Abstract
Bone morphogenetic proteins (BMPs) act as central regulators of ovarian physiology and may be involved in ovarian cancer development. In an effort to understand these processes we characterized TGFβ/BMP receptor and Smad expression in immortalised ovarian surface epithelial cells (IOSE) and a panel of ovarian cancer cell lines. These studies prompted us to evaluate the potential role of BMP9 signalling in ovarian cancer. Using siRNA, ligand trap, inhibitor and ligand stimulation approaches we demonstrate that BMP9 acts as a proliferative factor for IOSE and ovarian cancer cell lines, signalling predominantly via an ALK2/Smad1/Smad4 pathway rather than via ALK1, the major BMP9 receptor in endothelial cells. Importantly, we find that some ovarian cancer cell lines have gained autocrine BMP9 signalling which is required for proliferation. Furthermore, immunohistochemistry analysis of an ovarian cancer tissue microarray reveals that approximately 25% of epithelial ovarian cancers express BMP9 whereas normal human OSE specimens do not. Our data indicate that BMP9 signalling via ALK2 may be a novel therapeutic target in ovarian cancer.
Keywords: BMP9, ovarian surface epithelial cells, ovarian cancer, proliferation, Smad
Introduction
Bone morphogenetic proteins (BMPs) act as multifunctional regulators of development, adult tissue homeostasis and are implicated in a variety of pathophysiological processes (1-3). BMPs have emerged as central modulators in ovarian physiology and female fertility (4, 5). BMPs signal by binding to a heteromeric type I / type II receptor complex. BMPs utilise three distinct type II receptors, namely BMP type II receptor (BMPRII), activin type II receptors A and B (ActRIIA and ActRIIB). Four different type I receptors have been implicated in BMP signalling: activin receptor like kinase (ALK) 1, ALK2, ALK3 (BMPR-IA) and ALK6 (BMPR-IB). The combinatorial interactions of type I and II receptors allow for diversity and selectivity in ligand binding and intracellular signalling (6). Upon ligand binding the receptor complexes activate the canonical Smad pathway and several non-Smad signalling pathways. ALK1, ALK2, ALK3 and ALK6 phosphorylate the receptor regulated (R-Smad) Smads, Smad1, Smad5 and Smad8 which enables complex formation with the co-Smad, Smad4. These heteromeric Smad complexes then accumulate in the nucleus and regulate target gene expression by binding to gene regulatory elements and recruiting transcriptional co-activation and/or co-repression factors (7).
Epithelial ovarian cancer (EOC) accounts for 90% of malignant ovarian tumours, the remaining 10% are thought to originate in granulosa cells and more rarely in the stroma or germ cells. The majority of EOC is believed to arise from ovarian surface epithelial (OSE) cells, although other cells of origin have been proposed (8). OSE cells form a single layer that covers the surface of the ovary and actively participates in the cyclical ovulatory rupture and repair process (9). Despite the fact that ovarian cancer is the fifth most common cause of death from cancer among women in the Western world, there is a poor understanding of the underlying biology of EOC and its cells of origin.
A growing body of evidence implicates BMP signalling as a target of modulation in cancer including EOC (3). BMP4 and BMP2 have been found to be overexpressed in EOC when compared to normal OSE cells, and chordin an antagonist of BMP signalling, is downregulated in EOC suggesting a pro-tumourigenic role of BMPs in ovarian cancer (10-13). However, the role of other BMP family members, their signalling pathways and their potential roles in OSE biology and EOC pathogenesis remain to be explored.
Here we demonstrate that BMP9 functions as a proliferative factor for immortalised human OSE cells (IOSE) and EOC cell lines and that serum derived BMP9 is required for ovarian cells proliferation in vitro. In contrast to previous findings demonstrating that BMP9 can inhibit endothelial cell proliferation signalling through ALK1 (14, 15), we find that BMP9 promotes EOC and IOSE cell proliferation via an ALK2/Smad1/Smad4 pathway. Importantly, we show that EOC cells, but not IOSE cells, have autocrine BMP9 signalling and that their proliferation is severely impaired when this signalling is abolished. Furthermore, immunohistochemistry analysis of human ovarian cancer tissues indicates that 25% of EOC samples express BMP9 in vivo. Taken together our findings indicate that BMP9 acts as a positive regulator of IOSE and EOC cells proliferation and suggests that BMP9 signalling via an ALK2/Smad1/Smad4 pathway represents a novel target for therapeutic intervention in EOC.
Materials and Methods
Antibodies
The following antibodies were used for Western blot experiments: Phospho-Smad1 (Ser463/465)/ Smad5 (Ser463/465)/ Smad8 (Ser426/428) polyclonal antibody from Cell Signaling Technology (Danvers, MA). Smad1 polyclonal antibody from Upstate Biotechnology (Watford, UK). Smad3 and Smad1 polyclonal antibodies from Zymed (Paisley, UK). Smad2/3 monoclonal antibody from BD Biosciences (Franklin Lakes, NJ). Id1 polyclonal and Smad4 monoclonal antibodies from Santa Cruz Biotechnology. β-tubulin monoclonal antibody from Sigma. Monoclonal anti human BMP9 antibody from R&D systems (Abingdon, UK) was used in the BMP9 bioassay and polyclonal anti human BMP9 antibody from Abgent (San Diego, CA) was used in immunohistochemical analysis.
Reagents
BMP9 (1-10ng/ml), BMP4 (10ng/ml), ALK1 extracellular domain (ALK1ecd) and ALK3ecd (2-16 fold molar excess concentration, F.M.E, R&D Systems). Dorsomorphin (0.2-5μM, Calbiochem, Nottingham, UK). AccuMax tissue microarray of human ovarian cancer (A213II) (Stretton Scientific Ltd, Stretton, UK) and normal human ovarian tissues were used in concordance with local ethical committee guidelines.
