, we have shown that arctic species were recruited from a wide variety of ecosystems and habitats, but mainly from mountains and steppes, and that some lineages actually diversified later within the Arctic. This multiple evolution of taxa occurring in the Arctic appears to be a general pattern in many different genera (Hoffmann and Röser 2009). A molecular clock approach revealed that several lineages of arctic Artemisia originated contemporaneously with the arctic biome, whereas other lineages proved much older (Tkach et al. 2008a, 2008b).
A higher proportion of polyploid species relative to more southern species is a well-known pattern in the Arctic (Hagerup 1932; Johnson and Packer 1965). For example, in the Svalbard flora (lat. 80°N), 78% of the taxa are polyploid (Brochmann et al. 2004). However, in Artemisia, no higher levels of polyploidy were observed relative to the nonarctic sister groups (Tkach et al. 2008a), but morphological changes upon colonization of arctic habitats were observed.
Here we study the recruitment and evolution of taxa in another large genus of the arctic flora. Ranunculus comprises ~600 species distributed in most parts of the world (Tamura 1995). The genus shows a high morphological diversity, often related to adaptation to certain habitats or climatic conditions and its occurrence from near sea level up to the highest mountain zones or the high Arctic. Chromosome base numbers in Ranunculus are x=8 or, more rarely, x=7. Polyploidy is frequent and often connected with apomixis, as reviewed by Hörandl et al. (2005). Ranunculus is represented in the Arctic by ~42–48 species and subspecies (Tolmachev 1971; the Panarctic Flora Project outlined in Elvebakk et al. 1999; Nordal and Razzhivin 1999; cited in the following as Elven 2007). Previous molecular phylogenetic studies corroborated to exclude the arctic species R. pallasii and R. lapponicum from Ranunculus and supported their treatment under a separate genus Coptidium. Also, Halerpestes, represented in the Arctic by Halerpestes cymbalaria, has been shown to be a line-age separate from Ranunculus (Hörandl et al. 2005; Paun et al. 2005; Lehnebach et al. 2007; Hörandl, forthcoming). We included in this investigation most of the arctic taxa and other northern Asian species not sampled in previous molecular phylogenetic studies on Ranunculus and its close relatives of tribe Ranunculeae to address the following questions: Are the numerous arctic species a result of speciation or radiation of particular lineages in the Arctic or of parallel evolution starting from different lineages? Do lineages proliferating arctic species point to common biogeographical “centers of origin”? Are there recurrent patterns in life form, habitat preference, and ecological adaptations that help to explain success or failure under arctic environmental conditions? What is the approximate time frame in the diversification of clades comprising arctic species relative to the onset of arctic biomes?
Material and Methods
Sampling Strategy
Altogether 64 Ranunculus taxa from the arctic and south-erly bordering regions were selected, following the treatments of Tolmachev (1971) and Elven (2007) on the arctic flora. They were surveyed for sequence variation in the nuclear ITS (including the 5.8S rDNA gene) and in the chloroplast matK gene (including flanking regions of the trnK intron; table A1, available as an Excel data file in the online edition of the International Journal of Plant Sciences). This was intended to complement the data matrix generated for the same DNA markers by Hörandl et al. (2005) and Paun et al. (2005), which spanned most subgenera and sections of Ranunculus and its relatives acknowledged in the earlier systematic treatment of Tamura (1995). Trautvetteria was chosen as out-group (Hörandl et al. 2005; Paun et al. 2005). Our data set comprises 194 taxa.
Laboratory Methods and DNA Sequence Analysis
Total genomic DNA was isolated from herbarium specimens using DNA-binding columns (Macherey and Nagel, Düren, Germany). PCR amplification and sequencing reaction followed standard protocols (Tkach et al. 2008a), with primers listed in table 1. Editing of the sequences and alignments was done manually using Sequencher 4.6 (Genecodes Corporation, Ann Arbor, MI). The data sets (nuclear, chloroplast, and combined) were analyzed using PAUP* b10 (Swofford 2002) under maximum parsimony (200 bootstrap replicates, closest taxon addition, TBR, multrees on, 1000 maxtrees). The combined data were also analyzed by Bayesian inference with MrBayes, version 3.1.2. (Huelsenbeck and Ronquist 2006), with the following initial settings: nst = 6, rates = invgamma, ngen = 1,000,000, nchains = 6, nswaps = 2, samplefreq = 100. Tree building parameters were burn-in = 2500 and contype = half-compat. The branch lengths of the Bayesian tree were visualized using TreeView b1.6.6 (Page 1996).
Table 1. Primers Used in This Study.
| DNA region and primer name | Primer sequence 5′→3′ | Reference |
|---|---|---|
| matK gene–3′trnK exon: | ||
| trnK710f | GTATCGCACTATGTWTCATTTGA | Paun et al. 2005 |
| Ranu 44-F | AAAATGGARGAATTAMAAAGA | This study |
| Ranu460-F | CTACATTTCCMTTTTTAGAGGA | This study |
| Ranu548-R | GAGGCRTCTTGTATCCAA | This study |
| Ranu960-F | TTCTGATGAAGAAATGGAAA | This study |
| Ranu1070-R | TTKGTCGTACACYTGAAAGATAAC | This study |
| Ranu1450-F | RAAAAGRTKGGGTTCGGAATT | This study |
| Ranu1540-R | RATACRAATAATATCAAAATACCA | This study |
| trnK3r | GATTCGAACCCGGAACTAGTCGG | Paun et al. 2005 |
| ITS1-5.8S gene–ITS2: | ||
| ITS-A | GGAAGGAGAAGTCGTAACAAGG | Blattner 1999 |
| ITS-B | CTTTTCCTCCGCTTATTGATATG | Blattner 1999 |
| ITS-C | GCAATTCACACCAAGTATCGC | Blattner 1999 |
| ITS-D | CTCTCGGCAACGGATATCTCG | Blattner 1999 |
Time Estimates
The molecular clock analysis was performed using the penalized likelihood approach implemented in r8s, version 1.71 (Sanderson 2006), on the phylogram obtained from the Bayesian analysis on the combined data set. The calibration points used in this study (fig. 1) were 24 Myr for the clade of Ranunculus and 42 Myr for the split between Ranunculus, Ceratocephala, Myosurus, Coptidium, Ficaria, and the non-European genera of tribe Ranunculeae (Beckwithia, Callianthemoides, Peltocalathos, Halerpestes), taken from Paun et al. (2005). Additionally, confidence intervals using time estimates from 20 bootstrapped data sets were calculated according to the instructions of Sanderson (2006). The results of these molecular clock estimates were compared with those of two other methods, the clock-constrained likelihood tree score computed in PAUP* (Swofford 2002) for a GTR + G model on the topology derived from the Bayesian analysis and the uncorrelated lognormal model of a relaxed clock (Drummond et al. 2006) in BEAST 1.4.7 (Drummond and Rambaut 2007). For the latter we used the following settings: substitution model GTR, empirical base frequencies, gamma distribution of the site heterogeneity, and invariant sites with four rate categories; factory settings were used for other model parameters and priors. We ran 10,000,000 generations (burn-in = 10%) and saved every one-thousandth tree with fixing of the tree topology.
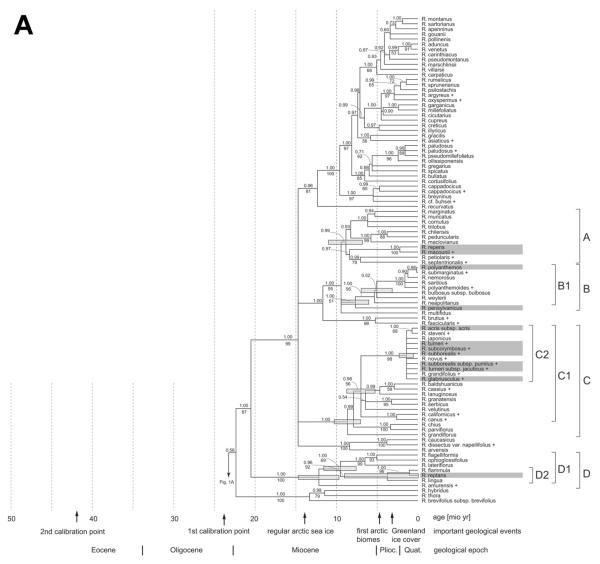
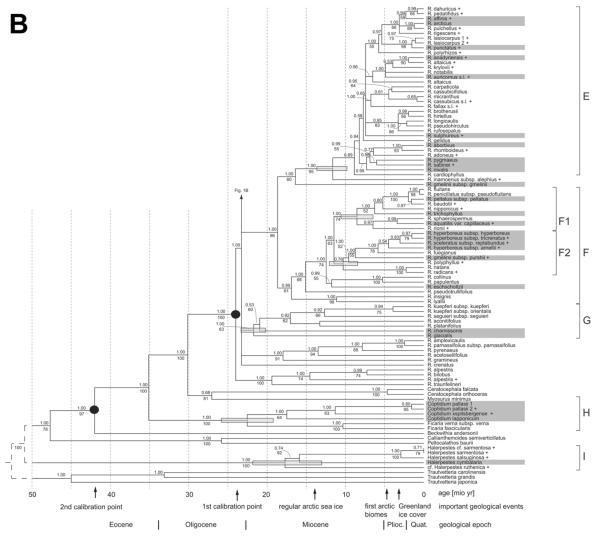
Fig. 1.
