Abstract
The freshwater prawn Macrobrachium rosenbergii is a tropical crustacean with characteristics similar to those of lobsters and crayfish. Adult males develop through three morphological types—small (SC), yellow (YC), and blue claws (BC)—with each representing a level in the dominance hierarchy of a group, BC males being the most dominant. We are interested in understanding the role played by neuropeptides in the mechanisms underlying aggressive behavior and the establishment of dominance hierarchies in this type of prawn. SIFamides are a family of arthropod peptides recently identified in the central nervous system of insects and crustaceans, where it has been linked to olfaction, sexual behavior, and gut endocrine functions. One of the six SIFamide isoforms, GYRKPPFNGSIFamide (Gly-SIFamide), is highly conserved among decapod crustaceans such as crabs and crayfish. We wanted to determine whether Gly-SIFamide plays a role in modulating aggression and dominant behavior in the prawn. To do this, we performed behavioral experiments in which interactions between BC/YC pairs were recorded and quantified before and after injecting Gly-SIFamide directly into the circulating hemolymph of the living animal. Behavioral data showed that aggression among interacting BC/YC prawns was enhanced by injection of Gly-SIFamide, suggesting that this neuropeptide does have a modulatory role for this type of behavior in the prawn.
Introduction
Crustaceans such as lobsters and crayfish have been used extensively as model systems for studying the neural basis of aggressive behavior. In these animals, status as dominant or subordinate during agonistic encounters is established on the basis of body size and experience. The giant freshwater prawn Macrobrachium rosenbergii (de Man, 1879) is a type of clawed shrimp that lives in some tropical and subtropical areas of the world and has characteristics similar to those of lobsters and crayfish concerning general body plan, internal anatomical organization, and some aspects of behavior (Huxley, 1973; Bliss, 1982). Sexually mature males develop through three distinct morphological types that differ in the ratio of claw to body length, growth rate, and claw color and morphology. The morphotypes are designated blue claw (BC), yellow claw (YC), and small claws (SC), each representing a level in the dominance hierarchy of the population, with BCs being the most dominant and SCs the most subordinate (Ra'anan and Cohen, 1985).
Freshwater prawn adult males also show behavioral differences according to morphotype in terms of territoriality and behavior toward females (Ra'anan and Cohen, 1985). The BCs are territorial, sexually active, and tend to protect females; the YCs are nonterritorial, sexually incompetent, and tend to attack females; the nonterritorial SCs are strongly attracted to females that are receptive to mating and occasionally succeed in copulating with a female by sneaking in between it and a BC (Ra'anan and Sagi, 1985).
Even though the most obvious characteristic that distinguishes the prawn morphotypes is claw size and coloration, this is not the only relevant cue in the establishment of the dominance hierarchy of a population. The relative proportions in an enclosed population of the three male morphotypes, SCs, YCs, and BCs, remain nearly constant at 5:4:1, respectively, under a wide range of environmental conditions (Brody et al., 1980; Cohen et al., 1981; Ra'anan et al., 1991). These ratios are maintained in a dynamic state, in which individual males are capable of undergoing a transformation from one morphotype to another, following an irreversible order: from SC to YC to BC. Such transformations occur whenever large individuals either die or are selectively removed from the local habitat (Ra'anan and Cohen, 1985). Thus, since the transition to a higher-order morphotype is influenced by the ratio of each morphotype already present in the animal's environment, it is possible that, in addition to the coloration of their claws, some form of chemical cues for status and size may be involved in the animal's assessment of its position within the hierarchy. Similar cues signaling sex, status, and size have been described in other crustaceans (e.g., Hazlett, 1985, 1990; Caldwell, 1987).
We are using this model system because the status of the males as dominant or subordinate is of a more fixed nature than in other crustaceans, being established on the basis of claw type first and body size second (Barki et al., 1992). Also, the other differences in behavior, in terms of territoriality and interactions with females, make this model system interesting for comparative behavioral studies.
An extensive body of evidence links biogenic amines, such as serotonin and octopamine, with actions on the crustacean nervous system that result in behaviors associated with dominance or subordination. Some studies have focused on understanding the role played by postural systems controlling abdomen muscles in agonistic behavioral interactions in lobsters and crayfish (Livingstone et al., 1980; Harris-Warrick, 1985; Kravitz, 1988; Ma et al., 1992; Sosa and Baro, 2002). Injections of biogenic amines induce specific types of postures linked to social status, such as serotonin-flexed abdomen and octopamine-hyperextended abdomen (Livingstone et al., 1980; Kravitz, 1988; Sosa and Baro, 2002). Moreover, injected serotonin induces aggression in crayfish and lobsters (Livingstone et al., 1980; Kravitz, 1988; Huber et al., 1997a, b; Huber and Delago, 1998; Tierney and Mangiamele, 2001; Tierney et al., 2004). We showed previously that injections of serotonin and octopamine can modulate the levels of aggression in the freshwater prawn (Sosa and Baro, 2002). Injections of serotonin in YC males resulted in increased aggression, while injections of octopamine in BC males resulted in increased submissiveness. This evidence suggests that biogenic amines play a role in modulating aggressive behavior in these animals.
Escape responses (e.g., tailflips) and their underlying neural basis have also been studied extensively in crustaceans (Wine and Krasne, 1972, 1982; Edwards et al., 1999; Krasne and Edwards, 2002; Herberholz et al., 2004; Espinoza et al., 2006). Evidence also supports the role of serotonin in escape systems of crustaceans (Glanzman and Krasne, 1983; Yeh et al., 1996, 1997; Krasne et al., 1997; Teshiba et al., 2001; Edwards et al., 2002; Antonsen and Edwards, 2007). In the crayfish, it has been found that modulatory actions of serotonin on synaptic responses within a circuit involved in the escape response depend on the initial social status of the animal (Yeh et al., 1996, 1997). In subordinates, serotonin inhibits escape, but in dominants it facilitates the lateral giant (LG) escape reflex. It has also been shown that burrowing, an activity not directly associated with fighting, is repressed in subordinate crayfish while in the presence of a dominant animal, but is reestablished when the animal is alone for a period of time after the determination of its status as subordinate (Herberholz et al., 2003). The simultaneous occurrence of the inhibition in burrowing with that of aggression in these subordinate animals suggests a common or similar underlying mechanism. Thus, while the neural mechanisms involved in determining social status and aggressive behavior initially are not well known, there is ample evidence demonstrating the link between neuromodulatory influences and maintenance or changes in said status.
Serotonin and octopamine play a primary role in modulating aggression and fighting behavior in crustaceans, including the freshwater prawn, but it is understood that they are not the sole determinant elements. Existing models proposed to explain the roles of biogenic amines cannot fully explain all aspects of aggressive behavior and how it can be adjusted to varying circumstances. In effect, no single chemical or transmitter system is responsible for generating or modulating these complex behaviors. The interaction and resulting balance of circulating transmitters is most probably responsible for controlling and regulating such behaviors (Wood et al., 1995). Neuropeptides are very likely candidates for acting as modulators of aggression. A thorough understanding of the roles neuropeptides are likely to be playing in modulating agonistic behaviors in the prawn will help to expand the inventory of potential target molecules, binding sites, messenger systems, and genes for controlling or regulating various forms of interactive behaviors. This knowledge will further our understanding of the neural basis of aggressive behavior.
Over the past decade, a number of studies have used techniques of mass spectrometry, immunohistochemistry, or bioinformatics to identify peptides in the nervous system of crustaceans (Nilsson et al., 1998; Yasuda-Kamatani and Yasuda, 2000, 2004; Skiebe et al., 2002, 2003; Li et al., 2002, 2003; Nichols, 2002; Huybrechts et al., 2003; Bulau et al., 2004; Yasuda et al., 2004; Messinger et al., 2005). These studies have identified a wide variety of novel neuropeptides whose distribution and physiological functions in nervous tissues are not yet known (Christie et al., 2006). One such peptide is GYRKPPFNGSIFamide (Gly-SIFamide), recently identified in the nervous system of various crustacean species (Sithigorngul et al., 2002; Huybrechts et al., 2003; Yasuda et al., 2004; Yasuda-Kamatani and Yasuda, 2006; Messinger et al., 2005; Sullivan and Beltz, 2005; Polanska et al., 2007; Gard et al., 2009; Ma et al., 2009a, b), including the freshwater prawn (Ma et al., 2008b). A related analog, VYRKPPFNGSIFamide (Val-SIFamide), has been described in the American lobster Homarus americanus (Christie et al., 2006; Cape et al., 2008; Ma et al., 2008a; Dickinson et al., 2008), and more recently in the European green crab Carcinus maenas (Ma et al., 2009a). A third analog, AYRKPPFNGSIFamide (Ala-SIFamide), appears to be the native form in most insects (Janssen et al., 1996; Vanden Broeck, 2001; Audsley and Weaver, 2006; Christie, 2008a, b; Roller et al., 2008; Weaver and Audsley, 2008). Another three isoforms have been described in insects and crustaceans (Verleyen et al., 2009).
