Abstract
The chromatin-remodelling complex SNF2-related CBP activator protein (SRCAP) regulates chromatin structure in yeast by modulating the exchange of histone H2A for the H2A.Z variant. Here, we have investigated the contribution of H2A.Z-mediated chromatin remodelling to mammalian cell differentiation reprogramming. We show that the SRCAP subunit named ZNHIT1 or p18Hamlet, which is a substrate of p38 MAPK, is recruited to the myogenin promoter at the onset of muscle differentiation, in a p38 MAPK-dependent manner. We also show that p18Hamlet is required for H2A.Z accumulation into this genomic region and for subsequent muscle gene transcriptional activation. Accordingly, downregulation of several subunits or the SRCAP complex impairs muscle gene expression. These results identify SRCAP/H2A.Z-mediated chromatin remodelling as a key early event in muscle differentiation-specific gene expression. We also propose a mechanism by which p38 MAPK-mediated signals are converted into chromatin structural changes, thereby facilitating transcriptional activation during mammalian cell differentiation.
Keywords: histone H2A.Z, myogenesis, p38 MAPK, SRCAP
Introduction
Cell lineage specification from pluripotent stem cells is central for development and organ formation. This process requires modifications throughout the genome (Cui et al, 2009) to allow expression or silencing of particular sets of genes. At the molecular level, this choice is brought about by specific changes in chromatin (de la Serna et al, 2006; Kouzarides, 2007). In fact, defined epigenetic changes characterize terminally differentiated cell types. Skeletal muscle differentiation-specific gene expression is coordinately regulated by transcription factors of the MyoD and MEF2 families and by chromatin-remodelling factors (Sartorelli and Caretti, 2005; Perdiguero et al, 2009). In particular, recruitment of the SWI/SNF ATP-ase Brg1 and the trithorax (TrxG) methyltransferase Ash2L to the regulatory regions of muscle genes is necessary for their expression (Simone et al, 2004; Rampalli et al, 2007).
Transcriptional activation may be also regulated by the replacement of canonical histones with histone variants. In yeast, the replacement of histone H2A by H2A.Z has been proposed to positively regulate gene transcription (Santisteban et al, 2000; Adam et al, 2001), and the majority of euchromatic gene promoters have been found to be enriched in H2A.Z (Raisner et al, 2005). In addition, yeast H2A.Z has been shown to antagonize the spread of the heterochromatin (mediated by Sir2 and Sir3), therefore acting as an anti-silencing factor (Meneghini et al, 2003). Genetic and biochemical approaches have further shown that the exchange of histone H2A.Z in yeast is specifically catalysed by the SWR1 chromatin-remodelling complex (Krogan et al, 2003; Meneghini et al, 2003; Kobor et al, 2004). Notably, H2A.Z is a highly conserved protein that has been found in many organisms from protozoan parasites to plants and humans. A role for H2A.Z in gene activation has also been documented in Caenorhabditis elegans, where H2A.Z is recruited to foregut promoters at the time of transcription onset and together with the transcription factor PHA-4 coordinates temporal gene expression (Updike and Mango, 2006). In mammals, H2A.Z is essential for embryonic development and chromosome segregation (Faast et al, 2001; Rangasamy et al, 2004). Interestingly, high-resolution profiling analysis of histone modifications in the human genome has shown the association of H2A.Z with functional regulatory elements, close to transcription start sites (Barski et al, 2007). There are two mammalian Swr1-like complexes that have been proposed to regulate H2A.Z deposition in cells, one is p400 (Gevry et al, 2007, 2009) and the other is Snf2-related CBP (CREB-binding protein) activator protein (SRCAP) (Wong et al, 2007). Moreover, SRCAP has been reported to catalyse the incorporation of H2A.Z into chromatin in vitro (Ruhl et al, 2006). However, little is known about the recruitment and function of H2A.Z at mammalian promoters during genomic reprogramming towards terminal cell differentiation. A recent report has shown increased levels of H2A.Z in genes that lose the H3K27me3 mark and become activated during differentiation of multipotent human primary hematopoietic stem cells into erythrocyte precursors (Cui et al, 2009).
We have characterized a protein named ZNHIT1 or p18Hamlet as a substrate of p38α and p38β MAPKs, which mediates p53-dependent transcriptional responses to genotoxic stress (Cuadrado et al, 2007; Lafarga et al, 2007). ZNHIT1/p18Hamlet has been also identified as a subunit of the human SRCAP complex (Cai et al, 2005; Sardiu et al, 2008). The p38 MAPK pathway is critical for the activation of the muscle differentiation gene program (Lluis et al, 2006), which involves the p38 MAPK-regulated recruitment of the SWI/SNF and TrxG chromatin-remodelling complexes to muscle-specific loci (Simone et al, 2004; Rampalli et al, 2007). Here, we show that p18Hamlet and the SRCAP complex regulate muscle differentiation. Our results show an important role for SRCAP and histone H2A.Z incorporation in the initiation of the muscle-specific gene expression program, through the recruitment of the p38 MAPK-regulated p18Hamlet protein to muscle promoters, ensuring the changes in chromatin structure necessary for transcriptional activation.
Results
p18Hamlet is upregulated during muscle differentiation in a p38 MAPK-dependent manner
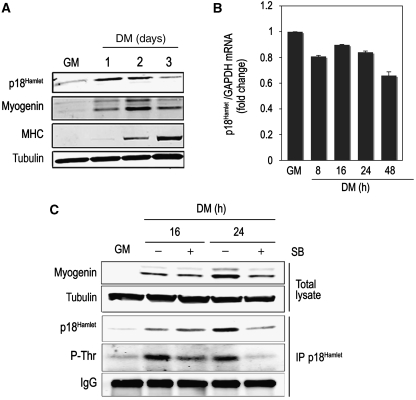
To analyse the potential contribution of the p38 MAPK substrate p18Hamlet to skeletal muscle differentiation, we first investigated its expression pattern in C2C12 myoblasts. We found that p18Hamlet protein levels increased early during the differentiation process (Figure 1A), whereas p18Hamlet mRNA levels were very similar in undifferentiated and differentiated myoblasts (compare growth medium (GM) with differentiation medium (DM)) (Figure 1B). Moreover, the p38α and p38β chemical inhibitor SB203580 inhibited the accumulation of p18Hamlet (Figure 1C), confirming the relationship between p38 MAPK activation and the stabilization of the p18Hamlet protein (Cuadrado et al, 2007). Furthermore, p18Hamlet was phosphorylated during myoblast differentiation in a p38 MAPK-dependent manner (Figure 1C). Altogether, these data link p38 MAPK activation with the phosphorylation and accumulation of p18Hamlet during skeletal myogenesis.
Figure 1.
p18Hamlet protein levels increase during muscle differentiation in a p38 MAPK-dependent manner. (A) p18Hamlet, myogenin and MHC protein levels were analysed by immunoblotting in proliferating C2C12 myoblasts (GM) and during the differentiation process (DM). Tubulin was used as a loading control. (B) p18Hamlet mRNA levels were analysed by qRT–PCR and normalized to GAPDH at the indicated time points during C2C12 differentiation. Data are shown as means±s.d. of samples from a representative experiment assayed in triplicates. (C) C2C12 myoblasts were incubated in DM for the indicated times, either in the absence or presence of SB203580 (SB). Endogenous p18Hamlet was immunoprecipitated and analysed by immunoblotting with phospho-Thr and p18Hamlet antibodies. Myogenin levels in total lysates were analysed by immunoblotting. Tubulin and IgG were used as loading controls in total lysates and immunoprecipitates, respectively.
