SOX5 controls cell cycle progression in neural progenitors by interfering with the WNT–β-catenin pathway
Activated WNT–β-catenin signalling drives cell proliferation in many cellular contexts. Morales and colleagues now show that the transcription factor SOX5 regulates the timing of cell cycle exit by opposing WNT–β-catenin activity on cell cycle progression during spinal cord neurogenesis.
Keywords: β-catenin, cell cycle, neurogenesis, SOX5, spinal cord
Abstract
Genes of the SOX family of high-mobility group transcription factors are essential during nervous system development. In this study, we show that SOX5 is expressed by neural progenitors in the chick spinal cord and is turned off as differentiation proceeds. The overexpression of SOX5 in neural progenitors causes premature cell cycle exit and prevents terminal differentiation. Conversely, knocking down SOX5 protein extends the proliferative period of neural progenitors and causes marked cell death in a dorsal interneuron (dI3) population. Furthermore, SOX5 reduces WNT–β-catenin signalling, thereby triggering the expression of the negative regulator of the pathway axin2. We propose that SOX5 regulates the timing of cell cycle exit by opposing WNT–β-catenin activity on cell cycle progression.
Introduction
During the development of the central nervous system, a large number of different neurons and glial cells are generated from a small population of self-renewing stem and progenitor cells. In the vertebrate spinal cord, mitotically active and post-mitotic cell populations are spatially segregated. Thus, neural progenitors are located in the medial ventricular zone and migrate laterally to the mantle zone on exiting the cell cycle, a site where differentiating cells accumulate.
A dorsal–ventral gradient of the WNT–β-catenin–TCF (T-cell factor) pathway positively regulates cell cycle progression of spinal neural progenitors through cyclin D1, cyclin D2 and N-MYC (Megason & McMahon, 2002).
However, it is still not clear how the progression of the proliferation programme, promoted by signals, such as WNT, can be counteracted to facilitate the initiation of the neurogenic programme. The high-mobility group box transcription factors of the SOX gene family could be at the core of some of those processes, as they have essential regulatory functions during neurogenesis in the central nervous system (Wegner & Stolt, 2005). In the spinal cord, SOX1–3 proteins (SOXB1 group) preserve cells in an undifferentiated state (Bylund et al, 2003). By contrast, SOXB2 factors promote the initiation of the differentiation programme (Sandberg et al, 2005). SOX5 belongs to the SOXD group and is involved in the formation of the cephalic neural crest (Perez-Alcala et al, 2004), and in the control of the cell fate of distinct corticofugal neurons (Lai et al, 2008).
In this study, we show that SOX5 is expressed in neural progenitors in the spinal cord, and in dorsal dI3 interneurons. Through gain- and loss-of-function analyses, we observed that SOX5 controls the timing of cell cycle exit by neural progenitors at the G1–S transition by counteracting the mitotic effect of the WNT–β-catenin pathway. We provide evidence to suggest that SOX5 does this by controlling the feedback repressor pathway regulating WNT signalling. Furthermore, we have observed that SOX5 downregulation in post-mitotic cells is necessary for the progression of the differentiation programme. Hence, these data indicate SOX5 as an important brake on WNT–β-catenin mitogenic activity during the progression of neurogenesis.
Results And Discussion
SOX5 is mainly expressed in neural progenitors
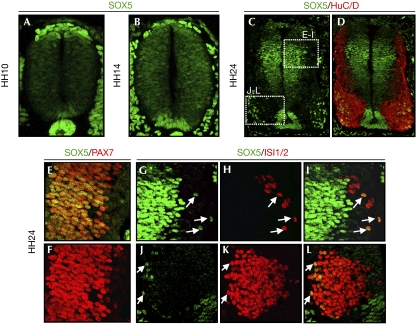
To determine the possible role of SOX5 in early neurogenesis, we defined the pattern of SOX5 expression at the mid-trunk level of the spinal cord in chicken embryos at Hamburguer and Hamilton stages 10–24 (HH10–24). At HH10, when most of the neuroepithelial cells are producing progenitors, very low levels of SOX5 were detected in these progenitors, whereas higher levels were observed in the dorsal premigratory neural crest cells (Fig 1A). By stage HH14, when around 12% of the neural progenitors have exited the cell cycle and have started differentiation at the marginal zone (Wilcock et al, 2007), SOX5 expression increased in progenitors (Fig 1B). Finally, from stages HH14 to HH24, when neural differentiation is more active and gliogenesis has not yet started, SOX5 expression disappeared from most of the interneurons that expressed the pan-neural marker HuC/D (Fig 1C,D). At stage HH24, SOX5 expression remained in neural progenitors expressing PAX7 (Fig 1E,F). Dorsally, only islet 1/2+ dorsal interneurons (dI3) expressed SOX5 (Fig 1G–I). Ventrally, SOX5 was expressed by a small subpopulation of the islet 1/2+ motorneurons (Fig 1J–L). This dynamic pattern of expression suggests a possible role for SOX5 in the control of the transition from proliferation to differentiation.
