Abstract
Purpose
βB1-crystallin is a putative target of an autoantibody observed in a subset of patients with uveitis. The purpose of this study was to determine whether seroreactivity against βB1 or other specific purified crystallin proteins is observed in patients with uveitis and whether this reactivity is associated with either cataract or active intraocular inflammation.
Methods
Sera from patients with uveitis were tested for IgG antibodies with reactivity against αA-, αB-, βB1-, or βB2-crystallin proteins using a modified slot-blot protocol. Ophthalmic evaluations included analysis of the degree of intraocular inflammation and assessment of lens opacity by the Lens Opacities Classification System (LOCS) III. Positive anti-crystallin reactivity was defined as greater than the mean + 2 SD of the reactivity of a commercially available control serum panel. Statistical analysis was performed with the Fisher exact test, Kruskal-Wallis test, and Student’s t-test.
Results
IgG antibodies against αA-, αB-, or βB1-crystallin were identified in 70% of 39 subjects; in contrast, only 30% of the control sera exhibited reactivity against one or more of these crystallin proteins (P ≤ 0.01). Seroreactivity against αA-, αB-, or βB1-, but not βB2-crystallin was related to active anterior segment inflammation. Seroreactivity against αB and βB1 was significantly related to cortical cataract (P ≤ 0.05).
Conclusions
Serum antibodies against specific crystallin proteins are present in most patients with uveitis. The relationship between the presence of specific anti-crystallin antibodies and active inflammation may indicate a role for these autoantibodies in uveitis pathogenesis.
Crystallins are major lens structural proteins expressed in the vertebrate eye that contribute to the transparency and refractive properties of the lens. In addition to its structural role, a variety of crystallin proteins are expressed in extralenticular locations. The best characterized are the α-crystallins, which are widely expressed throughout the body in tissues as diverse as the heart, skeletal muscle, and brain. These proteins act as intracellular molecular chaperones and heat shock proteins (HSP).1 In addition, α-crystallin may act to inhibit apoptotic cell death and is implicated in neuroprotection.2 Several investigators have suggested a role for α-crystallin protein in the pathophysiology of glaucomatous optic atrophy, multiple sclerosis, Alzheimer disease, and neuro-Behçet disease.3–5
Pathogenesis of noninfectious uveitis is thought to involve genetic predisposition, environmental exposures, and a T-lymphocyte–mediated, antigen-specific response.6 Complications of anterior uveitis include cataract, secondary glaucoma, and cystoid macular edema; the pathophysiology of which, in the context of inflammation, is not fully understood.7 Several autoimmune diseases, such as inflammatory bowel disease, diabetes, and Wegener’s granulomatosis, are associated with marker antibodies that may be used for diagnostic purposes, to identify clinically meaningful subgroups, and to determine the potential for disease complications.8,9 Identification of uveitis-associated marker antibodies may provide clues for understanding the specific pathogenesis of either the primary disease target or the secondary complications of the disease and may ultimately suggest tools for the modulation of specific self-destructive inflammatory responses.
Our previous work identified βB1-crystallin as a candidate uveitis-associated antigen and demonstrated its extralenticular expression in the ciliary body.10 βB1-crystallin is also identified in drusen.11 Purified crystallin proteins are immunogenetic in the Lewis rat, causing autoimmune ocular inflammation in preliminary studies in our laboratory (Chen L, Gordon LK, unpublished data, 2004). It has been reported that antibodies against lens proteins may be observed in patients with uveitis and in some patients with senile cataract.12,13 In the present study we hypothesize that crystallin may be an important autoantigen in human anterior uveitis and demonstrate that reactivity against specific crystallin proteins may be associated, either with the active intraocular inflammation or with secondary cataract pathogenesis.
Methods
Patients
Patients with uveitis were recruited from the Ocular Inflammatory Disease Center at the Jules Stein Eye Institute. This investigation was approved by the UCLA Institutional Review Board and was conducted in accordance with the Declaration of Helsinki. Patients were included if they had a history of noninfectious uveitis or had active noninfectious uveitis. Exclusion criteria was prior cataract surgery.
