Abstract
Information gleaned from learning and memory processes is essential in guiding behavior towards a specific goal. However, the neural mechanisms that determine how these processes are effectively utilized to guide goal-directed behavior are unknown. Here, we show that rats utilize retrospective and prospective memory and flexible switching between these two memory processes to guide behaviors to obtain rewards. We found that retrospective memory is mainly processed in the hippocampus (HPC), but that this retrospective information must be incorporated within the prefrontal cortex (PFC) to be used to switch to an anticipatory response strategy involving prospective memory. Furthermore, switching between memory processes is regulated by the mesocortical dopamine (DA) system. Thus, DA D1 and D2 receptor activation in the PFC differentially affects retrospective memory processing within the HPC via an indirect feedback pathway. In contrast, D1, but not D2, receptor activation is crucial for incorporation of HPC-based retrospective information into the PFC. However, once this takes place, D2 receptor activation is required for further processing of information to effect preparation of future actions. These results provide a unique perspective on the mechanism of memory-based goal-directed behavior.
Keywords: Hippocampus, Prefrontal Cortex, Dopamine, Episodic Memory, Future Planning, Goal-directed Behavior
Introduction
Learning and memory processes are critical for an organism to effectively achieve goals, and developing an effective response strategy must include the ability to alter behavior whenever an obstacle is encountered. For example, when driving to work we may find our normal route blocked; we then would use retrospective memory to recall an alternate route. On the next day, remembering that the normal route is blocked, we would use a prospective planning strategy to take the alternate route and avoid a delay. The prefrontal cortex (PFC) and the hippocampus (HPC) are the two major brain regions proposed to mediate such learning and memory processes (Kesner and Rogers 2004; Laroche et al 2000; McDonald et al 2004; Simons and Spiers 2003; Takehara et al 2003; Wiltgen et al 2004). Indeed, individuals with neurological and psychiatric disorders including Alzheimer’s disease and schizophrenia that are known to exhibit pathology within the HPC and PFC show deficits in control of their behaviors based on memory processing (Buckner 2004; Frith 1997; Heckers et al 1998; Jones et al 2006; Meyer-Lindenberg et al 2005; Perry and Hodges 1996; Shum et al 2004).
Anatomical and electrophysiological studies have shown that the HPC and PFC exhibit reciprocal interactions. The HPC sends direct excitatory afferents into the PFC, targeting mainly the deep layers (layer V–VI) to contact both pyramidal neurons and interneurons (Jay and Witter 1991; Sesack et al 1989). The PFC in turn sends feedback projections into the HPC (Fuster 1997). Although the PFC afferent feedback into the HPC is indirect through the temporal cortex (Fuster 1997), a previous study has revealed that PFC lesion causes alterations of HPC neural activity associated with spatial localization in animals (i.e. place cells) (Kyd and Bilkey 2003), suggesting that such indirect PFC feedback information is indeed important for HPC function.
Accumulating evidence suggests that the mesocortical dopamine (DA) projection to the PFC plays a critical role in the modulation of information processing by HPC-PFC interactions. Thus, disconnection of DA-modulated HPC afferents into the PFC (via unilateral inactivation of the HPC combined with contralateral injection of DA D1 receptor antagonist into the PFC) disrupts working memory function (Seamans et al 1998). Synaptic plasticity induction such as long-term potentiation (LTP) at HPC afferents into the PFC is also governed by D1 receptor activation and its interaction with NMDA channels (Gurden et al 2000; Gurden et al 1999). In addition, a recent functional human imaging study has revealed that genetic variance of catechol-o-methly-transferase (COMT), a major molecule involved in clearance of DA in the PFC, affects HPC-PFC interactions during memory processing (Bertolino et al 2006).
In this study, we use combined electrophysiological recordings and behavioral assessments in rats to provide evidence that the ability to utilize previous experience toward achievement of a specific goal is mediated by the DA-dependent exchange of information between the HPC and PFC through both direct (from the HPC to the PFC) and indirect (from the PFC to the HPC) reciprocal connectivity between these regions.
Materials and Methods
Subjects
All experiments were conducted in accordance with the USPHS Guide for the Care and Use of Laboratory Animals and were approved by the University of Pittsburgh Institutional Animal Care and Use Committee. Male adult Sprague-Dawley rats were individually housed in cages in a temperature-controlled environment.
Behavioral test
To test episodic memory and future planning strategies, we modified a task originally described in other studies (Kesner 1989). After 3 days of intensive handling (10 minutes each day) and another 3 days of habituation to an eight-arm radial maze, intracranial cannula implantation was done. Three groups of rats were anesthetized with sodium pentobarbitol (50 mg/kg) and placed in a stereotaxic apparatus. Twenty-two gauge guide cannulae were placed (i) bilaterally into the HPC (the posterior dorsal CA1 region; anteroposterior (AP), −5.6 mm from bregma; lateral (L), ±4.6 mm; dorsoventral (DV), −2.8 mm from the surface; coordinates determined by the stereotaxic atlas (Paxinos and Watson 1998). We chose the dorsal, rather than ventral, HPC for microinfusion, given that (a) the dorsal HPC appears to be more associated with cognitive information processing, whereas the ventral HPC is more related to affective information processing (Trivedi and Coover 2004); and (ii) the posterior dorsal HPC innervates the PFC (Izaki et al 2003)); (b) bilaterally into the dorsomedial portion of the PFC (the anterior cingulate Cg1/prelimbic cortex; AP, +3.0 mm; L, +0.7 mm; DV −2.4 mm); and (c) unilaterally into the HPC and into the contralateral PFC for disconnection of HPC-PFC interactions (Everitt et al 1991; Floresco et al 1999). Although anatomical studies have shown heavy connections between the ventral HPC and the medial PFC (Jay and Witter 1991), there is evidence that the posterior dorsal part of the HPC is also functionally connected with the medial PFC; thus, electrical stimulation of the dorsal HPC evokes monosynaptic field potential responses in the PFC (Izaki et al 2003; Kawashima et al 2006). Moreover, strong functional coupling of the dorsal HPC and the dorsomedial PFC, which is most likely relies on anatomical connection of these specific parts of the HPC and PFC, has also been revealed by simultaneous recordings from these areas (Siapas et al 2005; Hyman et al 2005). After 1 week of recovery, these animals were subjected to behavioral tests. Animals were food-restrained to maintain about 85% of normal body weight starting at handling until termination of the tests.