Bayesian molecular phylogenetic tree for Ranunculus and related genera of tribe Ranunculeae based on analysis of combined nuclear ITS and chloroplast matK-trnK intron data. Bayesian support >0.5 and bootstrap values >50% are above and below branches, respectively. Shaded bars on tree nodes are confidence intervals under penalized likelihood (r8s results) for the time estimates relative to the scale at the bottom. Filled circles denote the calibration points used. Names of taxa present in the Arctic are shaded, and newly sequenced taxa are marked with a plus sign.
Nonmolecular Data
Data on morphology, ecology, chromosome numbers, and distribution were collected from the systematic treatment of Tamura (1995) and current floristic literature covering most of the Northern Hemisphere, cited by Tkach et al. (2008c). Chromosome numbers were taken from Hörandl et al. (2005), the Index to Plant Chromosome Numbers database (http://mobot.mobot.org/W3T/Search/ipcn.html), and Elven (2007). In the absence of data from growth experiments under standard conditions or statistically supported average plant sizes, we used maximum plant height reported in the floristic literature for comparisons between arctic and nonarctic taxa in each phylogenetic lineage. We used t-tests, as implemented in SPSS (SPSS, Chicago), to check for differences in plant height and flower size.
The distribution of the species was mapped following the method of Tkach et al. (2008c). We recorded the presence and absence of the species in small areas, such as countries, states, or biogeographical regions used in floristic works of the Northern Hemisphere. Range sizes of the arctic Ranunculus taxa were assessed using the method of Hoffmann and Welk (1999). Briefly, total distribution range categories were estimated by comparisons of the species ranges with reference areas across the world, such as continents or countries. This compensates for differences of reports from the field. For range sizes the following categories were used: 8 => 10 million km2, 7 = 5–10 million km2, 6.7 = 1–5 million km2, and 5 = 0.1–1 million km2. This method is superior against calculations of range sizes by, e.g., geographical information systems, because distribution data of the species are sparse for some areas and heterogeneous among the species. The proportion of the Arctic relative to the total range of a taxon is given in categories of 1 =< 1%, 2 = 1%–20%, 3 = 20%–40%, 4 = 40%–60%, 5 = 60%–80%, and 6 = 80%–100%.
Results
The Arctic harbors 42–48 species or subspecies of Ranunculus (Tolmachev 1971; Elven 2007); 39 were included with our molecular phylogenetic analyses (shaded species in figs. 1, 2; table A1), also encompassing arctic representatives of the widely agamospermic species complexes of Ranunculus auricomus and R. monophyllos and, additionally, species of the genera Coptidium and Halerpestes. The aligned nuclear ITS sequence, which included the 5.8S rDNA, had a length of 641 nucleotide positions. Individual sequence lengths ranged from 596 to 602 bp. The aligned plastid DNA data set (matK gene and adjacent parts of trnK intron) had a length of 1981 nucleotide positions, with individual sequence lengths between 1781 and 1811 bp. The combined data set of nuclear and chloroplast sequences thus comprised 2622 positions.
Fig. 2.
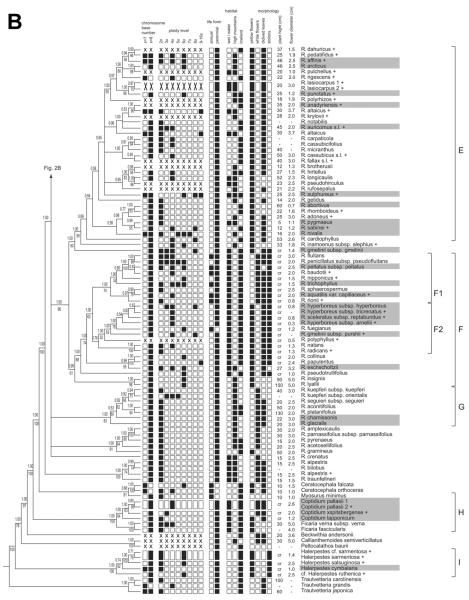
Mapping of characters obtained from the literature on the phylogenetic tree. Presence of a character state is indicated by a filled box. For habitat, “wet/water” indicates whether a species is almost confined to boggy places or is a water plant. “High mountains” and “lowland” show the species' centers of distribution. For morphology, if flower color was not yellow or white, the boxes are open. “Divided leaves” denotes divided or deeply lobed leaves (filled boxes) or entire leaves (open boxes). Maximum plant height and maximum flower diameter are listed in the last columns; “cr” refers to species that are creeping or floating and usually devoid of ascending shoots.
Parsimony analysis for the combined data set yielded 3234 most parsimonious trees, with a length of 3232 steps, a consistency index of 0.431, and a retention index of 0.875. Bayesian interference revealed a tree topology similar to that of the consensus tree with frequently high posterior probability support. Some branches with a low bootstrap values (<60%) received posterior probability >0.95.
We observed the same incongruities between the nuclear and the chloroplast data sets as discussed by Paun et al. (2005), which affected, among the arctic taxa, only R. gmelinii subsp. gmelinii and R. eschscholtzii. In the chloroplast and the combined data sets, R. gmelinii subsp. gmelinii was sister to clade E (fig. 1), though supported by only 60% bootstrap, whereas it was part of clade F2 in the nuclear data but again without support (not shown). The position of R. eschscholtzii switched from clade E, which had 97% bootstrap support in the nuclear data, to clade F2, supported by 88% bootstrap, which was unsupported in the combined data sets (fig. 1). Newly sequenced R. gmelinii subsp. purshii was placed by the nuclear data along with subsp. gmelinii in clade F2 and also kept this position in the chloroplast and combined sequence analyses, in contrast to subsp. gmelinii (fig. 1). The uncertain molecular phylogenetic position of R. gmelinii subsp. gmelinii and R. eschscholtzii led us to exclude them from further analyses of morphological, ecological, and biogeographic traits in arctic versus nonarctic lineages.
The partly low resolution of the phylogenetic tree precluded tracing character changes along the branches in detail and with confidence. Therefore, only supported clades with arctic and nonarctic taxa (fig. 1) were considered with respect to the morphological, karyological, ecological, and biogeographical characters scored for the entire taxon set (fig. 2). Arctic and nonarctic taxa within supported clades showed almost no significant differences in the scored characters (table 2), except for clade A, with a stoloniferous growth of R. repens and R. macounii, in comparison with an upright growth in the sister taxa.
Table 2. Summary of Characters Evaluated for Arctic Taxa of Tribe Ranunculeae in Comparison with Their Nonarctic Relatives.
| Clade | No. arctic taxa |
No. nonarctic taxa |
Consistent differences between arctic and nonarctic species |
Nonarctic sisters of arctic taxa confined to either forest or open places |
Altitudinal distribution of nonarctic sisters |
Arctic taxa polyploid |
Nonarctic sisters polyploid |
Total range size |
Proportion of arctic partial range |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Flower color |
Life forma |
Growth formb |
Stolons | Leaf formc |
Plant height (P) |
Flower diameter (P) |
|||||||||
| A | 2 | 9 | No | No d | No | Nod | No | Creeping | >.05 | No | Lowland | Yes | Yes | 7 | 1–2 |
| B | 2 | 7 | No | No | No | No | No | >.05 | >.05 | No | Lowland | No | No | 7 | 1 |
| B1 | 1 | 7 | No | No | No | No | No | >.05 | >.05 | No | Lowland | No | No | 7 | 1 |
| C1 | 7 | 12 | No | No | No | No | No | .007e | >.05 | No | Lowland, rarely alpine |
Yes | Yes | 6–8 | 1–6 |
| C2 | 7 | 4 | No | No | No | No | No | .045 | >.05 | No | Lowland, rarely alpine |
Yes | Yes | 6–8 | 1–6 |
| C3 | 7 | 15 | No | No | No | No | No | .025 | >.05 | No | Lowland, rarely alpine |
Yes | Yes | 6–8 | 1–6 |
| D | 1 | 6 | No | Yes/no f | No | No | No | Creeping | >.05 | No | Lowland | Yes | Yes | 7–8 | 1–2 |
| D2 | 1 | 2 | No | No | Yes | No | No | Creeping | Smaller in arctic species g | No | Lowland | Yes | Yes | 7–8 | 1–2 |
| E | 11 | 22 | No | No | No | Noh | No | >.05 | >.05 | No | Lowland to alpine |
Yes | Yes | 6.7–8 | 1–6 |
| F1 | 3 | 6 | No | No | No | No | No | >.05 | No | Lowland | Yes | Yes | 7–8 | 1–2 | |
| F2 | 5 | 4 | No | No | No | No | No | >.05 | No | Lowland | Yes | Yes | 6–8 | 2–6 | |
| G | 2 | 5 | No | No | No | No | No | >.05 | >.05 | No | Alpine or lowland |
No | No | 6–6.7 | 4, 6 |
| H | 3 | 2 | Yes/no | No | Yes | No? | No | Creeping | >.05 | No | Lowland | Yes | Yes | 6–8 | 3, 6 |
Annual or perennial.