While members of the SIFamide family have been identified in a number of arthropods and appear to be widely distributed in the central nervous system, very little is known about the physiological roles they play in any species. The fact that the sequence of SIFamide is extremely conserved among species suggests an important function for this neuropeptide (Verleyen et al., 2009). In Drosophila, Ala-SIFamide is restricted to only four neurons in the brain and appears to be involved in modulating sexual behavior (Terhzaz et al., 2007). In other insects and crayfish, the expression patterns of the SIFamide peptides in specific areas of the brain support involvement in the integration of visual, tactile, and olfactory information (Verleyen et al., 2004, 2009; Yasuda et al., 2004; Yasuda-Kamatani and Yasuda, 2006; Polanska et al., 2007). In the American lobster, immunohistochemical and physiological data are suggestive of modulatory actions of Val-SIFamide in the stomatogastric nervous system (Christie et al., 2006; Rehm et al., 2008). The recent identification of Gly-SIFamide in the crab midgut implies a possible gut endocrine/paracrine role for members of the SIFamide family (Christie et al., 2007). However, widespread expression patterns in other areas of the nervous systems of various arthropods clearly suggest that much remains to be learned about the functions of these neuropeptides.
Dominance and aggression are involved in the sexual behavior of many species, including the prawn. These elements of interactive behavior are also influenced directly by information the animal receives from its immediate external environment by means of visual, tactile, and olfactory cues. Since, as mentioned above, SIFamide has been suggested to be involved precisely in the integration of visual, tactile, and olfactory information (Verleyan et al., 2009), as well as in regulating sexual behavior (Terhzaz et al., 2007), we postulated that maybe this amide also functions in modulating dominance and aggressive behavior in the prawn.
The purpose of this study was thus to determine the role that Gly-SIFamide plays in modulating the prawn's aggressive behavior during agonistic encounters, as a means to start elucidating how neuropeptides function in the mechanisms underlying plasticity of interactive behaviors. We focused on the question of whether exogenous Gly-SIFamide increases or decreases the level of aggressiveness displayed by interacting prawns. To address this question, we performed behavioral observation experiments in which the interactions between BC/YC pairs were recorded and quantified before and after Gly-SIFamide was injected directly into the circulating hemolymph of the living animal.
Materials and Methods
Experimental animals
The freshwater prawn Macrobrachium rosenbergii is a crustacean model system characterized by adults with three claw morphotypes that differ in color, relative size, and spination, each morphotype correlating with a fixed dominance status and other defining features of the animal's modes of interaction with others, including territoriality and courtship behavior (Ra'anan and Cohen, 1985; Ra'anan and Sagi, 1985; Barki et al., 1992). The fixed nature of the dominance hierarchies established by the prawn makes this model interesting for behavioral studies.
Male prawns measuring 8–15 cm in length from eyestalk to telson were obtained from private aquaculture farms in Lajas and Canovanás, Puerto Rico. They were maintained in community tanks whose water was continuously filtered and aerated at the Animal Care Facility of the Institute of Neurobiology of the University of Puerto Rico Medical Sciences Campus under a photoperiod of 12-h light/12-h dark. Experiments were done at the end of the dark photoperiod (the animals are nocturnal). Water temperature was maintained at 26–28 °C and the pH adjusted to 7.4 (safe range is 6.9–8.5). Animals were fed a high-protein (>40%) pelleted Purina chow once every other day. All procedures involving the use of animals were approved by the University of Puerto Rico Medical Sciences Campus Institutional Animal Care and Use Committee (IACUC) prior to the start of the experiments.
Behavioral observations
Since the dominance hierarchy of this species of prawn is predetermined on the basis of the animal's morphotype (Ra'anan and Cohen, 1985; Ra'anan and Sagi, 1985; Barki et al., 1992), specific pairs of BC and YC prawns did not need to be placed together before experiments to establish dominance, as is required with other crustacean models. All animals used for behavioral observations had been previously kept in a communal tank containing male prawns of all morphotypes, as well as females, in the proportions they are normally found in their natural habitats (50% SC, 40% YCs, 10% BCs; Brody et al., 1980; Cohen et al., 1981; Ra'anan et al., 1991). Interactions were observed in an 80-l glass tank (24 × 12 × 16 in [≈60.9 × 30.5 × 40.6 cm]). All behavioral observations were recorded on videotape using a camcorder (Panasonic Palmcorder PVA228) or digital camera (SONY Handycam PC350) placed in front of the tank at the same time as parameters of interest were noted on paper. Observations (and their corresponding numerical values) during each session were registered at 30-s intervals when analyzing the video recordings. A grid of squares with sides of 4 cm was marked at the bottom of the tanks using white tape against a black background. All other sides of the tanks, except the front, were also covered with a black background to increase contrast. To minimize the possibility of the animals reacting to the presence or movements of the observer, a black curtain was placed in front of the observation tank with a small opening for the lens of the camera and another for the eyes of the observer. Water filters, heaters, and aeration tubes were temporarily removed during observation sessions to minimize sources of distraction for the animals. Before reuse, each test aquarium was thoroughly rinsed and filled with freshly prepared water.
Quantitative assessment of aggressive behavior
We measured parameters that serve as indicators of dominance/subordination in instances in which a BC and a YC are placed in the same tank. These parameters were the same as those used for prawns by Barki et al. (1992) and similar to those used for crayfish by Huber and Kravitz (1995), with some additions and modifications based on our own observations (Table 1). The values in the Level column designate the weight of the parameter as an indicator of dominance/subordination, 6 indicating the most highly dominant behavior and 0 indicating complete or total subordination (based on Barki et al., 1992). Thus, the weight values ascribed to each parameter at each instance of observation serve as a measure of the level of aggression. These values were used to calculate a dominance index (DI) for each individual tested in the paired interactions, calculated as the sum of the weights of all the instances recorded during the observation period, divided by the total number of instances. All DI values are shown as mean ± S.E.M. To determine whether specific parameters could be differentially affected by the injections in comparison to the others, the mean level of aggression reached for each parameter was also calculated for each animal during the observation periods.
Table 1. Parameters used to calculate a dominance index (DI) for each individual tested in the pair interactions.
| Parameter | Level |
|---|---|
| Movement toward or away from each other | |
| Rush toward other prawn while facing it | 6 |
| Walk toward other prawn while facing it | 5 |
| Turn toward other prawn but not walking to it | 4 |
| Turn away from facing other prawn, without walking away from it | 3 |
| Walk away from other prawn while facing it | 2 |
| Walk away from other prawn without facing it | 1 |
| Rush to move away from other prawn without facing it | 0 |
| Abdomen posture | |
| Fully flexed | 6 |
| Partially flexed | 4, 5 |
| Extended (horizontally) | 3 |
| Partially hyperextended | 1, 2 |
| Fully hyperextended | 0 |
| Position of body and walking legs | |
| Legs fully flexed/body touching bottom | 0, 1 |
| Legs partially flexed/body partially lifted | 2, 3, 4 |
| Legs fully extended/body fully lifted | 5, 6 |
| Movement and position of claws | |
| Crossed in front of eyes | 0 |
| Touching bottom while extended | 1 |
| Touching bottom while flexed | 2 |
| At body level | 3 |
| Above body level | 4 |
| Extended at or above body level | 5 |
| Quickly thrust forward | 6 |
| Used for lifting the other prawn | 6 |
| Movement of chela | |
| Closed/not touching other prawn | 0 |
| Closed/touching other prawn | 1 |
| Open/not touching other prawn | 2 |
| Open/touching other prawn | 3 |
| Scissoring in front of other prawn | 4 |
| Open, grabbing other prawn | 5 |
| Nipping or tearing | 6 |
The values in the Level column indicate the weight or level of the parameter as an indicator of dominance/subordination, 6 indicating the most highly dominant behavior, 0 indicating complete or total subordination. These parameters are based on those used by Barki and colleagues (1992) with the following modifications: (1) a more detailed specification of levels or weights by describing in words what each whole number in the scale represents; (2) subdividing the parameter of claw movement into the two parameters“movement and position of claws” and “movement of chelas”; and (3) changing the scale from one that included negative numbers (−3 through +3) to one with only positive numbers (0 through +6).
Previous studies carried out in our laboratory have shown that when a pair of BC and YC prawns are placed together, 30 min of recording is sufficient to obtain a reliable quantitative estimate of the behaviors of interest, and that further extending these observation periods does not significantly alter the resulting values of the DI (Sosa and Baro, 2002). Therefore, animals were observed and videotaped for 30 min before the injection (control or pre-injection behavior). A session following the same sequence was conducted after one of the animals was injected with Gly-SIFamide, or with prawn saline (vehicle solution) in the case of control injections (post-injection behavior). Tapes were watched in a random order, and data were registered by three trained observers who were blind to the injection status of the animals being observed.
