Abstract
We examined the in vitro anti-human immunodeficiency virus (HIV) activity of MRN-100, an iron-based compound derived from bivalent and tervalent ferrates. MRN-100 action against HIV-1 (SF strain) was tested in primary cultures of peripheral blood mononuclear cells (MNC) by analyzing p24 antigen production and percent survival of MNC infected with HIV. MRN-100 at a concentration of 10% (v/v) inhibited HIV-1 replication in 11 out of 14 samples (79%). The percentage of suppression of p24 antigen was −12.3 to 100% at 10 days post-treatment. MRN-100 also exhibited a significant protective effect in the survival of HIV-1-infected MNC. MNC survival post-treatment was dose dependent, 70.4% ± 8.4, 83.6% ± 10.7 and 90% ± 11.4, at concentrations 2.5, 5 and 10% (v/v), respectively, as compared with 53% ± 4 for HIV-1-infected MNC without treatment. The effect was detected as early as 4 days and continued up to 11 days. Treatment with MRN-100 caused no significant change in proliferative response of MNC alone or cocultured with different mitogens: PHA and Con-A (activators of T cell function) and PWM (activator of CD4+ T cell-dependent B cells). We concluded that MRN-100 possesses anti-HIV activity in vitro and without an increase in lymphocyte proliferation, MRN-100 may be a useful agent for treating patients with acquired immunodeficiency syndrome.
Keywords: B cells, HIV, MRN-100, p24 antigen, T cells
Introduction
Human immunodeficiency virus (HIV) is the causative agent of the acquired immunodeficiency syndrome (AIDS). HIV is one of the principle threats to human life and health worldwide. More than 25 million people have died and an estimated 39 million were living with HIV by the year 2005. A complex relationship exists between HIV and its cellular targets. An increase in immunodeficiency and the transition to AIDS is marked by a decrease in the number of CD4+ T lymphocytes as HIV exerts its cytolytic effect on these cells (1,2). However, as is the case with other Lentiviruses, the macrophage is also a major target cell for the HIV virus. Furthermore, the macrophage appears to be a more stable host, and is considered to be the primary reservoir for the virus in the host (3). CD4 molecules are not restricted to T cells but are also found on other cells (4–8). Therefore, infection by HIV-1 is also characterized by a disregulation of the immune system, which results in increased susceptibility to opportunistic infections, and increased frequency of certain neoplasmas (9–13). Another feature of HIV infection is anemia. Castaldo et al. (14) reported iron deficiency in 48% of HIV-infected children. In addition, Rosseau et al. (15) reported iron deficiency in 19% of adult HIV patients; they also noted no significant difference in the iron level between patients with CD4+ cell counts <250/mm3 and patients with CD4+ cells counts >250/mm3. HAART treatment did not change the level of iron as compared with non-HAART patients.
HAART is a frequent choice for first-line therapy that consists of a combination of protease inhibitors, nucleoside reverse transcriptase inhibitors and/or non-nucleoside reverse transcriptase inhibitors. Other HAART treatments that have proven immensely potent in the therapy of AIDS are Lopinavir/ritonavir (LPV/r) and tenofovir disoproxil fumarate (TDF). However, HAART, LPV/r and TDF all are expensive and cause severe side effects (16). Therefore, there is much interest and need to identify anti-HIV agents with minimal side effects, which are also affordable.
It is of great interest to note that iron ions possess anti-viral activity against several viruses, such as Junin virus, herpes simplex virus and several bacteriophages (17). In addition, iron ions inactivate HIV in vitro, as indicated by inhibition of the syncytial formation and the synthesis of virus-specific p24 antigen in HIV–infected cells (18). Current research for the use of iron in the development of a new anti-AIDS drug is underway. Several compounds including iron ions with anti-HIV activity have been reported, such as ferrocene (Fe(II)(C5H5)2), an organometallic chemical compound (19), and ferrovir, a trivalent iron in complex with native sturgeon milt DNA (20,21). The present study was undertaken to investigate the in vitro anti-HIV activity of MRN-100, which is obtained from the plant extract phytosin. MRN-100 is an iron-based compound derived from bivalent and tervalent ferrates and sold as a beverage in Japan. Results of this study demonstrated that MRN-100 inhibited HIV replication in peripheral blood mononuclear cells (MNC) in vitro without cytotoxicity.