Upright, creeping, or flooding/submersed.
Leaf blades either entire or divided into leaflets.
South American species variable; annual but some perennial with or without stolons.
Lower plant height in arctic taxa.
South American species annual but some perennial with stolons.
Too few samples for statistical tests.
One arctic species with stolons.
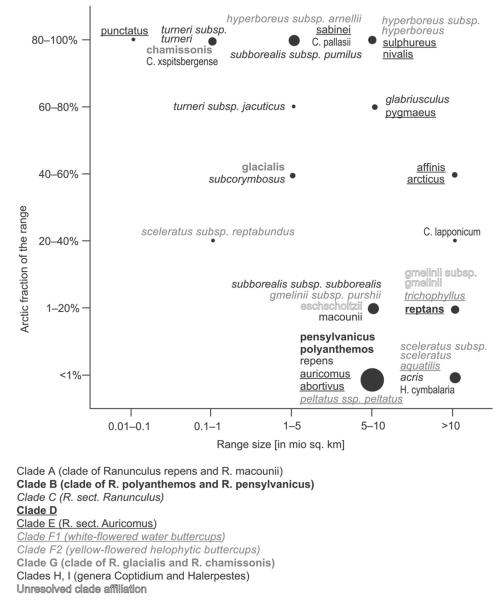
Range sizes and the percentage of the Arctic relative to the total range are given for the arctic taxa in figure 3. Many arctic Ranunculus species have a large distribution range, and only a small fraction of this range is situated in the Arctic (e.g., R. acris, R. repens). Some taxa are confined to or are concentrated in the Arctic (R. hyperboreus in clade F2, R. chamissonis in clade G, and Coptidium pallasii in clade H), but most of them belong to the taxonomically critical groups of clade C2 (R. acris species group: R. subborealis subsp. pumilus, R. turneri, R. glabriusculus)and clade E (R. sect. Auricomus: R. nivalis, R. punctatus, R. pygmaeus, R. sabinei, R. sulphureus, R. pygmaeus).
Fig. 3.
Total range sizes and proportions of their arctic partial ranges in taxa of Ranunculus, Coptidium, and Halerpestes represented in the flora of the Arctic, according to the size categories defined in “Material and Methods.” Circle sizes denote the number of taxa belonging to each combination of total range size/arctic partial range. Taxa with small entire ranges confined to the Arctic are shown in the top left-hand corner, and widespread circumpolar taxa with occurrence only at the margins of the Arctic are shown in the bottom right-hand corner. Taxa names are arranged close to their respective circles.
The three methods used for the calculation of the molecular clocks yielded largely similar age estimates. Age estimates of the crown group diversification of supported clades comprising the arctic Rancunculeae lineages are summarized in figure 1. The estimates ranged from ~22.5 Myr (clade H; Coptidium/Ficaria) to ~1.4 Myr (clade C; part of R. sect. Ranunculus). Confidence intervals ranged from 1.7 to 6.6 Myr. Most of the clades comprising arctic taxa emerged gradually during the evolution of the genus; most of them diversified between 11 and 6 Myr, i.e., before the onset of the arctic biome. The arctic taxa are usually younger than the whole clades they are nested in, except for clade G, with R. glacialis/R. chamissonis. Most of them are younger than 5 Myr, and diversification is of quite recent origin, e.g., in the R. acris species group (1.4 Myr; clade C2).
Discussion
Our extended sampling of Ranunculus, with 64 newly added taxa, yielded essentially a tree topology (fig. 1) similar to that reported by Hörandl et al. (2005) and Paun et al. (2005), who discussed it comprehensively in relation to the previous section classification of Tamura (1995). Therefore, in the following we focus on the clades comprising the arctic taxa of Ranunculus, which were sampled in depth (39 out of the 42–48 arctic taxa, 20 of which were sequenced for the first time), and the related genera Coptidium and Halerpestes, with several newly sampled taxa.
Lineages of Tribe Ranunculeae in the Arctic
Clade A (Ranunculus repens and R. macounii). This clade and clade B comprise a mixture of species treated previously under R. sectt. Acris and Echinella (Tamura 1995). Ranunculus repens and R. macounii are not typical arctic elements and appear to grow only in the low Arctic. They are distributed mainly in temperate regions and extend southward to the meridional zone. Ranunculus repens is native to Eurasia and is introduced in North America; R. macounii is widespread in North America (Whittemore 1997). These two species differ by stoloniferous growth and by vegetative propagation from nine further consistently nonarctic species of clade A (fig. 1; table 2). Stoloniferous habit with runners rooting at the nodes seemingly corresponds to the colonizing ability of R. repens and R. macounii on wet soils, and the plants are found in such habitats over a wide latitudinal range but only up to the low Arctic. Most species of clade A are polyploid. The estimated age of their split from the common ancestor of clade A dates back to about 8 Myr and precedes the formation of the arctic biome. The distribution of clade A contrasts widely with that of the following R. polyanthemos clade (clade B) because the clade A species are almost absent from eastern Eurasia and the austral Pacific area but occur instead in western Eurasia and North and South America.
Clade B (R. polyanthemos and R. pensylvanicus). The Eurasian R. polyanthemos is reported from the Russian Arctic on the basis of a couple of possibly synanthropic occurrences (Tolmachev 1971). Its main distribution lies in woodlands of lowlands in the temperate zone (fig. 2). A similar situation is encountered in R. pensylvanicus, which is widespread in North America in wet places of open and woodland areas and enters the Arctic only marginally in Alaska (Elven 2007; fig. 3). According to nuclear ITS data (Hörandl et al. 2005), R. pensylvanicus is of special biogeographical interest because it is sister to a large, mainly southern hemispheric clade (not included in this study) of mostly alpine species growing in open, wet places of the mountains of Southeast Asia, Australia, and New Zealand (Hörandl et al. 2005). This clade comprises an additional six East Asian species that seem to resemble R. pensylvanicus ecologically because they grow on wet grounds and mainly in forested areas. Biogeographically, the entire clade B, including the species studied by Hörandl et al. (2005), shows, first, an East Asian–North American disjunctive distribution that is also found multiple times in lineages of Ranunculus not protruding into the Arctic (Emadzadeh et al. 2008) and in the related genus Trautvetteria (Xiang et al. 1998). Second, the clade has an extremely wide and quite continuous distribution from North America to the Southern Hemisphere. In the morphological or karyological characters scored, the two species of clade B reaching the Arctic do not differ from their nonarctic sister taxa. Ranunculus pensylvanicus is probably the only arctic species of clades A and B that grows as a facultative annual; it also has the smallest flowers (fig. 2; table 2). Morphologically, it shares with some East Asian species branched stems bearing some cauline leaves. The southern hemispheric taxa have variable habit; e.g., scapose, creeping, and cushion plants occur in the Malesian mountains (Eichler 1958). The species of clade B, including the arctic taxa, are diploids, with only a few exceptions.
Clade C (R. sect. Ranunculus). This clade comprises most taxa of Tamura's (1995) R. sect. Acris, which must be named sect. Ranunculus for nomenclatural reasons (Hörandl et al. 2005). Clade C encompasses seven arctic taxa that belong to the molecularly well-supported but largely unresolved species group of R. acris (clade C2), whose taxonomy is still incompletely studied. Its taxa appearing in figure 1 are either considered separate species or treated as subspecies, sometimes only varieties of R. acris (Coles 1971). They differ slightly in size and characters of the indumentum and sometimes also in the division of the leaves, the length of the fruit beak, and the length of the rhizome, but they all appear to be devoid of stolons (table 2). There are mainly polyploids in the Arctic, but R. acris and R. subborealis comprise diploid and polyploid populations. Taxa occurring in nonarctic and arctic regions have, on average, smaller maximum plant height than do exclusively nonarctic taxa (table 2). This result was obtained, however, by recognizing all taxa of clade C2 as separate. If taxa represented in the Arctic would become amalgamated by future taxonomic treatments under more widespread species of clade C2, this difference is likely to disappear.
The low tree resolution within the R. acris species group (clade C2) also impacts the estimates of diversity of the arctic flora. Some of the species earlier recognized by Ovczinnikov (1937) and Tolmachev (1971) and considered endemics or subendemics of the Eurasian arctic regions (fig. 3) have already been amalgamated by Elven (2007). The molecular phylogenetic results on this species group obtained in this study seem to support this. However, a concise treatment of this group, including all of its taxa and using more sensitive molecular markers, is clearly needed.
