Abstract
Diabetic nephropathy is one of the most frequent and serious complications of diabetes mellitus. Soybeans have been shown to reduce urinary albumin excretion and total cholesterol in non-diabetic patients with nephrotic syndrome. However, reports focusing specifically on diabetic nephropathy are scarce and the available results are inconsistent. It was reported that soybean consumption reduced urinary protein excretion in type 1 diabetic patients with diabetic nephropathy, whereas it was found to elicit an increase in urinary protein excretion when soybeans were consumed by type 2 diabetic patients. This study aims to investigate the effects of soybean in diabetic nephropathy, particularly the effects of consuming soybeans on the histopathology of diabetic nephropathy, using aquaporin (AQP) and osteopontin (OPN) expression as diagnostic markers. Male Sprague-Dawley rats were assigned to one of three groups: control, diabetic with red chow diet and diabetic with soybean diet. For histological examination, the expression of OPN and AQP, renal function and hemoglobin A1c were evaluated at the end of the study. Improvements in glomerular and tubulointerstitial lesions were demonstrated in the diabetic rat group given a soybean diet. OPN and AQP expression were suppressed in the kidney specimens of diabetic rats with the soybean diet. In conclusion, soybeans may prevent the weight loss and morphological disruption of the kidney associated with diabetes mellitus. Soybeans also may improve glycemic control. It seems likely that long-term control of blood glucose levels using a soybean diet could prevent the progression of diabetes mellitus, and therefore, nephropathy could be prevented.
Keywords: aquaporin, diabetes mellitus, osteopontin, kidney
Introduction
Diabetic nephropathy is one of the most frequent complications of diabetes mellitus, developing in 30–40% of patients with type 1 and type 2 diabetes mellitus. Terminal renal failure occurs within 7 years after the onset of renal disease and diabetic nephropathy. Renal disease is usually attributed to metabolic consequences of abnormal glucose regulation manifested by elevated blood and tissue levels of glycosylated proteins and hemodynamic changes within the kidney tissue (1). Diabetic nephropathy is characterized by a progressive accumulation of extracellular matrix components in the glomerular mesangium and tubular interstitium, which eventually leads to proteinuria and renal failure (2). It is generally accepted that tubulointerstitial injury, along with glomerulosclerosis, is a major feature and an important predictor of renal dysfunction in diabetic nephropathy (3). Tubulointerstitial fibrosis has long been recognized as an important histological parameter that correlates with chronic renal failure in a variety of renal diseases including diabetic nephropathy (4,5).
The exact mechanisms underlying the evolution of diabetic nephropathy are complex and not well defined.
Aquaporins (AQPs) are ubiquitously expressed pore-forming proteins located in renal proximal tubules and are a part of the descending thin limbs. AQP play a highly important role in the reabsorption of water from the renal tubular fluid. Immunohistochemical studies of biopsy samples from a wide range of renal diseases revealed a substantial and striking upregulation of aquaporin-1 (AQP-1) in the glomeruli and also in the tubules of diseased kidneys (6).
Osteopontin (OPN) is an arginine-glycine-aspartate containing adhesive glycoprotein that is expressed in a variety of organs including bone, kidney, vasculature, and epithelia. A functional role for OPN in attracting macrophages has been recently documented in vivo and in vitro (7,8). OPN plays a pro-inflammatory role in the kidney. The up-regulation of OPN expression is also closely associated with macrophage influx in several models of kidney diseases (9). Moreover, the extent of up-regulation of OPN expression in tubules correlates with the degree of macrophage accumulation and the magnitude of tubulointerstitial fibrosis and renal dysfunction (10,11).
Restricting dietary protein intakes has long been known to reduce urinary albumin excretion. It is also beneficial for the prevention and treatment of diabetic nephropathy (12,13).
Recently, instead of reducing protein intake, some interest has been directed toward manipulating the dietary protein quality, specifically by replacing animal protein with soybeans (14). Soybeans have been shown to reduce urinary albumin excretion and total cholesterol in non-diabetic patients with nephrotic syndrome (15). Improvements in renal function were shown in animal models of polycystic kidney disease (16,17). Teixeira et al. (18) found that a high soybean diet was able to halt the increase in urinary albumin excretion typically seen in a type 2 diabetes mellitus mouse model, the db/db mouse. Therefore, there is a growing body of evidence indicating that soybean consumption may have beneficial effects for nephropathy in general.
However, reported studies focusing specifically on diabetic nephropathy are scarce, and the available results are inconsistent. Jibani et al. (19) and Kontessis et al. (20) found that soybean consumption reduced urinary protein excretion in type 1 diabetic patients with diabetic nephropathy, whereas Anderson et al. (21) found an increase in urinary protein excretion when soybeans were consumed by type 2 diabetic patients with urinary protein excretion <1000 mg/d and serum creatinine <176.8 µmol/l (<2 mg/dl).
