The SH3 domain of postsynaptic density 95 mediates inflammatory pain through phosphatidylinositol-3-kinase recruitment
Sensitization to inflammatory pain is a pathological form of neuronal plasticity that is poorly understood and treated. Here the authors report that the SH3 domain of the scaffold protein PSD-95 binds a lipid signaling enzyme, PI3K-C2a, that mediates inflammatory sensitization. The results show that different types of behavioural plasticity are mediated by specific domains of PSD-95 and suggest that PI3K-C2a is a potential drug target for inflammatory pain.
Keywords: nociception, inflammation, PSD95, SH3 domain
Abstract
Sensitization to inflammatory pain is a pathological form of neuronal plasticity that is poorly understood and treated. Here we examine the role of the SH3 domain of postsynaptic density 95 (PSD95) by using mice that carry a single amino-acid substitution in the polyproline-binding site. Testing multiple forms of plasticity we found sensitization to inflammation was specifically attenuated. The inflammatory response required recruitment of phosphatidylinositol-3-kinase-C2α to the SH3-binding site of PSD95. In wild-type mice, wortmannin or peptide competition attenuated the sensitization. These results show that different types of behavioural plasticity are mediated by specific domains of PSD95 and suggest novel therapeutic avenues for reducing inflammatory pain.
Introduction
Plasticity in different regions of the central nervous system underlies a diverse set of behaviours and pathologies. At excitatory synapses, the scaffolding molecule postsynaptic density 95 (PSD95) assembles signalling complexes with glutamate receptors and couples them to intracellular pathways mediating synaptic and neuronal changes (Migaud et al, 1998; Komiyama et al, 2002; Fernandez et al, 2009). Previous studies of mice lacking PSD95 show alterations in multiple forms of experience-dependent plasticity (Migaud et al, 1998; Komiyama et al, 2002; Fagiolini et al, 2003; Yao et al, 2004) including sensory processing (Garry et al, 2003). As the structure of PSD95 includes many protein interaction domains—three PDZ, a single SH3 and a single GK domain—it is possible that each domain controls specific behaviours by recruiting specific protein-binding partners. To investigate this issue we engineered a point mutation into the SH3 domain of PSD95 in mice and examined multiple forms of behavioural plasticity.
Results And Discussion
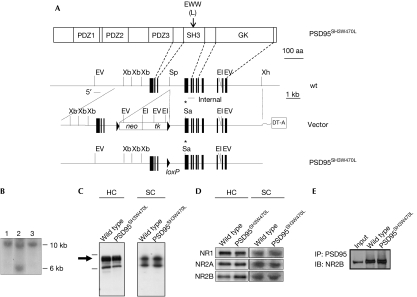
Using X-ray crystallographic data of several SH3 domains (Musacchio et al, 1992, 1994; Noble et al, 1993; Borchert et al, 1994; Guruprasad et al, 1995), we engineered a mutation that abrogates binding of proline-rich ligands (Lim & Richards, 1994; Erpel et al, 1995; Mayer & Eck, 1995). Trp 470 of PSD95 corresponds to the highly conserved tryptophan residue found in the hydrophobic binding surface of prototypical SH3 domains that mediates interaction with proline-containing peptides (Erpel et al, 1995). Leucine was substituted for this tryptophan in vivo using gene targeting, creating the PSD95SH3W470L line of mice (Fig 1A,B). Homozygous PSD95SH3W470L mice were fertile, showed normal Mendelian transmission, no obvious seizures, tremor, ataxia or other overt neurological abnormality, nor any discernible defects in gross neuroanatomy as determined histologically (data not shown). Here, all mutant mice are homozygous and are compared with wild-type (wt) littermates.
Figure 1.