The species of the whole clade C predominantly have the chromosome base number of x=7 and comprise diploids as well as polyploids, whereas most other taxa of Ranunculus have x=8 (Hörandl et al. 2005). The sister group relationships of the R. acris species group (clade C2) within clade C are not firmly established because it stands in a polytomy with other statistically supported lineages of morphologically similar American and southern Eurasian species distributed in the mountains of Europe to the Himalayas (clade C1). The species of clade C are morphologically highly diverse and also comprise annuals of the Mediterranean (R. chius, R. parviflorus). In summary, no morphological or ecological character is obvious in clade C that could be related to the occupation of the northernmost areas.
The age of clade C has been estimated as 2 Myr (Paun et al. 2005), an age obtained in this study for the diversification within the R. acris species group of clade C2 (1.4 Myr), whereas clades C1 (7.6 Myr) and C (8.5 Myr) are considerably older (fig. 1). The rather recent origin of clade C2 correlates with the weak morphological differences observed between its “microtaxa” and their eventually incomplete speciation processes.
Clade D. This clade comprises species of R. sect. Flammula (R. flammula, R. reptans, R. lingua, R. amurensis), which is not revealed as monophyletic (fig. 1; Hörandl et al. 2005), and, additionally, the Eurasian species R. lateriflorus (monotypic R. sect. Micranthus) and a well-supported clade of South American annuals of R. sect. Casalea (Hörandl et al. 2005), represented by R. flagelliformis in figure 1. The polyploid and widespread R. reptans, growing in moist to wet places, enters the Arctic frequently but is restricted to its southern margins. The range of R. flammula also extends far north but not into the Arctic. The species of clade D have variable, creeping to erect habit, are rooting at the nodes, and possess entire leaves. Sister to the creeping species pair in clade D2 is the western Eurasian R. lingua, which is nonstoloniferous, grows upright, and has larger flowers and a considerably higher chromosome number (fig. 2; table 2).
Presence of stolons is a character in clade D linked with occurrence in the Arctic, as similarly found in clade A (R. repens, R. macounii). Interestingly, stoloniferous habit is found also in the South American species R. flagelliformis of clade D1 (fig. 2; table 2), most likely reflecting long-distance dispersal of its ancestor from the Northern Hemisphere to the Southern Hemisphere, as likewise found in clade F2.
Clade E (R. sect. Auricomus). This clade is equivalent to Tamura's (1995) sect. Ranunculus, a name not applicable to clade E for nomenclatural reasons (Hörandl et al. 2005; see clade A). The whole clade is well supported and comprises, apart from sexually reproducing species (e.g., the grade of R. abortivus to R. cardiophyllus in figs. 1, 2), the R. auricomus species complex, which is prevailingly agamospermic. This complex is a taxonomically difficult group of >700 agamospermic microtaxa and few species with sexual reproduction (Ericsson 1992; Hörandl and Paun 2007). Hybrid origin and facultative recombination have resulted in a huge number of morphotypes in this complex (Hörandl 1998; Ericsson 2001) and contribute to a collapse of branches within clade E (fig. 1), due to conflicting phylogenetic signals caused by reticulate evolution and allopolyploidy (Hörandl et al. 2005). Three representatives of the main morphotypes used to subdivide the R. auricomus complex (R. auricomus s.l., R. cassubicus s.l., R. fallax s.l.) have been included in this study. No sexual taxon of the R. auricomus complex, whose angamospermic taxa probably originated from sexual species of temperate central Europe (R. notabilis, R. cassubicifolius, and R. carpaticola; Hörandl and Paun 2007), is known for the Arctic.
The weakly supported sister groups of the R. auricomus complex, i.e., the grade of R. abortivus to R. cardiophyllus in figures 1 and 2, comprise species with sexual reproduction, four of which occur in the Arctic (R. abortivus, R. pygmaeus, R. sabinei, R. nivalis). Most species of this grade are distributed in North America, but the arctic species are also present in Eurasia.
Arctic and nonarctic taxa of clade E do not significantly differ in any of the morphological and karyological characters scored (fig. 2; table 2). Most of the species are polyploid. Exclusively diploid taxa are rare but were reported for both arctic and nonarctic taxa. Some of the arctic species are nearly confined to the northernmost areas and could actually have originated in the Arctic (e.g., R. sabinei, R. sulphureus; fig. 3). Others have large ranges outside the Arctic (e.g., R. abortivus), and their place of origin is less clear because they could have reached the Arctic secondarily. Some taxa of this group, such as R. sulphureus and R. nivalis, are characteristic species of the Arctic (Tolmachev 1971). In terms of their ecological preferences, the taxa of clade E present in the Arctic seemingly do not differ from the nonarctic sister species (table 2). The species of clade E have an overall rather wide ecological amplitude, with species growing in forested areas as well as open habitats, from lowlands to alpine zones, usually under moist to wet soil conditions. Clade E is comparable in age to clades A and D, but its poorly structured and supported internal resolution currently precludes firm estimates on the age of its different lineages. Many of its taxa are probably of quite recent origin.
Clade F. This clade comprises mainly the white-flowered aquatic species of Ranunculus traditionally treated as a separate subgenus, R. subg. Batrachium (clade F1), and the yellow-flowered, helophytic species of R. sect. Xanthobatrachium and R. sect. Hecatonia (clade F2) as part of traditional R. subg. Ranunculus. The sectt. Xanthobatrachium and Hecatonia comprising the widespread R. sceleratus have been differently delineated (Tutin et al. 1993; Tamura 1995; Hörandl, forthcoming). According to nuclear ITS data (Hörandl et al. 2005), R. sceleratus subsp. sceleratus belongs to our clade F2. The same position of subsp. reptabundus, previously mostly considered a separate species and newly sampled here, corroborates the affiliation of R. sceleratus with this clade.
Clade F shows an enormous variation in shape and dissection of the leaves, including heterophylly with the production of entirely submerged leaves that deviate strongly in morphology from aerial or floating leaves by fine dissection serving for nutrition uptake from the water, otherwise a typical root function. None of the leaf characters reflect a special adaptation of certain lineages or taxa to arctic conditions (table 2); instead they are responses to aquatic or amphibious habitats. Except for upright-growing R. sceleratus, the plants are floating in lakes, ponds, or slowly running rivers or are creeping in swamps and the margins of lakes or rivers. Ranunculus sceleratus subsp. reptabundus can grow erect but is also creeping, with rooting runners. Most species of clade F have large ranges, a typical feature of aquatic or helophytic plants, and large parts of their ranges are situated outside the Arctic (fig. 3). Only the polymorphic R. hyperboreus is a near-endemic species of the arctic flora and shows signs of morphological differentiation in this region.
Species floating in water, typical of clade F1 (R. subg. Batrachium) but also occurring in clade F2 (R. sectt. Xanthobatrachium and Hecatonia), have a mainly Eurasian distribution, with the exception of some widespread taxa (R. aquatilis, R. trichophyllus). The creeping taxa of clade F2 are not centered in Eurasia. Ranunculus fuegianus from Patagonia is nested within an otherwise arctic clade (F2). It originated most likely after long-distance dispersal similarly encountered in clade D1. Ranunculus fuegianus occupies sites ecologically similar to those of its northern hemispheric sister taxa. The diversification of these two clades (F1 and F2) may have started before the appearance of the arctic biome. The species currently growing in the Arctic may have evolved early and occupied arctic areas upon their appearance because of their wide ecological amplitude.
Species of the sister groups to clades F1 and F2 are consistently southern hemispheric species such as R. lyalli to R. collinus, except for R. eschscholtzii, whose placement within clade F is uncertain. Many of them have yellow flowers and a creeping habit and occur in wet or boggy places. A well-supported and early-diverging lineage represented by R. insignis and R. lyallii in figure 1 consists of alpine species of New Zealand with a scapose growth form and predominantly large yellow or white flowers (R. sect. Pseudadonis; Tamura 1995). This species-rich lineage of seemingly polyploid species (Fisher 1965) was amply sampled for nuclear ITS by Hörandl et al. (2005) and represents a remarkable example of evolutionary radiation under geographical isolation and absence of competing taxa in New Zealand (Lockhart et al. 2001).
Clade G (R. glacialis and R. chamissonis). This small clade is geographically centered in the mountains of Europe. Ranunculus glacialis is additionally widespread in the Arctic and R. chamissonis even confined to it (Chukotka to Alaska). Both species were sometimes treated under genus Beckwithia (Elven 2007) or were assembled together with R. juniperinus and R. andersonii from the high mountains of North America under R. subg. Crymodes (Tamura 1995). They appear as part of Ranunculus with high support in the molecular phylogenetic data (fig. 1). This species pair assembles in clade G together with all three species recognized for R. sect. Aconitifolii (from R. subg. Ranunculus) and, additionally, R. kuepferi (fig. 1; Hörandl et al. 2005; Paun et al. 2005), though without good support. The latter are confined to the European mountains. All taxa of clade G are white flowered, produce no stolons, and have, with the exception of the European alpine R. kuepferi, divided leaves. They are sexual diploids, except for the polyploid and apomictic R. kuepferi subsp. orientalis (Huber 1988; Hörandl et al. 2008). Ranunculus glacialis and R. chamissonis form the earliest-diverging branches from the remainder of clade G but are apparently not monophyletic (fig. 1).