Figure 1.
Expression of SOX5 in the developing chick spinal cord. (A) At stage HH10, SOX5 is expressed dorsally in neural crest cells and in floor plate cells. (B) At stage HH14, SOX5 is expressed by most of the neuroepithelial cells. (C,D) SOX5 expression at HH24 is absent from most of the HuC/D-differentiating interneurons. (E,F) SOX5 is expressed in dorsal neural progenitors with PAX7. (G–I) A subpopulation of dorsal dI3 interneurons expresses SOX5 and islet 1/2 (arrows). (J–L) At HH24, SOX5 is expressed in a subpopulation of ISL1/2+ motorneurons (arrows). HH, Hamburguer and Hamilton.
SOX5 controls the timing of cell cycle exit
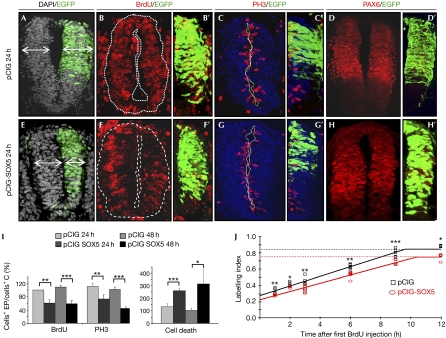
To explore the function of SOX5 in neurogenesis, we electroporated a pCAGGS-SOX5-IRES-GFP vector (pCIG-SOX5) and prematurely increased SOX5 levels (SOX5HIGH) in neural progenitors at stages HH10–13. SOX5HIGH provoked a 30.6±3.3% decrease in the size of the electroporated right hemi-tube at 24 h post-electroporation (PE; stages HH14–17) when compared with the left control side (Fig 2E; supplementary Table S1 online) or with an electroporated control neural tube (pCIG; Fig 2A). There were no significant changes in cell density in SOX5-electroporated neural tubes (supplementary Table S1 online). The change in the hemitube size was due, in part, to a substantial decrease in proliferation observed by a reduction in bromodeoxyuridine (BrdU) incorporation after a 40-min pulse (only 61±11% incorporated BrdU; Fig 2B,F,I). It was also due to the number of cells in M-phase expressing phospho-histone H3 (PH3; only 73±13%; Fig 2C,G,I) in comparison with control embryos. This reduction was even more marked at 48 h PE (at stages HH19–23) when the proportion of PH3+ cells decreased to 46±7% (Fig 2I) and that of BrdU+ cells fell to 59±9% (Fig 2I). The reduction in proliferation in SOX5HIGH cells was accompanied by an increase in apoptosis assessed by activated caspase 3 (Cas3*) expression at 24 h (260±20%; Fig 2I, supplementary Fig S1D online) and 48 h PE (314±114%; Fig 2I) in relation to control cells (Fig 2I; supplementary Fig S1A online). Consequently, a premature increase in SOX5 reduces the total number of neuroepithelial cells due to both a reduction in cell proliferation and activation of apoptosis.
Figure 2.