Injections
The approach we used to inject the agents of interest was similar to that used by Glanzman and Krasne (1983), with some modifications. An animal whose interaction with another had been observed and recorded was cold-anesthetized for 5–7 min and was then held, dorsal side up, on the edge of a deep preparation dish containing aerated tank water in such a manner that the animal was flexed at the thoracoabdominal junction and its cephalothorax was submerged in the water so it could breath. While held in this position, the prawn then received a single injection of Gly-SIFamide (GenScript, Piscataway, NJ) at a concentration of 1 × 10−3 mol l−1 (dissolved in prawn saline or Ringer) using a syringe needle (Hamilton, 30 gauge) inserted into the dorsal abdominal artery, just before it entered the heart. We also performed control experiments where the prawn received a single injection of vehicle solution (prawn saline) in the same manner as the Gly-SIFamide injections. The prawn saline solution had the following composition (in mmol l−1): 220 NaCl, 5.5 KCl, 13.5 CaCl2, 2.5 MgCl2, 2.0 NaHCO3, pH = 7.4. Only one animal in each pair received the injection, and each animal was only used once. Injections were carried out both in dominant BC and submissive YC animals. The injections were performed by a trained individual, and solutions were injected slowly (over a 10-s period). The animal was then placed in a 20-l glass tank (16 × 8 × 10 in [≈40.6 × 20.3 × 25.4 cm]) for 45 min to allow recovery from the injection (recovery period). The animal was not placed in contact with the same animal it had been tested with during the control sessions until its mobility level was reestablished to a level comparable to that observed before the injection (see below). After the 45-min recovery period, the animal was placed in the behavioral observation 80-l glass tank (24 × 12 × 16 in [≈60.9 × 30.5 × 40.6 cm]) in contact with the other prawn, and their interactions were recorded and compared with those of the pre-injection observation session. After testing, prawns were placed in isolated tanks and observed for 4 days to make sure they would not molt. Data from animals that molted during this period were discarded.
Number and duration of attacks
Besides measuring the overall DI and the DI level for individual parameters, we also counted the number of attacks and the duration of fights for each BC/YC pair, before and after injections. The beginning of an attack was registered when one of the animals thrust forward its chela towards the other animal. The attack was considered to be over once one of the animals retreated, escaped, or stopped pursuing the other. Retreating was defined as walking away from an attacking animal until being positioned out of reach of the latter. Escaping was defined as the triggering of reflexive flexion of abdominal muscles so that the animal quickly pulls itself away from the attacking party.
Locomotor activity
To assess possible changes in locomotion as a result of injections of vehicle (prawn saline) or Gly-SIFamide, we counted the number of lines demarcating a square pattern at the bottom of the tank that each prawn crossed during each consecutive 30-s interval for each 30-min recording period, before and after injections.
Statistical analysis
We used Sigma Stat ver. 3.0 (Systat Software, Inc., Chicago, IL) for all the statistical analysis of the behavioral data. Parametric one-way analysis of variance (ANOVA) was used to compare parameters of the DI between the different morphotypes, under control conditions and after injections. When significant differences were identified, a post hoc multiple comparison test (Tukey's test) was used to determine whether the observed significant differences were between the DIs of BCs and YCs either before or after injection, and when comparing pre- and post-injection DIs for BCs or pre- and post- injection DIs for YCs. We used the same statistical analysis to compare differences between how many times each animal performed each of the parameters used to calculate the DI. In all cases, results were considered significant when P < 0.05.
Results
The effect of Gly-SIFamide on aggressive behavior was assessed in the prawn by injecting this neuropeptide into the circulating haemolymph through the dorsal abdominal artery. To determine the potential effects of the injections on the interactive behavior between morphotypes, we paired a BC individual and a YC individual under various experimental conditions, described below. The animal's morphotype was determined on the basis of the coloration and relative size of its claws. The ratio of claw-to-body length of BCs is normally above 1.5, and that of YCs is normally above 0.5 and below 1.5. Values for animals used in the vehicle and Gly-SIFamide injections experiments are shown in Tables 2 and 3, respectively. A dominance index (DI) was calculated for each individual tested in all the paired interactions (the higher the DI value, the higher the dominance level).
Table 2. Mean and standard deviation of body and claw measurements of prawns used in vehicle injection experiments.
| Blue injected | Yellow injected | |||
|---|---|---|---|---|
| Parameter | BC (n = 5) |
YC (n = 5) |
BC (n = 5) |
YC (n = 5) |
| Body length (cm) | 14.7 ± 1.3 | 13.3 ± 0.7 | 13.9 ± 0.2 | 13.3 ± 0.9 |
| Claw length (cm) | 28.3 ± 0.5 | 15.8 ± 0.0 | 26.6 ± 0.3 | 14.9 ± 0.2 |
| Right | 27.9 ± 2.7 | 15.7 ± 1.5 | 26.8 ± 2.0 | 15.0 ± 2.7 |
| Left | 28.7 ± 4.1 | 15.8 ± 1.6 | 26.4 ±1.8 | 14.7 ± 2.5 |
| Ratio (Claw:Body) | 1.9 ± 0.1 | 1.2 ± 0.1 | 1.9 ± 0.1 | 1.1 ± 0.1 |
Table 3. Mean and standard deviation of body and claw measurements of prawns used in SIFamide injection experiments.
| Blue injected | Yellow injected | |||
|---|---|---|---|---|
| Parameter | BC (n = 5) |
YC (n = 5) |
BC (n = 5) |
YC (n = 5) |
| Body length (cm) | 14.8 ± 0.9 | 12.7 ± 1.5 | 14.1 ± 0.2 | 13.5 ± 1.7 |
| Claw length (cm) | 29.3 ± 0.8 | 14.3 ± 0.0 | 27.5 ± 0.4 | 16.4 ± 0.3 |
| Right | 29.9 ±1.7 | 14.3 ± 2.0 | 27.8 ±1.3 | 16.6 ± 3.5 |
| Left | 28.8 ± 2.1 | 14.2 ± 2.0 | 27.2 ± 1.1 | 16.2 ± 3.7 |
| Ratio (Claw:Body) | 2.0 ± 0.1 | 1.1 ± 0.1 | 2.0 ± 0.1 | 1.2 ± 0.1 |
Typical behavior of paired BC and YC prawns under normal conditions
Under normal (control) conditions, when a BC and a YC are placed together in an 80-l glass tank (24 × 12 × 16 in [≈60.9 × 30.5 × 40.6 cm]), the BC establishes itself as the dominant animal and tends to attack the YC. This behavior pattern continues until an escape response is elicited in the YC or the animals are separated. In control experiments prior to assessing the effect of injecting submissive animals with Gly-SIFamide (e.g., pre-injection behavior: Fig. 1), the mean DI for BCs was 2.10 ± 0.10 (n = 5), while that of YCs was 1.37 ± 0.09 (n = 5). A similar pattern was observed in the pre-injection behavior for other experiments carried out to assess the effect of injecting Gly-SIFamide in dominant animals (Fig. 2), as well as in experiments in which only prawn Ringer was injected to either morphotype (Figs. 3 and 4).
Figure 1.
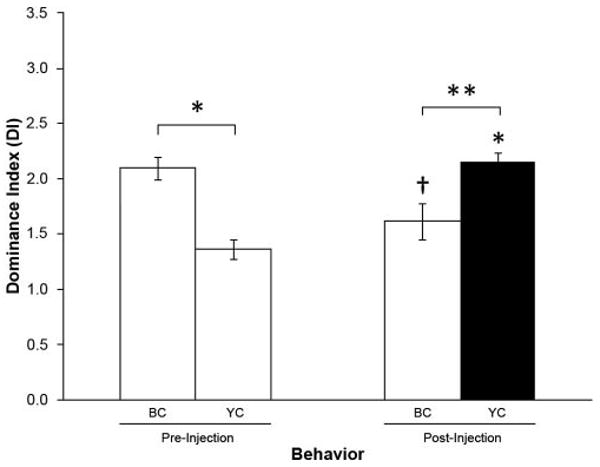
Gly-SIFamide injection into YCs. Effect on mean dominance index (DI) before (pre) and after (post) injection of the YCs with 1 × 10−3 mol l−1 Gly-SIFamide (n = 5). Before the injection, BCs established themselves as the dominant animals and YCs as the subordinates (one-way ANOVA, Tukey's test, P ≤ 0.001). When the YCs were injected with Gly-SIFamide, they behaved very much as do BCs under control conditions. The behavior of BCs confronted with Gly-SIFamide-injected YCs was also modified, showing a significant decrease in the levels of aggression compared to control conditions. The asterisk (*) over the YC post-injection group represents a significant difference from the YCs in control conditions (before the injection). The cross (†) over the BC post-injection group represents a significant difference from the BCs in control conditions. BC: blue clawed prawns; YC: yellow clawed prawns; filled bar indicates the mean DI of the injected (YC) prawns; *P ≤ 0.001; **P ≤ 0.025; † P ≤ 0.050.
Figure 2.
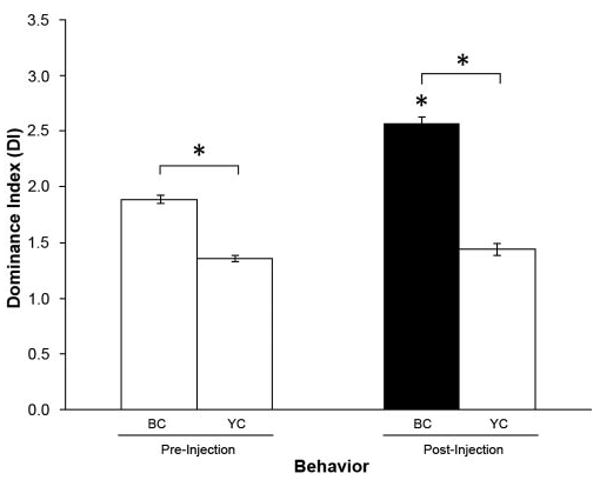
Gly-SIFamide injection into BCs. Effect on the mean dominance index (DI) before (pre) and after (post) injection of the BCs with 1 × 10−3 mol l−1 Gly-SIFamide (n = 5). When the BCs were injected, they further increased their aggression toward YCs when compared to control conditions (asterisk [*] over the BC post-injection group, P ≤ 0.001). BC: blue clawed prawns; YC: yellow clawed prawns; filled bar indicates the mean DI of the injected (BC) prawns; *P ≤ 0.001.