Ranunculus glacialis ranks among the three vascular plants reaching altitudes above 4200 m in the Alps (Hess 2001). It is a characteristic species of the alpine to nival altitudinal zones of the Alps and has been shown to be ecophysiologically narrowly adapted to low temperatures and light stress. The species is incapable of acclimatizing to increased temperatures, as corroborated in the field by transplantation experiments conducted within the Alps that demonstrated R. glacialis to fail at midaltitudes (Larcher et al. 1998; Totland and Alatalo 2002; Streb et al. 2003, 2005; Cooper 2004). Ranunculus glacialis even shows complicated patterns in the production of renewal buds, whose development may be retarded or suppressed, depending on whether the individual plant becomes free of snow cover during the favorable summer season or remains buried (Hess 2001). Ranunculus glacialis thus is an outstanding example within Ranunculus, owing to its experimentally proven narrow ecological adaptation and specialization to climatic conditions of arctic/high-alpine biomes. It would be interesting to know whether the related R. chamissonis or other arctic species of Ranunculus share ecophysiological or even growth rhythm patterns with R. glacialis; however, this has not been studied to date.
Clade G is biogeographically and ecologically rather narrowly defined by its restriction to the mountains of the temperate zone and the Arctic. It represents one of the earliest-diverging phylogenetic lineages in Ranunculus (fig. 1), which basically agrees with the results of Paun et al. (2005), but time estimates diverge. According to penalized likelihood calculation in this study, clade G dates back to the early Miocene (~22 Myr; fig. 1), whereas the uncorrelated lognormal model of relaxed clock yielded confidence intervals too large for accurate dating. Despite such uncertainty, it seems safe to conclude that clade G and its early-diverging R. glacialis/R. chamissonis grade or their ancestors originated long before the arctic ecosystem appeared.
Clade H (Coptidium and Ficaria). Coptidium is corroborated by the extended sampling in this study as a genus separate from Ranunculus, thus supporting the conclusions of Paun et al. (2005) and Lehnebach et al. (2007). Coptidium, with all of its species either protruding into or confined to the Arctic, is an old lineage that segregated from the southerly distributed sister genus Ficaria in the Miocene (fig. 1). Remnants of Coptidium plants have been reported from most fossil deposits of arctic floras (see “Introduction”), which means that Coptidium belonged to the arctic flora from its very beginning. Despite the age of Coptidium, its evolutionary diversification is low in comparison with that of Ranunculus when assuming an equivalent rate of extinction in both genera.
Tetraploid Coptidium pallasii is a species typical of the Arctic, with a comparatively wide range, but it hardly reaches the high Arctic, is rare in the southerly forest tundra (Tolmachev 1971), and is nearly absent outside of the Arctic. The diploid Coptidium lapponicum occurs in the southern Arctic and is also widespread in the boreal zone. Their triploid, seed-sterile hybrid C. × spitsbergense is morphologically intermediate (Elven and Murray 2008) and occupies a small range in the Eurasian Arctic, with scattered occurrences from Svalbard to Chukotka. The species of Coptidium are creeping in boggy places and thus are much different from the sister genus Ficaria distributed in temperate to meridional forest and grassland habitats. The strong adaptation to wetland is also seen in the presence of spongy tissue in the nutlets of Coptidium, which is unique within the tribe Ranunculeae and seems to reflect strong adaptation to water dispersal.
Clade I (Halerpestes). Judging from our molecular phylogenetic results on a broad sampling of Halerpestes species, this genus is likely monophyletic (fig. 1) and even more distant to Ranunculus than Coptidium, a position already suggested by Hörandl et al. (2005) and Paun et al. (2005), including only Halerpestes cymbalaria. Halerpestes comprises only a single species present in the Arctic, i.e., North American H. cymbalaria. Like its central Asian, prevailingly salinity-adapted congeners, H. cymbalaria is creeping and produces long stolons. Although taxonomy and species delineations are insufficiently studied in this genus, arctic H. cymbalaria appears sister to most of the entirely nonarctic remainder of the genus, except for Halerpestes ruthenica. The split in Halerpestes between arctic H. cymbalaria and the nonarctic remainder of its genus dates back to the Miocene and thus ranks among the earliest phylogenetic splits of this kind. It is comparable in age to the split between Coptidium and Ficaria (clade H) and that between R. glacialis/R. chamissonis and the remainder of clade G.
Phylogenetic Descent of Arctic Species in Tribe Ranunculeae
The molecular phylogenetic analysis reveals all taxa of Ranunculus with occurrences in the Arctic nested within otherwise nonarctic clades (fig. 1). The species of Ranunculus in the Arctic stem from at least seven different lineages. Indications of evolutionary radiation within the Arctic are confined to clade C2, with R. acris and its barely separable “microspecies,” and clade E, with R. sect. Auricomus, including a swarm of mostly agamospermic apomicts. Considering morphologically clear-cut sexual taxa in Ranunculus, R. hyperboreus has a distribution nearly confined to the Arctic but is part of the otherwise largely nonarctic clade F. Beringian R. chamissonis is the second example because it seems well delineated against R. glacialis (Elven 2007) but is nested similarly in a large lineage of prevailingly southern, nonarctic taxa (clade G). In the phylogeny, R. chamissonis represents an early segregate together with R. glacialis, much in contrast to R. hyperboreus, which is part of the terminal branches in clade F and clearly originated later. The small genus Halerpestes also contains predominantly nonarctic species; only H. cymbalaria reaches the Arctic in North America. In contrast to Ranunculus and Halerpestes, the genus Coptidium has a consistently northern center of distribution in the boreal to arctic zones and appears to be the only lineage of tribe Ranunculeae with a clear center in the North.
Time Frame and Southern Ancestors of Arctic Taxa
Occupation of arctic habitats occurred repeatedly not only in different phylogenetic lineages within Ranunculus and the related genera Coptidium and Halerpestes but also at different times. The earliest lineages of Ranunculus and its allies, currently represented in the Arctic, originated in the early to mid-Miocene (Halerpestes, Coptidium, R. glacialis/R. chamissonis; clades G–I in fig. 1). In most other clades the crown group age dates back to the late Miocene and is older than 5 Myr (clades A–C, C1, D–F), meaning that diversification and speciation of these clades started contemporaneously with a generally increased cooling of the climate but before the typical arctic biomes established (fig. 1). The only exception is the R. acris group (clade C2), most likely of Quaternary diversification (~1.4 Myr). Several lines of evidence suggest that some arctic representatives of Ranunculus/Coptidium/Halerpestes descended from ancestors with a southerly distribution. Other species of the Arctic are immigrants from southern areas also able to grow under arctic conditions.
The first applies in genus Ranunculus to R. hyperboreus and R. chamissonis but apparently also to clade C2, with signs of richer in situ evolution of arctic species. We suspect, however, that the latter is only an artifact caused by current classification with too strong of a splitting of “species” in R. acris and its close relatives. In clade E (R. sect. Auricomus), with a somewhat lower proportion of species in the Arctic than is the case in clade C2, a nonarctic origin of this group seems likely, but it is not proven. Firm progenitor-derivative relationships between arctic and nonarctic taxa cannot be identified in clade E on the base of our molecular phylogenetic data (see above for agamospermy, hybridization, allopolyploidy, and reticulate evolution in this clade; Hörandl 1998, 2006, 2008; Ericsson 2001; Hörandl and Paun 2007). Descent of arctic taxa from ancestors with southerly distributions is astonishingly clear at the genus level, considering Coptidium as sister to Ficaria and their firm nesting within nonarctic lineages (fig. 1). Coptidium shows signs of speciation even in the North. The second pattern, i.e., spatial expansion of southern species into arctic biomes, is much more common than phylogenetic radiation in the Arctic and accounts for more than three-fourths of the arctic species inventory in Ranunculus and Halerpestes.
Survival in the Arctic without Special Ecological and Lifestyle Features?
Morphological and ecological characters scored show no statistically significant differences between taxa occurring either within or exclusively outside the Arctic, except for clades A and D2 (table 2; fig. 2). Occupation of the northernmost ecosystem by the different lineages of Ranunculus/Coptidium/Halerpestes is seemingly not linked with typical or recurrent changes in life forms, ecological characters, habitat occupancy, and preferences for moisture or light. As a frequent pattern, the arctic species share adaptations to wet or aquatic habitats. Their nutlets usually do not sink in water and are frequently dispersed exo- or endozoochorously by birds (Müller-Schneider 1986). Zoochorous and hydrochorous long-distance dispersal of diaspores to and within the Arctic is thus likely, as reported for other species (Alsos et al. 2007). Fruits are frequently highly sensitive to desiccation, as shown for species of R. sect. Auricomus, and germinate best if transferred to wet soils immediately after maturity (Hörandl 2008). The arctic biome, with moist soils and partly wide wetlands arising each summer after melting of the snow, thus seems to allow for successful colonization by such plants. Special adaptations to low temperatures in physiology, and even growth rhythm known from R. glacialis, are seemingly not studied in other arctic species of Ranunculus. However, they are not unlikely and need to be studied in the future.