Forced SOX5 expression promotes cell cycle exit. In relation to control pCIG embryos (A–C′), SOX5 misexpression (pCIG-SOX5; GFP, green on right side; E–H′) in HH14–16 embryos caused a 30% reduction in the size of the hemitube (E), in the number of BrdU (F,F′) and PH3-positive cells (G,G′), and an increase in activated caspase 3 (Cas3*)-positive dying cells (I). Expression of the neural progenitor marker PAX6 is not altered in SOX5HIGH cells (H,H′, D,D′). (I) Quantification of the effect 24 or 48 h PE. *P<0.01; **P<0.005; ***P<0.001. (J) Cumulative BrdU labelling curves of pCIG (black squares) or pCIG-SOX5 (red circles) electroporated neural tube cells. Dashed lines indicate the reduction in growth fractions. Mean of three embryos per experimental point, s.d. and t-test was calculated; ***P<0.01; **P<0.025; *P<0.05. BrdU, bromodeoxyuridine; C, control; EP, electroporated; GFP, green fluorescent protein; HH, Hamburguer and Hamilton; pCIG, pCAGGS-IRES-GFP; PE, post-electroporation; PH3, phospho-histone H3.
To explore further whether the neural cells with SOX5HIGH had an altered cell cycle phenotype and not only an increased death rate, we analysed the cell cycle distribution of surviving cells by flow cytometry. After 24 h of SOX5 electroporation, the ratio of neuroepithelial cells accumulated in G0/G1 phases was increased by 23% with respect to control electroporated cells in G0/G1 (a 10% increase with respect to the total population; supplementary Fig S2A,B online). In accordance, the expression of cyclin D1 and N-MYC were severely reduced in neural progenitors (Fig 3H; data not shown).
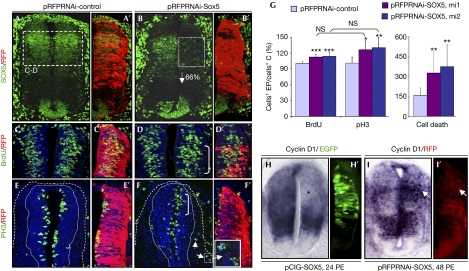
Figure 3.
SOX5 is necessary to control the timing of cell cycle exit. (A–G) Stage HH22, embryos analysed 48 h PE. (A–B′) SOX5-specific shRNA (pRFPRNAi-SOX5, mi2) caused a 66% decrease in the endogenous levels of SOX5 protein (B,B′) in relation to a pRFPRNAi-control (A,A′). (C–C′) Knocking down SOX5 expression with mi2 increases the number of BrdU- (D,D′) and PH3- (F,F′) positive cells with respect to control (C,C′, E,E′). Few proliferating RFP/PH3 double-positive cells are misallocated in the differentiated cell area (arrows in F, inset in F′). (G) Quantification of the effects using two shRNAs, mi1 and mi2. *P<0.05; **P<0.025; ***P<0.01. (H–I′) SOX5 overexpression decreases cyclin D1 expression at stage HH14 (asterisk in H), whereas reduction of SOX5 levels induces cyclin D1 expression at stage HH18 (arrow in I,I′). C, control; EP, electroporation; HH, Hamburguer and Hamilton; NS, not significant; pCIG, pCAGGS-IRES-GFP; PE, post-electroporation; PH3, phospho-histone H3; RFP, red fluorescent protein; shRNA, short hairpin RNA.
To clarify whether neural progenitors overexpressing SOX5 were exiting the cell cycle (accumulated in G0) or were being retained in a longer G1 phase due to the reduction in cyclin D1 levels (Lange et al, 2009), cumulative BrdU labelling was performed (Nowakowski et al, 1989; Fig 2J). We found that SOX5HIGH neural progenitors had a cell cycle length similar to pCIG control progenitors (14.8 and 14.4 h, respectively) and similar S-phase duration (4.5 and 4.7 h, respectively). However, there was a 12% decrease in the proportion of SOX5HIGH cycling cells with respect to the control (growth fraction of 0.75±0.03 versus 0.85±0.05; Fig 2J). In conclusion, SOX5 promotes premature cell cycle exit in neural progenitors without significantly affecting cell cycle length.