The behavioral task was conducted using an eight-arm radial maze. A trial of the task consisted of learning and test phases. During the learning phase, either 1, 3, 5, or 7 of pseudo-randomly selected arms were presented to animals and baited with a small reward (1/4 piece of Kellogg’s Froot Loops cereal) located at the end of each arm. Animals explored the maze freely during the learning phase until all rewards were obtained. After animals consumed each of the rewards, the rats were held in the center of the maze for 5 minutes. Then, a test phase was given to animals in which two arms were presented; one that animals had entered during the learning phase, and the other that animals had not. Animals had to choose the arm that they did not enter during a learning phase to obtain a large reward (a full piece of cereal). Each session consisted of 6 trials of either 1, 3, 5, or 7 arm conditions, and one session was given to the rats each day. The inter-trial interval was set to 30 minutes. One condition (one of 1, 3, 5, or 7 arm condition) has 4 sessions (and thereby taking 4 days) to complete. Initial 2 sessions of each arm condition were given to animals as pre-training in order to allow them to become familiar with the task, followed by another 2 sessions at each arm condition. These later 2 sessions consisting of each 1, 3, 5, and 7 arm condition were used for behavioral data analyses. All animals were started with Four sessions of the 1 arm condition, followed by another 4 sessions of the 3, 5, and 7 arm conditions (therefore, the entire task required 16 days to complete).
All drugs were administered via the 30 gauge infusion cannulae at 0.2 µl/min. starting 5 minutes before the first session of the day. Artificial cerebrospinal fluid (aCSF; 0.5 µl), muscimol (0.5 µg/0.5 µl in aCSF) or DA agonists and antagonists (SKF38393, 0.1 µg/0.5 µl; SCH23390, 1.0 µg/0.5 µl; quinpirole, 1.0 µg/0.5 µl; eticlopride, 2.0 µg/0.5 µl) was infused into the PFC or HPC. The doses of drugs were based on those used previously to selectively alter behaviors (Granon et al 2000; Olsen and Duvauchelle 2001; Swanson et al 1997). Statistical comparison of groups was evaluated using two-way ANOVA with post-hoc Tukey HSD test and significance was defined as P<0.05.
Electrophysiology
In vivo field potential and single unit recordings were sampled from the PFC and HPC, respectively. Rats were anesthetized with chloral hydrate (400 mg/kg) and placed in a stereotaxic apparatus. Extracellular electrodes pulled from glass micropipettes and filled with 2 M NaCl were lowered into the deep layer of the PFC (layer V–VI; the same coordinates for microinfusion in the behavioral test) or HPC (dorsal CA1; the same coordinates for microinfusion in the behavioral test). Field potential and single unit signals were amplified 1,000 and 10,000 times with an AC amplifier and band-pass filtered at 0.1–100 and 100–10,000 Hz, respectively. Recordings were digitized with an interface board at 10 kHz, and fed to a computer for off-line analysis. All data handling was performed using custom software (Neuroscope). Criteria for acceptable single unit sampling was defined as a signal-to-noise ratio of 3:1 or greater and at least 5 minutes of stable recording.
Concentric bipolar stimulation electrodes were placed in the HPC (dorsal CA1) or superficial layers of the PFC (AP, −3.0 mm; L, +0.2mm DV −2.4 mm) in the plasticity experiments. Single current pulses were delivered every 5 seconds to the HPC (0.2 ms; 0.2 to 0.8 mA) or the PFC (0.2 ms; 0.05 to 0.2 mA) for test pulses. Current intensity was adjusted to evoke approximately 50–60% of maximal responses. Two types of tetanic stimulation were used to induce short-term potentiation (STP). Theta burst stimulation consisted of 3, 4, or 5 trains of stimuli at 7 Hz (3, 4, or 5θ) with each train composed of 3 pulses at 100 Hz, and given into the dorsal CA1. The other tetanic stimulation consisted of either 2 or 3 trains of stimuli at 1 Hz (2 or 3γ) with each train composed of 5 pulses at 40 Hz, and given within the PFC. Changes in amplitudes of the potentials beginning 1 ms before the test pulse stimulation up to the first peak of negative (with HPC stimulation) or positive (with intra-PFC stimulation) shifts of evoked field potentials was measured before and after tetanization.
Drugs were administered intraperitonially or locally into the PFC via reverse microdialysis. Dialysis probes (2 mm exposed membrane) were advanced slowly into the PFC at the rate of 3–5 µm/sec to minimize any damage of brain tissues. All drugs were purchased from Sigma (St. Louis, MO) and were dissolved in aCSF (SKF38393, 10 µM; SCH23390, 10 µM; quinpirole, 10 µM; eticlopride, 20 µM) (Goto and Grace 2005). aCSF was continuously perfused throughout the experiments, and switched to drug administration during recordings. The D1 and D2 antagonists (SCH23390, 0.5 mg/kg; eticlopride, 1.0 mg/kg, dissolved in 1 ml aCSF) were also systemically administered 5 minutes before recordings were started.
Results
Flexible utilization of memory processing in goal-directed behavior
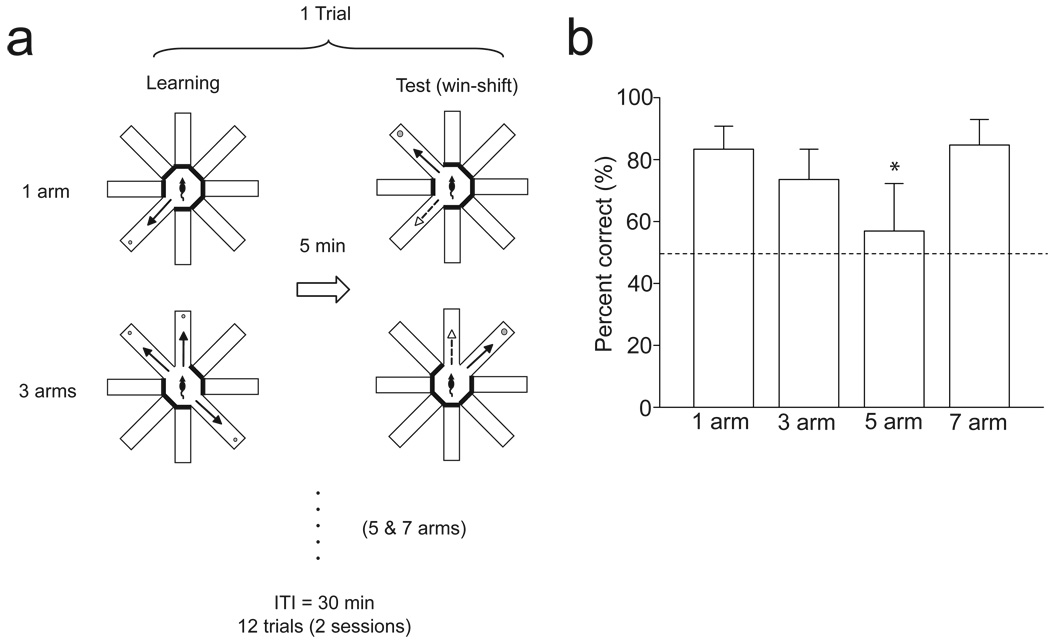
The ability of rats to use memory processes to appropriately guide their behaviors toward a specific goal was tested using a modified eight-arm radial maze task (Fig. 1A) (Kesner 1989). As described in the Methods, 1, 3, 5, or 7 arms were presented to rats first, and after 5 minutes of delay, two arms were presented; one that rats had entered, and the other that they had not. Animals were required to choose the unentered arm to obtain rewards. When control animals were previously exposed to one arm, they correctly chose the unentered arms 83.3 ± 7.5% (mean ± s.d.) of the time (n=6; Fig. 1B). This percentage declined as the number of pre-exposed arms increased (73.6 ± 9.7 and 57.0 ± 15.3% at 3 and 5 arms, respectively; p=0.002 comparing between 1 and 5 arm conditions, one-way ANOVA with post-hoc Tukey test; Fig. 1B). However, the percentage of correct responses was significantly increased when rats had been pre-exposed to the 7 arm condition (84.7 ± 8.2%; p=0.001 comparing between 5 and 7 arm conditions; Fig. 1B), suggesting that animals switched their response strategies between the 5 and 7 arm conditions. One explanation for these results is that animals might have taken into account the arms that they had entered previously to choose subsequent responses under the 1, 3, and 5 arm conditions. This is evidenced by the decline in the percentages of correct responses as the number of arms presented was increased. In contrast, in the 7 arm condition, animals may have utilized a more efficient response strategy from those used in the 1, 3, and 5 arm conditions; e.g., one possible explanation would be that, under this condition, the animals might extrapolate prospectively which the arm that they were going to enter for subsequent responses. This would be consistent with the observation that the percentage of correct responses in the 7 arm conditions was similar to that in the 1 arm condition. Another potential explanation for these results is that animals might utilize spatial cues to solve the behavioral task rather than utilizing retrospective and prospective memory. However, if this were the case, the behavior of the animal would be inordinately influenced by precisely which arms are presented in the test phase; e.g., if the correct and incorrect arms presented are spatially far apart, or located in close proximity to each other. Nevertheless, we found that correct responses were not influenced by the pattern of arm presentation.