Chromosome numbers do not differ consistently between arctic species and their nonarctic relatives in Ranunculus. If arctic species are polyploid, the nonarctic species of the same clade are likewise polyploid. Altogether, only two clades of Ranunculus comprise diploid taxa in the Arctic (clades B, G). In clade B, R. pensylvanicus and R. polyanthemos are not widespread in the Arctic but just reach its southern margin. Only clade G, with R. glacialis and R. chamissonis, is consistently diploid, except for the European alpine R. kuepferi subsp. orientalis, which is polyploid and agamospermic. Considering that many southerly species of tribe Ranunculeae are diploids, the prevalence of polyploid taxa of Ranunculus in the Arctic would seem to support the general observation that polyploid taxa frequently accumulated or originated in the Arctic (Hagerup 1932; Johnson and Packer 1965; reviewed in Brochmann et al. 2004). The phylogenetic context, however, reveals that polyploidy or diploidy in the arctic Ranunculeae taxa is strongly influenced by descent because polyploid or diploid arctic species have polyploid or diploid closest relatives, respectively, further south. Thus, neither was Polyploidy a prerequisite for successful colonization of arctic environments nor was diploidy an obstacle. It should be rewarding to test for this phylogenetic component by estimating polyploid/diploid ratios separately for different evolutionary lineages within other important genera of the Arctic.
Ranunculus Phylogenetic Lineages Show Only a Few Biogeographic Relations between the Arctic and Southern High Mountains
It is a long-standing issue that the arctic flora is at family, genus, and, sometimes, even species level highly similar to the flora of the southern high mountains of Eurasia and North America. This has led to the suggestion that the arctic flora was largely recruited from the old high-mountain floras of northern and central Asia, Europe, and North America (Hultén 1937, 1958; Tolmachev 1960; Weber 1965; Hedberg 1992). However, this hypothesis has rarely been tested using a molecular phylogenetic approach (Tkach et al. 2008a). The only example of firm connections at the species level between the Arctic and the southerly high mountains as a place of origin in Ranunculus is that of R. glacialis in clade G (fig. 1). On the basis of amplified fragment length polymorphism data, the present arctic distribution of R. glacialis is considered to rest on rapid postglacial recolonization of the Arctic, starting from the Alps (Schönswetter et al. 2003).
The nonarctic species of clade G are confined largely to the mountains of Europe, but there are also two species growing mainly in the forest zones of temperate Europe, i.e., R. aconitifolius and R. platanifolius. Age estimates suggested a diversification of this clade in the late Miocene/early Pliocene (Paun et al. 2005); here we estimate a crown group age around the early Miocene. Starting from the Tertiary ancestors, this clade shows an interesting ecological radiation in mostly mountainous habitats that geologically emerged during the Tertiary in western Eurasia, as seen in the adaptation of the recent species to quite different habitats and climatic conditions: R. platanifolius and R. aconitifolius are adapted to broad-leaved tall herb and riverside vegetation in the montane and subalpine zones, and R. seguieri and R. kuepferi are alpine species, the former growing in screes, the latter in grassland vegetation. Ranunculus glacialis/R. chamissonis became specialized to open pioneer habitats such as screes and glacier moraines in arctic/high-alpine biomes with extreme cold climates, short vegetation periods, and high light stress.
Further examples in Ranunculus, considered by Tolmachev (1960), in which southern high mountains acted as a “place of birth” of species present in the arctic flora were R. pygmaeus (Arctic and European high mountains), R. nivalis (only arctic Europe), and R. sabinei (Eurasian and American Arctic). These species, though morphologically comparatively well delineated, are nested within clade E, poorly resolved by molecular phylogenetics, and their respective sister taxa are uncertain (fig. 1). This clade encompasses further species from mountains or lowlands of Eurasia and, additionally, many North American lowland species (Emadzadeh et al. 2008) not sampled in this study. The place of birth of arctic R. pygmaeus, R. nivalis, and R. sabinei thus is unclear, and their suggested evolutionary origin in southern high mountains is uncertain. Tolmachev's (1960) hypothesis could be substantiated for only clade E if the actual sister species of R. pygmaeus, R. nivalis, and R. sabinei (1) can be identified reliably in future studies and (2) have their center of distribution in the mountain systems of the alpine orogenesis, equivalent to the situation in clade G.
Numerous clades in Ranunculus encompassing almost exclusively southern high–mountain species notably have not acted as a source of the arctic flora. Examples are the clades comprising R. hybridus/R. thora/R. brevifolius and R. alpestris/R. bilobus/R. traunfellneri and the clade from R. amplexicaulis to R. gramineus (in fig. 1, situated between clades D and E and between clades G and H, respectively).
Main Sources of the Arctic Ranunculaceae Flora
The most important constituents of Ranunculus in the arctic flora are wetland species (clades D, F1, F2). Wetland species in general were already considered by Tolmachev (1960) as significant in the taxon recruitment of the Arctic. Plants specialized to the salty plains of the coasts were a further principal source of the arctic flora discussed by Tolmachev (1960). This pattern is found not in Ranunculus, because only a few of its species occur on saline soils, but in Halerpestes, with the nonarctic species of this genus occurring at saline places only in the continental Asian interior instead of coastal regions.
The majority of arctic species in Ranunculus come from the large and species-rich clades C2 and E, which contain some nearly endemic arctic taxa, e.g., R. turneri, R. sulphureus, or R. sabinei, but the delineation of taxa is unsettled in parts of both clades. In clade C2 (R. acris species group), the acknowledged species and subspecies could be summarized under a single morphologically variable and widespread species (Elven 2007) with morphologically slightly different populations, eventually indicative of speciation at its infancy, as discussed for the Saxifraga rivularis species complex (Jørgensen et al. 2006). In clade E (R. sect. Auricomus) many species originate from reticulate evolution, including recurrent formation of new hybrids able to persist and spread via facultative agamospermy, thus enhancing the capability of such taxa to colonize devastated areas because of better founder abilities (Hörandl 2006; Hörandl and Paun 2007). The high diversity of this clade in previously glaciated areas within and outside of the Arctic points to postglacial colonization.
In summary, the only examples of more or less clear-cut taxa with likely in situ origin within the Arctic are R. chamissonis (clade G) and, by including the bordering boreal zone, R. hyperboreus (clade F2). The prevalent pattern of taxon recruitment by the arctic flora in Ranunculus seems to be immigration of otherwise southerly distributed species without significant changes in the ecological characters of the species. The clades comprising arctic taxa show a preference for wet or aquatic habitats. Plants with such adaptations from other plant families (e.g., in Cyperaceae, Poaceae) also tend to have wide distributions as part of the so-called azonal vegetation and are able to survive under climatically quite different conditions, including the arctic environment (Walter and Breckle 1991).
The Arctic Contains Genera with Different Rates of Immigration and Speciation
Immigration into the Arctic is typical of many other genera, e.g., Vaccinium, Empetrum, and Betula, that have a center of distribution in boreal forests but extend into the Arctic, where they become important and landscape-dominating plants. Other genera of the Arctic, such as Artemisia, have the origin of their arctic taxa predominantly in temperate or cold steppes and have colonized the arctic areas mainly from there (Tkach et al. 2008a, 2008b). In Artemisia, however, a greater proportion of species is nearly or completely restricted to the Arctic than in Ranunculus and has evolved most likely in situ. Other genera seem to diversify much more rapidly in the Arctic than Ranunculus to form clearly defined endemic or subendemic species (e.g., Draba, Primula; Grundt et al. 2006; Guggisberg et al. 2006; see also Hoffmann and Röser 2009). The frequent adaptation to wetland/aquatic habitats in Ranunculus and the almost ubiquitous availability of such habitats in the Arctic, resulting in the absence of efficient dispersal barriers, could have acted against or slowed speciation processes. The genus otherwise shows many examples of rapid and radiative speciation, e.g., in the Mediterranean, on oceanic islands, and in several high-mountain systems, including a striking radiation in the New Zealand Alps (Lockhart et al. 2001; Hörandl et al. 2005; Paun et al. 2005). In all of these radiations, geographical isolation, together with efficient dispersal barriers, has played a major role.