Construction of PSD95 SH3 point mutation in mice. (A) The top panel shows domain organization of PSD95 protein and the location of the point mutation. The lower three diagrams represent wt genomic DNA, the targeting vector and the targeted allele after excision of the selection cassette. Restriction enzyme sites: EI, EcoRI; EV, EcoRV; Sa, SacI; Sp, SpeI; Xb, XbaI; Xh, XhoI. Filled boxes, exons; asterisk, point mutation; neo and tk, neomycin and thymidine kinase genes; arrowheads, loxP sites; DT-A, diphtheria toxin-A cassette. (B) Southern blot of EcoRV-digested genomic DNA using a 5′ probe. Lane 1, wt; lane 2, cassette intact; lane 3, cassette excised. (C) PSD95 levels in the hippocampus (HC) and spinal cord (SC) of wt and mutant mice. (D) Immunoblots of NR1, NR2A and NR2B subunits of the NMDA receptor in wt and mutant mice in the HC and SC. Densitometric analysis of samples showed no significant differences (n=6, Student's t-test) (data not shown). (E) Co-immunoprecipitation of NR2B with PSD95 in hippocampal extracts shows the mutation does not affect association and thus the protein is folded. The input control is from wt mice indicating the correct molecular weight. NMDA, N-methyl-D-aspartate; NR, NMDA receptor; PSD95, postsynaptic density 95; wt, wild type.
PSD95 protein expression was examined in PSD95SH3W470L mutants by immunoblot analysis of mouse hippocampus and spinal cord extracts using a PSD95 antibody raised against the amino-terminus of the protein (Fig 1C). The expected full-length (∼95 kDa) PSD95 polypeptide was recognized at similar levels in both wt and PSD95SH3W470L mutant extracts. PSD95 binds to N-methyl-D-aspartate (NMDA) receptors through its first and second PDZ domains and we therefore tested the levels and association of NMDA receptor subunits. No detectable differences in NR1, NR2A and NR2B expression in either hippocampus or spinal cord extracts were found in PSD95SH3W470L mutants as compared with wt animals (Fig 1D). The PSD95SH3W470L mutant protein immunoprecipitated with NR2B to the same extent as wt PSD95 (Fig 1E), indicating that the mutant protein was normally folded and localized.
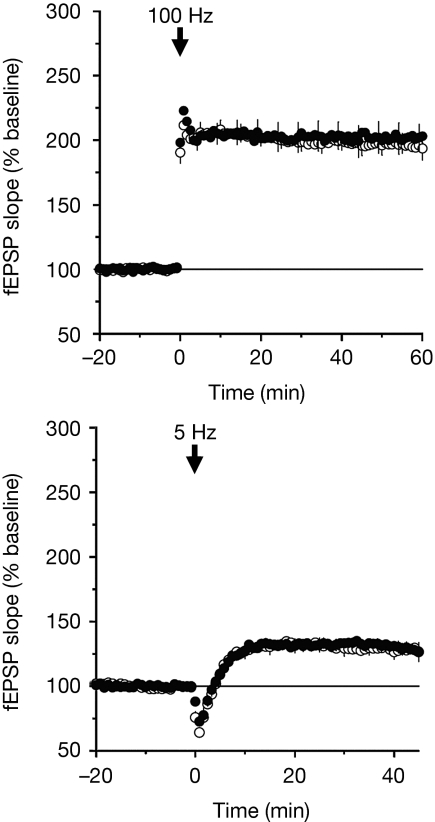
The most well-studied form of neuronal plasticity is long-term potentiation (LTP) in the hippocampus. Mice lacking PSD95 have severe impairments in LTP and we therefore tested the PSD95SH3W470L mice in this assay (Fig 2). As shown in Fig 2, we tested two forms of LTP using conventional high- or low-frequency protocols (100 and 5 Hz, respectively) and both of these were unaffected by the SH3 mutation. We therefore conclude that PSD95-mediated plasticity in the hippocampus does not require binding of ligands to the SH3 domain.
Figure 2.
Hippocampus synaptic plasticity unaltered in PSD95SH3W470L mutant mice. Conventional 100 Hz (top) and 5 Hz (bottom), high-frequency and low-frequency respectively, protocols were used that induce NMDA receptor-dependent LTP of synaptic transmission, which is greatly enhanced in PSD95-knockout mice (Migaud et al, 1998). We found no phenotype in PSD95SH3W470L mice (mean and s.e.m., n=4). Open symbols, wt; closed symbols, PSD95SH3W470L mice. LTP, long-term potentiation; NMDA, N-methyl-D-aspartate.