Figure 1.
Description of behavioral task. (A) Schematic diagram illustrating the behavioral test. ITI: inter-trial interval. (B) Bar graph showing percentages of correct responses at the 1, 3, 5, and 7 arm conditions. Higher correct responses were observed at the 1 and 7 arm conditions, suggesting that animals utilized retrospective and prospective memory in each condition, respectively (one way ANOVA with post hoc Tukey test, *P < 0.01). Error bars in this and all other figures indicate S.D.
HPC-PFC interactions in memory processing
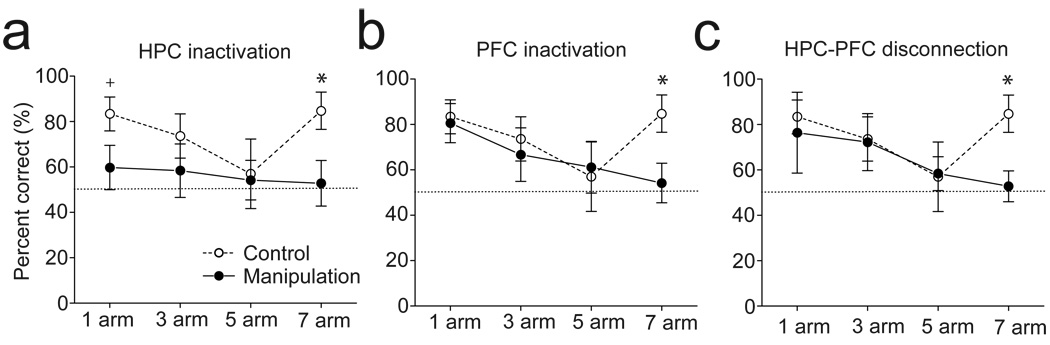
To test the dependence of HPC-PFC interactions that may underlie the flexible utilization of memory processing, muscimol was infused (i) bilaterally into the dorsal HPC, (ii) bilaterally into the dorsal portion of the medial PFC, and (iii) unilaterally into the HPC and contralaterally into the PFC. Inactivation of the HPC resulted in disruption of performance in both the 1 and 7 arm conditions (59.7 ± 9.7% in 1 arm; p=0.024 compared to control, two-way ANOVA with post-hoc Tukey test; 52.8 ± 10.1% in 7 arm; p=0.003; n=6; Fig. 2A), whereas PFC inactivation impaired performance in the 7 arm, but not the 1, 3, and 5 arm, condition (54.2 ± 8.7% in 7 arm; p=0.006; n=6; Fig. 2B). In contrast, when the HPC and PFC were disconnected by contralateral infusions, the animals exhibit impairment only in the 7 arm condition (52.8 ± 6.8% in 7 arm; p=0.003; n=6; Fig. 2C). These results suggest that a response strategy used in the 1, 3, and 5 arm condition involved information processing primarily in the HPC, and that HPC-based information could be incorporated in the PFC to provide the basis for switching to a new response strategy in the 7-arm condition.
Figure 2.
Disruption of HPC-PFC interactions altered the processing of retrospective and prospective memory. (A) Bilateral HPC inactivation disrupted performance at both the 1 and 7 arm conditions. (B) Bilateral PFC inactivation disrupted performance only at the 7 arm condition. (C) Unilateral PFC and contralateral HPC inactivation induced impairments similar to those produced by bilateral PFC inactivation (Two-way ANOVA with post hoc Tukey test, +P < 0.05, *P < 0.01).