Material and Methods
MRN-100
MRN-100 is an iron-based compound derived from bivalent and tervalent ferrates and was prepared in distilled water (DW) with the concentration of Fe2+ and Fe3+ ions at ∼2 × 10−12 mol/l. MRN-100 is obtained from phytosin, a plant extract that contains iron and neutral lipid compounds and is found in rice, wheat or radish seeds. When phytosin is dispersed in DW, ferric chloride is added, the lipid compounds are removed and the iron compound thus obtained is subjected to fractional determination with respect to bivalent ferrate and tervalent ferrate in order to generate MRN-100. MRN-100 was provided by ACM Co., Ltd., Japan.
Complete Medium (CM)
The CM consists of RPMI-1640 (Sigma, St. Louis, MO, USA) supplemented with 10% (v/v) fetal bovine serum and 100 µg/ml streptomycin and penicillin.
Preparation of Peripheral Blood MNCs
MNCs were prepared from heparinized peripheral venous blood by Ficoll-Hypaque density gradient centrifugation. Blood (20 ml) was gently overlaid on the surface of 6 ml Ficoll-Hypaque and centrifuged at 1400 rpm for 45 min; then the buffy coat which contained MNCs was aspirated using Pasteur pipette. The cells were washed twice with Hanks balance salt solution (HBSS) and resuspended in serum-free AIM V medium (GIBCO, Long Island, NY).
Production of HIV-1 p24 Antigen
MNCs from 14 healthy individuals were incubated with 5 µg/ml of PHA for 3 days (37°C) and then washed before incubation (37°C, 1 h) with HIV-1 SF strain (HIV-1 p24 of 3000 pg/106 cells). MNCs were then washed 3x with PBS to remove unbound viruses before incubation (37°C, 10 days) either with or without MRN-100 at concentration of 10% (v/v) in CM. Half of the medium was changed twice per week with corresponding MRN-100 concentrations. Another set of experiments was carried out to evaluate proper controls such as AZT (1 nmol/ml) for comparison. At the end of the incubation period (10 days), culture supernatants of HIV-infected-cells were collected and analyzed for viral production. HIV-1 p24 antigen was assayed using a commercially available ELISA kit, (DuPont NEN, Boston, MA, USA) according to manufacturer's protocol.
MNCs Survival
MNCs survival was determined using a colorimetric MTT assay. This assay is based on the reduction of tetrazolium salt MTT [3-(4,5-dimethylthiozol-3-yl)-2,5-diphenyltetrazolium bromide] by a mitochondrial dehydrogenase in viable cells from a colorless to a blue-colored formazan product that can be measured spectrometrically. The amount of formazan produced is proportional to the number of living cells. HIV-1 infected cells (1 × 104/well) were seeded in 96-well plates and cultured in triplicate in the presence or absence of MRN-100 at various concentrations 0–10% (v/v). A control group of MNC neither infected with HIV nor treated with MRN-100 was included. The final volume of medium in each well after addition of MRN-100 was 200 μl. The cultures were incubated at 37°C for 3 days, after which 50 μg of MTT was added to each well and the cultures incubated for an additional 4 h. The plates were centrifuged, the medium carefully removed, the formazan crystals solubilized with acid alcohol and the plates read at 590 nm using an ELISA plate reader (Molecular Devices, Menlo Park, CA, USA).
Cell Viability
Cell viability was measured using the propidium iodide technique and FACScan (Becton-Dickinson, San Jose, CA, USA). Briefly, MNCs were infected with HIV-1 and incubated in the presence of MRN-100 at various concentrations; 2.5, 5 and 10% (v/v). A control group of MNC neither infected with HIV nor treated with MRN-100 was included. At the end of incubation period (11 days), MNCs were stained with propidium iodide (5 µg/ml) for 30 min. Ten-thousand cells were acquired and analyzed with CellQuest software (Becton Dickinson). Experiments were done in triplicate with data expressed as percent viable cells.