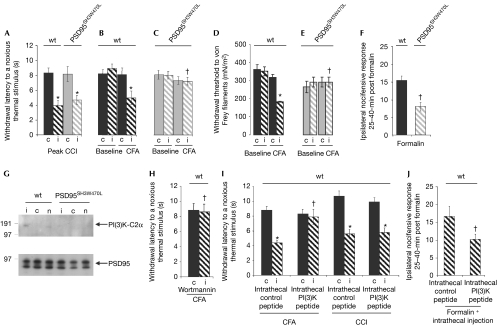
As PSD95 is known to have an important role in spinal cord plasticity (Garry et al, 2003), we examined sensitization to tissue inflammation and nerve injury. Chronic constriction injury to the peripheral nerve produces a sensitized state (reduced withdrawal latency to a noxious thermal stimulus), which was unaffected in the PSD95SH3W470L mice (Fig 3A). We next induced an inflammatory injury (injection of complete Freund's adjuvant, CFA), which in wt mice after 24 h leads to the development of sensitization (Fig 3B,D). In PSD95SH3W470L mice, sensitivity to thermal and mechanical stimuli was completely abrogated (Fig 3C,E). Similar results were obtained using formalin as a further inflammatory stimulus (Fig 3F). These behavioural studies show the PSD95 SH3 domain has an essential and specific role in the sensitized response to tissue inflammation.
Figure 3.
PI(3)K-C2α recruitment to PSD95 SH3 domain is required for inflammatory responses. (A) Development of ipsilateral (‘i') thermal hyperalgesia as compared with the contralateral side (‘c') after unilateral chronic constriction injury to the sciatic nerve occurred to a similar extent in wt and PSD95SH3W470L mice (*P<0.05, Student's paired t-test, n=5–7). (B,D) Wt mice developed ipsilateral thermal hyperalgesia (B) and mechanical allodynia (D) 24 h after inflammation evoked by hind-paw administration of CFA (*P<0.05, paired Student's t-test for thermal data and Wilcoxon test for mechanical data, n=4), but this was prevented (C,E) in PSD95SH3W470L mutant mice (†P<0.05, Student's t-test/Wilcoxon test, n=4). (F) Ipsilateral nocifensive responses taken over 25- to 40-min time points after administration of the noxious agent formalin by intraplantar injection of the hind paw were also significantly attenuated in PSD95SH3W470L mutants as compared with that in wt mice (†P<0.05, Student's t-test, n=8–9). Contralateral responses were negligible in each case. (G) Spinal cord PSD95 immunoprecipitates from wt and PSD95SH3W470L mice ipsilateral (‘i') and contralateral (‘c') to formalin challenge were collected at 35–40 min after formalin administration, in addition to naive groups (‘n') and probed for PI(3)K-C2α or PSD95 (n=14 mice in each group). PI(3)K-C2α was strongly associated with PSD95 in wt spinal cord ipsilateral to the formalin challenge. Densitometry from this and two further independent experimental groups shows ratios of PI(3)K-C2α/PSD95 were 0.053±0.007 (ipsilateral), 0.026±0.012 (contralateral) and 0.019±0.008 (naive) (means±s.e.m., Photoshop). PI(3)K-C2α was not detectable in control non-immune IgG immunoprecipitates. By contrast, PSD95SH3W470L mice (or naive wt mice) showed no association of PI(3)K-C2α with PSD95 (mean PI(3)K-C2α/PSD95 ratios were less than 0.02). Equivalent levels of PSD95 were found in all wt and PSD95SH3W470L samples. (H) In wt mice, intrathecal injection of wortmannin (7 nmol in 10 μl of saline with 1% dimethylformamide) reversed the enhancement of sensitivity to noxious thermal stimuli induced by CFA observed in panel B (†P<0.05 compared with the ipsilateral side without wortmannin, Student's t-test, n=6). Previous studies have shown that this vehicle alone has no effect on responses. (I) In wt mice, intrathecal injection of a myristoylated PI(3)K-C2α decoy peptide (but not a control peptide in which the three key proline residues were replaced with alanine (1 nmol in 10 μl of saline with 1% dimethylformamide)) reversed the enhanced sensitivity to noxious thermal stimuli induced ipsilateral to CFA, but not CCI (*P<0.05 compared with the contralateral side, paired Student's t-test, n=6; †P<0.05 compared with the ipsilateral side with control peptide, Student's t-test, n=6). (J) In wt mice, the intrathecal PI(3)K-C2α decoy peptide (but not control peptide) attenuated the nocifensive responses ipsilateral to formalin (†P<0.05 compared with the ipsilateral side with control peptide, Student's t-test, n=6). CCI, chronic constriction injury; CFA, complete Freund's adjuvant; PI(3)K, phosphatidylinositol-3-kinase; wt, wild type.