As for the increase in apoptosis, we have found that although 53±8% of the pCIG apoptotic cells were BrdU+ progenitors (after a 2-h pulse), only a 28±6% of the SOX5HIGH apoptotic cells were BrdU+. In addition, there were no changes in the number of apoptotic cells that expressed the differentiation marker HuC/D (15±6% for the pCIG and 13±4% for the SOX5HIGH apoptotic cells; data not shown). Furthermore, using the B-cell lymphoma 2 (BCL2) survival factor to rescue this cell death (Cayuso et al, 2006; supplementary Fig S1B–G online), we observed that neuroepithelial cells with high levels of SOX5 and BCL2 preferentially accumulated at G0/G1 (38% increase; supplementary Fig S2C,D online). Thus, apoptosis caused by SOX5 overexpression predominantly occurs in post-mitotic neural cells before they acquire definitive neuronal markers.
We next addressed whether SOX5 was not only sufficient but was also necessary to control the balance between cell proliferation and cell cycle exit. Knocking down SOX5 expression by specific interfering short hairpin RNAs (pRFPRNAi-SOX5) caused a marked 66±4% reduction in SOX5 protein levels at 48 h PE at HH19–22 (Fig 3A,B; supplementary Fig S3A online). Neural progenitors with reduced SOX5 levels, on using two different interfering RNAs (mi1 and mi2), presented a higher frequency of both BrdU incorporation (up to 114±7%; Fig 3C,D,G) and PH3 staining (up to 130±19%; Fig 3E–G). Furthermore, a small number of cells transfected with RNA-mediated interference (RNAi)-SOX5 and expressing PH3 were located in the mantle layer (insets in Fig 3F), suggesting that they entered mitosis in an ectopic position. In fact, reducing SOX5 levels increased cyclin D1 expression (n=3; Fig 3I). This could account for the appearance of ectopic mitosis as long-term forced expression of cyclin D1 leads to the appearance of proliferating cells in the differentiating field (Lobjois et al, 2008).
With respect to apoptosis, neural progenitors with low SOX5 levels presented up to a 2.4-fold increase in cell death when assessed by Cas3* expression and by the visualization of pyknotic nuclei (Fig 3G). It is possible that cells forced to proliferate when SOX5 expression is compromised initiate apoptosis while going through the G2/M phases. In neural tubes expressing BCL2 and pRFPRNAi-SOX5, we observed a 51% increase in the ratio of cells accumulated in G2/M with respect to control neural tubes by using flow cytometry analysis (supplementary Fig S3C,D online). Altogether, this suggests that neural progenitors with reduced SOX5 expression are maintained for longer in a proliferative state, and a fraction of them die by apoptosis.
In summary, SOX5 negatively regulates cell cycle progression and it is necessary and sufficient to promote cell cycle arrest at the G1–S transition.
SOX5 interferes with β-catenin transcriptional activity
The WNT–β-catenin signalling pathway favours neural tube progenitor proliferation by directly controlling the transcription of the cell cycle regulators cyclin D1 and N-MYC (Tetsu & McCormick, 1999; ten Berge et al, 2008; Fig 4A,B).
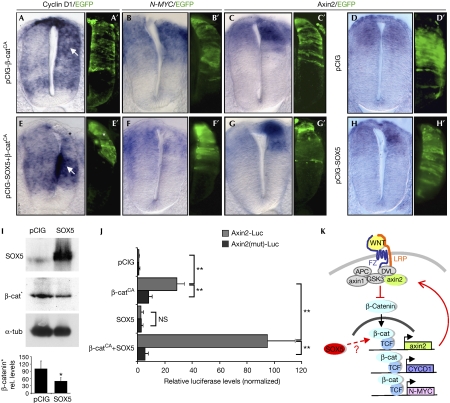
Figure 4.