In comparison, the phylogenies of Artemisia and Ranunculus, two important and similarly species-rich genera in the arctic flora (33 and 42–48 species, respectively), show several differences in the origin and evolution of their arctic taxa. The oldest lineages of both genera evolved in the Miocene; however, some of the arctic taxa of Ranunculus (clade G: R. glacialis/R. chamissonis; clade H: Coptidium) may be older than those of Artemisia (A. androsace/A. senjavinensis/A. glomerata clade: ~10.8 Myr). Clades of Artemisia seem to have split up in the Arctic more strongly into different taxa than have the clades of Ranunculus (A. globularia/A. furcata/A. flava/A. hyperborea clade, A. arctica/A. norvegica clade). The putative places of origins of their arctic lineages or species also differ in terms of habitat preferences. Ecologically, the arctic species of Artemisia are not centered on wet/aquatic places of the Arctic, as seen in Ranunculus (Tolmachev 1971; Tkach et al. 2008a), which accords to the general ecological preference of both genera: Artemisia is mainly a genus of dry habitats, comprising many species adapted to continental climates, occurring there as shrubs or semi-shrubs with xeromorphic leaves and wind dispersal, which are often dominant in the steppe vegetation of Eurasia and North America. In contrast, Ranunculus is consistently herbaceous, with a preference for humid to aquatic habitats, although it even occurs in regions with semiarid or arid climates. Such species show rapid flowering and fruiting during short, wet periods and survival of partly extended drought periods either by underground parts (perennials) or as seeds (annuals). Different ecological features and preadaptations inherited during their respective evolutionary history (e.g., life form, pollination, dispersal) have obviously shaped the representation of different plant genera in the arctic flora, as seen in the contrasting patterns of immigration and arctic in situ evolution of Ranunculus and Artemisia. Both genera show a multiple evolution of species currently present in the Arctic coming from evolutionary lineages that had already started to split in the Miocene. This points to a highly dynamic temporal and phylogenetic nature in taxon recruitment of the arctic ecosystem starting at its very beginning, but comparable data from other plant genera are needed to corroborate this.
Acknowledgments
The study was supported by grants from the German Academic Exchange Programme (DAAD) to N. V. Tkach and M. H. Hoffmann. A grant from the Austrian Academy of Sciences, Commission for Interdisciplinary and Ecological Studies, to E. Hörandl also supported this work. We are grateful for helpful comments of two anonymous reviewers.
Footnotes
Online enhancement: data file.
Literature Cited
- Abbott RJ, Brochmann C. History and evolution of the arctic flora: in the footsteps of Eric Hultén. Mol Ecol. 2003;12:299–313. doi: 10.1046/j.1365-294x.2003.01731.x. [DOI] [PubMed] [Google Scholar]
- Allen AP, Gillooly JG, Savage VM, Brown JH. Kinetic effects of temperature on rates of genetic divergence and speciation. Proc Natl Acad Sci USA. 2006;103:9130–9135. doi: 10.1073/pnas.0603587103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsos IG, Eidesen PB, Ehrich D, Skrede I, Westergaard K, Jacobsen GH, Landvik JY, Taberlet JY, Brochmann C. Frequent long-distance plant colonization in the changing Arctic. Science. 2007;316:1606–1609. doi: 10.1126/science.1139178. [DOI] [PubMed] [Google Scholar]
- Bennike O, Bøcher J. Forest-tundra neighbouring the North Pole: plant and insect remains from the Plio-Pleistocene Kap København Formation, North Greenland. Arctic. 1990;43:331–338. [Google Scholar]
- Blattner FR. Direct amplification of the entire ITS region from poorly preserved plant material using recombinant PCR. Biotechniques. 1999;27:1180–1186. doi: 10.2144/99276st04. [DOI] [PubMed] [Google Scholar]
- Brochmann C, Brysting AK, Alsos IG, Borgen L, Grundt HH, Scheen AC, Elven R. Polyploidy in arctic plants. Biol J Linn Soc. 2004;82:521–536. [Google Scholar]
- Coles SM. The Ranunculus acris L. complex in Europe. Watsonia. 1971;8:237–261. [Google Scholar]
- Cooper EJ. Out of sight, out of mind: thermal acclimation of root respiration in Arctic Ranunculus. Arct Alp Res. 2004;36:308–313. [Google Scholar]
- Drummond AJ, Ho SYW, Phillips MJ, Rambaut A. Relaxed phylogenetics and dating with confidence. PLoS Biology. 2006;4:e88. doi: 10.1371/journal.pbio.0040088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond AJ, Rambaut A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol Biol. 2007;7:214. doi: 10.1186/1471-2148-7-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichler HJ. Revision der Ranunculaceen Malesiens. Bibl Bot. 1958;124:1–110. [Google Scholar]
- Elvebakk A, Elven R, Razzhivin VY. Delimitation, zonal and sectorial subdivision of the Arctic for the Panarctic Flora Project. Skr Nor Vidensk-Akad Oslo I Mat-Naturvidensk Kl, NS. 1999;38:375–386. [Google Scholar]
- Elven R. Checklist of the panarctic flora (PAF) vascular plants. 2007. May 2007 version. http://www.binran.ru/infsys/paflist/index.htm.
- Elven R, Murray DF. New combinations in the panarctic vascular plant flora. J Bot Res Inst Tex. 2008;2:433–446. [Google Scholar]
- Emadzadeh K, Hörandl E, Lehnebach C, Lockhart P. Molecular phylogeny, biogeographical history and a revised classification of Ranunculus s.l. (Ranunculaceae) In: Gradstein S, editor. Systematics 2008: programme and abstracts. Universitaätsverlag Göttingen; Göttingen: 2008. p. 55. Göttingen, April 7–11. [Google Scholar]
- Ericsson S. The microspecies of the Ranunculus auricomus complex treated at the species level. Ann Bot Fenn. 1992;29:123–158. [Google Scholar]
- Ericsson S. Ranunculus auricomus complex. In: Jonsell B, Karlsson T, editors. Flora Nordica. Vol 2. Chenopodiaceae to Fumariaceae. Bergius Foundation; Stockholm: 2001. pp. 237–255. 382–397. [Google Scholar]
- Fisher FJF. The alpine Ranunculi of New Zealand. DSIR; Wellington: 1965. [Google Scholar]
- Grundt HH, Kjolner S, Borgen L, Rieseberg LH, Brochmann C. High biological species diversity in the arctic flora. Proc Natl Acad Sci USA. 2006;103:972–975. doi: 10.1073/pnas.0510270103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guggisberg A, Mansion G, Kelso S, Conti E. Evolution of biogeographic patterns, ploidy levels, and breeding systems in a diploid-polyploid species complex of Primula. New Phytol. 2006;171:617–632. doi: 10.1111/j.1469-8137.2006.01722.x. [DOI] [PubMed] [Google Scholar]
- Hagerup O. Über Polyploidie in Beziehung zu Klima, Ökologie und Phylogenie. Hereditas. 1932;16:19–40. [Google Scholar]
- Hedberg O. Taxonomic differentiation in Saxifraga hirculus L. (Saxifragaceae) Bot J Linn Soc. 1992;109:377–393. [Google Scholar]
- Hess D. Alpenpflanzen. Ulmer; Stuttgart: 2001. [Google Scholar]
- Hoffmann MH, Röser M. Taxon recruitment of the arctic flora: an analysis of phylogenies. New Phytol. 2009;182:774–780. doi: 10.1111/j.1469-8137.2009.02782.x. [DOI] [PubMed] [Google Scholar]
- Hoffmann MH, Welk E. A method for the estimation of the global population sizes of plant species: the area-abundance index. Global Ecol Biogeogr. 1999;8:39–46. [Google Scholar]
- Hörandl E. Species concepts in agamic complexes: applications in the Ranunculus auricomus complex and general perspectives. Folia Geobot. 1998;33:335–348. [Google Scholar]
- Hörandl E. The complex causality of geographical parthenogenesis. New Phytol. 2006;171:525–538. doi: 10.1111/j.1469-8137.2006.01769.x. [DOI] [PubMed] [Google Scholar]
- Hörandl E. Evolutionary implications of self-compatibility and reproductive fitness in the apomictic Ranunculus auricomus polyploid complex (Ranunculaceae) Int J Plant Sci. 2008;169:1219–1228. doi: 10.1086/591980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hörandl E. Melzheimer V, Jäger EJ, editors. Ranunculus: Taxonomie und Systematische Gliederung. Gustav Hegi Illustrierte Flora von Mittel-Europa. 3 Forthcoming. [Google Scholar]
- Hörandl E, Cosendai AC, Temsch E. Understanding the geographic distributions of apomictic plants: a case for a pluralistic approach. Plant Ecol Divers. 2008;1:309–320. doi: 10.1080/17550870802351175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hörandl E, Paun O, Grossniklaus U, Sharbel T. Patterns and sources of genetic diversity in apomictic plants: implications for evolutionary potentials. In: Hörandl E, van Dijk P, editors. Apomixis: evolution, mechanisms and perspectives. Regnum Vegetabile 147. Gantner; Ruggell: 2007. pp. 169–194. [Google Scholar]
- Hörandl E, Paun O, Johansson JT, Lehnebach C, Armstrong T, Chen L, Lockhart P. Phylogenetic relationships and evolutionary traits in Ranunculus s.l. (Ranunculaceae) inferred from ITS sequence analysis. Mol Phylogenet Evol. 2005;36:305–327. doi: 10.1016/j.ympev.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Huber W. Natürliche Bastardierungen zwischen weißblühenden Ranunculus-Arten in den Alpen (Natural hybridizations between white-flowered species of Ranunculus in the Alps) Veröff Geobot Inst ETH Stift Rübel Zür. 1988;100:1–160. [Google Scholar]
- Huelsenbeck JP, Ronquist F. MrBayes: Bayesian inference of phylogeny. Version 3.1.2. 2006. http://mrbayes.csit.fsu.edu.
