Abstract
Purpose
To evaluate the in vitro effects of an aerosolized cyclooxygenase-2 (COX-2) inhibitor, nimesulide, on the cytotoxicity and apoptotic response of doxorubicin against the human lung adenocarcinoma cell line A549.
Methods
Nimesulide was formulated into a metered dose inhaler (MDI) formulation and characterized for aerodynamic particle size and medication delivery. The in vitro cytotoxicity of nimesulide-MDI in the presence or absence of doxorubicin was assessed by using the six-stage viable impactor by an already standardized method. Induction of apoptosis in A549 cells by nimesulide (nonaerosolized or aerosolized) in combination with doxorubicin was evaluated by established techniques such as caspase-3 estimation and terminal deoxynucleotidyl transferase-mediated nick end labeling (TUNEL) staining. Finally, to understand the mechanism of action, the influence of different treatments on the expression of COX-2 and peroxisome proliferator-activated receptor-γ (PPAR-γ) in A549 cells was studied by immunoblotting.
Results
The nimesulide-MDI formulation had a mass median aerodynamic diameter (MMAD) of 1.1 μm, (GSD = 2.8) and a medication delivery of 51 μg/shot. Nimesulide-MDI (40 shots) in combination with doxorubicin (0.01 μg/ml) had a cell kill of more than 60% as determined by in vitro cytotoxicity assay. The specific caspase-3 activity in A549 cells treated with nimesulide (40 μg/ml) and doxorubicin (0.25 μg/ml) in combination was 3 and 5 times higher than doxorubicin and nimesulide, respectively. Further, TUNEL staining showed apoptosis in over 30% of A549 cells treated with aerosolized nimesulide and doxorubicin combination vs. negligible as seen in cells treated individually. The expression of COX-2 was not altered in control or treatments, whereas PPAR-γ was expressed only in the combination treatment.
Conclusion
Our results indicate that aerosolized nimesulide significantly enhances doxorubicin activity against A549 cells, and the enhanced cytotoxicity was probably mediated via a COX-2–independent mechanism.
Keywords: nimesulide, inhalation, doxorubicin, cytotoxicity, apoptosis
INTRODUCTION
Lung cancer is the leading cause of cancer-related deaths in the United States. In fact, lung cancer accounts for more solid tumor deaths than colorectal, breast, prostate, and pancreatic cancer combined. The lungs are also a primary site of metastases from other types of cancer, including breast, colon, and prostate cancer. Recent advances in molecular and cellular biology have provided a new foundation for novel treatment strategies. There is growing evidence that cyclooxygenase-2 (COX-2) is commonly overexpressed in premalignant tissues and malignant tumors, which suggests that COX-2 is mechanistically linked to the development of cancer (1). This is supported by both genetic and pharmacologic evidence. Selective inhibitors of COX-2 such as celecoxib and NS-398 reduced the formation of different forms of tumors such as breast, lungs, etc. (2,3) and suppressed the growth of established tumors including gut and prostate in animals (4,5). In addition, COX-2 has been expressed in newly formed blood vessels within tumors (6). The induction of COX-2 and its associated production of prostaglandin E2 (PGE2) from arachidonic acid are thought to play a role in the initiation and maintenance of cancer cell survival and growth (6).
Various investigations have shown synergistic cytotoxic effects of COX inhibitors with anticancer drugs in vitro. Nontoxic concentrations of indomethacin were found to enhance the sensitivity of etoposide and methotrexate in cultured Lewis lung carcinoma cells (7). It has been reported that indomethacin modulates the cytotoxicity of vincristine in a variety of pulmonary carcinoma cell lines (adenocarcinoma, large cell carcinoma, squamous cell carcinoma, and small cell carcinomas). Duffy et al. (8) reported that a subset of nonsteroidal antiinflammatory drugs such as indomethacin, sulindac, and tolmetin significantly improved the in vitro cytotoxicity of anthracyclines, teniposide, VP-16, and vincristine. The study also found that other COX inhibitors such as mefenamic acid, diclofenac, naproxen, fenoprofen, flurbiprofen, ketoprofen, and phenylbutazone were inactive in enhancing the cytotoxicity of anticancer drugs. Soriano et al. (9) have shown additive or synergistic cytotoxic effects of sulindac sulfide with cisplatin and paclitaxel against human lung cancer H460, SHP77, and A549 cell lines. More recently, it was demonstrated that a selective COX-2 inhibitor, nimesulide, at 10 to 30 μM concentrations, when used in combination, reduced the IC50 values of several anticancer drugs such as SN-38, docetaxel, VP-16, and cisplatin against a variety of non-small-cell lung cancer (NSCLC) cell lines. Moreover, the antiproliferative effect of nimesulide was found to be related to the expression of COX-2 in the lung cancer cell lines. Based on these findings, it may be said that selective COX-2 inhibitors may be used as chemopreventive agents and/or as an adjunct in the chemotherapy of cancer.
Localized delivery of drugs to the lungs by the inhalation route provides high local pulmonary concentrations while minimizing systemic exposure. Inhalation drug delivery for the treatment of lung cancer has received new attention from scientists for treatment of lung cancer, and nebulized liposomal formulations of 9-nitrocamptothecin and paclitaxel have been studied in the treatment of lung cancer in animal models. However, the most widely used and convenient inhalation device for the delivery of drugs to the lungs is the pressurized metered dose inhaler (MDI). Therefore, the aim of this study was to evaluate the feasibility and efficacy of delivering a COX-2 inhibitor using a hydrofluoroalkane (HFA) propellant-based MDI. Nimesulide, a nonsteroidal antiinflammatory drug (NSAID) and relatively selective COX-2 inhibitor with a COX-1/COX-2 IC50 ratio of 17.69, was used for this investigation. It is expected that inhaled delivery of the COX-2 inhibitor will provide effective tissue levels in lungs and provide a better synergistic cytotoxic response with intravenously administered cytotoxic drugs.
Among the potential targets of NSAIDs is the PPAR family of nuclear receptors that function as ligand-dependent transcription factors (10). Three isoforms have been described PPAR-α,-γ, and –δ, all of which bind to specific DNA sequences as heterodimers with the retinoic acid X-receptors (11). Whereas the function of PPAR-γ in the setting of human cancer is still not well studied, recent findings indicate that loss of PPAR-γ expression is associated with colon tumorigenesis, and activation of PPAR-γ leads to inhibition of anchorage-independent growth of colon cancer cells (12). It is known that activation of PPAR-γ regulates expression of genes regulating a myriad of physiologic and pathophysiologic states.
In the present study, we evaluated the effect of a COX-2 inhibitor, nimesulide, on the in vitro cytotoxicity of doxorubicin against human lung (A549 and H460) and colon (SW620) tumor cell lines, which are known to express COX-2. We observed that the combination of nimesulide with doxorubicin produces a substantial reduction in the IC50 value of doxorubicin against both lung cancer cell lines. Further, we evaluated the in vitro potentiation of the cytotoxicity of doxorubicin by aerosolized nimesulide (via MDI) against the human lung adenocarcinoma A549 cell line, as these cells have been extensively used in the literature and represent the histologic type of the majority of lung cancer patients (13). The objectives of this study were to (a) develop a MDI formulation of nimesulide using a HFA propellant, (b) assess the in vitro cytotoxicity of the aerosolized nimesulide via MDI alone or its combination with doxorubicin against A549 cells, (c) study the induction of apoptosis in A549 cells by the combination of nimesulide (aerosolized or nonaerosolized) with doxorubicin in comparison with that of nimesulide or doxorubicin alone, and (d) to investigate the possibility of a COX-2–independent and PPAR-γ–mediated mechanism involved in the enhanced cytotoxicity of the combination treatment.