To identify the molecular mechanism, we focused on the binding specificity of the SH3 domain and the possibility that particular proteins in the spinal cord interact with PSD95 through this domain. We probed 9,000 16-amino-acid-long peptide sequences, which encompassed the consensus SH3 ligand sequence (PxxP) immobilized on glass slides ( Jerini SH3-domain profiler detector peptide arrays) using a Cy5-labelled recombinant SH3 domain of PSD95 (supplementary Table S1 online). Among the strongest signals was the FPLWKLPGFPNRMVLG peptide from phosphatidylinositol-3-kinase-C2α (PI(3)K-C2α), a signalling molecule of the PI(3)K family, previously implicated in inflammatory processes (Foster et al, 2003; Pezet et al, 2008).
We next asked whether PI(3)K-C2α bound to PSD95 in the spinal cord and if association was increased with inflammatory sensitization. This was indeed the case and, importantly, recruitment of PI(3)K-C2α to PSD95 was absent in PSD95SH3W470L mutants. As shown in Fig 3G in wt mice, immunoprecipitation of PSD95 complexes recovered PI(3)K-C2α after inflammatory sensitization. In PSD95SH3W470L mutant mice this association was undetectable. Because this suggests that the activity of PI(3)K-C2α is required for inflammatory sensitization, wortmannin (a PI(3)K-C2α inhibitor; Wang et al, 2006) was administered intrathecally into wt mice and found to reverse sensitization (Fig 3H). To test if recruitment to PSD95 during the inflammatory response was required, we injected a peptide mimicking the PI(3)K-C2α SH3 target motif, which could compete with PI(3)K-C2α binding (and compared it with a control peptide lacking three principal proline residues replaced by alanine). The PI(3)K-C2α peptide prevented the sensitization after inflammation in wt mice, and as expected it did not affect sensitization to nerve injury (Fig 3I,J). The control peptide had no effect (Fig 3I,J).
These results show that peripheral tissue damage produces a neuronal response in the spinal cord involving recruitment of PI(3)K-C2α to the SH3 domain of PSD95. Blocking this recruitment with either a mutation or a peptide competitor abrogates the anticipated sensitized response, as does administration of a PI(3)K-C2α inhibitor. These data indicate that this recruitment is specifically required for inflammatory pain sensitization and not for two other forms of plasticity: peripheral nerve injury or hippocampus LTP. Furthermore, these other two forms of plasticity are impaired when the entire PSD95 is knocked out (Migaud et al, 1998; Fagiolini et al, 2003; Garry et al, 2003; Yao et al, 2004), indicating that specific domains of PSD95 have roles in specific forms of behaviour. The SH3-mediated recruitment of PI(3)K-C2α allows it to act in close proximity to other proteins binding to PSD95, including NR2B, calcium calmodulin kinase II and extracellular signal-regulated kinase, which are also involved in inflammatory responses.
Previous studies have shown that the signalling complexes associated with neurotransmitter receptors such as the NMDA receptor complex are composed of many proteins that are differentially expressed within the nervous system (Husi et al, 2000; Komiyama et al, 2002; Cuthbert et al, 2007; Emes et al, 2008; Fernandez et al, 2009). Differences in the composition of complexes between adaptor molecules and other proteins, mediated by specific protein interaction domains, could underlie different types of synaptic plasticity found in distinct types of neuron. Moreover, genetic disruption of these complexes in experimental animals and in heritable diseases will therefore alter plasticity to pain responses and other environmental responses including learning and memory (Migaud et al, 1998; Komiyama et al, 2002; Grant et al, 2005). Understanding the assembly of specific signalling complexes at different synapses might allow the development of therapeutics that target particular classes of synapse. The current studies suggest that PI(3)K-C2α is a potential drug target for inflammatory pain.