The following data were obtained: history of ocular surgery, uveitis duration, presence of uveitis-associated systemic diseases, and use of systemic immunomodulatory agents or topical anti-inflammatory medications at the time of enrollment. The best corrected Snellen visual acuity was recorded. Anterior chambers were evaluated by one of the uveitis specialists (RL, GNH); active uveitis was defined by the presence of at least occasional cells in the anterior chambers at the time of sampling. After dilation of the pupil, the degree of lens opacity was graded by the Lens Opacities Classification System (LOCS) III classification by one of the authors (RL). LOCS III is a method designed for classifying lens opacities for research purposes that involves a standardized classification of nuclear color at the slit lamp and cortical and posterior subcapsular (PSC) cataracts at the slit lamp with retroillumination.14 The LOCS III classification for cataract provided numerical values for the degree of cortical, subcapsular, and nuclear sclerosis in each subject. In this study, the presence of cortical cataract was defined as grade 2 or higher of cortical cataract. The presence of nuclear cataract was defined as grade 3 or higher of nuclear sclerosis. The presence of PSC cataract was defined as grade 2 or higher of PSC cataract. Serum was obtained from standard phlebotomy blood specimens, anonymously number coded, aliquoted, and stored at −20°C until use. Normal sera from 20 individuals were obtained from a commercially available panel for laboratory testing (Theratest, Chicago, IL). Individual sera were selected from the panel to reflect the gender and age characteristics of the uveitis patient population.
Crystallin Proteins
Human, recombinant βB1-crystallin was purified as previously described.10,15 Briefly, human recombinant βB1-crystallin in the pCR T7/V5 vector was expressed in Escherichia coli and purified by sequential cation and anion exchange chromatography. βB2-crystallin was prepared by using gel-exclusion chromatography.16 Recombinant αA- and αB-crystallins were prepared as described in a previous paper.17
Determination of Antibody Reactivity
Purified human or bovine crystallin protein was used as an antigenic target in a slot-blot quantitative analysis for antibody reactivity using modifications of a reported technique.18 Briefly, dilute crystallin protein (0.15 μg/100 μL) was applied to nitrocellulose filters and blocked with 1% bovine serum albumin (Invitrogen, Carlsbad, CA[b]). Initial studies with a panel of control normal test sera were performed with a range of dilutions, and a 1:200 dilution was determined to be optimal in producing some demonstrable reactivity (data not shown). Sera from patients or normal test sera were diluted at 1:200 in a physiologic buffered saline solution containing 0.05% Tween-20 and applied to the slot blots followed by extensive washing. Bound IgG was detected by using alkaline phosphatase-coupled goat anti-human IgG (Pierce, Rockford, IL) and identified colorimetrically with BCIP/NBT alkaline phosphatase (Vector Laboratories, Burlingame, CA). Care was taken to perform each experiment in an identical way, so that each blot contained internal controls in duplicate. These internal controls included no sera and known negative sera to test for nonspecific reactivities and known positive sera for use in internal standardization. The membrane was scanned into a computer, and quantitation of the reaction was performed by computer analysis of the color density. A relative intensity level of each sample was achieved by normalization with the internal positive controls. Samples were tested in duplicate and the results repeated on three occasions. Positive anti-crystallin reactivity was defined as greater than the mean + 2 SD of the normal sera from the standard test kit.
Analysis
Multiple analyses were performed to identify relationships between the presence of specific anti-crystallin antibodies or cataract and patient age, disease duration, or active uveitis at the time of sampling. A comparison of the presence of anti-crystallin antibodies and the type of cataract was also performed. In this analysis, positive cortical or PSC cataract was defined as a LOCS III grade ≥ 2 and a positive nuclear cataract was defined as a LOCS III grade ≥ 3. Statistical analysis was performed with the Fisher exact χ2, Kruskal-Wallis, and Student’s t-tests. P < 0.05 was considered significant. All probabilities associated with the data for cataracts were based on logistic regression models with the generalized estimating equation of the compound symmetry covariance structure, to account for correlations between the eyes of individual patients.
Results
A total of 39 subjects were enrolled in the study. Demographic and medical characteristics are shown in Table 1. There was a wide range of disease duration. Although the average duration was 76 months, the median was 48 months, reflecting several patients with long histories of uveitis spanning almost 30 years. Two eyes had undergone glaucoma filtration surgery. About two thirds of the patients had active disease and were on topical corticosteroid therapy at the time of enrollment. A few of the patients were on systemic corticosteroid or immunomodulatory therapy.
Table 1.