MATERIALS AND METHODS
Materials
HFA 134a and 227 were obtained from Du Pont (Ingleside, TX) and Solvay Fluorides Inc. (Hanover, Germany), respectively. Nimesulide was provided as a generous gift from Panacea Biotech Ltd. (New Delhi, India). Doxorubicin and all tissue culture chemicals were obtained from Sigma Chemical Company (St. Louis, MO). Acridine Orange Stain Dropper solution was obtained from Becton Dickinson and Company (Sparks, MD). DeadEnd™ Colorimetric Apoptosis Detection System and CaspACE™ Assay System colorimetric kits were obtained from Promega Corporation (Madison, WI). Apoptotic DNA ladder kit was purchased from Roche Diagnostic Corporation (Indianapolis, IN). DNA marker (100-bp ladder) was purchased from Fisher Scientific (Suwanee, GA). All other chemicals were of reagent grade. The six-stage viable impactor and eight-stage Andersen cascade impactor, Mark II, were obtained from Graseby Andersen (Smyrna, GA). Continuous and noncontinuous valves were kindly provided by 3M Pharmaceuticals (St. Paul, MN). The human lung tumor cell lines A549 and H460 and colon tumor cell line SW620 were obtained from American Type Culture Collection (Rockville, MD). A549 cells were grown in F12K medium supplemented with 10% fetal bovine serum. H460 and SW620 cells were grown in RPMI medium supplemented with 10% fetal bovine serum. All the tissue culture media contained penicillin (5000 U/ml), streptomycin (0.1 mg/ml), and neomycin (0.2 mg/ml). The tumor cells were grown in standard tissue culture conditions, passaged at 80–90% confluence, and cytotoxicity experiments were performed between 2 and 20 passages.
Solubility of Nimesulide in HFA 134a and 227
The solubility of nimesulide, at various concentrations of ethanol, was determined in the HFA propellants (134a and 227) after equilibration for 48 h. Excess nimesulide (at least 40 mg) was added to a clean 15-ml glass vial containing 0, 0.4, 0.8, 1.2, or 1.6 g of ethyl alcohol (200 proof) and crimped with a continuous valve. HFA 134a or 227 was then added from a pressure burette attached to a filling machine to bring the final weight in each vial up to 10 g. The vials were then placed on a platform shaker (Innova 2000, New Brunswick Scientific, Edison, NJ) at 150 rpm and allowed to shake for 48 h. The solubility was determined by transferring the contents to a chilled receiving vial via an assembly consisting of two transfer buttons connected to a 0.45-μm Acrodisc filter. The weight of the transferred portion in the receiving vial was recorded. Then the receiving vial was placed in dry ice (for about 30 min), the valve was decrimped, and the contents were poured into a clean, prechilled, volumetric flask through a glass funnel. After allowing time for the propellant to evaporate, the vial and the valve were rinsed, and the appropriate amount of methanol was added to the volumetric flask. The amount of nimesulide in methanol was then determined by using a spectrophotometer (Beckman DU 640) at a wavelength of 320 nm, and the solubility of nimesulide in the propellant system was calculated as percent weight.
Formulation of Nimesulide-MDI
Fifteen milligrams of nimesulide was placed in a clean 15-ml glass vial containing 2.25 g of ethyl alcohol (200 proof). The vial was immediately crimped with a continuous valve followed by the addition of HFA 134a as described above. The vial was then placed on a platform shaker at 150 rpm and allowed to shake overnight. The continuous valve was then replaced with a 50 μl noncontinuous valve.
Medication Delivery (Ex-Actuator Dose) of Nimesulide Formulation
The MDI formulation was primed by firing five shots into waste. The formulation was then fired once into the medication delivery device (MDD) with a glass wool filter under a flow rate of 30 L/min. The MDD chamber was then diluted with 25 ml of methanol and assayed with a spectrophotometer. The formulation was tested at least three times.
Aerodynamic Particle Size Distribution
An eight-stage Andersen cascade impactor, Mark II, was used to assess the aerodynamic size distribution of the nimesulide formulation. The formulation was primed by firing five shots into waste. Then five shots (at 5 s intervals) were fired into the cascade impactor under a flow rate of 28.3 L/min. The deposited nimesulide was determined from the actuator, throat, jet stage, impactor stages 0–7, and filter by transferring each component to individual polyethylene bags and rinsing with an appropriate volume of methanol. The samples were analyzed on a spectrophotometer at a wavelength of 320 nm. The mass median aerodynamic diameter (MMAD) and geometric standard deviation (GSD) were obtained, based on impaction data, using established software in our laboratory. Other parameters, such as percentage throat deposition, respirable mass, and respirable fraction, were calculated based on the known amount of drug deposited on the various components. A cutoff diameter of less than 4.7 μm was used to assess the respirable mass and fraction. Impaction experiments were conducted at least three times.
In Vitro Cytotoxicity of Nimesulide alone and in Combination with Doxorubicin against Various Cancer Cell Lines
The tumor cells (A549, H460, or SW620) were seeded at a density of 10,000 per well in 96-well plates and incubated overnight. Subsequently, they were treated with various concentrations of doxorubicin alone (0.01–1 μg/ml), nimesulide alone (20–100 μg/ml), and doxorubicin (0.005–0.5 μg/ml) combined with a fixed nontoxic concentration of nimesulide. For the combination treatments, nimesulide was used at 30 μg/ml in H460 and SW620 cells and at 40 μg/ml concentration in A549 cells. On treatment, the cells were incubated for 72 h, and the cytotoxicity was assayed by crystal violet dye uptake assay by measuring the absorbance at 540 nm.
In Vitro Cytotoxicity of Aerosolized Nimesulide-MDI alone and Its Combination with Doxorubicin against A549 Cells
A six-stage viable impactor was used to assess the in vitro cytotoxicity of aerosolized nimesulide, alone or in combination with a known concentration of doxorubicin (14). The viable impactor is similar to the Mark II impactor but allows sterile glass petri plates (outside diameter 9.5 cm) to be kept on its various stages. The impactor can be hooked up to the MDI through a USP throat and operated at a flow rate of 28.3 L/min. A549 cells (one million in 20 ml of medium per petri plate) were plated in petri plates and placed on stages 3, 4, 5, and 6 of the viable impactor. The cells were then exposed to the nimesulide formulation for 10, 20, and 40 shots. After the exposure, the petri plates were taken from the impactor, covered with sterile aluminum lids provided with the petri plate (Graseby Andersen, Smyrna, GA), and incubated at 37°C for 72 h. These operations (plating of cells in glass petri plates, assembly of petri plates on the various stages of viable impactor, exposure of cells to the aerosolized nimesulide, removal of petri plates from the impactor, and covering with the lids) were performed in a biologic safety cabinet (Class II, Type A/B3, NuAire, Inc., Plymouth, MN). The petri plate along with its lid assembly is similar to that of a standard tissue culture well assembly, and because all the operations were performed under a biologic safety cabinet using established tissue culture precautions, sterility can be maintained throughout the incubation period. At the end of incubation, the medium in the petri plate was discarded. The cells were rinsed three times with sterile PBS and detached by addition of trypsin. The cells were spun down with a centrifuge and resuspended in an appropriate amount of medium. The viable cells were then counted with a hemocytometer using trypan blue solution (0.4%). Untreated cells were used as control. The same procedure described above was used to assess the effect of combination therapy of nimesulide-MDI in conjunction with doxorubicin, except a fixed concentration of doxorubicin (0.01 μg/ml) was added to cells in the petri plate. To determine the aerosolized dose of the nimesulide-MDI in the six-stage viable impactor, 20 ml of 50% PEG 400 solution was used as the collection medium in petri plates in place of the tissue culture medium, and 40 shots were fired (at 5 s intervals) under a flow rate of 28.3 L/min. The concentration of nimesulide was determined by spectrophotometer, and the corresponding dose was calculated.
Detection of Apoptosis
Caspase-3 Activity
One million A549 cells were plated in 10 ml of medium in 25 cm2 flasks and incubated overnight. Subsequently, they were treated with nimesulide (40 μg/ml), doxorubicin (0.25 μg/ml), and nimesulide (40 μg/ml) with doxorubicin (0.25 μg/ml) for 48 h. Untreated cells were used as control. The activity of caspase-3 was estimated using the colorimetric caspase-3 detection kit (Promega Corporation, Madison, WI) as per the manufacturer's instructions.