Methods
Gene targeting. The PSD95SH3W470L targeting vector comprised two homology arms of genomic DNA (3.8 kb NotI–SpeI and 6.2 kb SpeI–XhoI fragments) flanking a floxed cassette containing neomycin and thymidine kinase selectable markers within a diphtheria toxin-A vector. A tryptophan-to-leucine amino-acid change (W470L) incorporating a SacI restriction site was introduced using the Stratagene Quik-Change Mutagenesis kit. The linearized targeting vector was transfected into embryonic stem (ES) cells (E14Tg2a clone), neomycin-resistant colonies were picked and subsequently transfected with Cre recombinase to excise the floxed cassette. Positive PSD95SH3W470L clones (with a targeting efficiency of 4%) were identified by Southern blot analysis: gels containing 20 μg of EcoRV- or SacI-digested genomic DNA were blotted and probed with a 700-bp internal and a 400-bp 5′ probe. Positive clones were injected into mouse blastocysts that were in turn inserted into recipient C57/Bl6 females. The resulting chimeric offspring were crossed onto the MF1 background for subsequent studies. Mice were genotyped at weaning by PCR using the following primers: forward primer 5′-CCGACTGCTCACTAGTATTTTCTCCC-3′ with reverse primer 5′-CTTGGCCTTTAAACTAGTCCACTCTCGT-3′. Wt and mutant alleles were distinguished by digesting the resulting 1,400-bp fragment with SacI to produce 1,000- and 400-bp mutant fragments. From 29 PSD95SH3W470L litters, 128 pups were genotyped resulting in 43 wt (34%), 43 heterozygotes (34%) and 42 homozygotes (32%). Fertility was not affected in homozygotes and no obvious seizure, tremor, ataxia or other overt neurological abnormality observed.
Immunoprecipitation. Mouse hippocampal extracts were homogenized at 4°C in 10 volumes of 1% sodium-deoxycholate, 1 mM NaF, 20 μM ZnCl2, 0.5 mM phenylmethylsulphonyl fluoride and 1 mM Na3VO4 in 50 mM Tris (pH 9.0) containing Complete EDTA-free protein inhibitors (Roche, Mannheim, Germany). Mouse spinal extracts were homogenized at 4°C in 10 volumes of phosphate-buffered saline containing 10% (v/v) glycerol, 1% (w/v) CHAPS, 0.5% (w/v) sodium-deoxycholate, 1 mM NaF, 1 mM Na3VO4, 5 mM sodium-molybdate and 1:100 dilution Protease Inhibitor Cocktail (Set III; Calbiochem, Darmstadt, Germany). After centrifugation at 25,000 r.p.m. for 15 min at 4°C, 1 mg aliquots of hippocampal protein extract diluted in 1 ml buffer were incubated overnight at 4°C with 4 μg of anti-PSD95 (Millipore, Billerica, MA, USA). Immunocomplexes were captured using 40 μl of a 50% solution of G-protein-coupled Sepharose (Amersham Pharmacia, Little Chalfont, Bucks, UK), incubated for 2 h at 4°C followed by several washes in 1 × lysis buffer. Spinal homogenates were allowed to solubilize for 1.5 h at 4°C with rolling followed by centrifugation at 11,000 r.p.m. for 15 min at 4°C. The resultant supernatant was pre-cleared in 20 μl/ml protein-G–Sepharose (Sigma, St Louis, MO, USA) for 1 h at 4°C. The pre-cleared supernatant was immunoprecipitated, for 16 h at 4°C, using 3.5 μg/ml of mouse anti-PSD95 (75-028, NeuroMab/Antibodies Inc., Davis, CA, USA) in the presence of 40 μl/ml protein-G–Sepharose. Hippocampal immunocomplexes and/or protein extracts (20 μg per lane) were separated by SDS–PAGE and standard procedures were used for immunoblotting.
