Abstract
Cercarial dermatitis or swimmer’s itch results when cercariae of schistosomes penetrate human skin and initiate inflammatory responses. The parasites typically die in the skin but in some cases may persist and infect other organs. Cercarial dermatitis is caused by a complex and poorly known assemblage of schistosome species, and can occur anywhere where people come in contact with water bodies harboring schistosome-infected snails. In North America, most cases are reported from the upper Midwest. In the southwest U.S., this phenomenon has not been well studied, and it is not known which schistosome species are present, or if cercarial dermatitis occurs with any regularity. As part of our ongoing studies of schistosome diversity, using morphological traits and sequence data to differentiate species, we have thus far identified eight schistosome genetic lineages from snails from New Mexico and Colorado. We have investigated two cercarial dermatitis outbreaks, one occurring in Stubblefield Lake in northern New Mexico, and one in Prospect Lake in the heart of Colorado Springs, Colorado. The New Mexico outbreak likely involved two different avian schistosome species, both transmitted by physid snails. The Colorado outbreak was due to Trichobilharzia brantae, a species transmitted by Canada geese and the snail Gyraulus parvus. These outbreaks are in contrast to those in northern states where schistosomes infecting snails of the family Lymnaeidae are more often responsible for outbreaks. Our survey suggests dermatitis-causing schistosomes are not rare in the southwest, and that there are plenty of opportunities for dermatitis outbreaks to occur in this region.
Introduction
Cercarial dermatitis, also known as “swimmer’s itch”, an ailment caused by the penetration of human skin by the cercariae of schistosome parasites, is a common, recurrent phenomenon in freshwater, brackish and marine habitats worldwide (Cort, 1950). Adult schistosomes typically live in the mesenteric blood vessels of birds and mammals, and produce eggs that pass from their host’s body in the feces, then hatch and release miracidia that penetrate and develop in snail intermediate hosts. Snail infections culminate in the production of numerous cercariae that are regularly released into the water where they seek to penetrate the skin of a vertebrate definitive host. Cort (1928) working at Douglas Lake, Michigan was the first to implicate schistosome cercariae emerging from freshwater snails as the causative agents of a dermatitis known around Michigan lakes as swimmer’s itch. He and others showed this ailment to be caused by the cercariae of several genera of schistosomes that normally use aquatic birds and non-primate mammals as their definitive hosts (e.g. Talbot, 1936; McMullen & Beaver, 1945; Macfarlane & Macy, 1946; McMullen & Brackett, 1948; Cort, 1950; Blankespoor & Reimink, 1991). Anyone who has spent summers swimming in freshwater ponds and lakes in North America is aware of this problem and may well have been a victim. Although cercarial dermatitis is unquestionably annoying, as the authors can attest, there has been a tendency to dismiss the problem because the duration of infection is limited, the schistosomes die in the skin, and it is non-fatal and not directly communicable. However, there are compelling reasons to reconsider this evaluation. As early as 1953, Olivier demonstrated that three species of the dermatitis-producing cercariae of Trichobilharzia Skrjabin and Zakharov, 1920 that normally develop in waterfowl, not only could penetrate the skin of mammals, but could migrate to the lungs and cause pulmonary hemorrhages in rabbits, guinea pigs, hamsters and monkeys (see also Penner, 1941; Olivier, 1949c; Horak & Kolarova, 2000; Bayssade-Dufour et al., 2001). Also, experimental exposures of primates to the cercariae of the North American rodent schistosome Schistosomatium douthitti (Cort, 1914) have shown that the cercariae do not inevitably die in the skin (Penner, 1941): at least some can continue to develop into schistosomules which can reach the liver and in some cases persist long enough to become non-gravid adult worms in monkeys (Kagan, 1953).
Most strikingly, results of more recent studies undertaken in Europe indicate that the avian schistosome Trichobilharzia regenti Horák, Kolářová, & Dvořák, 1998 migrates via the central nervous system to reach its unusual oviposition site, the nasal chambers of waterbirds. Cercariae of this species will also readily penetrate the skin of mice, enter the central nervous system and cause some paralysis (Horak et al., 1999; Kourilova et al., 2004). Furthermore, in immunocompromised mice exposed to T. regenti, even more parasites make it past the skin and enter the brain than in normal mice, again causing some paralysis (Kourilova, et al., 2004).