The objective of this study was to investigate the effects of a soybean diet on diabetic nephropathy. In particular, we chose to analyze the effects of soybean consumption on the histopathology of diabetic nephropathy, AQP and OPN expression as diagnostic markers.
Methods
Experiments were performed according to a protocol approved by the Yonsei University Animal Care and Use Committee in accordance with the Principles of Laboratory Animal Care (National Institutes of Health, 1985, revised version). Eight- to nine-week-old male Sprague-Dawley rats weighing 250–300 g (Samtako, Osan Korea) were fed a standard rat chow diet and had access to water ad libitum. At the beginning of the experimental period, 90 rats were assigned randomly into three subgroups: the control group, the diabetic group given a red chow diet and the diabetic group given a soybean diet. The composition of soybean diet is in Table 1. Diabetes was induced by a single intraperitoneal injection of streptozotocin (STZ, 50 mg/kg). One week after the STZ injection, blood glucose levels were determined in blood samples collected from the tail vein. Diabetes was confirmed by determining presence of hyperglycemia (blood glucose concentration >350 mg/dl). After diabetes was confirmed, soybean feeding was started. Animals were sacrificed four weeks after confirming high blood glucose level, and biochemical analyses were done before the sacrifice. They were perfused with 4% paraformaldehyde and kidneys were removed.
Table 1.
Comparison of composites in Sam Tako diet and soybean
| Ingredient | Unit:% | |
|---|---|---|
| Sam Tako Diet | Soybean | |
| Carbohydrate | 48.3 | 22.6 |
| Protein | 23.5 | 41.3 |
| Lipid | 5.9 | 17.6 |
| Calcium | 5.9 | 5.8 |
| Cellulose | 3.9 | 3.5 |
| Vitamin and Mineral | trace | trace |
Histology
The kidney specimens were fixed in 4% paraformaldehyde solution and embedded in paraffin. Sections were cut at 4 µm with a microtome and deparaffined with xylene. They were stained with Hematoxylin–Eosin (H–E) staining. Stained kidney sections were observed under a light microscope at magnifications of 200× and 400×.
Immunohistochemistry
Before paraffin embedding was performed, tissue blocks from whole kidneys were dehydrated in a graded series of ethanol (2 h each in 70, 96 and 99%) and xylene (overnight). Paraffin sections (4 µm thick) were cut on a Leica RM 2126 microtome and dried overnight at 37°C.
Sections were incubated with 10% normal goat serum for 20 min and incubated with the rabbit anti-mouse AQP-1 antibody (1:100 dilution) and OPN (1:100 dilution) at room temperature for 1 h. The immunoreactivity was visualized by incubation with a horseradish peroxidase-conjugated goat-anti-rabbit IgG antibody (kit from Zymed) for 30 min at room temperature, followed by treatment with 3′-diaminobenzidine (DAB) 0.01% hydrogen peroxide (Sigma, USA). Immunoreactivity was detected using the Zymed 2nd generation LAB-SA detection system (85-9043).
Electron Microscopy
Animals were perfused through the left ventricle with saline solution followed by 3% paraformaldehyde, 3% glutaraldehyde and 0.1% picric acid. Kidney tissues were excised in 1 × 1 × 1 mm blocks and immersed in fresh fixative for an additional 2 h at room temperature and then overnight at 4°C. After washing with PBS, the tissues were post-fixed with 1% OsO4 in the same buffer for 2 h at room temperature. Tissues were dehydrated in graded concentrations of ethanol and then embedded in Epon 812. Epon embedded samples were sectioned to a 1 µm thickness (semi-thin section) and stained with 1% toluidine blue. After observation with light microscope, selected portions were trimmed and sectioned with ultramicrotome to produce thin sections. Thin sections were stained with uranyl acetate and lead citrate and examined with Philips 500 electron microscope.
Biochemical Analyses
Blood samples for the measurement of blood chemistry were drawn into pre-chilled EDTA-containing tubes and immediately placed on ice. All tubes were centrifuged within several minutes of collection and stored at −70°C until assay. Serum samples were assayed for blood urea nitrogen (BUN) and creatinine using COBAS INTEGRA 400 (Roche, Sweden). Also, the hemoglobin A1c (Hb A1c) concentration was determined after hemolysis of the anticoagulated whole blood specimen. Hb A1c was determined immunoturbidimetrically. The ratio of both concentrations yields the final percent of Hb A1c result.
All the datas were analyzed statistically with Student-t-test.
Results
Rats that had received streptozotocin became diabetic at a frequency of 70%. Diabetes was associated with reduced body weight when compared with the control rats. The change of body weight is shown in Table 2.
Table 2.