Baseline Characteristics for 39 Patients with Uveitis
| Characteristics | Summary Statistics |
|---|---|
| Male sex, n (%) | 8 (21%) |
| Age (y) | |
| Mean ± SD | 46.7 ± 12.9 |
| Median (range) | 48 (16–71) |
| Duration of disease (mo) | |
| Mean ± SD | 75.7 ± 90.7 |
| Median (range) | 48 (1–350) |
| Laterality of uveitis, n (%) | |
| Unilateral | 12 (31%) |
| Bilateral | 27 (69%) |
| Active uveitis at time of sampling, n (%) | 26 (67%) |
| Medication at time of sampling, n (%)* | |
| None | 7 (18%) |
| Topical corticosteroids only | 25 (64%) |
| Systemic medication (with or without topical corticosteroids) | 7 (18%) |
| Prior intraocular surgery, n (%)† | 2 (5.1%) |
| Uveitis diagnosis, n (%) | |
| Idiopathic anterior uveitis | 27 (67%) |
| HLA-B27 associated anterior uveitis | 8 (21%) |
| Vogt-Koyanagi-Harada | 2 (5%) |
| Sarcoidosis | 1 (3%) |
| Juvenile idiopathic arthritis | 1 (3%) |
Including three patients on systemic corticosteroids, four patients on methotrexate, three patients on cyclosporine, and one patient on infliximab.
Including two patients who had drainage device implants.
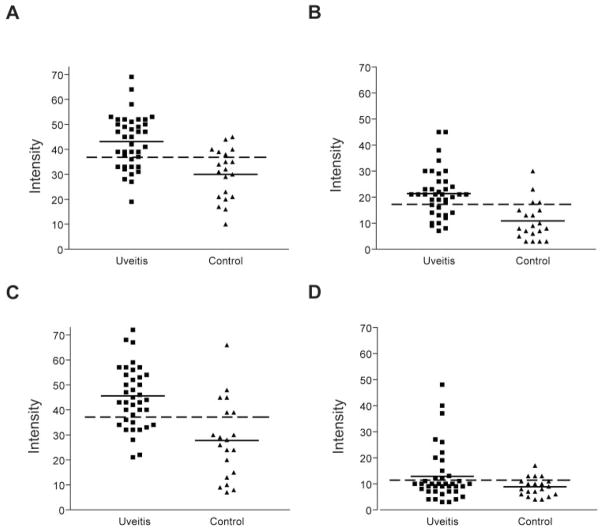
Of the 39 serum samples from patients with anterior uveitis, more than 70% exhibited substantial (greater than the mean + 2 SD of the control sera) antibody reactivity against αA-, αB-, and βB1-crystallins, whereas only 30% of the normal control samples did (P < 0.01; Fig. 1). Greater numerical reactivity against αA- and βB1-crystallin was observed in patients with uveitis compared to control subjects. In contrast, only a minority of patients with uveitis showed antibody reactivity against βB2-crystallin and there were no statistically significant differences between subjects and controls.
Figure 1.
Seroreactivity against crystallins in patients with uveitis and in normal control subjects. Horizontal dashed line: the mean + 2 SD of normal controls. Represented are seroreactivities against (A) αA-, (B) αB-, (C) βB1-, and (D) βB2-crystallins, respectively.
To address whether seropositivity against specific crystallins is associated with the level of uveitis activity, we compared reactivity in those subjects with active uveitis (n = 26) and those subjects who had a history of uveitis but in whom the disease was quiescent (n = 13) at the time of serum sampling (Table 2). A positive relationship was observed between uveitis activity and seroreactivity against the αA (P = 0.008), αB (P = 0.002), and βB1 crystallins (P = 0.022), but not in the anti-βB2-crystallin antibody responses (Table 2). Approximately 80% of the subjects with active uveitis exhibited reactivity against αA-, αB-, or βB1-crystallin, whereas less than 50% of subjects with quiescent uveitis exhibited seropositivity against these crystallins. No correlation was observed between the duration of disease or age of patients and seroreactivity against crystallins.
Table 2.