DNA Fragmentation
A549 cells were treated with (a) nimesulide (80 μg/ml), (b) doxorubicin (0.25 μg/ml), and (c) nimesulide (80 μg/ml) with doxorubicin (0.25 μg/ml) for 72 h. Untreated cells were used as control. About 2 × 106 cells (both viable and nonviable) from each of the treatments and control were used for the isolation of DNA using the Apoptotic DNA ladder kit (Roche Diagnostics, Indianapolis, IN) as per the manufacturer's instructions. Aliquots of DNA (equivalent to 1 μg) were resolved on 1.6% agarose gel impregnated with ethidium bromide (0.1 μg/ml). DNA was visualized by UV transillumination and photographed using Polaroid 667 film.
Assessment of Apoptosis in A549 Cells Induced by the Aerosolized Nimesulide in Combination with Doxorubicin by Acridine Orange and TUNEL Staining
A549 cells (one million in 20 ml of medium with or without 0.25 μg/ml doxorubicin) were exposed to aerosolized nimesulide on the fifth stage of the viable impactor, in the same way as described above for the in vitro cytotoxicity of aerosolized nimesulide. On exposure of the cells to the aerosolized nimesulide, the petri plate was incubated at 37°C for 15 min to provide sufficient time for the aerosolized drug to mix uniformly with the medium. Subsequently, the cell suspension was aspirated from the petri plate, and 1 ml of the cell suspension was then plated into Nunc Lab-Tek chamber slide, which was subsequently incubated for 72 h. The cells were then fixed with 0.25% glutaraldehyde, washed twice with PBS, stained with acridine orange, and, finally, observed with a fluorescent microscope (Olympus BX40, Olympus Optical Co., Ltd. Tokyo, Japan). The morphologic criteria used to detect apoptotic cells were (a) cytoplasmic and nuclear shrinkage, (b) chromatin condensation, and (c) cytoplasmic blebbing and the presence of apoptotic bodies. Alternatively, the slide was also studied for TUNEL staining using a DeadEnd™ Colorimetric Apoptosis Detection System kit (Promega) as per the manufacturer's instructions.
Western Blot Analysis
A549 cells were treated with nimesulide (40 μg/ml) alone, doxorubicin (0.25 μg/ml) alone and their combination. The control cells were treated with medium only. The cells were then lysed in RIPA B lysis buffer and centrifuged to obtain the cell lysate. The lysates from control and treatments (30 μg of protein) were then added to SDS-PAGE loading buffer with 5% 2-mercaptoethanol, heated for 5 min at 100°C, and loaded on an 8% gel. Separated proteins were electroblotted to nitrocellulose membrane (Osmonics Inc., Westborough, MA) at 100 V for 1 h. After the membrane was incubated for 2 h in a blocking solution containing 5% nonfat skimmed milk in TBS-T, it was incubated with primary monoclonal antihuman COX-2 and polyclonal antihuman PPAR-γ antibodies (Cayman Chemical, Ann Arbor, MI) overnight. The blot was then washed and incubated with antimouse and antirabbit peroxidase-conjugated secondary antibodies (Sigma Chemical Co., St. Louis, MO). The signal was detected by chemiluminescence using the SuperSignal® West Pico Chemiluminescent Substrate (Pierce, Rockford, IL) on Fluor-S MultiImager System (Bio-Rad Laboratories, Hercules, CA).
Effect of Exogenous PGE2 on the in Vitro Cytotoxicity of Nimesulide Alone and in Combination with Doxorubicin against A549 Cells
Ten thousand A549 cells were plated in 0.1 ml of medium in 96-well plates and incubated overnight. Subsequently, nimesulide (20–160 μg/ml) alone and in combination with PGE2 (1–5 μg/ml) were added to the plate. Similarly, nimesulide (20–160 μg/ml) in combination with doxorubicin (0.1–0.25 μg/ml) was added in the presence or absence of PGE2 (1–5 μg/ml) and incubated for 72 h, and the cytotoxicity was assessed by crystal violet dye uptake assay. Alternatively, one million A549 cells were plated in 10 ml of medium in 25 cm2 flasks and incubated overnight. They were treated with nimesulide (20 μg/ml) plus doxorubicin (0.25 μg/ml) in combination in the presence or absence of PGE2 (1 μg/ml) and incubated for 72 h. Finally, the medium was decanted, washed with PBS, cells were detached by trypsin, and viable cells were counted on trypan blue staining. Untreated cells were used as control.
Statistical Analysis
Students's t test was used to determine the significance of difference in the in vitro cytotoxicity of the combination of aerosolized nimesulide with doxorubicin vs. the combination of nimesulide (nonaerosolized) with doxorubicin. One-way ANOVA followed by Tukey's Multiple Comparison Test was performed to determine the significance of difference in the in vitro cytotoxicity and specific caspase-3 activity of different treatments. The statistical analysis was performed using GraphPad PRISM version 2.0 software (San Diego, CA).
RESULTS
Solubility of Nimesulide in HFA 134a and 227
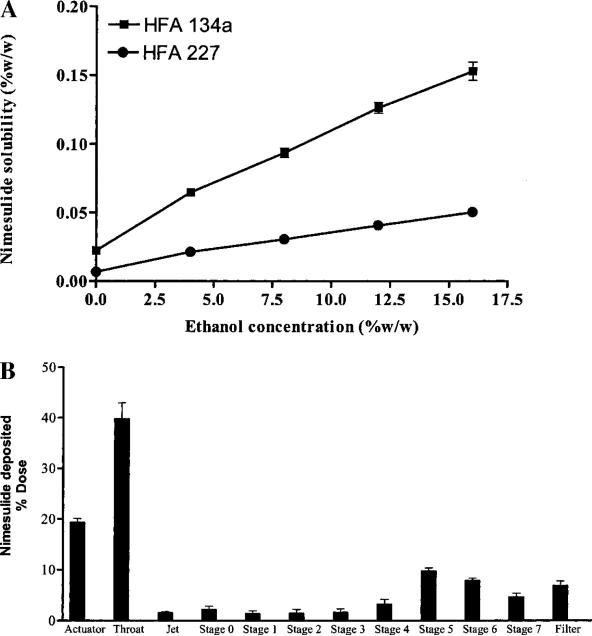
Figure 1A shows the solubility profiles of nimesulide in HFA propellants containing various concentrations (0–16% w/w) of ethyl alcohol. In all cases, the addition of ethanol increased the solubility of nimesulide in both HFA propellants (134a and 227). The highest solubility of nimesulide (0.16% w/w) was observed in HFA 134a with 16% ethyl alcohol. Therefore, a solution formulation of 0.1% w/w nimesulide, 15% w/w ethyl alcohol in 134a was prepared and used as a model MDI formulation. Attempts were made to formulate nimesulide as a suspension in HFA 227; however, initial experiments were not successful. Crystal growth was observed (confirmed by optical microscopy) after storage in cycling chamber (4–40°C, 6-h cycles) for 4 weeks. This was caused by the measurable solubility of nimesulide in HFA 227, which may support the crystal growth over time.
Fig. 1.
A, Solubility of nimesulide in HFA propellants as a function of ethyl alcohol concentration. Each point represents mean ± SD of three experiments. B, Andersen cascade impactor deposition profile of nimesulide-MDI. Data were expressed as the percentage of the total drug deposited on all stages of the impactor including actuator and throat and represent mean ± SD (n = 3).
Characterization of the Nimesulide-MDI Formulation
The aerodynamic characteristics of the nimesulide formulation are summarized in Table I. The deposition pattern of the aerosolized nimesulide from the Andersen cascade impactor is illustrated in Fig. 1B. In commercially available Proventil HFA actuators, the nimesulide-MDI had a medication delivery of 51.1 ± 3.3 μg/shot with 42.4 ± 4.2% of the dose being respirable. The MMAD of the formulation was 1.1 ± 0.3 μm. Further, when stored in glass and aluminum containers, the nimesulide formulation was found to be chemically stable at both room temperature and at 40°C for one month (data not shown).
Table I.