Weight changes of the rats
| Beginning (g) | After 4 weeks (g) | ||
|---|---|---|---|
| Control group | 273.5 ± 4.0 | 397.0 ± 13.6 | P < 0.001 |
| DM group | 270.3 ± 25.4 | 201.0 ± 32.5 | P < 0.01 |
| DM soy group | 275.5 ± 14.5 | 271.6 ± 30.5 |
Histopathological Finding
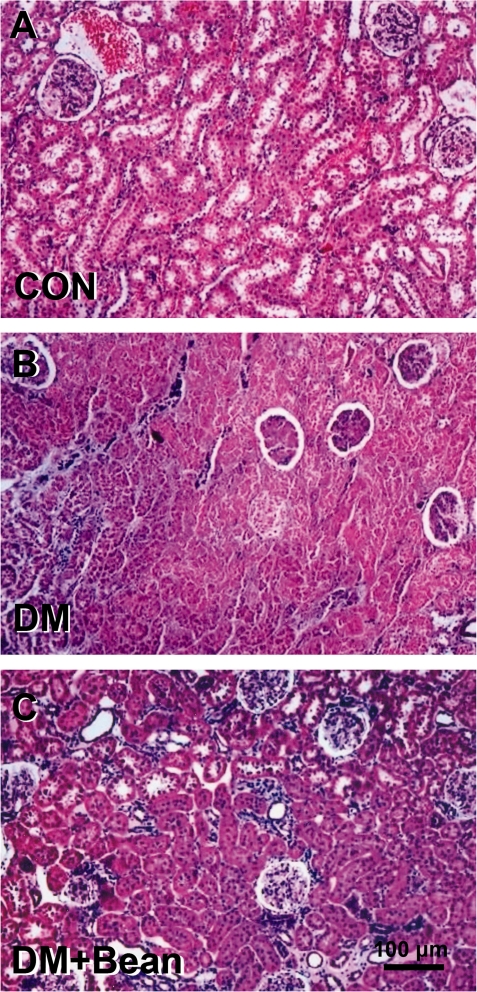
The kidney specimens of the diabetic group showed markedly severe destruction in glomerular and tubulointerstitial lesions such as glomerular sclerosis, atrophy, interstitial expansion, and interstitial cellular infiltration (Fig. 1B) as compared with those of the control group (Fig. 1A). Also, collagen deposition was prominent and tubular spaces were obstructed (Fig. 1B). In the diabetic rats with the soybean diet, general morphology of glomerulus and tubulointerstitial lesions were much improved and showed quite normal appearance (Fig. 1C).
Figure 1.
Photomicrographs of H–E staining in the kidney of each group. (A) Control rat. (B) Diabetic rat. (C) Diabetic rats with soybean. The kidney specimen of the diabetic group showed markedly severe destruction in glomerular and tubulointerstitial lesions such as glomerular sclerosis atrophy, interstitial expansion and interstitial cellular infiltration (B). General morphology of glomerulus and tubulointerstitial lesions were much improved and showed quite normal appearance.
Immunohistochemistry
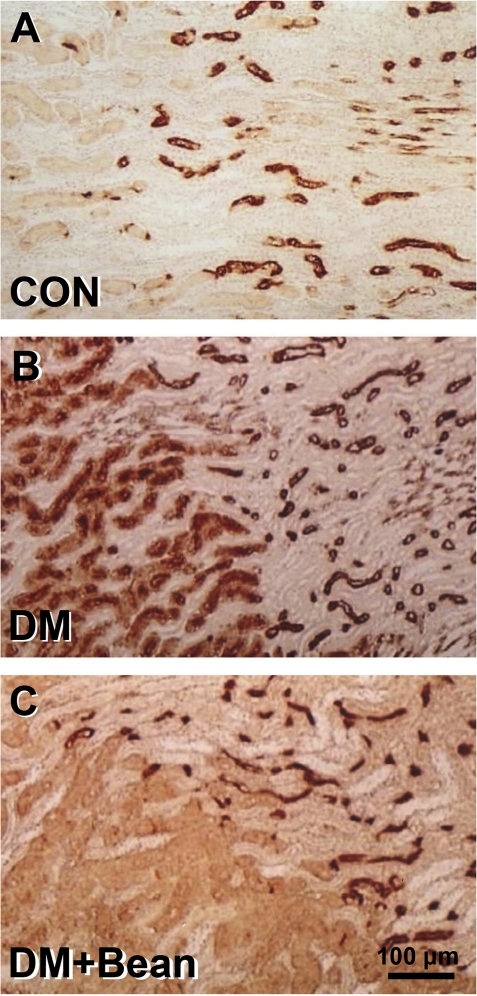
AQP-1 is located on the basolateral and apical membranes of the proximal tubules and descending thin limb of the loop of Henle in the control group. It is absent from other parts of the nephron and the collecting ducts (Fig. 2A).
Figure 2.