Different roles of D1 and D2 activation in memory processing
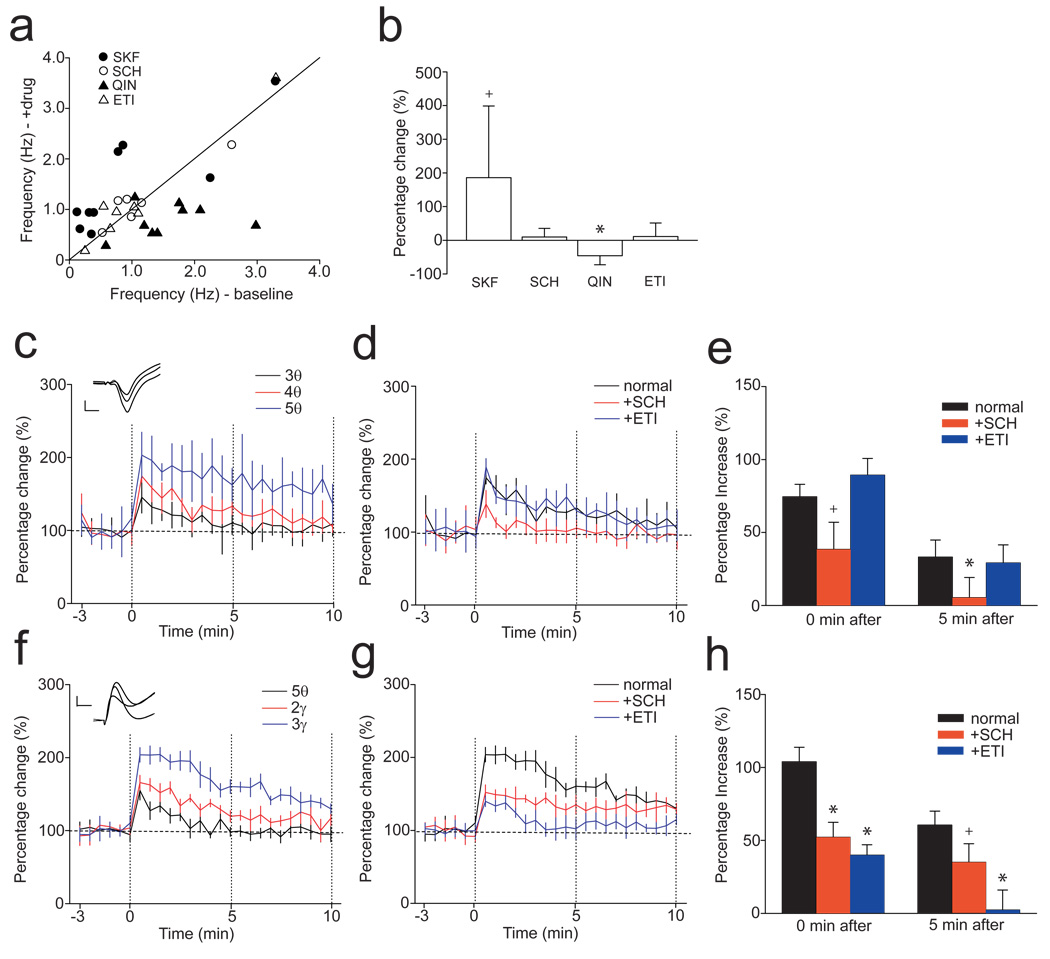
We next investigated the role of the mesocortical DA system in this flexible utilization of response strategies in guiding goal-directed behaviors using local infusion of D1 and D2 agonists and antagonists into the PFC. Local infusion of the D1 agonist SKF38393 facilitated performance at the 5 arm condition (75.0 ± 9.1% in 5 arm; p=0.005; n=6; Fig. 3A), whereas the D2 agonist quinpirole impaired performance at the 1 and 3 arm conditions (57.0 ± 8.2% in 1 arm; p=0.008; 45.9 ± 6.9% in 3 arm; p=0.004; n=6; Fig. 3B), but facilitated performance at the 5 arm condition (80.5 ± 4.3% in 5 arm; p=0.005; n=6; Fig. 3B). These drugs were infused into the PFC, yet affected HPC-mediated information processing, suggesting that D1 and D2 receptor activation in the PFC differentially affects HPC activity that mediates retrospective memory processes, possibly via indirect projections from the PFC into the HPC (PFC→HPC information processing) (Fuster 1997; Kyd and Bilkey 2003). Improved correct responses in the 5 arm condition were observed following both D1 and D2 agonist infusion. However, the mechanism of these improvements seems to be different. Improvement of performance in the 5 arm condition with D1 agonist may be simply due to facilitation of information processing in the HPC provided by PFC feedback. In contrast, impairments of performance at the 1 and 3 arm conditions but improved performance at the 5 arm condition with the D2 agonist suggest that D2 receptor activation in the PFC might interfere with HPC activity, possibly via the feedback projection from the PFC to the HPC. Thus, one possible explanation is that D2 receptor activation in the PFC could facilitate processing of a response strategy that is normally utilized only in the 7 arm condition, so that animals might have already switched their response strategy in the 5 arm condition.
Figure 3.
Altering DA stimulation within the PFC produces selective actions on the processing of retrospective and prospective memory. (A) Infusion of the D1 agonist SKF38393 (SKF) into the PFC selectively improved correct responses at the 5 arm condition. (B) The D2 agonist quinpirole (QIN) disrupted performance at the 1 and 3 arm conditions, but improved correct responses at the 5 arm condition. (C) The D1 antagonist SCH23390 (SCH) produced an impairment of performance at the 7 arm condition. (D) Similarly, the D2 antagonist eticlopride (ETI) also disrupted performance at the 7 arm condition (Two-way ANOVA with post hoc Tukey test, +P < 0.05, *P < 0.01).
Administration of either the D1 antagonist SCH23390, or the D2 antagonist eticlopride, into the PFC impaired performance in the 7 arm condition without affecting other arm conditions (62.5 ± 15.6% with SCH23390; p=0.012; n=6; 63.9 ± 11.4% with eticlopirde; p=0.028; n=6; Fig. 3C, D), suggesting that the D1 and D2 antagonists affect HPC→PFC information processing, and co-activation of D1 and D2 receptors in the PFC is required for utilization of HPC-based retrospective information to switch a response strategy in the 7 arm condition.
D1 and D2 regulation of reciprocal HPC-PFC interactions
The mechanisms underlying how D1 and D2 activation in the PFC regulate switching of response strategies were further examined using in vivo electrophysiological recordings. Local administration of the D1 and D2 agonists into the PFC via microdialysis probes produced significant augmentation and attenuation of HPC neuron spike firing frequency, respectively (185.9 ± 212.8% increase with SKF38393, p=0.028 comparing before and after drug administration, paired t-test; n=9; 45.8 ± 26.8% decrease with quinpirole, p=0.008; n=9; Fig. 4A, B). In contrast, administration of the D1 and D2 antagonists into the PFC did not significantly alter HPC neuron spike firing (9.6 ± 25.7% increase with SCH23390; n=6; 11.2 ± 40.1% increase with eticlopride; n=7; Fig. 4A, B). These results suggest that activation of D1 and D2 receptors in the PFC affected neural activities in the HPC via the PFC→HPC indirect pathway.
Figure 4.
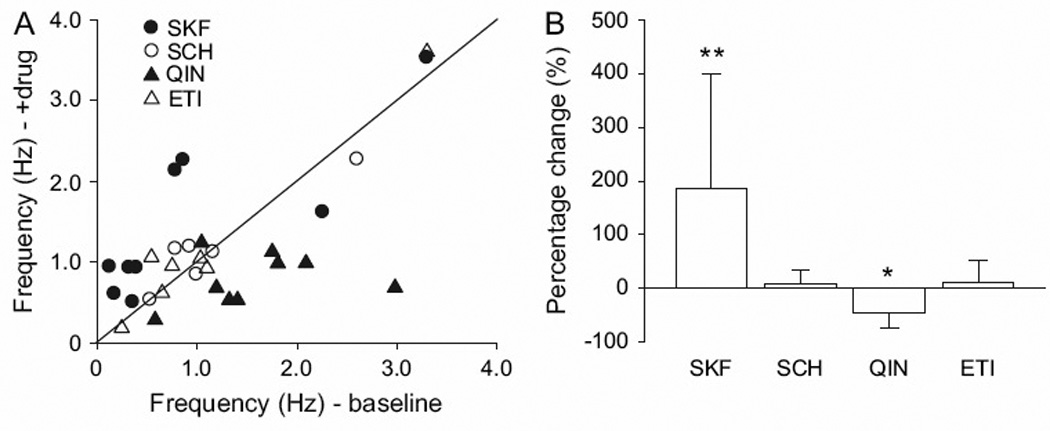
The effects of DA manipulation within the PFC on HPC neuron activity. (A, B) Infusion of the D1 agonist into the PFC increased HPC spike firing frequency, whereas D2 agonist infusion decreased HPC spike firing. In contrast, the D1 and D2 antagonists did not exert significant effects on HPC activity.