Recruitment of p18Hamlet and H2A.Z to the myogenin promoter at early stages of muscle differentiation
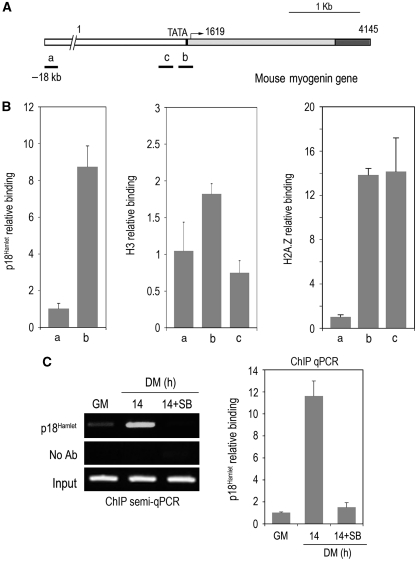
The yeast homolog of p18Hamlet, Vps71/Swc6, is essential for histone H2A.Z exchange catalysed by the SRW1 complex, enabling the association of the catalytic ATP-ase and histone H2A.Z interacting subunits (Wu et al, 2005). The Arabidopsis p18Hamlet homolog SEF is also required for the exchange of histone H2A for H2A.Z at the FLC promoter, which precedes FLC transcription (Deal et al, 2007; March-Diaz et al, 2007). However, the involvement of this chromatin-remodelling mechanism in mammalian cell differentiation remains unknown. Transcriptional activation of the myogenin gene is one of the earliest steps necessary for reprogramming undifferentiated myoblasts into fully differentiated muscle cells. We therefore investigated the potential binding of p18Hamlet and H2A.Z to the myogenin promoter at the onset of myoblast differentiation by using chromatin immunoprecipitation (ChIP) and quantitative PCR assays. First, we found that both proteins were highly enriched at the TATA box-containing region of the myogenin promoter compared with its binding to a non-coding DNA sequence located 18 kb upstream of the promoter, whereas histone H3 concentration was similar in the regions studied (Figure 2A and B). Moreover, the amount of p18Hamlet at the TATA box of the myogenin promoter substantially increased early in the differentiation process (Figure 2C). Importantly, the p38α and p38β inhibitor SB203580 impaired the recruitment of p18Hamlet to the myogenin TATA box (Figure 2C).
Figure 2.
p18Hamlet accumulates at the myogenin promoter at early stages of muscle differentiation. (A) Schematic representation of the mouse myogenin gene indicating the TATA box and the transcription start site. The positions of the DNA fragments amplified with three different pairs of primers are indicated. ‘a' refers to a non-coding region located 18 kb upstream, ‘b' to the TATA box-containing region, and ‘c' to a region close to the TATA box of the myogenin promoter. (B) ChIP analysis of p18Hamlet, Histone H3 and H2A.Z binding to the genomic regions ‘a', ‘b' and ‘c' of the myogenin promoter. qPCR data are shown as means±s.d. of samples from a representative experiment assayed in triplicates. In each case, relative binding to the ‘b' and ‘c' regions is referred to the binding to the ‘a' region, which is given the value of 1. (C) p18Hamlet binding to the myogenin promoter (region ‘b') was assayed by ChIP in proliferating C2C12 myoblasts (GM) and after 14 h in DM, either in the presence or absence of SB203580 (SB), as indicated. Both semiquantitative (left panel) and quantitative (right panel) PCRs were performed.
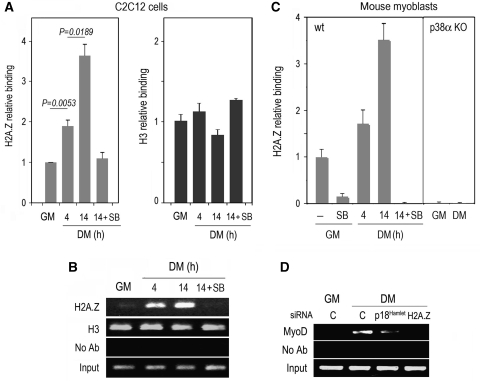
We then analysed whether p18Hamlet recruitment correlated with H2A.Z incorporation at the myogenin promoter during the differentiation process. We found that H2A.Z was already detectable at the myogenin TATA box under basal (GM) conditions (Figures 3B and 4B). Furthermore, H2A.Z began to significantly accumulate at this genomic location as soon as 4 h after the induction of differentiation, and the accumulation was more evident after 14 h (Figure 3A and B; Supplementary Figure S1). As a control, histone H3 binding to the myogenin promoter remained constant during the same kinetics. Interestingly, the p38α and p38β inhibitor SB203580 impaired H2A.Z incorporation at the myogenin TATA box (Figure 3A and B). To determine the physiological relevance of these observations, we analysed the presence of H2A.Z at the myogenin TATA box of mouse primary myoblasts under both basal and differentiation conditions (Figure 3C). We detected a significant amount of H2A.Z bound to this genomic region under GM conditions, and this binding was abrogated by SB203580. Moreover, H2A.Z accumulated at the myogenin TATA box during the differentiation of primary myoblasts to the same extent as in C2C12 cells. Importantly, p38α−/− primary myoblasts lacked H2A.Z at the myogenin promoter (Figure 3C), and the reintroduction of p38α in p38α−/− myoblasts partially restored H2A.Z binding to the myogenin promoter under differentiation conditions, and subsequent myogenin gene expression (Supplementary Figure S2). Overall, these data strongly support that during the early phases of muscle differentiation, histone H2A.Z accumulates at the myogenin TATA box in a p38 MAPK-dependent manner.
Figure 3.
Histone H2A.Z accumulates at the myogenin promoter early during muscle differentiation in a p18Hamlet-dependent manner. (A) ChIP analysis of histones H2A.Z and H3 binding to the TATA box-containing region of the myogenin promoter in proliferating C2C12 myoblasts (GM) and at early times during muscle differentiation (DM) either in the presence or absence of SB203580 (SB). qPCR data are shown as means±s.d. of two independent experiments performed in triplicates. Histone-binding values are normalized to the respective input DNA and are referred to the binding of each histone under GM conditions, which is given the value of 1. (B) C2C12 myoblasts were cultured as indicated in (A), and the binding of H2A.Z, and H3 to the myogenin TATA box was analysed by ChIP followed by semiquantitative PCR. (C) Mouse wt and p38α−/− (KO) primary myoblasts were grown in GM or DM in the presence or absence of SB203580 (SB) for the indicated times. H2A.Z binding to the myogenin TATA box-containing region was analysed by ChIP and qPCR. Relative-binding values are referred to the binding in wt myoblasts under GM conditions, which is given the value of 1. (D) C2C12 myoblasts were incubated with either p18Hamlet or H2A.Z siRNAs and then cultured in GM or incubated in DM for 14 h. MyoD binding to the myogenin TATA box region was analysed by ChIP followed by semiquantitative PCR. Analysis by qPCR of the same samples is shown in Supplementary Figure S3.