SOX5 interferes with β-catenin transcriptional activity in the control of axin2 expression. (A–J) Stages HH14–16 embryos analysed at 24 h post-electroporation. Activation of the canonical WNT pathway by overexpressing a stabilized form of β-catenin (pCIG-β-catCA) promotes an upregulation of cyclin D1 (arrow in A,A′), N-MYC (B,B′) and axin2 expression (C,C′). Forcing SOX5 expression together with pCIG–β-catCA represses cyclin D1 (asterisk versus arrow in E,E′) and N-MYC (F,F′) expression and induces axin2 expression (G,G′). SOX5HIGH alone also induces axin2 expression (D,D′ compared with H,H′). (I) SOX5 overexpression reduces active β-catenin levels (β-cat*) to 49% of those in pCIG-electroporated cells. Values were normalized using α-tubulin as a reference (α-tub). *P<0.005. (J) Quantitative analysis of the transcriptional activity of SOX5 alone or in combination with β-cateninCA on an intact axin2 promoter (lighter bars) or on a TCF-binding site mutated one (darker bars). Graphs show normalized luciferase units relative to the pCIG control. Each bar represents mean±s.e.m. of triplicate experiments. **P<0.001. (K) Model for SOX5 action on the WNT signalling pathway in the spinal cord (see main text). DVL, dishevelled; FZ, frizzled; GSK3, glycogen synthase kinase 3; HH, Hamburguer and Hamilton; pCIG, pCAGGS-IRES-GFP; TCF, T-cell factor.
To test whether SOX5 could control cell cycle exit by interfering with the WNT–β-catenin pathway, we electroporated SOX5 together with a more stable form of β-catenin (β-cateninCA; Tetsu & McCormick, 1999), lacking one of the four phosphorylation sites that mediate axin/adenomatous polyposis coli complex binding and degradation. As expected, neuroepithelial cells with β-cateninCA displayed a higher proportion of cells in G2/M with respect to the control (supplementary Fig S4A,B online). The expression of SOX5HIGH reverted this situation, causing a 33% reduction in the ratio of progenitors in G2/M phases with respect to cells expressing β-cateninCA alone (supplementary Fig S4B,C online). Furthermore, the increase in cyclin D1 and N-MYC expression mediated by β-cateninCA was prevented in a cell-autonomous manner (n=3; asterisk versus arrow in Fig 4E). In addition, cyclin D1 was expressed in adjacent non-electroporated cells probably by the induction of soluble signals coming from the cells electroporated with SOX5.
Conversely, knocking down SOX5 expression provoked a further 14% increase in the ratio of cells in the G2/M phase with respect to cells expressing β-cateninCA alone (supplementary Fig S4D online). Thus, in neural progenitors with reduced SOX5 levels, the proliferative potential of the WNT pathway is reinforced.
Next, we determined that SOX5-induced changes in gene expression (Fig 4A–H) were due to alterations in the WNT canonical pathway activity, as we observed a 51±15% decrease in the levels of the dephosphorylated active form of β-catenin (Fig 4I) in SOX5HIGH cells with respect to control cells. Surprisingly, using the TOPFLASH reporter of WNT/β-catenin transcriptional activity, in neural tube cells, we observed that SOX5 acted synergistically with β-cateninCA, increasing TCF reporter activity by 3.4-fold with respect to β-cateninCA, alone (supplementary Fig S5 online).
To reconcile these apparently opposing results, we analysed the transcription of negative regulators of the WNT pathway, such as axin2 (Leung et al, 2002). By using the luciferase reporter system under the control of 1 kb of the axin2 promoter (with a functional TCF-binding site; Leung et al, 2002), we found that SOX5 acted synergistically with β-cateninCA, increasing axin2 promoter activity by 3.3-fold with respect to β-cateninCA alone (Fig 4J). In fact, this SOX5 function is dependent on the TCF-binding site in the axin2 promoter (Fig 4J). Furthermore, axin2 was overexpressed in the dorsal progenitors expressing SOX5HIGH alone (Fig 4H) and/or with β-cateninCA (Fig 4G,C) in relation to the control (Fig 4D). In conclusion, SOX5 enhances TCF/β-catenin activity on the transcription of axin2 in neural progenitors.
The increased levels of axin2 could mediate the reduction in the active form of β-catenin observed in these cells and consequently downregulate the expression of cyclin D1 and N-MYC, leading to cell cycle exit (Fig 4K). Obviously, axin2 transcription would also be affected by a reduction in the active form of β-catenin. A plausible interpretation for the elevated levels of axin2 messenger RNA would be that SOX5 was enhancing/stabilizing TCF/β-catenin transcriptional activity preferentially in the context of the axin2 promoter, compensating for the reduction in active β-catenin. There are examples of SOX genes, such as SOX4, which enhance TCF/β-catenin activity and might function to stabilize β-catenin (Sinner et al, 2007). We cannot exclude that in the context of cyclin D1 or N-MYC promoter, SOX5 could reduce TCF/β-catenin activity, as the same SOX protein can exert a different transcriptional modulation in distinct genes, depending on the developmental scenario (Wegner & Stolt, 2005).