Photomicrographs of immunohistochemistry of AQP in the kidney of each group. (A) Control rat. (B). Diabetic rat. (C). Diabetic rat with soybean. AQP-1 is located on the basolateral and apical membranes of the proximal tubules and descending thin limb of the loop of Henle in the control group. It is absent from other parts of the nephron and the collecting ducts (A). In the diabetic rats, AQP-1 expression was greatly increased in the glomerular endothelium. AQP-1 was also evident in the sclerosed glomeruli. AQP-1 staining was widespread and prominent in all viable proximal tubules (B). AQP-1 expression was not found in glomeruli in a diabetic rat with soybean diet (C).
In the diabetic rats, AQP-1 expression was greatly increased in the glomerular endothelium. AQP-1 was also evident in the sclerosed glomeruli. AQP-1 staining was widespread and prominent in all viable proximal tubules (Fig. 2B).
AQP-1 expression was not found in glomeruli in diabetic rats fed the soybean diet (Fig. 2C).
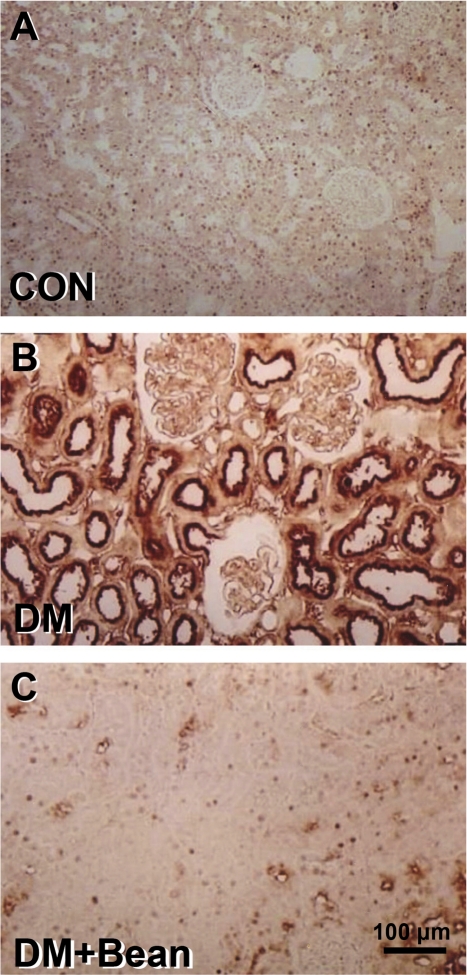
Immunostaining of OPN showed the typical distribution pattern observed in the renal cortex of control animals. OPN was expressed in parietal epithelial cells of Bowman's capsule and rarely in the tubular epithelium (Fig. 3A). In diabetic rats, the OPN expression was increased in the renal cortex. A strong staining for OPN protein was found in the tubular epithelial cells of STZ-diabetic rats. No OPN staining was observed in glomerular or interstitial areas (Fig. 3B). In diabetic rats with the soybean diet, OPN expression was not found in the tubular epithelium such as in control group (Fig. 3C).
Figure 3.
Photomicrographs of immunohistochemistry of OPN in the kidney of each group. (A) Control rat. (B) Diabetic rat. (C) Diabetic rat with soybean. Immunostaining of OPN showed the typical distribution pattern in the renal cortex of control rats. OPN was expressed in parietal epithelial cells of Bowman's capsule and rarely in the tubular epithelium (A). The OPN expression was increased in the renal cortex. A strong staining for OPN protein was found in the tubular epithelial cells of a diabetic rat (B). In diabetic rats with soybean diet, OPN expression was not found in the tubular epithelium (C).
Electron-microscopic Finding
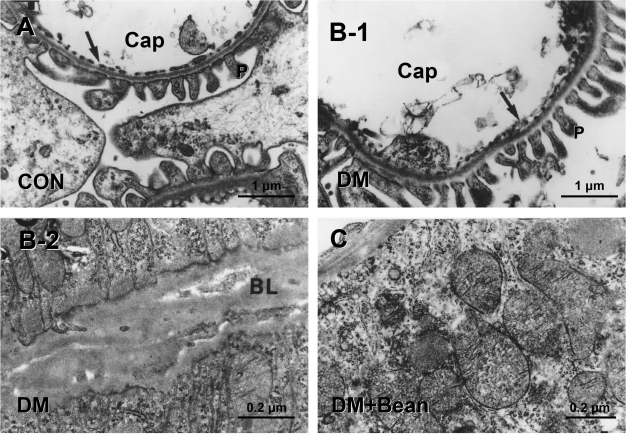
In the diabetic group, cells of the tubules and glomeruli showed general characteristics of disruption. Mitochondria were destroyed and formed vacuoles. Diaphragms of the endothelial cells of the glomerular capillaries showed irregularities, and they were obstructed with debris (Fig. 4B-1). Generally, all the basal lamina became thickened throughout the glomerulus and tubular cells (Fig. 4B-2).
Figure 4.