Aerodynamic Characteristics of Nimesulide-MDI
| Aerodynamic characteristics | Mean ± SD |
|---|---|
| Mass median aerodynamic diameter (MMAD) | 1.1 ± 0.3 μm |
| Geometric standard deviation (GSD) | 2.8 ± 0.6 |
| Throat deposition (%) | 49.5 ± 7.6 |
| Respirable mass (μg/shot) | 24.9 ± 6.8 |
| Respirable fraction (%) | 42.4 ± 4.2 |
| Medication delivery (μg/shot) | 51.1 ± 3.3 |
Note: Nimesulide impaction data on various stages of cascade impactor was used to calculate MMAD, GSD, throat deposition, respirable mass, and respirable fraction values as described in the Materials and Methods section. Data represent mean ± SD of three experiments.
Effect of Nimesulide on the in Vitro Cytotoxicity of Doxorubicin against Human Cancer Cell Lines
Table II shows the in vitro cytotoxicity data for nimesulide alone, doxorubicin alone, and the combination of nimesulide with doxorubicin against various cell lines. The combined treatment of nimesulide with doxorubicin reduced the IC50 values for doxorubicin by 33 to 67% in various cell lines.
Table II.
Effect of Nimesulide on the in Vitro Cytotoxicity of Doxorubicin against Various Human Cancer Cell Linesa
| Cell line | IC50 with nimesulide aloneb (μg/ml) | IC50 with doxorubicin alonec (μg/ml) | IC50 with nimesulide and doxorubicind (μg/ml) | % Reduction in IC50 of doxorubicin |
|---|---|---|---|---|
| A549 | 74.6 ± 13.5 | 0.24 ± 0.08 | 0.09 ± 0.02e | 62.5 |
| H460 | 54.9 ± 4.3 | 0.11 ± 0.02 | 0.04 ± 0.02e | 66.6 |
| SW620 | 62.0 ± 8.8 | 0.03 ± 0.005 | 0.02 ± 0.002e | 33.3 |
The human lung cancer (adenocarcinoma type A549 and large-cell carcinoma type H460) and colon adenocarcinoma (SW620) cell lines were obtained from American Type Culture Collection (Rockville, MD).
Nimesulide was employed in the concentration range of 20–100 μg/ml to determine the IC50 values.
Doxorubicin was employed in the concentration range of 0.01–1.0 μg/ml to determine the IC50 values.
In the combination of nimesulide with doxorubicin cytotoxicity experiments, nimesulide was employed at 30 μg/ml in H460 and SW620 cell lines and at 40 μg/ml concentration in A549 cells. Doxorubicin was varied between 0.005 and 0.5 μg/ml in these experiments.
The difference between the IC50 value of doxorubicin in the combination treatment and by doxorubicin alone was significant in A549 (p = 0.0307), H460 (p = 0.0133), and SW620 (p = 0.023) cell lines.
In Vitro Cytotoxicity of Aerosolized Nimesulide Formulation alone and as a Combination Therapy with Doxorubicin against A549 Cells
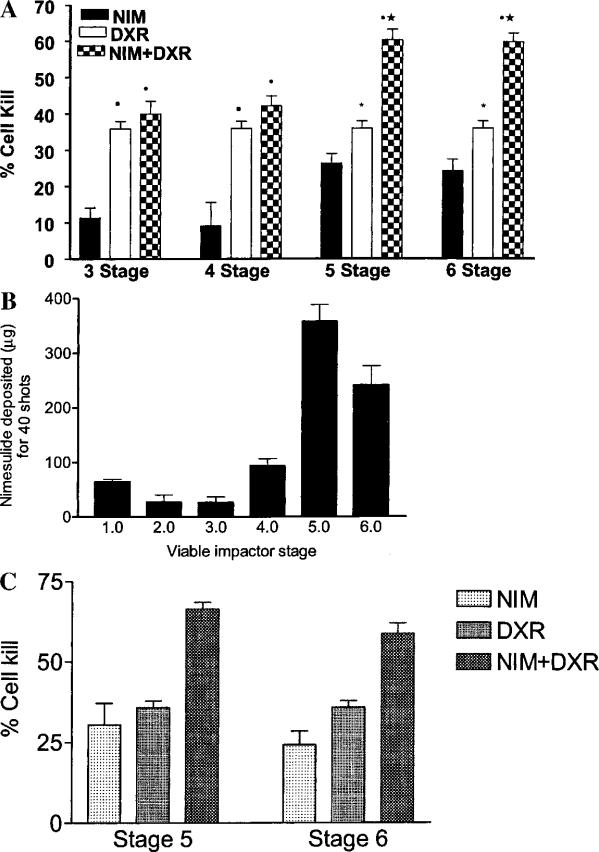
Figure 2A shows the in vitro cytotoxicity of the aerosolized nimesulide formulation alone and in combination with doxorubicin against A549 cells kept on stages 3–6 of the viable impactor. Stages 3–6 of the viable impactor were chosen because these stages correspond to a cutoff particle diameter of 4.7 μm used in the calculation of respirable mass and respirable fraction values from Andersen impactor data. Aerosolized nimesulide (40 shots) had a cell kill of 24.6% ± 1.8 on stage 5. Doxorubicin, when used alone at a concentration of 0.01 μg/ml, had a cell kill of 35.9% ± 4.9. However, when A549 cells were treated with 0.01 μg/ml doxorubicin and exposed to 40 shots of the aerosolized MDI, a cell kill as high as 60.2% ± 7.0 was observed on the fifth stage of the viable impactor. To ensure that the cell kill observed was caused by aerosolized nimesulide and not by formulation ingredients, two additional experiments were performed. First, the cells were exposed to 40 shots of a placebo formulation (15% ethyl alcohol in 134a), which showed a cell kill of about 5% on stages 3–6 of the viable impactor. Second, the amount of nimesulide deposited was quantified following the actuation of 40 shots of MDI formulation using 20 ml of 50% PEG 400 solution in place of tissue culture medium on stages 3–6 of the viable impactor as described above. Subsequently, the cytotoxicity was determined using the nonaerosolized nimesulide (dose equal to the amount deposited on stages 5 and 6 of the viable impactor) in combination with doxorubicin essentially in the same way as described above for the aerosolized nimesulide. Figure 2B shows the deposition profile of nimesulide on the various stages of the viable impactor after 40 shots of the MDI formulation. The experimentally determined dose of nimesulide deposited on stages 5 and 6 of the viable impactor following 40 actuations was 358 and 242 μg, respectively, which correspond to 17.9 and 12.1 μg/ml in 20 ml of collection medium used in petri plates (Fig. 2B). The comparison between in vitro cytotoxicity of the aerosolized vs. nonaerosolized nimesulide and its combination with doxorubicin was performed for stages 5 and 6 of the viable impactor because there was greater deposition of nimesulide on these stages as compared to the other stages (Fig. 2B). This is expected because the nimesulide-MDI used is a solution-type formulation. Further, the greater deposition of nimesulide on stages 5 and 6 of the viable impactor could significantly (p < 0.001) enhance the in vitro cytotoxicity of doxorubicin in comparison to doxorubicin alone, whereas such an enhanced cytotoxic response could not be obtained for the combination treatment on both stages 3 and 4 of the viable impactor (Fig. 2A). Figure 2C shows the cytotoxicity profiles of nonaerosolized dose of nimesulide (dose corresponding to that deposited on stages 5 and 6 of the viable impactor following 40 actuations of MDI formulation) alone and its combination with doxorubicin. It is evident from Figs. 2A and 2C that the enhanced cytotoxicity of aerosolized nimesulide with doxorubicin (Fig. 2A) is comparable to the combination of nonaerosolized nimesulide with doxorubicin (Fig. 2C), without any significant (p < 0.05) difference for stages 5 or 6 of the viable impactor. The results indicate that the process of aerosolization has not altered the activity of nimesulide and aerosolized nimesulide exhibits the same activity of as that of nonaerosolized form under equivalent dosing conditions.
Fig. 2.