Comparisons of Characteristics with Crystallin Antibody Activity among 39 Patients with Uveitis
| Characteristics | Crystallin Antibody Activity |
||
|---|---|---|---|
| Positive | Negative | P | |
| αA | |||
| Patients (n) | 27 | 12 | |
| Age (y) | |||
| Mean ± SD | 49.1 ± 11.9 | 41.3 ± 13.9 | |
| Median (range) | 50 (19–71) | 45 (16–62) | 0.100* |
| Duration of disease (mo) | |||
| Mean ± SD | 64.1 ± 83.4 | 102.0 ± 104.4 | |
| Median (range) | 25 (1–288) | 64 (3–350) | 0.135* |
| Active uveitis, n (%) | 22 (81%) | 4 (33%) | 0.008† |
| αB | |||
| Patients (n) | 28 | 11 | |
| Age (y) | |||
| Mean ± SD | 46.0 ± 12.9 | 48.4 ± 13.4 | |
| Median (range) | 47.5 (16–71) | 50 (25–67) | 0.492* |
| Duration of disease (mo) | |||
| Mean ± SD | 78.8 ± 98.0 | 68.1 ± 72.4 | |
| Median (range) | 35 (1–350) | 48 (1–240) | 0.888* |
| Active uveitis, n (%) | 23 (82%) | 3 (27%) | 0.002† |
| βB1 | |||
| Patients (n) | 28 | 11 | |
| Age (y) | |||
| Mean ± SD | 48.7 ± 12.2 | 41.5 ± 13.8 | |
| Median (range) | 49.5 (19–71) | 44 (16–62) | 0.122* |
| Duration of disease (mo) | |||
| Mean ± SD | 89.5 ± 101.3 | 40.8 ± 41.4 | |
| Median (range) | 48 (1–350) | 36 (3–144) | 0.453* |
| Active uveitis, n (%) | 22 (79%) | 4 (36%) | 0.022† |
| βB2 | |||
| Patients (n) | 14 | 25 | |
| Age (y) | |||
| Mean ± SD | 44.9 ± 13.7 | 47.7 ± 12.7 | |
| Median (range) | 47.5 (16–59) | 49 (19–71) | 0.736* |
| Duration of disease (mo) | |||
| Mean ± SD | 109.1 ± 116.7 | 57.1 ± 68.2 | |
| Median (range) | 78 (1–350) | 34 (1–288) | 0.387* |
| Active uveitis, n (%) | 9 (64%) | 17 (68%) | 1.00† |
Kruskal-Wallis test.
Fisher’s exact test, comparing the presence of anti-crystallin activity in individuals with active uveitis to those with inactive uveitis.
The association between cataracts, at equal to or greater than a specified LOCS III level (2 for cortical or posterior subcapsular and 3 for nuclear), and age, uveitis duration, and uveitis activity was also evaluated (Table 3). As expected, patients with any cataract tended to be older (median, 53.5 years; range, 31–71) than those without cataract (median, 42 years; range, 16–67). However, cortical cataract (P = 0.085) specifically was less related to the age of the patient, in contrast to either nuclear sclerotic (P < 0.001) or PSC cataract (P = 0.002). The duration of disease tended to be associated with the presence of any cataract (100.8 ± 103 months) compared with no cataract (47.2 ± 687 months; P = 0.056). No correlation was observed between active uveitis at the time of sampling and any type of cataract (P = 0.342).
Table 3.
Comparisons of Characteristics with the Status of Cataracts among 66 Eyes from 39 Patients with Uveitis
| Characteristics | Status of Cataract |
||
|---|---|---|---|
| Presence* | Absence | P† | |
| Any cataract | |||
| Eyes (n) | 34 | 32 | |
| Age (y) | 0.001 | ||
| Mean ± SD | 53.1 ± 8.5 | 39.3 ± 13.6 | |
| Median (range) | 53.5 (31–71) | 42.5 (16–67) | |
| Duration of disease (mo) | 0.056 | ||
| Mean ± SD | 100.8 ± 103.0 | 47.2 ± 68.7 | |
| Median (range) | 52 (1–350) | 12 (1–265) | |
| Active uveitis, n (%)‡ | 24 (71%) | 17 (53%) | 0.342 |
| Cortical cataract | |||
| Eyes (n) | 13 | 53 | |
| Age (y) | 0.085 | ||
| Mean ± SD | 53.9 ± 11.1 | 44.6 ± 13.0 | |
| Median (range) | 53 (31–71) | 46 (16–67) | |
| Duration of disease (mo) | 0.367 | ||
| Mean ± SD | 57.1 ± 54.8 | 79.2 ± 98.3 | |
| Median (range) | 48 (1–139) | 36 (1–350) | |
| Active uveitis, n (%)‡ | 10 (77%) | 31 (58%) | 0.214 |
| Nuclear cataract | |||
| Eyes (n) | 25 | 41 | |
| Age (y) | 0.0004 | ||
| Mean ± SD | 54.7 ± 7.6 | 41.4 ± 13.3 | |
| Median (range) | 54 (44–71) | 43 (16–67) | |
| Duration of disease (mo) | 0.141 | ||
| Mean ± SD | 108.0 ± 115.7 | 54.6 ± 66.8 | |
| Median (range) | 48 (1–350) | 13 (1–265) | |
| Active Uveitis, n (%)‡ | 17 (68%) | 24 (59%) | 0.351 |
| PSC cataract | |||
| Eyes (n) | 22 | 44 | |
| Age (y) | 0.002 | ||
| Mean ± SD | 54.8 ± 8.4 | 42.3 ± 13.2 | |
| Median (range) | 54 (42–71) | 45 (16–67) | |
| Duration of disease (mo) | 0.186 | ||
| Mean ± SD | 110.0 ± 115.9 | 57.2 ± 71.7 | |
| Median (range) | 52 (5–350) | 13 (1–265) | |
| Active uveitis, n (%)‡ | 18 (82%) | 23 (52%) | 0.087 |
Presence of cortical cataract is defined as grade 2 or higher, presence of nuclear cataract is defined as grade 3 or higher of nuclear sclerosis, and presence of PSC cataract is defined as grade 2 or higher of PSC cataract. Presence of any cataract is defined as the presence of cortical cataract, nuclear cataract, or PSC cataract, as described above.