Figure 3.
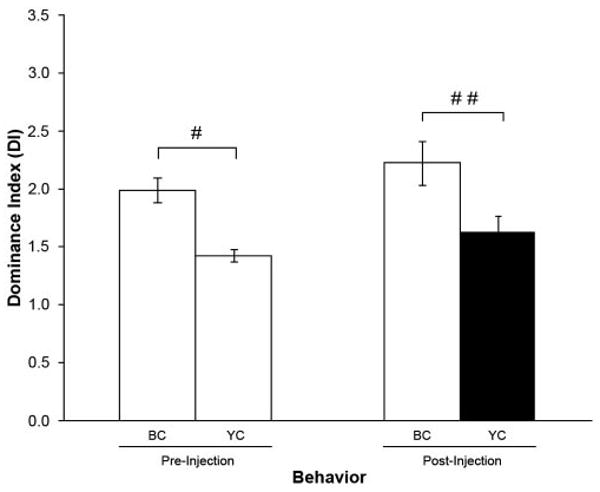
Vehicle injection into YCs. Effect on the mean dominance index (DI) before (pre) and after (post) injection of the YC with prawn saline (n = 5). Injection of vehicle solution alone did not change the DI for YCs. BC: blue clawed prawns; YC: yellow clawed prawns; filled bar indicates the mean DI of the injected (YC) prawns; #P ≤ 0.002; ##P ≤ 0.040.
Figure 4.
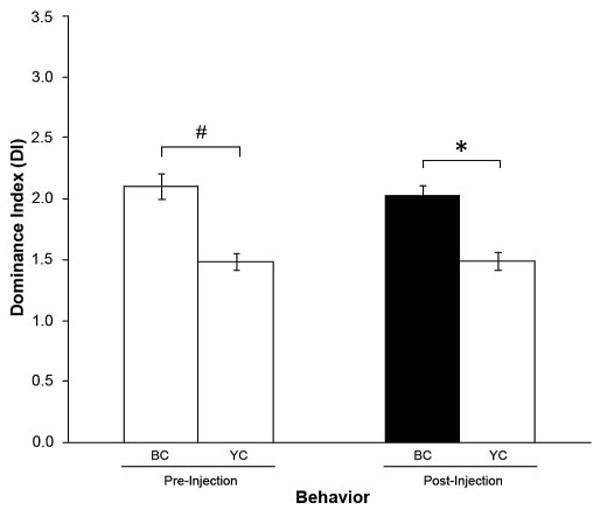
Vehicle injection into BCs. Effect on the mean dominance index (DI) before (pre) and after (post) injection of the BCs with prawn saline (n = 5). Injection of vehicle solution alone did not change the DI for BCs. BC: blue clawed prawns; YC: yellow clawed prawns; filled bar indicates the mean DI of the injected (BC) prawns; *P ≤ 0.001; #P ≤ 0.002.
Gly-SIFamide injection in the YC prawn
When the YC was injected with Gly-SIFamide (1 × 10−3 mol 1−1), the typical behavior of paired BC and YC prawns usually observed under normal conditions appeared to be reversed (Fig. 1). YCs injected with Gly-SIFamide behaved very much like BCs do under control conditions (mean DI of 2.16 ± 0.08; n = 5). Interestingly, this increase in aggression in the normally submissive prawn was accompanied by a statistically significant decrease (one-way ANOVA, Tukey's test, P = 0.041) in the levels of aggression of the normally dominant BC (mean DI of 1.62 ± 0.17, n = 5) when compared to control conditions (Fig. 1), even though the BC animal did not receive an injection.
Gly-SIFamide injection in the BC prawn
We also injected Gly-SIFamide into the circulating hemolymph of BC in other BC/YC pairs. Injections of Gly-SIFamide (1 × 10-3 mol l−1) increased even more the BC's normal aggression toward the YC (Fig. 2). The BC injected with Gly-SIFamide very quickly approached the YC and started pushing and grabbing him. The YC was always cornered and repeatedly attempted to push the BC away with its claws. The BC kept up this incessant attack until an escape response was elicited in the YC. Though the YC tried to get away, the BC kept fighting and would not retreat. The mean DI for BCs in these experiments was 2.57 ± 0.06 (n = 5), while that of YCs was 1.44 ± 0.06 (n = 5). Thus, levels of aggression were further enhanced in the BC by injection of Gly-SIFamide.
Vehicle injection in the BC and YC prawns
To rule out the possibility that the observed changes in behavior could be a result of the injection process, experiments were done in which the BC or the YC was injected only with vehicle solution (prawn saline). There were no significant changes in the level of aggression in these animals, as shown by comparing the DI before and after the injection of the vehicle into the YC (Fig. 3: P = 0.325 when comparing pre- and post-injection DIs for BCs; P = 0.215 when comparing pre- and post-injection DIs for YCs) or into the BC (Fig. 4: P = 0.621 when comparing pre- and post-injection DIs for BCs; P = 1.000 when comparing pre- and post-injection DIs for YCs). Thus, we can conclude that the injection process is not responsible for the changes in DI observed following injection of Gly-SIFamide into the BCs or YCs.
Effects of Gly-SIFamide on specific parameters of aggressive behavior
To determine which specific behavioral components of agonistic behavior increase or decrease after the Gly-SIFamide injection, we group our data in terms of how many times each animal performed each of the parameters used to calculate the DI before and after the injection of the neuropeptide. For data collected during pre- and post-injection sessions, a one-way ANOVA analysis found significant overall differences among the parameters for 4 of the 5 measured behavioral categories (n = 5; P ≤ 0.05). These included position of body and walking legs, movement and position of claws, movement of chela, and instances of movement toward or away from each other (Table 4). To determine the origin of the significant differences among groups, Tukey's tests were performed to compare BC and YC post-injection data to their corresponding control condition data. In general, these tests revealed that injection of Gly-SIFamide produced an increase in the number of times the animal performed a more dominant behavior, resulting in a decrease in the more submissive behaviors. We also found that the greater effects of the Gly-SIFamide injection were on the movement and position of claws, as well as on the movement of chela. Interestingly, we did not find any significant differences in the abdomen posture after the injection of the neuropeptide compared to control conditions.
Table 4. Significant differences in agonistic behaviors among BCs and YCs receiving Gly-SIFamide injections.
| Behavioral Parameters | BC Injected | YC injected | |||
|---|---|---|---|---|---|
| BC* | YC | BC | YC* | ||
| Position of Body and Walking Legs | |||||
| Legs fully flexed/body touching bottom | [0] | +(38) | −36 | ||
| Legs fully flexed/body touching bottom | [1] | −29 | |||
| Legs partially flexed/body partially lifted | [2] | +12 | |||
| Legs partially flexed/body partially lifted | [3] | ||||
| Legs partially flexed/body partially lifted | [4] | ||||
| Legs fully extended/body fully lifted | [5] | ||||
| Legs fully extended/body fully lifted | [6] | ||||
| Movement and Position of Claws | |||||
| Crossed in front of eyes | [0] | ||||
| Touching bottom while extended | [1] | −39 | |||
| Touching bottom while flexed | [2] | −35 | +(22) | ||
| At body level | [3] | +34 | +12 | −(24) | +34 |
| Above body level | [4] | ||||
| Extended at or above body level | [5] | ||||
| Quickly thrust forward/lifting other prawn | [6] | ||||
| Movement of Chela | |||||
| Closed/not touching the other prawn | [0] | −60 | +37 | −65 | |
| Closed/touching the other prawn | [1] | −11 | −17 | ||
| Open/not touching the other prawn | [2] | +58 | +68 | ||
| Open/touching the other prawn | [3] | ||||
| Scissoring in front of the other prawn | [4] | ||||
| Open, grabbing or lifting the other prawn | [5] | ||||
| Nipping or Tearing | [6] | ||||
| Movement Toward or Away From Each Other | |||||
| Rush to move away from other prawn w/o facing it | [0] | +1 | |||
| Walk away from other prawn w/o facing it | [1] | −(7) | |||
| Walk away from other prawn while facing it | [2] | ||||
| Turn away from other prawn, w/o walking away from it | [3] | −4 | |||
| Turn towards other prawn, w/o walking away from it | [4] | −(5) | |||
| Walk toward the other prawn while facing it | [5] | −6 | |||
| Rush toward the other prawn while facing it | [6] | ||||
The asterisk represents the animal injected with Gly-SIFamide in each pair interactions. Data values shown indicate where statistically significant differences were observed. These numbers are the mean percentage of the difference between how many times each animal performed each parameter before and after injection of the neuropeptide (the higher the number, the higher the magnitude of the effect). White spaces indicate parameters where nonsignificant differences were found. The values in brackets indicate the weight, or level, of the parameter as an indicator of dominance/subordination, 6 indicating the most highly dominant behavior, 0 indicating complete or total subordination. Numbers in brackets indicates a tendency. Plus and minus symbols represent a significant increase or decrease in the performed behavior after the injection compared to the control (before the injection), respectively.