T and B Lymphocyte Proliferation
We investigated the in vitro effects of MRN-100 on T and B cell proliferation as well as mitogen-induced proliferation, using 3H-thymidine uptake. MNCs (2 × 105 cells/ml) from six healthy individuals were treated with 10 μg/ml of one of the following mitogens: phytohemagglutinin (PHA), Concavalin A (Con A) or pokeweed mitogen (PWM) in the presence or absence of MRN-100 at concentration of 10% (v/v) for 3 days. One μCi of 3H-thymidine (New England Nuclear) was added to the cell cultures during the last 18 h. DNA was harvested and 3H-thymidine uptake was determined by scintillation counter. All experiments were done in triplicate and data expressed as counts per minute (cpm).
Statistical Analysis
For the percentage of MNC cell survival and the percent change of p24 antigen suppression, we analyzed the data using the Wilcoxon signed ranks test and the sign test to assess the significant differences between the groups. The survival related variables data were analyzed using a one-way analysis of variance (ANOVA) to test the hypothesis that the means of the groups are equal. We used the post hoc range tests and pair-wise multiple comparisons to determine which means differ. Pair-wise multiple comparisons test the difference between each pair of means, and yield a matrix where we identified the significantly different group means at an alpha level of 0.05. We used the multiple comparison tests, Bonferroni test and also detected the least significance difference between the groups using the LSD (least significant difference). The data were analyzed using SPSS software version 15.
Results
Production of HIV-1 p24 Antigen
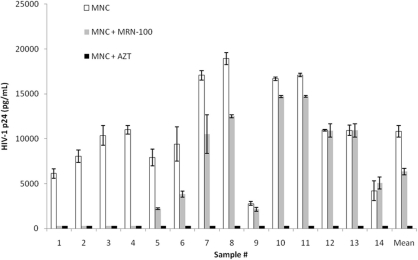
p24 antigen production was examined in MNC from 14 different HIV-1 infected samples that were treated with MRN-100 at a concentration of 10% (v/v) for 10 days (Fig. 1). The Wilcoxon signed ranks test showed a significant inhibition (P = 0.002) of HIV p24 antigen production between the MRN-100 group and the control group (MNC without MRN-100). A clear differential response can be seen among different individuals towards inhibitory effects by MRN-100. The percentage of suppression of p24 antigen for all samples was −12.3 to 100%: 100% suppression in four samples, 40–70% in four samples, 12–23% in three samples, no significant change in two samples and 10% increase in one sample. With respect to the control, data shows that treatment with AZT resulted in zero percent of p24 antigen production. Regarding the percent change of p24 antigen production, we had two groups: one group represented the percent of p24 suppression of the MRN-100 relative to the control group and the second group represented the percent of p24 suppression of the MRN-100 relative to the AZT group. The Wilcoxon signed ranks test showed a statistically significant difference between the two groups (P = 0.001).
Figure 1.
Inhibition of HIV-1 p24 antigen production by MRN-100. Data from 14 different samples were examined at 10 days post-treatment with MRN-100 in triplicate. AZT (1 nmol/ml) was used as a control. The data represents mean ± SD of triplicate counts for each sample.