The accurate identification of species involved in causing cercarial dermatitis is complicated by a number of factors: incomplete original descriptions, difficulties in recovering intact adult worms from birds, a reliance on morphological features that are not always informative, and unknown life cycles that make it difficult to connect adult and larval stages. The ability to acquire schistosome DNA sequence data from both cercariae from intermediate hosts and adult worms from definitive hosts provides a great opportunity to disentangle the etiology and epidemiology of cercarial dermatitis. The persistent accumulation of a schistosome database incorporating host use, sequence information, and available morphology will eventually provide a comprehensive overview of all schistosome species, and help to identify which species are most frequently implicated in causing dermatitis. These data will also help to validate unique morphological features that can be used for routine identification. Our extensive reference collection of schistosomes and the continued application of molecular methods to identify such worms (Brant et al., 2006; Brant, 2007; Brant & Loker, 2009) create exciting new opportunities to advance our understanding of this under-appreciated yet widespread public health problem.
The customary view is that most cases of cercarial dermatitis originate from schistosome species of waterfowl, primarily of the genus Trichobilharzia in North America (Cort, 1950; Lindblade, 1998). In North America, cercarial dermatitis is typically a problem encountered in more northern latitudes, particularly in the upper midwest (Cort, 1950; Jarcho & van Burkalow, 1952; Hunter, 1960; Oyoo et al., 1977; Verbrugge et al., 2004a; Coady et al., 2006), where large Trichobilharzia transmitting snails of the family Lymnaeidae Rafinesque, 1815 are common. While there is a considerable body of literature on swimmer’s itch in northern latitudes of North America, there is very little detailed knowledge of schistosome diversity in the southwestern portion of North America. In this study, we provide results of our snail survey work with avian schistosomes in New Mexico, an area that may seem inhospitable for schistosomes, but that nonetheless supports a diversity of species that potentially can cause dermatitis outbreaks. We also discuss two recent swimmer’s itch outbreaks in the southwest, one from New Mexico and one from Colorado.
Materials and methods
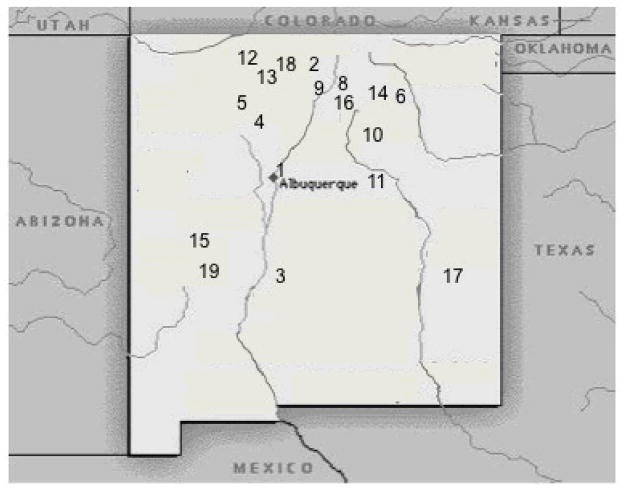
The focus of this survey from 2003 to 2008 was to examine snail intermediate hosts from a variety of habitats and elevations (fig. 1) to document what schistosomes are present and thus the potential for swimmer’s itch in New Mexico (table 1). Additionally, snails were collected from sites where dermatitis outbreaks were reported in July 2007: Stubblefield Lake, in northern New Mexico and Prospect Lake, in southern Colorado (table 1). Snails were collected by hand or wire mesh scoop and kept cool and moist until returned to the lab. Each snail was isolated in an individual well of a 24-well tissue culture plate in artificial spring water and placed in natural light to induce shedding. In most cases, snails shed cercariae within 30 minutes after being placed in natural light. All schistosome cercariae were first observed and photographed while alive and were then saved in 95% ethanol. Measurements were made on temporary mounts from these samples. Morphological features measured were length of body, tail, and furcae; width of body and tail, and number of flame cells.
Figure 1.
Map of the state of New Mexico, U.S.A. Numbers correspond to locality data in table 1.
Table 1.
Tally of snails screened and collection localities.