We also evaluated DA-dependent synaptic plasticity in the PFC to elucidate the effects of the D1 and D2 antagonists on HPC→PFC information processing. Previous studies have shown that HPC activation induces endogenous DA release in the PFC (Peleg-Raibstein et al 2005) and DA-dependent long-term potentiation (LTP) at HPC afferents into the PFC (Gurden et al 1999). In addition, recent studies have reported a coupling of PFC activities with HPC theta oscillations during cognitive task performance (Hyman et al 2005; Siapas et al 2005). Thus, we tested the effect of theta burst stimulation of the HPC on the induction of synaptic plasticity at these afferents into the PFC. We found that moderate strengths (4 or 5 trains of stimulation given at 7 Hz, 1 train consisting of 3 pulses at 100 Hz) of theta burst stimulation induced short-term potentiation (STP) of the amplitudes of evoked field potentials in the PFC that persisted for longer than 5 minutes (74.5 ± 8.6% and 33.2 ± 11.6% increase at 0 and 5 min. after 4 theta tetanic stimulation; n=8; Fig. 5A), which is the duration of a delay implemented in the behavioral test that we employed. This theta burst-induced STP was significantly attenuated by i.p. administration of the D1 antagonist SCH23390 (0.5 mg/kg; 38.4 ± 18.6% increase at 0 min. after 4 theta tetanic stimulation; p=0.012 compared to normal condition, two-way ANOVA with post hoc Tukey test; 5.3 ± 13.9% at 5 min. after; p=0.009; n=8; Fig. 5b, c), but not by the D2 antagonist eticolopride (1.0 mg/kg; 89.3 ± 11.4% and 29.1 ± 12.4% increase at 0 and 5 min. after 4 theta tetanic stimulation; n=8; Fig. 5B, C), suggesting that D1, but not D2, activation is critical for HPC→PFC information processing.
Figure 5.
The effects of DA manipulation on synaptic plasticity induced at HPC afferents into the PFC and within the PFC network. (A) Short term potentiation (STP) was induced at HPC afferents into the PFC by 3, 4, and 5 trains (3, 4, and 5θ)of theta burst stimulation. Traces in c show representative responses evoked by HPC stimulation before (the smallest amplitude) as well as 0 min. (the largest amplitude) and 5 min. (a median amplitude) after theta burst stimulation. Scale bar: 0.2 mV, 20 ms. (B, C) Systemic administration of the D1 (SCH), but not the D2 (ETI), antagonist significantly attenuated STP induced by 4θ stimulation. (D) Intra-PFC 5θ stimulation failed to induce STP that persisted for longer than 5 minutes, whereas 2 and 3 trains (2 and 3γ)of tetanic stimulation at gamma frequency given within the PFC induced STP that persisted for longer than 10 minutes. Traces in f show representative responses evoked by intra-PFC stimulation before (the smallest amplitude) as well as 0 min. (the largest amplitude) and 5 min. (a median amplitude) after gamma stimulation. Scale bar: 0.2 µV, 20 ms. (E, F) Pretreatment with ETI significantly attenuated STP induced by 3γ stimulation. In contrast, pretreatment of SCH moderately attenuated STP (two-way ANOVA with post hoc Tukey test, +P < 0.05, *P < 0.01).
The role of D2 activation on synaptic plasticity in the PFC was further examined using intra-PFC stimulation. Theta burst stimulation delivered to the superficial layer had only weak effects on STP obtained in the deep layer of the PFC (Fig. 5D). Even the strongest theta burst stimulation employed (5 trains) induced only a transient increase in the evoked field potentials, and this decayed within 5 minutes. In contrast, when the PFC was stimulated using a train of pulses at 40 Hz (2 or 3 trains of stimulation given at 1 Hz, 1 train consisting of 5 pulses at 40 Hz), which is in the range of the gamma frequency, the STP that was induced persisted for a duration of more than 5 minutes, which was similar to that observed with theta burst stimulation in the HPC (93.9 ± 9.9% and 50.5 ± 9.5% increase at 0 and 5 min. after 3 gamma tetanic stimulation; n=8; Fig. 5D). The STP induced by intra-PFC stimulation was significantly attenuated by systemic administration of the D2 antagonist (39.8 ± 7.2% (p=0.002) and 2.3 ± 13.8% (p=0.003) increase at 0 and 5 min. after 3 gamma tetanic stimulation; n=7; Fig. 5E, F), although the D1 antagonist also moderately attenuated STP at 0 min. (52.2 ± 10.2% increase; p=0.009; n=9; Fig. 5E, F) and 5 min. (35.0 ± 12.6% increase; p=0.030; Fig. 5E, F) after tetanic stimulation. These results suggest that short-term synaptic plasticity induced at HPC afferents to the PFC either directly or via local circuits within the PFC are differentially regulated by D1 and D2 receptor activation.
Discussion
Our data support the contention that the choice between different response strategies that utilize distinct memory processes is mediated by bi-directional interactions between the HPC and PFC, and that flexibility in utilizing these response strategies is dependent on mesocortical DA activation. Thus, our study suggests that retrospective memory is dependent on the HPC, but that such retrospective information has to be incorporated into the PFC in order to enable prospective information processing for anticipation of future actions. Furthermore, selection of HPC-dependent retrospective information appears to depend on D1, but not D2, receptor stimulation in the PFC. Thus, we found that D2 receptor activation impairs retrospective memory processing, suggesting that retrospective memory is processed independent from D2 receptor activation, but if D2 receptors are indeed activated, then it is disrupted. In contrast, the ability to generate prospective planning-like strategies based on this retrospective HPC information is largely dependent on D2 receptor modulation of PFC intrinsic circuits. This is consistent with previous results by others in which D2 receptor stimulation in the PFC is proposed to facilitate behavioral flexibility (Floresco et al 2006; Mehta et al 2004; Seamans and Yang 2004; Tost et al 2006; Winterer and Weinberger 2004).