Cell culture
TR175 ovarian carcinoma cell line was cultured in 10% FBS RPMI1640. OVCAR3, OVCA433, SKOV3 and IGROV ovarian cancer cell lines were cultured in 10% FBS DMEM and were obtained from the CRUK cell line bank, authenticated by STR profiling and utilised within 3 months of resusitation. Human umbilical vein endothelial cells (HUVEC) were cultured as described (14). The C2C12-BRE luciferase mouse myoblast cell line (16) was cultured in 10% FBS DMEM supplemented with 700μg/ml G418. Non tumourigenic SV40 large T antigen immortalized human ovarian surface epithelial cells (IOSE397 and IOSE398) were cultured in Medium 199 and MCDB105 (1:1) 5% FBS. Media was supplemented with 2mM L-glutamine and 100U/ml penicillin and streptomycin and cells were grown in 10% CO2.
Transfections and siRNA knockdowns
siRNA oligonucleotides were introduced into cells using HiPerFect transfection reagent (Qiagen, Crawley, UK) according to the manufacturer’s protocol and cells were analysed 48 hours after transfection. siRNA oliginucleotides targeting ALK1, ALK2, ALK5, ActRIIA, ActRIIB, BMP9 (Qiagen) and Smad1, Smad3, Smad4 (Ambion, Warrington, UK) were used at 20nM concentration, BMPRII siRNA (Qiagen) was used at 50nM.
Transcriptional reporter, western blotting, 125[I]BMP-9 binding assays and BMP9 bioassays
Transcriptional reporter assays, western blotting (17), 125[I]BMP-9 binding assays (14) and BMP9 bioassays (16) were performed as previously described.
Proliferation assays
10,000 or 15,000 cells/well in 12 well plates were plated in triplicate and serum starved prior treatment with different factors. At various time points, cells were harvested by trypsinisation and cell number was determined using a Casy cell counter.
RNA isolation, RT-PCR and quantitative RT-PCR (qRT-PCR)
RNA was isolated using TRIZOL reagent (Invitrogen). cDNA was prepared using the first strand cDNA synthesis kit (Roche, Burgess Hill, UK) for RT-PCR analysis or DyNAmo Sybr Green 2 step qRT-PCR kit (Finnzymes, Espoo, Findland) for qRT-PCR. Supplementary Table 1 shows the sense and antisense primers used in RT-PCR analysis and their annealing temperatures. PCR products were separated on 1.5% agarose gels. qRT-PCR was performed as described (18). The following primers were used: ALK2, ALK5, Id1, ActRIIA, ActRIIB, BMPRII, BMP9, Smad4, Smad1 and Smad3 (Qiagen) and ALK1 and β-actin (Supplementary Table 1). Amplified products were analysed by a Chromo4 continuous fluorescence detector (Biorad, Hemel Hempstead, UK) and Opticon Monitor3 software.
Retroviral infections
Oligonucleotides targeting human BMP9 or non-silencing oligos (Supplementary Table 2) were annealed and cloned into Xho-1/Eco-RI digested MSCV/LTRmiR30-PIGΔRI (LMP) (a kind gift of Ross Dickins and Scott Lowe). All constructs were sequenced prior to use and are referred to as non silencing (LMP-N.S), LMP-shBMP9#1, LMP-shBMP9#2 and LMP-shBMP9#3. Retrovirus was generated as described (18). Stable cell pools were generated after outgrowth in media containing 0.5μg/ml puromycin.
Immunohistochemistry
Formalin fixed paraffin embedded tissue sections were dewaxed in xylene and then rehydrated through graded alcohols to water and then subjected to a heat induced epitope retrieval method using a LabVision PT retrieval module. pH6 Sodium Citrate buffer (LabVision, TA-250PM1X) was heated to 98°C for 25 minutes in order to facilitate exposure of the epitopes. The sections were stained for 60 minutes at RT in a Dako Autostainer immunostaining facility for polyclonal anti human BMP9 antibody (Abgent, AP2064a 1/30). Immunoreactivity was visualised using DakoEnVision+system HRP kit following manufacturer’s instructions. Sections were counterstained with haematoxylin prior to microscopy.
Statistical analysis
Paired t-tests were used throughout comparing samples as indicated.
Results
Characterisation of TGFβ/BMP receptors and Smad expression in IOSE and EOC cell lines
To investigate the potential role of BMP/TGFβ signalling in IOSE and EOC cells, we determined the expression of ALKs 1-7 and downstream Smads by semi-quantitative RT-PCR analysis of total RNA isolated from early passage IOSE and a panel of EOC cell lines. The TGFβ signalling components (ALK5, Smad2, Smad3 and Smad4) were expressed widely in IOSE and EOC cells (Supplementary Table 3) according with previous reports (19). Of the BMP signalling components, Smad1, Smad5, Smad8 and the type I receptors ALK2, ALK3 and ALK4 were detected in all cell lines, whilst ALK6 and ALK7 presented a more restricted pattern of expression. Surprisingly we detected the expression of the endothelial specific type I receptor ALK1 (20) RNA in these cell lines (Supplementary Table 3). These results indicate that IOSE and EOC cells should be competent to respond to BMP and TGFβ signals.
BMP9 activates the Smad1,5,8 pathway
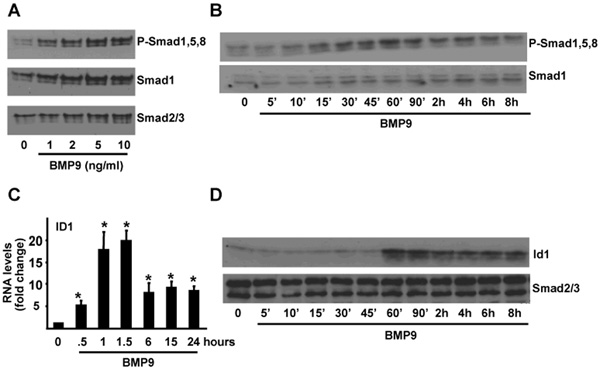
Recent studies indicate that BMP9 may act as the physiological ligand for ALK1 in endothelial cells (14, 15). We therefore tested the ability of BMP9 to stimulate Smad activation in IOSE397 cells. Western blotting experiments indicated that BMP9 induced Smad1,5,8 phosphorylation in a dose dependent manner (Fig. 1A). Phosphorylation could be observed after 10-15 min of BMP9 treatment, was maximal at 60 min and sustained for 8 hours (Fig. 1B). The Inhibitor of differentiation (Id)1 promoter-derived BMP reporter element (BRE)-luciferase reporter construct (BRE-Luc) acts as a readout system for transcriptional responses induced by Smad1 and/or Smad5 (21) and consistent with this, BMP9 treatment of IOSE397 cells lead to a dose dependent increase in BRE-Luc reporter activity (Supplementary Fig. 1). BMPRII, endoglin, Smad6 and Id1 are BMP9 target genes in endothelial cells (15) so we analyzed if BMP9 could regulate these genes in IOSE397 cells. No changes in BMPRII nor endoglin RNA levels were observed, however Smad6 and Id1 RNA levels were both increased in response to BMP9 with Id1 levels being increased by 20 fold after 90 minutes of treatment (Fig. 1C and data not shown). Western blotting analysis indicated that Id1 protein levels were also increased upon BMP9 treatment (Fig. 1D). These results indicate that BMP9 activates the canonical Smad1,5,8 pathway in IOSE397 cells to regulate gene transcription.