A, In vitro cytotoxicity profiles of aerosolized nimesulide (MDI-40 shots) alone and in combination with doxorubicin (0.01 μg/ml) against A549 cells on the third, fourth, fifth, and sixth stages of the viable impactor. NIM, nimesulide; DXR, doxorubicin. Data represent the mean ± SD of four experiments. Statistical significance of the difference in the cytotoxicity: •aerosolized nimesulide with doxorubicin vs. aerosolized nimesulide, p < 0.001; ★-aerosolized nimesulide with doxorubicin vs. doxorubicin, p < 0.001; ■-aerosolized nimesulide vs. doxorubicin, p < 0.001; *aerosolized nimesulide vs. doxorubicin, p < 0.05. B, Nimesulide deposited (μg) following 40 actuations of MDI on different stages of the viable impactor. Data represent the mean ± SD of three experiments. C, In vitro cytotoxicity of nimesulide alone (nonaerosolized form) and in combination with doxorubicin (0.01 μg/ml) against A549 cells. NIM, nimesulide; DXR, doxorubicin. The concentration of nimesulide used on stages 5 and 6 of the viable impactor corresponds to 17.9 and 12.1 μg/ml, respectively. The concentration of nimesulide was chosen based on the experimentally determined amount of nimesulide deposited for 40 shots of MDI formulation in 20 ml medium on stages 5 and 6 of the viable impactor as shown in B and described in Materials and Methods. Data represent the mean ± SD of three experiments.
Induction of Apoptosis in A549 Cells by the Combination of Nimesulide with Doxorubicin
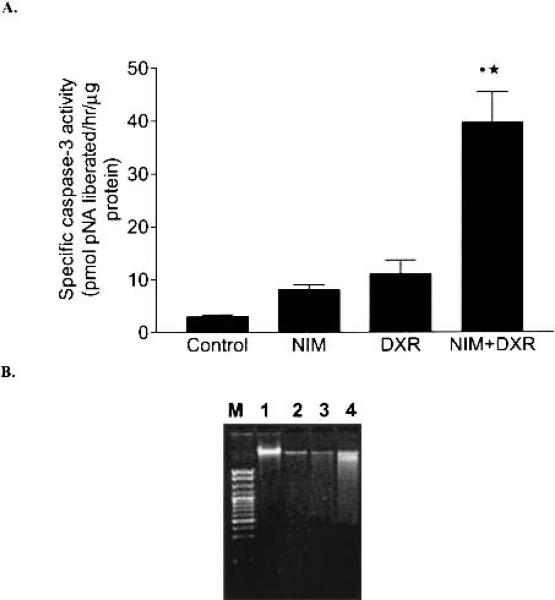
It is evident from Fig. 3A that the specific caspase-3 activity in A549 cells treated with nimesulide (40 μg/ml) plus doxorubicin (0.25 μg/ml) was five and three times more in comparison to nimesulide- (40 μg/ml) and doxorubicin- (0.25 μg/ml) treated cells, respectively. Further, the specific caspase-3 activity was not statistically significant in control and nimesulide- and doxorubicin-treated A549 cells (p > 0.05). However, the caspase-3 activity in A549 cells treated with the combination of nimesulide with doxorubicin was significantly higher in comparison to control (p < 0.001), nimesulide (p < 0.001) and doxorubicin (p < 0.01)-treated cells, respectively (Fig. 3A). The enhanced apoptotic response was also confirmed by the DNA degradation (Fig. 3B). The combination of nimesulide (80 μg/ml) with doxorubicin (0.25 μg/ml) showed evidence of DNA fragmentation in the form of a smear pattern (Lane 4, Fig. 3B). However, the control, nimesulide (80 μg/ml), and doxorubicin (0.25 μg/ml) treatments alone showed no such smearing.
Fig. 3.
A, Specific caspase-3 activity in A549 cells. Control, untreated cells; NIM, nimesulide (40 μg/ml); DXR, doxorubicin (0.25 μg/ml); NIM+DXR, nimesulide (40 μg/ml) + doxorubicin (0.25 μg/ml). Data represent the mean ± SD of three experiments. Statistical significance of the difference in the caspase-3 activity: •NIM+DXR vs. control; NIM+DXR vs. NIM, p < 0.001; ★-NIM+DXR vs. DXR, p < 0.01. B, Electrophoresis of DNA from A549 cells. Lane M, 100 bp DNA marker; lane 1, untreated control cells; lane 2, nimesulide (80 μg/ml); lane 3, doxorubicin (0.25 μg/ml); lane 4, nimesulide (80 μg/ml) + doxorubicin (0.25 μg/ml).
Apoptosis in A549 Cells by the Combination of Aerosolized Nimesulide with Doxorubicin
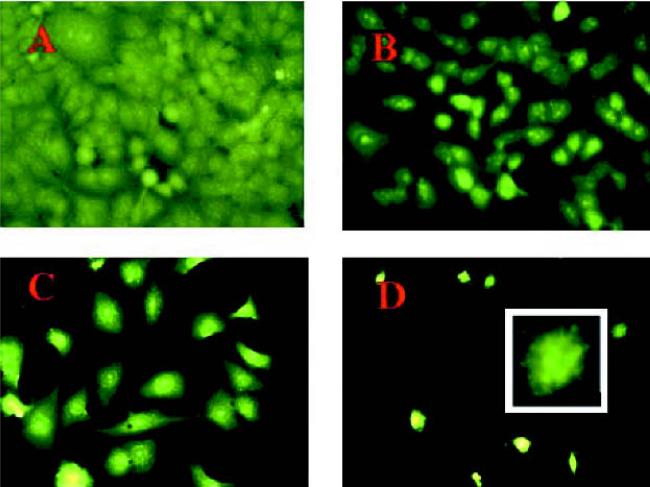
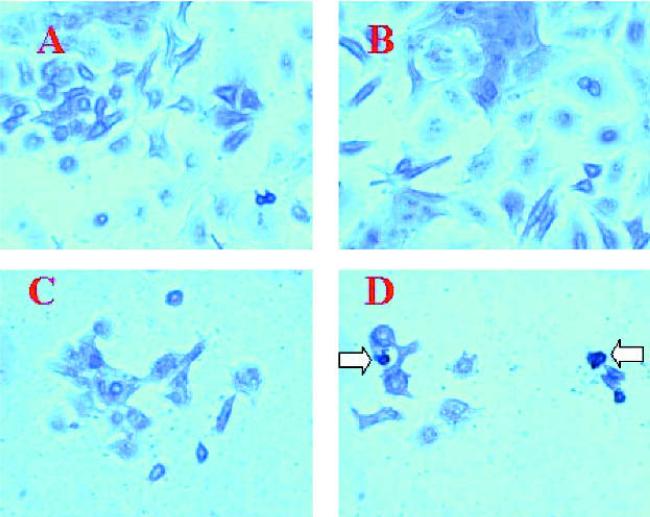
We evaluated the effect of the combined treatment of aerosolized nimesulide from MDI (40 shots) with doxorubicin on the apoptosis in A549 cells by acridine orange staining and TUNEL assay. Acridine orange staining showed that there was no appreciable apoptosis in control and aerosolized nimesulide (40 shots)-treated cells, whereas doxorubicin (0.25 μg/ml) treatment showed apoptosis in 25% cells (Fig. 4). However, the apoptotic response (40%) was greater in cells treated with aerosolized nimesulide (40 shots) with 0.25 μg/ml doxorubicin (Fig. 4). The results were further confirmed by TUNEL staining, which showed that there was negligible apoptosis in control, aerosolized nimesulide-, and doxorubicin-treated cells (Fig. 5). However, the combined treatment of aerosolized nimesulide with doxorubicin showed apoptotic response in 30% of cells (Fig. 5). Our results show that apoptosis could be induced in A549 cells by employing subapoptotic concentrations of doxorubicin with aerosolized nimesulide.
Fig. 4.
Acridine orange staining of A549 cells: A, untreated control cells; B, cells exposed to aerosolized nimesulide (40 shots); C, cells treated with doxorubicin (0.25 μg/ml); D, cells treated with aerosolized nimesulide (40 shots) + doxorubicin (0.25 μg/ml). Original magnification 40×. Inset to D, 100× magnification of an apoptotic cell. The cells were exposed to aerosolized nimesulide on the fifth stage of the viable impactor and incubated for 15 min, and 1 ml of cell suspension was transferred to chamber slide. The slide was incubated for 72 h and stained with acridine orange as described in the Materials and Methods.
Fig. 5.