The only significant differences seen in the experiments where the BCs were injected with Gly-SIFamide were in 2 of the 5 measured behavioral categories, movement and position of claws, and movement of chela (Table 2). In these experiments, the injected BCs and the non-injected YCs significantly increased the number of times they positioned their claws at body level. However, the difference in the injected BCs is higher than in the non-injected YCs. We also found that the above increase in BCs was accompanied by a significant decrease in the number of times they positioned claws touching the bottom of the tank while flexed.
The major significant differences were seen in the experiments where the YCs were injected with Gly-SIFamide (Table 4). In these experiments, injected YCs showed an increase in how many times the body was partially lifted relative to the bottom, the claws were at body levels and the chelae were open and touching the other prawn. We also found a significant reduction in the number of times injected YCs positioned the body touching the bottom of the tank, their claws touching the bottom while extended, and their chelae closed without touching the other prawn. Interestingly, the non-injected BCs exhibited more submissive behaviors after the injection of Gly-SIFamide into the YCs compared to control conditions (Table 4). These animals tended to maintain their bodies touching the bottom and their claws touching the bottom while flexed. Also, non-injected BCs showed a significant increase in instances of movement toward or away from each other such as turning away from the other prawn without walking away from it, and walking toward the other prawn while facing it. Thus, Gly-SIFamide acted to increase or decrease the performance of several important agonistic behaviors.
Effects of injections on number and duration of attacks
The effects of vehicle (prawn saline) and Gly-SIFamide injection were also examined in terms of number and duration of attacks between prawns. Data analysis showed no statistically significant effects when we compared the number of attacks and their duration before and after the injection of vehicle solution or Gly-SIFamide (Fig. 5). Nevertheless, nonsignificant trends toward an increase in the number of attacks and fighting duration were observed when BCs were injected with Gly-SIFamide (Fig. 5C, D; n = 5, P = 0.216 and P = 0.271, respectively). A similar nonsignificant trend toward an increase in fighting duration was also observed when BCs and YCs were injected with vehicle solution (Fig. 5B; n = 5, P = 0.264 and P = 0.053, respectively).
Figure 5.
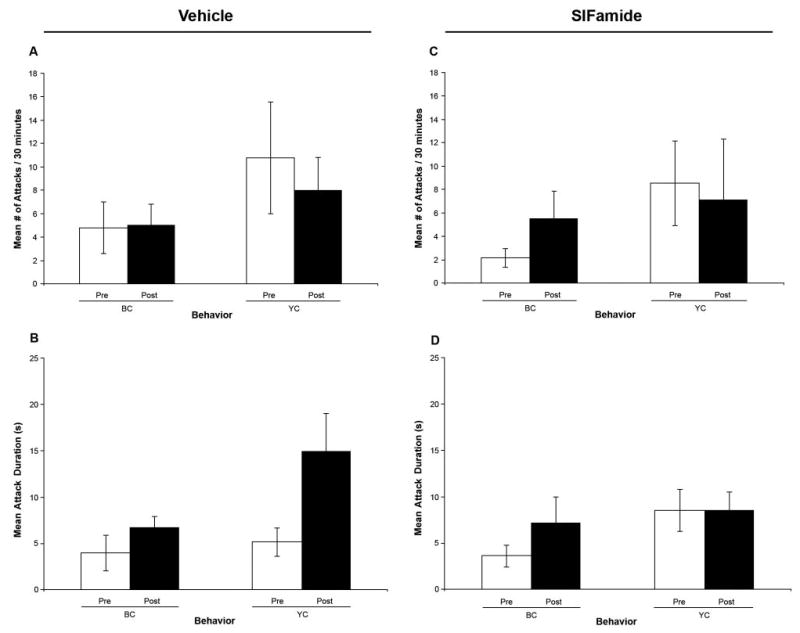
Effects of vehicle and SIFamide injections on number and duration of attacks between prawns. No statistically significant effects were observed on the number of attacks and fight duration for any of the injection experiments. Effect on the mean number of attacks (A) and attack duration (B) before (pre) and after (post) injection of the BC (n = 5) and YC (n = 5) with prawn saline, respectively. Effect on the mean number of attacks (C) and attack duration (D) before (pre) and after (post) injection of the BC (n = 5) and YC (n = 5) with Gly-SIFamide, respectively. BC: blue clawed prawns; YC: yellow clawed prawns; Pre: pre-injection behavior; Post: post-injection behavior; filled bar indicates the injected prawns in each set of experiments.
Effects of injections on locomotor activity
To examine the effects of vehicle (prawn saline) and Gly-SIFamide injection on locomotor activity, we assessed general locomotion by counting the number of lines crossed by each prawn during a 30-s period before and after the injection. Our data revealed that neither vehicle nor SIFamide injections had any statistically significant effect on locomotion of the animals (Fig. 6). However, in the experiments where we injected vehicle solution, we observed a nonsignificant trend toward a decrease in the number of lines crossed after injecting the BCs (Fig. 6A; n = 5, P = 0.195) and YCs (Fig. 6B; n = 4, P = 0.306). A similar nonsignificant trend toward a decrease in locomotion was also observed when YCs were injected with Gly-SIFamide (Fig. 6D; n = 5, P = 0.156). Interestingly, in the latter experiments, the non-injected BCs also showed a nonsignificant trend toward a decrease in locomotor activity (Fig. 6D; n = 5, P = 0.056).
Figure 6.
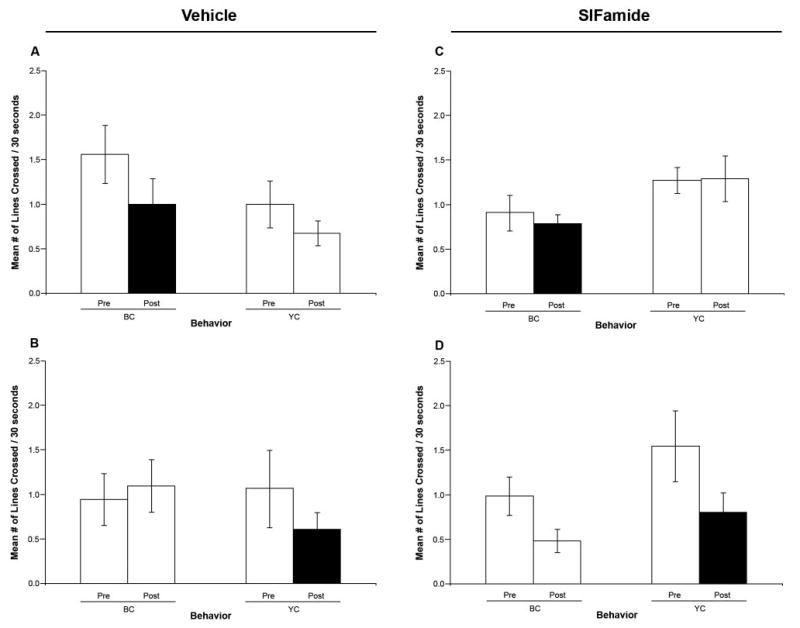
Effects of vehicle and SIFamide injections on the prawn's locomotion. No statistically significant effects were observed on locomotor activity for any of the injection experiments. (A) and (B) Effect on the mean number of lines crossed in 30-s intervals during a 30-min recording period, before (pre) and after (post) injection of the BCs (n = 5) and YCs (n = 4) with prawn saline, respectively. (C) and (D) Effect on the mean number of lines crossed in 30-s intervals during a 30-min recording period, pre- and post-injection of the BCs (n = 5) and YCs (n = 5) with Gly-SIFamide, respectively. BC: blue clawed prawns; YC: yellow clawed prawns; Pre: pre-injection behavior; Post: post-injection behavior; filled bar indicates the mean number of lines crossed in a 30-s interval by the injected (BC or YC) prawns.
Discussion
The different patterns of behavior associated with each of the three male morphotypes and their status within a fixed dominance hierarchy are the advantages of using the freshwater prawn as a model system to study aggressive behavior. In several studies on aggressive interactions among conspecifics in crustaceans, the parameters used to determine levels of aggression or degree of dominance have involved observing who initiates fighting behavior, who retreats first, how many times contact is established using claws, and the posture of the abdomen (Livingstone et al., 1980; Kravitz, 1988; Huber and Delago, 1998; Tierney and Mangiamele, 2001; Herberholz et al., 2003, 2004). Fighting behavior may be further characterized by breaking down the animal's behavioral sequences into individual actions, movements, or both that can then be assigned numerical values according to how important or relevant they are as indicators of aggression or submission. This approach has been used previously in determining the effect of size and morphotype on dominance hierarchies of Macrobrachium rosenbergii (Barki et al., 1992).