MNC Survival and Cell Viability
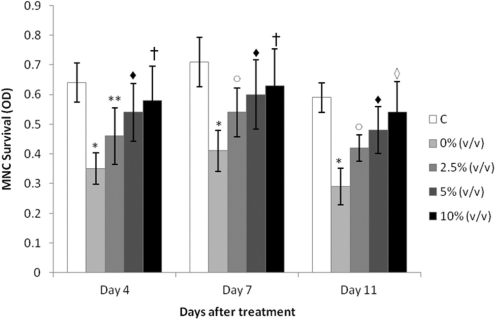
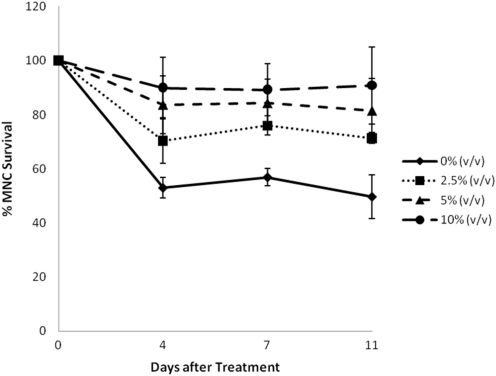
Effect of MRN-100 on the survival of HIV-1 infected MNCs was determined by MTT assay and the data is depicted in Figs 2 and 3. A control group of MNC neither infected with HIV nor treated with MRN-100 was included, and experimental values were normalized to this control. P-values reflected a comparison to the control group (Fig. 2). Results show that infection with HIV-1 resulted in 50% MNC survival. Treatment with MRN-100 increased survival of HIV-infected MNC in a dose dependent manner. MRN-100 at a concentration of 2.5% (v/v) significantly increased MNC survival to 70% as compared with the control group. Higher concentrations, 5 and 10% (v/v), resulted in a significant increase of cell survival, as compared with the HIV-infected group without MRN-100 treatment. The increase in the survival of HIV-infected cells was noted at 4, 7 and 11 days post-treatment with MRN-100.
Figure 2.
In vitro action of MRN-100 on the survival of HIV-1 infected MNC. MNC were infected with HIV-1 and cultured with MRN-100 at concentrations of 0–10% (v/v). Survival of MNC was examined at 4, 7 and 11 days by MTT assay. Data are expressed as mean ± SD of three samples in triplicate. OD, optical density. *P < 0.0001, **P = 0.01 and P = 0.02 (open circle) as compared with the control group (C) (no HIV-1 and no MRN-100 treatment). P < 0.01 (filled diamond), P = 0.005 (open square) and P < 0.001 (open diamond) as compared with the HIV-infected group.
Figure 3.
Percent survival of HIV-1 infected MNC post-treatment with MRN-100 in vitro. MNC infected cells were cultured with MRN-100 at different concentrations (v/v): 0% (filled diamond), 2.5% (filled square), 5% (filled triangle) and 10% (filled circle). Percent survival of MNC was examined at 4, 7 and 11 days. Data are expressed as mean ± SD of three samples carried in triplicate relative to a control group that was neither infected with HIV nor treated with MRN-100, which was set as 100%.
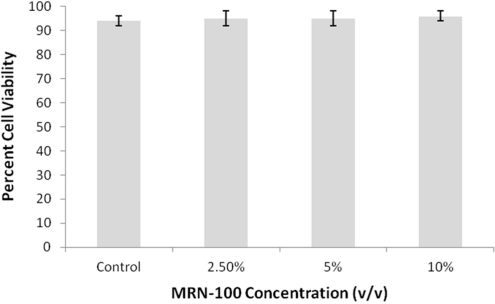
The effect of MRN-100 on the viability of HIV-1 infected cells was examined. Results in Fig. 4 show no significant change in the viability of the HIV-1 infected cells post-treatment with MRN-100 up until 11 days post-infection as compared with the control group of MNC neither infected with HIV nor treated with MRN-100.
Figure 4.
Effect of MRN-100 on cell viability. MNCs from six samples were infected with HIV-1 and incubated in the presence of MRN-100 at the various concentrations, 2.5, 5 and 10% (v/v), for 11 days. Cell viability was measured with the propidium iodide technique using FACScan. Experiments were done in triplicate with data expressed as percent viable cells. Results were compared with the control group of MNC neither infected with HIV nor treated with MRN-100.