Immunoblotting. Hippocampal or spinal cord samples from wt and mutant mice were homogenized in Laemmli buffer and denatured at 100°C for 5 min. Proteins were separated by SDS–PAGE electrophoresis on pre-cast 8% or 4–12% Bis–Tris Gels (Invitrogen Life Technologies, Carlsbad, CA, USA). Blots were probed with the following primary antibodies (spinal cord): NR1 (1:1,000; Millipore, or 1:100; Chemicon), NR2A (1:100; Upstate, or 1:1,000; Transduction Labs, Lexington, KY, USA), NR2B (1:100; Chemicon, or 1:250; Transduction Labs), PSD95 (1:10,000; Upstate, or Transduction Labs, UC Davis/NINDS/NIMH NeuroMab Facility (75-028: clone K28/43, amino-terminal, affinity purified; 1:100)) and mouse polyclonal PI(3)K-C2α (H00005286-A01; Abnova, Walnut, CA, USA), and detected by chemiluminescence. The specificity of the PI(3)K-C2α antibody was confirmed by pre-absorption experiments with either a glutathione-S-transferase (GST)-fusion protein of the antigenic segment of PI(3)K-C2α (Abnova) or, as a control, a GST-fusion protein of the small G-protein, RhoA (Cytoskeleton Inc., Denver, CO, USA). The PI(3)K-C2α antibody (1:100 in phosphate-buffered saline/0.1% Tween-20, 2% bovine serum albumin) was incubated overnight with 3 μg of the antigen (partial recombinant PI(3)K-C2α (1,577–1,687) as a GST-fusion protein (Abnova) or GST-RhoA (Cytoskeleton Inc.) for 16 h at 4°C with rolling. A 25 μl of glutathione–Sepharose was added and samples were incubated for 3 h at 4°C with rolling before the beads were removed by centrifugation (10,000 g, 10 min, 4°C) and the supernatants were used directly for primary antibody immunoblot incubations.
Peptide array interaction assays. Jerini SH3-domain profiler detector peptide arrays ( Jerini Peptide Technologies, Gmbh, Berlin, Germany) were used to analyse binding partners for the PSD95 SH3 domain. Nine-thousand-peptide sequences, 16-amino-acids long, which encompassed the consensus SH3 ligand sequence -PxxP-, were synthesized on cellulose membranes in a parallel manner using SPOT technology, deposited to glass slides and covalently immobilized to the surface. A series of positive and negative controls were also included. The glass slides were incubated with different concentrations of the recombinant PSD95 GST-SH3 domain (Panomics, FP3029) to obtain an appropriate signal-to-noise ratio; a 0.2 μg/ml concentration was selected for the array assays. For peptide array incubation, an Abgene incubation chamber was used (AB-0630); the chamber was previously treated with 0.1 mg/ml of polyvinylpyrrolidone for 6 h. The arrays were incubated at 4°C overnight, the chamber removed, washed five times with distilled water and incubated 4 h at 4°C with Cy5 anti-GST, 1 μg/ml (PA92002, Amersham GE Healthcare, Little Chalfont, Bucks, UK). Slides were washed five times with distilled water and scanned at a 5 nm resolution using ScanArray Express HT (Perkin-Elmer, Waltham, MA, USA). All of the assays were performed in triplicate. Image analysis and signal quantification were performed using ProscanArray Express (Perkin-Elmer) and positive signals were defined as significant (Student's t-test, P<0.001) after background subtraction was compared against peptides without SH3 consensus sequences. Peptides with positive signals were matched to the corresponding proteins shown in supplementary Table S1 online.
In vivo peptide studies. As the peptide (FPLWKLPGFPNRMVLG) encompassing the consensus proline-rich SH3 target site within PI(3)K-C2α was a strong binding partner for the GST-PSD95 SH3 domain construct, we designed an N-myristoylated version of the decoy peptide (myr-FPLWKLPGFPNRMVLG) together with a control (myr-FALWKLAGFANRMVLG) in which all crucial proline residues were substituted with alanine (Pepsyn Ltd, Liverpool, UK). The peptides were freshly dissolved in dimethylformamide (10 mM) and diluted into sterile saline, acidified to pH 4.0–4.5 with HCl to form 100 μM solutions, which were then re-titrated to pH 6.5 with dilute NaOH. Aliquots measuring 10 μl were then injected intrathecally. Nociceptive responses were recorded ipsilateral and contralateral to injury both before and after intrathecal injection of peptides.