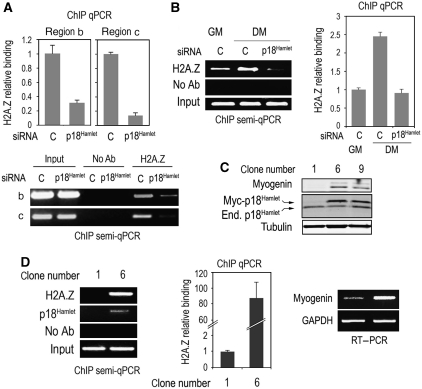
Figure 4.
p18Hamlet mediates histone H2A.Z binding to chromatin and induces muscle-specific gene expression. (A) C2C12 myoblasts were cultured in growing conditions and transfected with control (C) or p18Hamlet siRNA, and 72 h later H2A.Z binding to the indicated regions of the myogenin promoter (see Figure 2A) was analysed by ChIP and qPCR. Semiquantitative PCR analysis was also performed with samples of the same regions. (B) C2C12 myoblasts were incubated in DM for 14 h and the effect of p18Hamlet downregulation on H2A.Z binding to the myogenin promoter was analysed by ChIP and both semiquantitative and quantitative PCRs. (C) C2C12 myoblasts were transfected with a Myc-tagged p18Hamlet expression construct and clones were selected with G418. Myogenin and both endogenous and Myc-tagged p18Hamlet protein levels were analysed by immunoblotting in the indicated clones. (D) Binding of H2A.Z to the myogenin TATA box-containing region was determined by ChIP assays and both semiquantitative and quantitative PCR in control (number 1) and p18Hamlet overexpressing (number 6) C2C12 clones (left and middle panels). Myogenin mRNA levels were estimated by semiquantitative RT–PCR (right panel).
Finally, we investigated the importance of p18Hamlet recruitment and H2A.Z exchange for the assembly of the muscle-specific transcriptosome. One of the key pieces of this machinery is the transcription factor MyoD, which binds to the myogenin promoter at early stages during muscle differentiation (Berkes and Tapscott, 2005). Downregulation of either p18Hamlet or, specially, H2A.Z had a dramatic effect on MyoD binding to the myogenin promoter (Figure 3D; Supplementary Figure S3), suggesting that H2A.Z incorporation into chromatin is an essential step that precedes the transcriptional events taking place early during the process of muscle differentiation. It should be noted that p18Hamlet downregulation did not affect H2A.Z expression, and H2A.Z downregulation did not alter p18Hamlet protein levels (Supplementary Figure S4).
p18Hamlet requirement for H2A.Z recruitment at the myogenin promoter
Next, we investigated the relationship between p18Hamlet function and H2A.Z localization to chromatin using two different approaches. First, we designed siRNAs that reduced p18Hamlet levels in C2C12 cells by about 80% (Supplementary Figure S5). We observed that p18Hamlet downregulation abolished both basal (Figure 4A) as well as the differentiation-induced (Figure 4B) H2A.Z binding to the myogenin promoter. As a complementary experiment, we overexpressed p18Hamlet in C2C12 myoblasts grown in GM, and found a good correlation between p18Hamlet upregulation and myogenin induction (Figure 4C). Moreover, the myogenin protein levels in these clones were similar to those observed on the induction of differentiation in C2C12 myoblasts with DM (Supplementary Figure S6). Notably, forced expression of p18Hamlet promoted the recruitment of H2A.Z to the myogenin promoter in the differentiation-inhibitory conditions of GM, which correlated with enhanced transcription of myogenin (Figure 4D). These results indicate that p18Hamlet is required for H2A.Z recruitment to myogenin chromatin.
p38 MAPK-mediated p18Hamlet phosphorylation is required for myogenin induction and H2A.Z deposition at the myogenin promoter
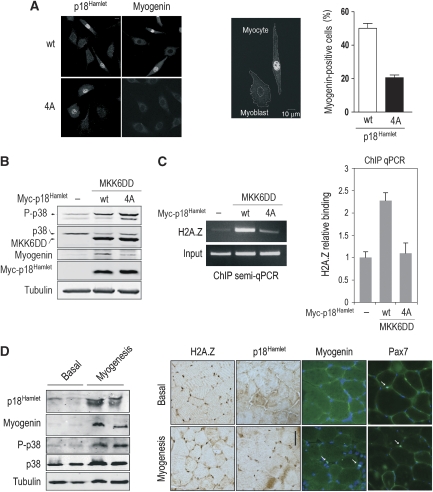
To determine the importance of p18Hamlet phosphorylation for its ability to activate myogenin transcription, we compared the effect of expressing at similar levels either wild type (wt) or a non-phosphorylatable p18Hamlet mutant (4A) in C2C12 cells grown in GM. By using both biochemical (myogenin expression) and morphological (cell shape) parameters, we observed that the p18Hamlet 4A mutant was less competent to activate myogenesis than its wt counterpart (Figure 5A). Indeed, ectopic expression of p18Hamlet wt in C2C12 myoblasts induced the expression of myogenin in 47% of the transfected cells in GM. In contrast, when cells were transfected with the non-phosphorylatable form of p18Hamlet, <20% of the transfected cells were positive for myogenin (Figure 5A). Furthermore, the myoblast elongation typical of prefusing myocytes was exclusively observed in p18Hamlet wt-transfected cells in GM differentiation-restricting conditions (Figure 5A). To further show that p38 MAPK-mediated phosphorylation of p18Hamlet is required for H2A.Z recruitment at the myogenin promoter, we ectopically activated p38 MAPK signalling in C2C12 myoblasts by cotransfection of constitutively activated MKK6DD (a specific p38 MAPK activator) with either p18Hamlet wt or the 4A mutant. MKK6DD in combination with overexpressed wt p18Hamlet was sufficient to induce myogenin expression under GM conditions to a much higher extent than the p18Hamlet 4A mutant (Figure 5B). Moreover, the non-phosphorylable p18Hamlet mutant was unable to mediate p38 MAPK-dependent enrichment of H2A.Z at the myogenin promoter (Figure 5C). Altogether, these results strongly support that accumulation and phosphorylation of p18Hamlet is sufficient to induce histone H2A.Z recruitment into promoters of differentiation-regulated mammalian genes. Consistent with this idea, using a model of de novo induced myogenesis in adult mouse skeletal muscle, we found upregulation of p18Hamlet expression during myogenesis in vivo, correlating with p38 MAPK activation and the induction of myogenin (Figure 5D).
Figure 5.
Accumulation and phosphorylation of p18Hamlet are necessary for H2A.Z chromatin enrichment and myogenin expression. (A) C2C12 myoblasts were transfected with plasmids encoding either wt or a non-phosphorylatable version (4A) of Myc-tagged p18Hamlet. Expression of p18Hamlet, myogenin induction and myocyte morphological changes were analysed by immunofluorescence (left panel) in at least 300 cells. The efficiency of transfection was 28 and 20% for the wt and 4A p18Hamlet constructs, respectively. The percentage of myogenin-positive cells for each transfection is represented (right panel). (B) C2C12 myoblasts were transfected with either MKK6DD alone or in combination with Myc-tagged p18Hamlet wt and 4A mutant, and 24 h later, the total lysates were analysed by immunoblotting using the indicated antibodies. Tubulin was used as loading control. Transfection efficiency was about 50%. (C) Chromatin from C2C12 myoblasts transfected as in (B) was collected 24 h after transfection, and H2A.Z binding to the myogenin TATA box-containing region was assayed by ChIP. Both semiquantitative and quantitative PCRs are shown. (D) Upregulation of p18Hamlet expression during myogenic differentiation in vivo correlates with p38 MAPK activation and myogenin induction. Extracts of plantaris muscles either in basal state or undergoing de novo myogenesis (3 days after overloading) were analysed by immunoblotting using the indicated antibodies. Myogenin is a marker of differentiating satellite cells/myoblasts (left panel). Sections of mouse plantaris muscles as above were immunostained with the indicated antibodies and detected with peroxidase (H2A.Z and p18Hamlet) or immunofluorescence (Myogenin and Pax7). Nuclei were detected with DAPI. Pax7 identifies satellite cells/myoblasts present both in basal and overloading conditions. Myogenin-positive satellite cells are exclusively detected in muscle with ongoing myogenesis/differentiation (right panel). Bar=50 μm.
p18Hamlet/SRCAP-mediated H2A.Z recruitment is necessary for muscle differentiation
Mammalian SRCAP is a multiprotein complex whose components have been well characterized in yeast. Five of these components are essential for nucleosomal histone H2A for histone H2A.Z exchange as well as for complex integrity, including the catalytic subunit Swr1 (mammalian SRCAP), the H2A.Z-binding protein Swc2 (mammalian YL1), and two small proteins necessary for complex formation and nucleosome binding, Arp6 and Swc6 (p18Hamlet) (Wu et al, 2005).