| Planorbidae | Locality | Latitude-Longitude |
|---|---|---|
| Gyraulus parvus Total 700 | 1. New Mexico: Bernalillo Co. Rio Grande Nature Center | 35.1305 N; 106.6822 W |
| 2. New Mexico: Taos Co. Latir Wilderness | 36.8467 N; 105.3794 W | |
| 3. New Mexico: Soccorro Co. San Marcial | 33.6887 N; 106.9921 W | |
| 4. New Mexico: Sandoval Co. Valles Caldera | 35.8485 N; 106.4907 W | |
| 5. New Mexico: Sandoval Co. Forest Rd 376 | 35.8645 N; 106.7188 W | |
| 6. New Mexico: Colfax Co. Maxwell NWR | 36.3487 N; 104.3537 W | |
| 7. Colorado: El Paso Co. Prospect Lake | 39.7759 N; 105.1269 W | |
| 8. New Mexico: Colfax Co. Pond off Hwy 64 | 36.4636 N; 105.2733 W | |
| 9. New Mexico: Taos Co. Eagle Nest Lake | 36.5087 N; 105.2717 W | |
| 10. New Mexico: Mora Co. Upper Charette Lakes | 36.1745 N; 104.8177 W | |
| Pecosorbis sp. Total 20 | 11. New Mexico: Guadalupe Co. N. Interstate 40 | 34.9690 N; 104.7442 W |
| Helisoma trivolvus Total 83 | 1. New Mexico: Bernalillo Co. Rio Grande Nature Center | 35.1305 N; 106.6822 W |
| 10. New Mexico: Mora Co. Upper Charette Lakes | 36.1745 N; 104.8177 W | |
| Physidae | ||
| Physa sp. Total 2045 | 1. New Mexico: Bernalillo Co. Rio Grande Nature Center | 35.1305 N; 106.6822 W |
| 2. New Mexico: Taos Co. Latir Wilderness | 36.8467 N; 105.3794 W | |
| 3. New Mexico: Soccorro Co. San Marcial | 33.6887 N; 106.9921 W | |
| 6. New Mexico: Colfax Co. Maxwell NWR | 36.3487 N; 104.3537W | |
| 7. Colorado: El Paso Co. Prospect Lake | 39.7759 N; 105.1269 W | |
| 8. New Mexico: Colfax Co. Pond off Hwy 64 | 36.4636 N; 105.2733 W | |
| 10. New Mexico: Mora Co. Upper Charette Lakes | 36.1745 N; 104.8177 W | |
| 12. New Mexico: Rio Arriba Co. Canjilon Lakes | 36.5636 N; 106.3286 W | |
| 13. New Mexico: Rio Arriba Co. Hopewell Lake | 36.7056 N; 106.2339 W | |
| 14. New Mexico: Colfax Co. Stubblefield Lake | 36.3372 N; 104.4079 W | |
| 15. New Mexico: Catron Co. Quemado Lake | 34.1384 N; 108.4973 W | |
| 16. New Mexico: San Miguel Co. Storrie Lake | 35.6559 N; 105.2354 W | |
| 17. New Mexico: Chavez Co. Bitter Lake NWR | 33.45 N; 104.4 W | |
| Lymnaeidae | ||
| Stagnicola elodes Total 640 | 2. New Mexico: Taos Co. Latir Wilderness | 36.8467 N; 105.3794 W |
| 4. New Mexico: Sandoval Co. Valles Caldera | 35.8485 N; 106.4907 W | |
| 5. New Mexico: Sandoval Co. Forest Rd 376 | 35.8645 N; 106.7188 W | |
| 8. New Mexico: Colfax Co. Pond off Hwy 64 | 36.4636 N; 105.2733 W | |
| 13. New Mexico: Rio Arriba Co. Hopewell Lake | 36.7056 N; 106.2339 W | |
| 15. New Mexico: Catron Co. Quemado Lake | 34.1384 N; 108.4973 W | |
| 18. New Mexico: Rio Arriba Co. Hwy 64 | 36.6414 N; 106.0660 W | |
| Lymnaea sp. Total 100 | 12. New Mexico: Rio Arriba Co. Canjilon Lakes | 36.5636 N; 106.3286 W |
| 19. New Mexico: Catron Co. Apache Creek | 33.8329 N; 108.6223 W | |
| Radix auricularia Total 111 | 8. New Mexico: Colfax Co. Pond off Hwy 64 | 36.4636 N; 105.2733 W |
| 19. New Mexico: Catron Co. Apache Creek | 33.8329 N; 108.6223 W | |
| Fossoria sp. Total 151 | 3. New Mexico: Soccorro Co. San Marcial | 33.6887 N; 106.9921 W |
| 8. New Mexico: Colfax Co. Pond off Hwy 64 | 36.4636 N; 105.2733 W | |
| 9. New Mexico: Taos Co. Eagle Nest Lake | 36.5087 N; 105.2717 W | |
| 19. New Mexico: Catron Co. Apache Creek | 33.8329 N; 108.6223 W | |
DNA was extracted from fresh or alcohol fixed worms with HotShot Lysis (Truett et al., 2000). DNA was amplified by polymerase chain reaction (Takara Ex Taq kit, Takara Biomedicals, Otsu, Japan) and sequenced using previously published primers. We used the mitochondrial cox1 and the nuclear marker ITS to distinguish species of avian schistosomes as described in Brant (2007). PCR products were purified with Montage Microcon columns (Millipore, Billerica, MD U.S.A.). Sequencing reactions were performed with Applied Biosystems BigDye direct sequencing kit, version 3.1 (Applied Biosystems, Foster City, CA, U.S.A.). Sequence data will be presented elsewhere. Sequences were used to identify distinct lineages as delineated by reference to our database of avian schistosomes and to GenBank (Brant et al., 2006; Brant, 2007; Brant & Loker, 2009).