Figure 1.
BMP9 activates the Smad1,5,8 pathway in IOSE397 cells. A-B, IOSE397 cells were incubated for 1 hour with increasing concentrations of BMP9 (A) or for the indicated periods of time −/+ BMP9 (5ng/ml) in 0.1% FBS media (B). Western blots were performed with the indicated antibodies. C, IOSE397 cells were incubated −/+ BMP9 (5ng/ml) for different periods of time in 0.1% FBS media and Id1 levels were analysed by qRT-PCR and normalized to β-actin. Fold changes relative to untreated samples were determined (mean ± S.E.M, n=3). D, Cells were treated as in C and analysed by western blotting with indicated antibodies. Statistical analysis compared treated to untreated samples. * = P< 0.05.
ALK2, ActRIIA and BMPRII are required for BMP9 signalling
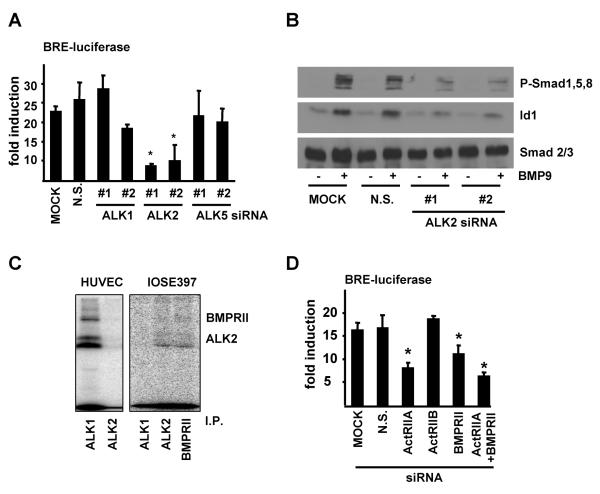
BMP9 can bind to recombinant ALK1 and BMPRII with high affinity but can also weakly bind to ALK2, ActRIIA and ActRIIB in-vitro (22). To define which receptors are required for BMP9 signalling we co-transfected IOSE397 cells with siRNAs targeting the type I receptors ALK1, ALK2 and ALK5 and the BRE-Luc reporter construct. The levels of silencing and the specificity of these reagents were analyzed by qRT-PCR (Supplementary Fig. 2A-C) and BRE-luciferase activity in response to BMP9 was determined (Fig. 2A). BMP9 stimulated BRE-luciferase activity was severely reduced when ALK2 was silenced, but not following ALK1 or ALK5 knockdown (Fig. 2A). Furthermore, ALK2 silencing impaired BMP9 induced Smad1,5,8 phosphorylation and Id1 RNA and protein up regulation (Fig. 2B, and Supplementary Fig. 2D-F). To further confirm this data, IOSE397 cells were incubated with 125[I]-BMP9 and crosslinked receptor ligand complexes were immunoprecipitated with antisera specific for ALK1, ALK2, ALK3, ALK6 and BMPRII. Immunoprecipitation of ALK2 and BMPRII readily precipitated 125[I]-BMP9 complexes whereas immunoprecipitation of ALK1, ALK3 and ALK6 failed to do so (Fig. 2C and data not shown). In contrast, BMP9 strongly bound to ALK1 and not ALK2 in endothelial (Huvec) cells as previously described (14). Consistent with this, ALK1 RNA levels were much lower than ALK2 RNA levels in IOSE397 and EOC cells and were very much lower than ALK1 RNA levels in HUVEC cells when analysed by qRT-PCR (Supplementary Fig. 3). siRNAs targeting ActRIIA, ActRIIB and BMPRII were also transiently transfected in IOSE397 cells together with the BRE-Luc reporter construct. Knockdown of ActRIIA and/or BMPRII reduced BMP9 induced BRE-luciferase reporter activity (Fig. 2D and Supplementary Fig. 2G-I). These results indicate that BMP9 signals via an ALK2/ActRIIA/BMPRII complex to trigger a phospho-Smad1,5,8 response in IOSE397 cells.
Figure 2.
BMP9 binds and signals via ALK2 in IOSE397 cells. A, IOSE397 cells were transiently transfected with siRNA oligonucleotides as indicated and with pGL3(BRE)-luciferase reporter gene and EF-LacZ and treated −/+ BMP9 (5ng/ml) for 15 hours. Normalised luciferase activity is shown as fold induction relative to untreated samples (mean ± S.E.M, n=3). B, IOSE397 cells were transiently transfected with siRNA oligonucleotides as indicated and treated for 1 hour −/+ BMP9 (5ng/ml) and western blots were performed with indicated antibodies. C, IOSE397 and Huvec cells were affinity labelled with 125[I]BMP9 and crosslinked ligand receptor complexes were immunoprecipitated (I.P.) with specific antisera as indicated and subjected to SDS-PAGE and autoradiography. D, IOSE397 cells were transiently transfected with siRNA oligonucleotides as indicated and treated as in A. Normalised luciferase activity is shown as fold activations relative to untreated samples (mean ± S.E.M., n=4). Statistical analysis compared siRNA treated to non silencing (N.S.) samples throughout. * = P< 0.05.