Photomicrographs of electron microscopic finding in the kidney of each group. (A) Control rat. (B-1,2) Diabetic rat. (C) Diabetic rat with soybean diet. Electron micrograph of a glomerular capillary, showing the fenestrated capillary endothelium C, capillary; P, Pedicle of podocyte; Arrow, fenestrated endothelium of type II capillary (A). Cells of the tubules and glomeruli showed general features of destruction. Diaphragms of the endothelial cells of the glomerular capillaries showed irregularities and were obstructed with debris (B-1). All the basal lamina became thickened through the glomerulus and tubular cells. BL, basal lamina (B-2). Tubular cells showed normal structure. In proximal tubular cells, interdigitation features remained normal and long microvilli were intact. BL, basal lamina (C).
In the DM with soybean group, tubular cells showed normal structure. In proximal tubular cells, severe interdigitation figures were kept normal and long microvilli were intact. In the glomerulus, podocyte pedicles were normal in shape and interpedicular diaphragms were intact (Fig. 4C).
Biochemical Finding
Blood glucose level was very high in DM group and DM soy group in 1 week after soybean diet, however 4 weeks later, the blood glucose level of DM soy group was makedly decreased (Table 3).
Table 3.
Change of Blood glucose
| 1 week (mg/dl) | 4 weeks (mg/dl) | ||
|---|---|---|---|
| Control group | 133.5 ± 7.6 | 173.0 ± 25.5 | |
| DM group | 427.8 ± 56.2 | 404.3 ± 64.0 | |
| DM soy group | 434.2 ± 19.8 | 268.0 ± 68.3 | P < 0.01 |
BUN, serum creatinine and Hb A1c were measured and the results are shown in Table 4. There was no significant difference in BUN and creatinine mean values between the groups. The level of Hb A1c was lower in the DM with soy group than in the DM group.
Table 4.
Biochemical analysis in blood
| Group | BUN (mg/dl) | Creatinine (mg/dl) | HbA1C (%) |
|---|---|---|---|
| Control group | 21.8 ± 1.2 | 0.5 ± 0.03 | 3.6 ± 0.1 |
| DM group | 24.3 ± 0.9 | 0.5 ± 0.02 | 8.2 ± 0.4 |
| DM soy group | 35.4 ± 2.7 | 0.5 ± 0.02 | 6.9 ± 0.8 |
Reference values: BUN: 7.8–21.4 mg/dl; Creatinine: 0.6–1.5 mg/dl, HbA1C: 4.5–5.7%.
Discussion
This study demonstrated that soybean altered disease progression in rats with diabetic nephropathy. There has been several works concerning some plants may ameliolate diabetic nephropathy. For example, red cabbage, Coscinium fenestratum and Argana spinosa seed extract improved the pathologic findings of diabetic rats (22–24). Soy fed rats had less renal fibrosis and less renal injury. The earliest morphologic abnormalities in diabetic nephropathy are a thickening of the glomerular basement membrane, an expansion of the mesangium and the accumulation of the extracellular matrix. With time, matrix accumulation becomes diffuse and is evident as eosinophilic, PAS positive glomerulosclerosis on renal biopsy (1). In this study, the general morphology of glomeruli and tubulointerstitial lesions of the diabetic rats with the soybean diet was much improved and seemed quite normal in appearance compared with that of diabetic rats. Soybean feeding is known to enhance the conversion of polyunsaturated fatty acids to docosahexaenoic acid (25). Increased production of this complex lipid has been linked to benefits in a variety of inflammatory models and diseases, including renal disease (26).
AQP-1 plays a critical role in the preservation of proximal tubule water handling and urinary concentration. AQP-1 is abundant in the proximal tubule and descending thin limb, where it is essential for constitutive water reabsorption at these sites. It is reported that AQP-1 deficient mice are polyuric and are unable to concentrate their urine in response to water deprivation or vasopressin administration (27). And there is also evidence that AQP-1 is overexpressed gene in diabetic nephropathy (28). It is suggested that renal injury, regardless of etiology, produces increased stress on cell integrity, and increased expression of AQP-1 is an adaptive response to this (29). The changes in AQP-1 immunostaining seen in this collection of rat renal biopsy specimens are similar to those documented in other studies. AQP-1 expression was increased in the diabetic rat kidney. It is an interesting finding that soybeans attenuate renal AQP expression in diabetic kidneys.