Indeed, a number of possible explanations could be made for observations of memory-guided response strategies to account for the behavior of the animals tested in this study. One potential scenario would be that these animals utilize a retrospective information processing mechanism similar to episodic memory for guiding their behaviors in the 1, 3, and 5 arm conditions, whereas their response strategy would switch at the 7 arm condition to a mechanism that may be similar to planning of future actions. Indeed, some other studies have shown that animals can utilize similar retrospective and prospective memory functions in driving goal-directed behavior (Dragoi and Buzsaki 2006; Eichenbaum 2001; Fortin et al 2002; Ingvar 1985; Mulder et al 2000; Mushiake et al 2006; Rainer et al 1999; Squire 1992). Electrophysiological recordings in rodents and primates have shown that sustained spike firing during the delay period of working memory serves to encode prospective information such as planned future responses at the level of PFC neurons (Ingvar 1985; Mulder et al 2000; Mushiake et al 2006; Rainer et al 1999). In contrast, a lesion of the HPC disrupts episodic memory function in humans and rodents (Eichenbaum 2001; Fortin et al 2002; Squire 1992), and moreover a recent study has shown that assemblies of HPC neurons encode episodic memory (Dragoi and Buzsaki 2006). Our study provides a new viewpoint suggesting that bidirectional interactions between the HPC and PFC may be essential for mediating the behaviorally relevant spike patterning associated with the ability of the HPC and PFC to optimally utilize retrospective and prospective memory processes.
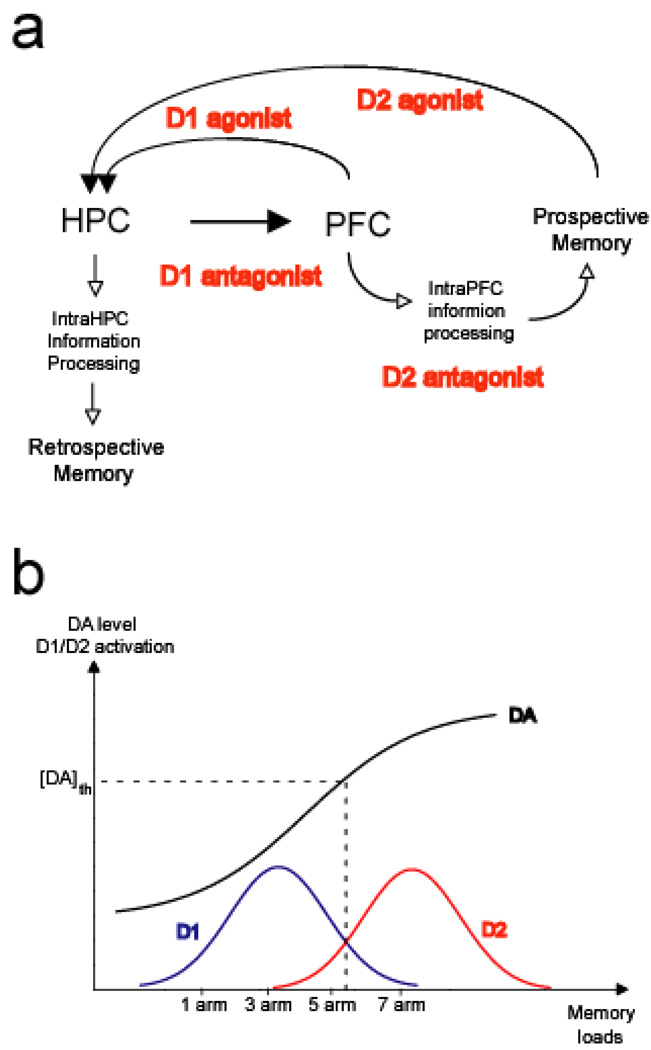
The PFC expresses both DA D1 and D2 receptors (Vincent et al 1993), and D1-dependent, but not D2-dependent, HPC-PFC interactions have been described in previous studies. Thus, disconnection of HPC-PFC interactions via unilateral D1 antagonist infusion into the PFC combined with inactivation of the contralateral HPC disrupts working memory (Seamans et al 1998). Similarly, synaptic plasticity such as LTP induction at HPC afferents into the PFC is selectively dependent on D1 activation (Gurden et al 2000). On the other hand, the function of D2 receptors in the PFC is relatively unclear. Nevertheless, a similar long-term synaptic plasticity induced within the PFC networks by stimulation of the superficial layers, where cortico-cortical afferents are located, requires both D1 and D2 activation (Matsuda et al 2006). These data suggest that different cellular mechanisms may underlie the induction of synaptic plasticity in the PFC network, in which DA receptor subtypes play distinct roles depending upon which afferent such synaptic plasticity is induced. Indeed, in support of this model of functional segregation, a recent study in primates shows that D1 and D2 receptor antagonists affect different aspects of PFC neuronal activity during working memory (Wang et al 2004). Our study is consistent with these previous findings in which D1, but not D2, activation modulates STP at HPC inputs, whereas D2 activation modulates STP induced within the PFC network. Thus, it appears that DA exerts its effects via a two-step process: D1 receptors select the information from the HPC that is to be incorporated into the PFC network. This incorporated information is further processed within the PFC by D2 receptors (Fig. 6A). Based on these results, we propose that increased DA release that is sufficient to stimulate both D1 and D2 receptors may be required to utilize future planning strategies (Fig. 6B). D1 activation could facilitate the use of episodic memory by facilitating PFC feedback onto the HPC via an indirect pathway, whereas D2 receptor activation could trigger a switch from the use of HPC-based episodic memory to a PFC-based employment of future planning strategies via suppression of HPC activity.
Figure 6.
Schematic diagram illustrating a proposed model of temporal flexibility of memory processing mediated by hippocampal (HPC) – prefrontal cortical (PFC) interaction and its regulation by the mesocortical dopamine (DA) system in the PFC. (A) Information processed in the HPC is optimally incorporated into the PFC in a manner that is dependent on D1 receptor activation. This information is further processed within the PFC via a D2 receptor-dependent mechanism. As a consequence, the D1 antagonist disrupts incorporation of HPC information into the PFC, whereas the D2 antagonist disrupts intra-PFC information processing, both of which lead to a deficit in prospective memory. In contrast, stimulation of D1 and D2 receptors in the PFC alters HPC-based retrospective memory processing, suggesting that these systems affect the feedback of information from the PFC into the HPC that occurs via an indirect pathway. (B) A model that accounts for PFC DA regulation of temporal flexibility of retrospective and prospective memory processing. A relationship between the inverted U shape of D1 and D2 receptor stimulation and the amount of DA release is advanced to explain the mechanism underlying the ability to switch from retrospective to prospective memory processing. Lower DA release activates primarily the extrasynaptic D1 receptors, whereas higher DA release preferentially activates D2 receptors. At the DA release [DA]th, D2 activation is larger than D1 activation, which may be a point at which retrospective memory is translated into prospective information processing. In this study, such a threshold of DA release may be reached in the 5 and 7 arm task conditions. DA agonists and antagonists shift this D1- and D2-dependent inverted U shape activation toward the left or right, respectively. Theta frequency coupling between the HPC and PFC is proposed to induce DA release that is sufficient to activate the D1 receptors, but is below the threshold for optimal D2 activation. On the other hand, gamma oscillations within the PFC is proposed to induce DA release that is sufficient to activate D2 receptors.