All probabilities were based on logistic regression models with generalized estimating equation (GEE) of compound symmetry covariance structure to account for correlations between two eyes of individual patients.
Percentage of eyes with active uveitis among eyes with or without cataracts.
To determine whether seropositivity against specific crystallins reflected the cataract severity, we analyzed the association of antibody reactivity with either nuclear sclerosis, cortical, or PSC cataract (Table 4). Significant relationships were observed between the presence of cortical cataract and seroreactivity against αB (P = 0.032) or βB1-crystallin (P = 0.028). No relationship was observed between the presence of either posterior subcapsular or nuclear sclerotic cataract and seroreactivity against either αB- or βB1-crystallin. No relationship was observed between seroreactivity against αA- or βB2-crystallin and any type of cataract.
Table 4.
Comparisons of Crystallin Antibody Activity with the Status of Cataract in 66 Eyes from 39 Patients with Uveitis
| Positive Crystallin Antibody Activity | Status of Cataract |
||
|---|---|---|---|
| Presence* | Absence | P† | |
| Any cataract | |||
| Eyes (n) | 34 | 32 | |
| Positive αA, n (%)‡ | 23 (68%) | 23 (72%) | 0.871 |
| Positive αB, n (%) | 27 (79%) | 21 (66%) | 0.395 |
| Positive βB1, n (%) | 27 (79%) | 19 (59%) | 0.125 |
| Positive βB2, n (%) | 11 (32%) | 11 (34%) | 0.945 |
| Cortical cataract | |||
| Eyes (n) | 13 | 53 | |
| Positive αA, n (%) | 11 (85%) | 35 (66%) | 0.234 |
| Positive αB, n (%) | 12 (92%) | 36 (68%) | 0.032 |
| Positive βB1, n (%) | 12 (92%) | 34 (64%) | 0.028 |
| Positive βB2, n (%) | 6 (46%) | 16 (30%) | 0.429 |
| Nuclear cataract | |||
| Eyes (n) | 25 | 41 | |
| Positive αA, n (%) | 17 (68%) | 29 (71%) | 0.858 |
| Positive αB, n (%) | 20 (80%) | 28 (68%) | 0.495 |
| Positive βB1, n (%) | 20 (80%) | 26 (63%) | 0.143 |
| Positive βB2, n (%) | 8 (32%) | 14 (34%) | 0.782 |
| PSC cataract | |||
| Eyes (n) | 22 | 44 | |
| Positive αA, n (%) | 16 (73%) | 30 (68%) | 0.812 |
| Positive αB, n (%) | 18 (82%) | 30 (68%) | 0.440 |
| Positive βB1, n (%) | 19 (86%) | 27 (61%) | 0.109 |
| Positive βB2, n (%) | 7 (32%) | 15 (34%) | 0.729 |
The presence of cortical cataract is defined as grade 2 or higher, the presence of nuclear cataract as grade 3 or higher of nuclear sclerosis, and the presence of PSC cataract as grade 2 or higher. The cataract subtypes are not mutually exclusive as, according to the LOCS grading system, one eye may have more than one type of cataract. Presence of any cataract is defined as the presence of cortical cataract, nuclear cataract, or PSC cataract, as described above.
All probabilities are based on logistic regression models with the generalized estimating equation (GEE) of the compound symmetry covariance structure used to account for correlations between the eyes of individual patients.
All percentages are those of eyes with positive crystallin antibody activity of all eyes with or without cataracts.