TUNEL staining of A549 cells. A, Untreated control cells; B, cells exposed to aerosolized nimesulide (40 shots); C, cells treated with doxorubicin (0.25 μg/ml); D, cells treated with aerosolized nimesulide (40 shots) + doxorubicin (0.25 μg/ml). Original magnification 40×. Arrows indicate apoptotic cells. The experimental conditions are similar to those described for Fig. 4.
Detection of COX-2 and PPAR-γ in A549 cells
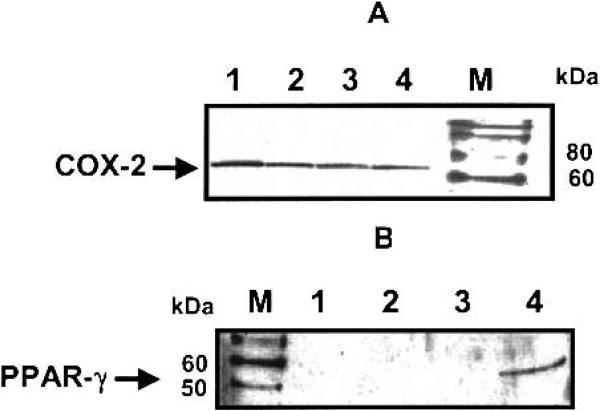
Western blot analysis of A549 cell lysates revealed equal protein expression of COX-2 in control (untreated) and treated (nimesulide alone, doxorubicin alone, and nimesulide with doxorubicin combination) cells (Fig. 6A). It is evident from Fig. 6B that PPAR-γ protein expression was found only in the combination treatment of nimesulide with doxorubicin.
Fig. 6.
Detection of (A) COX-2 and (B) PPAR-γ protein expression in A549 cells by Western blotting. Lane 1, untreated control cells; lane 2, nimesulide (40 μg/ml); lane 3, doxorubicin (0.25 μg/ml); lane 4, nimesulide (40 μg/ml) + doxorubicin (0.25 μg/ml). Lane M, Magic Mark™ Western molecular weight markers (Invitrogen, Carlsbad, CA). COX-2 is a 72-kDa protein, and a band was seen in lanes 1–4 localized between 60 and 80 kDa bands for the protein markers, thus confirming the COX-2 status (A). It is evident from A that the expression of COX-2 was not altered by treatment with nimesulide, doxorubicin, or the combination of nimesulide with doxorubicin. The human PPAR-γ1 and PPAR-γ2 proteins are 53 and 57 kDa proteins, respectively. In B, a band was seen in lane 4, located between 50 and 60 kDa for the protein markers, thus indicating the expression of PPAR-γ in A549 cells treated with the combination of nimesulide with doxorubicin.
Effect of Exogenous PGE2 on the in Vitro Cytotoxicity of Nimesulide alone and in Combination with Doxorubicin
In order to elucidate whether the antiproliferative effect was mediated via the COX pathway, we studied the effect of exogenous addition of PGE2 (1–5 μg/ml) on the in vitro cytotoxicity of nimesulide alone and its combination with doxorubicin against A549 cells. We observed that PGE2 could not reverse the growth inhibition of nimesulide alone and its combination with doxorubicin as assessed by the crystal violet dye uptake assay.
DISCUSSION
Nimesulide, 4-nitro-methanesulfonanilide, is an acidic NSAID having a reported pKa ranging from 6.4 to 6.8. It has a poor aqueous solubility (0.01 mg/ml) and is slightly soluble in ethanol. However, there is no cited report in the literature of the solubility of nimesulide in the HFA propellants 134a and 227. One of the most important factors dealing with the new HFA propellants is the need for ethanol in the system as a cosolvent to solubilize any surfactant or the drug itself. Therefore, initial preformulation work dealt with determining the solubility of nimesulide in the HFA propellants at various concentrations of ethanol. We developed an MDI formulation of nimesulide based on its maximum solubility in HFA 134a in the presence of ethyl alcohol (Fig. 1A). It is evident from Table I that the aerodynamic characteristics of the nimesulide-MDI formulation are similar to those of the approved HFA formulation of beclomethasone, having the same emitted dose. The physical stability of nimesulide-MDI was found to be satisfactory without any precipitation, crystal growth, or color change for 1 month at both room and elevated temperature (40°C). Further, the nimesulide formulation was found to be chemically stable under the abovementioned conditions.
Before evaluating the in vitro cytotoxicity enhancement effect of aerosolized nimesulide, we studied the effect of nimesulide on doxorubicin activity in a panel of cell lines, including lung and colon tumor cells. It is evident from the data presented in Table II that nimesulide potentiates the in vitro cytotoxicity of doxorubicin in all the cell lines studied. Further, the maximum reduction in IC50 of doxorubicin was observed in lung cancer cell lines (Table II). It is clear from Table II that nimesulide IC50 value in H460 cells (54.9 μg/ml) is smaller than the corresponding value in A549 cells (74.6 μg/ml). However, we obtained approximately same percentage reduction (about 60%) in the IC50 value of doxorubicin in both cell lines by the combination of nimesulide with doxorubicin. This is because we used nimesulide at 30 and 40 μg/ml concentrations for the combination of nimesulide with doxorubicin in H460 and A549 cells, respectively. The low IC50 value of nimesulide in H460 cells as compared to A549 cells was in agreement with the previously reported data, which showed that the IC50 values for the various chemosensitivity enhancers such as exisulind, cis-retinoic acid, and lipoxygenase inhibitor nordihydroguaiaretic acid in H460 cells were smaller than their corresponding values in A549 cells (9). Both A549 and H460 cell lines have been reported to weakly express multidrug resistance-associated protein (MRP) and strongly express lung resistance-related protein (LRP) (9). However, multidrug resistance protein (MDR1) expression was absent in A549 cells by RT-PCR (our unpublished data) and by immunofluorescent staining in A549 and H460 cell lines (9). Indomethacin has been shown to inhibit the efflux of doxorubicin from MRP-overexpressing lung sqaumous cell carcinoma, DLKP cells (8). Even though, LRP expression in A549 cells is thought to be responsible for its decreased sensitivity to doxorubicin, in comparison to MCF-7 cells (15), its basal expression is associated with resistance to cisplatin but not to doxorubicin (16). There has been no study reported so far on the effect of a COX inhibitor alone or in combination with anticancer drugs on the expression of MRP and or LRP in A549 and H460 cells, and therefore, it remains unclear whether a COX-2 inhibitor has any effect on the expression and activity of MRP and LRP in parental A549 and H460 cell lines (drug-sensitive cell lines).
We have previously used the six-stage viable impactor for determining the cytotoxicity of aerosolized methotrexate against HL-60 cells (14). In the present study, we used the same method for the exposure of A549 cells to aerosolized nimesulide, and we could demonstrate that nimesulide-MDI (40 shots) significantly enhances the in vitro cytotoxicity of doxorubicin on stages 5 and 6 of the viable impactor (Fig. 2A). We also studied the effect of aerosolized nimesulide (10 and 20 shots of MDI) on the in vitro cytotoxicity of doxorubicin (0.01 μg/ml) and found that there was no enhancement of doxorubicin cytotoxicity (data not shown). The in vitro cytotoxicity enhancement of doxorubicin by aerosolized nimesulide was corroborated by (a) negligible contribution to the cytotoxicity by MDI formulation ingredients (propellant and ethyl alcohol) as tested by placebo formulation (HFA 134a, 15% ethyl alcohol, 40 shots) and (b) no significant difference in cytotoxicity between aerosolized nimesulide and nonaerosolized nimesulide (dose equal to the 40 shots of nimesulide-MDI) in combination with doxorubicin (Figs. 2A and 2C). Based on the data presented in Figs. 2B and 2C, it is evident that it requires at least 12 μg/ml of nimesulide (240 μg dose) to significantly enhance the in vitro cytotoxicity of doxorubicin (0.01 μg/ml) against A549 cells under the experimental conditions studied. Nimesulide-MDI used in the present study could deliver such a dose in the sixth stage of viable impactor only by 40 actuations. This is because of the solubility limitation of nimesulide in HFA 134a containing 15% ethyl alcohol, which resulted in lower medication delivery. In order to reduce the number of actuations, we tried to develop a suspension-type formulation of nimesulide in HFA 134a and 227. However, initial experiments revealed that such formulations were physically unstable because of rapid crystal growth. Increasing the solubility of nimesulide in HFA 134a–ethyl alcohol system by cosolvents or altering the metered valve size from 50 μl (used in the present study) to 100 μl, might be attempted to decrease the number of actuations required to deliver the effective dose.