Although injecting substances such as biogenic amines or neuropeptides in the hemolymph of a living animal will most likely induce general systemic effects, these types of experiments can nevertheless be an essential initial step in elucidating the mechanisms that underlie a specific behavior, by narrowing down the range of agents that should be studied in more detail at the circuit or cellular level. We used this quantitative approach to measure the level of aggression/submission in interactions between BC/YC pairs before and after injecting Gly-SIFamide directly into the circulating hemolymph of the living animal.
In this study, we observed that YCs receiving a Gly-SIFamide injection showed a level of aggression that is normally characteristic of the BCs. These animals became so aggressive that they actively attacked BCs, a behavior that rarely occurred under normal circumstances. We also observed that Gly-SIFamide injections into the circulating hemolymph of BCs increased their aggressiveness toward YCs even more. Together, these behavioral data suggest that levels of aggression among interacting BC/YC prawns can be enhanced by injection of the neuropeptide SIFamide into the circulating hemolymph.
It is interesting that when only the YC received a Gly-SIFamide injection, the behavior of the BC was also modified. Under these circumstances, a significant decrease was observed in the levels of aggression of the normally dominant BC. This change in the behavior of BC could be explained as an effect of observation of the behavior of its rival or by influence from water-borne agents produced by the injected YC. In crayfish, injection of serotonin in a subordinate animal results in a reduction in the likelihood of the animal deciding to retreat during a fight (Huber et al., 1997a, b). Although this change can indeed be considered as less subordinate behavior, a complete reversal in status of the fighting animals, like that seen here in the prawn, has not been recorded. The results observed in our experiments following injection of Gly-SIFamide in a subordinate YC suggest a full reversal in status of both the injected and non-injected animals of a fighting BC/YC pair.
It has been reported that injection of serotonin in juvenile lobsters at concentrations required for observing an effect on aggressive behavior also inhibits locomotion, making interpretation of results more difficult (Peeke et al., 2000). This is not the case in the freshwater prawn, where injection of Gly-SIFamide at a concentration affecting aggression (1 × 10−3 mol l−1) does not affect the animal's mobility any more than does placing the animal on ice to perform a saline injection, a transient effect from which the animal recovers in a short time. Our results demonstrated that there were no statistically significant effects on locomotion after the injection of vehicle solution or Gly-SIFamide. However, a nonsignificant trend toward a decrease in locomotor activity was observed in YCs after the injection of either vehicle or Gly-SIFamide. YCs used in this study had a shorter body length than BCs (Tables 3 and 4) and may have had a smaller diameter in the dorsal abdominal artery, the site of injection. This may have resulted in a higher susceptibility to a reduction in general level of activity following injections. Nevertheless, these changes were not significant and thus did not mask the observed increase in aggressive behavior after injection of Gly-SIFamide. Interestingly, when YCs were injected with Gly-SIFamide, a nonsignificant reduction was also observed in the non-injected animal. Under these conditions, this reduction in the locomotor activity of BCs could be explained as an effect of observation of the behavior of its rival or by influence from water-borne agents produced by the injected YC. In the literature, we found only two functional studies on the physiological roles played by SIFamides, one in a crustacean and the other in an insect. In the American lobster Homarus americanus, exogenous application of Val-SIFamide to the isolated stomatogastric ganglion produced a profound modulation of the output of the pyloric neural circuit (Christie et al., 2006). In the fruit fly Drosophila melanogaster, results from studies using ablation of the SIFamide neurons and RNA interference have implicated Ala-SIFamide in the control of sexual behavior (Terhzaz et al., 2007). Also, several immunohistochemical and in situ hybridization studies suggest additional neuromodulatory roles for the SIFamides within the CNS, including modulation of the olfactory system (Yasuda et al., 2004; Yasuda-Kamatani and Yasuda, 2006). Recently, Christie et al. (2007) found that Gly-SIFamide is located in and released by midgut epithelial endocrine cells in several Cancer crabs, suggesting a possible gut paracrine/endocrine role for the SIFamide family. In the present study, we performed behavioral experiments in which the interactions between prawn pairs were recorded and quantified before and after injecting Gly-SIFamide directly into the circulating hemolymph of the living animal. Our data show that levels of aggression among interacting BC/YC prawns can be enhanced by injection of the Gly-SIFamide, suggesting a function in the modulation of aggression in the prawn. Our study thus constitutes the first report demonstrating a role for Gly-SIFamide in modulating aggressive behavior and, to our knowledge, the first description of the effects of injecting a neuropeptide in the circulatory system of a living animal. It remains to be determined through future experiments whether this neuropeptide may also play a part in other forms of the prawn's interactive behaviors (e.g., mating) or in regulating the mechanisms by which the animal progresses from one morphotype to the next. Further experiments are also needed to characterize the function of Gly-SIFamide at the circuit and molecular levels. A better understanding of the mechanisms involved in mediating and/or modulating interactive behaviors in simple model systems such as crustaceans will ultimately help enable the identification of novel sources for treatments of a wide variety of mental health disorders, through identification of new potential targets for drugs or gene therapies.
Acknowledgments
This investigation was supported by NIH/MBRS SCORE S06GM008224, NIH/MRISP MH48190, NIH-NCRR/RCMI G12RR03051, and NIH/MBRS RISE R25-GM061838.
Abbreviations
- Gly-SIFamide
GYRKPPFNGSIFamide
- Val-SIFamide
VYRKPPFNGSIFamide
- Ala-SIFamide
AYRKPPFNGSIFamide
- SC
male small claw
- YC
male yellow claw
- BC
male blue claw
- DI
dominance index
Literature Cited
- Antonsen BL, Edwards DH. Mechanisms of serotonergic facilitation of a command neuron. J Neurophysiol. 2007;98:3494–3504. doi: 10.1152/jn.00331.2007. [DOI] [PubMed] [Google Scholar]
- Audsley N, Weaver RJ. Analysis of peptides in the brain and corpora cardiaca-corpora allata of the honey bee, Apis mellifera using MALDI-TOF mass spectrometry. Peptides. 2006;27:512–520. doi: 10.1016/j.peptides.2005.08.022. [DOI] [PubMed] [Google Scholar]
- Barki A, Karplus I, Goren M. Effects of size and morphotype on dominance hierarchies and resource competition in the freshwater prawn Macrobrachium rosenbergii. Anim Behav. 1992;44:547–555. [Google Scholar]
- Bliss DE. Shrimps, Lobsters and Crabs. Their Fascinating Life Story. New Century Publishers; Piscataway, NJ: 1982. pp. 79–107. [Google Scholar]
- Brody T, Cohen D, Barnes A, Spector A. Yield characteristics of the prawn Macrobrachium rosenbergii in temperate zone aquaculture. Aquaculture. 1980;21:375–385. [Google Scholar]
- Bulau P, Meisen I, Schmitz T, Keller R, Peter-Katalinic J. Identification of neuropeptides from the sinus gland of the crayfish Orconectes limosus using nanoscale on-line liquid chromatography tandem mass spectrometry. Mol Cell Proteomics. 2004;3:558–564. doi: 10.1074/mcp.M300076-MCP200. [DOI] [PubMed] [Google Scholar]
- Caldwell RL. Assessment strategies in stomatopods. Bull Mar Sci. 1987;41:135–150. [Google Scholar]
- Cape SS, Rehm KJ, Ma M, Marder E, Li L. Mass spectral comparison of the neuropeptide complement of the stomatogastric ganglion and brain in the adult and embryonic lobster, Homarus americanus. J Neurochem. 2008;105:690–702. doi: 10.1111/j.1471-4159.2007.05154.x. [DOI] [PubMed] [Google Scholar]