Discussion
In this study, we demonstrated seroreactivities against αA-, αB-, and βB1-crystallin in most patients with uveitis, and obtained evidence of a relationship between the presence of these reactivities and active uveitis. Age was associated with any cataract and with either nuclear sclerotic or PSC cataracts, but not with cortical cataracts. Seroreactivity against αB- and βB1-crystallin was associated with cortical cataract. Although there is a known association between PSC cataract and use of corticosteroids; cortical cataracts, associated in this report with anti-crystallin antibodies, have not been associated with topical steroid use. Therefore, our results support a role for autoantibodies against αB- or βB1-crystallin and cortical cataract pathogenesis. However, this association could also be a coincidence as the absolute number of eyes with cortical cataract is relatively small. Confirmation of this association necessitates further evaluation in a larger series of patients.
Several studies have shown variable seroreactivity against lens proteins in patients with uveitis.12,19,20 Doycheva et al.12 demonstrated a high prevalence of antibodies against lens proteins in the sera of patients with uveitis. In contrast to our study, they showed a low prevalence of seroreactivity; 49% of patients with anterior uveitis, 32% of patients with intermediate uveitis and 22% of patients with posterior uveitis. Their reported prevalence of control seropositivity was also lower than that in our study, at approximately 12%. This disparity in results could reflect the different testing methods used and source of protein extract but underscores the potential relevance of crystallin proteins as candidate autoantigens in human uveitis.
There was discordance between the findings of Doycheva et al.12 and ours in terms of both uveitis activity levels and the presence of cataract. Their study included 165 patients with uveitis; 49% of those with anterior uveitis had antibodies against human lens proteins by ELISA testing. However, they did not find a correlation between anti-lens reactivity and either uveitis activity or the presence of cataract. There are several potential explanations for this difference. First, they identified the individuals as having reactivity against whole lens proteins and did not specifically examine the relationship between seroreactivity against specific crystallin proteins and either uveitis reactivity or cataract. Second, they did not evaluate cataracts according to a standardized protocol. It is possible that a careful analysis of cataract grading and type of cataract is necessary for the detection of a relationship between substantial cataracts of a particular type and seroreactivity against specific crystallin proteins. The present study is also skewed in a high percentage of female subjects, whereas sex is not reported in the other paper.12 Differences between the sexes in autoimmune disease and manifestations could also be a potential explanation for the differences between the two studies.
What is the potential relevance of seroreactivity against this subset of crystallin proteins in the context of human uveitis? α-Crystallin, a member of the small heat shock protein family (HSP), has been previously identified as a candidate autoantigen by serum antibodies associated with several autoimmune diseases such as multiple sclerosis and Alzheimer disease.3,5,21 These studies did not define reactivity against other crystallin isoforms. It is possible that specific crystallin isoforms are involved in the pathogenesis of inflammatory diseases such as uveitis or alternatively that this reactivity may simply be a bystander reaction observed in the setting of active disease. Seroreactivity against crystallins is also present in some healthy individuals as well as in patients with senile cataract,12,13 suggesting that antibody alone would not cause inflammation, and other factors may be requisite for disease development. Mechanisms underlying autoimmunity directed against ubiquitous self proteins in tissue-specific autoimmune diseases are not understood but are observed in a wide variety of diseases, ranging from diabetes mellitus to Crohn’s disease to Wegener’s granulomatosis, and in some cases, the levels of reactivity reflect the disease activity or their presence aids in disease diagnosis.8,9,22 It is intriguing that protein modifications may play an important role in disease pathogenesis in autoimmunity. It is well known that aging is associated with increasing changes in modifications of various crystallin proteins.15 In the setting of lipopolysaccharide-induced uveitis in the Lewis rat model, a proteomic approach was used to identify changes in the composition of the vitreous humor.23 That study clearly demonstrated both an increase in specific crystallin isoforms in the vitreous and a change in truncation of the crystallins in the uveitis model, supporting a potential role for modified crystallin proteins in uveitis pathogenesis. However, the present study did not examine specific autoantigenic epitopes or neoantigens that may arise during disease or aging.
βB1-crystallin was recently identified as a candidate uveitis-associated antigen, in which extralenticular expression was observed in the ciliary body and in drusen.10,11,24 In this study, we observe a positive association between the presence of serum anti-βB1-crystallin antibody and inflammation activity or the presence of substantial cataract. Although this result does not prove causation of the antibodies in cataract pathogenesis, it is known that proliferation of lens epithelial cells plays an important role in lens homeostasis, and cytotoxicity experiments suggest that β-crystallins may be associated with the plasma membrane of lens epithelial cells.25 Antibodies against β-crystallins may also induce lens epithelial cell damage and cell migration in cortical cataract in mice.25,26 These studies support a possible role for anti-crystallin antibodies in cataract pathogenesis in the setting of inflammatory disease. Further longitudinal studies and detailed examination of these antibodies in a larger number of patients with uveitis is needed to determine whether the presence of these antibodies is predictive of development of cataract over time.