BMP9 promotes proliferation of IOSE397 cells and ovarian cancer cell lines in low serum
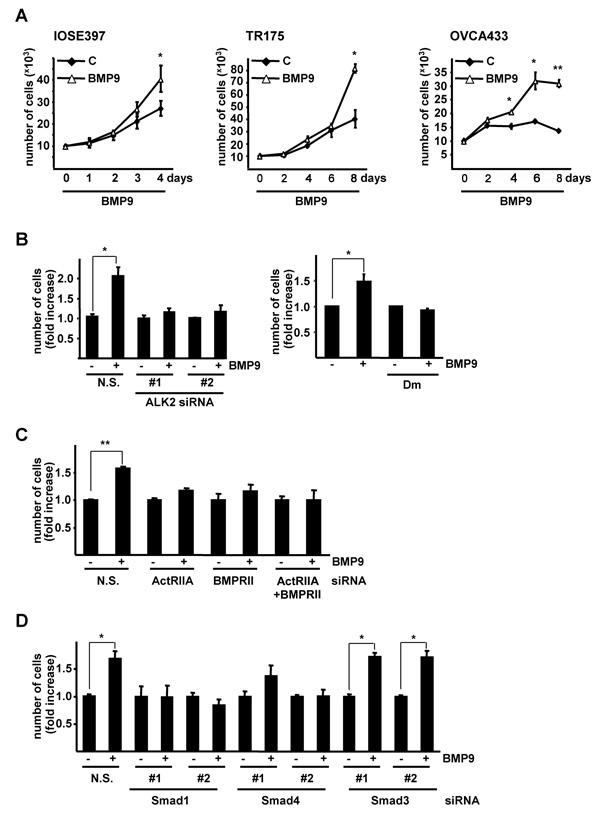
BMP9 has been shown to both positively and negatively control cell proliferation in different cell types (14, 15, 22-24). We therefore investigated whether BMP9 affected proliferation of IOSE397 cells. BMP9 had no affect on IOSE397 cell proliferation when cells were cultured in 5% FBS (Supplementary Fig. 4). However BMP9 was able to promote cell proliferation when cells were grown in serum starved (0.1% FBS) conditions. Similarly, BMP9 readily stimulated proliferation of the TR175 and OVCA433 EOC cell lines when they were grown in 0.1% FBS (Fig. 3A).
Figure 3.
BMP9 promotes proliferation of IOSE397 cells and ovarian cancer cell lines via an ALK2/ActRIIA/BMPRII/Smad1/Smad4 pathway. A, Proliferation curves of IOSE397, TR175 and OVCA433 cells incubated for different periods of time −/+ BMP9 (5ng/ml) in 0.1% FBS media (mean ± S.E.M., n=3). B left panel, OVCA433 cells were transiently transfected with siRNA oligonucleotides as indicated, treated −/+ BMP9 (5ng/ml) in 0.1% FBS for 4 days and counted (mean ± S.D, n=3). B right panel, OVCA433 cells were treated −/+ BMP9 (5ng/ml) and −/+ dorsomorphin (Dm, 1μM) in 0.1% FBS media for 4 days and counted. (mean ± S.E.M, n=5). C, OVCA433 cells were transiently transfected with siRNA oligonucleotides as indicated, treated −/+ BMP9 (5ng/ml) in 0.1% FBS for 6 days and counted (mean ± S.D, n=2). D, OVCA433 cells were transiently transfected with siRNA oligonucleotides as indicated and treated as in B. (mean ± S.D, n=3). B ,C, D, Data are displayed as fold increase relative to untreated samples. * = P< 0.05, ** = P < 0.005.
BMP9 exerts its proliferative effect via an ALK2/Smad1/Smad4 pathway
As BMP9 could stimulate proliferation in EOC cell lines we determined if BMP9 also signals via ALK2 in these cells. siRNA mediated knockdown experiments indicated that BMP9 mediated activation of Smad1,5,8 is also ALK2 dependent in TR175 and OVCA433 cells (Supplementary Fig. 5). We next assessed whether the ALK2/Smad1 pathway was involved in the proliferative effect induced by BMP9. Knockdown of ALK2 using siRNA efficiently inhibited BMP9 mediated proliferation of OVCA433 cells (Fig. 3B left panel and Supplementary Fig. 6A). We extended these observations by utilising the ALK2, ALK3 and ALK6 inhibitor dorsomorphin (25). Treatment of cells with 1μM dorsomorphin (a concentration that completely blocks BMP9 mediated BRE-Luc induction, Supplementary Fig. 6B) prevented BMP9-mediated stimulation of OVCA433 cell proliferation (Fig. 3B, right panel). Next we analyzed which type II receptor was implicated in the BMP9 proliferative response in EOC cells. siRNAs mediated knockdown of ActRIIA or/and BMPRII blocked BMP9 induced proliferation in OVCA433 cells (Fig. 3C and Supplementary Fig. 6C,D ). As dorsomorphin has been described to act as a specific Smad activation inhibitor (25), our results using this inhibitor suggested that the BMP9 mediated proliferative effect in OVCA433 cells may be Smad dependent. Consistent with this, siRNA mediated knockdown of Smad1 and Smad4 but not Smad3 resulted in the efficient inhibition of BMP9 induced proliferation in OVCA433 cells (Fig. 3D and Supplementary Fig. 6E-J). Taken together our data indicate that BMP9 induces cell proliferation via an ALK2/ActRIIA/BMPRII/Smad1/Smad4 pathway.