Effect of MRN-100 on T and B Cell Proliferation
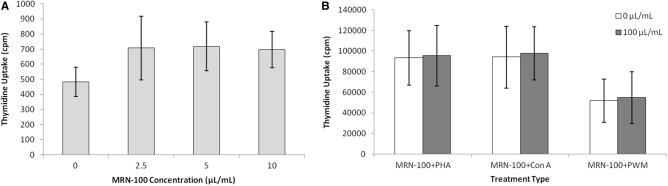
MNC from six healthy samples were cultured with MRN-100 for 3 days and cell proliferation was examined. Fig. 5A shows treatment with MRN-100 resulted in a slight elevation in cell proliferation at different concentrations [2.5–10% (v/v)] as compared with control untreated cells. Fig. 5B shows that MRN-100 at concentration of 10% (v/v) caused no significant change in proliferative response of MNC cocultured with different mitogens: PHA and ConA (T cell function activators) and PWM (B cell activator).
Figure 5.
Effect of MRN-100 on lymphocyte function. MNCs were incubated for 3 days with various concentrations of MRN-100 alone (A) and in the absence or presence of PHA and Con A (activators of T cell function) and PWM (activator of CD4+ T cell-dependent B cells). (B) Thymidine uptake was used to measure cell number. Data are expressed as mean ± SD of six individuals carried in triplicate.
Discussion
The results of this study showed that MRN-100, an iron-based compound derived from bivalent and tervalent ferrates, caused a significant increase in the survival of HIV-1 infected MNC and inhibition of p24 antigen production without an adverse affect on lymphocyte functions. Recently, there has been much research on the role of iron ions in anti-HIV activity. Sagripanti and Lightfoote reported that ferric ions inactivate HIV when cells are infected with the virus and when the virus was free in solution (18). da Silva et al. (19) and Nossik et al. (20,21) have shown that ferrovir and ferrocene inhibit HIV replication. Our results are in agreement with the above studies, and taken together, suggest that iron based compounds may provide an effective complementary and alternative treatment for subjects infected with HIV virus.
The use of complementary and alternative medicine (CAM) for the treatment of HIV is becoming more common in the USA (22,23), Canada (24) and Europe (25). For CAM in relation to HAART, studies in the USA revealed that CAM therapies complement, rather than replace, HAART (26). In Canada, nearly all patients used CAM in conjunction with antiretroviral medications (24), while in Europe the patients were administered CAM after reporting HAART-related side effects (25). Several studies indicate that many CAM therapies may improve the quality of life of people living with HIV-AIDS (26–28); on the other hand, the effectiveness of CAM against HIV is inconclusive (29). Clearly, further investigations on CAM therapies are needed to prove their effectiveness both with respect to clinical and basic research settings. Recent studies show that several CAM therapies have anti-HIV activity in vitro. Polysaccharides from different sources such as those found in rice bran (arabinoxylan) (30), pine cones (Pinus parvifloria Sieb Zucc) (31) and the plant Hyssop officinalis (32) inhibit the production of HIV antigen in HIV-infected MNC. A phase II controlled study of a combination of lentinan, beta-glucans from the Shiitake mushroom (Lentinula edodes), with the antiviral drug didanosine (DDI) was carried out in HIV patients. A significant increase in CD4 levels was observed post-treatment with this combination (38 weeks) when compared with DDI alone (14 weeks) (33).
The mechanisms by which MRN-100 inactivates HIV-1 are not fully understood. Sagripanti and Lightfoote (18) reported complete inhibition in the formation of syncytia and synthesis of virus-specific p24 antigen in HIV-infected cells post-treatment with Fe3+. Ferrovir (20,21) has been shown to influence DNA and RNA synthesis during early stages of HIV-1 replication by blocking the virus’ action on cell metabolism. Ferrocene has been reported to inhibit the activity of integrase, and thus block integration and subsequent viral replication. It is possible that both cellular and viral enzymes may be the targets for MRN-100 action. Alternatively, MRN-100 may exert its anti-HIV activity via NK immune modulation. AIDS is associated with abnormalities in NK cell function (11,12). Moreover, NK cells may play a role in host depletion of HIV-1 infected cells (34,35). In this regard, preliminary studies show that MRN-100 activates NK activity of healthy control samples in vivo (36).