Deficits in both episodic memory and future planning are observed in disorders such as Alzheimer’s disease and schizophrenia; disorders that are also known to exhibit structural and functional abnormalities in the HPC and PFC (Buckner 2004; Frith 1997; Heckers et al 1998; Jones et al 2006; Meyer-Lindenberg et al 2005; Perry and Hodges 1996; Shum et al 2004). Moreover, impairments of functional connectivity between the PFC and limbic structures (Frith 1997; Meyer-Lindenberg et al 2005) and its modulation by the DA system (Smolka et al 2005) have been described specifically in schizophrenia. Considered in light of the current results, deficits in the flexible utilization of episodic memory and future planning observed in these disorders may be a direct consequence of impairment of limbic and PFC interactions and their modulation by the DA system.
Acknowledgments
This work was supported by grants from the National Institute of Mental Health MH57440 (A.A.G.) and National Alliance for Research on Schizophrenia and Depression Young Investigator Award (Y.G.). We thank Ms. N. Macmurdo and C. Smolak for technical assistance, and Drs. H. Eichenbaum for reading and invaluable comments on the manuscript.
References
- Bertolino A, Rubino V, Sambataro F, et al. Prefrontal-hippocampal coupling during memory processing is modulated by COMT val158met genotype. Biol Psychiatry. 2006;60:1250–1258. doi: 10.1016/j.biopsych.2006.03.078. [DOI] [PubMed] [Google Scholar]
- Buckner RL. Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron. 2004;44:195–208. doi: 10.1016/j.neuron.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Dragoi G, Buzsaki G. Temporal encoding of place sequences by hippocampal cell assemblies. Neuron. 2006;50:145–157. doi: 10.1016/j.neuron.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. The hippocampus and declarative memory: cognitive mechanisms and neural codes. Behav Brain Res. 2001;127:199–207. doi: 10.1016/s0166-4328(01)00365-5. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Morris KA, O'Brien A, Robbins TW. The basolateral amygdala-ventral striatal system and conditioned place preference: further evidence of limbic-striatal interactions underlying reward-related processes. Neuroscience. 1991;42:1–18. doi: 10.1016/0306-4522(91)90145-e. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Braaksma DN, Phillips AG. Thalamic-cortical-striatal circuitry subserves working memory during delayed responding on a radial arm maze. J Neurosci. 1999;19:11061–11071. doi: 10.1523/JNEUROSCI.19-24-11061.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Magyar O, Ghods-Sharifi S, Vexelman C, Tse MT. Multiple dopamine receptor subtypes in the medial prefrontal cortex of the rat regulate set-shifting. Neuropsychopharmacology. 2006;31:297–309. doi: 10.1038/sj.npp.1300825. [DOI] [PubMed] [Google Scholar]
- Fortin NJ, Agster KL, Eichenbaum HB. Critical role of the hippocampus in memory for sequences of events. Nat Neurosci. 2002;5:458–462. doi: 10.1038/nn834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith CD. Functional brain imaging and the neuropathology of schizophrenia. Schizophr Bull. 1997;23:525–527. doi: 10.1093/schbul/23.3.525. [DOI] [PubMed] [Google Scholar]
- Fuster JM. The prefrontal cortex: Anatomy, physiology, and neuropsychology of the frontal lobe. 3rd ed. Philadelphia: Lippincott-Raven; 1997. [Google Scholar]
- Goto Y, Grace AA. Dopaminergic modulation of limbic and cortical drive of nucleus accumbens in goal-directed behavior. Nat Neurosci. 2005;8:805–812. doi: 10.1038/nn1471. [DOI] [PubMed] [Google Scholar]
- Granon S, Passetti F, Thomas KL, Dalley JW, Everitt BJ, Robbins TW. Enhanced and impaired attentional performance after infusion of D1 dopaminergic receptor agents into rat prefrontal cortex. J Neurosci. 2000;20:1208–1215. doi: 10.1523/JNEUROSCI.20-03-01208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurden H, Takita M, Jay TM. Essential role of D1 but not D2 receptors in the NMDA receptor- dependent long-term potentiation at hippocampal-prefrontal cortex synapses in vivo. J Neurosci. 2000;20:RC106. doi: 10.1523/JNEUROSCI.20-22-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurden H, Tassin J-P, Jay TM. Integrity of the mesocortical dopaminergic system is necessary for complete expression of in vivo hippocampal-prefrontal cortex long-term potentiation. Neuroscience. 1999;94:1019–1027. doi: 10.1016/s0306-4522(99)00395-4. [DOI] [PubMed] [Google Scholar]
- Heckers S, Rauch SL, Goff D, et al. Impaired recruitment of the hippocampus during conscious recollection in schizophrenia. Nat Neurosci. 1998;1:318–323. doi: 10.1038/1137. [DOI] [PubMed] [Google Scholar]
- Hyman JM, Zilli EA, Paley AM, Hasselmo ME. Medial prefrontal cortex cells show dynamic modulation with the hippocampal theta rhythm dependent on behavior. Hippocampus. 2005;15:739–749. doi: 10.1002/hipo.20106. [DOI] [PubMed] [Google Scholar]
- Ingvar DH. "Memory of the future": an essay on the temporal organization of conscious awareness. Hum Neurobiol. 1985;4:127–136. [PubMed] [Google Scholar]
- Izaki Y, Takita M, Nomura M, Akema T. Differences between paired-pulse facilitation and long-term potentiation in the dorsal and ventral hippocampal CA1-prefrontal pathways of rats. Brain Res. 2003;992:142–145. doi: 10.1016/s0006-8993(03)03538-8. [DOI] [PubMed] [Google Scholar]
- Jay TM, Witter MP. Distribution of hippocampal CA1 and subicular efferents in the prefrontal cortex of the rat studied by means of anterograde transport of Phaseolus vulgaris-leucoagglutinin. J Comp Neurol. 1991;313:574–586. doi: 10.1002/cne.903130404. [DOI] [PubMed] [Google Scholar]
- Jones S, Livner A, Backman L. Patterns of prospective and retrospective memory impairment in preclinical Alzheimer's disease. Neuropsychology. 2006;20:144–152. doi: 10.1037/0894-4105.20.2.144. [DOI] [PubMed] [Google Scholar]
- Kawashima H, Izaki Y, Grace AA, Takita M. Cooperativity between hippocampal-prefrontal short-term plasticity through associative long-term potentiation. Brain Res. 2006;1109:37–44. doi: 10.1016/j.brainres.2006.06.034. [DOI] [PubMed] [Google Scholar]
- Kesner RP. Retrospective and prospective coding of information: role of the medial prefrontal cortex. Exp Brain Res. 1989;74:163–167. doi: 10.1007/BF00248289. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Rogers J. An analysis of independence and interactions of brain substrates that subserve multiple attributes, memory systems, and underlying processes. Neurobiol Learn Mem. 2004;82:199–215. doi: 10.1016/j.nlm.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Kyd RJ, Bilkey DK. Prefrontal cortex lesions modify the spatial properties of hippocampal place cells. Cereb Cortex. 2003;13:444–451. doi: 10.1093/cercor/13.5.444. [DOI] [PubMed] [Google Scholar]