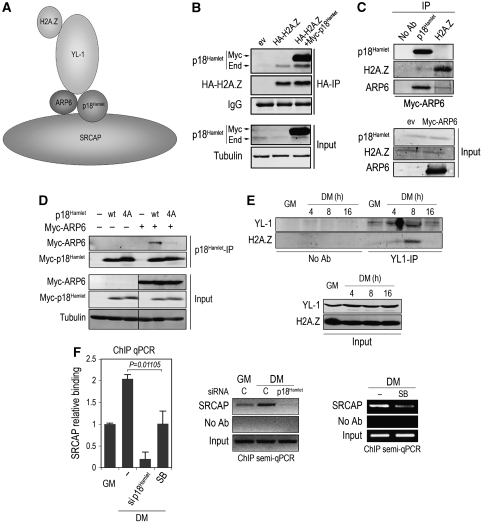
Although several groups have reported ZNHIT1/p18Hamlet as a member of the mammalian SRCAP complex, its specific role remains unclear. On the basis of the observed interactions between the essential components of the yeast SWR1 complex (Figure 6A), and due to the high homology of these proteins with their mammalian counterparts, we performed immunoprecipitation experiments to determine the ability of p18Hamlet to interact with other SRCAP complex members. We observed a clear interaction between p18Hamlet and H2A.Z when either both or only one of the proteins were overexpressed (Figure 6B). However, the interaction between the endogenous proteins, although detectable, was weak, consistent with the yeast model proposing that such interaction is not direct (Wu et al, 2005). Interestingly, we found that human Arp6 bound strongly to p18Hamlet (Figure 6C). To determine the importance of p18Hamlet phosphorylation for its interaction with Arp6, we transfected C2C12 cells with similar amounts of p18Hamlet wt or the mutant 4A. We observed that the interaction with Arp6 was markedly impaired in the case of the p18Hamlet 4A mutant (Figure 6D), indicating that p18Hamlet phosphorylation has an essential role in its interaction with Arp6 and presumably SRCAP complex integrity. According to the proposed model, H2A.Z recruitment to the SRCAP complex is mediated through its binding to the YL-1 subunit. To validate this hypothesis in the context of myogenesis, we analysed the coimmunoprecipitation of H2A.Z and YL-1 under GM and DM conditions. We found that H2A.Z binding to YL-1 was very low under proliferation conditions and increased at early stages of myogenesis, to dramatically decrease after 16 hours in differentiation media (Figure 6E). This result suggests that H2A.Z binding to the SRCAP complex occurs during muscle differentiation and that it is a dynamic process. To further support a role for SRCAP in H2A.Z recruitment at the myogenin promoter, we performed ChIP analysis of the SRCAP catalytic subunit in C2C12 cells under both proliferation and differentiation conditions, and found that SRCAP was already bound to chromatin in GM and accumulated in DM (Figure 6F). Interestingly, DM-induced binding of SRCAP to the myogenin TATA box-containing region was impaired both in the absence of p18Hamlet or upon p38 MAPK inhibition (Figure 6F).
Figure 6.
p18Hamlet phosphorylation is required for its interaction with Arp6, and regulation of H2A.Z binding to YL-1 during myogenesis. (A) Proposed interactions between essential components of SWR1, the yeast homolog of the SRCAP complex. (B) U2OS cells were transfected with 1 μg of HA-tagged H2A.Z and Myc-p18Hamlet expression vectors, and 48 h later, the total lysates were immunoprecipitated (IP) with HA antibodies to evaluate the interaction of H2A.Z with both overexpressed (Myc) and endogenous (End) p18Hamlet. Input represents 10% of the total lysate used for the IPs. (C) U2OS cells were transfected with 1 μg of either empty vector or a Myc-tagged Arp6 expression vector, and the total lysates were analysed by IP of p18Hamlet or H2A.Z endogenous proteins followed by Myc immunoblotting. Input corresponds to 5% of the total lysates used for the IPs. (D) C2C12 cells were transfected with p18Hamlet wt and mutant 4A alone or in combination with Myc-Arp6, and 48 h later, the total lysates were IP with a p18Hamlet antibody followed by Myc immunoblotting. Input corresponds to 10% of the total lysate used for IPs. (E) C2C12 cells were cultured in GM or in DM for the indicated times, and the total lysates were analysed by IP with an YL-1 antibody followed by H2A.Z immunoblotting. Input corresponds to 5% of the total lysates used for the IPs. (F) The presence of SRCAP in the myogenin TATA box-containing region was analysed by ChIP using chromatin obtained from C2C12 cells grown in GM or after 14 h in DM and either treated with p18Hamlet siRNA or SB203580 as indicated. Both quantitative and semiquantitative PCR analysis are shown.
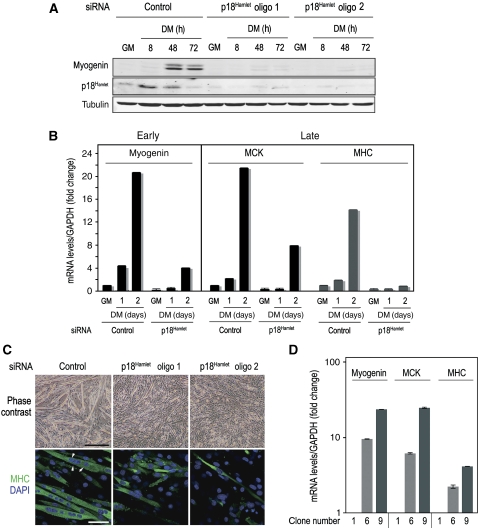
To assess the importance of the p18Hamlet/SRCAP-mediated chromatin remodelling in skeletal muscle differentiation, we analysed the consequences of downregulating p18Hamlet, YL-1, SRCAP and H2A.Z on muscle gene expression and myoblast fusion. Downregulation of p18Hamlet prevented the DM-induced myogenin expression (Figure 7A), in agreement with the impaired recruitment of H2A.Z observed at the myogenin promoter (see above, Figure 5A and B). Induction of late muscle differentiation-specific genes, such as myosin heavy chain (MHC) and muscle creatine kinase (MCK), was also impaired on p18Hamlet depletion in differentiation-promoting conditions (Figure 7B; Supplementary Figure S7). Moreover, myoblasts treated with two different p18Hamlet siRNAs remained undifferentiated, showing a severe defect in their ability to fuse into long multinucleated myotubes, even after 4 days in DM (Figure 7C). Conversely, overexpression of p18Hamlet sufficed to induce the expression of myogenin, MCK and MHC in proliferating C2C12 myoblasts (Figure 7D). These results indicate an essential role of the SRCAP component p18Hamlet in skeletal muscle differentiation.