Adverse side effects are one of the problems with using anti-HIV agents for treatment. Prolonged use of several anti-HIV drugs is associated with severe toxicity and development of drug resistance (37,38). MRN-100 is a beverage that is sold in Japan. In vitro studies have demonstrated that MRN-100 has no suppressive effect on lymphocyte function. In conclusion, we propose that MRN-100 possesses anti-HIV activity in vitro, indicating that it may be a useful agent for treating patients with AIDS.
References
- 1.Gougeon ML, Montagnier L. Apoptosis in AIDS. Science. 1993;260:1269–70. doi: 10.1126/science.8098552. [DOI] [PubMed] [Google Scholar]
- 2.Stevenson M, Stanwick TL, Dempsey MP, Lamonica CA. HIV-1 replication is controlled at the level of T cell activation and proviral integration. EMBO J. 1990;9:1551–60. doi: 10.1002/j.1460-2075.1990.tb08274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orenstein JM. Replication of HIV-1 in vivo and in vitro. Ultrastruct Pathol. 2007;31:151–67. doi: 10.1080/01913120701344343. [DOI] [PubMed] [Google Scholar]
- 4.McCoombe SG, Short RV. Potential HIV-1 target cells in the human penis. AIDS. 2006;20:1491–5. doi: 10.1097/01.aids.0000237364.11123.98. [DOI] [PubMed] [Google Scholar]
- 5.Donoval BA, Landay AL, Moses S, Agot K, Ndinya-Achola JO, Nyagaya EA, et al. HIV-1 target cells in foreskins of African men with varying histories of sexually transmitted infections. Am J Clin Pathol. 2006;125:386–91. [PubMed] [Google Scholar]
- 6.Shaw GM, Harper ME, Hahn BH, Epstein LG, Gajdusek DC, Price RW, et al. HTLV-III infection in brains of children and adults with AIDS encephalopathy. Science. 1985;227:177–82. doi: 10.1126/science.2981429. [DOI] [PubMed] [Google Scholar]
- 7.Dybul M, Weissman D, Rubbert A, Machado E, Cohn M, Ehler L, et al. The role of dendritic cells in the infection of CD4+ T cells with the human immunodeficiency virus: use of dendritic cells from individuals homozygous for the delta32CCR5 allele as a model. AIDS Res Hum Retroviruses. 1998;14:1109–13. doi: 10.1089/aid.1998.14.1109. [DOI] [PubMed] [Google Scholar]
- 8.Bhoopat L, Rithaporn TS, Khunamornpong S, Bhoopat T, Taylor CR, Thorner PS. Cell reservoirs in lymph nodes infected with HIV-1 subtype E differ from subtype B: identification by combined in situ polymerase chain reaction and immunohistochemistry. Mod Pathol. 2006;19:255–63. doi: 10.1038/modpathol.3800527. [DOI] [PubMed] [Google Scholar]
- 9.Fauci AS. The human immunodeficiency virus: infectivity and mechanisms of pathogenesis. Science. 1988;239:617–22. doi: 10.1126/science.3277274. [DOI] [PubMed] [Google Scholar]
- 10.Tersmette M, Miedema F, Huisman HG, Goudsmit J, Melief CJ. Productive HTLV-III infection of human B cell lines. Lancet. 1985;1:815–6. doi: 10.1016/s0140-6736(85)91469-2. [DOI] [PubMed] [Google Scholar]
- 11.Cerboni C, Neri F, Casartelli N, Zingoni A, Cosman D, Rossi P, et al. Human immunodeficiency virus 1 Nef protein downmodulates the ligands of the activating receptor NKG2D and inhibits natural killer cell-mediated cytotoxicity. J Gen Virol. 2007;88:242–50. doi: 10.1099/vir.0.82125-0. [DOI] [PubMed] [Google Scholar]