In agreement with previous studies, we found OPN expression was increased in the tubular epithelium of the diabetic rat kidney. OPN is a potent chemotactic and adhesive factor for macrophages (30). There is overwhelming evidence that the up-regulation of tubular OPN expression is strongly associated with macrophage infiltration subsequent to tubulointerstitial injury in experimental studies (31) and in human patients with kidney diseases (32). Diabetic nephropathy is not only a glomerular disease but also is characterized by impaired tubular function as well (33). The urinary excretion of low molecular weight proteins and tubular enzymes has been suggested to reflect disturbance and injury of proximal tubules and to precede microalbuminuria (34,35). Interstitial fibrosis occurs during the pathogenesis of diabetic nephropathy and has been shown to correlate with the development of reduced GFR (36). Li et al. suggested that up-regulation of OPN expression may play a role in tubulointerstitial injury associated with diabetic nephropathy and that blockade of the renin angiotensin system by ramipril may confer renoprotection by decreasing OPN expression in non-insulin dependent diabetic nephropathy (37). Since the up-regulation of OPN expression in the tubular epithelium in diabetic rat kidneys was significantly suppressed and the general morphology of glomerulus and tubulointerstitial lesions was much improved by soybean intake, it is tempting to speculate that one of the mechanism of the renoprotective effect of soybeans may be related to decreasing OPN expression in diabetic nephropathy.
In the present study, the level of Hb A1c was lower in the DM-Bean group than in the DM group. A soybean diet improves serum glucose and insulin levels, as well as insulin sensitivity in diabetes (38,39). Although the exact mechanism has yet to be elucidated, it is possible that the soluble fiber component of soybeans may be the most important factor. Approximately 15% of the soybean is composed of insoluble carbohydrates and over 30% of the fiber in soy is of the soluble variety. Moreover, soybeans are slowly digested and have a low glycemic index (40). Since factors implicated as triggers for increased matrix production in DM include the direct effects of hyperglycemia on mesangial cells, advanced glycosylation end-products and cell sorbitol accumulation (1), one of the mechanisms of the renoprotective effect of soybean may be related to glycemic control in diabetic nephropathy.
In conclusion, the results from this study show that soybeans may prevent morphological destruction of kidney due to diabetes mellitus. Further studies are required to determine the exact mechanism of the renoprotective effect of soybeans.
Conclusion
Based on our experiment, there is reason to believe that soybeans may prevent weight loss associated with diabetes mellitus. Also, soybeans may prevent the morphological destruction of the kidney that is associated with diabetes mellitus and may improve glycemic control. We suggest that long-term control of blood glucose levels using a soybean diet may prevent the progression of diabetes mellitus and prevent nephropathy, therefore.
References
- 1.Tuttle KR, Bruton JL, Puresek MC, Lancaster JL, Kopp DT, DeFronzo RA. Effect of strict glycemic control on renal hemodynamic responses to amino acids and renal enlargement in insulin-dependent diabetes mellitus. N Eng J Med. 1991;324:1626–32. doi: 10.1056/NEJM199106063242304. [DOI] [PubMed] [Google Scholar]
- 2.Lane PH, Steffes MW, Fioretto P, Mauer SM. Renal interstitial expansion in insulin-dependent diabetes mellitus. Kidney Int. 1993;43:661–7. doi: 10.1038/ki.1993.95. [DOI] [PubMed] [Google Scholar]
- 3.Bader R, Bader H, Grund KE, Mackensen-Haen S, Christ H, Bohle A. Structure and function of the kidney in diabetic glomerulosclerosis. Correlations between morphological and functional parameters. Pathol Res Pract. 1980;167:204–16. doi: 10.1016/S0344-0338(80)80051-3. [DOI] [PubMed] [Google Scholar]
- 4.Ziyadeh FN. The extracellular matrix in diabetic nephropathy. Am J Kidney Dis. 1993;22:735–42. doi: 10.1016/s0272-6386(12)80440-9. [DOI] [PubMed] [Google Scholar]
- 5.Taft JL, Nolan CJ, Yeung SP, Hewitson TD, Martin FI. Clinical and histological correlations of decline in renal function in diabetic patients with proteinuria. Diabetes. 1994;43:1046–51. doi: 10.2337/diab.43.8.1046. [DOI] [PubMed] [Google Scholar]