Figure 7.
p18Hamlet downregulation prevents differentiation of C2C12 myoblasts, and its overexpression is sufficient to induce muscle-specific gene expression. (A) C2C12 myoblasts were transfected with control or two different p18Hamlet siRNAs, and 48 h later were seeded at 70% of confluency and incubated for up to 3 days in DM. Expression levels of p18Hamlet, myogenin and tubulin proteins were analysed by immunoblotting. (B) RNA was purified from C2C12 myoblasts that were transfected with control or p18Hamlet-oligo 1 siRNAs and then maintained in DM for the indicated times or in GM. Myogenin (early gene), and MCK and MHC (late genes) mRNA levels were analysed by qRT–PCR. GAPDH mRNA levels were used for normalization. The histogram shows values of triplicate qRT–PCR reactions. Error bars represent s.d. Each experiment was performed at least twice with independent RNA samples and yielded similar results. (C) Downregulation of p18Hamlet abrogates the formation of the characteristic multinucleated muscle fibres (upper panel). Bar is 250 μm. Formation of muscle fibres was confirmed by immunofluorescence with MHC antibodies. White arrowheads indicate the presence of multiple nuclei (stained with DAPI) in each fibre (lower panel). Bar is 10 μm. (D) C2C12 myoblasts were transfected with a p18Hamlet expression vector and incubated with G418 to select clones (see Figure 4C). The mRNA levels of the indicated early and late muscle differentiation markers were analysed by qRT–PCR and normalized to GAPDH. Average values of triplicate qRT–PCR are shown. Error bars represent s.d.
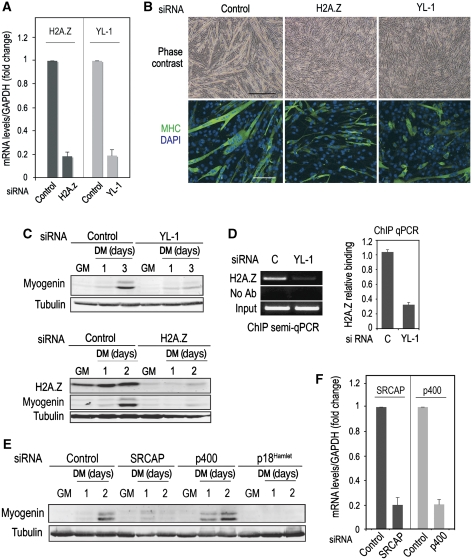
Downregulation of either YL-1 or H2A.Z also blocked the expression of muscle-specific genes as well as the fusion of myoblasts into myotubes (Figure 8A–C), strongly supporting that the function of p18Hamlet in muscle differentiation is connected with H2A.Z recruitment mediated by SRCAP. Accordingly, reduced YL1 expression severely impaired H2A.Z recruitment to myogenin promoter (Figure 8D). As the p400 chromatin-remodelling complex has been also proposed to incorporate H2A.Z into mammalian chromatin (Gevry et al, 2007), we investigated its possible implication in muscle differentiation. We downregulated (about 80%) the expression of either SRCAP or p400 catalytic subunits in C2C12 cells and found that reduced SRCAP, but not p400, levels significantly impaired myogenin induction under differentiation conditions (Figure 8E and F).
Figure 8.
Mammalian SRCAP is required for muscle differentiation. (A) C2C12 myoblasts were transfected with siRNAs to downregulate YL-1 or H2A.Z expression. Cyclophylin B siRNA was used as a control. The efficiency of siRNA-induced target mRNA downregulation was analysed 48 h after transfection by qRT–PCR. (B) C2C12 myoblasts were transfected with the indicated siRNAs and 48 h after transfection, cells were placed in DM. The formation of muscle fibres was analysed 4 days later, both by phase contrast microscopy (upper panel, bar=250 μm) and by immunofluorescence with MHC antibodies (lower panel, bar=30 μm). (C) C2C12 myoblasts were transfected with H2A.Z or YL-1 siRNAs and either cultured in GM or incubated in DM for the indicated times. Total cell lysates were analysed by immunoblotting with the indicated antibodies. (D) C2C12 myoblasts were cultured in GM and transfected with either control or YL-1 siRNAs. Chromatin was obtained 72 h after transfection, and H2A.Z binding to the myogenin-containing TATA region was assayed by ChIP. Both quantitative and semiquantitative PCR results are shown. (E) C2C12 myoblasts were transfected with siRNAs to downregulate the expression of SRCAP and p400 ATPases and cultured in GM or incubated in DM for the indicated times. Myogenin expression was analysed by immunoblotting using tubulin as a loading control. (F) C2C12 myoblasts were transfected with SRCAP or p400 siRNAs and the expression of the target mRNAs was analysed 48 h after transfection by qRT–PCR.
Discussion
Understanding the mechanisms that determine how undifferentiated cells activate genes at the appropriate time and place to generate different cell types is a key question in Biology. The chromatin environment that defines the specific transcription factors bound to chromatin, the composition and enzymatic activities of chromatin-bound protein complexes and the chromatin architecture in a certain genomic region determines whether a specific gene will be transcribed or not. During muscle differentiation, a complex series of chromatin remodelling and modification events take place and, as a result, the chromatin conformation of undifferentiated myoblasts becomes permissive for transcription at the onset of the differentiation program. Our results indicate that p18Hamlet-mediated incorporation of the H2A.Z histone variant into the myogenin promoter is an essential early step in myogenesis. We also show that the p18Hamlet effect on H2A.Z deposition is regulated by p38 MAPK, further supporting the essential role of this pathway in muscle differentiation. These results show an important role of the SRCAP chromatin-remodelling complex and H2A.Z exchange in a mammalian cell differentiation program.
In agreement with the enrichment of H2A.Z at promoter regions of human genes that are actively transcribed (Barski et al, 2007), we have found that H2A.Z accumulates in the TATA box region of the myogenin promoter at early stages of muscle differentiation. It should be noted that H2A.Z is already bound to this genomic region in the repressed state before the induction of differentiation. This result is consistent with recent studies showing that H2A.Z is present at promoters of inducible genes under basal expression conditions marking them for transcriptional activation; for example, in the case of H2A.Z enrichment at the glucocorticoid receptor binding sites in both constitutive and hormone-induced gene promoters (John et al, 2008). Moreover, a recent study of the chromatin signatures that characterize multipotent human hematopoietic stem cells indicate that certain genes that become activated during differentiation are associated with increased levels of H2A.Z (Cui et al, 2009). The role of H2A.Z in transcription regulation is probably related to chromatin changes caused by H2A substitution. Structural studies on H2A and H2A.Z-containing nucleosomes support a role for H2A.Z in reducing chromatin compactness, thereby facilitating the subsequent binding of transcription factors (Suto et al, 2000; Zlatanova and Thakar, 2008). Consistent with this idea, we found that H2A.Z incorporation into chromatin precedes the recruitment of the transcription factor MyoD. Interestingly, the yeast histone H2A.Z variant Htz1 protects genes from the silencing activity of the Sir2 and Sir3 histone deacetylases (Meneghini et al, 2003). H2A.Z and Sir2 have also opposing roles during cardiomyocyte hypertrophy. H2A.Z is essential for the expression of growth-related genes such as CDK7 in the hypertrophyc heart, whereas Sir2-mediated deacetylation results in ubiquitination and degradation of H2A.Z (Chen et al, 2006). As Sir2 inhibits muscle gene expression and differentiation (Fulco et al, 2003), it is tempting to propose that H2A.Z recruitment might also contribute to myogenesis by counteracting the Sir2 deacetylase activity at the differentiation onset.