Regulated of SOX5 expression is required for differentiation
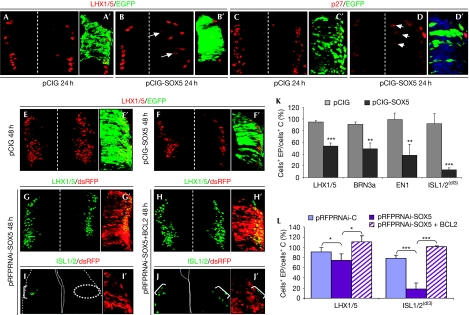
Proliferation and differentiation are highly coordinated events that can be uncoupled on occasion. To determine whether this was the case after SOX5 premature expression, specific markers of dorsal and ventral interneurons were analysed (Jessell, 2000). Neural progenitors with SOX5HIGH generated neurons that prematurely expressed LHX1/5 (dI2, dI4 and dI6 dorsal and V0 and V2 ventral interneurons; Martí et al, 2006), before reaching the mantle zone (n=4/8; Fig 5B). Furthermore, they ectopically expressed the cell cycle inhibitor p27kip1+ (n=3; Fig 5C,D). However, there was a marked reduction in LHX1/5+ interneurons located in the mantle zone 24 h (n=8/8; Fig 5B) and 48 h (54±5% remained; n=4; Fig 5E,F,K) after maintaining SOX5HIGH. A similar reduction was observed for BRN3a+ interneurons (dI3 and dI5; 49±10%; n=3; Fig 5K), and for the EN1+ V1 interneurons (38±19%; n=4; Fig 5K). More markedly, only 13±4% of the ISL1/2+ interneurons (dI3) remained (n=3; Fig 5K). Reducing apoptosis with the anti-apoptotic protein BCL2 did not significantly recover the reduced populations of LHX1/5+ and BRN3a+ interneurons, and only partly rescued that of ISL1/2+ interneurons (35±7% remained; supplementary Fig S6A–E online). In conclusion, progenitors with SOX5HIGH were forced to exit the cell cycle, generated neurons that prematurely expressed differentiation markers and around half of neurons fail to complete the differentiation programme.
Figure 5.
Downregulation of SOX5 expression is required for the progression of dorsal interneuron differentiation. (A–D′) Sustained elevation of SOX5 in stage HH14–16 embryos induces ectopic activation of LHX1/5 (arrows; B,B′) and p27kip1 (arrows in D,D′) in neurons before they reach the mantle zone, with respect to control pCIG cells (A,A′, C,C′). (E,F,K) At stages HH18–22, there was a reduction in the number of LHX1/5+ (F,F′), BRN3a+, EN1+ and ISL1/2+ interneurons (K). (G,I,L) At stages HH18–22, knocking down SOX5 expression (mi2, red) affects the number of LHX1/5+ (G,G′,L) and ISL1/2+ interneurons (I,I′,L). (H–J,L) Co-electroporation with BCL2 rescues the total number of (K) LHX1/5+ and (brackets in L) ISL1/2+ interneurons. Quantification of the number of cells expressing a given neuronal marker at 48 h PE with the indicated construct. *P<0.05; **P<0.003; ***P<0.001. C, control; EP, electroporation; HH, Hamburguer and Hamilton; ISL1/2, islet 1/2; pCIG, pCAGGS-IRES-GFP; PE, post-electroporation.