Chronic pain models. Peripheral nerve injury and formalin challenges have been described previously together with all assessment criteria (Garry et al, 2003). Formalin challenges were characterized over the first (5–15 min) and second (25–45 min) phases of the response. Inflammation using CFA was induced by intraplantar injection of the hind paw, under brief halothane anaesthesia (Chaplan et al, 1997). All behavioural and physiological analyses were performed using male and female mice ranging from 4 to 14 months of age. There were no differences in responses between male and female mice.
Hippocampal synaptic plasticity. Hippocampal slice electrophysiology was performed as described previously (Komiyama et al, 2002). Briefly, 400 μm-thick hippocampal slices were prepared from 14- to 20-week-old mice using standard techniques. The slices were maintained in an interface-type recording chamber perfused (1–3 ml/min) with warmed (30°C) oxygenated (95% O2/5% CO2) artificial cerebrospinal fluid containing 124 mM NaCl, 4.4 mM KCl, 25 mM Na2HCO3, 1 mM NaH2PO4, 1.2 mM MgSO4, 2 mM CaCl2 and 10 mM glucose. Low-resistance glass microelectrodes (5–10 MΩ) filled with artificial cerebrospinal fluid were placed into the stratum radiatum of the hippocampal CA1 region to record field excitatory postsynaptic potentials evoked by presynaptic stimulation pulses delivered once every 50 s to the Schaeffer collateral/commissural fibres through a bipolar nichrome wire stimulating electrode. The magnitude of the postsynaptic field excitatory postsynaptic potentials was measured using the initial slope of the field excitatory postsynaptic potentials. After a 20 min period of baseline recording, LTP was induced using either a high-frequency stimulation protocol (two trains of 100 Hz stimulation, each 1 s in duration and separated by 10 s) or a low-frequency stimulation protocol consisting of 900 stimulation pulses delivered at 5 Hz.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
SGNG, SMF-W, RM, NHK, MC, MIA and LHF were supported by the Wellcome Trust and Wellcome Trust Genes to Cognition Programme, and AD was supported by the Medical Research Council. We thank V.J. Robinson and K. Elsegood for technical support and Mike Croning for the website and G2Cdb (Croning et al, 2009) support.
Footnotes
The authors declare that they have no conflict of interest.
References
- Borchert TV, Mathieu M, Zeelen JP, Courtneidge SA, Wierenga RK (1994) The crystal structure of human CskSH3: structural diversity near the RT-Src and n-Src loop. FEBS Lett 341: 79–85 [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Malmberg AB, Yaksh TL (1997) Efficacy of spinal NMDA receptor antagonism in formalin hyperalgesia and nerve injury evoked allodynia in the rat. J Pharmacol Exp Ther 280: 829–838 [PubMed] [Google Scholar]
- Croning MD, Marshall MC, McLaren P, Armstrong JD, Grant SG (2009) G2Cdb: the genes to cognition database. Nucleic Acids Res 37: D846–D851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert PC, Stanford LE, Coba MP, Ainge JA, Fink AE, Opazo P, Delgado JY, Komiyama NH, O'Dell TJ, Grant SG (2007) Synapse-associated protein 102/dlgh3 couples the NMDA receptor to specific plasticity pathways and learning strategies. J Neurosci 27: 2673–2682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emes RD et al. (2008) Evolutionary expansion and anatomical specialization of synapse proteome complexity. Nat Neurosci 11: 799–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erpel T, Superti-Furga G, Courtneidge SA (1995) Mutational analysis of the Src SH3 domain: the same residues of the ligand binding surface are important for intra- and intermolecular interactions. EMBO J 14: 963–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagiolini M, Katagiri H, Miyamoto H, Mori