Acknowledgments
Supported in part by National Eye Institute Grants EY13708 (LKG), EY012239 (KJL), and EY3897 (JH); Research to Prevent Blindness (RPB), Inc., New York, NY (LKG, GNH); the MacDonald Family Foundation, Los Angeles, CA (RDL); the Skirball Foundation, New York, NY (GNH); and the Vernon O. Underwood Family Endowed Professorship (GNH). LKG is an RPB James S. Adams Scholar, GNH is the recipient of an RPB Physician-Scientist Award, and JH holds the Oppenheimer Brothers Chair in Ophthalmology. Additional support was provided by the Emily Plumb Estate and Trust Gift for resources utilized in the Jules Stein Eye Institute Clinical Research Center.
Footnotes
Disclosure: L. Chen, None; G.N. Holland, None; F. Yu, None; R.D. Levinson, None; K.J. Lampi, None; J. Horwitz, None; L.K. Gordon, None
References
- 1.Horwitz J. Alpha-crystallin. Exp Eye Res. 2003;76:145–153. doi: 10.1016/s0014-4835(02)00278-6. [DOI] [PubMed] [Google Scholar]
- 2.Kamradt MC, Lu M, Werner ME, et al. The small heat shock protein alpha B-crystallin is a novel inhibitor of TRAIL-induced apoptosis that suppresses the activation of caspase-3. J Biol Chem. 2005;280:11059–11066. doi: 10.1074/jbc.M413382200. [DOI] [PubMed] [Google Scholar]
- 3.Ousman SS, Tomooka BH, van Noort JM, et al. Protective and therapeutic role for alphaB-crystallin in autoimmune demyelination. Nature. 2007;448:474–479. doi: 10.1038/nature05935. [DOI] [PubMed] [Google Scholar]
- 4.Tezel G, Seigel GM, Wax MB. Autoantibodies to small heat shock proteins in glaucoma. Invest Ophthalmol Vis Sci. 1998;39:2277–2287. [PubMed] [Google Scholar]
- 5.Celet B, Akman-Demir G, Serdaroglu P, et al. Anti-alpha B-crystallin immunoreactivity in inflammatory nervous system diseases. J Neurol. 2000;247:935–939. doi: 10.1007/s004150070049. [DOI] [PubMed] [Google Scholar]
- 6.Caspi RR, Silver PB, Chan CC, et al. Genetic susceptibility to experimental autoimmune uveoretinitis in the rat is associated with an elevated Th1 response. J Immunol. 1996;157:2668–2675. [PubMed] [Google Scholar]
- 7.Woreta F, Thorne JE, Jabs DA, Kedhar SR, Dunn JP. Risk factors for ocular complications and poor visual acuity at presentation among patients with uveitis associated with juvenile idiopathic arthritis. Am J Ophthalmol. 2007;143:647–655. doi: 10.1016/j.ajo.2006.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saibeni S, Ciscato C, Vecchi M, et al. Antibodies to tissue-type plasminogen activator (t-PA) in patients with inflammatory bowel disease: high prevalence, interactions with functional domains of t-PA and possible implications in thrombosis. J Thromb Haemost. 2006;4:1510–1516. doi: 10.1111/j.1538-7836.2006.01970.x. [DOI] [PubMed] [Google Scholar]
- 9.Sebastian JK, Mahr AD, Ahmed SS, et al. Antiendothelial cell antibodies in patients with Wegener’s granulomatosis: prevalence and correlation with disease activity and manifestations. J Rheumatol. 2007;34:1027–1031. [PubMed] [Google Scholar]
- 10.Stempel D, Sandusky H, Lampi K, et al. BetaB1-crystallin: identification of a candidate ciliary body uveitis antigen. Invest Ophthalmol Vis Sci. 2003;44:203–209. doi: 10.1167/iovs.01-1261. [DOI] [PubMed] [Google Scholar]
- 11.Crabb JW, Miyagi M, Gu X, et al. Drusen proteome analysis: an approach to the etiology of age-related macular degeneration. Proc Natl Acad Sci U S A. 2002;99:14682–14687. doi: 10.1073/pnas.222551899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doycheva D, Preuss B, Klein R, Zierhut M. High incidence of antibodies to lens proteins in sera from patients with uveitis. Graefes Arch Clin Exp Ophthalmol. 2007;245:683–688. doi: 10.1007/s00417-006-0407-2. [DOI] [PubMed] [Google Scholar]