- Christie AE. Neuropeptide discovery in Ixodoidea: an in silico investigation using publicly accessible expressed sequence tags. Gen Comp Endocrinol. 2008a;157:174–185. doi: 10.1016/j.ygcen.2008.03.027. [DOI] [PubMed] [Google Scholar]
- Christie AE. In silico analyses of peptide paracrines/hormones in Aphidoidea. Gen Comp Endocrinol. 2008b;159:67–79. doi: 10.1016/j.ygcen.2008.07.022. [DOI] [PubMed] [Google Scholar]
- Christie AE, Stemmler EA, Peguero B, Messinger DI, Provencher HL, Scheerlinck P, Hsu YWA, Guiney ME, De la Iglesia HO, Dickinson PS. Identification, physiological actions, and distribution of VYRKPPFNGSIFamide (Val1-SIFamide) in the stomatogastric nervous system of the American lobster Homarus americanus. J Comp Neurol. 2006;496:406–421. doi: 10.1002/cne.20932. [DOI] [PubMed] [Google Scholar]
- Christie AE, Kutz-Naber KK, Stemmler EA, Klein A, Messinger DI, Goiney CC, Conterato AJ, Bruns EA, Hsu YW, Li L, Dickinson PS. Midgut epithelial endocrine cells are a rich source of the neuropeptides APSGFLGMRamide (Cancer borealis tachykinin-related peptide Ia) and GYRKPPFNGSIFamide (Gly1-SIFamide) in the crabs Cancer borealis, Cancer magister and Cancer productus. J Exp Biol. 2007;210:699–714. doi: 10.1242/jeb.02696. [DOI] [PubMed] [Google Scholar]
- Cohen D, Ra'anan Z, Brody T. Population profile development and morphotypic differentiation in the giant freshwater prawn Macrobrachium rosenbergii (de Man) J World Maricult Soc. 1981;12:231–243. [Google Scholar]
- De Man JG. Notes from the Royal Zoological Museum of the Netherlands at Leyden. Brill; Leyden: 1879. On some species of the genus Palaemon Fabr. with descriptions of two new forms; pp. 165–184. [Google Scholar]
- Dickinson PS, Stemmler EA, Cashman CR, Brennan HR, Dennison B, Huber KE, Peguero B, Rabacal W, Goiney CC, Smith CM, Towle DW, Christie AE. SIFamide peptides in clawed lobsters and freshwater crayfish (Crustacea, Decapoda, Astacidea): a combined molecular, mass spectrometric and electrophysiological investigation. Gen Comp Endocrinol. 2008;156:347–360. doi: 10.1016/j.ygcen.2008.01.011. [DOI] [PubMed] [Google Scholar]
- Edwards DH, Heitler WJ, Krasne FB. Fifty years of a command neuron: the neurobiology of escape behavior in the crayfish. Trends Neurosci. 1999;22:153–161. doi: 10.1016/s0166-2236(98)01340-x. [DOI] [PubMed] [Google Scholar]
- Edwards DH, Yeh SR, Musolf BE, Antonsen BL, Krasne FB. Metamodulation of the crayfish escape circuit. Brain Behav Evol. 2002;60:360–369. doi: 10.1159/000067789. [DOI] [PubMed] [Google Scholar]
- Espinoza SY, Breen L, Varghese N, Faulkes Z. Loss of escape-related giant neurons in a spiny lobster, Panulirus argus. Biol Bull. 2006;211:223–231. doi: 10.2307/4134545. [DOI] [PubMed] [Google Scholar]
- Gard AL, Lenz PH, Shaw JR, Christie AE. Identification of putative peptide paracrines/hormones in the water flea Daphnia pulex (Crustacea; Branchiopoda; Cladocera) using transcriptomics and immunohistochemistry. Gen Comp Endocrinol. 2009;160:271–287. doi: 10.1016/j.ygcen.2008.12.014. [DOI] [PubMed] [Google Scholar]
- Glanzman DL, Krasne FB. Serotonin and octopamine have opposite modulatory effects on the crayfish's lateral giant escape reaction. J Neurosci. 1983;3:2263–2269. doi: 10.1523/JNEUROSCI.03-11-02263.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris-Warrick RM. Amine modulation of extension command element-evoked motor activity in lobster abdomen. J Comp Physiol A. 1985;156:875–884. [Google Scholar]
- Hazlett BA. Chemical detection of sex and condition in the crayfish Orconectes virilis. J Chem Ecol. 1985;11:181–189. doi: 10.1007/BF00988201. [DOI] [PubMed] [Google Scholar]
- Hazlett BA. Disturbance pheromone in the hermit crab Calcinus laevimanus (Randall, 1840) Crustaceana. 1990;58:314–316. [Google Scholar]
- Herberholz J, Sen MM, Edwards DH. Parallel changes in agonistic and non-agonistic behaviors during dominance hierarchy formation in crayfish. J Comp Physiol A. 2003;189:321–325. doi: 10.1007/s00359-003-0409-z. [DOI] [PubMed] [Google Scholar]
- Herberholz J, Sen MM, Edwards DH. Escape behavior and escape circuit activation in juvenile crayfish during prey-predator interactions. J Exp Biol. 2004;207:1855–1863. doi: 10.1242/jeb.00992. [DOI] [PubMed] [Google Scholar]
- Huber R, Delago A. Serotonin alters decisions to withdraw in fighting crayfish, Astacus astacus: the motivational concept revisited. J Comp Physiol A. 1998;182:573–583. [Google Scholar]
- Huber R, Kravitz EA. A quantitative analysis of agonistic behavior in juvenile American lobsters (Homarus americanus L.) Brain Behav Evol. 1995;46:72–83. doi: 10.1159/000113260. [DOI] [PubMed] [Google Scholar]
- Huber R, Smith K, Delago A, Isaksson K, Kravitz EA. Serotonin and aggressive motivation in crustaceans: altering the decision to retreat. Proc Natl Acad Sci USA. 1997a;94:5939–5942. doi: 10.1073/pnas.94.11.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber R, Orzeszyna M, Pokorny N, Kravitz EA. Biogenic amines and aggression: experimental approaches in crustaceans. Brain Behav Evol. 1997b;50 1:60–68. doi: 10.1159/000113355. [DOI] [PubMed] [Google Scholar]
- Huxley TH. The Crayfish. An Introduction to the Study of Zoology. MIT Press; Cambridge: 1973. [Google Scholar]
- Huybrechts J, Nusbaum MP, Bosch LV, Baggerman G, De Loof A, Schoofs L. Neuropeptidomic analysis of the brain and thoracic ganglion from the Jonah crab, Cancer borealis. Biochem Biophys Res Commun. 2003;208:535–544. doi: 10.1016/s0006-291x(03)01426-8. [DOI] [PubMed] [Google Scholar]
- Janssen I, Schoofs L, Spittaels K, Neven H, Vanden Broeck J, Devreese B, Van Beeumen J, Shabanowitz J, Hunt DF, De Loof A. Isolation of NEB-LFamide, a novel myotropic neuropeptide from the grey fleshfly. Mol Cell Endocrinol. 1996;117:157–165. doi: 10.1016/0303-7207(95)03746-2. [DOI] [PubMed] [Google Scholar]
- Krasne FB, Edwards DH. Crayfish escape behavior: lessons learned. In: Wiese K, editor. Crustacean Experimental Systems in Neurobiology. Springer-Verlag; Berlin: 2002. pp. 3–22. [Google Scholar]
- Krasne FB, Shamsian A, Kulkarni R. Altered excitability of the crayfish lateral giant escape reflex during agonistic encounters. J Neurosci. 1997;17:709–716. doi: 10.1523/JNEUROSCI.17-02-00709.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz EA. Hormonal control of behavior: amines and the biasing of behavioral output in lobsters. Science. 1988;241:1775–1781. doi: 10.1126/science.2902685. [DOI] [PubMed] [Google Scholar]
- Li L, Pulver SR, Kelley WP, Thirumalai V, Sweedler JV, Marder E. Orcokinin peptides in developing and adult crustacean stomatogastric nervous systems and pericardial organs. J Comp Neurol. 2002;444:227–244. doi: 10.1002/cne.10139. [DOI] [PubMed] [Google Scholar]
- Li LJ, Kelley WP, Billimoria CP, Christie AE, Pulver SR, Sweedler JV, Marder E. Mass spectrometric investigation of the neuropeptide complement and release in the pericardial organs of the crab, Cancer borealis. J Neurochem. 2003;87:642–656. doi: 10.1046/j.1471-4159.2003.02031.x. [DOI] [PubMed] [Google Scholar]
- Livingstone MS, Harris-Warrick RM, Kravitz EA. Serotonin and octopamine produce opposite postures in lobsters. Science. 1980;208:76–79. doi: 10.1126/science.208.4439.76. [DOI] [PubMed] [Google Scholar]
- Ma PM, Beltz BS, Kravitz EA. Serotonin-containing neurons in lobsters: their role as gain-setters in postural control mechanisms. J Neurophysiol. 1992;68:36–54. doi: 10.1152/jn.1992.68.1.36. [DOI] [PubMed] [Google Scholar]
- Ma M, Chen R, Sousa GL, Bors EK, Kwiatkowski MA, Goiney CC, Goy MF, Christie AE, Li L. Mass spectral characterization of peptide transmitters/hormones in the nervous system and neuroendocrine organs of the American lobster Homarus americanus. Gen Comp Endocrinol. 2008a;156:395–409. doi: 10.1016/j.ygcen.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M, Christie AE, Li L. Peptidomic analyses of the nervous systems of the prawn Macrobrachium rosenbergii and the white shrimp Litopenaeus vannamei. Soc Neurosci Abstracts. 2008b;494.19 [Online]. Available: http://www.sfn.org/index.aspx?pagename=abstracts_ampublications [23 Sept. 2009]