Results
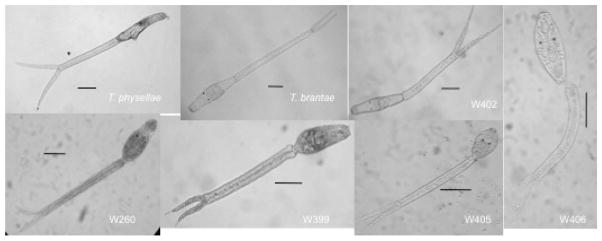
From 2003 – 2008, May–September, approximately 4,000 snails of three families and eight species were examined (table 1). Most were collected in New Mexico (fig. 1), but one collection was made from Prospect Lake, in Colorado Springs, Colorado, in response to a dermatitis outbreak. We found a total of 17 snails infected with avian schistosomes (table 2). Note the importance of Gyraulus parvus (Say, 1817) in transmission of the greatest diversity of avian schistosomes in our survey. We found four species previously recognized by both genetic and morphological criteria that are also known to cause dermatitis. They were Trichobilharzia physellae (Talbot, 1936), T. stagnicolae (Talbot, 1936), T. brantaeFarr & Blankemeyer, 1956, and Gigantobilharzia huronensis Najim, 1950. Additionally, we found three genetically distinct lineages, which we were not able to match unequivocally to any species or genus previously described either with morphological features or by matches with DNA sequence markers (tables 2, 3; fig. 2). We did find that, morphologically, the cercariae of W404 and W405 most closely matched those of Gigantobilharzia gyrauli Brackett, 1940 (Brackett, 1942). In total, there are at least eight lineages of avian schistosomes occurring in four common species of snails potentially capable of causing cercarial dermatitis in New Mexico.
Table 2.
List of the snail hosts harboring avian schistosomes. Numbers in parentheses following each snail species name represent number of individuals infected per locality listed. Schistosomes for which a species name could not be assigned are labeled with the sample number and correspond to designations in figure 2.
| Snail Host | Schistosome | Locality | Lat-Long |
|---|---|---|---|
| Physidae | |||
| Physa gyrina (2) | Trichobilharzia physellae | 1. New Mexico: Bernalillo Co. Rio Grande Nature Center | 35.1305 N; 106.6822 W |
| (1) | 4. New Mexico: Sandoval Co. Valles Caldera | 35.8485 N; 106.4907 W | |
| Physa sp. (1) | Gigantobilharzia huronensis | 1. New Mexico: Bernalillo Co. Rio Grande Nature Center | 35.1305 N; 106.6822 W |
| Physa acuta (1) | W260 | 10. New Mexico: Mora Co. Upper Charette Lakes | 36.1745 N; 104.8177 W |
| (1) | W399 | 14. New Mexico: Colfax Co. Stubblefield Lake | 36.3372 N; 104.4079 W |
| Lymnaeidae | |||
| Stagnicola elodes (1) | Trichobilharzia stagnicolae | 2. New Mexico: Taos Co. Latir Wilderness | 36.8467 N; 105.3794 W |
| Planorbidae | |||
| Gyraulus parvus (3) | Trichobilharzia brantae | 7. Colorado: El Paso Co. Prospect Lake | 39.7759 N; 105.1269 W |
| (1) | W237 | 19. New Mexico: Catron Co. Apache Creek | 33.8329 N; 108.6223 W |
| (1) | W307 | 10. New Mexico: Mora Co. Upper Charette Lakes | 36.1745 N; 104.8177 W |
| (2) | W402, W403 | 1. New Mexico: Bernalillo Co. Rio Grande Nature Center | 35.1305 N; 106.6822 W |
| (2) | W404, W405 | 1. New Mexico: Bernalillo Co. Rio Grande Nature Center | 35.1305 N; 106.6822 W |
| (1) | W406 | 1. New Mexico: Bernalillo Co. Rio Grande Nature Center | 35.1305 N; 106.6822 W |
Table 3.
Cercarial measurements from ethanol preserved specimens.
| T. brantae | W237 | W402 | W406 | W405 | W260 | W399 | |
|---|---|---|---|---|---|---|---|
| snail host | G. parvus | G. parvus | G. parvus | G. parvus | G. parvus | P. gyrina | P. gyrina |
| length body | 290 – 350 | 230 | 270 | 176 – 200 | 125 | 235 | 245 |
| width body | 75 – 120 | 80 | 90 | 80 | 65 | 90 | 130 |
| length tail | 550 – 570 | 330 | 380 | 345 | 300 | 440 | 390 |
| width tail | 40 – 60 | 30 | 60 | 33 | 25 | 45 | 40 |
| length furcae | 220 – 250 | 108 | 318 | 170 | 150 | 135 | 135 |
| body:tail | 0.55 | 0.7 | 0.7 | 0.55 | 0.4 | 0.5 | 0.63 |
| flame cells | 5 + 1 | - | 5 + 1 | 5 + 1 | 6 + 1 | - | - |
Figure 2.