- 6.Jennifer J, Bedford, John PL, Robert J. Walker aquaporin expression in normal human kidney and in renal disese. J Am Soc Nephrol. 2003;14:2581–7. doi: 10.1097/01.asn.0000089566.28106.f6. [DOI] [PubMed] [Google Scholar]
- 7.Butler WT. Structural and functional domains of osteopontin. Ann N Y Acad Sci. 1995;760:6–11. doi: 10.1111/j.1749-6632.1995.tb44615.x. [DOI] [PubMed] [Google Scholar]
- 8.Singh RP, Patarca R, Schwartz J, Singh P, Cantor H. Definition of a specific interaction between the early T lymphocyte activation 1 (Eta-1) protein and murine macrophages in vitro and its effect upon macrophages in vivo. J Exp Med. 1990;171:1931–42. doi: 10.1084/jem.171.6.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pichler R, Giachelli CM, Lombardi D, Pippin J, Gordon K, Alpers CE, et al. Tubulointerstitial disease in glomerulonephritis. Potential role of osteopontin. Am J Pathol. 1994;144:915–26. [PMC free article] [PubMed] [Google Scholar]
- 10.Kawano K, Hirachima T, Mori S, Saitoh Y, Kurosumi M, Natori T. Spontaneous long-term hyperglycemic rat with diabetic complications. Otsuka Long-Evans Tokushima Fatty(OLETF) strain. Diabetes. 1992;41:1422–8. doi: 10.2337/diab.41.11.1422. [DOI] [PubMed] [Google Scholar]
- 11.Fischer JW, Tschope C, Reinecke A. Giachelli CM, Unger T. Upregulation of osteopontin expression in renal cortex of streptozotocin-induced diabetic rats is mediated by Bradykinin. Diabetes. 1998;47:1512–8. doi: 10.2337/diabetes.47.9.1512. [DOI] [PubMed] [Google Scholar]
- 12.Fouque D, Laville M, Boissel JP, Chifflet R, Labeeuw M, Zech PY. Controlled low protein diets in chronic renal insufficiency:meta-analysis. Br Med J. 1992;304:216–20. doi: 10.1136/bmj.304.6821.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pedrini MT, Levey AS, Lau J, Chalmers TC, Wang PH. The effect of dietary protein restriction on the progression of diabetic and non-diabetic renal diseases: a meta-analysis. Ann Intern Med. 1996;124:627–32. doi: 10.7326/0003-4819-124-7-199604010-00002. [DOI] [PubMed] [Google Scholar]
- 14.Velasquez MT, Bhathena SJ. Dietary phytoestrogens: a possible role in renal disease protection. Am J Kidney Dis. 2001;37:1056–68. doi: 10.1016/s0272-6386(05)80025-3. [DOI] [PubMed] [Google Scholar]
- 15.D’Amico G, Gentile MG, Manna G, Fellin G, Ciceri R, Cofano F, et al. Effect of vegetarian soy diet on hyperlipidemia in nephrotic syndrome. Lancet. 1992;339:1131–4. doi: 10.1016/0140-6736(92)90731-h. [DOI] [PubMed] [Google Scholar]
- 16.Ogborn MR, Nitschmann E, Weiler HA, Bankovid-Calic N. Modification of polycystic kidney disease and fatty acid status by soy bean diet. Kidney Int. 2000;57:159–66. doi: 10.1046/j.1523-1755.2000.00835.x. [DOI] [PubMed] [Google Scholar]
- 17.Tomobe K, Philbrick DJ, Ogborn MR, Takahashi H, Holub BJ. Effect of dietary soy bean and genistein on disease progression in mice with polycystic kidney disease. Am J Kidney Dis. 1998;31:55–61. doi: 10.1053/ajkd.1998.v31.pm9428452. [DOI] [PubMed] [Google Scholar]
- 18.Teixeira SR, Tappenden KA, Erdman JW. Altering dietary protein type and quantity reduces urinary albumin excretion without affecting plasma glucose concentrations in BKS.cg-m+Lepr db/+Lepr db(db/db) mice. J Nutr. 2003;133:673–8. doi: 10.1093/jn/133.3.673. [DOI] [PubMed] [Google Scholar]
- 19.Jibani M, Bloodworth L, Foden E, Griffiths K. Predominatly vegetarian diet in patients with incipient and early clinical diabetic nephropahty: effects on albumin excretion rate and nutritional stuatus. Diabet Med. 1991;8:949–53. doi: 10.1111/j.1464-5491.1991.tb01535.x. [DOI] [PubMed] [Google Scholar]
- 20.Kontessis P, Bossinakou I, Sarika L, Iliopoulou E, Papantoniou A, Trevisan R, et al. Renal, metabolic, and hormonal responses to proteins of different origin in normotensive, nonproteinuric type 1 diabetic patients. Diabetes Care. 1995;18:1233–40. doi: 10.2337/diacare.18.9.1233. [DOI] [PubMed] [Google Scholar]
- 21.Anderson J, Blake J, Turner J, Smith B. Effects of soy bean on renal function and proteinuria in patients with Type 2 diabetes. Am J Clin Nutr. 1998;68:1347s–53s. doi: 10.1093/ajcn/68.6.1347S. [DOI] [PubMed] [Google Scholar]