- Laroche S, Davis S, Jay TM. Plasticity at hippocampal to prefrontal cortex synapses: dual roles in working memory and consolidation. Hippocampus. 2000;10:438–446. doi: 10.1002/1098-1063(2000)10:4<438::AID-HIPO10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Matsuda Y, Marzo A, Otani S. The presence of background dopamine signal converts long-term synaptic depression to potentiation in rat prefrontal cortex. J Neurosci. 2006;26:4803–3810. doi: 10.1523/JNEUROSCI.5312-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald RJ, Devan BD, Hong NS. Multiple memory systems: the power of interactions. Neurobiol Learn Mem. 2004;82:333–346. doi: 10.1016/j.nlm.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Mehta MA, Manes FF, Magnolfi G, Sahakian BJ, Robbins TW. Impaired set-shifting and dissociable effects on tests of spatial working memory following the dopamine D2 receptor antagonist sulpiride in human volunteers. Psychopharmacology (Berl) 2004;176:331–342. doi: 10.1007/s00213-004-1899-2. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg AS, Olsen RK, Kohn PD, et al. Regionally specific disturbance of dorsolateral prefrontal-hippocampal functional connectivity in schizophrenia. Arch Gen Psychiatry. 2005;62:379–386. doi: 10.1001/archpsyc.62.4.379. [DOI] [PubMed] [Google Scholar]
- Mulder AB, Nordquist R, Orgut O, Pennartz CM. Plasticity of neuronal firing in deep layers of the medial prefrontal cortex in rats engaged in operant conditioning. Prog Brain Res. 2000;126:287–301. doi: 10.1016/S0079-6123(00)26020-2. [DOI] [PubMed] [Google Scholar]
- Mushiake H, Saito N, Sakamoto K, Itoyama Y, Tanji J. Activity in the lateral prefrontal cortex reflects multiple steps of future events in action plans. Neuron. 2006;50:631–641. doi: 10.1016/j.neuron.2006.03.045. [DOI] [PubMed] [Google Scholar]
- Olsen CM, Duvauchelle CL. Intra-prefrontal cortex injections of SCH 23390 influence nucleus accumbens dopamine levels 24 h post-infusion. Brain Res. 2001;922:80–86. doi: 10.1016/s0006-8993(01)03152-3. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego, CA: Academic Press; 1998. [Google Scholar]
- Peleg-Raibstein D, Pezze MA, Ferger B, et al. Activation of dopaminergic neurotransmission in the medial prefrontal cortex by N-methyl-d-aspartate stimulation of the ventral hippocampus in rats. Neuroscience. 2005;132:219–232. doi: 10.1016/j.neuroscience.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Perry RJ, Hodges JR. Spectrum of memory dysfunction in degenerative disease. Curr Opin Neurol. 1996;9:281–285. doi: 10.1097/00019052-199608000-00007. [DOI] [PubMed] [Google Scholar]
- Rainer G, Rao SC, Miller EK. Prospective coding for objects in primate prefrontal cortex. J Neurosci. 1999;19:5493–5505. doi: 10.1523/JNEUROSCI.19-13-05493.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamans JK, Floresco SB, Phillips AG. D1 receptor modulation of hippocampal-prefrontal cortical circuits integrating spatial memory with executive functions in the rat. J Neurosci. 1998;18:1613–1621. doi: 10.1523/JNEUROSCI.18-04-01613.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol. 2004;74:1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol. 1989;290:213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- Shum D, Ungvari GS, Tang WK, Leung JP. Performance of schizophrenia patients on time-, event-, and activity-based prospective memory tasks. Schizophr Bull. 2004;30:693–701. doi: 10.1093/oxfordjournals.schbul.a007123. [DOI] [PubMed] [Google Scholar]
- Siapas AG, Lubenov EV, Wilson MA. Prefrontal phase locking to hippocampal theta oscillations. Neuron. 2005;46:141–151. doi: 10.1016/j.neuron.2005.02.028. [DOI] [PubMed] [Google Scholar]
- Simons JS, Spiers HJ. Prefrontal and medial temporal lobe interactions in long-term memory. Nat Rev Neurosci. 2003;4:637–648. doi: 10.1038/nrn1178. [DOI] [PubMed] [Google Scholar]
- Smolka MN, Schumann G, Wrase J, et al. Catechol-O-methyltransferase val158met genotype affects processing of emotional stimuli in the amygdala and prefrontal cortex. J Neurosci. 2005;25:836–842. doi: 10.1523/JNEUROSCI.1792-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Swanson CJ, Heath S, Stratford TR, Kelley AE. Differential behavioral responses to dopaminergic stimulation of nucleus accumbens subregions in the rat. Pharmacol Biochem Behav. 1997;58:933–945. doi: 10.1016/s0091-3057(97)00043-9. [DOI] [PubMed] [Google Scholar]
- Takehara K, Kawahara S, Kirino Y. Time-dependent reorganization of the brain components underlying memory retention in trace eyeblink conditioning. J Neurosci. 2003;23:9897–9905. doi: 10.1523/JNEUROSCI.23-30-09897.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tost H, Meyer-Lindenberg A, Klein S, Schmitt A, Hohn F, Tenckhoff A, Ruf M, Ende G, Rietschel M, Henn FA, Braus DF. D2 antidopaminergic modulation of frontal lobe function in healthy human subjects. Biol Psychiatry. 2006;60:1196–1205. doi: 10.1016/j.biopsych.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Trivedi MA, Coover GD. Lesions of the ventral hippocampus, but not the dorsal hippocampus, impair conditioned fear expression and inhibitory avoidance on the elevated T-maze. Neurobiol Learn Mem. 2004;81:172–184. doi: 10.1016/j.nlm.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Vincent SL, Khan Y, Benes FM. Cellular distribution of dopamine D1 and D2 receptors in rat medial prefrontal cortex. J Neurosci. 1993;13:2551–2564. doi: 10.1523/JNEUROSCI.13-06-02551.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Vijayraghavan S, Goldman-Rakic PS. Selective D2 receptor actions on the functional circuitry of working memory. Science. 2004;303:853–856. doi: 10.1126/science.1091162. [DOI] [PubMed] [Google Scholar]
- Wiltgen BJ, Brown RA, Talton LE, Silva AJ. New circuits for old memories: the role of the neocortex in consolidation. Neuron. 2004;44:101–108. doi: 10.1016/j.neuron.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Winterer G, Weinberger DR. Genes, dopamine and cortical signal-to-noise ratio in schizophrenia. Trends Neurosci. 2004;27:683–690. doi: 10.1016/j.tins.2004.08.002. [DOI] [PubMed] [Google Scholar]