However, it has been shown that forcing progenitors to continue to cycle does not prevent cells from differentiating into the right cell type (Dyer & Cepko, 2000; Lobjois et al, 2008). In fact, knocking down SOX5 levels does not alter the hemitube thickness and only mildly alters the gross pattern of differentiation, as it slightly reduced the number of LHX1/5+ interneurons (16% reduction; n=5; Fig 5G,L) and reduced the number of the small population of ISL1/2+ dI3 interneurons (by 82%; n=3; Fig 5I,L). The reduction in the number of LHX1/5+ and ISL1/2+ neurons was totally rescued by BCL2 protein (Fig 5H,J,L). As the apoptosis is not fully overcome by BCL2 expression (supplementary Fig S3D online), these results would suggest that progenitors with reduced levels of SOX5 remain cycling for longer and probably generate an increased number of LHX1/5+ differentiated interneurons, similar to cyclin D1-transfected neural progenitors (Lobjois et al, 2008). The possible excess of LHX1/5+ interneurons would have been overcompensated by apoptosis.
In summary, these results suggest that (i) SOX5 is sufficient to induce premature cell cycle exit but it prevents the progression of the interneuron differentiation programme; (ii) SOX5 is required for the timing of cell cycle exit and for the correct final number of dorsal interneurons; and (iii) SOX5 is essential for the survival of dI3 interneurons that normally express high level of SOX5.
Our data, to our knowledge, establish, for the first time in a neural context, both the role of a SOX transcription factor in the timing of cell cycle exit and in the modulation of the WNT–β-catenin pathway to control that function. By increasing the levels of the negative regulator axin2, SOX5 would control the feedback repressor pathway regulating WNT signalling (Leung et al, 2002).
Several SOX proteins fulfil crucial roles in the context of neurogenesis. SOXB1 promotes progenitor cell maintenance (Bylund et al, 2003), whereas SOXB2 promotes the onset of neuronal differentiation (Sandberg et al, 2005). Our studies assign a role for SOX5 between the activity of SOXB1 and SOXB2 proteins, as SOX5 promotes cell cycle exit of neural progenitors and its downregulation is required for the progression of neuronal differentiation.
Methods
Chick in ovo electroporation. Embryos were electroporated at stages HH10–13 and processed 24 h PE (HH14–17) or 48 h PE (HH19–22) as previously described (Perez-Alcala et al, 2004), by using immunohistochemistry, in situ hybridization, western blotting, fluorescent associated cell sorting or luciferase assays.
Fluorescent associated cell sorting (FACS). Electroporated neural tubes, carrying green fluorescent protein as the reporter, were dissociated into a single-cell suspension 24–48 h later as described previously (Cayuso et al, 2006). Nuclei were labelled with propidium iodide to estimate DNA content in GFP+ cells. Flow cytometry data were collected and multiparameter analysis was performed in an EPICS XL Coulter Cytometer (Beckman Coulter, Brea, CA, USA).
Luciferase reporter assay. Transcriptional activity assays were performed in embryos electroporated with the indicated DNAs, together with 1 kb of axin2 promoter in a luciferase reporter construct (Leung et al, 2002) and two Renilla luciferase reporter constructs each carrying the cytomegalovirus and the simian virus 40 promoter (Promega, Southampton, UK) for normalization. Luciferase activities were measured by the Dual Luciferase Reporter Assay System (Promega).
Statistical analysis. All data presented here are number of cells in the electroporated (EP) area that expresses a marker with respect to the cells expressing the same marker in an equivalent area (control; C) on the non-electroporated side (% cells+ EP/cells+ C). Quantitative data were expressed as mean±s.d. or s.e.m.; n⩾3 embryos per experimental point. Significant differences were tested by Student's t-test.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
We thank B. Lázaro and A. Arias for technical assistance; P. Bovolenta for her support and together with A.M. Nieto and R. Diez del Corral for discussions; E. Martí for β-cateninCA and BCL2 constructs, E. Fearon for the axin2 promoter, R. Nowakowski for the Excel spreadsheet to calculate cell cycle length, H. Kondoh for N-MYC complementary DNA and S.A. Wilson for the pRFPRNAi vector. This work is supported by the Spanish Ministry of Science grants to A.V.M. (BFU2005-00762 and BFU2008-02963). A.V.M. was supported by Ramon y Cajal Programme, P.L.M.-M. and A.C.Q. by Spanish Ministry of Science fellowships.
Footnotes
The authors declare that they have no conflict of interest.