We have shown that p18Hamlet, a key subunit of the SRCAP complex, is crucial for the transcription of muscle differentiation-specific genes such as myogenin, MHC and MCK. Several pieces of evidence support that p18Hamlet regulates H2A.Z deposition on chromatin during muscle differentiation. First, p18Hamlet and H2A.Z showed similar patterns of binding to specific genomic regions. Second, p18Hamlet downregulation almost totally abrogates H2A.Z basal binding as well as its differentiation-induced incorporation into the myogenin promoter. Third, p18Hamlet overexpression is sufficient by itself to enhance H2A.Z binding to the myogenin promoter, which in turn correlates with increased myogenin transcription. Our results therefore indicate a key role for p18Hamlet in histone H2A.Z exchange in mammalian cells, extending earlier observations in Saccharomyces cerevisiae (Wu et al, 2005) and Arabidopsis (Deal et al, 2007; March-Diaz et al, 2007). These results are also in agreement with a recent study showing that SRCAP downregulation impairs H2A.Z binding to several gene promoters in human adenocarcinoma cells (Wong et al, 2007). Interestingly, we found no evidence that SRCAP regulates expression of the cell-cycle inhibitor p21Cip1 (data not shown), whose levels also increase during muscle differentiation, suggesting a specific effect of this chromatin-remodelling complex on muscle differentiation-specific genes. Consistent herewith, differentiation-induced p21Cip1 expression is not affected by p38α inactivation in primary myoblasts (Perdiguero et al, 2007).
Our results provide several lines of evidence supporting a role for the SRCAP complex in myogenesis. First, the behaviour of p18Hamlet and the SRCAP catalytic subunit in terms of myogenin promoter recruitment is very similar. In addition, H2A.Z binding to YL-1, the SRCAP complex histone-binding subunit, is modulated early during myoblast differentiation. Finally, loss of function experiments confirm that several SRCAP complex subunits, including p18Hamlet, YL-1, SRCAP and H2A.Z itself are required for proper establishment of the muscle-specific expression program.
In mammals, two Swr1-like complexes have been reported to catalyse the incorporation of H2A.Z into chromatin. One of these complexes is p400, which has been proposed to participate in the targeting of H2A.Z to the p21Cip1 gene promoter, thereby suppressing its activation by p53 (Gevry et al, 2007). This work supports a suppressor role of H2A.Z in transcriptional regulation. However, p400-mediated H2A.Z chromatin incorporation has also been reported to be essential for estrogen receptor induced transcription (Gevry et al, 2009), in agreement with our results showing that H2A.Z recruitment into chromatin is necessary for the activation of specific transcriptional programs. The second complex that can exchange H2A for H2A.Z is SRCAP, whose depletion has been reported to impair H2A.Z binding to chromatin (Wong et al, 2007). Our work supports different roles for p400 and SRCAP chromatin-remodelling complexes in skeletal muscle differentiation.
p18Hamlet protein levels are low in proliferating myoblasts, but significantly increase during differentiation. In agreement with our earlier findings, the upregulation of p18Hamlet during muscle differentiation correlates with its phosphorylation by p38 MAPK. Notably, the correlation between p18Hamlet accumulation and the activation of p38 MAPK is also observed during myogenic differentiation in vivo. Importantly, ectopic activation of p38 MAPK signalling in proliferating myoblasts promotes H2A.Z recruitment at myogenin promoter in the presence of wt but not the non-phosphorylable p18Hamlet mutant. Moreover, inhibition of p38 MAPK impairs H2A.Z exchange at the myogenin promoter. Our results suggest that p18Hamlet phosphorylation by p38 MAPK is likely to be important for the binding of this protein to the SRCAP complex through Arp6. This illustrates a new mechanism by which extracellular signals can regulate gene expression changes during cell differentiation, namely the phosphorylation of an SRCAP subunit by p38 MAPK leads to the induction of H2A.Z-mediated chromatin remodelling at specific loci. Altogether, our results indicate that SRCAP-mediated histone H2A.Z deposition has a key role ensuring the proper chromatin structure necessary for myogenesis-specific transcriptional activation.
Materials and methods
Cell culture, transfection and retroviral infection
C2C12 murine myoblasts were obtained from the European Collection of Cell Cultures and were cultured in DMEM supplemented with 10% FBS (GM). Differentiation was induced by switching cultures at 70% of confluency to DMEM supplemented with 2% horse serum and 1 μg/ml of human insulin from Roche (DM). When indicated, SB203580 (5 μM, Calbiochem) was added to cell cultures every 24 h. Primary myoblasts were derived from satellite cells obtained from muscles of wt and p38α-deficient mice and were cultured as described (Perdiguero et al, 2007). Reintroduction of p38α into p38α−/− myoblasts was performed by retroviral infection as described (Perdiguero et al, 2007).
Cells expressing the Myc-tagged versions of p18Hamlet wt and the 4A mutant (Cuadrado et al, 2007) were generated by transfection of C2C12 cells with LipofectamineTM LTX and PLUSTM reagents (Invitrogen) followed by 3 weeks of selection with 1 mg/ml of G418. For expression in mammalian cells of N-terminally Myc-tagged Arp6, the human cDNA was cloned into the pCS2 vector as an EcoRI fragment obtained by partial digestion. For the expression of N-terminally HA-tagged H2A.Z, the human cDNA was amplified by PCR, cloned into pCRII and then subcloned as a BamHI/EcoRI fragment into the HA-pCDNA3 vector (provided by Giulio Superti-Furga, CeMM, Vienna, Austria). The construct expressing the constitutively activated MKK6DD was already described (Ambrosino et al, 2003).
siRNA
siRNA oligonucleotides were obtained from Dharmacon (Lafayette, CO) and transfected into C2C12 cells at 50–100 nM concentration with Dharmafect reagent 1 according to the manufacturer's protocol. The oligonucleotide sequences were as follows: p18Hamlet oligo 1: AACTCGAGGTGACCACTTTTT; p18Hamlet oligo 2: GAGCAGAACCTGAGTGCATTT; H2A.Z: ACAAATCGCTGATCGGGAATT; YL-1: TCATCCGTGAGGCGTACAATT; p400: AGGAATATCTGGAAGGAAATT. SRCAP was knockeddown by using ON-TARGETplus SMARTpool L-068483 siRNA. Cyclophilin B siRNA from Dharmacon was used as a control.
Immunofluorescence
Cells were rinsed in PBS, fixed in 3.7% paraformaldehyde for 15 min and washed with PBS. Non-specific sites were blocked by incubation with PBS containing 1% BSA and 0.5% Triton-X100 for 30 min at RT. Cells were then washed three times in PBS and incubated for 2 h at RT with monoclonal antibodies anti-MHC (1:10; clone MF20), anti-myc (1:200; 9E10 Santa Cruz Biotechnology) or anti-myogenin (1:10; clone F5D). After three washes with PBS, cells were incubated with Alexa Fluor 594 anti-mouse IgG secondary antibody (Molecular Probes) for 30 min at RT, stained with DAPI (0.1 μg/ml) for 5 min and mounted in mowiol. The preparations were visualized by confocal microscopy (Leica Mycrosystems).
mRNA isolation and quantitative real-time PCR (qRT–PCR) analysis
Total RNA was isolated from C2C12 cells using Qiagen RNeasy Kit (Qiagen) and cDNA was synthesized with SuperScript II reverse transcriptase (Invitrogen) using random hexamer primers following the manufacturer's indications. An Applied Biosystems 7900HT Fast qRT–PCR was used to determine the mRNA levels. Primers used for mRNA amplification are described in the Supplementary Figure S8.