- 22.Kataya AH, Hamza AA. Red cabbage (Brassica oleracea) ameliorates diabetic nephropathy in rats. eCAM. 2007;3:1–7. doi: 10.1093/ecam/nem029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Punitha SR, Rajendran K, Shirwaikar A, Shirwaikar A. Alcoholic stem extract of Coscinium fenestratum regulates carbohydrate metabolism and improves antioxidant status in streptozotocin-nicotinamide induced diabetic rats. eCAM. 2005;2:375–81. doi: 10.1093/ecam/neh099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Samane S, Noel J, Charrouf Z, Amarouch H, Haddad PS. Insulin-sensitizing and anti-proliferative effects of Argania Spinosa seed extracts. eCAM. 2006;3:317–27. doi: 10.1093/ecam/nel015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shimokawa I, Higami Y, Hubbard GB, McMahan CE, Masoro EJ, Yu BP. Diet and the suitability of the male Fischer 344 rat as a model for aging research. J Gerontol A Biol Sci Med Sci. 2003;48:B27–32. doi: 10.1093/geronj/48.1.b27. [DOI] [PubMed] [Google Scholar]
- 26.Clark WF, Parbtani A, Philbrick DJ, Spanner E, Huff MW, Holub BJ. Dietary protein restriction versus fish oil supplementation in the chronic remnant nephron model. Clin Nephrol. 1993;39:295–304. [PubMed] [Google Scholar]
- 27.Verkman AS. Physiological importance of aquaporin: lessons from knockout mice. Curr Opin Nephrol Hypertens. 2000;9:517–22. doi: 10.1097/00041552-200009000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Baelde HJ, Eikmans M, Doran PP, Lappin DW, de Heer E, Bruijn JA. Gene expression profiling in glomeruli from human kidneys with diabetic nephropathy. Am J Kidney Dis. 2004;43:636–50. doi: 10.1053/j.ajkd.2003.12.028. [DOI] [PubMed] [Google Scholar]
- 29.Bedford JJ, Leader JP, Walkers RJ. Aquaporin expression in normal human kidney and in renal disease. J Am Soc Nephrol. 2003;14:2581–7. doi: 10.1097/01.asn.0000089566.28106.f6. [DOI] [PubMed] [Google Scholar]
- 30.Weber GF, Ashkar S, Glimcher MJ, Cantor H. Receptor-ligand interaction between CD44 and osteopontin(Eta-1) Science. 1996;271:509–12. doi: 10.1126/science.271.5248.509. [DOI] [PubMed] [Google Scholar]
- 31.Pichler R, Giachelli CM, Lombardi D. Tubulointerstitial disease in glomerulonephritis. Potential role of osteopontin. Am J Pathol. 1994;28:139–50. [PMC free article] [PubMed] [Google Scholar]
- 32.Hudkins KL, Giachelli CM, Eitner F. Osteopontin expression in human crescentic glomerulonephritis. Kidney Int. 2000;57:105–16. doi: 10.1046/j.1523-1755.2000.00813.x. [DOI] [PubMed] [Google Scholar]
- 33.Turner G, Coates P, Warren S, Woodhead JS, Peters JR. Proximal tubular reabsorption of growth hormone and sodium/fluid in normo- and microalbuminuric insulin-dependent diabetes mellitus. Acta Diabetol. 1997;34:27–32. doi: 10.1007/s005920050061. [DOI] [PubMed] [Google Scholar]
- 34.Galanti LM, Jamart J, Dell’omo J, Donckier J. Comparison of urinary excretion of albumin, alpha 1 microglobulin and retinol-binding protein in diabetic patients. Diabetes Metab. 1996;22:324–30. [PubMed] [Google Scholar]
- 35.O’Brien SF, Watts GF, Powrie JK, Shaw KM, Miller NJ. Lipids, lipoproteins, antioxidants and glomerular and tubular dysfunction in type 1 diabetes. Diabetes Res Clin Pract. 1996;32:81–90. doi: 10.1016/0168-8227(96)01252-1. [DOI] [PubMed] [Google Scholar]
- 36.Mauer SM, Steffes MW, Ellis EN, Sutherland DE, Brown DM, Goetz FC. Structural-functional relationships in diabetic nephropathy. J Clin Invest. 1984;74:1143–55. doi: 10.1172/JCI111523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li C, Yang CW, Park CW, Ahn HJ, Kim WY, Yoon KH, et al. Long term treatment with ramipril attenuates renal osteopontin expression in diabetc rat. Kidney Int. 2003;63:454–63. doi: 10.1046/j.1523-1755.2003.00751.x. [DOI] [PubMed] [Google Scholar]
- 38.Iritani N, Sugimoto T, Fukada H, Komiya M, Ikeda H. Dietary soybean protein increases insulin receptor gene expression in Wistar fatty rats when dietary polyunsaturated fatty acid level is low. J Nutr. 1997;127:1077–83. doi: 10.1093/jn/127.6.1077. [DOI] [PubMed] [Google Scholar]
- 39.Lavigne C, Marette A, Jacques H. Cod and soy beans compared with casein improve glucose tolerance and insulin sensitivity in rats. Am J Physiol Endocrinol Metab. 2000;278:491–500. doi: 10.1152/ajpendo.2000.278.3.E491. [DOI] [PubMed] [Google Scholar]
- 40.Jenkins DJ, Wolever TM, Taylor RH, Barker H, Fielden H, Baldwin JM, et al. Glycemic index of foods: a physiological basis for carbohydrate exchange. Am J Clin Nutr. 1981;134:362–6. doi: 10.1093/ajcn/34.3.362. [DOI] [PubMed] [Google Scholar]