References
- Bylund M, Andersson E, Novitch BG, Muhr J (2003) Vertebrate neurogenesis is counteracted by Sox1–3 activity. Nat Neurosci 6: 1162–1168 [DOI] [PubMed] [Google Scholar]
- Cayuso J, Ulloa F, Cox B, Briscoe J, Martí E (2006) The Sonic hedgehog pathway independently controls the patterning, proliferation and survival of neuroepithelial cells by regulating Gli activity. Development 133: 517–528 [DOI] [PubMed] [Google Scholar]
- Dyer MA, Cepko CL (2000) p57(Kip2) regulates progenitor cell proliferation and amacrine interneuron development in the mouse retina. Development 127: 3593–3605 [DOI] [PubMed] [Google Scholar]
- Jessell TM (2000) Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat Rev Genet 1: 20–29 [DOI] [PubMed] [Google Scholar]
- Lai T, Jabaudon D, Molyneaux BJ, Azim E, Arlotta P, Menezes JR, Macklis JD (2008) SOX5 controls the sequential generation of distinct corticofugal neuron subtypes. Neuron 57: 232–247 [DOI] [PubMed] [Google Scholar]
- Lange C, Huttner WB, Calegari F (2009) Cdk4/cyclinD1 overexpression in neural stem cells shortens G1, delays neurogenesis, and promotes the generation and expansion of basal progenitors. Cell Stem Cell 5: 320–331 [DOI] [PubMed] [Google Scholar]
- Leung JY, Kolligs FT, Wu R, Zhai Y, Kuick R, Hanash S, Cho KR, Fearon ER (2002) Activation of AXIN2 expression by beta-catenin–T cell factor. J Biol Chem 277: 21657–21665 [DOI] [PubMed] [Google Scholar]
- Lobjois V, Bel-Vialar S, Trousse F, Pituello F (2008) Forcing neural progenitor cells to cycle is insufficient to alter cell-fate decision and timing of neuronal differentiation in the spinal cord. Neural Dev 3: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martí E, García-Campmany L, Bovolenta P (2006) Dorso-Ventral Patterning of the Vertebrate Central Nervous System. Weinheim, Germany: Wiley-VCH [Google Scholar]
- Megason SG, McMahon AP (2002) A mitogen gradient of dorsal midline Wnts organizes growth in the CNS. Development 129: 2087–2098 [DOI] [PubMed] [Google Scholar]
- Nowakowski RS, Lewin SB, Miller MW (1989) Bromodeoxyuridine immunohistochemical determination of the lengths of the cell cycle and the DNA-synthetic phase for an anatomically defined population. J Neurocytol 18: 311–318 [DOI] [PubMed] [Google Scholar]
- Perez-Alcala S, Nieto MA, Barbas JA (2004) LSox5 regulates RhoB expression in the neural tube and promotes generation of the neural crest. Development 131: 4455–4465 [DOI] [PubMed] [Google Scholar]
- Sandberg M, Kallstrom M, Muhr J (2005) Sox21 promotes the progression of vertebrate neurogenesis. Nat Neurosci 8: 995–1001 [DOI] [PubMed] [Google Scholar]
- Sinner D, Kordich JJ, Spence JR, Opoka R, Rankin S, Lin SC, Jonatan D, Zorn AM, Wells JM (2007) Sox17 and Sox4 differentially regulate beta-catenin/T-cell factor activity and proliferation of colon carcinoma cells. Mol Cell Biol 27: 7802–7815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Berge D, Brugmann SA, Helms JA, Nusse R (2008) Wnt and FGF signals interact to coordinate growth with cell fate specification during limb development. Development 135: 3247–3257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetsu O, McCormick F (1999) Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 398: 422–426 [DOI] [PubMed] [Google Scholar]
- Wegner M, Stolt CC (2005) From stem cells to neurons and glia: a Soxist's view of neural development. Trends Neurosci 28: 583–588 [DOI] [PubMed] [Google Scholar]
- Wilcock AC, Swedlow JR, Storey KG (2007) Mitotic spindle orientation distinguishes stem cell and terminal modes of neuron production in the early spinal cord. Development 134: 1943–1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.