Induction of de novo myogenesis in adult skeletal muscle
Activation of quiescent satellite cells and further differentiation was induced in mouse plantaris muscle by overloading resulting from incapacitation of the gastrocnemius muscle, as described (Serrano et al, 2008). Mice (12 weeks of age) were anesthetized with ketamine/xylazine (80/10 mg/kg, intraperitoneally) before surgery. Muscle extract preparation both in basal conditions and 3 days after overloading, immunoblotting and immunohistochemical analyses of muscle cryosections were performed as described (Serrano et al, 2008). The antibodies against H2A.Z (07-1594, Upstate) and p18Hamlet (Cuadrado et al, 2007) were detected with peroxidase-conjugated secondary antibodies, and those against myogenin (clone F5D) and Pax7 (hybridoma supernatant; Developmental Studies Hybridoma Bank) with the fluorescein M.O.M. Kit (Vector Laboratories). Micrographs were obtained using a Leica DMR microscope.
Immunoblotting and immunoprecipitations
Cell lysates were prepared in IP buffer (50 mM Tris–HCl pH 7.5, 150 mM NaCl, 1% NP-40, 5 mM EGTA, 5 mM EDTA, 20 mM NaF) supplemented with protease and phosphatase inhibitors (0.1 mM sodium orthovanadate, 1 mM PMSF, 2.5 mM benzamidine, 2 μM microcystin and 10 μg/ml of both leupeptin and aprotinin). Cleared lysates containing 40–60 μg of protein were separated by SDS–PAGE and transferred to nitrocellulose membranes. The commercial antibodies used for immunoblotting are described in the Supplementary Figure S9. For p18Hamlet, we used an affinity-purified rabbit polyclonal antibody (Cuadrado et al, 2007). Immunoblots were developed and quantified using Alexa Fluor 680 (Molecular probes) or Li-Cor IRDye 800 (Rockland) labelled antibodies with the Odissey Infrared Imaging System (Li-Cor Biosciences).
For immunoprecipitations, 10 μl of affinity-purified p18Hamlet or 5 μg of H2A.Z and YL-1 antibodies were incubated with 1 mg of protein lysates for 14 h at 4°C followed by 1 h of incubation with 20 μl of agarose-conjugated protein A. The beads were then washed three times in IP buffer and analysed by immunoblotting.
Chromatin immunoprecipitation
For ChIP assays, C2C12 cells (5 × 106) and wt or p38α KO mouse primary myoblasts were seeded in 15 cm dishes and 8 h later were shifted to DM, either alone or in combination with SB203580, and further incubated for the time indicated in each experiment. In the case of clones 1 and 6, cells were collected when reached 70% of confluency in GM. Experimental details are indicated as Supplementary data.
For semiquantitative PCR analysis, DNA content was analysed in the exponential range of amplification that varied from 23 (in the case of the inputs) to 32 cycles. Amplification products (all in the size range of 200–300 bp) were analysed by electrophoresis in 2% agarose gels and visualized by ethidium bromide. A measure of 1 μl from a 1:40 dilution of input was amplified in each case to ensure equal initial genomic DNA material. Analysis by qRT–PCR was performed using the SybrGreen Master Mix (Invitrogen) with triplicates of each sample. Values were normalized to the respective input DNAs and were represented in figures relative to the respective controls, as described in the figure legends. P values were obtained from a two-tailed Student's t-test.
The primers used in both semiquantitative and qRT–PCR were as follows:
−18 kb (a): fw 5′-CTAAAGGCAGAGGCAGATGG-3′ rev 5′-AGGACAAGAGACCTGCCTTCA-3′ Myogenin TATA (b): fw 5′-AGAGGGAAGGGGAATCACAT-3′ rev 5′-ATAGAAGTGGGGCTCCTGGT-3′
Myogenin (c): fw 5′-GGATTTTCAAGACCCCTTCC-3′ rev 5′-CCGTCGGCTGTAATTTGATT-3′.
Supplementary Material
Acknowledgments
We are grateful to AL Serrano (UPF) for advice on in vivo analysis of de novo myogenesis and to M Berciano (Universidad de Cantabria) for help with immunofluorescence studies. NC was supported by the Marie Curie-FP6 training project Onco-Train. This work was funded by CNIO and by grants from the Spanish Ministerio de Ciencia e Innovacion (MICINN) (BFU2007-60575, RD06/0020/0083 and SAF2009-09782), the European Commission FP7 program grant ‘INFLA-CARE' (EC contract number 223151), Fundación Científica de la AECC and Fundación La Caixa.
Footnotes
The authors declare that they have no conflict of interest.
References
- Adam M, Robert F, Larochelle M, Gaudreau L (2001) H2A.Z is required for global chromatin integrity and for recruitment of RNA polymerase II under specific conditions. Mol Cell Biol 21: 6270–6279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosino C, Mace G, Galban S, Fritsch C, Vintersten K, Black E, Gorospe M, Nebreda AR (2003) Negative feedback regulation of MKK6 mRNA stability by p38alpha mitogen-activated protein kinase. Mol Cell Biol 23: 370–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barski A, Cuddapah S, Cui K, Roh TY, Schones DE, Wang Z, Wei G, Chepelev I, Zhao K (2007) High-resolution profiling of histone methylations in the human genome. Cell 129: 823–837 [DOI] [PubMed] [Google Scholar]
- Berkes CA, Tapscott SJ (2005) MyoD and the transcriptional control of myogenesis. Semin Cell Dev Biol 16: 585–595 [DOI] [PubMed] [Google Scholar]
- Cai Y, Jin J, Florens L, Swanson SK, Kusch T, Li B, Workman JL, Washburn MP, Conaway RC, Conaway JW (2005) The mammalian YL1 protein is a shared subunit of the TRRAP/TIP60 histone acetyltransferase and SRCAP complexes. J Biol Chem 280: 13665–13670 [DOI] [PubMed] [Google Scholar]
- Chen IY, Lypowy J, Pain J, Sayed D, Grinberg S, Alcendor RR, Sadoshima J, Abdellatif M (2006) Histone H2A.z is essential for cardiac myocyte hypertrophy but opposed by silent information regulator 2alpha. J Biol Chem 281: 19369–19377 [DOI] [PubMed] [Google Scholar]
- Cuadrado A, Lafarga V, Cheung PC, Dolado I, Llanos S, Cohen P, Nebreda AR (2007) A new p38 MAP kinase-regulated transcriptional coactivator that stimulates p53-dependent apoptosis. EMBO J 26: 2115–2126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui K, Zang C, Roh TY, Schones DE, Childs RW, Peng W, Zhao K (2009) Chromatin signatures in multipotent human hematopoietic stem cells indicate the fate of bivalent genes during differentiation. Cell Stem Cell 4: 80–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Serna IL, Ohkawa Y, Imbalzano AN (2006) Chromatin remodelling in mammalian differentiation: lessons from ATP-dependent remodellers. Nat Rev Genet 7: 461–473 [DOI] [PubMed] [Google Scholar]