- Hultén E. Outline of the history of arctic and boreal biota during the Quaternary period. Bokförlags Aktiebolaget Thule; Stockholm: 1937. [Google Scholar]
- Hultén E. The amphi-Atlantic plants and their phytogeographical connections. K Sven Vetenskapsakad Handl. 1958;7:1–340. [Google Scholar]
- Johnson AW, Packer JG. Polyploidy and environment in arctic Alaska. Science. 1965;148:237–239. doi: 10.1126/science.148.3667.237. [DOI] [PubMed] [Google Scholar]
- Jørgensen MH, Elven R, Tribsch A, Gabrielsen TM, Stedje B, Brochmann C. Taxonomy and evolutionary relationships in the Saxifraga rivularis complex. Syst Bot. 2006;31:702–729. [Google Scholar]
- Larcher W, Wagner J, Lutz C. The effect of heat on photosynthesis, dark respiration and cellular ultrastructure of the arctic-alpine psychrophyte Ranunculus glacialis. Photosynthetica. 1998;34:219–232. [Google Scholar]
- Lehnebach C, Cano A, Monsalve C, McLenachan P, Hörandl E, Lockhart P. Phylogenetic relationships of the monotypic Peruvian genus Laccopetalum (Ranunculaceae) Plant Syst Evol. 2007;264:109–116. [Google Scholar]
- Lockhart P, McLechnanan PA, Havell D, Glenny D, Huson D, Jensen U. Phylogeny, dispersal and radiation of New Zealand alpine buttercups: molecular evidence under split decomposition. Ann Mo Bot Gard. 2001;88:458–477. [Google Scholar]
- Mai DH. Tertiaäre Vegetationsgeschichte Europas. Fischer; Jena: 1995. [Google Scholar]
- Matthews JR, Ovenden LE. Late Tertiary plant macrofossils from localities in Arctic/Subarctic North America: a review of the data. Arctic. 1990;43:384–392. [Google Scholar]
- Matthews JV. Tertiary and Quaternary environments: historical background for an analysis of the Canadian insect fauna. In: Danks HV, editor. Canada and its insect fauna. Entomological Society of Canada; Ottawa: 1979. pp. 31–86. [Google Scholar]
- Moran K, Backman J, Brinkhuis H, Clemens SC, Cronin T, Dickens GR, Eynaud F, et al. The Cenozoic palaeoenvironment of the Arctic Ocean. Nature. 2006;441:601–605. doi: 10.1038/nature04800. [DOI] [PubMed] [Google Scholar]
- Müller-Schneider P. Verbreitungsbiologie der Blütenpflanzen Graubündens. Veröff Geobot Inst ETH Stift Rüb Zür. 1986;85 [Google Scholar]
- Murray DF. Causes of arctic plant diversity: origin and evolution. In: Chapin SS, Körner C, editors. Arctic and alpine biodiversity: patterns, causes, and ecosystem consequences. Vol 13. Ecological studies. Springer; Berlin: 1995. pp. 21–32. [Google Scholar]
- Nordal I, Razzhivin VY. The species concept in the High North: a panarctic flora initiative. Skr Nor Vidensk-Akad Oslo I Mat-Naturvidensk Kl. 1999;38 [Google Scholar]
- Ovczinnikov PN. In: Flora USSR. Komarov VL, Schischkin BK, editors. Vol. 7. Izd Akad Nauka SSSR; Moscow: 1937. pp. 351–509. [Google Scholar]
- Page RDM. TreeView v1.6.6 (update from 2001): an application to display phylogenetic trees on personal computers. Comput Appl Biosci. 1996;12:357–358. doi: 10.1093/bioinformatics/12.4.357. [DOI] [PubMed] [Google Scholar]
- Paun O, Lehnebach C, Johansson JT, Lockhart P, Hörandl E. Phylogenetic relationships and biogeography of Ranunculus and allied genera (Ranunculaceae) in the Mediterranean region and in the European alpine system. Taxon. 2005;54:911–930. [Google Scholar]
- Sanderson MJ. r8s, version 1.71: analysis of rates of evolution. 2006 Computer program and manual distributed by the author. [Google Scholar]
- Schönswetter P, Paun O, Tribsch A, Niklfeld H. Colonization of northern Europe by East Alpine populations of the glacier buttercup Ranunculus glacialis. Mol Ecol. 2003;12:3373–3381. doi: 10.1046/j.1365-294x.2003.01984.x. [DOI] [PubMed] [Google Scholar]
- Streb P, Aubert S, Gout E, Bligny R. Reversibility of cold- and light-stress tolerance and accompanying changes of metabolite and antioxidant levels in the two high mountain plant species Soldanella alpina and Ranunculus glacialis. J Exp Bot. 2003;54:405–418. doi: 10.1093/jxb/erg048. [DOI] [PubMed] [Google Scholar]
- Streb P, Josse EM, Gallouet E, Baptist F, Kuntz M, Cornic G. Evidence for alternative electron sinks to photosynthetic carbon assimilation in the high mountain plant species Ranunculus glacialis. Plant Cell Environ. 2005;28:1123–1135. [Google Scholar]
- Svendsen JI, Alexanderson H, Astakhov VI, Demidov I, Dowdeswell JA, Funder S, Gataullin V, et al. Late Quaternary ice sheet history of northern Eurasia. Quat Sci Rev. 2004;23:1229–1271. [Google Scholar]
- Swofford DL. PAUP*: phylogenetic analysis using parsimony (*and other methods). Version 4. Sinauer; Sunderland, MA: 2002. [Google Scholar]
- Tamura M. Angiospermae: Ordnung Ranunculales, Fam. Ranunculaceae. II. Systematic Part. In: Hiepko P, editor. Natürliche Pflanzenfamilien. 17aIV. Duncker & Humblot; Berlin: 1995. pp. 223–519. [Google Scholar]
- Tkach NV, Hoffmann MH, Röser M, Korobkov AA, von Hagen KB. Parallel evolutionary patterns in multiple lineages of arctic Artemisia L. (Asteraceae) Evolution. 2008a;62:184–198. doi: 10.1111/j.1558-5646.2007.00270.x. [DOI] [PubMed] [Google Scholar]
- Tkach NV, Hoffmann MH, Röser M, von Hagen KB. Temporal patterns of evolution in the Arctic explored in Artemisia L. (Asteraceae) lineages of different age. Plant Ecol Divers. 2008b;1:161–169. [Google Scholar]
- Tkach NV, Röser M, Hoffmann MH. Range size variation and diversity distribution in the vascular plant flora of the Eurasian Arctic. Org Divers Evol. 2008c;8:251–266. [Google Scholar]
- Tolmachev AI. Der autochthone Grundstock der arktischen Flora und ihre Beziehungen zu den Hochgebirgsfloren Nord- und Zentralasiens. Bot Tidsskr. 1960;55:269–276. [Google Scholar]
- Tolmachev AI. Arkticheskaya Flora SSSR. Vol. 6. Nauka; Leningrad: 1971. [Google Scholar]
- Totland O, Alatalo JM. Effects of temperature and date of snowmelt on growth, reproduction, and flowering phenology in the arctic/alpine herb Ranunculus glacialis. Oecologia. 2002;133:168–175. doi: 10.1007/s00442-002-1028-z. [DOI] [PubMed] [Google Scholar]
- Tutin TG, Akeroyd JR, Cook CDK. Ranunculus L. In: Tutin TG, Heywood VH, Burges NA, Moore DM, Valentine DH, Walters SM, Webb DA, editors. Flora Europaea. Vol. 1. Cambridge University Press; Cambridge: 1993. pp. 269–286. [Google Scholar]
- Walter H, Breckle S-W. Grundlagen. Vol. 1. Fischer; Stuttgart: 1991. Ökologie der Erde. [Google Scholar]
- Weber WA. Plant geography in the southern Rocky Mountains. In: Wright HE, Frey DG, editors. The Quaternary of the United States. Princeton University Press; Princeton, NJ: 1965. pp. 453–468. [Google Scholar]
- Whittemore A. Ranunculus. In: Flora of North America Committee, editor. Flora of North America North of Mexico. Vol 3. Magnoliophyta: Magnoliidae and Hamamelidae. Oxford University Press; New York: 1997. pp. 88–135. [Google Scholar]
- Xiang QY, Soltis DE, Soltis PS. The eastern Asian and eastern and western North American floristic disjunction: congruent phylogenetic patterns in seven diverse genera. Mol Phylogenet Evol. 1998;10:178–190. doi: 10.1006/mpev.1998.0524. [DOI] [PubMed] [Google Scholar]