Images of live cercariae collected during the survey. The sample number on each image corresponds to the samples listed in table 2. Measurements for these cercariae can be found in table 3. Bar = 100 μm.
Cercarial dermatitis at Stubblefield Lake, Maxwell, New Mexico 2007 and 2008
In July of 2007 there was an outbreak of cercarial dermatitis in Stubblefield Lake, a large impoundment in northern New Mexico. The Centers for Disease Control and Prevention (CDCP) received snails collected from the lake during the outbreak and collected schistosome cercariae from Physa sp. They later sent us an image of the Stubblefield Lake cercaria, which resembles Trichobilharzia physellae (Talbot, 1936) based on size and shape (Talbot, 1936; McMullen & Beaver, 1945) and our own reference collection (Brant & Loker, 2009). Cercarial measurements and snail host of origin are not by themselves sufficient to provide a definitive diagnosis, so this identification is considered provisional. We learned of the outbreak five days after the CDCP collections had been made, and at that time we collected 448 Physa sp. from the lake as well as 92 Physa sp. and 62 G. parvus from the neighboring Maxwell National Wildlife Refuge. None of the snails were infected with schistosome cercariae.
In July, 2008, we returned to Stubblefield Lake and collected 125 Physa acuta Draparnaud, 1805. No G. parvus were found. We found one P. acuta (0.8% prevalence) infected with schistosomes, but they were distinctive in morphology and size from the cercariae collected in 2007. Based on genetic data, the cercariae collected in 2008 are of an unknown lineage that thus far has not matched any sequences obtained from adult worms from birds (table 3; fig. 2). However, these cercariae do match (in sequence and morphology) cercariae collected from snails we have collected in other locations in New Mexico as well as Minnesota (Brant & Loker, 2009).
Cercarial dermatitis at Prospect Lake, Colorado Springs, Colorado 2007
On 19 July, 2007, we collected 111 Physa sp. and 442 Gyraulus parvus from Prospect Lake. We had recently received reports of an outbreak of cercarial dermatitis at the lake, which lies in the heart of the City of Colorado Springs, Colorado. A public swimming area is present on the west shore of the lake. A flock of Canada geese (Branta canadensis L.) was on the east shore at the time of these collections. Three (0.7% prevalence) G. parvus subsequently shed schistosome cercariae (table 3, fig. 2) that were genetically identical to ITS sequences of morphologically and genetically identified Trichobilharzia brantae adults (Brant & Loker, 2009). Adult worms of this species were originally described from B. canadensis from the eastern U.S. (Farr & Blankemeyer, 1956), and we have since found adult worms in the same host species in New Mexico, and in Churchill, Manitoba, Canada (Brant & Loker, 2009). None of the physid snails collected harbored schistosome infections.
Discussion
This survey provides new information regarding the distribution, host-use and diversity of avian schistosomes in the southwest of North America. One surprising outcome was the high diversity of avian schistosomes lineages collected in a relatively low diversity of snail species examined, and the major role of the planorbid snail Gyraulus parvus in harboring that diversity. Other records of schistosomes from Gyraulus Charpentier, 1837, or related small planorbid species in particular, imply this host family has been heavily utilized by avian schistosomes, not just in North America, but across multiple continents (e.g. Cawston, 1917; Faust, 1926; Brackett, 1940; Tanabe, 1951; Khalifa, 1972, 1976; Laman et al., 1984; Nassi, 1987, Rind, 1991; Kolářová et al., 1997; Brant et al. 2006). With the increasing use of gene sequencing to provide consistent and solid markers for species identification, we are increasingly able to recognize the full spectrum of schistosome diversity that will be invaluable to evaluating informative morphological characteristics essential for species diagnosis.
Compared to the northern latitudes of the U.S. where cercarial dermatitis is a common summer time occurrence (Cort, 1950), the relatively arid southwest hardly seems a likely place for this water-borne ailment to occur with any frequency. However, the last several years have seen reports outbreaks of swimmer’s itch in the southwest (though not in the primary literature), with the most notable outbreak occurring in Lake Mead in Arizona/Nevada (National Park Service). Recently, in 2007, outbreaks were reported in northern New Mexico and in central Colorado. There is nothing about the circumstances in these areas to suggest that such outbreaks will not reoccur: many freshwater habitats, mostly man-made, are present that support both the necessary vertebrate and snail hosts in abundance, and human water contact with such habitats is extensive and likely to become even more so as we continue to alter our water systems. Our survey has uncovered a diversity of avian schistosomes from such habitats, implying the requirements needed for future dermatitis outbreaks in the southwest are present.