- Deal RB, Topp CN, McKinney EC, Meagher RB (2007) Repression of flowering in Arabidopsis requires activation of FLOWERING LOCUS C expression by the histone variant H2A.Z. Plant Cell 19: 74–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faast R, Thonglairoam V, Schulz TC, Beall J, Wells JR, Taylor H, Matthaei K, Rathjen PD, Tremethick DJ, Lyons I (2001) Histone variant H2A.Z is required for early mammalian development. Curr Biol 11: 1183–1187 [DOI] [PubMed] [Google Scholar]
- Fulco M, Schiltz RL, Iezzi S, King MT, Zhao P, Kashiwaya Y, Hoffman E, Veech RL, Sartorelli V (2003) Sir2 regulates skeletal muscle differentiation as a potential sensor of the redox state. Mol Cell 12: 51–62 [DOI] [PubMed] [Google Scholar]
- Gevry N, Chan HM, Laflamme L, Livingston DM, Gaudreau L (2007) p21 transcription is regulated by differential localization of histone H2A.Z. Genes Dev 21: 1869–1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gevry N, Hardy S, Jacques PE, Laflamme L, Svotelis A, Robert F, Gaudreau L (2009) Histone H2A.Z is essential for estrogen receptor signaling. Genes Dev 23: 1522–1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- John S, Sabo PJ, Johnson TA, Sung MH, Biddie SC, Lightman SL, Voss TC, Davis SR, Meltzer P, Stamatoyannopoulos JA, Hager GL (2008) Interaction of the glucocorticoid receptor with the chromatin landscape. Mol Cell 29: 611–624 [DOI] [PubMed] [Google Scholar]
- Kobor MS, Venkatasubrahmanyam S, Meneghini MD, Gin JW, Jennings JL, Link AJ, Madhani HD, Rine J (2004) A protein complex containing the conserved Swi2/Snf2-related ATPase Swr1p deposits histone variant H2A.Z into euchromatin. PLoS Biol 2: E131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouzarides T (2007) Chromatin modifications and their function. Cell 128: 693–705 [DOI] [PubMed] [Google Scholar]
- Krogan NJ, Keogh MC, Datta N, Sawa C, Ryan OW, Ding H, Haw RA, Pootoolal J, Tong A, Canadien V, Richards DP, Wu X, Emili A, Hughes TR, Buratowski S, Greenblatt JF (2003) A Snf2 family ATPase complex required for recruitment of the histone H2A variant Htz1. Mol Cell 12: 1565–1576 [DOI] [PubMed] [Google Scholar]
- Lafarga V, Cuadrado A, Nebreda AR (2007) p18(Hamlet) mediates different p53-dependent responses to DNA-damage inducing agents. Cell Cycle 6: 2319–2322 [DOI] [PubMed] [Google Scholar]
- Lluis F, Perdiguero E, Nebreda AR, Munoz-Canoves P (2006) Regulation of skeletal muscle gene expression by p38 MAP kinases. Trends Cell Biol 16: 36–44 [DOI] [PubMed] [Google Scholar]
- March-Diaz R, Garcia-Dominguez M, Florencio FJ, Reyes JC (2007) SEF, a new protein required for flowering repression in Arabidopsis, interacts with PIE1 and ARP6. Plant Physiol 143: 893–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meneghini MD, Wu M, Madhani HD (2003) Conserved histone variant H2A.Z protects euchromatin from the ectopic spread of silent heterochromatin. Cell 112: 725–736 [DOI] [PubMed] [Google Scholar]
- Perdiguero E, Ruiz-Bonilla V, Gresh L, Hui L, Ballestar E, Sousa-Victor P, Baeza-Raja B, Jardi M, Bosch-Comas A, Esteller M, Caelles C, Serrano AL, Wagner EF, Munoz-Canoves P (2007) Genetic analysis of p38 MAP kinases in myogenesis: fundamental role of p38alpha in abrogating myoblast proliferation. EMBO J 26: 1245–1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdiguero E, Sousa-Victor P, Ballestar E, Munoz-Canoves P (2009) Epigenetic regulation of myogenesis. Epigenetics 4: 541–550 [DOI] [PubMed] [Google Scholar]
- Raisner RM, Hartley PD, Meneghini MD, Bao MZ, Liu CL, Schreiber SL, Rando OJ, Madhani HD (2005) Histone variant H2A.Z marks the 5′ ends of both active and inactive genes in euchromatin. Cell 123: 233–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampalli S, Li L, Mak E, Ge K, Brand M, Tapscott SJ, Dilworth FJ (2007) p38 MAPK signaling regulates recruitment of Ash2 L-containing methyltransferase complexes to specific genes during differentiation. Nat Struct Mol Biol 14: 1150–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangasamy D, Greaves I, Tremethick DJ (2004) RNA interference demonstrates a novel role for H2A.Z in chromosome segregation. Nat Struct Mol Biol 11: 650–655 [DOI] [PubMed] [Google Scholar]
- Ruhl DD, Jin J, Cai Y, Swanson S, Florens L, Washburn MP, Conaway RC, Conaway JW, Chrivia JC (2006) Purification of a human SRCAP complex that remodels chromatin by incorporating the histone variant H2A.Z into nucleosomes. Biochemistry 45: 5671–5677 [DOI] [PubMed] [Google Scholar]
- Santisteban MS, Kalashnikova T, Smith MM (2000) Histone H2A.Z regulats transcription and is partially redundant with nucleosome remodeling complexes. Cell 103: 411–422 [DOI] [PubMed] [Google Scholar]
- Sardiu ME, Cai Y, Jin J, Swanson SK, Conaway RC, Conaway JW, Florens L, Washburn MP (2008) Probabilistic assembly of human protein interaction networks from label-free quantitative proteomics. Proc Natl Acad Sci USA 105: 1454–1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartorelli V, Caretti G (2005) Mechanisms underlying the transcriptional regulation of skeletal myogenesis. Curr Opin Genet Dev 15: 528–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano AL, Baeza-Raja B, Perdiguero E, Jardí M, Muñoz-Cánoves P (2008) Interleukin-6 is an essential regulator of satellite cell-mediated skeletal muscle hypertrophy. Cell Metab 7: 33–44 [DOI] [PubMed] [Google Scholar]
- Simone C, Forcales SV, Hill DA, Imbalzano AN, Latella L, Puri PL (2004) p38 pathway targets SWI-SNF chromatin-remodeling complex to muscle-specific loci. Nat Genet 36: 738–743 [DOI] [PubMed] [Google Scholar]
- Suto RK, Clarkson MJ, Tremethick DJ, Luger K (2000) Crystal structure of a nucleosome core particle containing the variant histone H2A.Z. Nat Struct Biol 7: 1121–1124 [DOI] [PubMed] [Google Scholar]
- Updike DL, Mango SE (2006) Temporal regulation of foregut development by HTZ-1/H2A.Z and PHA-4/FoxA. PLoS Genet 2: e161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong MM, Cox LK, Chrivia JC (2007) The chromatin remodeling protein, SRCAP, is critical for deposition of the histone variant H2A.Z at promoters. J Biol Chem 282: 26132–26139 [DOI] [PubMed] [Google Scholar]
- Wu WH, Alami S, Luk E, Wu CH, Sen S, Mizuguchi G, Wei D, Wu C (2005) Swc2 is a widely conserved H2AZ-binding module essential for ATP-dependent histone exchange. Nat Struct Mol Biol 12: 1064–1071 [DOI] [PubMed] [Google Scholar]
- Zlatanova J, Thakar A (2008) H2A.Z: view from the top. Structure 16: 166–179 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.