Serum derived BMP9 promotes proliferation
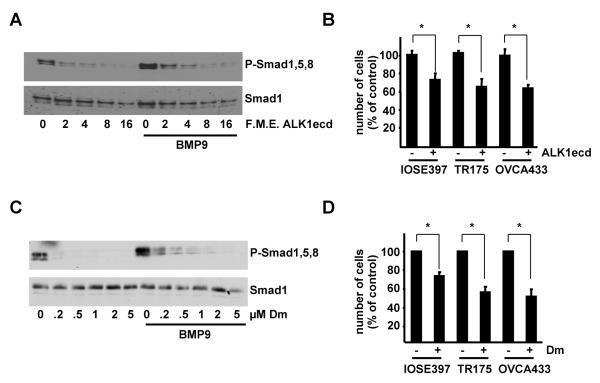
We and others have recently demonstrated that BMP9 is present in human (16, 26) and bovine serum (16) at physiologically active concentrations. As BMP9 proliferative effects are only manifest in serum starved cells we reasoned that serum derived BMP9 may mask the effect of recombinant BMP9. To test this hypothesis, we employed purified Fc-coupled extracellular domain of ALK1 (ALK1ecd), which binds to BMP9 with high affinity (22), and therefore may be considered as a specific ligand trap for BMP9 and consequently as a specific BMP9 inhibitor. Specificity controls indicated that the ALK1ecd had no effect on BMP4 mediated induction of BRE-Luc and that ALK3ecd had no effect on BMP9 responses (Supplementary Fig. 7A-C). ALK1ecd was able to inhibit exogenous BMP9 induced phosphorylation of Smad1,5,8 (Fig. 4A), activation of BRE-Luc and upregulation of Id1 protein (Supplementary Fig. 7D-E) in a dose dependent manner in IOSE397 cells. Interestingly ALK1ecd also decreased basal phosphorylation of Smad1,5,8 presumably by blocking serum derived BMP9 signalling (Fig. 4A). Importantly, treatment of IOSE397, TR175 and OVCA433 cells growing in the presence of serum with the ALK1ecd reduced the proliferation rates of all three cell lines (Fig. 4B). Furthermore, a BMP9 blocking antibody was able to reproduce this effect when added to OVCA433 cells growing in 10% FBS medium (Supplementary Fig. 7F). Dorsomorphin treatment efficiently inhibited both basal and BMP9 stimulated phosphorylation of Smad1,5,8 in IOSE397 cells (Fig. 4C) and had equivalent effects to ALK1ecd treatment on cell proliferation (Fig. 4D).
Figure 4.
Serum derived BMP9 promotes proliferation of IOSE397 cells and ovarian cancer cell lines. A, IOSE397 cells were incubated for 1 hour with increasing concentrations of ALK1ecd −/+ BMP9 (5ng/ml) in 5% FBS media. Western blots were performed with indicated antibodies. B, cells were incubated −/+ ALK1ecd (16 F.M.E.) in 5% FBS (IOSE397) or 10% FBS (TR175 and OVCA433) and counted at day 6. Data are displayed as percent of untreated cells (mean ± S.D, n≥3). C, IOSE397 cells were incubated for 1 hour with increasing concentrations dorsomorphin (Dm) −/+ BMP9 (5ng/ml) in 5% FBS media. Western blots were performed with indicated antibodies. D, Cells were incubated −/+ dorsomorphin (Dm, 1μM) in the same conditions as in C and counted at day 4. Data are displayed as percentage of untreated samples (mean ± S.E.M, n=3). * = P< 0.05.
Autocrine BMP9 promotes the proliferation of ovarian cancer cell lines
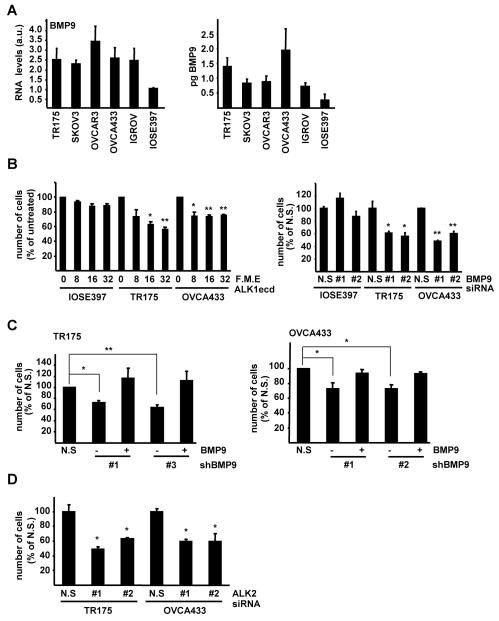
We next investigated if IOSE and EOC cell lines produce BMP9 by qRTPCR analysis. Notably all EOC cell lines produced more BMP9 mRNA than IOSE397 cells (Fig. 5A, left panel). In agreement with this result, bioassay analysis indicated that EOC cell lines release greater quantities of bioactive BMP9 into their culture media than IOSE397 cells (Fig. 5A right panel). Treatment of IOSE397, TR175 and OVCA433 cells with ALK1ecd in serum starved conditions reduced the proliferation rates in OVCA433 and TR175 but not in IOSE397 cells (Fig. 5B, left panel). Furthermore, transient knockdown of BMP9 using siRNAs (approximately 80% reduction) had no effect on proliferation of IOSE397 cells. In stark contrast to these results we observed that transient knockdown of BMP9 in TR175 cells (by 30-50%) or OVCA433 cells (by 40-50%) was sufficient to inhibit proliferation of both cell lines by 40-50% when cultured in low serum. (Fig. 5B right panel and Supplementary Fig. 8A-C). To confirm these data, we generated a non-silencing control (LMP-NS) and BMP9 shRNA pMir based retroviral vectors (LMP-shBMP#1, #2 and #3) and established stable cell lines in OVCA433 and TR175 cells. These vectors were capable of efficiently reducing BMP9 mRNA levels by approximately 80% (Supplementary Fig. 8D-E). In agreement with the transient knockdown experiments, TR175 and OVCA433 cell lines with reduced levels of BMP9 exhibited a decrease in cell proliferation in low serum when compared to non-silencing control lines. Importantly, addition of exogenous BMP9 was able to rescue this effect and restore proliferation rates (Fig. 5C). Finally, we confirmed that ALK2 was required for the autocrine BMP9 proliferative effect, as ALK2 silencing also compromised the proliferation of TR175 and OVCA433 when cultured in serum starved conditions (Figure 5D and Supplementary Fig. 8F,G). Taken together these findings indicate that unlike IOSE cells EOC cell lines exhibit autocrine BMP9 signalling which supports proliferation in low serum conditions.
Figure 5.