- 12.Fontana L, Sirianni MC, de Sanctis G, Carbonari M, Ensoli B, Aiuti F. Deficiency of natural killer activity, but not of natural killer binding, in patients with lymphoadenopathy syndrome positive for antibodies to HTLV-III. Immunobiology. 1986;171:425–35. doi: 10.1016/S0171-2985(86)80074-2. [DOI] [PubMed] [Google Scholar]
- 13.Martinelli E, Cicala C, Van Ryk D, Goode DJ, Macleod K, Arthos J, et al. HIV-1 gp120 inhibits TLR9-mediated activation and IFN-{alpha} secretion in plasmacytoid dendritic cells. Proc Natl Acad Sci USA. 2007;104:3396–401. doi: 10.1073/pnas.0611353104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castaldo A, Tarallo L, Palomba E, Albano F, Russo S, Zuin G, et al. Iron deficiency and intestinal malabsorption in HIV disease. J Pediatr Gastroenterol Nutr. 1996;22:359–63. doi: 10.1097/00005176-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Rousseau MC, Molines C, Moreau J, Delmont J. Influence of highly active antiretroviral therapy on micronutrient profiles in HIV-infected patients. Ann Nutr Metab. 2000;44:212–6. doi: 10.1159/000046686. [DOI] [PubMed] [Google Scholar]
- 16.Esser S, Helbig D, Hillen U, Dissemond J, Grabbe S. Side effects of HIV therapy. JDDG. 2005;5:745–54. doi: 10.1111/j.1610-0387.2007.06322.x. [DOI] [PubMed] [Google Scholar]
- 17.Sagripanti J-L, Routson LB, Lytle CD. Virus inactivation by copper or iron ions alone and in the presence of peroxide. Appl Environ Microbiol. 1993;59:4374–6. doi: 10.1128/aem.59.12.4374-4376.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sagripanti J-L, Lightfoote MM. Cupric and ferric ions inactivate HIV. AIDS Res Human Retroviruses. 1996;12:333–6. doi: 10.1089/aid.1996.12.333. [DOI] [PubMed] [Google Scholar]
- 19.da Silva CH, Del Ponte G, Neto AF, Taft CA. Rational design of novel diketoacid-containing ferrocene inhibitors of HIV-1 integrase. Bioorg Chem. 2005;33:274–84. doi: 10.1016/j.bioorg.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 20.Nossik D, Kaplina E, Nossik N, Sato S, Fomin Y, Voronin E. Ferrovir: a new antiviral drug for the treatment of HIV. Am Clin Lab. 2001;20:36–8. [PubMed] [Google Scholar]
- 21.Nosik DN, Nosik NN, Kaplina EN, Kalnina LB, Kiseleva IA, Kondrashina NG, et al. Activity of ‘Ferrovir’ preparation towards RNA and DNA viruses. 1. Vopr Virusol. 2002;47:21–3. [PubMed] [Google Scholar]
- 22.Owen-Smith A, Diclemente R, Wingood G. Complementary and alternative medicine use decreases adherence to HAART in HIV-positive women. AIDS Care. 2007;19:589–93. doi: 10.1080/09540120701203279. [DOI] [PubMed] [Google Scholar]
- 23.Mikhail IS, DiClemente R, Person S, Davies S, Elliott E, Wingood G, et al. Association of complementary and alternative medicines with HIV clinical disease among a cohort of women living with HIV/AIDS. J Acquir Immune Defic Syndr. 2004;37:1415–22. doi: 10.1097/01.qai.0000130549.65946.3d. [DOI] [PubMed] [Google Scholar]
- 24.Furler MD, Einarson TR, Walmsley S, Millson M, Bendayan R. Use of complementary and alternative medicine by HIV-infected outpatients in Ontario, Canada. AIDS Patient Care STDS. 2003;17:155–68. doi: 10.1089/108729103321619764. [DOI] [PubMed] [Google Scholar]