Based on our observations to date, there are some notable contrasts in the epidemiology of cercarial dermatitis in northern versus southwestern areas of North America, much of this due to differences in the snail host species most often implicated in outbreaks. Cercarial dermatitis in the northern latitudes is typically caused by schistosomes that use snails of the family Lymnaeidae as intermediate hosts (Cort, 1950). In New Mexico, we found that Physa gyrina (Say, 1821), P. acuta, and Gyraulus parvus are the snails used most commonly as intermediate hosts, but we did collect a single lymnaeid snail (Stagnicola elodes Say, 1821) with a schistosome infection (Brant & Loker, 2009). Comparing schistosome prevalence in snails we collected in northern states (MN, MI, MT) to the southwestern states (NM, CO, CA, NV) shows the following values: for lymnaeids 4.5% vs. 0.11%, physids 0.3% vs. 2.2% and planorbids 1.1% vs. 2.8%, respectively. Here it is important to note that both physids and gyraulids can also be involved in cercarial dermatitis outbreaks in northern locations as they are also abundant there (Brant unpublished). Physids are ubiquitous and speciose throughout North America and a few species have a cosmopolitan distribution (Taylor, 2003).
Another important difference between northern and southwestern areas has to do with the body sizes of the snails supporting schistosome development, and the aggregate numbers of cercariae they release. Physa sp. and especially Gyraulus sp. are smaller snails than species of Lymnaea Lamark, 1799 and Stagnicola Jeffreys, 1830 more commonly implicated in the north. For example, the greatest shell dimension for G. parvus, P. gyrina, Stagnicola elodes and Lymnaea stagnalis L. are about 0.5 cm, 2.7 cm, 3.6 cm, and 5.0 cm, respectively (Burch, 1989). While screening the snails, we noticed that smaller snails shed fewer cercariae per snail than the larger bodied lymnaeid snails. For example, our experience with infected G. parvus indicates they will release fewer than 15 cercariae per day whereas L. stagnalis will consistently release several hundred cercariae per day. From an epidemiological point of view, for an outbreak to occur resulting from schistosomes transmitted by either species of Physa or Gyraulus, either the densities of infected snails present must be much greater than required for species of Lymnaea or Stagnicola, or the water volumes have to be considerably reduced or transmission confined to a restricted location where human water contact happens to be high (as around a dock or boat ramp). For example, in Prospect Lake, the tiny G. parvus was present by the hundreds on rocks and concrete surfaces near the swimming area.
Definitive hosts also play an important role in establishing the outbreak potential of a particular area. All of the avian schistosomes collected so far in North America that use lymnaeid snails as intermediate hosts use ducks as a definitive host (Brant & Loker, 2009). This differs from schistosomes dependent on physid and planorbid snails for which the definitive hosts are ducks, but also geese, passerine birds (e.g. red-winged blackbirds), and a diversity of other water birds (e.g. grebes). Ducks and geese often aggregate on particular lakes (many used for recreaction) in huge numbers during migratory seasons and release massive numbers of eggs resulting eventually in infection of many snails and a concomitantly higher potential for dermatitis outbreaks, whereas other water birds such as grebes do not attain large aggregate numbers, especially in the southwest.
Numerous additional factors also influence the occurrence, timing and length of outbreaks (e.g. Leighton et al., 2000; Levesque et al., 2002; Verbrugge et al., 2004a, b). Contributing factors are eutrophication that increases algae or aquatic plant populations thus favoring increases in snail densities (Lindblade, 1998) and concentrated use by both waterfowl and humans. Water shortages in the future could create greater need for irrigation, particularly in arid regions. The increase in man-made lakes and ponds in the southwest (many of which do not freeze in the winter) has increased the number of resident ducks and geese. These resident birds are further encouraged to stay by people feeding them, especially during the winter. The prolonged presence of birds increases both the likelihood that schistosome life cycles become established in snails and the amount of feces deposited in the water, which contributes to habitat eutrophication (Unckless & Makarewicz, 2007) and increases in snail populations. The role of birds as “nutrient vectors” has been noted in New Mexico (Post et al., 1998). Given our increased understanding of the potential health impacts of avian schistosomes and that changing ecological circumstances are likely to favor continued and increased exposure, it is important that we continue to apply new approaches to understanding the constellations of hosts and parasites involved so we can achieve a more complete and accurate picture of the epizootiology of cercarial dermatitis.