Autocrine BMP9 promotes ovarian cancer cell lines proliferation. A left panel, BMP9 RNA levels of IOSE and EOC cell lines were analysed by qRT-PCR and normalized to β-actin. IOSE397 BMP9 RNA content was assigned an arbitrary value of 1 (mean ± S.E.M, n=3). A right panel, Bioassay for the analysis of BMP9 production in EOC and IOSE397 cell lines. Cells were serum starved (0.1% FBS media) and after 15 hours, counted and media collected and assayed for BMP9 content. Results are expressed as pg of BMP9 /100,000 cells/ hour (mean ± S.D., n=6). B left panel IOSE397, TR175 and OVCA433 cells were incubated with different concentrations of ALK1ecd (F.M.E.) in 0.1% FBS and counted at day 6. Data are displayed as percent of untreated cells (mean ± S.E.M, n=3). B right panel, IOSE397, TR175 and OVCA433 cells were transiently transfected with BMP9 siRNA oligonucleotides, serum starved and counted 6 days later. Data are displayed as percent of non silencing siRNA control transfected (N.S) cells, (mean ± S.D, n≥3). C, Independent stable cell lines expressing non-silencing (N.S) and two different shRNAs targeted against BMP9 were generated by retroviral infection of TR175 and OVCA433 cells. Cells were serum starved in 0.1% FBS, treated −/+ BMP9 (5ng/ml) and counted at day 6 (mean ± S.E.M, n=3). D, TR175 and OVCA433 cells were transiently transfected with ALK2 siRNA oligonucleotides as indicated, serum starved and counted 4 days later. Data are displayed as percent of non silencing control siRNA transfected (N.S) cells (mean ± S.D, n≥3)* = P< 0.05, ** = P < 0.005.
BMP9 is expressed in human epithelial ovarian carcinoma but not in normal human OSE cells
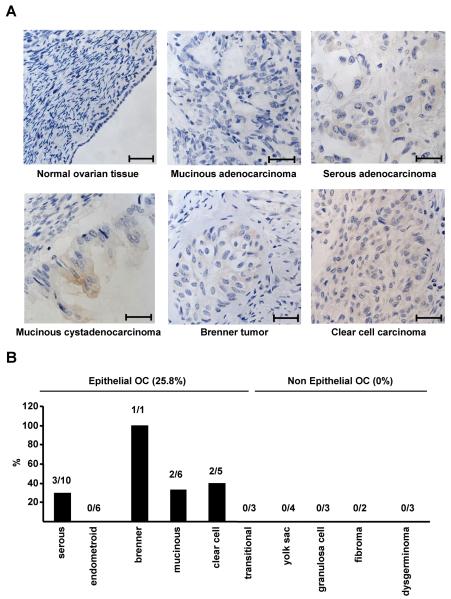
Having demonstrated that ovarian cancer cell lines have gained an autocrine proliferative BMP9 pathway we wished to determine if BMP9 expression could be detected in human normal OSE and primary ovarian cancer specimens. We performed immunohistochemistry analysis on four normal ovary specimens with detectable OSE cells and on a commercial ovarian cancer tissue microarray (TMA). We could not detect BMP9 expression in any of the normal OSE specimens (Fig. 6A and data not shown). In contrast we were readily able to detect BMP9 expression in some (Fig. 6A) but not all of the tumor specimens (Fig. 6A, upper middle panel and data not shown). All of the BMP9 positive samples belonged to the EOC type, while no BMP9 staining was observed in non epithelial ovarian tumours. 25.8% of the EOC samples analysed presented BMP9 staining (Fig. 6B). Of the different subtypes of EOC, no BMP9 staining was detected in endometroid and transitional EOCs, while in serous, mucinous and clear cell EOC subtypes BMP9 expression was observed in 30-40% of the cases. BMP9 staining was also observed in the single Brenner tumour present on the TMA. These results indicate that BMP9 protein can be detected in EOC cells but not in normal human OSE cells, suggesting that during EOC development, cells may acquire BMP9 autocrine production to support their proliferation.
Figure 6.
BMP9 is expressed in human epithelial ovarian cancer tissue and not in normal OSE cells. Sections of formalin fixed paraffin embedded human ovarian tissues were stained with BMP9 antibody and counterstained with haematoxylin. A, Representative images of normal human OSE cells and EOCs. Scale bars represent 500 μm. B, Summary of BMP9 IHC of a human ovarian cancer TMA. Staining was scored as positive or negative for BMP9 staining and percentage positive samples for each ovarian cancer subtype are shown.
Discussion
BMP9 was first isolated in the developing mouse liver (27), and it has been shown to be expressed in the non parenchymal cells in adult liver as well as other tissues as the central nervous system and normal human bone (28-30). Recent studies of ours and others have demonstrated the presence of active BMP9 in normal human serum (16, 26). BMP9 has been described to mediate cholinergic differentiation in neurons in the central nervous system (28, 31), to promote chondrogenic differentiation and promote bone formation in vivo (32) and to participate in both glucose and iron homeostasis (23, 33, 34). In addition, recent studies have demonstrated that BMP9 has a role in endothelial cell physiology (14, 15). With the exception of endothelial cells, where BMP9 signalling occurs through ALK1, it is unknown which receptors mediate BMP9 signalling. Here we show that BMP9 binds to ALK2 and signals via Smad1/Smad4 to regulate gene transcription in normal and transformed ovarian epithelial cells. We also determined that BMP9 signals through BMPRII and/or ActRIIA type II receptors in IOSE and EOC cells. This is in concurrence with previous studies indicating that BMPRII and/or ActRIIA are the type II receptors required for BMP9 signalling in endothelial cells (15).
BMPs control a wide variety of cellular processes in both fetal and adult tissues, and several reports have suggested a role for BMP9 in both positively and negatively controlling cell proliferation in different cell types (14, 15, 22-24), although the involvement of the Smad pathways in these processes has yet to be elucidated. Dorsomorphin has been shown to functionally separate Smad-dependent and non-Smad pathways triggered by BMPs (25). Dorsomorphin completely abrogated BMP9 induced proliferation in ovarian cancer cells, suggesting that this may be mediated by Smad pathways, and indeed, knockdown of Smad1 and Smad4 impaired BMP9 induced proliferation. Taken together our data indicates that BMP9 promotes proliferation by signalling via an ALK2/ActRIIA/BMPRII/Smad1/Smad4 pathway.
BMP9 induction of proliferation was only observed in serum starved conditions since it was masked by the proliferative effect of physiologically relevant concentrations of serum derived BMP9. It will be interesting to determine if the proliferation of many other cell lines in culture requires serum borne BMP9. It will also be interesting to investigate whether BMP9 concentration in serum is altered in ovarian cancer patients or if it can be found in ascitic fluids and if so, if BMP9 levels correlate with the outcome of the disease.