There have been few studies on the in vivo potentiation of cytotoxicity of anticancer drugs by COX inhibitors (13,17,18). In all these studies, the COX inhibitor was administered either by oral route (13,18) or by intraperitoneal route (17). To the best of our knowledge, this is the first study considering the potential of aerosolized COX-2 inhibitor for the potentiation of the cytotoxic drugs. However, the concept of inhaled COX inhibitors has been previously pursued in the treatment of asthma and bronchorrhea (19,20). Based on the studies by Homma et al. (19) and Tamaoki et al. (20), which considered the potential of inhaled COX inhibitors in patients with lung cancer and the enhanced in vivo response with the combination of COX inhibitors with cytotoxic drugs (13,17,18), it may be worth pursuing the inhalation delivery approach for the COX-2 inhibitors to optimize their therapeutic effect in the treatment of lung cancer.
The antiproliferative effects of COX-2 inhibitors are thought to be mediated in part through induction of apoptosis (21). Nimesulide was shown to induce apoptosis in a non-small-cell lung carcinoma cell line, ACC-LC-91, without having any effect on Bcl-2. Initially, we studied apoptosis in A549 cells by using nonaerosolized nimesulide with or without doxorubicin. In our study, we demonstrate that nimesulide at concentrations equal to or below its IC50 value enhanced the apoptotic response of doxorubicin against A549 cells by caspase-3 activity and DNA degradation assays (Fig. 3). Caspase-3 activation is an early event in the apoptosis cascade, where its activation triggers PARP cleavage, which is parallel to apoptosis detection by DNA fragmentation and TUNEL assays (22). The increase in the caspase-3 activity in A549 cells by the combination of nimesulide with doxorubicin is in agreement with that of a recent report (23). We also showed that aerosolized nimesulide enhances the apoptotic response of doxorubicin against A549 cells by acridine orange and TUNEL staining methods (Figs. 4 and 5). Enhancement of apoptotic response of docetaxel by exisulind against A549 cells was recently reported, and both compounds were used at concentrations below their apoptotic concentrations (13).
We showed by Western analysis that COX-2 protein, reflecting the COX-2 expression, is not altered in the different treatments over the control in A549 cells (Fig. 6A). The downstream product of the COX-2–catalyzed metabolism of arachidonic acid, PGE2, has been shown to promote the growth of colorectal carcinoma cells (24) and reverse the growth-inhibitory effect of the selective COX-2 inhibitor SC58125 in colon cancer HCA-7 cells (25). In our studies, we found that exogenous addition of PGE2 (1–5 μg/ml) did not reverse the antiproliferative effect of nimesulide (20–80 μg/ml) alone and in combination with doxorubicin (0.1–0.25 μg/ml). Our result is supported by the data of Duffy et al. (8), who showed that exogenous PGE2 did not reverse the synergistic cytotoxicity of doxorubicin with indomethacin against DLKP cell line. Similarly, PGE2 and the prostaglandin precursor arachidonic acids were shown not to reverse the growth-inhibitory effects of sulindac sulfide against HT-29 cells (21). These observations point to COX-independent mechanisms for the in vitro growth-inhibitory effects of these compounds.
Further, we looked into the expression of PPAR-γ as numerous studies have implicated a role of PPAR-γ in cancer, although its role in lung cancer is not well investigated. PPAR-γ is a nuclear receptor that plays a pivotal role in the regulation of gene transcription and cellular differentiation. It increases transcription of genes whose products are either growth inhibitors, tumor suppressors, or proapoptotic (10). Our studies showed that doxorubicin plus nimesulide combination treatment resulted in PPAR-γ expression, whereas there was none in doxorubicin or nimesulide treatments alone or in controls (Fig. 6B). This finding strongly supports our earlier stated hypothesis of a COX-2–independent mechanism involved in A549 growth inhibition. PPAR-γ was reported to be the target of NSAIDs such as sulindac sulfide that accounts for COX-independent inhibition of lung cancer cell growth (10).
Our results indicate the potential of inhalation delivery of COX-2 inhibitors for the enhancement of the activity of cytotoxic drugs used in the treatment of lung cancer. We used a nimesulide MDI formulation to demonstrate in vitro the proof-of-principle for the inhalation delivery approach for COX inhibitors. We used doxorubicin as a model drug in our study because it is highly effective in vitro against various lung cancer cell lines and has been studied extensively as a model drug in various in vitro conditions. However, doxorubicin is not currently the drug of choice in the treatment of lung cancer because of the poor response rates associated with it (26,27). This is mostly because of the dose-related toxicity of doxorubicin and development of drug resistance to doxorubicin. In order to overcome these drawbacks, new delivery systems for doxorubcin such as long-circulating liposomes and inhalation approaches have been studied recently in the treatment of lung cancer (28–30). Although the development of a proper inhalation device in lung cancer patients is essential, further preclinical studies are needed to evaluate the advantages associated with inhalation delivery of COX-2 inhibitors for in vivo cytotoxicity enhancement, and currently studies are in progress in our laboratory to address this aspect.
ACKNOWLEDGMENTS
The authors acknowledge the financial support provided by RCMI award G12RR03020-11 from NIH.
ABBREVIATIONS
- COX-2
cyclooxygenase-2
- HFA
hydrofluoroalkane
- GSD
geometric standard deviation
- MDD
medication delivery device
- MDI
metered dose inhaler
- MMAD
mass median aerodynamic diameter
- NSAID
nonsteroidal antiinflammatory drug
- NSCLC
non-small-cell lung cancer
- PGE2
prostaglandin E2
- PPAR
peroxisome proliferator-activated receptor
- TUNEL
terminal deoxynucleotidyl transferase-mediated nick end labeling
REFERENCES
- 1.Dannenberg AJ, Altorki NK, Boyle JO, Dang C, Howe LR, Weksler BB, Subbaramaiah K. Cyclo-oxygenase 2: a pharmacological target for the prevention of cancer. Lancet Oncol. 2001;2:544–551. doi: 10.1016/S1470-2045(01)00488-0. [DOI] [PubMed] [Google Scholar]
- 2.Harris RE, Alshafie GA, Abou-Issa H, Seibert K. Chemoprevention of breast cancer in rats by celecoxib, a cyclooxygenase 2 inhibitor. Cancer Res. 2000;60:2101–2103. [PubMed] [Google Scholar]
- 3.Rioux N, Castonguay A. Prevention of NNK-induced lung tumourigenesis in A/J mice by acetylsalicylic acid and NS-398. Cancer Res. 1998;58:5354–5360. [PubMed] [Google Scholar]
- 4.Williams CS, Watson AJM, Sheng H, Helou R, Shao J, DuBois RN. Celecoxib prevents tumor growth in vivo without toxicity to normal gut: Lack of correlation between in vitro and in vivo models. Cancer Res. 2000;60:6045–6051. [PubMed] [Google Scholar]