- 13.Merck KB, de Haard-Hoekman WA, Cruysberg JR, Bloemendal H, de Jong WW. Characterization of anti-crystallin autoantibodies in patients with cataract. Mol Biol Rep. 1993;17:93–99. doi: 10.1007/BF00996216. [DOI] [PubMed] [Google Scholar]
- 14.Chylack LT, Jr, Wolfe JK, Singer DM, et al. The Lens Opacities Classification System III. The Longitudinal Study of Cataract Study Group. Arch Ophthalmol. 1993;111:831–836. doi: 10.1001/archopht.1993.01090060119035. [DOI] [PubMed] [Google Scholar]
- 15.Lampi KJ, Oxford JT, Bachinger HP, Shearer TR, David LL, Kapfer DM. Deamidation of human beta B1 alters the elongated structure of the dimer. Exp Eye Res. 2001;72:279–288. doi: 10.1006/exer.2000.0950. [DOI] [PubMed] [Google Scholar]
- 16.McFall-Ngai M, Horwitz J, Ding LL, Lacey L. Age-dependent changes in the heat-stable crystallin, beta Bp, of the human lens. Curr Eye Res. 1986;5:387–394. doi: 10.3109/02713688609025178. [DOI] [PubMed] [Google Scholar]
- 17.Horwitz J, Huang QL, Ding L, Bova MP. Lens alpha-crystallin: chaperone-like properties. Methods Enzymol. 1998;290:365–383. doi: 10.1016/s0076-6879(98)90032-5. [DOI] [PubMed] [Google Scholar]
- 18.Marlow SJ, Handa AK. Immuno slot-blot assay using a membrane which covalently binds protein. J Immunol Methods. 1987;101:133–139. doi: 10.1016/0022-1759(87)90226-2. [DOI] [PubMed] [Google Scholar]
- 19.Luntz MH. Anti-uveal and anti-lens antibodies in uveitis and their significance. Exp Eye Res. 1968;7:561–569. doi: 10.1016/s0014-4835(68)80010-7. [DOI] [PubMed] [Google Scholar]
- 20.Patel M, Shine B, Murray PI. Antilens antibodies in cataract and inflammatory eye disease: an evaluation of a new technique. Int Ophthalmol. 1990;14:97–100. doi: 10.1007/BF00154208. [DOI] [PubMed] [Google Scholar]
- 21.Wilhelmus MM, Otte-Holler I, Wesseling P, de Waal RM, Boelens WC, Verbeek MM. Specific association of small heat shock proteins with the pathological hallmarks of Alzheimer’s disease brains. Neuropathol Appl Neurobiol. 2006;32:119–130. doi: 10.1111/j.1365-2990.2006.00689.x. [DOI] [PubMed] [Google Scholar]
- 22.Kumar PA, Suryanarayana P, Reddy PY, Reddy GB. Modulation of alpha-crystallin chaperone activity in diabetic rat lens by curcumin. Mol Vis. 2005;11:561–568. [PubMed] [Google Scholar]
- 23.Bahk SC, Lee SH, Jang JU, et al. Identification of crystallin family proteins in vitreous body in rat endotoxin-induced uveitis: involvement of crystallin truncation in uveitis pathogenesis. Proteomics. 2006;6:3436–3444. doi: 10.1002/pmic.200500779. [DOI] [PubMed] [Google Scholar]
- 24.Nakata K, Crabb JW, Hollyfield JG. Crystallin distribution in Bruch’s membrane-choroid complex from AMD and age-matched donor eyes. Exp Eye Res. 2005;80:821–826. doi: 10.1016/j.exer.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 25.Ibaraki N, Lin LR, Dang L, et al. Anti-beta-crystallin antibodies (mouse) or sera from humans with age-related cataract are cytotoxic for lens epithelial cells in culture. Exp Eye Res. 1997;64:229–238. doi: 10.1006/exer.1996.0203. [DOI] [PubMed] [Google Scholar]
- 26.Sueno T, Inoue E, Singh DP, Awata T, Chylack LT, Jr, Shinohara T. Oral administration of lens homogenate suppresses antibody production in mice injected with beta-crystallin emulsified in CFA. Exp Eye Res. 1997;64:379–385. doi: 10.1006/exer.1996.0218. [DOI] [PubMed] [Google Scholar]