Acknowledgments
We appreciate the cooperation and help from Judy Espinoza of New Mexico State Department of Health and Bernadette Albanese of El Paso County Department of Health and Environment Colorado. We thank people in the Loker lab and Robin Loker for their participation in collecting snails. We thank two anonymous reviewers for their helpful suggestions that improved this manuscript. We acknowledge technical support from the University of New Mexico’s Molecular Biology Facility, which is supported by NIH Grant Number 1P20RR18754 from the Institute Development Award (IDeA) Program of the National Center for Research Resources. This study was supported by funds provided by the College of Arts and Sciences at UNM and NIH grant RO1 AI44913 and P20 RR018754.
References
- Blankespoor HD, Reimink RL. The control of swimmer’s itch in Michigan: Past, present and future. Michigan Academician. 1991;24:7–23. [Google Scholar]
- Brackett S. Two new species of schistosome cercariae from Wisconsin. Journal of Parasitology. 1940;26:195–200. [Google Scholar]
- Brackett S. Five new species of avian schistosomes from Wisconsin and Michigan with the life cycle of Gigantobilharzia gyrauli (Brackett, 1940) Journal of Parasitology. 1942;28:25–42. [Google Scholar]
- Brant SV. The occurrence of the avian schistosome Allobilharzia visceralis Kolarova, Rudolfova, Hampl, Skirnisson, 2006 (Schistosomatidae) in the tundra swan, Cygnus columbianus, (Anatidae) from North America. Folia Parasitologica. 2007;54:99–104. doi: 10.14411/fp.2007.013. [DOI] [PubMed] [Google Scholar]
- Brant SV, Loker ES. Systematics of the avian schistosome genus Trichobilharzia (Trematoda: Schistosomatidae) in North America. Journal of Parasitology. 2009 doi: 10.1645/GE-1870.1. In PRESS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brant SV, Morgan JAT, Mkoji GM, Snyder SD, Rajapakse RPVJ, Loker ES. An approach to revealing blood fluke life cycles, taxonomy, and diversity: provision of key reference data including DNA sequence from single life cycle stages. Journal of Parasitology. 2006;92:77–88. doi: 10.1645/GE-3515.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch JB. North American Freshwater Snails. Hamburg: Malacological Publications; 1989. p. 365. [Google Scholar]
- Coady NR, Muzzall PM, Burton TM, Snider RJ, Saxton J, Sergeant M, Sommers A. Ubiquitous variability in the prevalence of Trichobilharzia stagnicolae (Schistosomatidae) infecting Stagnicola emarginata in three northern Michigan lakes. Journal of Parasitology. 2006;92:10–15. doi: 10.1645/GE-3336.1. [DOI] [PubMed] [Google Scholar]
- Cawston FG. The cercariae of Natal. Journal of Parasitology. 1917;3:131–135. [Google Scholar]
- Cort WW. Schistosome dermatitis in the United States (Michigan) Journal of the American Medical Association. 1928;90:1027–1029. [Google Scholar]
- Cort WW. Studies on schistosome dermatitis XI. Status of knowledge after more than twenty years. American Journal of Hygiene. 1950;52:251–307. [PubMed] [Google Scholar]
- Faust EC. Further observations on South African larval trematodes. Parasitology. 1926;18:101–127. [Google Scholar]
- Farr MM, Blankemeyer VG. Trichobilharzia brantae n. sp. (Trematoda: Schistosomatidae) from the Canada goose (Branta canadensis L.) Journal of Parasitology. 1956;42:320–325. [PubMed] [Google Scholar]
- Horak P, Dvořák J, Kolářová L, Trefil L. Trichobilharzia regenti, a pathogen of the avian and mammalian central nervous system. Parasitology. 1999;119:577–581. doi: 10.1017/s0031182099005132. [DOI] [PubMed] [Google Scholar]
- Hunter GW. Studies on schistosomiasis. XIII. Schistosome dermatitis in Colorado. Journal of Parasitology. 1960;46:231–233. [PubMed] [Google Scholar]
- Jarcho S, van Burkalow A. A geographical study of “Swimmer’s Itch” in the United States and Canada. Geographical Review. 1952;42:212–226. [Google Scholar]
- Khalifa R. Studies on Schistosomatidae Looss, 1899 (Trematoda) of aquatic birds of Poland. I. On the life cycle of Bilharziella polonica Kowalewski, 1895, with a discussion of the subfamily Bilharziellinae Price, 1929. Acta Parasitologica Polonica. 1972;20:343–365. [Google Scholar]
- Khalifa R. Studies on Schistosomatidae Looss, 1899 (Trematoda) of aquatic birds of Poland. III. Notes on the morphology and life cycle of Dendritobilharzia pulverulenta (Brauwn, 1901) Acta Parasitologica Polonica. 1976;24:1–9. [Google Scholar]