H, Grant SG, Mishina M, Hensch TK (2003) Separable features of visual cortical plasticity revealed by N-methyl-D-aspartate receptor 2A signaling. Proc Natl Acad Sci USA 100: 2854–2859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez E, Collins MO, Uren RT, Kopanitsa MV, Komiyama NH, Croning MD, Zografos L, Armstrong JD, Choudhary JS, Grant SG (2009) Targeted tandem affinity purification of PSD-95 recovers core postsynaptic complexes and schizophrenia susceptibility proteins. Mol Syst Biol 5: 269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster FM, Traer CJ, Abraham SM, Fry MJ (2003) The phosphoinositide (PI) 3-kinase family. J Cell Sci 116: 3037–3040 [DOI] [PubMed] [Google Scholar]
- Garry EM, Moss A, Delaney A, O'Neill F, Blakemore J, Bowen J, Husi H, Mitchell R, Grant SG, Fleetwood-Walker SM (2003) Neuropathic sensitization of behavioral reflexes and spinal NMDA receptor/CaM kinase II interactions are disrupted in PSD-95 mutant mice. Curr Biol 13: 321–328 [DOI] [PubMed] [Google Scholar]
- Grant SG, Marshall MC, Page KL, Cumiskey MA, Armstrong JD (2005) Synapse proteomics of multiprotein complexes: en route from genes to nervous system diseases. Hum Mol Genet 14 (Spec No. 2): R225–R234 [DOI] [PubMed] [Google Scholar]
- Guruprasad L, Dhanaraj V, Timm D, Blundell TL, Gout I, Waterfield MD (1995) The crystal structure of the N-terminal SH3 domain of Grb2. J Mol Biol 248: 856–866 [DOI] [PubMed] [Google Scholar]
- Husi H, Ward MA, Choudhary JS, Blackstock WP, Grant SG (2000) Proteomic analysis of NMDA receptor–adhesion protein signaling complexes. Nat Neurosci 3: 661–669 [DOI] [PubMed] [Google Scholar]
- Komiyama NH et al. (2002) SynGAP regulates ERK/MAPK signaling, synaptic plasticity, and learning in the complex with postsynaptic density 95 and NMDA receptor. J Neurosci 22: 9721–9732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim WA, Richards FM (1994) Critical residues in an SH3 domain from Sem-5 suggest a mechanism for proline-rich peptide recognition. Nat Struct Biol 1: 221–225 [DOI] [PubMed] [Google Scholar]
- Mayer BJ, Eck MJ (1995) SH3 domains. Minding your p's and q's. Curr Biol 5: 364–367 [DOI] [PubMed] [Google Scholar]
- Migaud M et al. (1998) Enhanced long-term potentiation and impaired learning in mice with mutant postsynaptic density-95 protein. Nature 396: 433–439 [DOI] [PubMed] [Google Scholar]
- Musacchio A, Noble M, Pauptit R, Wierenga R, Saraste M (1992) Crystal structure of a Src-homology 3 (SH3) domain. Nature 359: 851–855 [DOI] [PubMed] [Google Scholar]
- Musacchio A, Saraste M, Wilmanns M (1994) High-resolution crystal structures of tyrosine kinase SH3 domains complexed with proline-rich peptides. Nat Struct Biol 1: 546–551 [DOI] [PubMed] [Google Scholar]
- Noble ME, Musacchio A, Saraste M, Courtneidge SA, Wierenga RK (1993) Crystal structure of the SH3 domain in human Fyn; comparison of the three-dimensional structures of SH3 domains in tyrosine kinases and spectrin. EMBO J 12: 2617–2624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezet S, Marchand F, D'Mello R, Grist J, Clark AK, Malcangio M, Dickenson AH, Williams RJ, McMahon SB (2008) Phosphatidylinositol 3-kinase is a key mediator of central sensitization in painful inflammatory conditions. J Neurosci 28: 4261–4270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y et al. (2006) Class II phosphoinositide 3-kinase alpha-isoform regulates Rho, myosin phosphatase and contraction in vascular smooth muscle. Biochem J 394: 581–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao WD, Gainetdinov RR, Arbuckle MI, Sotnikova TD, Cyr M, Beaulieu JM, Torres GE, Grant SG, Caron MG (2004) Identification of PSD-95 as a regulator of dopamine-mediated synaptic and behavioral plasticity. Neuron 41: 625–638 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.