Our results suggest that whereas normal OSE cells are restricted to serum derived BMP9, ovarian cancer cells have an autocrine BMP9 pathway which supports their proliferation. To our knowledge, this is the first time that a bone-fide functional autocrine BMP pathway verified by knockdown analysis has been linked to cancer cell biology. Furthermore, immunohistochemical analysis of BMP9 performed in a human ovarian cancer TMA revealed that BMP9 is present in EOC cells but not in non-EOC tissues and normal human OSE cells. These results suggest that BMP9 may have a role in EOC tumorigenesis in vivo. In which stage of tumour development and how ovarian cancer cells acquire the ability to over-produce BMP9 remains to be investigated.
Our study adds to a growing body of evidence that implicates aberrant BMP signalling in ovarian cancer pathology. BMP4 and BMP2 have been shown to be produced by ovarian cancer cell lines (10, 12, 13, 35) and BMP2 has recently been detected by immunohistochemistry in primary ovarian cancer specimens (13). Chordin is an antagonist of BMP activity (36) and it has been found to be downregulated in ovarian cancer (11) which further suggests a pro-tumourogenic role for BMPs in EOC. Targeting BMP signalling in ovarian cancer may offer a novel therapeutic strategy to add to the oncologists’ armoury in the fight against this devastating disease. Our results described here indicate that the ALK2, ALK3, ALK6 kinase inhibitor dorsomorphin can restrict the proliferation of ovarian cancer cell lines. This indicates that the use of this and more specific next generation inhibitors of ALKs maybe efficacious in the treatment of ovarian cancers. We would also like to speculate that immunohistochemistry and/or bioassay analysis of BMP production and BMP activity may provide selection criteria for the use of these inhibitors both preclinically and ultimately in the clinic.
Supplementary Material
Acknowledgements
We thank Dr. Nelly Auersperg for IOSE cells, Dr Tim Crook for cell lines and Dr Karin Oien for normal human ovary tissue. We thank Colin Nixon for immunohistochemical staining, Ross Dickins and Scott Lowe for retroviral shRNAmir plasmids. We also thank Drs Lindsay Spender, Adèle Hannigan, John Ferguson and Darren O’Brien for critically reading the manuscript and for helpful discussions. B.H. and G.J.I. are supported by CRUK and an AICR fellowship to G.J.I. P.tD. is supported by the Netherlands Organization for Scientific Research and Centre for Biomedical Genetics. B.H. and G.J.I. designed and performed research, analysed and interpreted data and drafted the manuscript. M.vD. performed research and P.tD. designed experiments, analysed and interpreted data.
Footnotes
The authors have no conflicting interests.
References
- 1.ten Dijke P, Korchynskyi O, Valdimarsdottir G, Goumans MJ. Controlling cell fate by bone morphogenetic protein receptors. Mol Cell Endocrinol. 2003;211:105–13. doi: 10.1016/j.mce.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 2.Xiao YT, Xiang LX, Shao JZ. Bone morphogenetic protein. Biochem Biophys Res Commun. 2007;362:550–3. doi: 10.1016/j.bbrc.2007.08.045. [DOI] [PubMed] [Google Scholar]
- 3.Gordon KJ, Blobe GC. Role of transforming growth factor-beta superfamily signaling pathways in human disease. Biochim Biophys Acta. 2008;1782:197–228. doi: 10.1016/j.bbadis.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Shimasaki S, Moore RK, Erickson GF, Otsuka F. The role of bone morphogenetic proteins in ovarian function. Reprod Suppl. 2003;61:323–37. [PubMed] [Google Scholar]
- 5.Knight PG, Glister C. TGF-beta superfamily members and ovarian follicle development. Reproduction. 2006;132:191–206. doi: 10.1530/rep.1.01074. [DOI] [PubMed] [Google Scholar]
- 6.Feng XH, Derynck R. Specificity and versatility in tgf-beta signaling through Smads. Annu Rev Cell Dev Biol. 2005;21:659–93. doi: 10.1146/annurev.cellbio.21.022404.142018. [DOI] [PubMed] [Google Scholar]
- 7.Ross S, Hill CS. How the Smads regulate transcription. Int J Biochem Cell Biol. 2008;40:383–408. doi: 10.1016/j.biocel.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Dubeau L. The cell of origin of ovarian epithelial tumours. Lancet Oncol. 2008;9:1191–7. doi: 10.1016/S1470-2045(08)70308-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Auersperg N, Wong AS, Choi KC, Kang SK, Leung PC. Ovarian surface epithelium: biology, endocrinology, and pathology. Endocr Rev. 2001;22:255–88. doi: 10.1210/edrv.22.2.0422. [DOI] [PubMed] [Google Scholar]
- 10.Le Page C, Ouellet V, Madore J, et al. Gene expression profiling of primary cultures of ovarian epithelial cells identifies novel molecular classifiers of ovarian cancer. Br J Cancer. 2006;94:436–45. doi: 10.1038/sj.bjc.6602933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moll F, Millet C, Noel D, et al. Chordin is underexpressed in ovarian tumors and reduces tumor cell motility. Faseb J. 2006;20:240–50. doi: 10.1096/fj.05-4126com. [DOI] [PubMed] [Google Scholar]
- 12.Shepherd TG, Nachtigal MW. Identification of a putative autocrine bone morphogenetic protein-signaling pathway in human ovarian surface epithelium and ovarian cancer cells. Endocrinology. 2003;144:3306–14. doi: 10.1210/en.2003-0185. [DOI] [PubMed] [Google Scholar]
- 13.Le Page C, Puiffe ML, Meunier L, et al. BMP-2 signaling in ovarian cancer and its association with poor prognosis. J Ovarian Res. 2009;2:4. doi: 10.1186/1757-2215-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scharpfenecker M, van Dinther M, Liu Z, et al. BMP-9 signals via ALK1 and inhibits bFGF-induced endothelial cell proliferation and VEGF-stimulated angiogenesis. J Cell Sci. 2007;120:964–72. doi: 10.1242/jcs.002949. [DOI] [PubMed] [Google Scholar]