- Kolářová L, Horák P, Sitko J. Cercarial dermatitis in focus: schistosomes in the Czech Republic. Helminthologica. 1997;34:127–139. [Google Scholar]
- Kouřilová P, Syrůček M, Kolářová L. The severity of mouse pathologies caused by the bird schistosome Trichobilharzia regenti in relation to host immune status. Parasitology Research. 2004;93:8–16. doi: 10.1007/s00436-004-1079-7. [DOI] [PubMed] [Google Scholar]
- Laman TG, Daniell DL, Blankespoor HD. The role of Gyraulus parvus as an intermediate host for avian schistosomes. Proceedings of the Helminthological Society of Washington. 1984;51:267–269. [Google Scholar]
- Leighton BJ, Zervos S, Webster JM. Ecological factors in schistosome transmission, and an environmentally benign method for controlling snails in a recreational lake with a record of schistosome dermatitis. Parasitology International. 2000;49:9–17. doi: 10.1016/s1383-5769(99)00034-3. [DOI] [PubMed] [Google Scholar]
- Levesque B, Giovenazzo P, Guerrier P, Laverdiere D, Prud’fHomme H. Investigation of an outbreak of cercarial dermatitis. Epidemiology and Infections. 2002;129:379–386. doi: 10.1017/s0950268802007379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindblade KA. The epidemiology of cercarial dermatitis and its association with limnological characteristics of a northern Michigan Lake. Journal of Parasitology. 1998;84:19–23. [PubMed] [Google Scholar]
- McMullen DB, Beaver PC. Studies on schistosome dermatitis IX. The life cycle of three dermatitis producing schistosomes from birds and a discussion of the subfamily Bilharziellinae (Trematoda: Schistosomatidae) American Journal of Hygiene. 1945;42:128–154. [Google Scholar]
- Macfarlane DG, Macy RW. Cercaria oregonensis, n. sp., a dermatitis-producing schistosome cercaria from the Pacific Northwest. Journal of Parasitology. 1946;32:281–285. [PubMed] [Google Scholar]
- McMullen DB, Brackett S. Studies on schistosome dermatitis. X. Distribution and epidemiology in Michigan. American Journal of Hygiene. 1948;47:259–270. doi: 10.1093/oxfordjournals.aje.a119204. [DOI] [PubMed] [Google Scholar]
- Nassi H. Sur quatre furcocercaires emises par Biomphalaria glabrata en Guadeloupe. Annales Parasitologie Humaine et Comparee. 1987;62:17–35. [Google Scholar]
- Olivier L. Observations on the migration of avian schistosomes in mammals previously unexposed to cercariae. Journal of Parasitology. 1953;39:237–243. [PubMed] [Google Scholar]
- Oyoo AO, Donnell D, Prendergast TJ., Jr Epidemic of ‘swimmer’s itch’ traced to cercarial trematodes. Missouri Medicine. 1977;74:218–219. [PubMed] [Google Scholar]
- Post DM, Taylor JP, Kitchell JF, Olson MH, Schindler DE, Herwig BR. The role of migratory waterfowl as nutrient vectors in a managed wetland. Conservation Biology. 1998;12:910–920. [Google Scholar]
- Rind S. Three ocellate schistosome cercariae (Trematodea: Schistosomatidae) in Gyraulus corinna, with reference to Cercaria longicauda MacFarlane, 1944 in Lymnaea tomentosa. New Zealand Journal of Zoology. 1991;18:53–62. [Google Scholar]
- Unckless RL, Makarewicz JC. The impact of nutrient loading from Canada Geese (Branta canadensis) on water quality, a mesocosm approach. Hydrobiologia. 2007;586:393–401. [Google Scholar]
- Talbot SB. Studies on schistosome dermatitis. II. Morphological and life history studies on three dermatitis-producing schistosome cercariae, C. elvae Miller, 1923, C. stagnicolae n. sp., and C. physellae n. sp. American Journal of Hygiene. 1936;23:372–384. [Google Scholar]
- Tanabe H. Cercaria segmintinae Tanable 1948, a homonym of Cercaria sturniae. Journal of Parasitology. 1951;37:321. [Google Scholar]
- Taylor DW. Introduction to Physidae (Gastropoda: Hygrophila); biogeography, classification, morphology. Revista de Biologia Tropical. 2003;51:1–287. [PubMed] [Google Scholar]
- Verbrugge LM, Rainey JJ, Reimink RL, Blankespoor HD. Swimmer’s itch: incidence and risk factors. American Journal of Public Health. 2004a;94:738–741. doi: 10.2105/ajph.94.5.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbrugge LM, Rainey JJ, Reimink RL, Blankespoor HD. Prospective study of swimmer’s itch incidence and severity. Journal of Parasitology. 2004b;90:697–704. doi: 10.1645/GE-237R. [DOI] [PubMed] [Google Scholar]