- 5.Liu XH, Kirschenbaum A, Yao S, Lee R, Holland JF, Levine AC. Inhibition of cyclooxgenase-2 suppresses angiogenesis and the growth of prostate cancer in vivo. J. Urol. 2000;164:820–825. doi: 10.1097/00005392-200009010-00056. [DOI] [PubMed] [Google Scholar]
- 6.Masferrer JL, Leahy KM, Koki AT, Zweifel BS, Settle SL, Woerner BM, Edwards DA, Flickinger AG, Moore RJ, Seibert K. Antiangiogenic and antitumor activities of cyclooxygenase-2 inhibitors. Cancer Res. 2000;60:1306–1311. [PubMed] [Google Scholar]
- 7.Maca RD. Enhancement of etoposide and methotrexate sensitivity by indomethacin in vitro. Anticancer Drug Des. 1991;6:453–466. [PubMed] [Google Scholar]
- 8.Duffy CP, Elliott CJ, O'Connor RA, Heenan MM, Coyle S, Cleary IM, Kavanagh K, Verhaegen S, O'Loughlin CM, NicAmhlaoibh R, Clynes M. Enhancement of chemotherapeutic drug toxicity to human tumor cells in vitro by a subset of non-steroidal anti-inflammatory drugs (NSAIDs). Eur. J. Cancer. 1998;34:1250–1259. doi: 10.1016/s0959-8049(98)00045-8. [DOI] [PubMed] [Google Scholar]
- 9.Soriano AF, Helfrich B, Chan DC, Heasley LE, Bunn PA, Jr., Chou T-C. Synergistic effects of new chemopreventive agents and conventional cytotoxic agents against human lung cancer cell lines. Cancer Res. 1999;59:6178–6184. [PubMed] [Google Scholar]
- 10.Wick M, Hurteau G, Dessev C, Chan D, Geraci MW, Winn RA, Heasley LE, Nemenoff RA. Peroxisome proliferator-activated receptor-γ is a target of nonsteroidal anti-inflammatory drugs mediating cyclooxygenase-independent inhibition of lung cancer cell growth. Mol. Pharmacol. 2002;64:1207–1214. doi: 10.1124/mol.62.5.1207. [DOI] [PubMed] [Google Scholar]
- 11.Kubota T, Koshizuka K, Williamson EA, Asou H, Said JW, Holden S, Miyoshi I, Koeffler HP. Ligand for peroxisome proliferator-activated receptor γ (troglitazone has potent antitumor effect against human prostrate cancer both in vitro and in vivo. Cancer Res. 1998;58:3344–3352. [PubMed] [Google Scholar]
- 12.Brockman JA, Gupta RA, Dubois N. Activation of PPAR gamma leads to inhibition of anchorage-independent growth of human colorectal cancer cells. Gastroenterology. 1998;115:1049–1055. doi: 10.1016/s0016-5085(98)70072-1. [DOI] [PubMed] [Google Scholar]
- 13.Chan DC, Earle KA, Zhao TLM, Helfrich B, Zeng C, Baron A, Whitehead CM, Piazza G, Pamukcu R, Thompson WJ, Alila H, Nelson P, Bunn PA., Jr Exisulind in combination with docetaxel inhibits growth and metastasis of human lung cancer and prolongs survival in athymic nude rats with orthotopic lung tumors. Clin. Cancer Res. 2002;8:904–912. [PubMed] [Google Scholar]
- 14.Shaik MS, Haynes A, McSween J, Ikediobi O, Kanikkannan N, Singh M. Inhalation delivery of anticancer agents via HFA-based metered dose inhaler using methotrexate as a model drug. J. Aerosol Med. 2002;15:261–270. doi: 10.1089/089426802760292609. [DOI] [PubMed] [Google Scholar]
- 15.Meschini S, Marra M, Calcabrini A, Monti E, Gariboldi M, Dolfini E, Arancia G. Role of the lung resistance-related protein (LRP) in the drug sensitivity of cultured tumor cells. Toxicology in Vitro. 2002;16:389–398. doi: 10.1016/s0887-2333(02)00035-8. [DOI] [PubMed] [Google Scholar]
- 16.Berger W, Elbling L, Micksche M. Expression of the major vault protein LRP in human non-small cell lung cancer cells: activation by short-term exposure to antineoplastic drugs. Int. J. Cancer. 2000;88:293–300. [PubMed] [Google Scholar]
- 17.Teicher BA, Korbt TT, Menon K, Holden SA, Ara G. Cyclooxygenase and lipoxygenase inhibitors as modulators of cancer therapies. Cancer Chemother. Pharmacol. 1994;33:515–522. doi: 10.1007/BF00686511. [DOI] [PubMed] [Google Scholar]
- 18.Hida T, Kozaki K-I, Ito H, Miyaishi O, Tatematsu Y, Suzuki T, Matsuo K, Sugiura T, Ogawa M, Takahashi T, Takahashi T. Significant growth inhibition of human lung cancer cells both in vitro and in vivo by the combined use of a selective cyclooxygenase-2 inhibitor, JTE-522 and conventional anticancer agents. Clin. Cancer Res. 2002;8:2443–2447. [PubMed] [Google Scholar]
- 19.Homma S, Kawabata M, Kishi K, Tsuboi E, Narui K, Nakatami T, Nakata K. Successful treatment of refractory bronchorrhea by inhaled indomethacin in two patients with bronchioloalveolar carcinoma. Chest. 1999;115:1465–1468. doi: 10.1378/chest.115.5.1465. [DOI] [PubMed] [Google Scholar]
- 20.Tamaoki J, Kohri K, Isono K, Nagai A. Inhaled indomethacin in bronchorrhea in bronchioloalveolar carcinoma: role of cyclooxygenase. Chest. 2000;117:1213–1214. doi: 10.1378/chest.117.4.1213. [DOI] [PubMed] [Google Scholar]
- 21.Piazza GA, Rahm AK, Finn TS, Fryer BH, Li H, Stoumen AL, Pamukcu R, Ahnen DJ. Apoptosis primarily accounts for the growth-inhibitory properties of sulindac metabolites and involve a mechanism that is independent of cyclooxygenase inhibition, cell cycle arrest and p53 induction. Cancer Res. 1997;57:2452–2459. [PubMed] [Google Scholar]
- 22.Li M, Wu X, Xu X-C. Induction of apoptosis by cyclooxygenase-2 inhibitor NS398 through a cytochrome C-dependent pathway in esophageal cancer cells. Int. J. Cancer. 2001;93:218–223. doi: 10.1002/ijc.1322. [DOI] [PubMed] [Google Scholar]
- 23.Swamy MV, Cooma I, Reddy BS, Rao CV. Lamin B, caspase-3 activity, and apoptosis induction by a combination of HMG-CoA reductase inhibitor and C0X-2 inhibitors: a novel approach in developing effective chemopreventive regimens. Int. J. Oncol. 2002;20:753–759. [PubMed] [Google Scholar]
- 24.Sheng H, Shao J, Washington MK, DuBois RN. Pros-taglandin E2 increases growth and motility of colorectal carcinoma cells. J. Biol. Chem. 2001;276:18075–18081. doi: 10.1074/jbc.M009689200. [DOI] [PubMed] [Google Scholar]
- 25.Sheng H, Shao J, Morrow JD, Beauchamp RD, DuBois RN. Modulation of apoptosis and Bcl-2 expression by pros-taglandin E2 in human colon cancer cells. Cancer Res. 1998;58:362–366. [PubMed] [Google Scholar]
- 26.Non-Small Cell Lung Cancer Collaborative Group Chemo-therapy in non-small cell lung cancer: meta-analysis using updated data in individual patients from 52 randomized clinical trials. BMJ. 1995;311:899–900. [PMC free article] [PubMed] [Google Scholar]
- 27.Bunn PA, Jr., Kelly K. New combinations in the treatment of lung cancer. A time for optimism. Chest. 2000;117:138S–143S. doi: 10.1378/chest.117.4_suppl_1.138s. [DOI] [PubMed] [Google Scholar]
- 28.Koukourakis MI, Romanidis K, Froudarakis M, Kyrgias G, Koukourakis GV, Retalis G, Bahlitzanakis N. Concurrent administration of docetaxel and stealth liposomal doxorubicin with radiotherapy in non-small cell lung cancer: excellent tolerance using subcutaneous amifostine for cytoprotection. Br. J. Cancer. 2002;87:385–392. doi: 10.1038/sj.bjc.6600486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.White D, Kris M, Otterson GA, Imondi A, Allen JN, Sharma S. Phase I clinical and pharmacologic study of inhaled doxorubicin in adults with advanced solid tumors affecting lungs. Proc. Am. Soc. Clin. Oncol. 2001 Abstract 2739. [Google Scholar]
- 30.Sharma S, White D, Kris M, Otterson G, Allen J, Murren J, Schiller J, Sandler A, Ryan C, Ratain M, Ramirez J, Imondi A. A multicenter phase I clinical and pharmacologic study of inhaled doxorubicin (Resmycin) in adults with advanced solid tumors affecting the lungs. Proc. Am. Soc. Clin. Oncol. 2001 Abstract 1204. [Google Scholar]