- 15.David L, Mallet C, Mazerbourg S, Feige JJ, Bailly S. Identification of BMP9 and BMP10 as functional activators of the orphan activin receptor-like kinase 1 (ALK1) in endothelial cells. Blood. 2007;109:1953–61. doi: 10.1182/blood-2006-07-034124. [DOI] [PubMed] [Google Scholar]
- 16.Herrera B, Inman GJ. A rapid and sensitive bioassay for the simultaneous measurement of multiple bone morphogenetic proteins. Identification and quantification of BMP4, BMP6 and BMP9 in bovine and human serum. BMC Cell Biol. 2009;10:20. doi: 10.1186/1471-2121-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inman GJ, Nicolas FJ, Hill CS. Nucleocytoplasmic shuttling of Smads 2, 3, and 4 permits sensing of TGF-beta receptor activity. Mol Cell. 2002;10:283–94. doi: 10.1016/s1097-2765(02)00585-3. [DOI] [PubMed] [Google Scholar]
- 18.Spender LC, O’Brien DI, Simpson D, et al. TGF-beta induces apoptosis in human B cells by transcriptional regulation of BIK and BCL-XL. Cell Death Differ. 2009;16:593–602. doi: 10.1038/cdd.2008.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henriksen R, Gobl A, Wilander E, Oberg K, Miyazono K, Funa K. Expression and prognostic significance of TGF-beta isotypes, latent TGF-beta 1 binding protein, TGF-beta type I and type II receptors, and endoglin in normal ovary and ovarian neoplasms. Lab Invest. 1995;73:213–20. [PubMed] [Google Scholar]
- 20.ten Dijke P, Ichijo H, Franzen P, et al. Activin receptor-like kinases: a novel subclass of cell-surface receptors with predicted serine/threonine kinase activity. Oncogene. 1993;8:2879–87. [PubMed] [Google Scholar]
- 21.Korchynskyi O, ten Dijke P. Identification and functional characterization of distinct critically important bone morphogenetic protein-specific response elements in the Id1 promoter. J Biol Chem. 2002;277:4883–91. doi: 10.1074/jbc.M111023200. [DOI] [PubMed] [Google Scholar]
- 22.Brown MA, Zhao Q, Baker KA, et al. Crystal structure of BMP-9 and functional interactions with pro-region and receptors. J Biol Chem. 2005;280:25111–8. doi: 10.1074/jbc.M503328200. [DOI] [PubMed] [Google Scholar]
- 23.Chen C, Grzegorzewski KJ, Barash S, et al. An integrated functional genomics screening program reveals a role for BMP-9 in glucose homeostasis. Nat Biotechnol. 2003;21:294–301. doi: 10.1038/nbt795. [DOI] [PubMed] [Google Scholar]
- 24.Song JJ, Celeste AJ, Kong FM, Jirtle RL, Rosen V, Thies RS. Bone morphogenetic protein-9 binds to liver cells and stimulates proliferation. Endocrinology. 1995;136:4293–7. doi: 10.1210/endo.136.10.7664647. [DOI] [PubMed] [Google Scholar]
- 25.Yu PB, Hong CC, Sachidanandan C, et al. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat Chem Biol. 2008;4:33–41. doi: 10.1038/nchembio.2007.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.David L, Mallet C, Keramidas M, et al. Bone morphogenetic protein-9 is a circulating vascular quiescence factor. Circ Res. 2008;102:914–22. doi: 10.1161/CIRCRESAHA.107.165530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Celeste AJ, Song JJ, Cox K, Rosen V, Wozney JM. Bone morphogenetic protein-9, a new member of the TGF-beta superfamily. J Bone Min Res. 1994;(Suppl 1):136. [Google Scholar]
- 28.Lopez-Coviella I, Berse B, Krauss R, Thies RS, Blusztajn JK. Induction and maintenance of the neuronal cholinergic phenotype in the central nervous system by BMP-9. Science. 2000;289:313–6. doi: 10.1126/science.289.5477.313. [DOI] [PubMed] [Google Scholar]
- 29.Miller AF, Harvey SA, Thies RS, Olson MS. Bone morphogenetic protein-9. An autocrine/paracrine cytokine in the liver. J Biol Chem. 2000;275:17937–45. doi: 10.1074/jbc.275.24.17937. [DOI] [PubMed] [Google Scholar]
- 30.Suttapreyasri S, Koontongkaew S, Phongdara A, Leggat U. Expression of bone morphogenetic proteins in normal human intramembranous and endochondral bones. Int J Oral Maxillofac Surg. 2006;35:444–52. doi: 10.1016/j.ijom.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 31.Lopez-Coviella I, Follettie MT, Mellott TJ, et al. Bone morphogenetic protein 9 induces the transcriptome of basal forebrain cholinergic neurons. Proc Natl Acad Sci U S A. 2005;102:6984–9. doi: 10.1073/pnas.0502097102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Majumdar MK, Wang E, Morris EA. BMP-2 and BMP-9 promotes chondrogenic differentiation of human multipotential mesenchymal cells and overcomes the inhibitory effect of IL-1. J Cell Physiol. 2001;189:275–84. doi: 10.1002/jcp.10025. [DOI] [PubMed] [Google Scholar]
- 33.Truksa J, Peng H, Lee P, Beutler E. Bone morphogenetic proteins 2, 4, and 9 stimulate murine hepcidin 1 expression independently of Hfe, transferrin receptor 2 (Tfr2), and IL-6. Proc Natl Acad Sci U S A. 2006;103:10289–93. doi: 10.1073/pnas.0603124103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caperuto LC, Anhe GF, Cambiaghi TD, et al. Modulation of BMP-9 expression and processing by insulin, glucose and glucocorticoids: possible candidate for HISS. Endocrinology. 2008 doi: 10.1210/en.2008-0655. [DOI] [PubMed] [Google Scholar]
- 35.Shepherd TG, Theriault BL, Nachtigal MW. Autocrine BMP4 signalling regulates ID3 proto-oncogene expression in human ovarian cancer cells. Gene. 2008;414:95–105. doi: 10.1016/j.gene.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 36.Piccolo S, Sasai Y, Lu B, De Robertis EM. Dorsoventral patterning in Xenopus: inhibition of ventral signals by direct binding of chordin to BMP-4. Cell. 1996;86:589–98. doi: 10.1016/s0092-8674(00)80132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.