- Ma M, Bors EK, Dickinson ES, Kwiatkowski MA, Sousa GL, Henry RP, Smith CM, Towle DW, Christie AE, Li L. Characterization of the Carcinus maenas neuropeptidome by mass spectrometry and functional genomics. Gen Comp Endocrinol. 2009a;161:320–334. doi: 10.1016/j.ygcen.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M, Wang J, Chen R, Li L. Expanding the crustacean neuropeptidome using a multifaceted mass spectrometric approach. J Proteome Res. 2009b;8:2426–2437. doi: 10.1021/pr801047v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messinger DI, Kutz KK, Le T, Verley DR, Hsu YA, Ngo CT, Cain SD, Birmingham JT, Li L, Christie AE. Identification and characterization of a tachykinin-containing neuroendocrine organ in the commissural ganglion of the crab Cancer productus. J Exp Biol. 2005;208:3303–3319. doi: 10.1242/jeb.01787. [DOI] [PubMed] [Google Scholar]
- Nichols R. The discovery of novel neuropeptides takes flight. Genome Biol. 2002;3:1032. doi: 10.1186/gb-2002-3-11-reviews1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson CL, Karlsson G, Bergquist J, Westman A, Ekman R. Mass spectrometry of peptides in neuroscience. Peptides. 1998;19:781–789. doi: 10.1016/s0196-9781(97)00471-3. [DOI] [PubMed] [Google Scholar]
- Peeke HV, Blank GS, Figler MH, Chang ES. Effects of exogenous serotonin on a motor behavior and shelter competition in juvenile lobsters (Homarus americanus) J Comp Physiol A. 2000;186:575–582. doi: 10.1007/s003590000113. [DOI] [PubMed] [Google Scholar]
- Polanska MA, Yasuda A, Harzsch S. Immunolocalisation of crustacean-SIFamide in the median brain and eyestalk neuropils of the marbled crayfish. Cell Tissue Res. 2007;330:331–344. doi: 10.1007/s00441-007-0473-8. [DOI] [PubMed] [Google Scholar]
- Ra'anan Z, Cohen D. The ontogeny of social structure and population dynamics in the freshwater prawn Macrobrachium rosenbergii (de Man) In: Schram FM, Wenner A, editors. Crustacean Issues II. Crustacean Growth. Balkema; Rotterdam: 1985. pp. 227–311. [Google Scholar]
- Ra'anan Z, Sagi A. Alternative mating strategies in male morphotypes of the freshwater prawn Macrobrachium rosenbergii (De Man) Biol Bull. 1985;169:592–601. [Google Scholar]
- Ra'anan Z, Sagi A, Wax Y, Karplus I, Hulata G, Kuris A. Growth, size rank, and maturation of the freshwater prawn, Macrobrachium rosenbergii: analysis of marked prawns in an experimental population. Biol Bull. 1991;181:379–386. doi: 10.2307/1542358. [DOI] [PubMed] [Google Scholar]
- Rehm KJ, Deeg KE, Marder E. Developmental regulation of neuromodulator function in the stomatogastric ganglion of the lobster, Homarus americanus. J Neurosci. 2008;28:9828–9839. doi: 10.1523/JNEUROSCI.2328-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roller L, Yamanaka N, Watanabe K, Daubnerová I, Zitnan D, Kataoka H, Tanaka Y. The unique evolution of neuropeptide genes in the silkworm Bombyx mori. Insect Biochem Mol Biol. 2008;38:1147–1157. doi: 10.1016/j.ibmb.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Sithigorngul P, Pupuem J, Krungkasem C, Longyant S, Chaivisuthangkura PW, Sithigorngul W, Petsom A. Seven novel FMRFamide-like neuropeptide sequences from the eyestalk of the giant tiger prawn Penaeus monodon. Comp Biochem Physiol. 2002;131B:325–337. doi: 10.1016/s1096-4959(01)00499-7. [DOI] [PubMed] [Google Scholar]
- Skiebe P, Dreger M, Meseke M, Evers JF, Hucho F. Identification of orcokinins in single neurons in the stomatogastric nervous system of the crayfish, Cherax destructor. J Comp Neurol. 2002;444:245–259. doi: 10.1002/cne.10145. [DOI] [PubMed] [Google Scholar]
- Skiebe P, Dreger M, Borner J, Meseke M, Weckwerth W. Immunocytochemical and molecular data guide peptide identification by mass spectrometry: orcokinin and orcomyotropin-related peptides in the stomatogastric nervous system of several crustacean species. Cell Mol Biol. 2003;49:851–871. [PubMed] [Google Scholar]
- Sosa MA, Baro DJ. Amine effects on aggression in the giant tropical freshwater prawn Macrobrachium rosenbergii. In: Wiese K, editor. The Crustacean Nervous System. Springer-Verlag; Berlin: 2002. pp. 143–155. [Google Scholar]
- Sullivan JM, Beltz BS. Newborn cells in the adult crayfish brain differentiate into distinct neuronal types. J Neurobiol. 2005;65:157–170. doi: 10.1002/neu.20195. [DOI] [PubMed] [Google Scholar]
- Terhzaz S, Rosay P, Goodwin SF, Veenstra JA. The neuropeptide SIFamide modulates sexual behavior in Drosophila. Biochem Biophys Res Commun. 2007;352:305–310. doi: 10.1016/j.bbrc.2006.11.030. [DOI] [PubMed] [Google Scholar]
- Teshiba TM, Shamsian A, Yashar B, Yeh SR, Edwards DH, Krasne FB. Dual and opposing modulatory effects of serotonin on crayfish lateral giant escape command neurons. J Neurosci. 2001;21:4523–4529. doi: 10.1523/JNEUROSCI.21-12-04523.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney AJ, Mangiamele LA. Effects of serotonin and serotonin analogs on posture and agonistic behavior in crayfish. J Comp Physiol A. 2001;187:757–767. doi: 10.1007/s00359-001-0246-x. [DOI] [PubMed] [Google Scholar]
- Tierney AJ, Greenlaw MA, Dams-O'Connor K, Aig SD, Perna AM. Behavioral effects of serotonin and serotonin agonists in two crayfish species, Procambarus clarkii and Orconectes rusticus. Comp Biochem Physiol A. 2004;139:495–502. doi: 10.1016/j.cbpb.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Vanden Broeck J. Neuropeptides and their precursors in the fruitfly, Drosophila melanogaster. Peptides. 2001;22:241–254. doi: 10.1016/s0196-9781(00)00376-4. [DOI] [PubMed] [Google Scholar]
- Verleyen P, Huybrechts J, Baggerman G, Van Lommel A, De Loof A, Schoofs L. SIFamide is a highly conserved neuropeptide: a comparative study in different insect species. Biochem Biophys Res Commun. 2004;320:334–341. doi: 10.1016/j.bbrc.2004.05.173. [DOI] [PubMed] [Google Scholar]
- Verleyen P, Huybrechts J, Schoofs L. SIFamide illustrates the rapid evolution in Arthropod neuropeptide research. Gen Comp Endocrinol. 2009;162:27–35. doi: 10.1016/j.ygcen.2008.10.020. [DOI] [PubMed] [Google Scholar]
- Weaver RJ, Audsley N. Neuropeptides of the beetle, Tenebrio molitor identified using MALDI-TOF mass spectrometry and deduced sequences from the Tribolium castaneum genome. Peptides. 2008;29:168–178. doi: 10.1016/j.peptides.2007.09.020. [DOI] [PubMed] [Google Scholar]
- Wine JJ, Krasne FB. The organization of escape behaviour in the crayfish. J Exp Biol. 1972;56:1–18. doi: 10.1242/jeb.56.1.1. [DOI] [PubMed] [Google Scholar]
- Wine JJ, Krasne FB. The cellular organization of crayfish escape behavior. In: Sandeman DC, Atwood HL, editors. Neural Integration and Behavior. Academic Press; New York: 1982. pp. 241–292. [Google Scholar]
- Wood DE, Gleeson RA, Derby CD. Modulation of behavior by biogenic amines and peptides in the blue crab, Callinectes sapidus. J Comp Physiol A. 1995;177:321–333. doi: 10.1007/BF00192421. [DOI] [PubMed] [Google Scholar]
- Yasuda A, Yasuda-Kamatani Y, Nozaki M, Nakajima T. Identification of GYRKPPFNGSIFamide (crustacean-SIFamide) in the crayfish Procambarus clarkii by topological mass spectrometry analysis. Gen Comp Endocrinol. 2004;135:391–400. doi: 10.1016/j.ygcen.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Yasuda-Kamatani Y, Yasuda A. Identification of orcokinin gene-related peptides in the brain of the crayfish Procambarus clarkii by the combination of MADLI-TOF and on-line capillary HPLC/Q-T of mass spectrometries and molecular cloning. Gen Comp Endocrinol. 2000;118:161–172. doi: 10.1006/gcen.1999.7453. [DOI] [PubMed] [Google Scholar]
- Yasuda-Kamatani Y, Yasuda A. APSGFLGMRamide is a unique tachykinin-related peptide in crustacean. Eur J Biochem. 2004;271:1546–1556. doi: 10.1111/j.1432-1033.2004.04065.x. [DOI] [PubMed] [Google Scholar]
- Yasuda-Kamatani Y, Yasuda A. Characteristic expression patterns of allatostatin-like peptide, FMRFamide-related peptide, orcokinin, tachykinin-related peptide, and SIFamide in the olfactory system of crayfish Procambarus clarkii. J Comp Neurol. 2006;496:135–147. doi: 10.1002/cne.20903. [DOI] [PubMed] [Google Scholar]
- Yeh SR, Fricke RA, Edwards DH. The effect of social experience on serotonergic modulation of the escape circuit of crayfish. Science. 1996;271:366–369. doi: 10.1126/science.271.5247.366. [DOI] [PubMed] [Google Scholar]
- Yeh SR, Musolf BE, Edwards DH. Neuronal adaptations to changes in the social dominance status of crayfish. J Neurosci. 1997;17:697–708. doi: 10.1523/JNEUROSCI.17-02-00697.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]