- 25.Agnoletto V, Chiaffarino F, Nasta P, Rossi R, Parazzini F. Use of complementary and alternative medicine in HIV-infected subjects. Complement Ther Med. 2006;14:193–9. doi: 10.1016/j.ctim.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 26.Bica I, Tang AM, Skinner S, Spiegelman D, Knox T, Gorbach S, et al. Use of complementary and alternative therapies by patients with human immunodeficiency virus disease in the era of highly active antiretroviral therapy. J Altern Complement Med. 2003;9:65–76. doi: 10.1089/107555303321222955. [DOI] [PubMed] [Google Scholar]
- 27.Power R, Gore-Felton C, Vosvick M, Israelski DM, Spiegel D. HIV: effectiveness of complementary and alternative medicine. Prim Care. 2002;29:361–78. doi: 10.1016/s0095-4543(01)00013-6. [DOI] [PubMed] [Google Scholar]
- 28.Kirksey KM, Goodroad BK, Kemppainen JK, Holzemer WL, Bunch EH, Corless IB, et al. Complementary therapy use in persons with HIV/AIDS. J Holist Nurs. 2002;20:264–78. doi: 10.1177/089801010202000306. [DOI] [PubMed] [Google Scholar]
- 29.Mills E, Wu P, Ernst E. Complementary therapies for the treatment of HIV: in search of the evidence. Int J STD AIDS. 2005;16:395–403. doi: 10.1258/0956462054093962. [DOI] [PubMed] [Google Scholar]
- 30.Ghoneum M. Anti-HIV activity in vitro of MGN-3, an activated arabinoxylane from rice bran. Biochem Biophys Res Commun. 1998;243:25–9. doi: 10.1006/bbrc.1997.8047. [DOI] [PubMed] [Google Scholar]
- 31.Lai PK, Donovan J, Takayama H, Sakagami H, Tanaka A, Konno K, et al. Modification of human immunodeficiency viral replication by pine cone extracts. AIDS Res Hum Retroviruses. 1990;6:205–17. doi: 10.1089/aid.1990.6.205. [DOI] [PubMed] [Google Scholar]
- 32.Gollapudi S, Sharma HA, Agrawal S, Byers LD, Ensley HE, Gupta S. Isolation of a previously unidentified polysaccharide (MAR-10) from Hyssop officinalis that exhibits strong activity against human immunodeficiency virus type 1. Biochem Biophys Res Commun. 1995;210:145–51. doi: 10.1006/bbrc.1995.1639. [DOI] [PubMed] [Google Scholar]
- 33.Gordon M, Guralnik M, Kaneko Y, Mimura T, Goodgame J, DeMarzo C, et al. A phase II controlled study of a combination of the immune modulator, lentinan, with didanosine (ddI) in HIV patients with CD4 cells of 200-500/mm3. J Med. 1995;26:193–207. [PubMed] [Google Scholar]
- 34.Loubeau M, Ahmad A, Toma E, Menezes J. Enhancement of natural killer and antibody-dependent cytolytic activities of the peripheral blood mononuclear cells of HIV-infected patients by recombinant IL-15. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;16:137–45. doi: 10.1097/00042560-199711010-00001. [DOI] [PubMed] [Google Scholar]
- 35.Chehimi J, Marshall JD, Salvucci O, Frank I, Chehimi S, Kawecki S, et al. IL-15 enhances immune functions during HIV infection. J Immunol. 1997;158:5978–87. [PubMed] [Google Scholar]
- 36.Ghoneum M. Proceedings of the 4th Internatl. Symposium on Predictive Oncology and Therapy; October 24–27, 1998; Nice, France. [Google Scholar]
- 37.Hofman P, Nelson AM. The pathology induced by highly active antiretroviral therapy against human immunodeficiency virus: an update. Curr Med Chem. 2006;13:3121–32. doi: 10.2174/092986706778742891. [DOI] [PubMed] [Google Scholar]
- 38.Sension MG. Long-Term suppression of HIV infection: benefits and limitations of current treatment options. J Assoc Nurses AIDS Care. 2007;18:S2–10. doi: 10.1016/j.jana.2006.11.012. [DOI] [PubMed] [Google Scholar]